1. Introduction
Cyclic tetrapeptides are a class of natural products with significant potential in drug discovery, characterized by their unique structural features and diverse biological activities [
1,
2]. Their cyclic structure confers stability and contributes to their potency, while their low molecular weight and favorable lipophilicity enhance drug-like properties. According to Lipinski’s rule of five, cyclic tetrapeptides typically exhibit manageable hydrophobicity and an appropriate balance of hydrogen-bond donors and acceptors, facilitating cellular uptake and solubility [
3]. The presence of hydrogen-bond acceptors and donors contributes significantly to the drug-like properties of cyclic tetrapeptides, enabling their diverse biological activities such as potent inhibition of histone deacetylase, broad-spectrum antimicrobial activity, efficacy against dinoflagellates, and cytotoxic effects on various cell lines, etc. [
4,
5]. Cyclic tetrapeptides as medium-sized peptides, often derived from microorganism sources, contain amino acids such as leucine, isoleucine, phenylalanine, valine, and proline [
4]. Endolides A and B, from a sponge-derived fungus
Stachylidium sp. were found to be vasopressin and serotonin-receptor interacting
N-methylated peptides [
6]. Asperterrestide A, a novel cyclic tetrapeptide isolated from the marine fungus
Aspergillus terreus SCSGAF0162 showed low IC
50 values (6.2 and 6.4 μM) against two human carcinoma cell lines and also inhibited the influenza strains H1N1 (15 μM) and H3N2 (8.1 μM) [
7]. As for now, a natural cyclic tetrapeptide, romidepsin (Istodax), as an HDAC inhibitor, was approved by FDA in 2009 for the treatment of T-cell lymphoma [
8].
The chemical synthesis of cyclic tetrapeptides remains one of the most formidable challenges in peptide chemistry, primarily due to the extreme ring strain inherent in the 12/13/14-membered macrocycle [
4,
9]. This strain arises from unfavorable transannular interactions and the high entropic cost of forcing the
N- and
C-termini into close proximity, a prerequisite for efficient head-to-tail cyclization. Consequently, even seemingly simple sequences often fail to cyclize or give poor yields, especially when composed of
l-amino acids lacking turn-inducing elements [
9,
10]. Racemization at the
C-terminal residue during activation is another persistent risk, particularly when
N-methylated amino acids are involved [
11]. Despite s tactics like the use of
d-amino acids, or proline,
N-methylation, and ring-contraction strategies and recent advances in coupling reagents, microwave-assisted cyclization, and transition-metal-mediated C–H activation, have been developed to lower the activation barrier, a universal protocol remains elusive and every cyclic tetrapeptides still requires case-by-case optimization [
12,
13,
14].
During our continuous exploration of cyclic peptide molecules from marine-derived fungi [
15,
16,
17], a chemical investigation of a sponge-associated
Penicillium sp. SCSIO41035 resulted in the isolation and identification of three novel cyclic tetrapeptides (
1–
3), one novel spiro-quinazolines (
4), three cyclic dipeptide natural products (
5–
7), and three known spiro-quinazoline analogues (
8–
10), as shown in
Figure 1. Bioactivity screening results showed compounds
