Evaluation of Antioxidant Activity of Residue from Bioethanol Production Using Seaweed Biomass
Abstract
1. Introduction
2. Results
2.1. Characterization of the Biomass
| Species | Seaweed | Composition | |||
|---|---|---|---|---|---|
| Carbohydrate | Crude Protein | Crude Lipid | Crude Ash | ||
| Red seaweed | Gelidium amansii [25] | 74.40 | 7.27 | 0.03 | 18.30 |
| Gloiopeltis furcata [26] | 62.56 | 24.47 | 0.23 | 12.74 | |
| Pyropia tenera | 52.04 | 33.77 | 2.15 | 12.04 | |
| Brown seaweed | Ascophyllum nodosum [27] | 69.70 | 23.30 | 4.20 | 2.80 |
| Sacchrina japonica [28] | 66.00 | 10.60 | 1.60 | 21.80 | |
| Undaria pinnatifida | 43.20 | 23.80 | 3.50 | 29.50 | |
| Green seaweed | Codium fragile | 34.24 | 10.64 | 2.23 | 52.89 |
| Ulvaintestinalis | 31.60 | 29.20 | 1.80 | 37.40 | |
| Ulva prolifera | 45.30 | 30.84 | 0.78 | 23.08 | |
2.2. Bioethanol Production from Nine Seaweeds
2.3. Antioxidant Activity of Bioethanol Residue Extracts (BRE)
2.4. Cell Viability in the Presence of BRE
3. Materials and Methods
3.1. Biomass
3.2. Ethanol Production
3.3. Seaweed Extraction
3.4. Antioxidant Analyses
3.4.1. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Assay
3.4.2. Ferric Reducing Antioxidant Power (FRAP) Assay
3.5. Cytotoxicity and Antioxidant Evaluation
3.5.1. Cell Culture
3.5.2. Cell Viability Assay
3.5.3. Determination of Intracellular ROS Scavenging Activity
3.6. Analysis
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Androniceanu, A.; Sabie, O.M. Overview of green energy as a real strategic option for sustainable development. Energies 2022, 15, 8573. [Google Scholar] [CrossRef]
- Srivastava, R.K.; Shetti, N.P.; Reddy, K.R.; Aminabhavi, T.M. Biofuels, biodiesel and biohydrogen production using bioprocesses. A review. Environ. Chem. Lett. 2020, 18, 1049–1072. [Google Scholar] [CrossRef]
- Londono-Pulgarin, D.; Cardona-Montoya, G.; Restrepo, J.C.; Munoz-Leiva, F. Fossil or bioenergy? Global fuel market trends. Renew. Sustain. Energy Rev. 2021, 143, 110905. [Google Scholar] [CrossRef]
