Abstract
Abalone is a rich source of nutrition, the viscera of which are discarded as by-product during processing. This study explored the biological activities of peptides derived from abalone viscera (AV). Trypsin-hydrolysate of AV (TAV) was purified into three fractions using a Sephadex G-10 column. Nine bioactive peptides (VAR, NYER, LGPY, VTPGLQY, QFPVGR, LGEW, QLQFPVGR, LDW, and NLGEW) derived from TAV-F2 were sequenced. LGPY, VTPGLQY, LGEW, LDW, and NLGEW exhibited antioxidant properties, with IC50 values of 0.213, 0.297, 0.289, 0.363, and 0.303 mg/mL, respectively. In vitro analysis determined that the peptides VAR, NYER, VTPGLQY, QFPVGR, LGEW, QLQFPVGR, and NLGEW inhibited ACE, with IC50 values of 0.104, 0.107, 0.023, 0.023, 0.165, 0.004, and 0.146 mg/mL, respectively. The binding interactions of ACE-bioactive peptide complexes were investigated using docking analysis with the ZDCOK server. VTPGLQT interacted with HIS513 and TYR523, and QLQFPVGR interacted with HIS353, ALA354, GLU384, HIS513, and TYR523, contributing to the inhibition of ACE activity. They also interacted with amino acids that contribute to stability by binding to zinc ions. QFPVGR may form complexes with ACE surface sites, suggesting indirect inhibition. These results indicate that AV is a potential source of bioactive peptides with dual antioxidant and anti-hypertensive dual effects.
1. Introduction
Bioactive peptide consists of 2–20 amino acids that are important for regulating the essential activities of human metabolism [1,2]. Bioactive peptides have various activities, such as antioxidant, anti-inflammatory, and anti-hypertension activities, and skin improvement [3,4,5,6]. Food-derived bioactive peptides are safe and have health values [7]. Seafood is a strategic source of bioactive peptides and has, therefore, attracted research attention [8].
Reactive oxygen species (ROS) are physiological metabolites that cause oxidative damage to macromolecules in cells and are eliminated by the antioxidant defense system. Hydrogen peroxide (H2O2), a representative ROS, interacts with the peroxidase enzyme in humans [9]. Recent studies have reported that bioactive peptides with H2O2 resistance exhibit dual anti-hypertensive properties [10]. Hypertension is a cardiovascular condition that affects 30–45% of the population (2023) [11]. The World Health Organization (WHO) reported that hypertension is the most important cause of death [12]. Angiotensin-I converting enzyme (ACE) inhibition is an effective anti-hypertensive therapy.
Previous studies have reported that bioactive peptides isolated from proteins supplied by digestion disturb ACE [13]. Marine peptides derived from fish, jellyfish, sea cucumber, sponge, and oysters demonstrate excellent anti-hypertensive properties [14]. Indeed, previous studies have reported the dual effects of antioxidant and anti-hypertensive bioactive peptides derived from marine resources such as seaweeds and marine diatoms; however, detailed data remain lacking [15,16,17].
The sea has enormous diversity, making it an important source of novel compounds. As marine consumption increases, fishery by-products are produced during processing [18]. These by-products are difficult to dispose of owing to their rapidly decomposing vulnerabilities and are accompanied by environmental and economic problems [19,20]. Marine molluscs include 48,584 species and are a rich source of proteins [21,22,23]. Abalone is a seafood consisting of gastropod molluscs which are highly produced and consumed as the dominant source of nutrition, especially in Asia [24]. Abalone viscera (AV) account for 15–25% of the body weight and cannot be disposed of directly due to their high organic content [25,26]. With industrial development, AV are drawing attention as fishery by-products, with the possibility of becoming affordable, economical, and high-value-added products [27]. Viscera contain abundant proteins that serve as sources of bioactive peptides [28]. Enzymatic hydrolysis is the most beneficial and frequently used method for extracting bioactive peptides from materials [29].
In this study, to illustrate fishery by-products as a source of protein for bioactive peptides, the dual AV antioxidant and anti-hypertensive effects and the interaction with ACE are confirmed through docking analysis [30,31].
2. Results and Discussion
2.1. Proximate Compositions of TAV
The proximate compositions of the trypsin-hydrolysate of AV (TAV) are summarized in Table 1. The yield was 75.44 ± 1.22%, calculated by subtracting the dry weight of the residue from the TAV and expressed as a percentage. The protein, polysaccharide, and total polyphenol contents of the TAV were 48.19 ± 0.58, 6.30 ± 0.26, and 2.36 ± 0.03%, respectively. TAV’s primary chemical component is protein; hence, it was selected. The yield of enzymatic hydrolysate assisted by another protease (Alcalase, Flavourzyme, Neutrase, and Protamex) of AV previously reported was 28.02–34.64%, with a protein content of 19.30% [32]. Therefore, compared with the results of this study, trypsin-hydrolysis can be adopted as an efficient procedure to obtain a high protein yield by achieving a content difference of approximately double.

Table 1.
Proximate compositions of TAV.
2.2. Separation of Peptides from TAV and Determination of Their H2O2 Radical Scavenging Activity
Enzyme hydrolysates purified according to molecular size exhibit antioxidant properties as the molecular weight decreases [33]. Therefore, we conducted purification to investigate the antioxidant properties of the TAV according to molecular size. The TAV was purified using GFC on a Sephadex G-10 column to separate the active peptides. As shown in Figure 1, the TAV was separated into three fractions according to molecular size. When the highest concentration (4 mg/mL) of all fractions was treated, the TAV and TAV-F1 showed H2O2 scavenging effects close to 90%, and TAV-F2 and TAV-F3 showed scavenging effects close to 100%. The IC50 value of the scavenging effects of the TAV was 1.34 ± 0.01 mg/mL, which was not significantly different from that of TAV-F1 of 1.37 ± 0.19 mg/mL. However, the IC50 values of TAV-F2 and TAV-F3 were 0.41 ± 0.08 and 0.47 ± 0.04 mg/mL, respectively, which were significantly low (Figure 2).