1–
10 exhibited varying levels of neuraminidase inhibitory activity. Herein, the details of the isolation, structural identification and biological activities of
1–
10 are described.
2. Results and Discussion
Penicopeptide B (
1) was obtained as a yellow oil and its molecular formula was established as C
36H
36N
4O
6 by the HR-ESI-MS data at
m/z: 621.2711 ([M + H]
+, calcd. for C
36H
37N
4O
6+, 621.2708). The
1H NMR data (
Table 1) displayed two overlapped methoxy signals at
δH 3.73 (6H, s, 39/40-OCH
3), two amide
N-methyl signals at
δH 2.93 (3H, s, H-38) and
δH 3.14 (3H, s, H-37), two amino proton signals at
δH 9.60 (1H, s, NH-2) and 9.26 (1H, s, NH-20), and two amino acid protons at
δH 4.28 (1H, dd,
J = 8.5, 6.1 Hz, H-11) and
δH 4.22 (1H, dd,
J = 11.0, 6.3 Hz, H-29). The
13C NMR and DEPT spectra indicated that compound
1 possessed 36 carbon signals, involving two methoxyls, two
N-methyls, two methylenes, eighteen methines and twelve quaternary carbons (
Table 2). Analyses of the 2D NMR data indicated compound
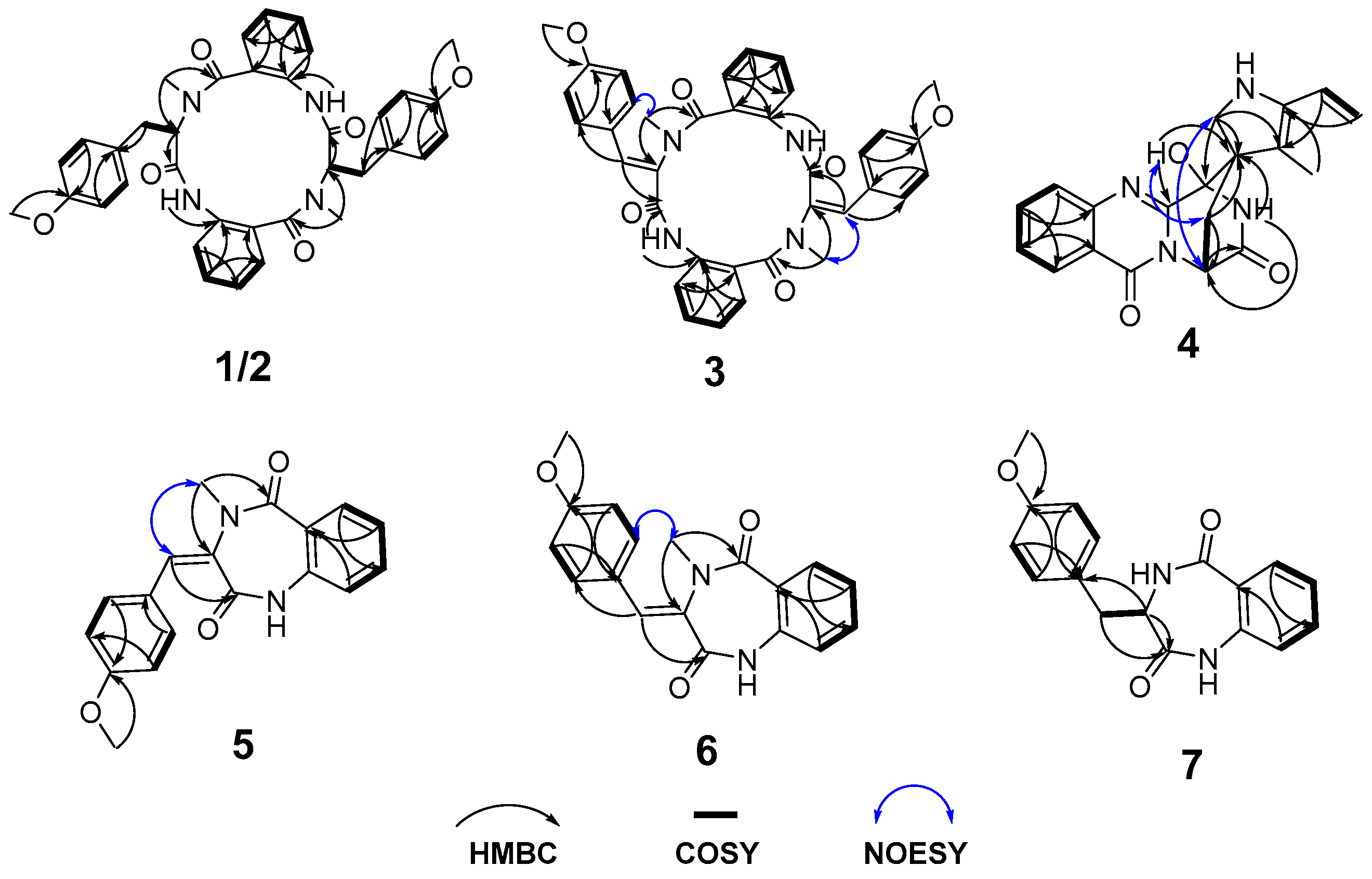
1 was a cyclic tetrapeptide skeleton compound containing two tyrosine groups and two aminobenzoic acid moities, which was confirmed by HMBC signals (
Figure 2) from NH-2 to C-1 (
δC 172.0) and C-3 (
δC 126.9), from H
3-37 to C-9 (
δC 168.5) and C-11 (
δC 56.7), from H-11 to C-19 (
δC 170.3), from NH-20 to C-19 and C-21 (
δC 127.5), from H
3-38 to C-27 (
δC 166.1) and C-29 (
δC 68.7). The cyclic tetrapeptide skeleton of
1 was identical to that of penicopeptide A by comparison of their
1H and
13C NMR data [
18]. Compared with penicopeptide A, two additional methoxy groups at C-16 (
δC 158.6) and C-36 (
δC 158.8) in compound
1 were confirmed by HMBC signals from the overlapped methoxy hydrogen signals to C-16 and C-36, respectively. Thus, the planar structure of
1 was identified as a novel cyclic tetrapeptide composed of two units, each unit being formed by the condensation of
N-methyl-7-methoxy-tyrosine with
O-aminobenzoic acid (
Figure 1).
Penicopeptide C (
2) was obtained as a yellow oil and assigned the same molecular formula of C
36H
36N
4O
6 as
1 on the basis of its HRESIMS data. Both NMR data of
1 and
2 (
Table 1 and
Table 2) were collected in the same CDCl
3 solvent and showed the same number of carbon atoms and chemical shifts in
13C NMR spectrum, but slight differences chemical shifts were observed in
1H NMR spectrum. Therefore, compound
2 was identified to possess the same cyclic tetrapeptide skeleton and might be an epimer of compound
1. To further determine the absolute configuration, 0.6 mg of compounds
1 and
2 were dissolved in 1.0 mL of 6 N HCl and heated to 110 °C for 1 h. The hydrolysis products were dissolved in methanol and their specific rotation was measured. The structure of compound
1 was determined as a mesomere, containing two
N-methyl-7-methoxy-tyrosines with opposite configurations by the specific optical rotation value of its hydrolysis product ([
α= 0.001°,
c 0.1, MeOH) [
19], while compound
2 contained only L-
N-methyl-7-methoxy-tyrosine residue as deduced by the specific optical rotation value of its hydrolysis product ([
α= −21.1°,
c 0.1, MeOH). Thus, the absolute configurations of compounds
1 and
2 were determined to be 11
R, 29
S and 11
R, 29
R, respectively.
