Targeting YY1-DR5 Axis by Pyripyropene O as a Novel Therapeutic Strategy Against Prostate Cancer: Molecular Mechanisms and In Vivo Zebrafish Validation
Abstract
1. Introduction
2. Results
2.1. Pyripyropene O Suppressed Prostate Cancer Cell Proliferation
2.2. Pyripyropene O Blocks the Cell Cycle and Induces Apoptosis in PC-3 Cells
2.3. Pyripyropene O Induces Apoptosis by Targeting the YY1/DR5 Axis
2.4. Virtual Pharmacokinetic Characterization of Pyripyropene O
2.5. In Vivo Anti-Prostate Cancer Activity of Pyripyropene O
3. Discussion
4. Materials and Methods
4.1. Compound Preparation
4.2. Reagents and Antibodies
4.3. Cell Culture
4.4. Cell Viability and Growth Analysis
4.5. Cell Migration Assay
4.6. Apoptosis and Cell Cycle Assay
4.7. Transmission Electron Microscopy
4.8. Cellular Reactive Oxygen Species Assay
4.9. Proteome Profiler Human Apoptosis Array
4.10. Molecular Docking Study
4.11. Immunofluorescence Assay
4.12. Realtime Fluorescence Quantitative PCR
4.13. Western Blot
4.14. Cellular Thermal Shift Assay (CETSA)
4.15. ADMET Analysis of Pyripyropene O
4.16. Prostate Cancer Xenograft Model in Zebrafish
4.17. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADMET | Absorption, Distribution, Metabolism, Excretion, Toxicity |
| AMdock | Assisted molecular docking |
| AR | Androgen receptor |
| BCA | Bicinchoninic acid |
| CETSA | Cellular thermal shift assay |
| CRPC | Castration-resistant prostate cancer |
| DMSO | Dimethyl sulfoxide |
| DR5 | Death receptor 5 |
| FBS | Fetal bovine serum |
| KD | Dissociation affinity constant |
| mCRPC | Metastatic castration resistant prostate cancer |
| mHSPC | Metastatic hormone-sensitive prostate cancer |
| MNP | Marine natural products |
| mRNA | Messenger RNA |
| MTT | 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide |
| PBS | Phosphate buffered saline |
| PCa | prostate cancer |
| PDB | Protein data bank |
| PMSF | Phenylmethanesulfonyl fluoride |
| PyrO | Pyripyropene O |
| PVDF | Polyvinylidene fluoride |
| RIPA | Radio immunoprecipitation assay |
| RTCA | Real time cellular analysis |
| YY1 | Yin And Yang 1 Protein |
References
- Cirulli, G.O.; Davis, M.; Stephens, A.; Chiarelli, G.; Finati, M.; Chase, M.; Tinsley, S.; Arora, S.; Sood, A.; Lughezzani, G.; et al. Midlife baseline prostate-specific antigen, velocity, and doubling time association with lethal prostate cancer and mortality. Cancer 2025, 131, e35563. [Google Scholar] [CrossRef]
- Li, C.; Zeng, X.; Qiu, S.; Gu, Y.; Zhang, Y. Nanomedicine for urologic cancers: Diagnosis and management. Semin. Cancer Biol. 2022, 86, 463–475. [Google Scholar] [CrossRef]
- Unger, J.M.; Till, C.; Tangen, C.M.; Hershman, D.L.; Goodman, P.J.; LeBlanc, M.; Barlow, W.E.; Vaidya, R.; Minasian, L.M.; Parnes, H.L.; et al. Long-Term Adverse Effects and Complications After Prostate Cancer Treatment. JAMA Oncol. 2024, 10, 1654–1662. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Wang, L.; Bernards, R. Rational combinations of targeted cancer therapies: Background, advances and challenges. Nat. Rev. Drug Discov. 2023, 22, 213–234. [Google Scholar] [CrossRef]
