Subcritical Water Extraction of Undaria pinnatifida: Comparative Study of the Chemical Properties and Biological Activities across Different Parts
Abstract
1. Introduction
2. Results and Discussion
2.1. Proximate Composition
2.2. Extraction Efficiency
2.3. Color and Maillard Reaction Products (MRPs)
2.4. Total Phenolic Contents (TPC) and Total Flavonoid Contents (TFC)
2.5. Total Sugar Content (TSC), Reducing Sugar Content (RSC), and Total Protein Content (TPrC)
2.6. Monosaccharide Composition, Sulfate Content, and Molecular Weight Analysis
2.7. GC–MS Analysis
2.7.1. Sugar Alcohols and Monosaccharides
2.7.2. Fatty Acids and Fatty Amides
2.7.3. Amino Acids
2.7.4. Glycosides and Monoglycerides
2.8. Biological Activity
2.8.1. Antioxidant Activity
2.8.2. α-Glucosidase Inhibitory
2.8.3. Antihypertensive Activity
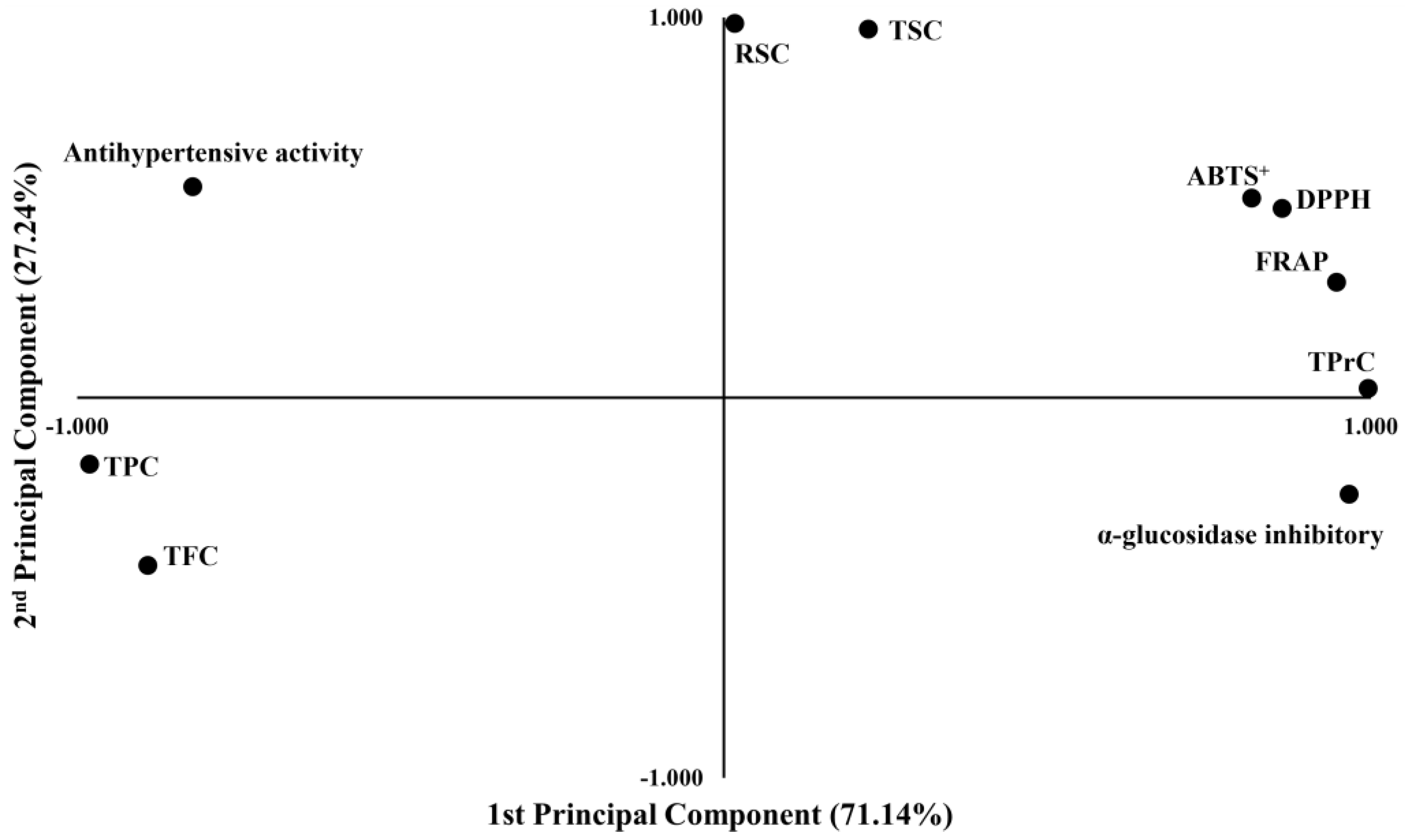
2.9. Principal Component Analysis
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Proximate Composition
3.3. Subcritical Water Extraction
3.4. Chemical Properties of USE
3.4.1. Color, pH, and MRPs
3.4.2. Total Phenolic and Total Flavonoid Contents
3.4.3. Total Sugar and Reducing Sugar Content
3.4.4. Total Protein Content
3.4.5. Monosaccharide Analysis
3.4.6. Sulfate Content
3.4.7. Molecular Weight Analysis
3.4.8. GC–MS
Sample Preparation
Analysis
3.5. Biological Activity
3.5.1. Antioxidant Activity (DPPH, ABTS+, and FRAP Assay)
3.5.2. Antihypertensive Activity
3.5.3. α-Glucosidase Inhibitory Activity
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviation
References
- Zeng, J.; Luan, F.; Hu, J.; Liu, Y.; Zhang, X.; Qin, T.; Zhang, X.; Liu, R.; Zeng, N. Recent research advances in polysaccharides from Undaria pinnatifida: Isolation, structures, bioactivities, and applications. Int. J. Biol. Macromol. 2022, 206, 325–354. [Google Scholar] [CrossRef] [PubMed]
- Arijón, M.; Raffo, M.P.; Sánchez, N.; Dellatorre, F.G. Photosynthetic pigments and color of wild Undaria pinnatifida for wakame production (Chubut, Patagonia Argentina). Algal Res. 2023, 69, 102918. [Google Scholar] [CrossRef]
- Boulom, S.; Robertson, J.; Hamid, N.; Ma, Q.; Lu, J. Seasonal changes in lipid, fatty acid, α-tocopherol and phytosterol contents of seaweed, Undaria pinnatifida, in the Marlborough Sounds, New Zealand. Food Chem. 2014, 161, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Park, Y.-J.; Jeon, Y.-J.; Ryu, B. Bioactivities of the edible brown seaweed, Undaria pinnatifida: A review. Aquaculture 2018, 495, 873–880. [Google Scholar] [CrossRef]
- Pedro, B.; Guedes, L.; André, R.; Gaspar, H.; Vaz, P.; Ascensão, L.; Melo, R.; Serralheiro, M.L. Undaria pinnatifida (U. pinnatifida) bioactivity: Antioxidant, gastro-intestinal motility, cholesterol biosynthesis and liver cell lines proteome. J. Funct. Foods 2021, 83, 104567. [Google Scholar] [CrossRef]
- Queffelec, J.; Flórez-Fernández, N.; Domínguez, H.; Torres, M. Microwave hydrothermal processing of Undaria pinnatifida for bioactive peptides. Bioresour. Technol. 2021, 342, 125882. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.-W.; Zhou, T.-S.; Qiu, W.-H.; Wang, Y.-K.; Xu, Q.-L.; Ke, S.-Z.; Wang, S.-J.; Jin, W.-H.; Chen, J.-W.; Zhang, H.-W. Characterization and hypoglycemic effects of sulfated polysaccharides derived from brown seaweed Undaria pinnatifida. Food Chem. 2021, 341, 128148. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Lin, J.; Yi, J. Medication rules of anti-tumor formulas containing marine Chinese medicinals. J. Beijing Univ. TCM 2018, 41, 253–258. [Google Scholar]