- Stančin, H.; Mikulčić, H.; Wang, X.; Duić, N. A review on alternative fuels in future energy system. Renew. Sustain. Energy Rev. 2020, 128, 109927. [Google Scholar] [CrossRef]
- Chadar, S.N.; Ahirwar, A.K. Biofuel from biomass as an alternative energy source for sustainable development. Open Access Res. J. Sci. Technol. 2022, 6, 071–074. [Google Scholar] [CrossRef]
- Edwin, M.; Nila, J.N.; Nair, M.S. Biofuel production: An initiative of environmentally sound technologies (EST’s) or Green technologies. In Environmental Sustainability of Biofuels; Elsevier: Amsterdam, The Netherlands, 2023; pp. 99–136. [Google Scholar]
- Ceylan, S.; Potuk, K.; Bayraktar, O. High-value–added products from microalgae production integrated with bioethanol process. In Bioenergy Engineering; Elsevier: Amsterdam, The Netherlands, 2023; pp. 315–332. [Google Scholar]
- Liu, J.J.; Dickson, R.; Niaz, H.; Van Hal, J.W.; Dijkstra, J.; Fasahati, P. Production of fuels and chemicals from macroalgal biomass: Current status, potentials, challenges, and prospects. Renew. Sustain. Energy Rev. 2022, 169, 112954. [Google Scholar] [CrossRef]
- Zafar, S.U.; Mehra, A.; Nesamma, A.A.; Jutur, P.P. Innovations in algal biorefineries for production of sustainable value chain biochemicals from the photosynthetic cell factories. Algal Res. 2023, 69, 102949. [Google Scholar] [CrossRef]
- Yun, J.H.; Archer, S.D.; Price, N.N. Valorization of waste materials from seaweed industry: An industry survey based biorefinery approach. Rev. Aquac. 2023, 15, 1020–1027. [Google Scholar] [CrossRef]
- Kumar, S.; Gupta, R.; Kumar, G.; Sahoo, D.; Kuhad, R.C. Bioethanol production from Gracilaria verrucosa, a red alga, in a biorefinery approach. Bioresour. Technol. 2013, 135, 150–156. [Google Scholar] [CrossRef]
- Prabhu, M.S.; Israel, A.; Palatnik, R.R.; Zilberman, D.; Golberg, A. Integrated biorefinery process for sustainable fractionation of Ulva ohnoi (Chlorophyta): Process optimization and revenue analysis. J. Appl. Phycol. 2020, 32, 2271–2282. [Google Scholar] [CrossRef]