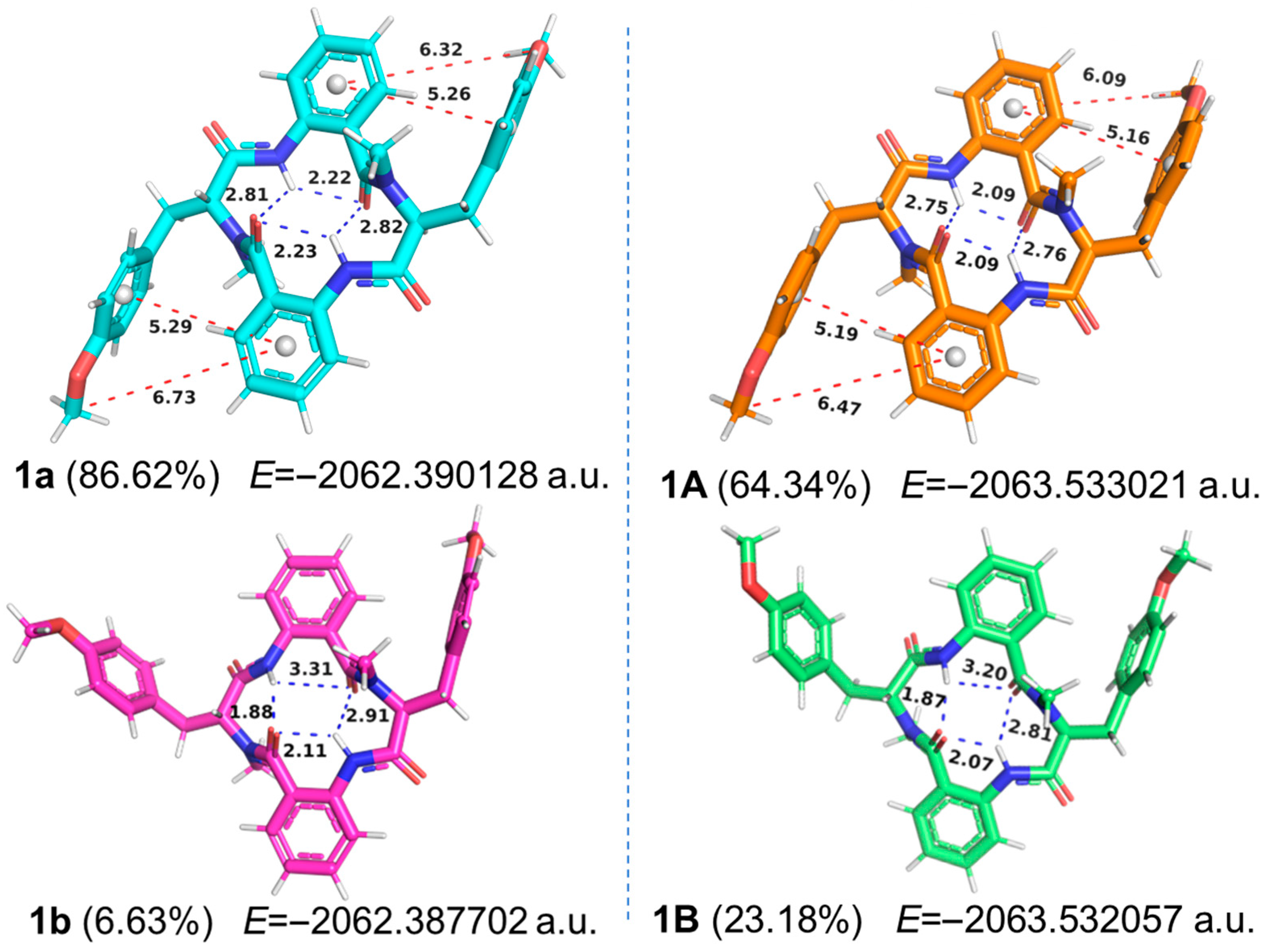
Theoretical analysis indicated that compounds
1 and
2, as structurally symmetrical cyclic tetrapeptides, should exhibit overlapping NMR signals for their equivalent positions. However, experimental results reveal distinct chemical shift patterns for the two compounds. This apparent discrepancy may arise from asymmetrical conformational preferences adopted by these macrocyclic structures in solution-phase dynamics, as supported by comprehensive conformational analysis using molecular modeling. Initially, conformational search and energy minimization were conducted for the structures of compounds
1 and
2 in a vacuum environment, and subsequently performed the same procedures in chloroform solvent model (
Supplementary Materials). The optimal conformation (
1a) of
1 in vacuum accounted for 86.62% of the Boltzmann distribution. From the three-dimensional structure and the strength of the intramolecular hydrogen bonds (2.22, 2.23, 2.81, and 2.82 Å, blue dashed lines,
Figure 3), the overall molecular framework appeared symmetrical. In contrast, within the solvation model, the proportion of the best conformation (
1A) decreased to 64.34%, even though the intramolecular hydrogen bonds 2.09, 2.09, 2.75, and 2.76 Å still suggested that the overall framework maintained a symmetrical state. The above result indicated that the conformation of the three-dimensional structure of the cyclic tetrapeptide molecule was significantly affected under the solvation model, consistent with previous reports [
4]. Additionally, through more detailed analysis, it was found that in both vacuum and solvation models, the distances (red dash lines) between adjacent phenyl rings (π-π interactions), as well as the distances (red dash lines) from the methoxy group to the center of the phenyl ring of the
o-aminobenzoic acid within
1a and
1A, were not equal, which indicated that the overall structures of
1a and
1A were in an asymmetric state.