- Wu, D.-Y. Analysis of varieties and characteristics of marine Chinese medicines recorded in Compendium of Materia Medica. Chin. Tradit. Herb. Drugs 2020, 24, 4338–4347. [Google Scholar]
- Park, J.-S.; Han, J.-M.; Shin, Y.-N.; Park, Y.-S.; Shin, Y.-R.; Park, S.-W.; Roy, V.C.; Lee, H.-J.; Kumagai, Y.; Kishimura, H. Exploring bioactive compounds in brown seaweeds using subcritical water: A comprehensive analysis. Mar. Drugs 2023, 21, 328. [Google Scholar] [CrossRef]
- Álvarez-Viñas, M.; Rodríguez-Seoane, P.; Flórez-Fernández, N.; Torres, M.D.; Díaz-Reinoso, B.; Moure, A.; Domínguez, H. Subcritical water for the extraction and hydrolysis of protein and other fractions in biorefineries from agro-food wastes and algae: A review. Food Bioprocess Technol. 2021, 14, 373–387. [Google Scholar] [CrossRef]
- Zhang, J.; Wen, C.; Zhang, H.; Duan, Y.; Ma, H. Recent advances in the extraction of bioactive compounds with subcritical water: A review. Trends Food Sci. Technol. 2020, 95, 183–195. [Google Scholar] [CrossRef]
- Hernández-Hernández, H.; Moreno-Vilet, L.; Villanueva-Rodríguez, S. Current status of emerging food processing technologies in Latin America: Novel non-thermal processing. Innov. Food Sci. Emerg. Technol. 2019, 58, 102233. [Google Scholar] [CrossRef]
- Park, J.-S.; Jeong, Y.-R.; Chun, B.-S. Physiological activities and bioactive compound from laver (Pyropia yezoensis) hydrolysates by using subcritical water hydrolysis. J. Supercrit. Fluids 2019, 148, 130–136. [Google Scholar] [CrossRef]
- Lee, H.-J.; Chae, S.-J.; Saravana, P.S.; Chun, B.-S. Physical and functional properties of tunicate (Styela clava) hydrolysate obtained from pressurized hydrothermal process. Fish. Aquat. Sci. 2017, 20, 14. [Google Scholar] [CrossRef]
- Zhao, Y.; Zheng, Y.; Wang, J.; Ma, S.; Yu, Y.; White, W.L.; Yang, S.; Yang, F.; Lu, J. Fucoidan extracted from Undaria pinnatifida: Source for nutraceuticals/functional foods. Mar. Drugs 2018, 16, 321. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hawboldt, K.; MacQuarrie, S.; Thomas, R.; Gebregiworgis, T. Alkaline subcritical water extraction of bioactive compounds and antioxidants from beach-cast brown algae (Ascophyllum Nodosum). Chem. Eng. J. 2024, 494, 153109. [Google Scholar] [CrossRef]
- Thakuri, L.S.; Park, C.M.; Park, J.W.; Kim, H.-A.; Rhyu, D.Y.; Thakuri, L.S.; Park, C.M.; Park, J.W.; Kim, H.-A.; Rhyu, D.Y. Subcritical water extraction of Gracilaria chorda abbreviates lipid accumulation and obesity-induced inflammation. Algae 2023, 38, 81–92. [Google Scholar] [CrossRef]
- Bordoloi, A.; Goosen, N.J. A greener alternative using subcritical water extraction to valorize the brown macroalgae Ecklonia maxima for bioactive compounds. J. Appl. Phycol. 2020, 32, 2307–2319. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, Y.; Hu, C.; Zou, X.; Lin, Y.; Xia, Y.; You, L. Chemistry and immunostimulatory activity of a polysaccharide from Undaria pinnatifida. Food Chem. Toxicol. 2019, 128, 119–128. [Google Scholar] [CrossRef]
