Marine Delivery Vehicles: Molecular Components and Applications of Bacterial Extracellular Vesicles
Abstract
1. Introduction
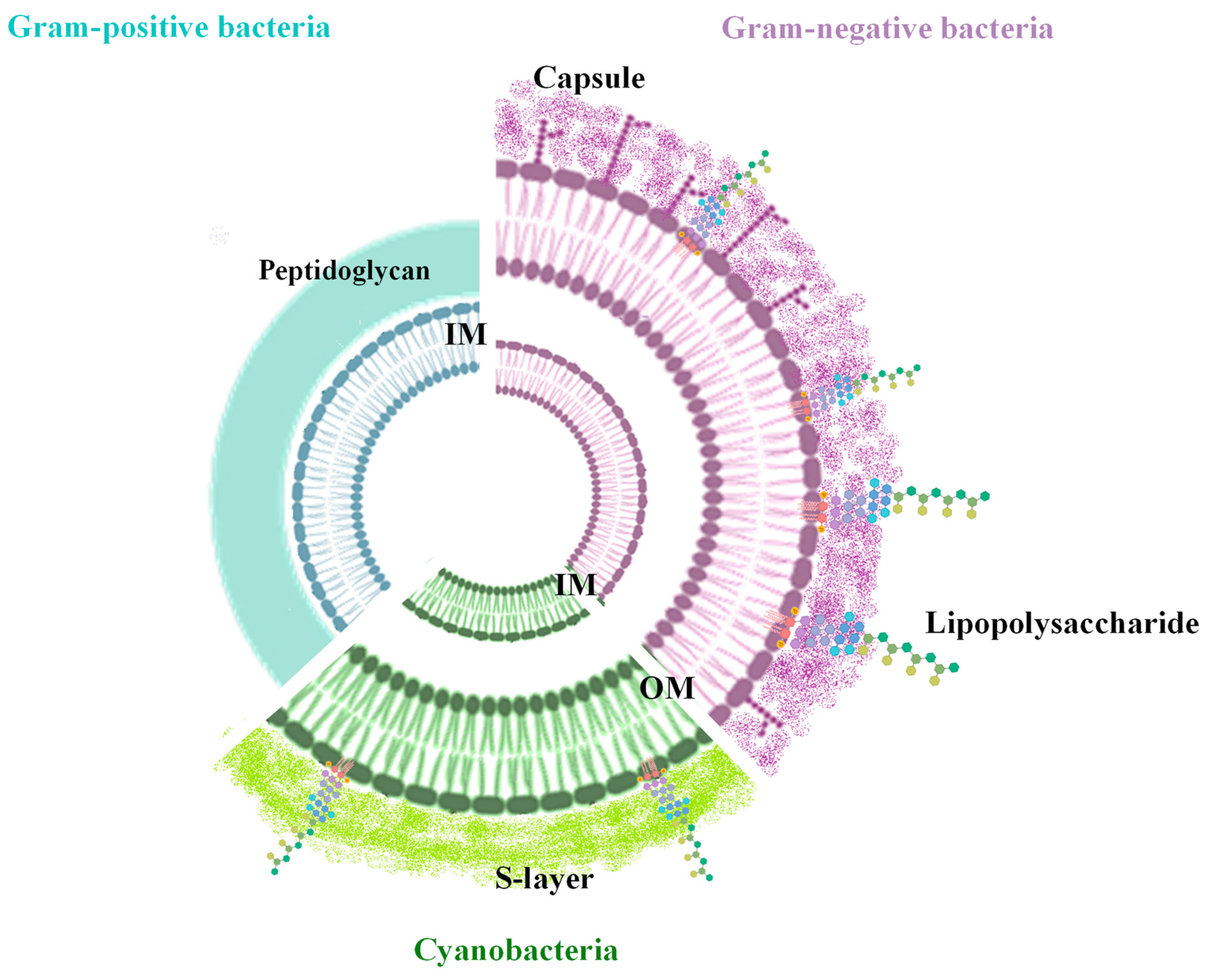
2. Biogenesis of Bacterial Vesicles
3. Size and Molecular Components of the BEVs
3.1. Proteins
3.2. Nucleic Acids
3.3. Phospholipids
3.4. Lipopolysaccharides
3.5. Capsular Polysaccharide
4. Conventional Techniques for BEV Visualization, Purification, and Characterization
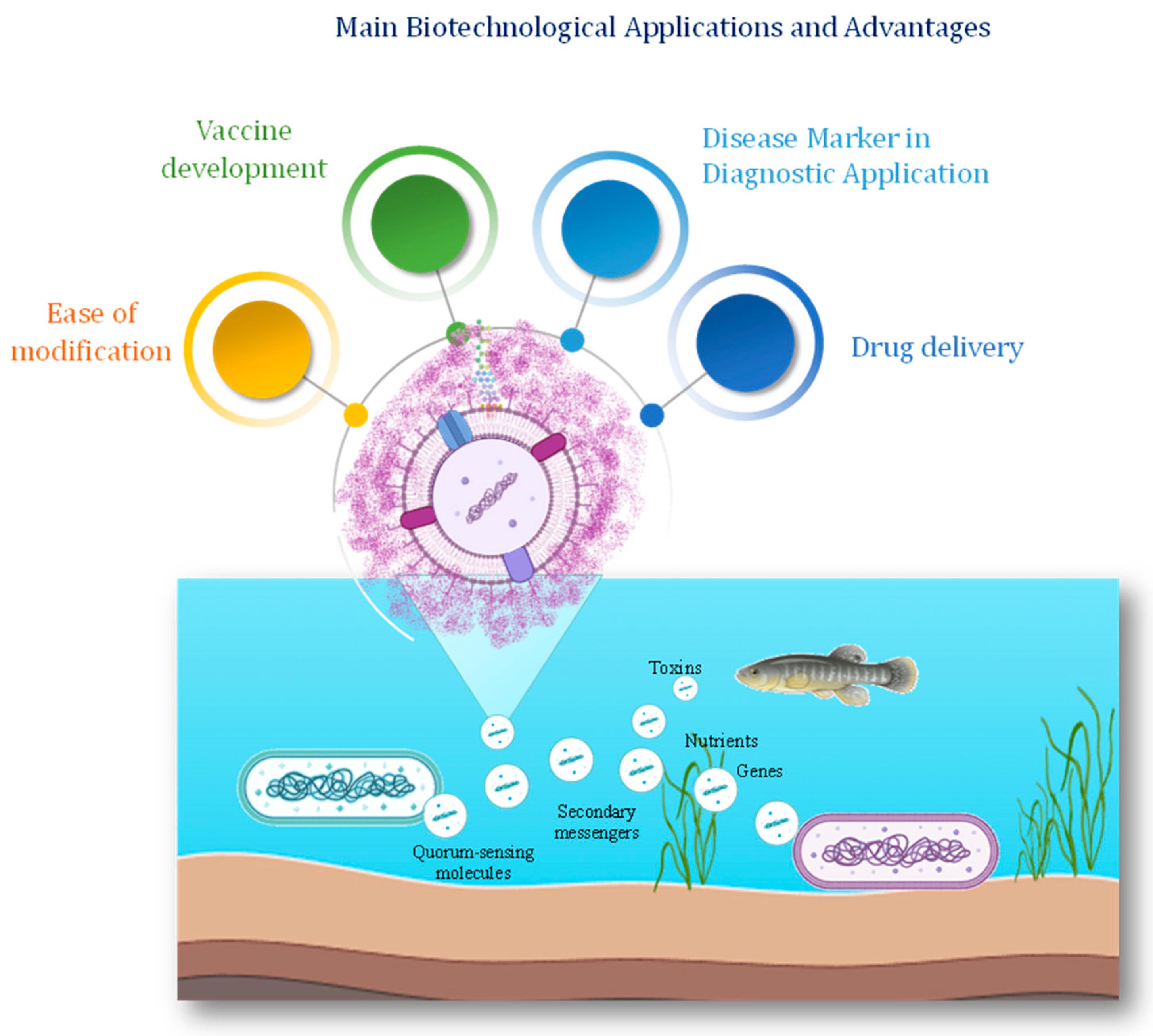
5. Functional Significance and Biotechnology Applications of Vesicles
6. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Deatherage, B.L.; Brad, T.C. Membrane vesicle release in bacteria, eukaryotes, and archaea: A conserved yet underappreciated aspect of microbial life. Infect. Immun. 2012, 80, 1948–1957. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.; Catchpole, R.; Forterre, P. Extracellular membrane vesicles in the three domains of life and beyond. FEMS Microbiol. Rev. 2019, 43, 273–303. [Google Scholar] [CrossRef] [PubMed]
- Buzas, E.I. The roles of extracellular vesicles in the immune system. Nat. Rev. Immunol. 2023, 23, 236–250. [Google Scholar] [CrossRef] [PubMed]
- Couch, Y.; Buzàs, E.I.; Di Vizio, D.; Gho, Y.S.; Harrison, P.; Hill, A.F.; Lötvall, J.; Raposo, G.; Stahl, P.D.; Théry, C.; et al. A brief history of nearly EV-erything–The rise and rise of extracellular vesicles. J. Extracell. Vesicles 2021, 10, e12144. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Zhang, Q.; Li, W.; Yuan, M.; Zhou, J.; Hua, L.; Chen, Y.; Ye, C.; Ma, Y. Development of novel nanoantibiotics using an outer membrane vesicle-based drug efflux mechanism. J. Control. Release 2020, 317, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Masaharu, S.; Yoshioka, Y.; Ochiya, T. Drug delivery application of extracellular vesicles; insight into production, drug loading, targeting, and pharmacokinetics. AIMS Bioeng. 2017, 4, 73–92. [Google Scholar]
- Chen, Q.; Bai, H.; Wu, W.; Huang, G.; Li, Y.; Wu, M.; Tang, G.; Ping, Y. Bioengineering bacterial vesicle-coated polymeric nanomedicine for enhanced cancer immunotherapy and metastasis prevention. Nano Lett. 2019, 20, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Bar-On, Y.M.; Milo, R. The biomass composition of the oceans: A blueprint of our blue planet. Cell 2019, 179, 1451–1454. [Google Scholar] [CrossRef]
- Toyofuku, M.; Nomura, N.; Eberl, L. Types and origins of bacterial membrane vesicles. Nat. Rev. Microbiol. 2019, 17, 13–24. [Google Scholar] [CrossRef]
- Zlatkov, N.; Nadeem, A.; Uhlin, B.E.; Wai, S.N. Eco-evolutionary feedbacks mediated by bacterial membrane vesicles. FEMS Microbiol. Rev. 2021, 45, fuaa047. [Google Scholar] [CrossRef]
- Biller, S.J.; Lundeen, R.A.; Hmelo, L.R.; Becker, K.W.; Arellano, A.A.; Dooley, K.; Chisholm, S.W. Prochlorococcus extracellular vesicles: Molecular composition and adsorption to diverse microbes. Environ. Microbiol. 2022, 24, 420–435. [Google Scholar] [CrossRef] [PubMed]
- Kadurugamuwa, J.L.; Beveridge, T.J. Virulence factors are released from Pseudomonas aeruginosa in association with membrane vesicles during normal growth and exposure to gentamicin: A novel mechanism of enzyme secretion. J. Bacteriol. 1995, 177, 3998–4008. [Google Scholar] [CrossRef]
- Biller, S.J.; Schubotz, F.; Roggensack, S.E.; Thompson, A.W.; Summons, R.E.; Chisholm, S.W. Bacterial vesicles in marine ecosystems. Science 2014, 343, 183–186. [Google Scholar] [CrossRef]
- Puca, V.; Marinacci, B.; Pellegrini, B.; Campanile, F.; Santagati, M.; Grande, R. Biofilm and bacterial membrane vesicles: Recent advances. Expert Opin. Ther. Pat. 2024, 34, 475–491. [Google Scholar] [CrossRef]
- Manning, A.J.; Kuehn, M.J. Contribution of bacterial outer membrane vesicles to innate bacterial defense. BMC Microbiol. 2011, 11, 258. [Google Scholar] [CrossRef]
- Pérez-Cruz, C.; Delgado, L.; López-Iglesias, C.; Mercade, E. Outer-inner membrane vesicles naturally secreted by gram-negative pathogenic bacteria. PLoS ONE 2015, 10, e0116896. [Google Scholar] [CrossRef]
- Li, J.; Liu, K.; Chen, H.; Li, R.; Drechsler, M.; Bai, F.; Yan, Y. Functional built-in template directed siliceous fluorescent supramolecular vesicles as diagnostics. ACS Appl. Mater. Interfaces 2017, 9, 21706–21714. [Google Scholar] [CrossRef] [PubMed]