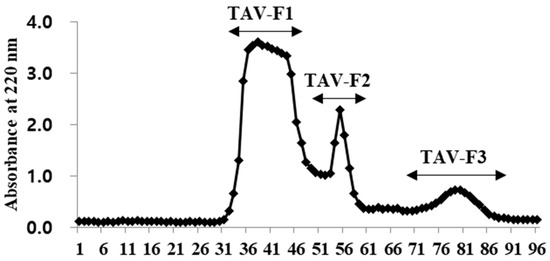
Figure 1.
Separation chromatogram of TAV by gel filtration chromatography on Sephadex G-10 Column.
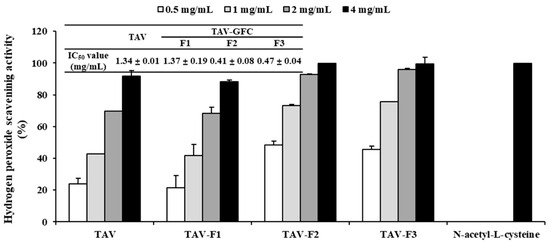
Figure 2.
Determination of H2O2 scavenging activity of TAV and gel filtration chromatography fractions with IC50 Values.
In a previous study, the DPPH and hydroxyl scavenging effects of the TAV showed IC50 values of 4 and 23 mg/mL, respectively [34]. Conversely, this study investigated the TAV at a significantly lower concentration for H2O2 scavenging activity. In addition, the antioxidant properties of the low-molecular-weight peptides were determined by measuring the IC50 values of TAV-F2 and TAV-F3, which were significantly lower than that of the TAV.
2.3. Identification of Separated Active Peptides and Determination of Their H2O2 Radical Scavenging Activity
The efficacy of the separated peptides was evaluated to identify bioactive peptides with antioxidant properties. TAV-F2, which was preferred, considering its effects and yield, was analyzed using a Micro Q-TOF mass spectrometer as a fraction of the amino acid sequence. Nine main peaks were detected in the chromatogram (Figure 3), which corresponded to the following peptides: VAR, NYER, LGPY, VTPGLQY, QFPVGR, LGEW, QLQFPVGR, LDW, and NLGEW. To compare the efficacy of the fraction, peptides with the same sequence were synthesized to measure H2O2 scavenging activity (Figure 4 and Table 2). The H2O2 scavenging activity of the LGPY was 0.213 ± 0.010 mg/mL, which was the lowest IC50 value, and VAR, QFPVGR, and QLQFPVGR activity were not detected. The IC50 value of NYER exceeded 0.4 mg/mL, while the IC50 values of VTPGLQY, LGEW, LDW, and NLGEW were 0.297 ± 0.016, 0.289 ± 0.012, 0.363 ± 0.020, and 0.303 ± 0.001 mg/mL, respectively, which were lower than the IC50 value of TAV-F2. Previous studies have shown that the presence of tryptophan in functional peptides is closely related to antioxidant efficacy [35]. Additionally, C-terminus residues contribute to the improvement in the antioxidant ability of peptides containing aromatic amino acid Tyrosine, and the presence of Leucine in the N-terminus has also been reported to have antioxidant efficacy [36]. The average concentration of AV-derived bioactive peptides isolated using different protocols has been reported to be 0.611 mg/mL [37]. The bioactive peptides derived from TAV-F2 presented a clear scavenging effect and may serve as a factor that allows for the utilization of the TAV as an antioxidant source for bioactive peptides from fishery by-products [38,39].
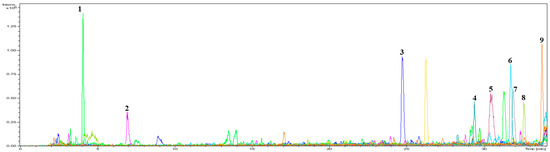
Figure 3.
UHPLC analysis chromatogram by C18 column obtained from TAV-F2.
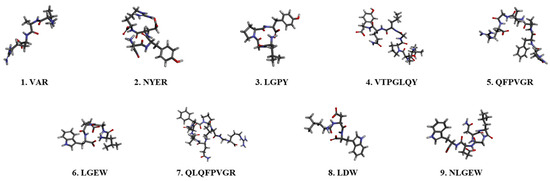
Figure 4.
Sequencing results and 3D structures of bioactive peptides derived from TAV-F2.

Table 2.
Determination of mass-to-charge ratio using LC-MS/MS and H2O2 scavenging activity IC50 values of bioactive peptides derived from TAV-F2.
2.4. In Vitro Analysis of Active Peptides on ACE Inhibition
Recently, materials that possess both antioxidant and anti-hypertensive properties have been designed for the functional food industry, focusing on bioactive peptides [40]. Previous studies have revealed the ACE-inhibitory activity of bioactive peptides, including their antioxidant effects [10]. Marine gastropods have also been studied as the sources of the dual effects [41]. However, only limited attempts have been made to use marine resources for this purpose, and there has been no access to AV, a by-product of fisheries. Marine products are receiving increasing attention as functional materials which have the effects of anti-hypertension, and the proteins derived from them are important sources of physiological activities [42]. Research on ACE inhibition has demonstrated that peptides capable of inhibiting ACE are short-sequenced [43,44]. Various studies have been conducted on ACE inhibition by bioactive peptides derived from hydrolysates of fishery by-product enzymes obtained using proteases such as trypsin [45].
Therefore, this study evaluated the ACE-inhibitory effects of bioactive peptides derived from TAV-F2 through in vitro analysis to investigate their dual effects. The ACE inhibition activity IC50 values are listed in Table 3. Except for LGPY and LDW, the peptides of IC50 values were VAR (0.104 ± 0.010), NYER (0.107 ± 0.004), LGEW (0.165 ± 0.011), and NLGEW (0.146 ± 0.009 mg/mL). QLQFPVGR showed the best efficacy with a value of 0.004 ± 0.001 mg/mL, followed by VTPGLQY and QFPVGR at 0.023 ± 0.001 and 0.023 ± 0.003 mg/mL, respectively. The ACE inhibitory efficacy of the isolated seahorse-derived bioactive peptides was measured via comparable protocols for IC50 values of 0.088–0.171 mg/mL in an in vitro study [40]. Through comparison with these results, the inhibition efficacy of QLQFPVGR, VTPGLQY, and QFPVGR was confirmed.