- Arias, A.; Feijoo, G.; Moreira, M.T. Macroalgae biorefineries as a sustainable resource in the extraction of value-added compounds. Algal Res. 2023, 69, 102954. [Google Scholar] [CrossRef]
- Kassim, M.A.; Meng, T.K.; Serri, N.A.; Yusoff, S.B.; Shahrin, N.A.M.; Seng, K.Y.; Bakar, M.H.A.; Keong, L.C. Sustainable biorefinery concept for industrial bioprocessing. In Biorefinery Production Technologies for Chemicals and Energy; John Wiley & Sons: Hoboken, NJ, USA, 2020; pp. 15–53. [Google Scholar]
- Trivedi, N.; Mondal, A.S.; Sharma, R.; Mone, D. Marine macroalgal biorefinery: Recent developments and future perspectives. In Algal Biorefineries and the Circular Bioeconomy; CRC Press: Boca Raton, FL, USA, 2022; pp. 1–36. [Google Scholar]
- Ismail, M.M.; El Zokm, G.M.; Lopez, J.M.M. Nutritional, bioactive compounds content, and antioxidant activity of brown seaweeds from the Red Sea. Front. Nutr. 2023, 10, 1210934. [Google Scholar] [CrossRef] [PubMed]
- John, R.P.; Anisha, G.; Nampoothiri, K.M.; Pandey, A. Micro and macroalgal biomass: A renewable source for bioethanol. Bioresour. Technol. 2011, 102, 186–193. [Google Scholar] [CrossRef]
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Samarathunga, J.; Wijesekara, I.; Jayasinghe, M. Seaweed proteins as a novel protein alternative: Types, extractions, and functional food applications. Food Rev. Int. 2023, 39, 4236–4261. [Google Scholar] [CrossRef]
- Fleurence, J. Seaweed proteins: Biochemical, nutritional aspects and potential uses. Trends Food Sci. Technol. 1999, 10, 25–28. [Google Scholar] [CrossRef]
- Premarathna, A.D.; Tuvikene, R.; Fernando, P.; Adhikari, R.; Perera, M.; Ranahewa, T.; Howlader, M.M.; Wangchuk, P.; Jayasooriya, A.P.; Rajapakse, R. Comparative analysis of proximate compositions, mineral and functional chemical groups of 15 different seaweed species. Sci. Rep. 2022, 12, 19610. [Google Scholar] [CrossRef] [PubMed]