- de Jesus Raposo, M.F.; De Morais, R.M.S.C.; de Morais, A.M.M.B. Bioactivity and applications of sulphated polysaccharides from marine microalgae. Mar. Drugs 2013, 11, 233–252. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Roy, V.C.; Park, J.-S.; Chun, B.-S. Extraction and characterization of bioactive compounds from brown seaweed (Undaria pinnatifida) sporophyll using two sequential green extraction techniques. Algal Res. 2024, 77, 103330. [Google Scholar] [CrossRef]
- Aryee, A.N.; Agyei, D.; Akanbi, T.O. Recovery and utilization of seaweed pigments in food processing. Curr. Opin. Food Sci. 2018, 19, 113–119. [Google Scholar] [CrossRef]
- Gereniu, C.R.N.; Saravana, P.S.; Getachew, A.T.; Chun, B.-S. Characteristics of functional materials recovered from Solomon Islands red seaweed (Kappaphycus alvarezii) using pressurized hot water extraction. J. Appl. Phycol. 2017, 29, 1609–1621. [Google Scholar] [CrossRef]
- Zhang, N.; Fan, D.; Zhao, Y.; Wu, Y.; Yan, B.; Zhao, J.; Wang, M.; Zhang, H. Dielectric loss mediated promotion of microwave heating in the Maillard reaction. LWT 2019, 101, 559–566. [Google Scholar] [CrossRef]
- Wang, X.P.; Zhao, X.H. Using an enzymatic galactose assay to detect lactose glycation extents of two proteins caseinate and soybean protein isolate via the Maillard reaction. J. Sci. Food Agric. 2017, 97, 2617–2622. [Google Scholar] [CrossRef]
- Naik, R.R.; Ye, Q.; Wang, Y.; Selomulya, C. Assessing the effect of Maillard reaction products on the functionality and antioxidant properties of Amaranth-red seaweed blends. Food Res. Int. 2024, 175, 113759. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Agar, O.T.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A. Improving potential strategies for biological activities of phlorotannins derived from seaweeds. Crit. Rev. Food Sci. Nutr. 2023, 1–23. [Google Scholar] [CrossRef]
- Subbiah, V.; Xie, C.; Dunshea, F.R.; Barrow, C.J.; Suleria, H.A. The quest for phenolic compounds from seaweed: Nutrition, biological activities and applications. Food Rev. Int. 2023, 39, 5786–5813. [Google Scholar] [CrossRef]
- Phang, S.J.; Teh, H.X.; Looi, M.L.; Arumugam, B.; Fauzi, M.B.; Kuppusamy, U.R. Phlorotannins from brown algae: A review on their antioxidant mechanisms and applications in oxidative stress-mediated diseases. J. Appl. Phycol. 2023, 35, 867–892. [Google Scholar] [CrossRef]
- Gan, A.; Baroutian, S. Subcritical water extraction for recovery of phenolics and fucoidan from New Zealand Wakame (Undaria pinnatifida) seaweed. J. Supercrit. Fluids 2022, 190, 105732. [Google Scholar] [CrossRef]
- Xie, C.; Lee, Z.J.; Ye, S.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A. A review on seaweeds and seaweed-derived polysaccharides: Nutrition, chemistry, bioactivities, and applications. Food Rev. Int. 2024, 40, 1312–1347. [Google Scholar] [CrossRef]
- Park, Y.-S.; Roy, V.C.; Park, J.-S.; Han, J.-M.; Shin, Y.-N.; Shin, Y.-R.; Park, S.-W.; Chun, B.-S. Bioactive compounds obtained via subcritical water hydrolysis of Ecklonia stolonifera: Characterization and potential application. J. Appl. Phycol. 2024, 36, 897–905. [Google Scholar] [CrossRef]
- Pavlicevic, M.; Maestri, E.; Marmiroli, M. Marine bioactive peptides—An overview of generation, structure and application with a focus on food sources. Mar. Drugs 2020, 18, 424. [Google Scholar] [CrossRef]