Table 3.
In vitro and in silico results of ACE inhibitory activity of bioactive peptides derived from TAV-F2.
2.5. In Silico Analysis of Active Peptides on ACE Inhibition
Computer prediction simulations were used to investigate the interactions between the bioactive peptides derived from TAV-F2 and ACE. ZDOCK is an automated simulation that selects poses by clustering according to ligand position, and has a high level of understanding in the modeling and analysis of protein–protein complexes essential for physiological activity [46,47,48]. The ZRANK score, which showed an accurate hit rate, was adopted and its efficacy was assessed [49]. Automated docking analysis was used to predict the binding interaction between peptides with short amino acid sequences and the target protein (Figure S1). The positive control group (captopril) used in vitro is a macromolecule compound that inhibits it as an ACE target and has not been studied using ZDOCK, which focuses on protein–protein binding in this paper. In particular, QLQFPVGR, VTPGLQY, and QFPVGR were evaluated as having the lowest ZRANK scores of −82.468, −81.500, and −78.016, respectively, whereas the peptides, except for VAR, had scores lower than −50.000 (Table 3). The in vitro and in silico results for the three peptides with the best efficacy were equivalent. The docking poses are shown as 2D diagrams and 3D crystalline structures to investigate the interaction of the combined docking complexes (Figure 5). In the predicted binding complex, VTPGLQY interacted with TYR51, TRP59, ASN66, ILE88, HIS91, THR92, LYS 118, TRP357, TYR360, HIS387, PHE391, VAL399, ARG402, GLU403, HIS513, PRO519, PROG522, and TYR523. QFPVGR was predicted to interact with ALA170, SER284, ASN285, THR302, VAL373, ASN374, LEU375, and GLU376, and QLQFPVGR was predicted to interact with ASN70, GLU123, HIS353, ALA354, SER355, ALA356, TRP357, HUS383, GLU384, HIS387, PRO407, HIS410, GLU411, PHE457, HIS513, SER517, ARG522, TYR523, and GLY2000 residues.
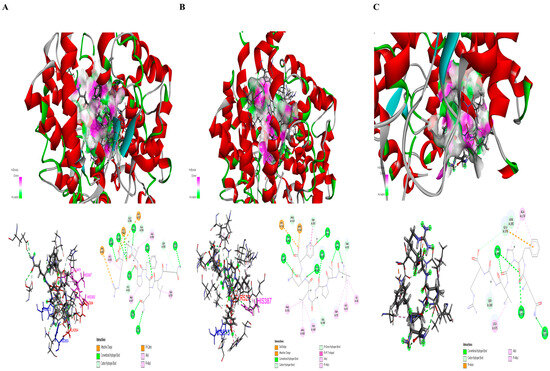
Figure 5.
The prediction of the binding site of ACE-bioactive peptide complexes. The ACE-bioactive peptide complexes are shown by favorable hydrogen bond interactions at specific points and represented as stick models of residues with amino acid names and 2D diagrams of the binding complex. QLQFPVGR (A), VTPGLQY (B), and QFPVGR (C) are shown. The amino acid interaction in the active site pockets S1 and S2 are marked in red and blue, respectively, while the amino acid interaction in the zinc ion binding is marked in pink.
ACE comprises three major active site pockets that interact with residues: the S1 pocket (ALA354, GLU384, and TYR 523), S1’ pocket (GLU162), and S2 pocket (GLN281, HIS353, LYS511, HIS513, and TYR520) [50]. HIS383, HIS387, and GLU411 in ACE and their binding to zinc ions play a significant role in protein activity by coordinating tetrahedrons [51]. The sites of interaction between these residues are shown in Figure S2. QLQFPVGR forms hydrogen bonds with ALA354, GLU384, and TYR523, which are in the S1 pocket, and express an attractive charge with HIS353 and HIS513, which are in the S2 pocket. VTPGLQY forms a hydrogen bond with TYR523, which is in the S1 pocket, and shows that HIS513, in the S2 pocket, is an attractive charge. VTPGLQY forms a hydrogen bond with HIS387, and QLQFPVGR forms a pi-alkyl bond with HIS383 and hydrogen bonds with HIS387 and GLU411, where the residues interact with zinc ions (Figure 5A,B). The HIS513 and TYR523 residues interacting with VTPGLQY and the residues interacting with HIS353, ALA354, GLU411, HIS513, and TYR523, and those interacting with QLQFPVGR, are included in the residues comprising the complex binding of lisinopril, which is used as an ACE target inhibitor [52]. Moreover, it interacts with VTPGLQY, HIS513, and TRY523 residues, and QLQFPVGR interacts with HIS353, ALA354, HIS513, and TYR523 residues, and provides evidence for strong activity involved in predictive binding to its ACE-inhibition compound captopril [53]. The predicted ACE-lisinopril and captopril binding complexes are shown in Figure S3. These results suggest that these bioactive peptides directly interact with the active site in the S1 and S2 pockets and can influence ACE activity by interfering with zinc-binding motifs [54].
Hydrogen bonds play an important role as catalytic cavities for the stability of ACE and peptides in the complex [55]. QLQFPVGR, VTPGLQY, and QFPVGR interacted with the ACE complex through 19, 18, and 8 binding sites, of which 12 residues (ASN70, GLU123, ALA354, SER355, GLU384, HIS387, HIS410, GLU411, SER517, ARG522, TYR523, and GLY2000), 10 residues (TYR51, ASN66, THR92, LYS118, TRP357, HIS387, VAL399, GLU403, PRO519, and TYR523), and 6 residues (SER284, ASN285, THR302, VAL373, ASN374, and GLU376) were hydrogen bonds, respectively. The relatively weak non-covalent bond, which allows for its transformation into different forms of chains on all protein surfaces, can act as a binding site for peptides of different structures [56]. Unlike the other peptides, QFPVGR formed hydrogen and pi-alkyl bonds at the ACE surface site (Figure 5C). It is speculated that it inhibits activity by preventing interactions at the internal acting site, as this binding complex was formed to surround the surface of ACE. The role of the binding forms in inhibiting the activity by interfering with the interactions between other structures warrants further investigation.