- Habeebullah, S.F.K.; Alagarsamy, S.; Al-Haddad, S.; Al-Yamani, F. Composition, in vitro antioxidant and angiotensin-converting enzyme inhibitory effects of lipids isolated from fifteen species of seaweeds. Food Chem. Adv. 2023, 3, 100352. [Google Scholar] [CrossRef]
- Plaza, M.; Cifuentes, A.; Ibáñez, E. In the search of new functional food ingredients from algae. Trends Food Sci. Technol. 2008, 19, 31–39. [Google Scholar] [CrossRef]
- Sharma, S.; Neves, L.; Funderud, J.; Mydland, L.T.; Øverland, M.; Horn, S.J. Seasonal and depth variations in the chemical composition of cultivated Saccharina latissima. Algal Res. 2018, 32, 107–112. [Google Scholar] [CrossRef]
- Sunwoo, I.Y.; Ra, C.H.; Jeong, G.-T.; Kim, S.-K. Evaluation of ethanol production and bioadsorption of heavy metals by various red seaweeds. Bioprocess Biosyst. Eng. 2016, 39, 915–923. [Google Scholar] [CrossRef]
- Park, Y.R.; Yang, J.W.; Sunwoo, I.Y.; Jang, B.-K.; Kim, S.R.; Jeong, G.-T.; Kim, S.-K. Enhancement of catabolite regulatory genes in Saccharomyces cerevisiae to increase ethanol production using hydrolysate from red seaweed Gloiopeltis furcata. J. Biotechnol. 2021, 333, 1–9. [Google Scholar] [CrossRef]
- Sunwoo, I.; Kwon, J.E.; Jeong, G.-T.; Kim, S.-K. Optimization of hyper-thermal acid hydrolysis and enzymatic saccharification of Ascophyllum nodosum for ethanol production with mannitol-adapted yeasts. Bioprocess Biosyst. Eng. 2019, 42, 1255–1262. [Google Scholar] [CrossRef] [PubMed]
- Ra, C.H.; Sunwoo, I.Y.; Nguyen, T.H.; Sukwang, P.; Sirisuk, P.; Jeong, G.-T.; Kim, S.-K. Butanol and butyric acid production from Saccharina japonica by Clostridium acetobutylicum and Clostridium tyrobutyricum with adaptive evolution. Bioprocess Biosyst. Eng. 2019, 42, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Kadimpati, K.K.; Thadikamala, S.; Devarapalli, K.; Banoth, L.; Uppuluri, K.B. Characterization and hydrolysis optimization of Sargassum cinereum for the fermentative production of 3G bioethanol. Biomass Convers. Biorefinery 2021, 13, 1831–1841. [Google Scholar] [CrossRef]