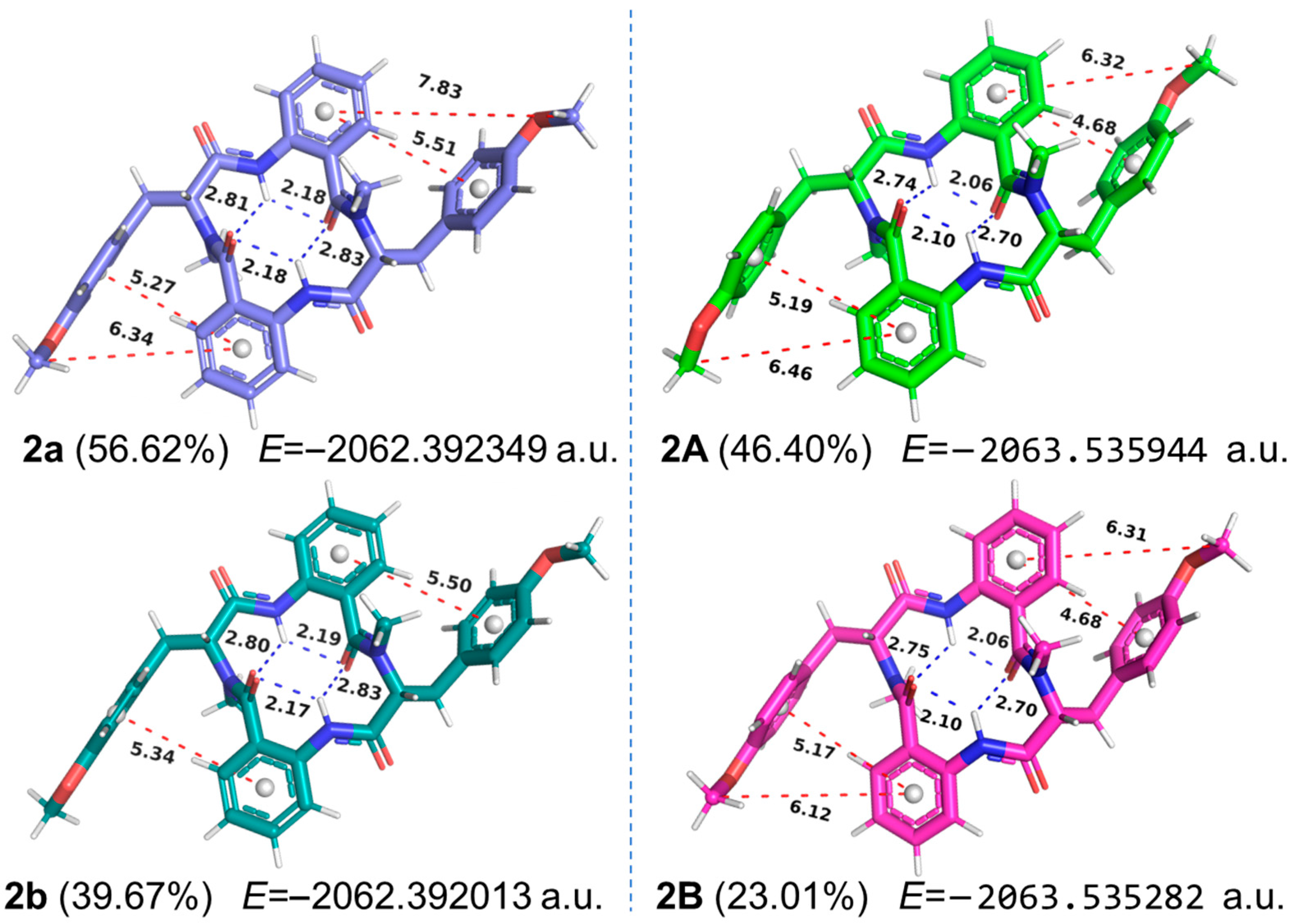
As for
2 (
Figure 4), the proportion of the first two relatively symmetrical conformations decreased from 96.29% in vacuum (
2a and
2b) to 69.41% in the solvation mode (
2A and
2B). Meanwhile, due to the differences in the strength of intramolecular hydrogen bonds and the distances between adjacent phenyl rings, compound
2 was also in an asymmetric state in the solvation model, as the substantial electron density of tyrosine phenyl side chain significantly influenced the NMR signals of nearby groups. Thus, in chloroform solution, the conformational distributions of compounds
1 and
2 were significantly influenced by solvation effects and the inherent flexibility of the tyrosine’s aromatic side chains, resulting in an asymmetric molecular structure and giving rise to distinct NMR chemical shifts. The NMR-derived asymmetric characteristics of compounds
1 and
2 have also been reported in another cyclic tetrapeptide molecule, penicopeptide A [
18], which was similarly investigated through molecular modeling, but without considering intramolecular hydrogen bonds and π-π interactions between phenyl rings.
Penicopeptide D (
3) was also obtained as a white solid and assigned the molecular formula of C
36H
32N
4O
6 on the basis of its HRESIMS data ([M + H]
+ m/
z 617.2422, calcd for C
36H
33N
4O
6+, 617.2395). The cyclic tetrapeptide nature of
3 was inferred from the high similarity of its NMR data to that of compounds
1 and
2 (
Table 1 and
Table 2). The main difference was that compound
3 possessed two additional double bonds at
δH/C 6.65 (s)/133.1 (CH-12);
δC 130.6 (C-11) and
δH/C 6.90 (s)/133.5 (CH-30);
δC 131.1 (C-29) replacing two amino acid protons, as confirmed by HMBC signals from H
3-37 (
δH 3.48, s) to C-11, from H-12 to C-19 (
δC 168.3) and C-14 (
δC 130.7), from H
3-38 (
δH 3.22, s) to C-29, and from CH-30 to C-1 (
δC 172.0) (
Figure 2). The 11
E, 29
Z configurations of
3 were determined by NOESY correlations of H-12/H-37 and H3-38/H-32 (
δH 7.32, d,
J = 8.8 Hz), respectively. Thus, compound
3 was elucidated as a novel cyclic tetrapeptide containing two units that formed by the condensation of
N-methyl-2-ene-7-methoxy-tyrosine with
o-aminobenzoic acid.