- Sato, M.; Oba, T.; Yamaguchi, T.; Nakano, T.; Kahara, T.; Funayama, K.; Kobayashi, A.; Nakano, T. Antihypertensive effects of hydrolysates of wakame (Undaria pinnatifida) and their angiotensin-I-converting enzyme inhibitory activity. Ann. Nutr. Metab. 2002, 46, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Lafarga, T.; Acién-Fernández, F.G.; Garcia-Vaquero, M. Bioactive peptides and carbohydrates from seaweed for food applications: Natural occurrence, isolation, purification, and identification. Algal Res. 2020, 48, 101909. [Google Scholar] [CrossRef]
- Kloareg, B.; Quatrano, R. Structure of the cell walls of marine algae and ecophysiological functions of the matrix polysaccharides. In Oceanography and Marine Biology: An Annual Review; Aberdeen University Press: Aberdeen, UK, 1988; Volume 26, pp. 259–315. [Google Scholar]
- Sasaki, C.; Tamura, S.; Suzuki, M.; Etomi, K.; Nii, N.; Hayashi, J.; Kanemaru, K. Continuous microwave-assisted step-by-step extraction of bioactive water-soluble materials and fucoidan from brown seaweed Undaria pinnatifida waste. Biomass Convers. Biorefinery 2024, 14, 7673–7682. [Google Scholar] [CrossRef]
- Skriptsova, A.V.; Shevchenko, N.M.; Zvyagintseva, T.N.; Imbs, T.I. Monthly changes in the content and monosaccharide composition of fucoidan from Undaria pinnatifida (Laminariales, Phaeophyta). J. Appl. Phycol. 2010, 22, 79–86. [Google Scholar] [CrossRef]
- Arokiarajan, M.S.; Thirunavukkarasu, R.; Joseph, J.; Ekaterina, O.; Aruni, W. Advance research in biomedical applications on marine sulfated polysaccharide. Int. J. Biol. Macromol. 2022, 194, 870–881. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Jia, X.; Wang, N.; Xiao, M.; Song, S.; Wu, S.; Li, Z.; Wang, S.; Cui, S.W.; Guo, Q. Insights into the structure-bioactivity relationships of marine sulfated polysaccharides: A review. Food Hydrocoll. 2022, 123, 107049. [Google Scholar] [CrossRef]
- Saravana, P.S.; Tilahun, A.; Gerenew, C.; Tri, V.D.; Kim, N.H.; Kim, G.-D.; Woo, H.-C.; Chun, B.-S. Subcritical water extraction of fucoidan from Saccharina japonica: Optimization, characterization and biological studies. J. Appl. Phycol. 2018, 30, 579–590. [Google Scholar] [CrossRef]
- Sun, L.; Wang, L.; Zhou, Y. Immunomodulation and antitumor activities of different-molecular-weight polysaccharides from Porphyridium cruentum. Carbohydr. Polym. 2012, 87, 1206–1210. [Google Scholar] [CrossRef]
- Ali, M.S.; Ho, T.C.; Razack, S.A.; Haq, M.; Roy, V.C.; Park, J.-S.; Kang, H.W.; Chun, B.-S. Oligochitosan recovered from shrimp shells through subcritical water hydrolysis: Molecular size reduction and biological activities. J. Supercrit. Fluids 2023, 196, 105868. [Google Scholar] [CrossRef]
- Fitton, J.H. Therapies from fucoidan; multifunctional marine polymers. Mar. Drugs 2011, 9, 1731–1760. [Google Scholar] [CrossRef] [PubMed]
- Awuchi, C.G.; Echeta, K.C. Current developments in sugar alcohols: Chemistry, nutrition, and health concerns of sorbitol, xylitol, glycerol, arabitol, inositol, maltitol, and lactitol. Int. J. Adv. Acad. Res. 2019, 5, 1–33. [Google Scholar]
- Fluhr, J.; Darlenski, R.; Surber, C. Glycerol and the skin: Holistic approach to its origin and functions. Br. J. Dermatol. 2008, 159, 23–34. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Li, Y.; Han, C.; Lin, R.; Qian, W.; Hou, X. L-Fucose ameliorates DSS-induced acute colitis via inhibiting macrophage M1 polarization and inhibiting NLRP3 inflammasome and NF-kB activation. Int. J. Immunopharmacol. 2019, 73, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Torres, D.P.; Gonçalves, M.d.P.F.; Teixeira, J.A.; Rodrigues, L.R. Galacto-oligosaccharides: Production, properties, applications, and significance as prebiotics. Compr. Rev. Food Sci. Food Saf. 2010, 9, 438–454. [Google Scholar] [CrossRef] [PubMed]