- Meng, R.; Zeng, M.; Ji, Y.; Huang, X.; Xu, M. The potential role of gut microbiota outer membrane vesicles in colorectal cancer. Front. Microbiol. 2023, 14, 1270158. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi Badi, S.; Moshiri, A.; Fateh, A.; Rahimi Jamnani, F.; Sarshar, M.; Vaziri, F.; Siadat, S.D. Microbiota-derived extracellular vesicles as new systemic regulators. Front. Microbiol. 2017, 8, 279325. [Google Scholar] [CrossRef]
- Arntzen, M.Ø.; Várnai, A.; Mackie, R.I.; Eijsink, V.G.; Pope, P.B. Outer membrane vesicles from Fibrobacter succinogenes S85 contain an array of carbohydrate-active enzymes with versatile polysaccharide-degrading capacity. Environ. Microbiol. 2017, 19, 2701–2714. [Google Scholar] [CrossRef]
- Abe, K.; Nomura, N.; Suzuki, S. Biofilms: Hot spots of horizontal gene transfer (HGT) in aquatic environments, with a focus on a new HGT mechanism. FEMS Microbiol. Ecol. 2020, 96, fiaa031. [Google Scholar] [CrossRef] [PubMed]
- Schatz, D.; Vardi, A. Extracellular vesicles—New players in cell–cell communication in aquatic environments. Curr. Opin. Microbiol. 2018, 43, 148–154. [Google Scholar] [CrossRef]
- Janke, U.; Mitlehner, A.; Weide, A.; Gutmann, T.; Delcea, M. Reconstitution of Functional Integrin αIIbβ3 and Its Activation in Plasma Membrane-Mimetic Lipid Environments. Membranes 2021, 11, 499. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, W.; Wang, X.; Hu, X.; Peng, L.; Yang, J.L.; Liang, X. Mussel settlement mediated by bacterial VgrG proteins via extracellular outer membrane vesicles. Int. Biodeterior. Biodegrad. 2023, 180, 105595. [Google Scholar] [CrossRef]
- Linney, M.D.; Eppley, J.M.; Romano, A.E.; Luo, E.; DeLong, E.F.; Karl, D.M. Microbial sources of exocellular DNA in the ocean. Appl. Environ. Microbiol. 2022, 88, e02093-21. [Google Scholar] [CrossRef]
- Lynch, J.B.; Alegado, R.A. Spheres of hope, packets of doom: The good and bad of outer membrane vesicles in interspecies and ecological dynamics. J. Bacteriol. 2017, 199, e00012-17. [Google Scholar] [CrossRef] [PubMed]
- Azam, F.; Malfatti, F. Microbial structuring of marine ecosystems. Nat. Rev. Microbiol. 2007, 5, 782–791. [Google Scholar] [CrossRef]
- Toyofuku, M.; Schild, S.; Kaparakis-Liaskos, M.; Eberl, L. Composition and functions of bacterial membrane vesicles. Nat. Rev. Microbiol. 2023, 21, 415–430. [Google Scholar] [CrossRef]
- Zarantonello, V.; Silva, T.P.; Noyma, N.P.; Gamalier, J.P.; Mello, M.M.; Marinho, M.M.; Melo, R.C. The cyanobacterium Cylindrospermopsis raciborskii (CYRF-01) responds to environmental stresses with increased vesiculation detected at single-cell resolution. Front. Microbiol. 2018, 9, 327786. [Google Scholar] [CrossRef]
- Beveridge, T.J. Structures of gram-negative cell walls and their derived membrane vesicles. J. Bacteriol. 1999, 181, 4725–4733. [Google Scholar] [CrossRef]
- Schwechheimer, C.; Kuehn, M.J. Outer-membrane vesicles from Gram-negative bacteria: Biogenesis and functions. Nat. Rev. Microbiol. 2015, 13, 605–619. [Google Scholar] [CrossRef]
- Turnbull, L.; Toyofuku, M.; Hynen, A.L.; Kurosawa, M.; Pessi, G.; Petty, N.K.; Osvath, S.R.; Cárcamo-Oyarce, G.; Gloag, E.S.; Shimoni, R.; et al. Explosive cell lysis as a mechanism for the biogenesis of bacterial membrane vesicles and biofilms. Nat. Commun. 2016, 7, 11220. [Google Scholar] [CrossRef]
- Juodeikis, R.; Carding, S.R. Outer membrane vesicles: Biogenesis, functions, and issues. Microbiol. Mol. Biol. Rev. 2022, 86, e00032-22. [Google Scholar] [CrossRef]
- Roier, S.; Zingl, F.G.; Cakar, F.; Schild, S. Bacterial outer membrane vesicle biogenesis: A new mechanism and its implications. Microb. Cell 2016, 3, 257. [Google Scholar] [CrossRef]
- Briaud, P.; Carroll, R.K. Extracellular vesicle biogenesis and functions in gram-positive bacteria. Infect. Immun. 2020, 88, e00433-20. [Google Scholar] [CrossRef]
- Du, R.; Wang, C.; Zhu, L.; Yang, Y. Extracellular Vesicles as Delivery Vehicles for Therapeutic Nucleic Acids in Cancer Gene Therapy: Progress and Challenges. Pharmaceutics 2022, 14, 2236. [Google Scholar] [CrossRef]
- Biller, S.J.; McDaniel, L.D.; Breitbart, M.; Rogers, E.; Paul, J.H.; Chisholm, S.W. Membrane vesicles in sea water: Heterogeneous DNA content and implications for viral abundance estimates. ISME J. 2017, 11, 394–404. [Google Scholar] [CrossRef]
- Chen, Y.; Vojtech, L.N.; Hong, J.; Norris, M.H. Infection-Induced pH and Voltage Changes Contribute to Vesicle Content Mobilization in Pseudomonas aeruginosa. mBio 2021, 12, e03329-20. [Google Scholar]