The molecular formula of compound
4, a white solid, was confirmed as C
20H
16N
4O
3 based on the HRESIMS analysis at
m/z 361.1300 ([M + H]
+, calcd. for C
20H
17N
4O
3+ 361.1295) indicating fifteen degrees of unsaturation. The 1D NMR spectra (
Table 3 and
Table 4) and
1H-
1H COSY spectrum of
4 showed the presences of two 1,2-disubstituted benzene ring systems and a -
N-CH-CH
2- sequence, indicating a tryptophan residue as those in alantrypinone (
8) [
20], a spiroquinazoline from
Penicillium thymicola. Compared to alantrypinone (
8), the methyl group was replaced by a hydroxyl group (
δH 6.31, d,
J = 3.8 Hz, 3-OH) in
4, as evidenced by the chemical shift in C-3 (
δC 70.7) and the HMBC signals from 3-OH to C-4 (
δC 153.8) and C-16 (
δC 59.3). The carbonyl group in the indole ring of
8, was reduced to a methylene group (
δC/H 40.0/2.79, dd,
J = 14.5, 1.9 Hz; 2.48, dd,
J = 14.5, 4.3 Hz, CH
2-17) of
4, as confirmed by the HMBC signals from H
2-17 to C-3 (
δC 70.7), C-16 (
δC 59.3) and C-24 (
δC 123.7) (
Figure 2). Thus, the planar structure of
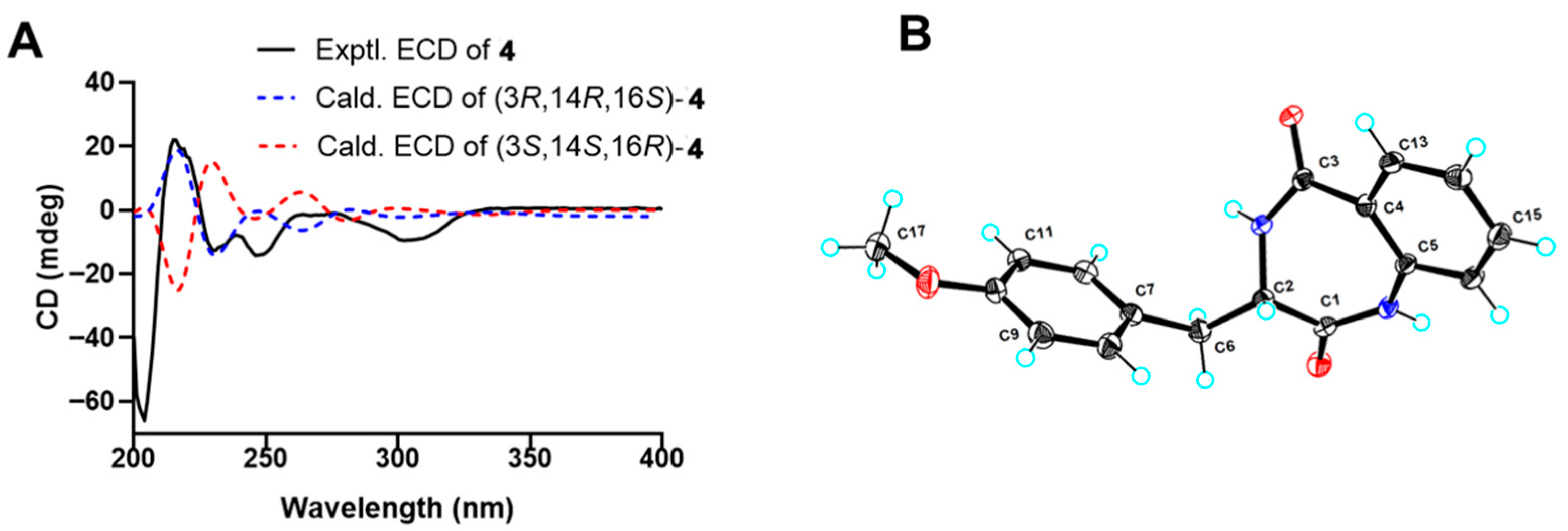
4 was determined as shown and named as penicopeptide E The relative configuration of
4 was determined as 3
R*, 14
R*, 16
S* based on the NOESY correlations between 3-OH and H
2-15 (
δH 4.07, d,
J = 12.6 Hz; 3.58, dd,
J = 12.6, 3.8 Hz), as well as between H-14 (
δH 5.40, m) and H
2-17. Two possible diastereoisomers, (3
R,14
R,16
S)-
4 and (3
S,14
S,16
R)-
4, were then subjected to time-dependent density functional theory (TDDFT) ECD calculations. The absolute configuration of
4 was determined as 3
R,14
R,16
S, as the experimental curve coincided with the calculated curve of (3
R,14
R,16
S)-
4 (
Figure 5A).
Penicopeptides F and G (
5 and
6) were obtained as white solid and assigned the same molecular formula of C
18H
16N
2O
3 based on their HRESIMS data at
m/
z 309.1233 and 309.1240 ([M + H]
+, calcd for C
18H
17N
2O
3+, 309.1234), respectively. At the same time, their almost identical NMR data (
Table 3 and
Table 4) displayed the feature of a
ortho-disubstituted and a
para-disubstituted benzene ring systems at
δC/H 120.7/6.97 (d,
J = 8.0 Hz, CH-7), 132.4/7.43 (td,
J = 7.7, 1.6 Hz, CH-8), 125.6/7.25 (overlapped, CH-9), 131.8/7.94 (dd,
J = 8.0, 1.6 Hz, CH-10), 130.8/7.34 (d,
J = 8.8 Hz, 2CH-14/18), and 114.3/6.83 (d,
J = 8.8 Hz, 2CH-15/17) for
5, while at
δC/H 120.4/6.94 (d,
J = 8.0 Hz, CH-7), 132.8/7.45 (td,
J = 8.0, 1.6 Hz, CH-8), 125.2/7.24 (t,
J = 7.6 Hz, CH-9), 131.6/8.00 (dd,
J = 8.0, 1.6 Hz, CH-10), 131.5/7.33 (d,
J = 8.8 Hz, 2CH-14/18), and 114.6/6.83 (d,
J = 8.8 Hz, 2CH-15/17) for
6. The cyclic dipeptide skeleton of
5 and
6 composed of
N-methyl-2-ene-7-methoxy-tyrosine and
o-aminobenzoic acid was determined by HMBC signals from
N-CH
3-19 to C-1 and C-3, and from the olefinic proton H-12 (
δH 6.65, s, in
5; 7.79, s, in
6) to C-4 (
Figure 1). The 3
E configuration of
5 and 3
Z configuration of
6 were confirmed by NOESY correlations between H-12 and H
3-19 (
δH 3.49, d,
J =3.2 Hz) of
5, and between H-14/18 and H3-19 (
δH 3.22, s) of
6. Compounds
5 and
6, characterized as dehydro-4-methoxycyclopeptines, have been previously reported as synthetic intermediates of cyclopeptin [
21] and as substrates for elucidating the mechanism of oxoglutarate-dependent oxygenase [
22]. This is the first report of compounds
5 and
6 being isolated as new natural products from a sponge-associated
Penicillium sp. SCSIO41035.