- Grewal, K.; Dorff, T.B.; Mukhida, S.S.; Agarwal, N.; Hahn, A.W. Advances in Targeted Therapy for Metastatic Prostate Cancer. Curr. Treat. Options Oncol. 2025, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ryan, C.J.; Smith, M.R.; de Bono, J.S.; Molina, A.; Logothetis, C.J.; de Souza, P.; Fizazi, K.; Mainwaring, P.; Piulats, J.M.; Ng, S.; et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N. Engl. J. Med. 2013, 368, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Beer, T.M.; Armstrong, A.J.; Rathkopf, D.E.; Loriot, Y.; Sternberg, C.N.; Higano, C.S.; Iversen, P.; Bhattacharya, S.; Carles, J.; Chowdhury, S.; et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N. Engl. J. Med. 2014, 371, 424–433. [Google Scholar] [CrossRef]
- de Bono, J.; Mateo, J.; Fizazi, K.; Saad, F.; Shore, N.; Sandhu, S.; Chi, K.N.; Sartor, O.; Agarwal, N.; Olmos, D.; et al. Olaparib for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2020, 382, 2091–2102. [Google Scholar] [CrossRef]
- Glaviano, A.; Foo, A.S.C.; Lam, H.Y.; Yap, K.C.H.; Jacot, W.; Jones, R.H.; Eng, H.; Nair, M.G.; Makvandi, P.; Geoerger, B.; et al. PI3K/AKT/mTOR signaling transduction pathway and targeted therapies in cancer. Mol. Cancer 2023, 22, 138. [Google Scholar] [CrossRef]
- Morris, M.J.; Castellano, D.; Herrmann, K.; de Bono, J.S.; Shore, N.D.; Chi, K.N.; Crosby, M.; Piulats, J.M.; Fléchon, A.; Wei, X.X.; et al. 177Lu-PSMA-617 versus a change of androgen receptor pathway inhibitor therapy for taxane-naive patients with progressive metastatic castration-resistant prostate cancer (PSMAfore): A phase 3, randomised, controlled trial. Lancet 2024, 404, 1227–1239. [Google Scholar] [CrossRef]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2021, 38, 362–413. [Google Scholar] [CrossRef] [PubMed]
- Montuori, E.; Hyde, C.A.C.; Crea, F.; Golding, J.; Lauritano, C. Marine Natural Products with Activities against Prostate Cancer: Recent Discoveries. Int. J. Mol. Sci. 2023, 24, 1435. [Google Scholar] [CrossRef]
- Fan, M.; Nath, A.K.; Tang, Y.; Choi, Y.J.; Debnath, T.; Choi, E.J.; Kim, E.K. Investigation of the Anti-Prostate Cancer Properties of Marine-Derived Compounds. Mar. Drugs 2018, 16, 160. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Luo, X.; Gan, X.; Chen, C.; Yang, Z.; Wen, J.; Fang, W.; Huang, H.; Gao, C.; Zhou, X.; et al. Analysis of regulating activities of 5′-epiequisetin on proliferation, apoptosis, and migration of prostate cancer cells in vitro and in vivo. Front. Pharmacol. 2022, 13, 920554. [Google Scholar] [CrossRef]
- Cai, J.; Wang, X.; Yang, Z.; Tan, Y.; Peng, B.; Liu, Y.; Zhou, X. Thiodiketopiperazines and Alkane Derivatives Produced by the Mangrove Sediment-Derived Fungus Penicillium ludwigii SCSIO 41408. Front. Microbiol. 2022, 13, 857041. [Google Scholar] [CrossRef]
- Chen, C.; Wang, X.; Fang, W.; Liang, J.; Cai, J.; Yang, D.; Luo, X.; Gao, C.; Yi, X.; Liu, Y.; et al. Talaketides A-G, linear polyketides with prostate cancer cytotoxic activity from the mangrove sediment-derived fungus Talaromyces sp. SCSIO 41027. Chin. J. Nat. Med. 2024, 22, 1047–1056. [Google Scholar] [CrossRef]