- Zhang, W.; Liu, H.; Tian, L. Extracellular Vesicles in Marine Microbial Ecology: Diversity, Functions, and Applications. Front. Microbiol. 2021, 12, 663543. [Google Scholar]
- Kulk, G.; MacIntyre, H.L.; Hickman, A.E.; Lewis, M.R.; Bouman, H.A.; Beardall, J. The role of trade-offs in the evolution of phytoplankton cell size and elemental stoichiometry. Am. Nat. 2020, 195, 315–329. [Google Scholar]
- Schooling, S.R.; Beveridge, T.J. Membrane Vesicles: An Overlooked Component of the Matrices of Biofilms. J. Bacteriol. 2006, 188, 5945–5957. [Google Scholar] [CrossRef]
- Gasperini, G.; Biagini, M.; Arato, V.; Gianfaldoni, C.; Vadi, A.; Norais, N.; Bensi, G.; Delany, I.; Pizza, M.; Aricò, B.; et al. Outer membrane vesicles (OMV)-based and proteomics-driven antigen selection identifies novel factors contributing to Bordetella pertussis adhesion to epithelial cells. Mol. Cell. Proteom. 2018, 17, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Socorro Ruiz-Palma, M.D.; Avila-Calderón, E.D.; Aguilera-Arreola, M.G.; López-Merino, A.; Ruiz, E.A.; Morales-García, M.D.R.; López-Villegas, E.O.; Gomez-Lunar, Z.; Arellano-Reynoso, B.; Contreras-Rodríguez, A. Comparative proteomic analysis of outer membrane vesicles from Brucella suis, Brucella ovis, Brucella canis and Brucella neotomae. Arch. Microbiol. 2021, 203, 1611–1626. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Ma, B.; Xu, M.; Xu, H.; Yan, Z.; Wang, F.; Wang, Y.; Huang, Z.; Yin, S.; Zhao, Y.; et al. Comparative proteomics study of exosomes in Vibrio harveyi and Vibrio anguillarum. Microb. Pathog. 2023, 181, 106174. [Google Scholar] [CrossRef] [PubMed]
- McMillan, H.M.; Kuehn, M.J. Proteomic profiling reveals distinct bacterial extracellular vesicle subpopulations with possibly unique functionality. Appl. Environ. Microbiol. 2023, 89, e01686-22. [Google Scholar] [CrossRef] [PubMed]
- Naval, P.; Chandra, T.S. Characterization of membrane vesicles secreted by seaweed associated bacterium Alteromonas macleodii KS62. Biochem. Biophys. Res. Commun. 2019, 514, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Elhenawy, W.; Debelyy, M.O.; Feldman, M.F. Preferential packing of acidic glycosidases and proteases into Bacteroides outer membrane vesicles. mBio 2014, 5, e00909-14. [Google Scholar] [CrossRef] [PubMed]
- Fadeev, E.; Carpaneto Bastos, C.; Hennenfeind, J.H.; Biller, S.J.; Sher, D.; Wietz, M.; Herndl, G.J. Characterization of membrane vesicles in Alteromonas macleodii indicates potential roles in their copiotrophic lifestyle. Microlife 2023, 4, uqac025. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.H.; Lee, S.Y.; Choi, C.W.; Lee, H.; Ro, H.J.; Jun, S.; Kwon, Y.M.; Kwon, K.K.; Kim, S.-J.; Kim, G.-H.; et al. Proteomic characterization of the outer membrane vesicle of the halophilic marine bacterium Novosphingobium pentaromativorans US6-1. J. Microbiol. 2017, 55, 56–62. [Google Scholar] [CrossRef]
- Provenzano, D.; Klose, K.E. Altered expression of the ToxR-regulated porins OmpU and OmpT diminishes Vibrio cholerae bile resistance, virulence factor expression, and intestinal colonization. Proc. Nat. Acad. Sci. USA 2000, 97, 10220–10224. [Google Scholar] [CrossRef]
- Langlete, P.; Krabberød, A.K.; Winther-Larsen, H.C. Vesicles from Vibrio cholerae contain AT-rich DNA and shorter mRNAs that do not correlate with their protein products. Front. Microbiol. 2019, 10, 2708. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Guo, A.; Huang, S.; Azam, F.; Sun, X.; Zhang, J.; Long, L.; Zhang, S. Outer membrane vesicles produced by coral-associated Vibrio coralliilyticus inhibit bacteriophage infection and its ecological implications. Microbiol. Res. 2024, 281, 127607. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, H.M.; Swamy, C.V.; Jagannadham, M.V. Molecular characterization and functional analysis of outer membrane vesicles from the antarctic bacterium Pseudomonas syringae suggest a possible response to environmental conditions. J. Prot. Res. 2014, 13, 1345–1358. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, F.; Kawamoto, J.; Imai, T.; Kurihara, T. Characterization of extracellular membrane vesicles of an Antarctic bacterium, Shewanella livingstonensis Ac10, and their enhanced production by alteration of phospholipid composition. Extremophiles 2017, 21, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Bozal, N.; Montes, M.J.; Minana-Galbis, D.; Manresa, A.; Mercade, E. Shewanella vesiculosa sp. nov., a psychrotolerant bacterium isolated from an Antarctic coastal area. Int. J. Syst. Evol. Microbiol. 