Halocins and C50 Carotenoids from Haloarchaea: Potential Natural Tools against Cancer
Abstract
1. Introduction
2. Halocins: Description and Potential Uses as Antibiotics and Antitumoral Molecules
3. C50 Carotenoids: Description and Potential Uses against Cancer
4. Future Perspectives
5. Conclusions
Funding
Conflicts of Interest
References
- Abaramak, G.; Kırtel, O.; Öner, E.T. Fructanogenic halophiles: A new perspective on extremophiles. In Physiological and Biotechnological Aspects of Extremophiles; Salwan, R., Sharma, V., Eds.; Elsevier: Amsterdam, The Netherlands; Academic Press: London, UK, 2020; pp. 123–130. [Google Scholar] [CrossRef]
- Antón, J.; Peña, A.; Santos, F.; Martínez-García, M.; Schmitt-Kopplin, P.; Rosselló-Mora, R. Distribution, abundance and diversity of the extremely halophilic bacterium Salinibacter ruber. Saline Syst. 2008, 4, 15. [Google Scholar] [CrossRef]
- Oren, A. The microbiology of red brines. Adv. Appl. Microbiol. 2020, 113, 57–110. [Google Scholar] [CrossRef] [PubMed]
- Oren, A. Life at high salt concentrations, intracellular KCl concentrations, and acidic proteomes. Front. Microbiol. 2013, 4, 315. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.D. Life at low water activity. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2004, 359, 1249–1267. [Google Scholar] [CrossRef]
- Coker, J.A.; DasSarma, P.; Kumar, J.; Müller, J.A.; DasSarma, S. Transcriptional profiling of the model Archaeon Halobacterium sp. NRC-1: Responses to changes in salinity and temperature. Saline Syst. 2007, 3, 6. [Google Scholar] [CrossRef] [PubMed]
- Andrei, A.S.; Banciu, H.L.; Oren, A. Living with salt: Metabolic and phylogenetic diversity of archaea inhabiting saline ecosystems. FEMS Microbiol. Lett. 2012, 330, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Moopantakath, J.; Imchen, M.; Anju, V.T.; Busi, S.; Dyavaiah, M.; Martínez-Espinosa, R.M.; Kumavath, R. Bioactive molecules from haloarchaea: Scope and prospects for industrial and therapeutic applications. Front. Microbiol. 2023, 14, 1113540. [Google Scholar] [CrossRef]
- Martínez, G.M.; Pire, C.; Martínez-Espinosa, R.M. Hypersaline environments as natural sources of microbes with potential applications in biotechnology: The case of solar evaporation systems to produce salt in Alicante County (Spain). Curr. Res. Microb. Sci. 2022, 3, 100136. [Google Scholar] [CrossRef]
- Litchfield, C.D. Potential for industrial products from the halophilic Archaea. J. Ind. Microbiol. Biotechnol. 2011, 38, 1635–1647. [Google Scholar] [CrossRef]
- Kasirajan, L.; Maupin-Furlow, J.A. Halophilic archaea and their potential to generate renewable fuels and chemicals. Biotechnol. Bioeng. 2021, 118, 1066–1090. [Google Scholar] [CrossRef]
- Giani, M.; Montero-Lobato, Z.; Garbayo, I.; Vílchez, C.; Vega, J.M.; Martínez-Espinosa, R.M. Haloferax mediterranei cells as C50 carotenoid factories. Mar. Drugs 2021, 19, 100. [Google Scholar] [CrossRef] [PubMed]
- Simó-Cabrera, L.; García-Chumillas, S.; Hagagy, N.; Saddiq, A.; Tag, H.; Selim, S.; AbdElgawad, H.; Arribas Agüero, A.; Monzó Sánchez, F.; Cánovas, V.; et al. Haloarchaea as cell factories to produce bioplastics. Mar. Drugs 2021, 19, 159. [Google Scholar] [CrossRef] [PubMed]
- Charlesworth, J.C.; Burns, B.P. Untapped Resources: Biotechnological potential of peptides and secondary metabolites in Archaea. Archaea 2015, 282035. [Google Scholar] [CrossRef]