- Chen, Y.; Cai, J.; Xia, Z.; Chen, C.; Liu, Y.; Jayasinghe, L.; Wang, X.; Zhou, X. New Bioactive Polyketides from the Mangrove-Derived Fungus Penicillium sp. SCSIO 41411. Mar. Drugs 2024, 22, 384. [Google Scholar] [CrossRef]
- Gan, X.; Luo, X.; Chen, J.; Fang, W.; Nie, M.; Lu, H.; Liu, Y.; Wang, X. Ilicicolin C suppresses the progression of prostate cancer by inhibiting PI3K/AKT/mTOR pathway. Mol. Cell. Biochem. 2024, 480, 1089–1104. [Google Scholar] [CrossRef] [PubMed]
- Gan, X.; Nie, M.; Cai, S.; Liu, Y.; Zhang, F.; Feng, X.; Li, Y.; Yang, B.; Wang, X. Dankasterone A induces prostate cancer cell death by inducing oxidative stress. Eur. J. Pharmacol. 2023, 957, 175988. [Google Scholar] [CrossRef]
- Yang, B.; Shao, S.; Nie, M.; Tie, Q.; Pang, X.; Lin, X.; Zhou, X.; Liu, Y.; Wang, X.; Li, Y. Novel Metabolites from the Marine-Derived Fungus Peniophora sp. SCSIO41203 Show Promising In Vitro Antitumor Activity as Methuosis Inducers in PC-3 Cells. Mar. Drugs 2024, 22, 218. [Google Scholar] [CrossRef]
- Wang, J.; Lu, H.; Fang, W.; Lin, M.; Feng, Y.; Qi, X.; Gao, C.; Liu, Y.; Wang, X.; Luo, X. Antiproliferative effects of resorcylic acid lactones from the Beibu Gulf coral-derived fungus Curvularia lunata GXIMD 02512 on prostate cancer cells. RSC Adv. 2024, 14, 38697–38705. [Google Scholar] [CrossRef]
- Tomoda, H.; Tabata, N.; Yang, D.J.; Namatame, I.; Tanaka, H.; Omura, S.; Kaneko, T. Pyripyropenes, novel ACAT inhibitors produced by Aspergillus fumigatus. IV. Structure elucidation of pyripyropenes M to R. J. Antibiot. 1996, 49, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Hu, J.; Su, H.; Yu, Q.; Lang, Y.; Yang, M.; Fan, X.; Liu, Y.; Liu, B.; Zhao, Y.; et al. TRAIL induces podocyte PANoptosis via death receptor 5 in diabetic kidney disease. Kidney Int. 2024, 107, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Attardi, L.D. A Balancing Act: p53 Activity from Tumor Suppression to Pathology and Therapeutic Implications. Annu. Rev. Pathol. 2022, 17, 205–226. [Google Scholar] [CrossRef]
- Liu, Y.; Su, Z.; Tavana, O.; Gu, W. Understanding the complexity of p53 in a new era of tumor suppression. Cancer Cell 2024, 42, 946–967. [Google Scholar] [CrossRef] [PubMed]
- Casalini, P.; Iorio, M.V.; Berno, V.; Bergamaschi, A.; Dale, A.L.B.; Gasparini, P.; Orlandi, R.; Casati, B.; Tagliabue, E.; Ménard, S. Relationship between p53 and p27 expression following HER2 signaling. Breast 2007, 16, 597–605. [Google Scholar] [CrossRef]
- Zha, W.; Hu, W.; Ge, C.; Chen, J.; Cao, Z. Zebrafish as a model system for studying reproductive diseases. Front. Cell Dev. Biol. 2024, 12, 1481634. [Google Scholar] [CrossRef]
- Xiao, J.; Glasgow, E.; Agarwal, S. Zebrafish Xenografts for Drug Discovery and Personalized Medicine. Trends Cancer 2020, 6, 569–579. [Google Scholar] [CrossRef]