2009, 59, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Cruz, C.; Carrión, O.; Delgado, L.; Martinez, G.; López-Iglesias, C.; Mercade, E. New type of outer membrane vesicle produced by the Gram-negative bacterium Shewanella vesiculosa M7T: Implications for DNA content. Appl. Environ. Microbiol. 2013, 79, 1874–1881. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Kawamoto, J.; Kawai, S.; Tame, A.; Kato, C.; Imai, T.; Kurihara, T. Isolation of a novel bacterial strain capable of producing abundant extracellular membrane vesicles carrying a single major cargo protein and analysis of its transport mechanism. Front. Microbiol. 2020, 10, 3001. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, F.; Imai, T.; Aoki, W.; Ueda, M.; Kawamoto, J.; Kurihara, T. Identification of a putative sensor protein involved in regulation of vesicle production by a hypervesiculating bacterium, Shewanella vesiculosa HM13. Front. Microbiol. 2021, 12, 629023. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.D.; Smith, J.K.; Brown, S.A.; Johnson, L.M. Mechanisms of nucleic acid incorporation into vesicles produced by marine bacteria and cyanobacteria. Mar. Mol. Biol. Rev. 2019, 10, 205–220. [Google Scholar]
- Liu, Q.; Zhao, X.; Liu, Q.; Xu, L.; Yan, Q.; Han, Y.; Li, Y. The Diversity of Resistome in the Microbial Community in the Colored Snow from the Fildes Peninsula, King George Island, Maritime Antarctica. Front. Microbiol. 2018, 9, 2846. [Google Scholar]
- Kim, Y.S.; Lee, J.H.; Kim, S.W.; Kim, S. Exosomal microRNAs as potential biomarkers in diagnosis and prognosis of colorectal cancer. World J. Gastroenterol. 2018, 23, 3117–3126. [Google Scholar]
- Sartorio, M.G.; Valguarnera, E.; Hsu, F.F.; Feldman, M.F. Lipidomics analysis of outer membrane vesicles and elucidation of the inositol phosphoceramide biosynthetic pathway in Bacteroides thetaiotaomicron. Microbiol. Spectr. 2022, 10, e00634-21. [Google Scholar] [CrossRef] [PubMed]
- Corsaro, M.M.; Lanzetta, R.; Parrilli, E.; Parrilli, M.; Tutino, M.L.; Ummarino, S. Influence of growth temperature on lipid and phosphate contents of surface polysaccharides from the Antarctic bacterium Pseudoalteromonas haloplanktis TAC 125. J. Bacteriol. 2004, 186, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Nevot, M.; Deroncelé, V.; Messner, P.; Guinea, J.; Mercadé, E. Characterization of outer membrane vesicles released by the psychrotolerant bacterium Pseudoalteromonas antarctica NF3. Environ. Microbiol. 2006, 8, 1523–1533. [Google Scholar] [CrossRef] [PubMed]
- Zingl, F.G.; Kohl, P.; Cakar, F.; Leitner, D.R.; Mitterer, F.; Bonnington, K.E.; Rechberger, G.N.; Kuehn, M.J.; Guan, Z.; Reidl, J.; et al. Outer membrane vesiculation facilitates surface exchange and in vivo adaptation of Vibrio cholerae. Cell Host Microbe 2020, 27, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Baeza, N.; Delgado, L.; Comas, J.; Mercade, E. Phage-mediated explosive cell lysis induces the formation of a different type of O-IMV in Shewanella vesiculosa M7T. Front. Microbiol. 2021, 12, 713669. [Google Scholar] [CrossRef]
- Ganin, H.; Danin-Poleg, Y.; Kashi, Y.; Meijler, M.M. Vibrio cholerae autoinducer CAI-1 interferes with Pseudomonas aeruginosa quorum sensing and inhibits its growth. ACS Chem. Biol. 2012, 7, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Brameyer, S.; Plener, L.; Müller, A.; Klingl, A.; Wanner, G.; Jung, K. Outer membrane vesicles facilitate trafficking of the hydrophobic signaling molecule CAI-1 between Vibrio harveyi cells. J. Bacteriol. 2018, 200, e00740-17. [Google Scholar] [CrossRef] [PubMed]
- Lüderitz, O.; Freudenberg, M.A.; Galanos, C.; Lehmann, V.; Rietschel, E.T.; Shaw, D.H. Lipopolysaccharides of gram-negative bacteria. In Current Topics in Membranes and Transport; Academic Press: Cambridge, MA, USA, 1982; Volume 17, pp. 79–151. [Google Scholar]
- Raetz, C.R.H.; Whitfield, C. Lipopolysaccharide Endotoxins. Annu. Rev. Biochem. 2002, 71, 635–700. [Google Scholar] [CrossRef]
- Simpson, B.W.; Trent, M.S. Pushing the envelope: LPS modifications and their consequences. Nat. Rev. Microbiol. 2019, 17, 403–416. [Google Scholar] [CrossRef]