Compound
7 was assigned the molecular formula of C
17H
16N
2O
3 based on the HRESIMS analysis at
m/
z 297.1235 ([M + H]
+, calcd. for C
17H
17N
2O
3+, 297.1234). Analysis of the NMR data (
Table 3 and
Table 4) revealed that
7 was a cyclic dipeptide analogue closely related to
6, with the structural difference being the absences of an
N-methyl group and an olefinic moiety in
7. The amino acid proton at
δH 4.00 (1H, dt,
J = 8.4, 6.1 Hz, H-3) and a methylene signal
δC/H 33.7/3.01 (dd,
J =14.6, 8.4 Hz, H-12a); 3.35 (dd,
J =14.6, 6.1 Hz, H-12b) were observed in 1D NMR data. The 3
R configuration of
7 was confirmed by the single-crystal diffraction result (
Figure 5B, CCDC number 2453921). Thus, compound
7 was determined as a cyclic dipeptide composed of a
l-7-methoxy-tyrosine and
o-aminobenzoic acid. Compound
7, named penicopeptide H, together with its crystal structure, was first reported as a natural product from a sponge-associated
Penicillium sp. SCSIO41035. It has also been previously described as a substrate for investigating the non-enzymatic rearrangement synthesis of 4′-methoxyviridicatin [
22].
The other known compounds were identified as spiroquinazoline analogs alantrypinone (
8) [
20], serantrypinone (
9) [
23], and aurantiomide C (
10) [
24], respectively, by comparisons of NMR and MS data with literature data. The isolated compounds were evaluated for antiviral effects against HSV-1/2, dengue, and Zika viruses and antitumor effects against cancer cell lines PC-3, 22Rv1, and A549. However, all tested compounds were inactive to both viruses and cancer cell lines at concentrations of 20.0 μM. All these isolates were also tested for their enzyme inhibitory activity against pancreatic lipase, acetylcholinesterase, and neuraminidase. The results showed that under a concentration of 100 µg/mL, ten compounds only demonstrated good neuraminidase inhibitory activity, with inhibition rates ranging from 43.16% to 85.40% (
Table 5), while the inhibition rates for the other two enzymes were both below 35%. Regarding structure-activity relationship, cyclic dipeptides (63.65–85.40%) generally showed superior inhibitory activity compared to cyclic tetrapeptides (43.16–58.67%). Additionally, the presence of the double bond at C-3 further enhanced the neuraminidase activity, as demonstrated by the following comparisons: 58.67% for
3 vs 43.16% for
2 and 85.40% for
5 vs 63.65% for
6.
4. Materials and Methods
4.1. General Experimental Procedures
Optical rotations were measured in a PerkinElmer MPC 500 (Waltham, MA, USA) polarimeter at 25 °C. UV spectra were recorded in MeOH using Shimadzu UV-2600 spectrophotometer (Shimadzu, Kyoto, Japan). ECD data were acquired by the Chirascan circular dichroism spectrometer (Applied Photophysics, Leatherhead Surrey, UK). Crystallographic data were collected on an XtaLAB AFC12 (RINC): a Kappa single diffractometer using Cu Kα radiation. NMR spectra were obtained at 500 MHz using a Bruker Avance spectrometer (Bruker, Billerica, MA, USA). HRESIMS spectra were generated on a Bruker miXis TOF-QII mass spectrometer (Bruker, Billerica, MA, USA). Thin-layer chromatography (TLC) and column chromatography (CC) were performed using silica gel GF254 (10–40 µm) and silica gel (200–300 mesh) from Qingdao Marine Chemical Factory (Qingdao, China). TLC spots were visualized under 254 nm UV light. Semipreparative high-performance liquid chromatography (HPLC) was conducted using an ODS column (NanoChrom, ChromCore 120-C18, 10 × 250 mm, 5 µm).
4.2. Fungal Material
The fungal strain SCSIO41035 was isolated from an unidentified sponge species collected at Zhongsha Islands in the South China Sea. The ITS gene region (ITS1-5.8S-ITS2) of strain SCSIO41035 was amplified by PCR. DNA sequencing showed it shared a significant homology (99.24%) to the sequence of Penicillium albocoremium IBT 10682 (accession No. NR_138271.1), so it was designated as Penicillium sp. and named as Penicillium sp. SCSIO41035. The producing strain was stored on MB agar (malt extract 15 g, artificial sea salt 10 g, agar 16 g, H2O 1 L, pH 7.4−7.8) slants at 4 °C and deposited in the CAS Key Laboratory of Tropical Marine Bioresources and Ecology, South China Sea Institute of Oceanology, Chinese Academy of Sciences, Guangzhou, China.
4.3. Fermentation and Extraction
The seed culture was prepared by inoculating spores of strain SCSIO41035 into three 150 mL flasks, each containing 30 mL seed medium (malt extract: 15 g, sea salt: 2.5 g, distilled water: 1 L and pH: 7.4–7.8), and incubated at 25 °C on a rotary shaker (178 rpm) for 3 days. The seed culture was then transferred into 1 L × 36 conical flasks with solid rice medium (each flask contained 200 g rice, 3 g sea salt and 200 mL naturally sourced water), and the large-scale fermentation of the strain was carried out at 25 °C for 30 days. The total rice culture was crushed and extracted with EtOAc for five times to yield 64.3 g crude gum.