- Moore, E.M.; Wagner, C.; Komarnytsky, S. The enigma of bioactivity and toxicity of botanical oils for skin care. Front. Pharmacol. 2020, 11, 785. [Google Scholar] [CrossRef]
- Pereira-Leite, C.; Bom, M.; Ribeiro, A.; Almeida, C.; Rosado, C. Exploring stearic-acid-based nanoparticles for skin applications—Focusing on stability and cosmetic benefits. Cosmetics 2023, 10, 99. [Google Scholar] [CrossRef]
- Cheng, M.-C.; Ker, Y.-B.; Yu, T.-H.; Lin, L.-Y.; Peng, R.Y.; Peng, C.-H. Chemical synthesis of 9 (Z)-octadecenamide and its hypolipidemic effect: A bioactive agent found in the essential oil of mountain celery seeds. J. Agric. Food Chem. 2010, 58, 1502–1508. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.H. Alternative to Glycerine in Cosmetics. In Glycerine: A Key Cosmetic Ingredient; Jungermann, E., Sonntag, N.O., Eds.; CRC Press: Boca Raton, FL, USA, 1991; Volume 11, pp. 407–434. [Google Scholar]
- Holeček, M. Aspartic acid in health and disease. Nutrients 2023, 15, 4023. [Google Scholar] [CrossRef] [PubMed]
- Schrader, A.; Siefken, W.; Kueper, T.; Breitenbach, U.; Gatermann, C.; Sperling, G.; Biernoth, T.; Scherner, C.; Stäb, F.; Wenck, H. Effects of glyceryl glucoside on AQP3 expression, barrier function and hydration of human skin. Ski. Pharmacol. Physiol. 2012, 25, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Konishi, T.; Satsu, H.; Hatsugai, Y.; Aizawa, K.; Inakuma, T.; Nagata, S.; Sakuda, S.H.; Nagasawa, H.; Shimizu, M. Inhibitory effect of a bitter melon extract on the P-glycoprotein activity in intestinal Caco-2 cells. Br. J. Pharmacol. 2004, 143, 379–387. [Google Scholar] [CrossRef]
- Jacobsen, C.; Sørensen, A.-D.M.; Holdt, S.L.; Akoh, C.C.; Hermund, D.B. Source, extraction, characterization, and applications of novel antioxidants from seaweed. Annu. Rev. Food Sci. Technol. 2019, 10, 541–568. [Google Scholar] [CrossRef] [PubMed]
- Zhong, B.; Robinson, N.A.; Warner, R.D.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A. LC-ESI-QTOF-MS/MS characterization of seaweed phenolics and their antioxidant potential. Mar. Drugs 2020, 18, 331. [Google Scholar] [CrossRef]
- Chan, P.T.; Matanjun, P.; Yasir, S.M.; Tan, T.S. Antioxidant activities and polyphenolics of various solvent extracts of red seaweed, Gracilaria changii. J. Appl. Phycol. 2015, 27, 2377–2386. [Google Scholar] [CrossRef]
- Park, J.-S.; Han, J.-M.; Surendhiran, D.; Chun, B.-S. Physicochemical and biofunctional properties of Sargassum thunbergii extracts obtained from subcritical water extraction and conventional solvent extraction. J. Supercrit. Fluids 2022, 182, 105535. [Google Scholar] [CrossRef]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef]
- Zhao, G.; Hou, X.; Li, X.; Qu, M.; Tong, C.; Li, W. Metabolomics analysis of alloxan-induced diabetes in mice using UPLC–Q-TOF-MS after Crassostrea gigas polysaccharide treatment. Int. J. Biol. Macromol. 2018, 108, 550–557. [Google Scholar] [CrossRef]
- Xie, X.; Chen, C.; Fu, X. Screening α-glucosidase inhibitors from four edible brown seaweed extracts by ultra-filtration and molecular docking. LWT 2021, 138, 110654. [Google Scholar] [CrossRef]