- Park, B.S.; Song, D.H.; Kim, H.M.; Choi, B.S.; Lee, H.; Lee, J.O. The structural basis of lipopolysaccharide recognition by the TLR4–MD-2 complex. Nature 2009, 458, 1191–1195. [Google Scholar] [CrossRef] [PubMed]
- Holst, O.; Brade, H. Chemical structure of the core region of lipopolysaccharides. In Bacterial Endotoxic Lipopolysaccharides; CRC Press: Boca Raton, FL, USA, 1992; pp. 171–205. [Google Scholar]
- Caroff, M.; Karibian, D. Structure of bacterial lipopolysaccharides. Carbohydr. Res. 2003, 338, 2431–2447. [Google Scholar] [CrossRef]
- Casillo, A.; Parrilli, E.; Tutino, M.L.; Corsaro, M.M. The outer membrane glycolipids of bacteria from cold environments: Isolation, characterization, and biological activity. FEMS Microbiol. Ecol. 2019, 95, fiz094. [Google Scholar] [CrossRef] [PubMed]
- Bryant, C.E.; Spring, D.R.; Gangloff, M.; Gay, N.J. The molecular basis of the host response to lipopolysaccharide. Nat. Rev. Microbiol. 2010, 8, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Elhenawy, W.; Bording-Jorgensen, M.; Valguarnera, E.; Haurat, M.F.; Wine, E.; Feldman, M.F. LPS remodeling triggers formation of outer membrane vesicles in Salmonella. mBio 2016, 7, e00940-16. [Google Scholar] [CrossRef] [PubMed]
- Haurat, M.F.; Aduse-Opoku, J.; Rangarajan, M.; Dorobantu, L.; Gray, M.R.; Curtis, M.A.; Feldman, M.F. Selective sorting of cargo proteins into bacterial membrane vesicles. J. Biol. Chem. 2011, 286, 1269–1276. [Google Scholar] [CrossRef] [PubMed]
- Casillo, A.; Di Guida, R.; Carillo, S.; Chen, C.; Kamasaka, K.; Kawamoto, J.; Kurihara, T.; Corsaro, M.M. Structural elucidation of a novel lipooligosaccharide from the Antarctic bacterium OMVs producer Shewanella sp. HM13. Mar. Drugs 2019, 17, 34. [Google Scholar] [CrossRef] [PubMed]
- Di Guida, R.; Casillo, A.; Yokoyama, F.; Kawamoto, J.; Kurihara, T.; Corsaro, M.M. Detailed structural characterization of the lipooligosaccharide from the extracellular membrane vesicles of Shewanella vesiculosa HM13. Mar. Drugs 2020, 18, 231. [Google Scholar]
- Frias, A.; Manresa, A.; de Oliveira, E.; López-Iglesias, C.; Mercade, E. Membrane Vesicles: A Common Feature in the Extracellular Matter of Cold-Adapted Antarctic Bacteria. Microb. Ecol. 2010, 59, 476–486. [Google Scholar] [CrossRef]
- Freckelton, M.L.; Nedved, B.T.; Cai, Y.S.; Cao, S.; Turano, H.; Alegado, R.A.; Hadfield, M.G. Bacterial lipopolysaccharide induces settlement and metamorphosis in a marine larva. Proc. Nat. Acad. Sci. USA 2022, 119, e2200795119. [Google Scholar] [CrossRef]
- Limoli, D.H.; Jones, C.J.; Wozniak, D.J. Bacterial extracellular polysaccharides in biofilm formation and function. Microb. Biof. 2015, 223–247. [Google Scholar] [CrossRef]
- Willis, L.M.; Whitfield, C. Structure, biosynthesis, and function of bacterial capsular polysaccharides synthesized by ABC transporter-dependent pathways. Carbohydr. Res. 2013, 378, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Nagar, S.; Antony, R.; Thamban, M. Extracellular polymeric substances in Antarctic environments: A review of their ecological roles and impact on glacier biogeochemical cycles. Polar Sci. 2021, 30, 100686. [Google Scholar] [CrossRef]
- Whitfield, C.; Wear, S.S.; Sande, C. Assembly of bacterial capsular polysaccharides and exopolysaccharides. Annu. Rev. Microbiol. 2020, 74, 521–543. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Widmalm, G.; Im, W. Modeling and simulation of bacterial outer membranes with lipopolysaccharides and capsular polysaccharides. J. Chem. Inf. Model. 2023, 63, 1592–1601. [Google Scholar] [CrossRef] [PubMed]
- Fresno, S.; Jimenez, N.; Izquierdo, L.; Merino, S.; Corsaro, M.M.; De Castro, C.; Parrilli, M.; Naldi, T.; Regué, M.; Tomás, J.M. The ionic interaction of Klebsiella pneumoniae K2 capsule and core lipopolysaccharide. Microbiology 2006, 152, 1807–1818. [Google Scholar] [CrossRef] [PubMed]
- Casillo, A.; Lanzetta, R.; Parrilli, M.; Corsaro, M.M. Exopolysaccharides from marine and marine extremophilic bacteria: Structures, properties, ecological roles and applications. Mar. Drugs 2018, 16, 69. [Google Scholar] [CrossRef]