4.4. Isolation and Purification
The EtOAc extract was subjected to vacuum liquid chromatography on a silica gel column using step gradient elution with MeOH−CH2Cl2 (0−100%) to separate into six fractions based on TLC properties. Fraction 2 was divided into three parts (Frs 2-1–2-3) followed by a Sephadex LH-20 column eluted with MeOH. Fr. 2-1 was purified by semipreparative HPLC (65% MeOH−H2O, 2 mL/min) to yield 1 (4.1 mg, tR 20.5 min), 2 (3.8 mg, tR 22.6 min), and 6 (8.2 mg, tR 13.9 min). Fr. 2-2 was separated by semipreparative HPLC (75% MeOH–H2O with 0.1% TFA, 2 mL/min) to afford 3 (3.3 mg, tR 18.3 min), 9 (6.1 mg, tR 23.5 min), and 10 (5.0 mg, tR 26.2 min). Fraction 3 was divided into four parts (Frs 3-1−3-4) followed by a Sephadex LH-20 column eluted with MeOH. Fr. 3-2 was purified by semipreparative HPLC (60% MeOH−H2O, 2 mL/min) to yield 4 (3.3 mg, tR 14.5 min) and 5 (2.9 mg, tR 16.3 min). and 6 (5.2 mg, tR 13.9 min). Fraction 5 was also subjected to semi-preparative HPLC eluting with 58% CH3OH/H2O (0.1% TFA) to give 7 (3.2 mg, tR 15.5 min, 2 mL/min) and a subfraction 5-2. Compound 8 (10.5 mg, tR 18.5 min) was obtained from the subfraction 5-2 by semi-preparative HPLC eluting with 54% CH3OH/H2O (2 mL/min).
Penicopeptide B (
1): a yellow oil, [
α = −2.41 (
c 0.1, MeOH); UV (MeOH)
λmax (log
ε) 215 (3.98), 227.6 (3.90), 283 (3.85) nm; ECD (
c 0.40 mM, MeOH)
λ (
Δε) 215 (0.77), 217 (0.69), 231 (−2.44), 243 (0.18), 246 (0.17), 255 (0.67), 282 (−0.48) nm;
1H NMR (CDCl
3, 500 MHz) and
13C NMR (CDCl
3, 125 MHz) NMR data, see
Table 1 and
Table 2; HRESIMS
m/
z 621.2711 [M + H]
+ (calcd. for C
36H
37N
4O
6, 621.2708), 643.2517 [M + Na]
+ (calcd. for C
36H
36N
4NaO
6, 643.2527).
Penicopeptide C (
2): a yellow oil, [
α = −38.7 (
c 0.1, MeOH); UV (MeOH)
λmax (log
ε) 213 (3.99), 283 (3.06) nm; ECD (
c 0.48 mM, MeOH)
λ (
Δε) 221 (−2.79), 235 (−14.56), 246 (0.17), 258 (4.21), 286 (−8.55) nm;
1H NMR (CDCl
3, 500 MHz) and
13C NMR (CDCl
3, 125 MHz) NMR data, see
Table 1 and
Table 2; HRESIMS
m/
z 621.2711 [M + H]
+ (calcd. for C
36H
37N
4O
6, 621.2708), 643.2517 [M + Na]
+ (calcd. for C
36H
36N
4NaO
6, 643.2527).
Penicopeptide D (
3); a white solid, UV(MeOH)
λmax (log
ε) 205.0 (3.78), 210.0 (3.78), 281.8 (3.17) nm;
1H NMR (CDCl
3, 500 MHz) and
13C NMR (CDCl
3, 125 MHz) NMR data, see
Table 1 and
Table 2; HRESIMS
m/
z 617.2422 [M + H]
+ (calcd. for C
36H
33N
4O
6, 617.2395), 639.2233 [M + Na]
+ (calcd. for C
36H
32N
4NaO
6, 639.2214).
Penicopeptide E (
4): a white solid, [
α = 42.45 (
c 0.1, MeOH), UV (MeOH)
λmax (log
ε) 210 (3.88), 228 (3.05), 266 (3.07), 277 (3.08), 305 (2.97), 316 (2.82) nm; ECD (
c 0.45 mM, MeOH)
λ (
Δε) 215 (+21.96), 230 (−12.67), 246 (−14.20), 275 (−0.98), 305 (−9.32) nm;
1H NMR (DMSO-
d6, 500 MHz) and
13C NMR (DMSO-
d6, 125 MHz) NMR data, see
Table 3 and
Table 4; HRESIMS
m/z: 361.1300 [M + H]
+ (calcd. for C
20H
17N
4O
3: 361.1295), 383.1111 [M + Na]
+ (calcd. for C
20H
16N
4NaO
3: 383.1115), 721.2531 [2M + H]
+ (calcd. for C
40H
33N
8O
6: 721.2518), 743.2321 [2M + Na]
+ (calcd. for C
40H
33N
8NaO
6: 743.2337).
Penicopeptide F (
5): a white solid, UV(MeOH)
λmax (log
ε) 213.6 (4.50), 286.6 (4.17) nm;
1H NMR (CDCl
3, 500 MHz) and
13C NMR (CDCl
3, 125 MHz) NMR data, see
Table 3 and
Table 4; HRESIMS
m/
z 309.1240 [M + H]
+ (calcd. for C
18H
17N
2O
3, 309.1234), 331.1060 [M + Na]
+ (calcd. for C
18H
16N
2NaO
3, 331.1053).