- Kostas, E.T.; White, D.A.; Cook, D.J. Bioethanol production from UK seaweeds: Investigating variable pre-treatment and enzyme hydrolysis parameters. Bioenergy Res. 2020, 13, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, A.W.; Wunderlich, J.; Müller, L.; Buchner, G.A.; Marxen, A.; Michailos, S.; Armstrong, K.; Naims, H.; McCord, S.; Styring, P. Techno-economic assessment guidelines for CO2 utilization. Front. Energy Res. 2020, 8, 5. [Google Scholar] [CrossRef]
- Ramachandra, T.; Hebbale, D. Bioethanol from macroalgae: Prospects and challenges. Renew. Sustain. Energy Rev. 2020, 117, 109479. [Google Scholar] [CrossRef]
- Ha, N.T.; Ha, C.H.; Hayakawa, N.; Chujo, R.; Kawahara, S. Relationship between structure and some physico-chemical properties of funori from red seaweed Gloiopeltis. J. Cult. Herit. 2021, 51, 14–20. [Google Scholar] [CrossRef]
- Chan, P.T.; Matanjun, P.; Yasir, S.M.; Tan, T.S. Antioxidant activities and polyphenolics of various solvent extracts of red seaweed, Gracilaria changii. J. Appl. Phycol. 2015, 27, 2377–2386. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Q.; Luo, H.; Deng, S.; Tian, Y.; Wang, S. Mechanisms of the ethanol extract of Gelidium amansii for slow aging in high-fat male Drosophila by metabolomic analysis. Food Funct. 2022, 13, 10110–10120. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.-S.; Do Thi, N. Effects of extraction and processing methods on antioxidant compound contents and radical scavenging activities of laver (Porphyra tenera). Prev. Nutr. Food Sci. 2014, 19, 40. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, W.; Latimer, G.W. Official Methods of Analysis of AOAC International; AOAC International Gaithersburg: Rockville, MD, USA, 2000; Volume 1. [Google Scholar]
- Graikini, D.; Soro, A.B.; Sivagnanam, S.P.; Tiwari, B.K.; Sánchez, L. Bioactivity of Fucoidan-Rich Extracts from Fucus vesiculosus against Rotavirus and Foodborne Pathogens. Mar. Drugs 2023, 21, 478. [Google Scholar] [CrossRef] [PubMed]