3. Materials and Methods
3.1. Materials
Abalones were purchased from a fishing village market on Jeju Island. AV was obtained as the shells and muscles were removed. The AV was washed with tap water, and dried for 72 h at 40 °C using far-infrared drying equipment. The dried AV was ground and stored in a freezer at −20 °C. Trypsin were purchased from Sigma-Aldrich (St. Louis, MO, USA). Peptides, identified based on amino acid sequences, were synthesized by Anygen Co., Ltd. (Gwangju, Republic of Korea). All the other chemicals were of analytical grades.
3.2. Preparation of Trypsin-Enzymatic Hydrolysate of AV
Enzymatic hydrolysis was performed by slightly modifying the method described in a previous study [57]. Briefly, 50 g of AV powder was blended with 500 mL of distilled water, and the pH was adjusted to 8.0. Trypsin was then added to the mixture at a 1:100 ratio of substrate to enzyme. Then, the reaction was performed for 24 h in a shaking incubator set at 37 °C. At the end of the reaction, the hydrolysate was inactivated at 100 °C for 10 min. The hydrolysate was clarified via centrifugation, filtered, and dried via lyophilization to obtain trypsin-hydrolysate of AV (TAV).
3.3. Compositional Analysis of TAV
Protein content was measured using a bicinchoninic acid (BCA) assay with bovine serum albumin as the reference standard [58]. Total polysaccharide content was determined using glucose as a standard in the phenol-sulfuric acid method, as described by DuBois et al., with modifications [59]. The total polyphenolic content was determined using the Folin–Ciocalteu method with gallic acid as a reference standard, as described by Chandler et al., with slight modifications [60].
3.4. Purification of Peptides from TAV
The active properties of TAV were isolated using a slight modification of a previous study [61]. The hydrolysate was dissolved in distilled water and loaded onto a Sephadex G-10 gel filtration column (2.5 × 100 cm) equilibrated with distilled water. The elution was performed in each 10 mL tube at a flow rate of 1.2 mL/min, and the elution peaks measured at 220 nm were freeze-dried for the next experiment.
3.5. H2O2 Radical Scavenging Activity
H2O2 radical scavenging activity was measured as previously described, with slight modifications [62]. Briefly, 20 μL hydrolysate solution at different concentrations was mixed with 100 μL of 0.1 M phosphate buffer (pH 5.0), and 20 μL of 10 mM hydrogen peroxide (in deionized water) was added to a 96-well plate and incubated at 37 °C for 5 min. After incubation, 1.25 mM ABTS of 30 μL and 30 μL (1 unit/mL) of peroxidase were added and kept at 37 °C for 10 min. The absorbance was read at 405 nm using a microplate reader.
3.6. Sequencing of Amino Acid from Separated Active Properties
The purified peptides from the TAV were separated on a Zorbax Eclipse Plus C18 column using ultimate 3000 ultra-high-performance liquid chromatography (UHPLC, Thermo Fisher Scientific, Waltham, MA, USA). The masses and amino acid sequences of the separated peaks were identified using quadruple time-of-flight mass spectrometry (Micro Q-TOF III mass spectrometer; Bruker Daltonics, Bremen, Germany) coupled with electrospray ionization (ESI). Molecular masses were determined with a single-charge ([M + H]+) or doubly charged ([M + 2H]+) positive states in the mass spectra obtained from the isolated peaks. Peptides were automatically selected for fragmentation and their amino acid sequences were confirmed using tandem mass spectrometry analysis.
3.7. ACE Inhibition Activity Assay
ACE inhibitory activity was determined as previously described [63]. Inhibition was measured using an ACE kit-WST (Dojindo Inc., Kumamoto, Japan), a colorimetric method. Briefly, the inhibitory effect was determined using enzyme activity and the amount of 3-hydroxybutyric acid made from 3-hydroxybutyryl-Gly-Gly-Gly.
3.8. Molecular Docking by Computer Simulation
3.8.1. Preparation of ACE 3D Structure
For molecular docking analysis, the crystal structure of ACE (ID:1O86) was obtained from the Protein Data Bank (PDB). To predict the interactions between the bioactive peptides of interest and the protein, the ACE crystal structure was prepared for docking with the “prepare protein” protocol from the Discovery Studio 2024 (Biovia, San Diego, CA, USA) tool by removing all water molecules, inhibitor lisinopril, and free glycine molecules [64,65].
3.8.2. Preparation of Peptide 3D Structure
Active peptides were manually prepared using Discovery Studio 2024. The molecular dynamics (MD) simulations were performed to select the structures by reducing the absence of solvation during docking and alleviating the influence of packing effects [66]. MD simulation for each step was performed as previously described, with modifications [67]. Briefly, the solvation process was performed using the explicit peripheral of the cubic box cell shape of the peptide, in which the “prepare protein” protocol was followed. Next, simulation was performed with the steepest descent algorithm set to a maximum number of 1000 steps and a root mean squared (RMS) gradient of 1, and the first minimization process was performed, and then the second minimization process was performed with a max number of 2000 steps and an RMS gradient of 0.1 using an adopted-basis Newton–Raphson algorithm. Then, 20 ns of MD was conducted under standard particle number, pressure, and temperature conditions for the peptides, with a 300 ps heating process at 300 K, and the structure of the stable pose was obtained from the final simulation step.
3.8.3. Docking Analysis
The docking of bioactive peptides and ACE was performed using the ZDOCK algorithm to confirm the clustering of poses and binding actions [68]. To allow rapid clustering, the root mean square division (RMSD) and interface cutoff were set to 3° and 6°, respectively. The maximum number of clusters and poses were limited to 30 and 2000, respectively.
3.9. Statistical Analysis
Quantitative data were represented as the mean ± standard deviation from triplicate determinations. Statistical comparisons of mean values were performed using one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test. Differences were considered statistically significant at p < 0.05 (* p < 0.05, ** p < 0.01).