- Hu, J.; Okawa, H.; Yamamoto, K.; Oyama, K.; Mitomi, M.; Anzai, H. Characterization of two cytochrome P450 monooxygenase genes of the pyripyropene biosynthetic gene cluster from Penicillium coprobium. J. Antibiot. 2011, 64, 221–227. [Google Scholar] [CrossRef]
- Kwiatkowska, D.; Mazur, E.; Reich, A. YY1 Is a Key Player in Melanoma Immunotherapy/Targeted Treatment Resistance. Front. Oncol. 2022, 12, 856963. [Google Scholar] [CrossRef]
- Hernandez-Cueto, A.; Hernandez-Cueto, D.; Antonio-Andres, G.; Mendoza-Marin, M.; Jimenez-Gutierrez, C.; Sandoval-Mejia, A.L.; Mora-Campos, R.; Gonzalez-Bonilla, C.; Vega, M.I.; Bonavida, B.; et al. Death receptor 5 expression is inversely correlated with prostate cancer progression. Mol. Med. Rep. 2014, 10, 2279–2286. [Google Scholar] [CrossRef]
- Nengroo, M.A.; Maheshwari, S.; Singh, A.; Verma, A.; Arya, R.K.; Chaturvedi, P.; Saini, K.K.; Singh, A.K.; Sinha, A.; Meena, S.; et al. CXCR4 intracellular protein promotes drug resistance and tumorigenic potential by inversely regulating the expression of Death Receptor 5. Cell Death Dis. 2021, 12, 464. [Google Scholar] [CrossRef]
- Bonavida, B.; Garban, H. Nitric oxide-mediated sensitization of resistant tumor cells to apoptosis by chemo-immunotherapeutics. Redox Biol. 2015, 6, 486–494. [Google Scholar] [CrossRef]
- Yang, C.; Xu, H.; Yang, D.; Xie, Y.; Xiong, M.; Fan, Y.; Liu, X.; Zhang, Y.; Xiao, Y.; Chen, Y.; et al. A renal YY1-KIM1-DR5 axis regulates the progression of acute kidney injury. Nat. Commun. 2023, 14, 4261. [Google Scholar] [CrossRef]
- Bonavida, B. Rituximab-induced inhibition of antiapoptotic cell survival pathways: Implications in chemo/immunoresistance, rituximab unresponsiveness, prognostic and novel therapeutic interventions. Oncogene 2007, 26, 3629–3636. [Google Scholar] [CrossRef]
- Ishii, Y.; Kolluri, K.K.; Pennycuick, A.; Zhang, X.; Nigro, E.; Alrifai, D.; Borg, E.; Falzon, M.; Shah, K.; Kumar, N.; et al. BAP1 and YY1 regulate expression of death receptors in malignant pleural mesothelioma. J. Biol. Chem. 2021, 297, 101223. [Google Scholar] [CrossRef]
- Baritaki, S.; Huerta-Yepez, S.; Sakai, T.; Spandidos, D.A.; Bonavida, B. Chemotherapeutic drugs sensitize cancer cells to TRAIL-mediated apoptosis: Up-regulation of DR5 and inhibition of Yin Yang 1. Mol. Cancer Ther. 2007, 6, 1387–1399. [Google Scholar] [CrossRef]
- Lee, J.Y.; Huerta-Yepez, S.; Vega, M.; Baritaki, S.; Spandidos, D.A.; Bonavida, B. The NO TRAIL to YES TRAIL in cancer therapy (review). Int. J. Oncol. 2007, 31, 685–691. [Google Scholar] [CrossRef]
- Martínez-Paniagua, M.A.; Baritaki, S.; Huerta-Yepez, S.; Ortiz-Navarrete, V.F.; González-Bonilla, C.; Bonavida, B.; Vega, M.I. Mcl-1 and YY1 inhibition and induction of DR5 by the BH3-mimetic Obatoclax (GX15-070) contribute in the sensitization of B-NHL cells to TRAIL apoptosis. Cell Cycle 2011, 10, 2792–2805. [Google Scholar] [CrossRef]
- Park, A.; Lee, J.; Mun, S.; Kim, D.J.; Cha, B.H.; Moon, K.T.; Yoo, T.K.; Kang, H.G. Identification of Transcription Factor YY1 as a Regulator of a Prostate Cancer-Specific Pathway Using Proteomic Analysis. J. Cancer 2017, 8, 2303–2311. [Google Scholar] [CrossRef]