- Admassu, H.; Gasmalla, M.A.A.; Yang, R.; Zhao, W. Bioactive peptides derived from seaweed protein and their health benefits: Antihypertensive, antioxidant, and antidiabetic properties. J. Food Sci. 2018, 83, 6–16. [Google Scholar] [CrossRef]
- Lee, S.Y.; Hur, S.J. Antihypertensive peptides from animal products, marine organisms, and plants. Food Chem. 2017, 228, 506–517. [Google Scholar] [CrossRef]
- Kumagai, Y.; Toji, K.; Katsukura, S.; Morikawa, R.; Uji, T.; Yasui, H.; Shimizu, T.; Kishimura, H. Characterization of ACE inhibitory peptides prepared from Pyropia pseudolinearis protein. Mar. Drugs 2021, 19, 200. [Google Scholar] [CrossRef] [PubMed]
- Nasri, M. Protein hydrolysates and biopeptides: Production, biological activities, and applications in foods and health benefits. A review. In Advances in Food and Nutrition Research; Elsevier: Amsterdam, The Netherlands, 2017; Volume 81, pp. 109–159. [Google Scholar]
- Nkurunziza, D.; Pendleton, P.; Sivagnanam, S.P.; Park, J.-S.; Chun, B.S. Subcritical water enhances hydrolytic conversions of isoflavones and recovery of phenolic antioxidants from soybean byproducts (okara). J. Ind. Eng. Chem. 2019, 80, 696–703. [Google Scholar] [CrossRef]
- Dinh, T.V.; Saravana, P.S.; Woo, H.C.; Chun, B.S. Ionic liquid-assisted subcritical water enhances the extraction of phenolics from brown seaweed and its antioxidant activity. Sep. Purif. Technol. 2018, 196, 287–299. [Google Scholar] [CrossRef]
- Chamika, W.A.S.; Ho, T.C.; Roy, V.C.; Kiddane, A.T.; Park, J.-S.; Kim, G.-D.; Chun, B.-S. In vitro characterization of bioactive compounds extracted from sea urchin (Stomopneustes variolaris) using green and conventional techniques. Food Chem. 2021, 361, 129866. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.-R.; Park, J.-S.; Nkurunziza, D.; Cho, Y.-J.; Chun, B.-S. Valorization of blue mussel for the recovery of free amino acids rich products by subcritical water hydrolysis. J. Supercrit. Fluids 2021, 169, 105135. [Google Scholar] [CrossRef]
- Martínez-Sanz, M.; Gómez-Mascaraque, L.G.; Ballester, A.R.; Martínez-Abad, A.; Brodkorb, A.; López-Rubio, A. Production of unpurified agar-based extracts from red seaweed Gelidium sesquipedale by means of simplified extraction protocols. Algal Res. 2019, 38, 101420. [Google Scholar] [CrossRef]
- NIST NIST20: Updates to the NIST Tandem and Electron Ionization Spectral Libraries. Available online: https://www.nist.gov/programs-projects/nist20-updates-nist-tandem-and-electron-ionization-spectral-libraries (accessed on 30 March 2024).
- Chun, B.-S.; Lee, S.-C.; Ho, T.-C.; Micomyiza, J.-B.; Park, J.-S.; Nkurunziza, D.; Lee, H.-J. Subcritical water hydrolysis of comb pen shell (Atrina pectinata) edible parts to produce high-value amino acid products. Mar. Drugs 2022, 20, 357. [Google Scholar] [CrossRef]
- Dojindo Laboratories Co., Ltd. ACE Kit—WST: A502 Product Manual. Available online: https://www.dojindo.com/manual/A502/ (accessed on 23 July 2024).
- Saravana, P.S.; Cho, Y.-N.; Patil, M.P.; Cho, Y.-J.; Kim, G.-D.; Park, Y.B.; Woo, H.-C.; Chun, B.-S. Hydrothermal degradation of seaweed polysaccharide: Characterization and biological activities. Food Chem. 2018, 268, 179–187. [Google Scholar] [CrossRef] [PubMed]
| Parts | Moisture | Ash | Crude Lipid | Crude Protein | Carbohydrate |
|---|---|---|---|---|---|
| Blade | 7.58 ± 0.01 b | 36.63 ± 0.16 b | 2.42 ± 0.62 c | 13.92 ± 0.88 a | 39.45 ± 0.42 b |