4. Conclusions
In this study, the potential efficacy of fishery by-products was revealed by verifying the antioxidant effects of functional peptides derived from AV and demonstrating the dual effects of simultaneous ACE inhibition activity. Trypsin hydrolysis and the purification of GFC yielded nine bioactive peptides, the H2O2 scavenging activities of which were measured. The peptide IC50 values of LGPY, VTPGLQY, LGEW, LDW, and NLGEW were 0.213, 0.297, 0.289, 0.363, and 0.303 mg/mL, respectively, which showed distinct antioxidant efficacy. The bioactive peptides with dual efficacy showed anti-hypertensive activity, as demonstrated using ACE-inhibitory efficacy assessments in in vitro and in silico analysis. Peptide IC50 values of the ACE inhibition of VAR, NYER, VTPGLQY, QFPVGR, LGEW, QLQFPVGR, and NLGEW were 0.104, 0.107, 0.023, 0.023, 0.165, 0.004, and 0.146 mg/mL, respectively, and had low concentrate values, indicating anti-hypertensive effects. QLQFPVGR, VTPGLQY, and QFPVGR displayed superior ZRANK scores based on the docking analysis of ACE inhibition. QLQFPVGR, VTPGLQY, and QFPVGR displayed superior ZRANK scores based on the docking analysis of ACE inhibition. VTPGLQY had a superior dual efficacy in both antioxidant and anti-hypertensive activities, which offers a specialized potential for use as a functional material with both effects. Our results suggest that both LGEW and NLGEW peptides have distinct dual efficacies. In conclusion, TAV, a by-product of fisheries, is a potential bioactive resource with antioxidant and anti-hypertensive properties. Further studies should establish mass-production processes and evaluate their efficacy for industrial applications.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/md22100461/s1, Figure S1. The prediction of the binding site of ACE-bioactive peptide complexes; Figure S2. Sites formed by selecting residues involved in the interaction; Figure S3. The prediction of the binding site of ACE-lisinopril and captopril complexes.
Author Contributions
Conceptualization, methodology, writing—original draft, J.-H.H.; validation, investigation, E.-A.K.; data curation, methodology, N.K.; software, S.-Y.H.; formal analysis, G.A.; writing—review and editing, project administration, supervision, S.-J.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Korea Institute of Marine Science & Technology Promotion (KIMST) and funded by the Ministry of Oceans and Fisheries (20220128) and research grants from the Korea Institute of Ocean Science and Technology (grant. No. PEA0215).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article; further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Görgüç, A.; Gençdağ, E.; Yılmaz, F.M. Bioactive peptides derived from plant origin by-products: Biological activities and techno-functional utilizations in food developments—A review. Food Res. Int. 2020, 136, 109504. [Google Scholar] [CrossRef]
- Domínguez-Pérez, L.A.; Beltrán-Barrientos, L.M.; González-Córdova, A.F.; Hernández-Mendoza, A.; Vallejo-Cordoba, B. Artisanal cocoa bean fermentation: From cocoa bean proteins to bioactive peptides with potential health benefits. J. Funct. Foods 2020, 73, 104134. [Google Scholar] [CrossRef]
- González-Serrano, D.J.; Hadidi, M.; Varcheh, M.; Jelyani, A.Z.; Moreno, A.; Lorenzo, J.M. Bioactive peptide fractions from collagen hydrolysate of common carp fish byproduct: Antioxidant and functional properties. Antioxidants 2022, 11, 509. [Google Scholar] [CrossRef]
- Li, T.; Zhang, X.; Ren, Y.; Zeng, Y.; Huang, Q.; Wang, C. Antihypertensive effect of soybean bioactive peptides: A review. Curr. Opin. Pharmacol. 2022, 62, 74–81. [Google Scholar] [CrossRef]
- Zhu, W.; Ren, L.; Zhang, L.; Qiao, Q.; Farooq, M.Z.; Xu, Q. The Potential of Food Protein-Derived Bioactive Peptides against Chronic Intestinal Inflammation. Mediat. Inflamm. 2020, 2020, 6817156. [Google Scholar] [CrossRef]
- Kong, J.; Hu, X.-M.; Cai, W.-W.; Wang, Y.-M.; Chi, C.-F.; Wang, B. Bioactive peptides from skipjack tuna cardiac arterial bulbs (II): Protective function on UVB-irradiated HaCaT cells through antioxidant and anti-apoptotic mechanisms. Mar. Drugs 2023, 21, 105. [Google Scholar] [CrossRef]
- Yang, F.-J.; Xu, C.; Huang, M.-C.; Qian, Y.; Cai, X.-X.; Xuan, C.; Ming, D.; Huang, J.-L.; Wang, S.-Y. Molecular characteristics and structure–activity relationships of food-derived bioactive peptides. J. Integr. Agric. 2021, 20, 2313–2332. [Google Scholar] [CrossRef]
- Xing, L.; Wang, Z.; Hao, Y.; Zhang, W. Marine products as a promising resource of bioactive peptides: Update of extraction strategies and their physiological regulatory effects. J. Agric. Food Chem. 2022, 70, 3081–3095. [Google Scholar] [CrossRef]
- Ko, S.-C.; Kim, D.; Jeon, Y.-J. Protective effect of a novel antioxidative peptide purified from a marine Chlorella ellipsoidea protein against free radical-induced oxidative stress. Food Chem. Toxicol. 2012, 50, 2294–2302. [Google Scholar] [CrossRef]