Penicopeptide G (
6): a white solid, UV(MeOH)
λmax (log
ε) 212.6 (4.67), 294.4 (4.35) nm;
1H NMR (CDCl
3, 500 MHz) and
13C NMR (CDCl
3, 125 MHz) NMR data, see
Table 3 and
Table 4; HRESIMS
m/
z 309.1233 [M + H]
+ (calcd. for C
18H
17N
2O
3, 309.1234), 331.1050 [M + Na]
+ (calcd. for C
18H
16N
2NaO
3, 331.1053).
Penicopeptide H (
7), a light-yellow oil, [
α = 6.19 (
c 0.1, MeOH); UV(MeOH)
λmax (log
ε) 216 (3.88), 283 (2.68) nm; ECD (c 0.35 mM, MeOH)
λ (
Δε) 212 (+14.72), 231 (–16.46), 250 (+32.57), 292 (−3.57) nm;
1H NMR (CDCl
3, 500 MHz) and
13C NMR (CDCl
3, 125 MHz) NMR data, see
Table 3 and
Table 4; HRESIMS
m/
z 297.1235 [M + H]
+ (calcd. for C
17H
17N
2O
3, 297.1234), 319.1072 [M + Na]
+ (calcd. for C
17H
17N
2NaO
3, 319.1053), 593.2380 [2M + H]
+ (calcd. for C
34H
33N
4O
6, 593.2395), 615.2156 [2M + Na]
+ (calcd. for C
34H
33N
4NaO
6, 615.2214).
4.5. Crystallographic Data for Penicopeptide H (7)
Moiety formula: C17H18N2O4 (M = 314.33 g/mol), monoclinic, Crystal size = 0.13 × 0.04 × 0.04 mm3, Space group = P21; unit cell dimensions: a = 7.03720(10) Å, b = 8.6825(2) Å, c = 12.8359(3) Å, V = 754.45(3) Å3, ρcalcd = 1.384 g/cm3, Z = 2, T = 100 (10) K, μ (CuKα) = 0.822 mm−1, A total of 7956 reflections were measured (7.16° ≤ 2Θ ≤148.314°) with 2928 independent reflections (Rint = 0.0306, Rsigma = 0.0334). Final R indexes [I ≥ 2σ (I)]: R1 = 0.0303, wR2 = 0.0745. Final R indexes [all data]: R1 = 0.0334, wR2 = 0.0759. Largest diff. peak and hole = 0.18 and −0.18 eÅ−3. Goodness-of-fit on F2 = 1.063. Flack parameter = −0.04(10). Crystallographic data for structure 7 have been deposited with the Cambridge Crystallographic Data Centre as supplementary publication number CCDC 2453921. Copies of the data can be obtained, free of charge, on application to CCDC, 12 Union Road, Cambridge CB21EZ, UK [fax: +44(0)-1223-336033 or e-mail: deposit@ccdc.cam.ac.uk].
4.6. Conformational Analyses of 1 and 2
The conformational searches for compounds
1 and
2 were carried out by the Confab programs in OpenBabel software (version 3-1-1) [
25], which can systematically generate diverse low-energy conformations that are proposed to be close to crystal structures. The parameters were set as follows: RMSD cutoff = 2, Energy cutoff = 30, Conformer cutoff = 100,000. The generated conformers were subjected to preliminary optimization at the semi-empirical PM7 level using the MOPAC software (version 2016), which was invoked by the Molclus program [
26]. The conformers with a Boltzmann population of over 1% were reoptimized using density functional theory (DFT) at the B3LYP/6-31G* (GD3BJ) level under vacuum or solvent condition using the ORCA 5.0.3 program [
27].
4.7. ECD Calculation of Compound 4
The preliminary conformational distribution search was also performed using the Confab algorithm in OpenBabel software. Due to the absence of flexible side chains, only one conformation per configuration was reoptimized using density functional theory (DFT) at the B97-3c level under vacuum condition using the ORCA 5.0.3 program [
27]. Subsequently, frequency calculations were performed following geometry optimization to verify that all the structures correspond to energy minima and have no imaginary frequency. The overall theoretical calculation of ECD was conducted in MeOH using time dependent density functional theory (TD-DFT) at the PBE0/def2-TZVP level for the optimized conformers. Rotatory strengths for a total of 30 excited states were calculated. The ECD spectra of different conformers were generated using the Multiwfn program [
28] with a half-bandwidth of 0.3–0.4 eV, according to the Boltzmann calculated contribution of each conformer. The calculated ECD spectrum was corrected for offset based on the difference between the calculated and experimental UV spectra.
4.8. Neuraminidase Inhibitory Assay
The neuraminidase inhibitory activity was evaluated using a Neuraminidase In-hibitor Screening Kit (Beyotime Institute of Biotechnology, Shanghai, China). Assays were carried out in 96-well plates. For each well, 70 μL of neuraminidase assay buffer, 10 μL of neuraminidase solution, and 10 μL of test compound (dissolved in DMSO; final con-centration 100 μg/mL) were gently mixed. The reaction was initiated by adding 10 μL of the fluorogenic substrate to each well, and the plate was incubated at 37 °C for 30 min. Fluorescence was measured on an EnSpire microplate reader with excitation at 322 nm and emission at 450 nm. The fluorescence intensity of neuraminidase was measured at several concentrations to generate a standard curve relating fluorescence signal to enzyme concentration. The fluorescence corresponding to the highest neuraminidase concentration was set as 0% inhibition. Each sample well initially contained neuraminidase at this concentration, and the residual neuraminidase concentration after the reaction was determined from the measured fluorescence using the standard-curve equation. The inhibition percentages were then calculated. Oseltamivir acid and reaction buffer without inhibitor were used as positive and negative controls, respectively. Oseltamivir acid at 100 μg/mL inhibited neuraminidase activity by approximately 94%.