- Price, N.L.; Goyette-Desjardins, G.; Nothaft, H.; Valguarnera, E.; Szymanski, C.M.; Segura, M.; Feldman, M.F. Glycoengineered outer membrane vesicles: A novel platform for bacterial vaccines. Sci. Rep. 2016, 6, 24931. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, T.C.; Cywes-Bentley, C.; Moeller, T.D.; Weyant, K.B.; Putnam, D.; Chang, Y.F.; Jones, B.D.; Pier, G.B.; DeLisa, M.P. Immunization with outer membrane vesicles displaying conserved surface polysaccharide antigen elicits broadly antimicrobial antibodies. Proc. Nat. Acad. Sci. USA 2018, 115, E3106–E3115. [Google Scholar] [CrossRef]
- Korenevsky, A.; Beveridge, T.J. The surface physicochemistry and adhesiveness of Shewanella are affected by their surface polysaccharides. Microbiology 2007, 153, 1872–1883. [Google Scholar] [CrossRef]
- Carillo, S.; Casillo, A.; Pieretti, G.; Parrilli, E.; Sannino, F.; Bayer-Giraldi, M.; Cosconati, S.; Novellino, E.; Ewert, M.; Deming, J.W.; et al. A unique capsular polysaccharide structure from the psychrophilic marine bacterium Colwellia psychrerythraea 34H that mimics antifreeze (glyco) proteins. J. Am. Chem. Soc. 2015, 137, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Casillo, A.; Parrilli, E.; Sannino, F.; Mitchell, D.E.; Gibson, M.I.; Marino, G.; Lanzetta, R.; Parrilli, M.; Cosconati, S.; Novellino, E.; et al. Structure-activity relationship of the exopolysaccharide from a psychrophilic bacterium: A strategy for cryoprotection. Carbohydr. Polym. 2017, 156, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Deming, J.W.; Young, J.N. The role of exopolysaccharides in microbial adaptation to cold habitats. In Psychrophiles: From Biodiversity to Biotechnology; Springer: Cham, Switzerland, 2017; pp. 259–284. [Google Scholar]
- Casillo, A.; Di Guida, R.; Cavasso, D.; Stellavato, A.; Rai, D.; Yokoyama, F.; Kamasaka, K.; Kawamoto, J.; Kurihara, T.; Schiraldi, C.; et al. Polysaccharide corona: The acetyl-rich envelope wraps the extracellular membrane vesicles and the cells of Shewanella vesiculosa providing adhesiveness. Carbohydr. Polym. 2022, 297, 120036–120047. [Google Scholar] [CrossRef] [PubMed]
- Di Guida, R.; Casillo, A.; Stellavato, A.; Kawai, S.; Ogawa, T.; di Meo, C.; Kawamoto, J.; Kurihara, T.; Schiraldi, C.; Corsaro, M.M. Capsular Polysaccharide from a fish-gut bacterium induces/promotes apoptosis of colon cancer cells in vitro through Caspases’ pathway activation. Carbohydr. Polym. 2022, 278, 118908. [Google Scholar] [CrossRef] [PubMed]
- Kokoulin, M.S.; Kuzmich, A.S.; Romanenko, L.A.; Chikalovets, I.V. Structure and in vitro antiproliferative activity of the acidic capsular polysaccharide from the deep-sea bacterium Psychrobacter submarinus KMM 225T. Carbohydr. Polym. 2021, 262, 117941. [Google Scholar] [CrossRef] [PubMed]
- Kokoulin, M.S.; Kuzmich, A.S.; Romanenko, L.A.; Chikalovets, I.V. Sulfated capsular polysaccharide from the marine bacterium Kangiella japonica inhibits T-47D cells growth in vitro. Carbohydr. Polym. 2022, 290, 119477. [Google Scholar] [CrossRef] [PubMed]
- Baeza, N.; Mercade, E. Relationship between membrane vesicles, extracellular ATP and biofilm formation in Antarctic Gram-negative bacteria. Microb. Ecol. 2021, 81, 645–656. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Chanda, W.; Zhong, M. The relationship between biofilm and outer membrane vesicles: A novel therapy overview. FEMS Microbiol. Lett. 2015, 362, fnv117. [Google Scholar] [CrossRef]
- Orench-Rivera, N.; Kuehn, M.J. Environmentally controlled bacterial vesicle-mediated export. Cell. Microbiol. 2016, 18, 1525–1536. [Google Scholar] [CrossRef]
- Poupardin, R.; Wolf, M.; Maeding, N.; Paniushkina, L.; Geissler, S.; Bergese, P.; Witwer, K.W.; Schallmoser, K.; Fuhrmann, G.; Strunk, D. Advances in extracellular vesicle research over the past decade: Source and isolation method are connected with cargo and function. Adv. Healthc. Mater. 2024, 13, e2303941. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Seike, S.; Kobayashi, H.; Ueda, M.; Takahashi, E.; Okamoto, K.; Yamanaka, H. Outer membrane vesicles released from Aeromonas strains are involved in the biofilm formation. Front. Microbiol. 2021, 11, 613650. [Google Scholar] [CrossRef]
- Koteska, D.; Wang, H.; Wagner-Döbler, I.; Schulz, S. Outer membrane vesicles of Dinoroseobacter shibae transport a volatile aldehyde. Front. Ecol. Evol. 2023, 11, 1102159. [Google Scholar] [CrossRef]
- Lin, L.; Wang, Y.; Srinivasan, R.; Zhang, L.; Song, H.; Song, Q.; Wang, G.; Lin, X. Quantitative proteomics reveals that the protein components of outer membrane vesicles (OMVs) in Aeromonas hydrophila play protective roles in antibiotic resistance. J. Prot. Res. 2022, 21, 1707–1717. [Google Scholar] [CrossRef]