- Zhan, J.; Li, G.; Dang, Y.; Pan, D. Purification and identification of a novel hypotensive and antioxidant peptide from porcine plasma. J. Sci. Food Agric. 2022, 102, 4933–4941. [Google Scholar] [CrossRef]
- Ahmad, H.; Khan, H.; Haque, S.; Ahmad, S.; Srivastava, N.; Khan, A. Angiotensin-converting enzyme and hypertension: A systemic analysis of various ACE inhibitors, their side effects, and bioactive peptides as a putative therapy for hypertension. J. Renin-Angiotensin-Aldosterone Syst. 2023, 2023, 7890188. [Google Scholar] [CrossRef]
- Mancia, G.; De Backer, G.; Dominiczak, A.; Cifkova, R.; Fagard, R.; Germano, G.; Grassi, G.; Heagerty, A.M.; Kjeldsen, S.E.; Laurent, S. 2007 Guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur. Heart J. 2007, 28, 1462–1536. [Google Scholar] [CrossRef]
- Pfeffer, M.A.; Frohlich, E.D. Improvements in clinical outcomes with the use of angiotensin-converting enzyme inhibitors: Cross-fertilization between clinical and basic investigation. Am. J. Physiol. -Heart Circ. Physiol. 2006, 291, H2021–H2025. [Google Scholar] [CrossRef]
- Walquist, M.J.; Eilertsen, K.-E.; Elvevoll, E.O.; Jensen, I.-J. Marine-Derived Peptides with Anti-Hypertensive Properties: Prospects for Pharmaceuticals, Supplements, and Functional Food. Mar. Drugs 2024, 22, 140. [Google Scholar] [CrossRef]
- Mäkinen, S.; Johannson, T.; Gerd, E.V.; Pihlava, J.M.; Pihlanto, A. Angiotensin I-converting enzyme inhibitory and antioxidant properties of rapeseed hydrolysates. J. Funct. Foods 2012, 4, 575–583. [Google Scholar] [CrossRef]
- Admassu, H.; Gasmalla, M.A.A.; Yang, R.; Zhao, W. Bioactive peptides derived from seaweed protein and their health benefits: Antihypertensive, antioxidant, and antidiabetic properties. J. Food Sci. 2018, 83, 6–16. [Google Scholar] [CrossRef]
- Barkia, I.; Al-Haj, L.; Abdul Hamid, A.; Zakaria, M.; Saari, N.; Zadjali, F. Indigenous marine diatoms as novel sources of bioactive peptides with antihypertensive and antioxidant properties. Int. J. Food Sci. Technol. 2019, 54, 1514–1522. [Google Scholar] [CrossRef]
- Lu, W.-C.; Chiu, C.-S.; Chan, Y.-J.; Mulio, A.T.; Li, P.-H. Characterization and biological properties of marine by-product collagen through ultrasound-assisted extraction. Aquac. Rep. 2023, 29, 101514. [Google Scholar] [CrossRef]
- Food and Agriculture Organization. The State of World Fisheries and Aquaculture 2020: Sustainability in Action; Food and Agriculture Organization of the United Nations: Rome, Italy, 2020. [Google Scholar]
- Nikoo, M.; Regenstein, J.M.; Yasemi, M. Protein hydrolysates from fishery processing by-products: Production, characteristics, food applications, and challenges. Foods 2023, 12, 4470. [Google Scholar] [CrossRef]
- Agrawal, S.; Acharya, D.; Adholeya, A.; Barrow, C.J.; Deshmukh, S.K. Nonribosomal peptides from marine microbes and their antimicrobial and anticancer potential. Front. Pharmacol. 2017, 8, 828. [Google Scholar] [CrossRef]
- Harnedy, P.A.; FitzGerald, R.J. Bioactive peptides from marine processing waste and shellfish: A review. J. Funct. Foods 2012, 4, 6–24. [Google Scholar] [CrossRef]
- Costello, M.J.; Bouchet, P.; Boxshall, G.; Fauchald, K.; Gordon, D.; Hoeksema, B.; Poore, G.; Van Soest, R.; Stöhr, S.; Walter, T.; et al. Global Coordination and Standardisation in Marine Biodiversity, World Register of Marine Species (WoRMS) and Related Databases. PLoS ONE 2013, 8, e51629. [Google Scholar] [CrossRef]
- Rivera-Pérez, C.; Ponce González, X.P.; Hernández-Savedra, N.Y. Antimicrobial and anticarcinogenic activity of bioactive peptides derived from abalone viscera (Haliotis fulgens and Haliotis corrugata). Sci. Rep. 2023, 13, 15185. [Google Scholar] [CrossRef]
- Zhou, D.-Y.; Zhu, B.-W.; Qiao, L.; Wu, H.-T.; Li, D.-M.; Yang, J.-F.; Murata, Y. In vitro antioxidant activity of enzymatic hydrolysates prepared from abalone (Haliotis discus hannai Ino) viscera. Food Bioprod. Process. 2012, 90, 148–154. [Google Scholar] [CrossRef]
- Gu, J.; Zhang, H.; Wen, C.; Zhang, J.; He, Y.; Ma, H.; Duan, Y. Purification, characterization, antioxidant and immunological activity of polysaccharide from Sagittaria sagittifolia L. Food Res. Int. 2020, 136, 109345. [Google Scholar] [CrossRef]
- Guo, S.; Wang, J.; He, C.; Wei, H.; Ma, Y.; Xiong, H. Preparation and antioxidant activities of polysaccharides obtained from abalone viscera by combination of enzymolysis and multiple separation methods. J. Food Sci. 2020, 85, 4260–4270. [Google Scholar] [CrossRef]
- Zhou, D.Y.; Ma, D.D.; Zhao, J.; Wan, X.L.; Tong, L.; Song, S.; Yang, J.F.; Zhu, B.W. Simultaneous Recovery of Protein and Polysaccharide from Abalone (Haliotis discus hannai I no) Gonad Using Enzymatic Hydrolysis Method. J. Food Process. Preserv. 2016, 40, 119–130. [Google Scholar] [CrossRef]
- Pimentel, F.B.; Alves, R.C.; Harnedy, P.A.; FitzGerald, R.J.; Oliveira, M.B.P. Macroalgal-derived protein hydrolysates and bioactive peptides: Enzymatic release and potential health enhancing properties. Trends Food Sci. Technol. 2019, 93, 106–124. [Google Scholar] [CrossRef]