| Sporophyll | 8.28 ± 0.29 a | 20.31 ± 0.31 c | 6.34 ± 0.29 a | 13.74 ± 0.67 a | 51.33 ± 0.39 a |
| Root | 5.18 ± 0.27 c | 41.73 ± 0.05 a | 4.25 ± 0.31 b | 9.60 ± 0.79 b | 39.24 ± 0.36 b |
| Parts | Extraction Efficiency (%) | Color | MRPs | ||||||
|---|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | C* | H° | 294 nm | 420 nm | 294/420 | ||
| USE-B | 80.40 ± 0.65 a | 32.30 ± 0.73 a | 13.32 ± 1.26 b | 13.65 ± 1.54 a | 19.07 ± 1.96 a | 45.65 ± 1.16 a | 2.580 ± 0.050 b | 0.188 ± 0.002 b | 13.703 ± 0.367 b |
| USE-S | 73.87 ± 0.32 b | 30.42 ± 0.42 b | 14.00 ± 0.45 a | 10.29 ± 0.36 b | 17.35 ± 0.46 b | 36.27 ± 1.30 b | 2.879 ± 0.068 a | 0.158 ± 0.004 c | 18.185 ± 0.279 a |
| USE-R | 65.93 ± 0.39 c | 27.68 ± 0.87 c | 11.56 ± 0.44 c | 6.96 ± 0.56 c | 13.28 ± 0.56 c | 30.67 ± 1.40 c | 2.886 ± 0.062 a | 0.225 ± 0.003 a | 12.844 ± 0.188 c |
| Parts | Chemical Properties | ||||
|---|---|---|---|---|---|
| Total Phenolic (mg PGE/g of Dry Sample) | Total Flavonoid (mg QE/g of Dry Sample) | Total Sugar (mg Glucose/g of Dry Sample) | Reducing Sugar (mg Glucose/g of Dry Sample) | Total Protein (mg BSA/g of Dry Sample) | |
| USE-B | 33.13 ± 0.14 b | 19.91 ± 0.54 b | 36.43 ± 0.75 c | 21.33 ± 0.51 c | 83.47 ± 1.76 a |
| USE-S | 30.11 ± 0.35 c | 9.22 ± 0.54 c | 97.35 ± 4.23 a | 56.44 ± 3.10 a | 84.93 ± 2.82 a |
| USE-R | 43.32 ± 0.19 a | 31.54 ± 1.63 a | 57.04 ± 1.39 b | 39.44 ± 3.61 b | 65.91 ± 3.53 b |
| Parts | Monosaccharide Composition (%) | Sulfate Content (%) | Molecular Weight (Da) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fucose | Galactose | Glucose | Xylose | Mannose | Peak No. | Mn | Mw | PI | ||
| USE-B | 20.62 ± 1.07 b | 48.57 ± 0.27 a | 17.27 ± 0.73 a | 4.15 ± 0.13 c | 9.41 ± 0.19 c | 2.50 ± 0.10 b | 1 | 2263 | 2924 | 1.29 |
| 2 | 609 | 664 | 1.09 | |||||||
| 3 | 192 | 208 | 1.08 | |||||||
| USE-S | 41.99 ± 0.09 a | 25.04 ± 0.98 c | 14.20 ± 1.20 b | 8.41 ± 0.09 b | 10.37 ± 0.23 b | 7.76 ± 0.17 a | 1 | 2287 | 2914 | 1.27 |
| 2 | 699 | 764 | 1.09 | |||||||
| 3 | 199 | 214 | 1.08 | |||||||
| USE-R | 25.65 ± 2.25 c | 27.45 ± 0.05 b | 14.80 ± 0.30 b | 14.70 ± 2.60 a | 17.40 ± 0.60 a | 2.41 ± 0.20 c | 1 | 2307 | 3025 | 1.31 |
| 2 | 606 | 663 | 1.09 | |||||||
| 3 | 182 | 201 | 1.10 | |||||||
| Parts | Name | Classification | Sample (%) |
|---|---|---|---|
| 1 | Silanol, phosphate | Silane derivative | USE-S (0.75) |
| 2 | Glycerol | Sugar alcohol | USE-B (1.55), USE-S (1.08) |
| 3 | Butanedioic acid | Dicarboxylic acid | USE-B (1.70), USE-S (0.85), USE-R (2.85) |
| 4 | 2-Butenedioic acid | Dicarboxylic acid | USE-S (0.35), USE-B (0.20) |
| 5 | Butanoic acid | Fatty acids | USE-B (0.20), USE-R (0.30) |
| 6 | DL-Pyroglutamic acid | Amino acid derivative | USE-B (2.15), USE-S (1.79) |
| 7 | L-Aspartic acid | α-Amino acid | USE-B (0.49) |
| 8 | N-heneicosane | Alkane | USE-B (0.50) |
| 9 | L-5-Oxoproline | α-Amino acid | USE-R (1.78) |
| 10 | Pentanedioic acid | Dicarboxylic acid | USE-B (0.71), USE-S (0.25), USE-R (1.09) |
| 11 | L-Fucose | Monosaccharide | USE-S (2.93), USE-R (0.48) |
| 12 | Galactopyranose | Monosaccharide | USE-S (0.47) |
| 13 | Citric acid | Tricarboxylic acid | USE-B (1.78), USE-S (1.93), USE-R (1.11) |
| 14 | Talose | Monosaccharide | USE-R (0.60) |
| 15 | Galactose | Monosaccharide | USE-S (0.67) |
| 16 | Sorbitol | Sugar alcohol | USE-B (53.57), USE-S (49.55), USE-R (33.82) |