- Xu, C.; Tsai, Y.H.; Galbo, P.M.; Gong, W.; Storey, A.J.; Xu, Y.; Byrum, S.D.; Xu, L.; Whang, Y.E.; Parker, J.S.; et al. Cistrome analysis of YY1 uncovers a regulatory axis of YY1:BRD2/4-PFKP during tumorigenesis of advanced prostate cancer. Nucleic Acids Res. 2021, 49, 4971–4988. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.J.; Xu, Z.P.; Huang, X.; Xu, B.; Chen, M. Yin Yang 1 promotes the neuroendocrine differentiation of prostate cancer cells via the non-canonical WNT pathway (FYN/STAT3). Clin. Transl. Med. 2023, 13, e1422. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Lu, K.; Hou, Y.; You, Z.; Shu, C.; Wei, X.; Wu, T.; Shi, N.; Zhang, G.; Wu, J.; et al. YY1 complex in M2 macrophage promotes prostate cancer progression by upregulating IL-6. J. Immunother. Cancer 2023, 11, e006020. [Google Scholar] [CrossRef]
- Hu, J.M.; Zhang, F.; Qin, X.M.; Nong, X.; Shi, X.; Zhou, X.M.; Qin, Y.M. Oxymatrine Inhibits Liver Cancer Progression by Regulating SIRT1/YY1/GPX4 Axis-Mediated Ferroptosis. Chem. Res. Toxicol. 2025, 38, 46–57. [Google Scholar] [CrossRef]
- Zhao, J.Q.; Zhou, Q.Q.; Sun, Y.; Yu, T.; Jiang, Y.; Li, H.J. The anti-non-small cell lung cancer effect of Diosbulbin B: Targeting YY1 induced cell cycle arrest and apoptosis. Phytomedicine Int. J. Phytother. Phytopharm. 2024, 130, 155734. [Google Scholar] [CrossRef]
- Zhai, Z.; Ren, Y.; Shu, C.; Chen, D.; Liu, X.; Liang, Y.; Li, A.; Zhou, J. JAC1 targets YY1 mediated JWA/p38 MAPK signaling to inhibit proliferation and induce apoptosis in TNBC. Cell Death Discov. 2022, 8, 169. [Google Scholar] [CrossRef]
- Chow, K.H.; Liu, J.; Sun, R.W.; Vanhoutte, P.M.; Xu, A.; Chen, J.; Che, C.M.; Wang, Y. The gold (III) porphyrin complex, gold-2a, suppresses WNT1 expression in breast cancer cells by enhancing the promoter association of YY1. Am. J. Transl. Res. 2011, 3, 479–491. [Google Scholar]
- Bonavida, B.; Baritaki, S. Dual role of NO donors in the reversal of tumor cell resistance and EMT: Downregulation of the NF-κB/Snail/YY1/RKIP circuitry. Nitric Oxide Biol. Chem. 2011, 24, 1–7. [Google Scholar] [CrossRef]
- Mijatovic, S.; Maksimovic-Ivanic, D.; Mojic, M.; Malaponte, G.; Libra, M.; Cardile, V.; Miljkovic, D.; Harhaji, L.; Dabideen, D.; Cheng, K.F.; et al. Novel nitric oxide-donating compound (S,R)-3-phenyl-4,5-dihydro-5-isoxazole acetic acid-nitric oxide (GIT-27NO) induces p53 mediated apoptosis in human A375 melanoma cells. Nitric Oxide Biol. Chem. 2008, 19, 177–183. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Xu, X.; Chen, S.; Gao, F. Picroside II alleviates renal fibrosis through YY1-dependent transcriptional inhibition of TGFβ1. Metab. Open 2024, 23, 100316. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.; Zhang, Y.; Song, J.; Ma, X.; Luo, Y.; Fei, X.; Jiang, J.; Ru, Y.; Tai, Z.; Zhu, Q.; et al. Liquiritin exerts psoriasis therapy and prevention by regulating the YY1/RBP3 axis. Phytomedicine Int. J. Phytother. Phytopharm. 2024, 134, 155951. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Wang, Y.; Cao, X.; Peng, Y.; Huang, J.; Chen, L.; Pang, J.; Jiang, Z.; Qian, S.; Liu, Y.; et al. Targeting mTOR/YY1 signaling pathway by quercetin through CYP7A1-mediated cholesterol-to-bile acids conversion alleviated type 2 diabetes mellitus induced hepatic lipid accumulation. Phytomedicine Int. J. Phytother. Phytopharm. 2023, 113, 154703. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Fan, X.D.; Qu, H.; Bai, R.N.; Shi, D.Z. Berberine Protects against TNF-α-Induced Injury of Human Umbilical Vein Endothelial Cells via the AMPK/NF-κB/YY1 Signaling Pathway. Evid.-Based Complement. Altern. Med. Ecam 2021, 2021, 6518355. [Google Scholar] [CrossRef]
- Du, J.; Zhang, P.; Luo, J.; Shen, L.; Zhang, S.; Gu, H.; He, J.; Wang, L.; Zhao, X.; Gan, M.; et al. Dietary betaine prevents obesity through gut microbiota-drived microRNA-378a family. Gut Microbes 2021, 13, 1862612. [Google Scholar] [CrossRef]
- Huerta-Yepez, S.; Vega, M.; Escoto-Chavez, S.E.; Murdock, B.; Sakai, T.; Baritaki, S.; Bonavida, B. Nitric oxide sensitizes tumor cells to TRAIL-induced apoptosis via inhibition of the DR5 transcription repressor Yin Yang 1. Nitric Oxide Biol. Chem. 2009, 20, 39–52. [Google Scholar] [CrossRef]
- Gan, X.; Huang, H.; Wen, J.; Liu, K.; Yang, Y.; Li, X.; Fang, G.; Liu, Y.; Wang, X. α-Terthienyl induces prostate cancer cell death through inhibiting androgen receptor expression. Biomed. Pharmacother. Biomed. Pharmacother. 2022, 152, 113266. [Google Scholar] [CrossRef]
- Garje, R.; Riaz, I.B.; Naqvi, S.A.A.; Rumble, R.B.; Taplin, M.E.; Kungel, T.M.; Herchenhorn, D.; Zhang, T.; Beckermann, K.E.; Vapiwala, N.; et al. Systemic Therapy in Patients with Metastatic Castration-Resistant Prostate Cancer: ASCO Guideline Update. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2025, Jco2500007. [Google Scholar] [CrossRef]
- Ghandi, M.; Huang, F.W.; Jané-Valbuena, J.; Kryukov, G.V.; Lo, C.C.; McDonald, E.R., 3rd; Barretina, J.; Gelfand, E.T.; Bielski, C.M.; Li, H.; et al. Next-generation characterization of the Cancer Cell Line Encyclopedia. Nature 2019, 569, 503–508. [Google Scholar] [CrossRef]
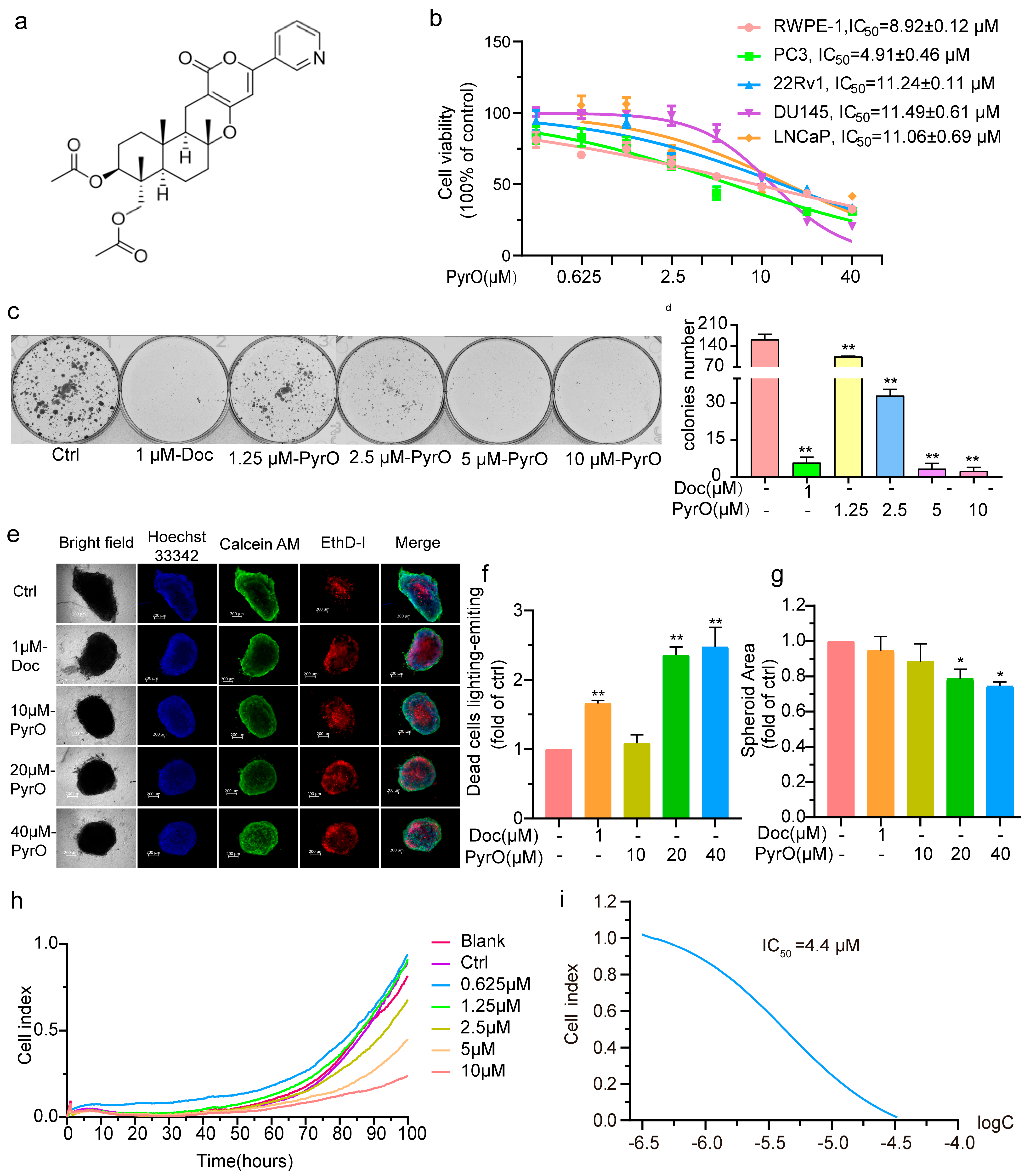

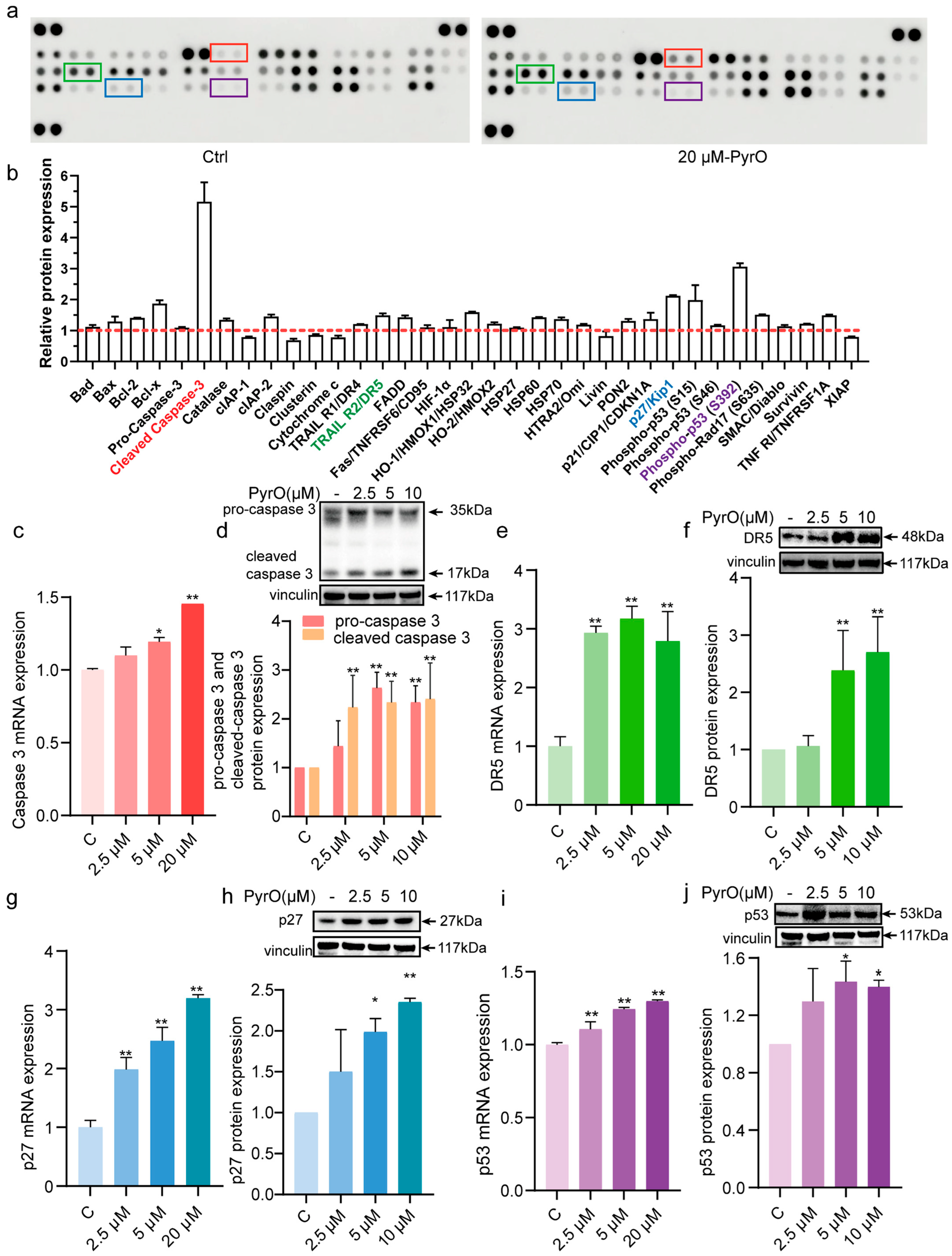
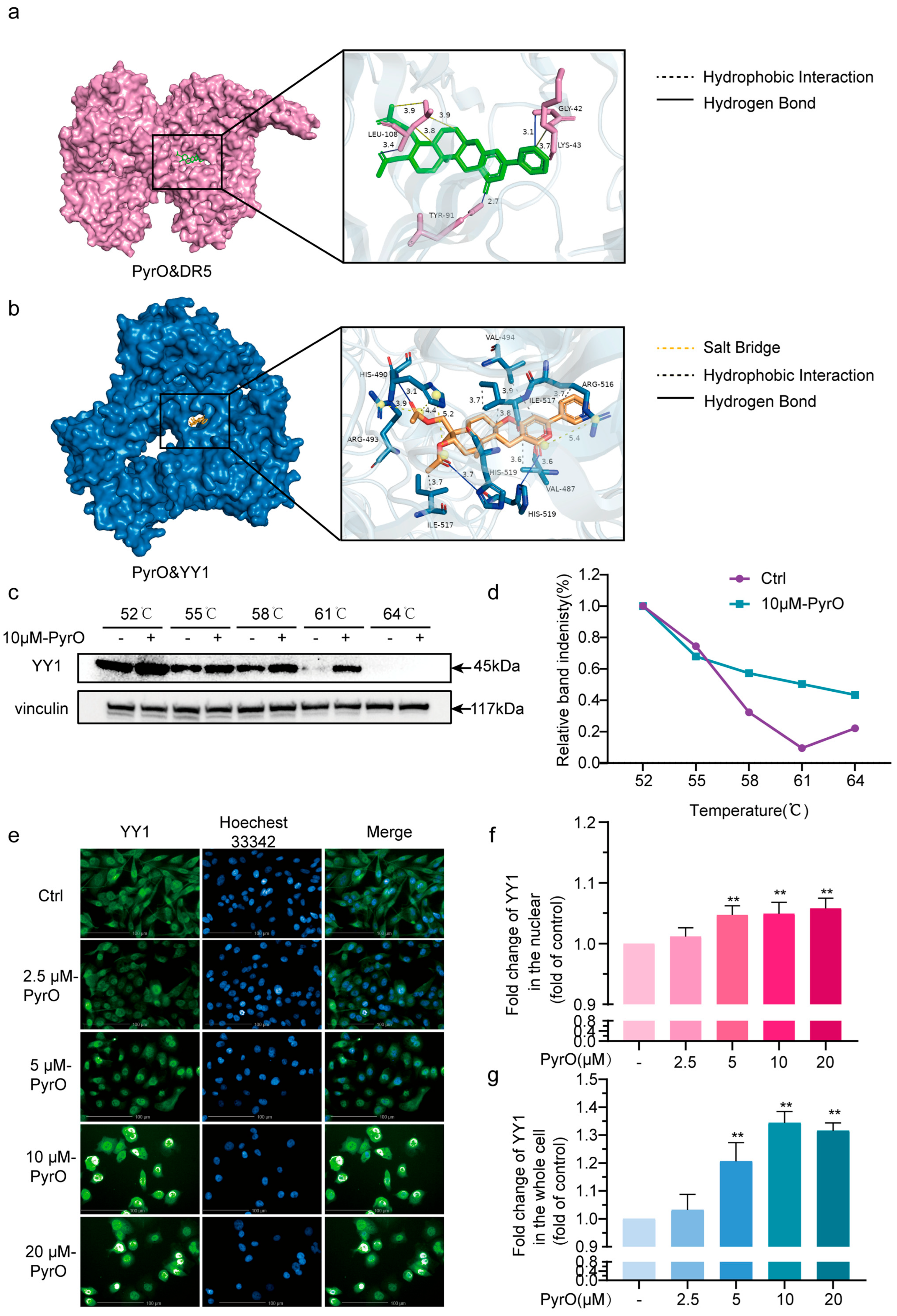
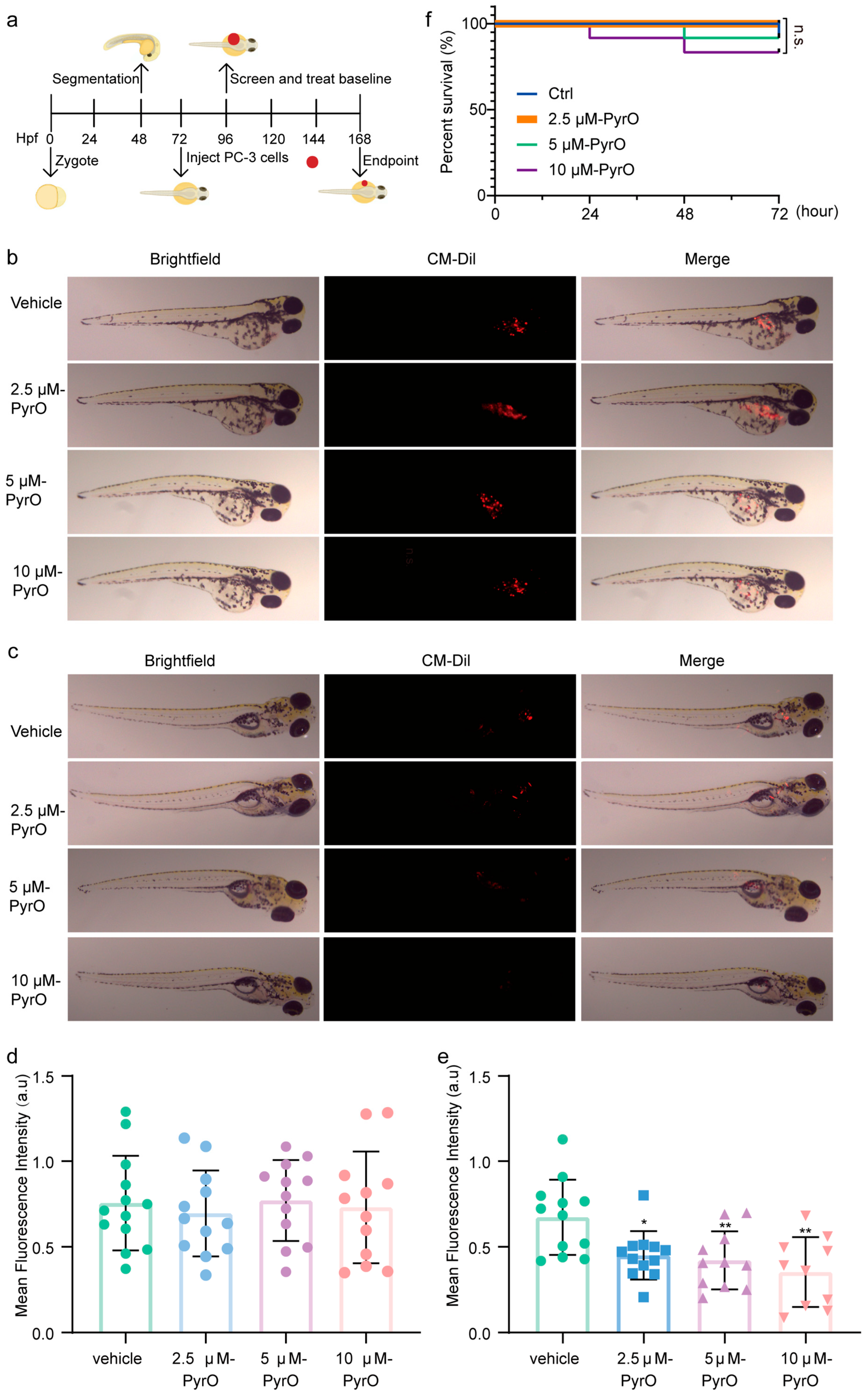
| Protein Name | PDB ID | Affinity (kcal/mol) | Estimated Ki | Ligand Efficiency |
|---|---|---|---|---|
| DR5 | 2H9G | −8.9 | 299.41 nM | −0.24 |
| YY1 | 4C5I | −11.2 | 6.17 nM | −0.3 |
| SP1 | 1VA1 | −6.9 | 8.76 μM | −0.19 |
| AP1 | 1A02 | −9.2 | 180.45 nM | −0.25 |
| TP53 | 5HOU | −7.4 | 3.77 μM | −0.20 |
| NF-κB | 1SVC | −7.5 | 3.18 μM | −0.20 |
| CEBP | 6DC0 | −8.3 | 824.26 nM | −0.22 |
| Property | Value | Decision | Comment |
|---|---|---|---|
| Caco-2 Permeability | −5.028 | ● | Optimal: higher than −5.15 Log unit |
| MDCK Permeability | 2.1 × 10−5 | ● | ■ low permeability: <2 × 10−6 cm/s ■ medium permeability: 2–20 × 10−6 cm/s ■ high passive permeability: >20 × 10−6 cm/s |
| Pgp-inhibitor | 0.996 | ● | ■ Category 1: Inhibitor; Category 0: Non inhibitor; ■ The output value is the probability of being Pgp-inhibitor |