- Kang, N.; Kim, E.-A.; Kim, J.; Lee, S.-H.; Heo, S.-J. Identifying potential antioxidant properties from the viscera of sea snails (Turbo cornutus). Mar. Drugs 2021, 19, 567. [Google Scholar] [CrossRef]
- Cunha, S.A.; Pintado, M.E. Bioactive peptides derived from marine sources: Biological and functional properties. Trends Food Sci. Technol. 2022, 119, 348–370. [Google Scholar] [CrossRef]
- Je, J.-Y.; Park, S.Y.; Hwang, J.-Y.; Ahn, C.-B. Amino acid composition and in vitro antioxidant and cytoprotective activity of abalone viscera hydrolysate. J. Funct. Foods 2015, 16, 94–103. [Google Scholar] [CrossRef]
- He, S.; Zhang, Y.; Sun, H.; Du, M.; Qiu, J.; Tang, M.; Sun, X.; Zhu, B. Antioxidative peptides from proteolytic hydrolysates of false abalone (Volutharpa ampullacea perryi): Characterization, identification, and molecular docking. Mar. Drugs 2019, 17, 116. [Google Scholar] [CrossRef] [PubMed]
- Pratama, I.S.; Putra, Y.; Pangestuti, R.; Kim, S.-K.; Siahaan, E.A. Bioactive peptides-derived from marine by-products: Development, health benefits and potential application in biomedicine. Fish. Aquat. Sci. 2022, 25, 357–379. [Google Scholar] [CrossRef]
- Li, Q.; Shi, C.; Wang, M.; Zhou, M.; Liang, M.; Zhang, T.; Yuan, E.; Wang, Z.; Yao, M.; Ren, J. Tryptophan residue enhances in vitro walnut protein-derived peptides exerting xanthine oxidase inhibition and antioxidant activities. J. Funct. Foods 2019, 53, 276–285. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, D.; Liu, M.; Li, Y.; Lv, R.; Li, X.; Wang, Q.; Ren, D.; Wu, L.; Zhou, H. Identification of antioxidant peptides derived from tilapia (Oreochromis niloticus) skin and their mechanism of action by molecular docking. Foods 2022, 11, 2576. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wu, G.; Yang, J.; He, C.; Xiong, H.; Ma, Y. Abalone visceral peptides containing Cys and Tyr exhibit strong in vitro antioxidant activity and cytoprotective effects against oxidative damage. Food Chem. X 2023, 17, 100582. [Google Scholar] [CrossRef]
- Chakniramol, S.; Wierschem, A.; Cho, M.-G.; Bashir, K.M.I. Physiological and clinical aspects of bioactive peptides from marine animals. Antioxidants 2022, 11, 1021. [Google Scholar] [CrossRef]
- Tao, J.; Zhao, Y.-Q.; Chi, C.-F.; Wang, B. Bioactive peptides from cartilage protein hydrolysate of spotless smoothhound and their antioxidant activity in vitro. Mar. Drugs 2018, 16, 100. [Google Scholar] [CrossRef]
- García-Mora, P.; Martín-Martínez, M.; Bonache, M.A.; González-Múniz, R.; Peñas, E.; Frias, J.; Martinez-Villaluenga, C. Identification, functional gastrointestinal stability and molecular docking studies of lentil peptides with dual antioxidant and angiotensin I converting enzyme inhibitory activities. Food Chem. 2017, 221, 464–472. [Google Scholar] [CrossRef]
- Wang, C.-X.; Song, C.-C.; Liu, X.-T.; Qiao, B.-W.; Song, S.; Fu, Y.-H. ACE inhibitory activities of two peptides derived from Volutharpa ampullacea perryi hydrolysate and their protective effects on H2O2 induced HUVECs injury. Food Res. Int. 2022, 157, 111402. [Google Scholar] [CrossRef]
- Je, J.-G.; Kim, H.-S.; Lee, H.-G.; Oh, J.-Y.; Lu, Y.A.; Wang, L.; Rho, S.; Jeon, Y.-J. Low-molecular weight peptides isolated from seahorse (Hippocampus abdominalis) improve vasodilation via inhibition of angiotensin-converting enzyme in vivo and in vitro. Process Biochem. 2020, 95, 30–35. [Google Scholar] [CrossRef]
- Ngo, D.-H.; Ryu, B.; Kim, S.-K. Active peptides from skate (Okamejei kenojei) skin gelatin diminish angiotensin-I converting enzyme activity and intracellular free radical-mediated oxidation. Food Chem. 2014, 143, 246–255. [Google Scholar] [CrossRef]
- Abdelhedi, O.; Nasri, R.; Jridi, M.; Mora, L.; Oseguera-Toledo, M.E.; Aristoy, M.-C.; Amara, I.B.; Toldrá, F.; Nasri, M. In silico analysis and antihypertensive effect of ACE-inhibitory peptides from smooth-hound viscera protein hydrolysate: Enzyme-peptide interaction study using molecular docking simulation. Process Biochem. 2017, 58, 145–159. [Google Scholar] [CrossRef]
- Manikkam, V.; Vasiljevic, T.; Donkor, O.; Mathai, M. A review of potential marine-derived hypotensive and anti-obesity peptides. Crit. Rev. Food Sci. Nutr. 2016, 56, 92–112. [Google Scholar] [CrossRef]
- Launay, G.; Ohue, M.; Prieto Santero, J.; Matsuzaki, Y.; Hilpert, C.; Uchikoga, N.; Hayashi, T.; Martin, J. Evaluation of consrank-like scoring functions for rescoring ensembles of protein–protein docking poses. Front. Mol. Biosci. 2020, 7, 559005. [Google Scholar] [CrossRef] [PubMed]
- Aramyan, S.; McGregor, K.; Sandeep, S.; Haczku, A. SP-A binding to the SARS-CoV-2 spike protein using hybrid quantum and classical in silico modeling and molecular pruning by Quantum Approximate Optimization Algorithm (QAOA) Based MaxCut with ZDOCK. Front. Immunol. 2022, 13, 945317. [Google Scholar] [CrossRef]
- Pierce, B.G.; Wiehe, K.; Hwang, H.; Kim, B.-H.; Vreven, T.; Weng, Z. ZDOCK server: Interactive docking prediction of protein–protein complexes and symmetric multimers. Bioinformatics 2014, 30, 1771–1773. [Google Scholar] [CrossRef]