- Ale, M.T.; Mikkelsen, J.D.; Meyer, A.S. Important determinants for fucoidan bioactivity: A critical review of structure-function relations and extraction methods for fucose-containing sulfated polysaccharides from brown seaweeds. Mar. Drugs 2011, 9, 2106–2130. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, Y.; Yang, H.; Li, H.; Xu, W.; Chen, G.; Zhu, H. Physicochemical characterization, antioxidant and immunostimulatory activities of sulfated polysaccharides extracted from Ascophyllum nodosum. Molecules 2018, 23, 1912. [Google Scholar] [CrossRef] [PubMed]
- Abd El Hafez, M.S.M.; ElKomy, R.G.; Saleh, H.; Aboul-Ela, H.M. Extracts of the green algae Ulva prolifera possess antioxidant and antibacterial activities in vitro. Egypt. J. Aquat. Biol. Fish. 2020, 24, 267–280. [Google Scholar] [CrossRef]
- Hansen, M.B.; Nielsen, S.E.; Berg, K. Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J. Immunol. Methods 1989, 119, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Sunwoo, I.; Kim, Y.; Kim, J.; Cho, H.; Jeong, G.-T. Optimization, Scale-Up, and Economic Analysis of the Ethanol Production Process Using Sargassum horneri. Fermentation 2023, 9, 1004. [Google Scholar] [CrossRef]
- Sunwoo, I.Y.; Sukwong, P.; Park, Y.R.; Jeong, D.Y.; Kim, S.R.; Jeong, G.-T.; Kim, S.-K. Enhancement of galactose uptake from Kappaphycus alvarezii using Saccharomyces cerevisiae through deletion of negative regulators of GAL genes. Appl. Biochem. Biotechnol. 2021, 193, 577–588. [Google Scholar] [CrossRef]
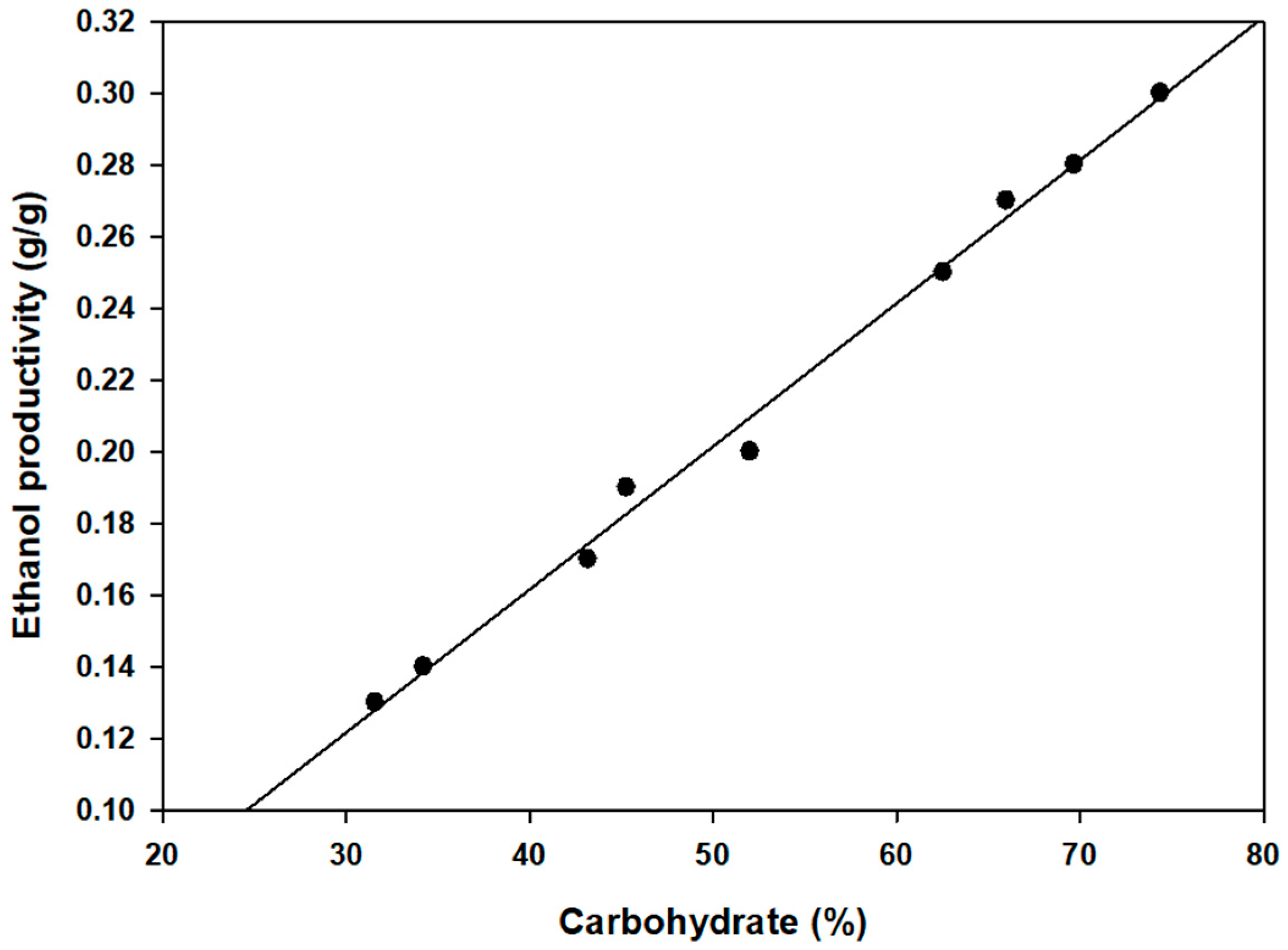
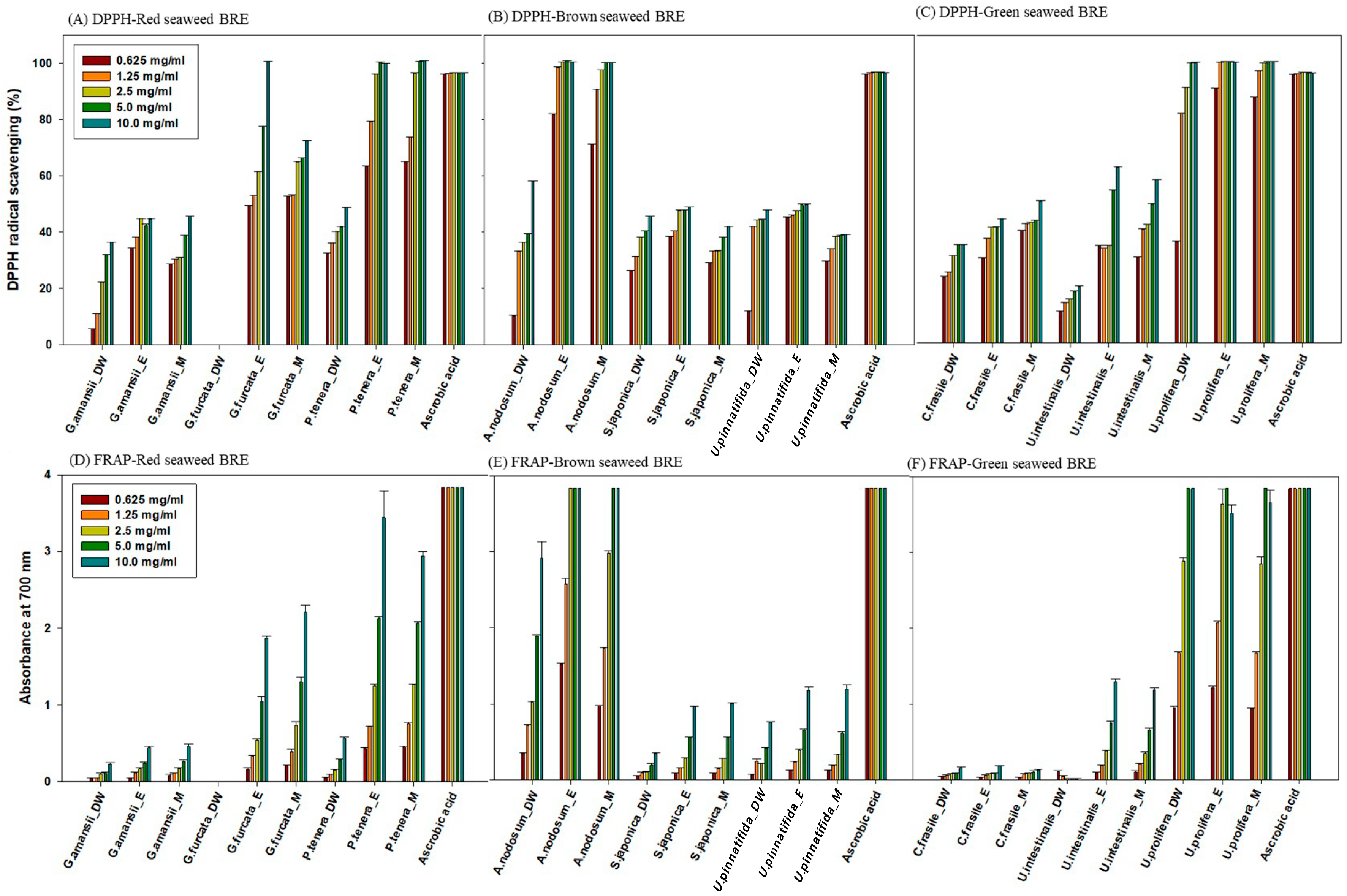
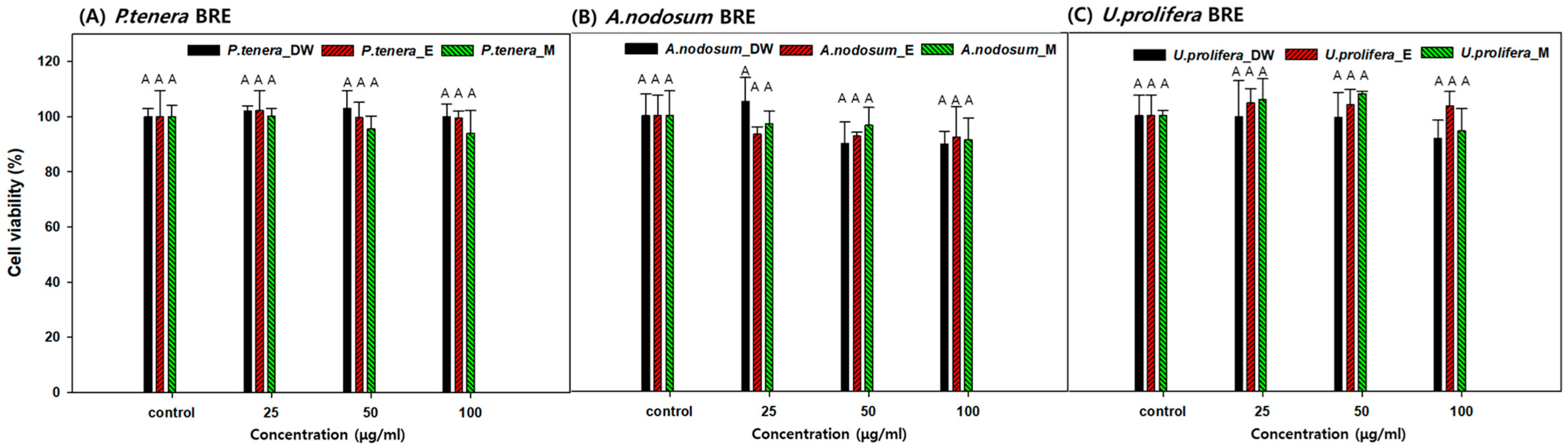
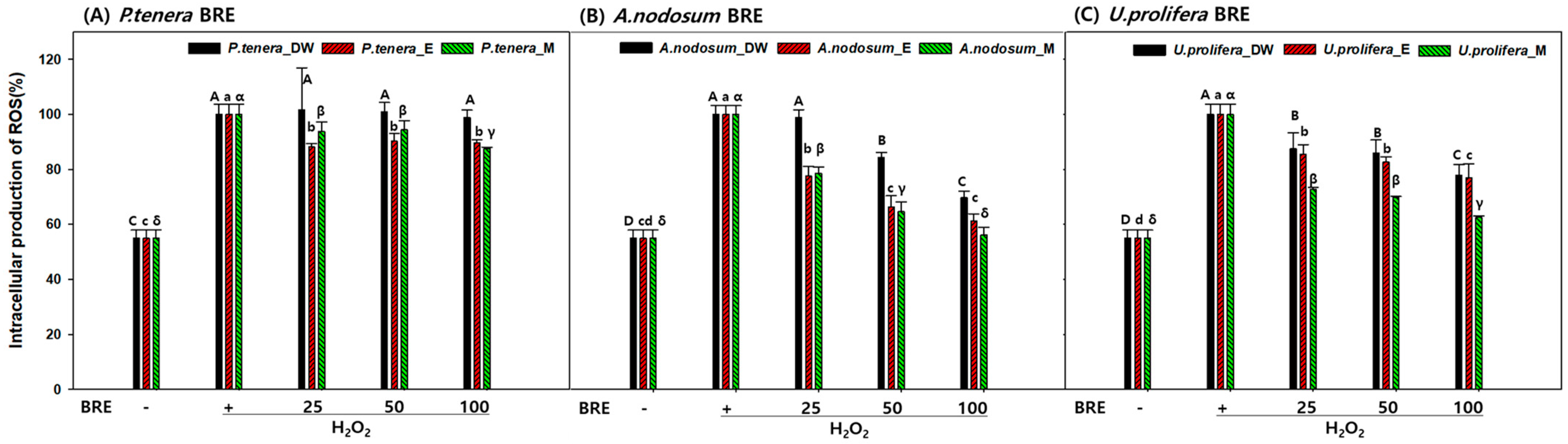
| Species | Seaweed | Efficiency of Pretreatment (%) | Initial Monosaccharides (g/L) | Ethanol (g/L) | Productivity (g EtOH/ g Biomass) | ||
|---|---|---|---|---|---|---|---|
| Glucose | Galactose | Mannitol | |||||