- Teixeira, A.; Loureiro, I.; Lisboa, J.; Oliveira, P.N.; Azevedo, J.E.; Dos Santos, N.M.; do Vale, A. Characterization and vaccine potential of outer membrane vesicles from Photobacterium damselae subsp. piscicida. Int. J. Mol. Sci. 2023, 24, 5138. [Google Scholar] [CrossRef]
- Fischer, T.; Schorb, M.; Reintjes, G.; Kolovou, A.; Santarella-Mellwig, R.; Markert, S.; Rhiel, E.; Littmann, S.; Becher, D.; Schweder, T.; et al. Biopearling of interconnected outer membrane vesicle chains by a marine flavobacterium. Appl. Environ. Microbiol. 2019, 85, e00829-19. [Google Scholar] [CrossRef]
- Kwon, Y.M.; Patra, A.K.; Chiura, H.X.; Kim, S.J. Production of extracellular vesicles with light-induced proton pump activity by proteorhodopsin-containing marine bacteria. Microbiologyopen 2019, 8, e00808. [Google Scholar] [CrossRef]
- Dragovic, R.A.; Gardiner, C.; Brooks, A.S.; Tannetta, D.S.; Ferguson, D.J.; Hole, P.; Carr, B.; Redman, C.W.; Harris, A.L.; Dobson, P.J.; et al. Sizing and Phenotyping of Cellular Vesicles Using Nanoparticle Tracking Analysis. Nanomedicine 2011, 7, 780–788. [Google Scholar] [CrossRef]
- Gardiner, C.; Ferreira, Y.J.; Dragovic, R.A.; Redman, C.W.; Sargent, I.L. Extracellular Vesicle Sizing and Enumeration by Nanoparticle Tracking Analysis. J. Extracell. Vesicles 2013, 2, 19671. [Google Scholar] [CrossRef]
- Lawrie, A.S.; Albanyan, A.; Cardigan, R.A.; Mackie, I.J.; Harrison, P. Microparticle Sizing by Dynamic Light Scattering in Fresh Frozen Plasma. Vox Sang. 2009, 96, 206–212. [Google Scholar] [CrossRef]
- Shao, H.; Im, H.; Castro, C.M.; Breakefield, X.; Weissleder, R.; Lee, H. New technologies for analysis of extracellular vesicles. Chem. Rev. 2018, 118, 1917–1950. [Google Scholar] [CrossRef] [PubMed]
- Mobarak, H.; Javid, F.; Narmi, M.T.; Mardi, N.; Sadeghsoltani, F.; Khanicheragh, P.; Narimani, S.; Mahdipour, M.; Sokullu, E.; Valioglu, F.; et al. Prokaryotic microvesicles Ortholog of eukaryotic extracellular vesicles in biomedical fields. Cell Commun. Sign. 2024, 22, 80. [Google Scholar] [CrossRef] [PubMed]
- McBroom, A.J.; Johnson, A.P.; Vemulapalli, S.; Kuehn, M.J. Outer membrane vesicle production by Escherichia coli is independent of membrane instability. J. Bacteriol. 2006, 188, 5385–5392. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, C.; Di Vizio, D.; Sahoo, S.; Thery, C.; Witwer, K.W.; Wauben, M.; Hill, A.F. Techniques Used for the Isolation and Characterization of Extracellular Vesicles: Results of a Worldwide Survey. J. Extracell. Vesicles 2016, 5, 32945. [Google Scholar] [CrossRef] [PubMed]
- Klimentová, J.; Stulík, J. Methods of isolation and purification of outer membrane vesicles from gram-negative bacteria. Microbiol. Res. 2015, 170, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Marsh, J.B.; Weinstein, D.B. Simple charring method for determination of lipids. J. Lip. Res. 1966, 7, 574–576. [Google Scholar] [CrossRef]
- Christopoulos, T.K. Nucleic acid analysis. Anal. Chem. 1999, 71, 425–438. [Google Scholar] [CrossRef]
- Nowotny, A.; Nowotny, A. Carbohydrate determination by phenol-sulfuric acid. In Basic Exercises in Immunochemistry; Springer: Berlin/Heidelberg, Germany, 1979. [Google Scholar]
- Biermann, C.J.; McGinnis, G.D. Analysis of Carbohydrates by GLC and MS; CRC Press: Boca Raton, FL, USA, 1988. [Google Scholar]
- Tian, C.M.; Yang, M.F.; Xu, H.M.; Zhu, M.Z.; Zhang, Y.; Yao, J.; Wang, L.-S.; Liang, Y.-J.; Li, D.-F. Emerging role of bacterial outer membrane vesicle in gastrointestinal tract. Gut Pathog. 2023, 15, 20. [Google Scholar] [CrossRef]
- Shen, Y.; Torchia, M.L.G.; Lawson, G.W.; Karp, C.L.; Ashwell, J.D.; Mazmanian, S.K. Outer membrane vesicles of a human commensal mediate immune regulation and disease protection. Cell Host Microbe 2012, 12, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Kulp, A.; Kuehn, M.J. Biological functions and biogenesis of secreted bacterial outer membrane vesicles. Annu. Rev. Microbiol. 2010, 64, 163–184. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Li, H.; Deng, J.; Zhou, R.; Zeng, Q. Biological interactions with Prochlorococcus: Implications for the marine carbon cycle. Trends Microbiol. 2023, 32, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Bergman, B.; Chen, B.; Zheng, S.; Xiang, G.; Rasmussen, U. Cellular responses in the cyanobacterial symbiont during its vertical transfer between plant generations in the Azolla microphylla symbiosis. New Phytol. 2009, 181, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.H.; Lee, S.H. Marine bacterial vesicles as a novel class of nanomaterials. Front. Mar. Sci. 2019, 6, 457. [Google Scholar]