- Pierce, B.; Weng, Z. ZRANK: Reranking protein docking predictions with an optimized energy function. Proteins Struct. Funct. Bioinform. 2007, 67, 1078–1086. [Google Scholar] [CrossRef]
- Li, X.; Feng, C.; Hong, H.; Zhang, Y.; Luo, Z.; Wang, Q.; Luo, Y.; Tan, Y. Novel ACE inhibitory peptides derived from whey protein hydrolysates: Identification and molecular docking analysis. Food Biosci. 2022, 48, 101737. [Google Scholar] [CrossRef]
- Renjuan, L.; Xiuli, Z.; Liping, S.; Yongliang, Z. Identification, in silico screening, and molecular docking of novel ACE inhibitory peptides isolated from the edible symbiot Boletus griseus-Hypomyces chrysospermus. LWT 2022, 169, 114008. [Google Scholar] [CrossRef]
- Kang, N.; Ko, S.-C.; Kim, H.-S.; Yang, H.-W.; Ahn, G.; Lee, S.-C.; Lee, T.-G.; Lee, J.-S.; Jeon, Y.-J. Structural evidence for antihypertensive effect of an antioxidant peptide purified from the edible marine animal Styela clava. J. Med. Food 2020, 23, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Arámburo-Gálvez, J.G.; Arvizu-Flores, A.A.; Cárdenas-Torres, F.I.; Cabrera-Chávez, F.; Ramírez-Torres, G.I.; Flores-Mendoza, L.K.; Gastelum-Acosta, P.E.; Figueroa-Salcido, O.G.; Ontiveros, N. Prediction of ACE-I inhibitory peptides derived from chickpea (Cicer arietinum L.): In silico assessments using simulated enzymatic hydrolysis, molecular docking and ADMET evaluation. Foods 2022, 11, 1576. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.-Y.; Kang, N.; Kim, E.-A.; Kim, J.; Lee, S.-H.; Ahn, G.; Oh, J.H.; Shin, A.Y.; Kim, D.; Heo, S.-J. Purification and Molecular Docking Study on the Angiotensin I-Converting Enzyme (ACE)-Inhibitory Peptide Isolated from Hydrolysates of the Deep-Sea Mussel Gigantidas vrijenhoeki. Mar. Drugs 2023, 21, 458. [Google Scholar] [CrossRef] [PubMed]
- Tu, M.; Wang, C.; Chen, C.; Zhang, R.; Liu, H.; Lu, W.; Jiang, L.; Du, M. Identification of a novel ACE-inhibitory peptide from casein and evaluation of the inhibitory mechanisms. Food Chem. 2018, 256, 98–104. [Google Scholar] [CrossRef]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. The shape and structure of proteins. In Molecular Biology of the Cell, 4th ed.; Garland Science: New York, NY, USA, 2002. [Google Scholar]
- Ko, J.-Y.; Lee, J.-H.; Samarakoon, K.; Kim, J.-S.; Jeon, Y.-J. Purification and determination of two novel antioxidant peptides from flounder fish (Paralichthys olivaceus) using digestive proteases. Food Chem. Toxicol. 2013, 52, 113–120. [Google Scholar] [CrossRef]
- Walker, J.M. The bicinchoninic acid (BCA) assay for protein quantitation. In The Protein Protocols Handbook; Springer: Berlin/Heidelberg, Germany, 2009; pp. 11–15. [Google Scholar]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.t.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Chandler, S.; Dodds, J. The effect of phosphate, nitrogen and sucrose on the production of phenolics and solasodine in callus cultures of Solanum laciniatum. Plant Cell Rep. 1983, 2, 205–208. [Google Scholar] [CrossRef]
- Kang, N.; Ko, S.-C.; Samarakoon, K.; Kim, E.-A.; Kang, M.-C.; Lee, S.-C.; Kim, J.; Kim, Y.-T.; Kim, J.-S.; Kim, H. Purification of antioxidative peptide from peptic hydrolysates of Mideodeok (Styela clava) flesh tissue. Food Sci. Biotechnol. 2013, 22, 541–547. [Google Scholar] [CrossRef]
- Heo, J.-H.; Je, J.-G.; Sim, J.-H.; Ryu, B.; Heo, S.-J.; Jeon, Y.-J. Quantitative analysis of fucose in fucoidans from Sargassum spp. in Jeju Island, South Korea using 3-methyl-1-phenyl-5-pyrazolone derivatization and RP-HPLC-UV method. Algal Res. 2024, 79, 103441. [Google Scholar] [CrossRef]
- Kang, N.; Lee, J.-H.; Lee, W.; Ko, J.-Y.; Kim, E.-A.; Kim, J.-S.; Heu, M.-S.; Kim, G.H.; Jeon, Y.-J. Gallic acid isolated from Spirogyra sp. improves cardiovascular disease through a vasorelaxant and antihypertensive effect. Environ. Toxicol. Pharmacol. 2015, 39, 764–772. [Google Scholar] [CrossRef]
- De Oliveira, T.V.; Polêto, M.D.; De Oliveira, M.R.; Silva, T.J.; Barros, E.; Guimarães, V.M.; Baracat-Pereira, M.C.; Eller, M.R.; Coimbra, J.S.d.R.; De Oliveira, E.B. Casein-derived peptides with antihypertensive potential: Production, identification and assessment of complex formation with angiotensin I-converting enzyme (ACE) through molecular docking studies. Food Biophys. 2020, 15, 162–172. [Google Scholar] [CrossRef]
- Kang, N.; Kim, E.-A.; Heo, S.-Y.; Heo, S.-J. Structure-based in silico screening of marine phlorotannins for potential walrus calicivirus inhibitor. Int. J. Mol. Sci. 2023, 24, 15774. [Google Scholar] [CrossRef] [PubMed]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1, 19–25. [Google Scholar] [CrossRef]
- Kang, N.; Kim, E.-A.; Park, A.; Heo, S.-Y.; Heo, J.-H.; Heo, S.-J. Antiviral Potential of Fucoxanthin, an Edible Carotenoid Purified from Sargassum siliquastrum, against Zika Virus. Mar. Drugs 2024, 22, 247. [Google Scholar] [CrossRef]
- Chen, R.; Li, L.; Weng, Z. ZDOCK: An initial-stage protein-docking algorithm. Proteins Struct. Funct. Bioinform. 2003, 52, 80–87. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).