| Pgp-substrate | 0.001 | ● | ■ Category 1: substrate; Category 0: Non-substrate; ■ The output value is the probability of being Pgp-substrate |
| HIA | 0.004 | ● | ■ Human Intestinal Absorption ■ Category 1: HIA+ (HIA < 30%); Category 0: HIA−(HIA < 30%); The output value is the probability of being HIA+ |
| F20% | 0.51 | ● | ■ 20% Bioavailability ■ Category 1: F20%+ (bioavailability < 20%); Category 0: F20−(bioavailability ≥ 20%); The output The output value is the probability of being F20% |
| F30% | 0.988 | ● | ■ 30% Bioavailability ■ Category 1: F30%+ (bioavailability < 30%); Category 0: F30%−(bioavailability ≥ 30%); The output value is the probability of being F30% |
| Property | Value | Decision | Comment |
|---|---|---|---|
| PPB | 80.78% | ● | ■ Plasma Protein Binding ■ Optimal: < 90%. Drugs with high protein-bound may have a low therapeutic index. |
| VD | 1.0 | ● | ■ Volume Distribution ■ Optimal: 0.04–20 L/kg |
| BBB Penetration | 0.176 | ● | ■ Blood-Brain Barrier Penetration ■ Category 1: BBB+; Category 0: BBB−; The output value is the probability of being BBB+ |
| Fu | 21.33% | ● | ■ The fraction unbound in plasms ■ Low: <5%; Middle: 5~20%; High: > 20% |
| Property | Value | Comment |
|---|---|---|
| CYP1A2 inhibitor | 0.205 | ■ Category 1: Inhibitor; Category 0: Non-inhibitor; ■ The output value is the probability of being inhibitor. |
| CYP1A2 substrate | 0.288 | ■ Category 1: Substrate; Category 0: Non-substrate; ■ The output value is the probability of being substrate. |
| CYP2C19 inhibitor | 0.177 | ■ Category 1: Inhibitor; Category 0: Non-inhibitor; ■ The output value is the probability of being inhibitor. |
| CYP2C19 substrate | 0.553 | ■ Category 1: Substrate; Category 0: Non-substrate; ■ The output value is the probability of being substrate. |
| CYP2C9 inhibitor | 0.326 | ■ Category 1: Inhibitor; Category 0: Non-inhibitor; ■ The output value is the probability of being inhibitor. |
| CYP2C9 substrate | 0.157 | ■ Category 1: Substrate; Category 0: Non-substrate; ■ The output value is the probability of being substrate. |
| CYP2D6 inhibitor | 0.077 | ■ Category 1: Inhibitor; Category 0: Non-inhibitor; ■ The output value is the probability of being inhibitor. |
| CYP2D6 substrate | 0.147 | ■ Category 1: Substrate; Category 0: Non-substrate; ■ The output value is the probability of being substrate. |
| CYP3A4 inhibitor | 0.81 | ■ Category 1: Inhibitor; Category 0: Non-inhibitor; ■ The output value is the probability of being inhibitor. |
| CYP3A4 substrate | 0.478 | ■ Category 1: Substrate; Category 0: Non-substrate; ■ The output value is the probability of being substrate. |
| Property | Value | Decision | Comment |
|---|---|---|---|
| CL | 2.193 | ● | ■ Clearance ■ High: >15 mL/min/kg; moderate: 5–15 mL/min/kg; low: <5 mL/min/kg |
| T1/2 | 0.278 | - | ■ Category 1: long half-life; Category 0: short half-life; ■ long half-life: >3 h; short half-life: <3 h ■ The output value is the probability of having long half-life. |