- Ellis, T.N.; Kuehn, M.J. Virulence and immunomodulatory roles of bacterial outer membrane vesicles. Microbiol. Mol. Biol. Rev. 2010, 74, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Devoe, I.W.; Gilchrist, J.E. Release of alkaline phosphatase by Pseudomonas aeruginosa during growth. J. Bacteriol. 1073, 116, 395–401. [Google Scholar]
- Alves, N.J.; Turner, K.B.; DiVito, K.A.; Walper, S.A.; Zabetakis, D. Bacterial nanobiotechnology: Bioengineered bacterial outer membrane vesicles for therapeutic applications. Curr. Opin. Biotechnol. 2015, 35, 76–84. [Google Scholar]
- Turner, L.; Bitto, N.J.; Steer, D.L.; Lo, C.; D’Costa, K.; Ramm, G.; Kaparakis-Liaskos, M. Helicobacter pylori outer membrane vesicle size determines their mechanisms of host cell entry and protein content. Front. Immunol. 2018, 9, 1466. [Google Scholar] [CrossRef]
- Ferreira, M.; Ogren, M.; Dias, J.N.R.; Silva, M.; Gil, S.; Tavares, L.; Aires-da-Silva, F.; Gaspar, M.M.; Aguiar, S.I. Liposomes as Antibiotic Delivery Systems: A Promising Nanotechnological Strategy against Antimicrobial Resistance. Molecules 2021, 26, 2047. [Google Scholar] [CrossRef]
- Caracciolo, G.; Farokhzad, O.C.; Mahmoudi, M. Biological identity of nanoparticles in vivo: Clinical implications of the protein corona. Trends Biotechnol. 2017, 35, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Dong, Z.; Ma, C.; Wu, R.; Lv, R.; Liu, S.; Liu, J. High-Yield, Magnetic Harvesting of Extracellular Outer-Membrane Vesicles from Escherichia coli. Small 2022, 18, 2204350. [Google Scholar] [CrossRef] [PubMed]
- Tzipilevich, E.; Habusha, M.; Ben-Yehuda, S. Acquisition of phage sensitivity by bacteria through exchange of phage receptors. Cell 2017, 168, 186–199. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Dou, Y.; Liu, Y.; Di, M.; Bian, H.; Sun, X.; Yang, Q. Advances in therapeutic applications of extracellular vesicles. Int. J. Nanomed. 2023, 18, 3285–3307. [Google Scholar] [CrossRef] [PubMed]



| Vesicle Size | Microorganisms | Vesicle Type | Functions | References |
|---|---|---|---|---|
| 30–100 nm | Prochlorococcus, Nostoc, Synechocystis, Gloeocapsa, Trichodesmium | Small Vesicles (Exosomes) | Intercellular communication, carrying proteins, lipids, nucleic acids | [13,36,38,39] |
| 100–300 nm | Synechococcus, Anabaena, Microcystis, Spirulina, Oscillatoria | Medium-sized Vesicles (Microvesicles/Ectosomes) | Nutrient acquisition, environmental signaling | [9,29,31,40] |
| 300–700 nm | V. cholerae, P. aeruginosa, B. subtilis, S. aureus, M. xanthus, Shewanella, E. coli, Rhodobacter sphaeroides, A. baumannii, H. pylori | Large Vesicles | Cellular waste removal, containing fragmented DNA, extracellular polymeric substances, biofilm formation, environmental adaptation | [4,5,9,36,38,40,41] |
| Field | Function | |
|---|---|---|
| Drug Delivery |
| [127,130] |
| Vaccine Development |
| [31,131] |
| Diagnostics |
| [2,24] |
| Bioremediation |
| [132,133] |
| Nutraceuticals and Functional Foods |
| [134] |
| Cosmetics |
| [130] |
| Agriculture |
| [31,130] |
| Nanotechnology |
| [24,127] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casillo, A.; D’Amico, R.; Lanzetta, R.; Corsaro, M.M. Marine Delivery Vehicles: Molecular Components and Applications of Bacterial Extracellular Vesicles. Mar. Drugs 2024, 22, 363. https://doi.org/10.3390/md22080363
Casillo A, D’Amico R, Lanzetta R, Corsaro MM. Marine Delivery Vehicles: Molecular Components and Applications of Bacterial Extracellular Vesicles. Marine Drugs. 2024; 22(8):363. https://doi.org/10.3390/md22080363
Chicago/Turabian StyleCasillo, Angela, Raffaele D’Amico, Rosa Lanzetta, and Maria Michela Corsaro. 2024. "Marine Delivery Vehicles: Molecular Components and Applications of Bacterial Extracellular Vesicles" Marine Drugs 22, no. 8: 363. https://doi.org/10.3390/md22080363
APA StyleCasillo, A., D’Amico, R., Lanzetta, R., & Corsaro, M. M. (2024). Marine Delivery Vehicles: Molecular Components and Applications of Bacterial Extracellular Vesicles. Marine Drugs, 22(8), 363. https://doi.org/10.3390/md22080363