| 17 | Palmitic Acid, | Fatty acids | USE-B (6.79), USE-S (4.25), USE-R (11.49) |
| 18 | Myo-Inositol | Sugar alcohol | USE-B (0.52), USE-S (1.79) USE-R (0.52) |
| 19 | Stearic acid | Fatty acid | USE-B (4.74), USE-S (2.80), USE-R (7.73) |
| 20 | Glyceryl-glycoside | Glycoside | USE-B (2.69), USE-S (0.27), USE-R (0.27) |
| 21 | 9-Octadecenamide | Fatty amide | USE-S (1.46) |
| 22 | 1-Monopalmitin | Monoacylglycerol | USE-S (2.40) |
| 23 | Glycerol monostearate | Monoacylglycerol | USE-S (2.75) |
| Parts | Antioxidant Activities | Antidiabetic Activity | Antihypertensive Activity | ||
|---|---|---|---|---|---|
| ABTS+ | DPPH | FRAP | α-Glucosidase Inhibitory | ||
| IC50 Value (mg/mL) | EC50 Value (mg/mL) | IC50 Value (mg/mL) | IC50 Value (mg/mL) | ||
| USE-B | 2.44 ± 0.23 b | 4.54 ± 0.14 b | 3.40 ± 0.17 b | 17.52 ± 0.71 a | 0.62 ± 0.01 c |
| USE-S | 3.70 ± 0.62 a | 5.96 ± 0.24 a | 4.02 ± 0.22 a | 14.69 ± 0.59 b | 0.76 ± 0.01 b |
| USE-R | 1.51 ± 0.12 c | 3.31 ± 0.24 c | 2.23 ± 0.13 c | 5.07 ± 0.45 c | 0.90 ± 0.02 a |
| Standard | 0.19 ± 0.01 d | 0.19 ± 0.01 d | 0.27 ± 0.01 d | 0.04 ± 0.01 d | 4.35 × 10−5 ± 0.01 d |
| Trait | TPC | TFC | TSC | RSC | TPrC | ABTS+ | DPPH | FRAP | α-Glucosidase Inhibitory | Antihypertensive Activity |
|---|---|---|---|---|---|---|---|---|---|---|
| TPC | 1 | 0.961 ** | −0.394 ns | −0.200 ns | −0.989 ** | −0.925 ** | −0.941 ** | −0.992 ** | −0.905 ** | 0.736 * |
| TFC | 1 | −0.632 ns | −0.463 ns | −0.909 ** | −0.994 ** | −0.998 ** | −0.973 ** | −0.753 ** | 0.521 * | |
| TSC | 1 | 0.975 ** | 0.513 ns | 0.628 * | 0.576 * | 0.825 ** | −0.034 ns | 0.333 ns | ||
| RSC | 1 | 0.665 ns | 0.839 ** | 0.795 ** | 0.930 ** | −0.235 ns | 0.516 * | |||
| TPrC | 1 | −0.974 ** | −0.986 ** | −0.926 ** | 0.959 ** | −0.829 ** | ||||
| ABTS+ | 1 | 0.999 ** | 0.980 ** | 0.676 * | −0.423 ns | |||||
| DPPH | 1 | 0.993 ** | 0.708 * | −0.464 ns | ||||||
| FRAP | 1 | 0.844 * | −0.644 ns | |||||||
| α-glucosidase inhibitory | 1 | −0.954 ** | ||||||||
| Antihypertensive activity | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.-S.; Han, J.-M.; Park, S.-W.; Kim, J.-W.; Choi, M.-S.; Lee, S.-M.; Haq, M.; Zhang, W.; Chun, B.-S. Subcritical Water Extraction of Undaria pinnatifida: Comparative Study of the Chemical Properties and Biological Activities across Different Parts. Mar. Drugs 2024, 22, 344. https://doi.org/10.3390/md22080344
Park J-S, Han J-M, Park S-W, Kim J-W, Choi M-S, Lee S-M, Haq M, Zhang W, Chun B-S. Subcritical Water Extraction of Undaria pinnatifida: Comparative Study of the Chemical Properties and Biological Activities across Different Parts. Marine Drugs. 2024; 22(8):344. https://doi.org/10.3390/md22080344
Chicago/Turabian StylePark, Jin-Seok, Ji-Min Han, Sin-Won Park, Jang-Woo Kim, Min-Seo Choi, Sang-Min Lee, Monjurul Haq, Wei Zhang, and Byung-Soo Chun. 2024. "Subcritical Water Extraction of Undaria pinnatifida: Comparative Study of the Chemical Properties and Biological Activities across Different Parts" Marine Drugs 22, no. 8: 344. https://doi.org/10.3390/md22080344
APA StylePark, J.-S., Han, J.-M., Park, S.-W., Kim, J.-W., Choi, M.-S., Lee, S.-M., Haq, M., Zhang, W., & Chun, B.-S. (2024). Subcritical Water Extraction of Undaria pinnatifida: Comparative Study of the Chemical Properties and Biological Activities across Different Parts. Marine Drugs, 22(8), 344. https://doi.org/10.3390/md22080344