| Property | Value | Decision | Comment |
|---|---|---|---|
| hERG Blockers | 0.583 | ● | ■ Category 1: active; Category 0: inactive. ■ The output value is the probability of being active. |
| H-HT | 0.777 | ● | ■ Human Hepatotoxicity ■ Category 1: H-HT positive (+); Category 0: H-HT negative (−). ■ The output value is the probability of being toxic. |
| DILI | 0.822 | ● | ■ Drug Induced Liver Injury. ■ Category 1: drugs with a high risk of DILI; Category 0: drugs with no risk of DILI. The output value is the probability of being toxic. |
| AMESToxicity | 0.011 | ● | ■ Category 1: Ames positive (+); Category 0: Ames negative (−); ■ The output value is the probability of being toxic. |
| Rat Oral Acute Toxicity | 0.104 | ● | ■ Category 0: low-toxicity; Category 1: high-toxicity. ■ The output value is the probability of being highly toxic. |
| FDAMDD | 0.454 | ● | ■ Maximum Recommended Daily Dose ■ Category 1: FDAMDD (+); Category 0: FDAMDD (−) ■ The output value is the probability of being positive. |
| Skin Sensiti zation | 0.566 | ● | ■ Category 1: Sensitizer; Category 0: Non-sensitizer. ■ The output value is the probability of being sensitizer. |
| Carcinogen city | 0.067 | ● | ■ Category 1: carcinogens; Category 0: non-carcinogens. ■ The output value is the probability of being toxic. |
| Eye Corrosion | 0.003 | ● | ■ Category 1: corrosives; Category 0: noncorrosives ■ The output value is the probability of being corrosives. |
| Eye Irritation | 0.009 | ● | ■ Category 1: irritants; Category 0: nonirritants ■ The output value is the probability of being irritants. |
| Respiratory Toxicity | 0.929 | ● | ■ Category 1: respiratory toxicants; Category 0: respiratory nontoxicants ■ The output value is the probability of being toxic. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, W.; Chen, Y.; Nie, M.; Zhou, X.; Liu, Y.; Tao, H.; Yang, B.; Wang, X. Targeting YY1-DR5 Axis by Pyripyropene O as a Novel Therapeutic Strategy Against Prostate Cancer: Molecular Mechanisms and In Vivo Zebrafish Validation. Mar. Drugs 2025, 23, 214. https://doi.org/10.3390/md23050214
Fang W, Chen Y, Nie M, Zhou X, Liu Y, Tao H, Yang B, Wang X. Targeting YY1-DR5 Axis by Pyripyropene O as a Novel Therapeutic Strategy Against Prostate Cancer: Molecular Mechanisms and In Vivo Zebrafish Validation. Marine Drugs. 2025; 23(5):214. https://doi.org/10.3390/md23050214
Chicago/Turabian StyleFang, Wenxuan, Ying Chen, Mingyi Nie, Xuefeng Zhou, Yonghong Liu, Huaming Tao, Bin Yang, and Xueni Wang. 2025. "Targeting YY1-DR5 Axis by Pyripyropene O as a Novel Therapeutic Strategy Against Prostate Cancer: Molecular Mechanisms and In Vivo Zebrafish Validation" Marine Drugs 23, no. 5: 214. https://doi.org/10.3390/md23050214
APA StyleFang, W., Chen, Y., Nie, M., Zhou, X., Liu, Y., Tao, H., Yang, B., & Wang, X. (2025). Targeting YY1-DR5 Axis by Pyripyropene O as a Novel Therapeutic Strategy Against Prostate Cancer: Molecular Mechanisms and In Vivo Zebrafish Validation. Marine Drugs, 23(5), 214. https://doi.org/10.3390/md23050214