| Red seaweed | G. amansii | 82.19 | 27.11 | 21.81 | - | 23.97 | 0.30 |
| G. furcata | 81.12 | 22.43 | 18.17 | - | 19.89 | 0.25 | |
| P. tenera | 78.76 | 20.23 | 12.56 | - | 16.07 | 0.20 | |
| Brown seaweed | A. nodosum | 81.12 | 31.12 | - | 14.11 | 22.16 | 0.28 |
| S. japonica | 82.45 | 32.12 | - | 11.41 | 21.33 | 0.27 | |
| U. pinnatifida | 79.12 | 21.12 | - | 6.22 | 13.40 | 0.17 | |
| Green seaweed | C. fragile | 80.12 | 20.00 | - | - | 11.19 | 0.14 |
| U. intestinalis | 79.45 | 18.90 | - | - | 10.24 | 0.13 | |
| U. prolifera | 83.12 | 28.53 | - | - | 15.36 | 0.19 | |
| DPPH IC50 (mg/mL) | Red Seaweed | Brown Seaweed | Green Seaweed | ||||||
|---|---|---|---|---|---|---|---|---|---|
| G. amansii | G. furcata | P. tenera | A. nodosum | S. japonica | U. pinnatifida | C. fragile | U. intestinalis | U. prolifera | |
| H2O | 10.62 | - | 5.69 | 6.41 | 6.51 | 5.47 | 7.96 | 17.07 | 2.07 |
| Ethanol | 5.14 | 2.55 | 1.79 | 1.80 | 4.69 | 4.21 | 5.62 | 5.10 | 1.85 |
| Methanol | 6.97 | 3.07 | 1.78 | 1.77 | 6.63 | 6.02 | 4.79 | 5.06 | 1.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sunwoo, I.-Y.; Cho, H.; Kim, T.; Koh, E.-J.; Jeong, G.-T. Evaluation of Antioxidant Activity of Residue from Bioethanol Production Using Seaweed Biomass. Mar. Drugs 2024, 22, 340. https://doi.org/10.3390/md22080340
Sunwoo I-Y, Cho H, Kim T, Koh E-J, Jeong G-T. Evaluation of Antioxidant Activity of Residue from Bioethanol Production Using Seaweed Biomass. Marine Drugs. 2024; 22(8):340. https://doi.org/10.3390/md22080340
Chicago/Turabian StyleSunwoo, In-Yung, Hyunjin Cho, Taeho Kim, Eun-Jeong Koh, and Gwi-Taek Jeong. 2024. "Evaluation of Antioxidant Activity of Residue from Bioethanol Production Using Seaweed Biomass" Marine Drugs 22, no. 8: 340. https://doi.org/10.3390/md22080340
APA StyleSunwoo, I.-Y., Cho, H., Kim, T., Koh, E.-J., & Jeong, G.-T. (2024). Evaluation of Antioxidant Activity of Residue from Bioethanol Production Using Seaweed Biomass. Marine Drugs, 22(8), 340. https://doi.org/10.3390/md22080340