- Rodríguez-Valera, F.; Juez, G.; Kushner, D.J. Halocins: Salt-dependent bacteriocins produced by extremely halophilic rods. Can. J. Microbiol. 1982, 28, 151–154. [Google Scholar] [CrossRef]
- Sun, C.; Li, Y.; Mei, S.; Lu, Q.; Zhou, L.; Xiang, H. A single gene directs both the production and immunity of halocin C8 in a haloarchaeal strain AS7092. Mol. Microbiol. 2005, 57, 537–549. [Google Scholar] [CrossRef] [PubMed]
- Meseguer, I.; Rodríguez, V.F.; Ventosa, A. Antagonistic interactions among halobacteria due to halocin production. FEMS Microbiol. Lett. 1986, 36, 177–182. [Google Scholar] [CrossRef]
- Torreblanca, M.; Meseguer, I.; Ventosa, A. Production of halocin is a practically universal feature of archaeal halophilic rods. Lett. Appl. Microbiol. 1994, 19, 201–205. [Google Scholar] [CrossRef]
- Besse, A.; Vandervennet, M.; Goulard, C.; Peduzzi, J.; Isaac, S.; Rebuffat, S.; Carré-Mlouka, A. Halocin C8: An antimicrobial peptide distributed among four halophilic archaeal genera: Natrinema, Haloterrigena, Haloferax, and Halobacterium. Extremophiles 2017, 21, 623–638. [Google Scholar] [CrossRef]
- Kumar, V.; Singh, B.; van Belkum, M.J.; Diep, D.B.; Chikindas, M.L.; Ermakov, A.M.; Tiwari, S.K. Halocins, natural antimicrobials of Archaea: Exotic or special or both? Biotechnol. Adv. 2021, 53, 107834. [Google Scholar] [CrossRef]
- Li, Y.; Xiang, H.; Liu, J.; Zhou, M.; Tan, H. Purification and biological characterization of halocin C8, a novel peptide antibiotic from Halobacterium strain AS7092. Extremophiles 2003, 7, 401–407. [Google Scholar] [CrossRef]
- Mei, S.; Sun, C.; Liu, X.; Lu, Q.; Cai, L.; Li, Y.; Xiang, H. The helix-loop-helix motif at the N terminus of halI is essential for its immunity function against halocin C8. J. Bacteriol. 2008, 190, 6501–6508. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ri, C.H.; Li, S.R.; Paek, C.I.; Kim, Y.S. Functional expression of an antimicrobial peptide, belonging to halocin C8 family, from Natrinema sp. RNS21 in Escherichia coli. Extremophiles 2023, 27, 21. [Google Scholar] [CrossRef] [PubMed]
- Platas, G.; Meseguer, I.; Amils, R. Optimization of the production of a bacteriocin from Haloferax mediterranei Xia3. Microbiologia 1996, 12, 75–84. [Google Scholar] [PubMed]
- Platas, G.; Meseguer, I.; Amils, R. Purification and biological characterization of halocin H1 from Haloferax mediterranei M2a. Int. Microbiol. 2002, 5, 15–19. [Google Scholar] [CrossRef]
- Cheung, J.; Danna, K.J.; O’Connor, E.M.; Price, L.B.; Shand, R.F. Isolation, sequence, and expression of the gene encoding halocin H4, a bacteriocin from the halophilic archaeon Haloferax mediterranei R4. J. Bacteriol. 1997, 179, 548–551. [Google Scholar] [CrossRef]
- Chen, S.; Sun, S.; Korfanty, G.A.; Liu, J.; Xiang, H. A halocin promotes DNA uptake in Haloferax mediterranei. Front. Microbiol. 2019, 10, 1960. [Google Scholar] [CrossRef]
- Meseguer, I.; Torreblanca, M.T.; Konishi, T. Specific inhibition of the halobacterial Na+/H+ antiporter by halocin H6. J. Biol. Chem. 1995, 270, 6450–6455. [Google Scholar] [CrossRef]
- Kumar, V.; Saxena, J.; Tiwari, S.K. Description of a halocin-producing Haloferax larsenii HA1 isolated from Pachpadra salt lake in Rajasthan. Arch. Microbiol. 2016, 198, 181–192. [Google Scholar] [CrossRef]
- Kumar, V.; Tiwari, S.K. Activity-guided separation and characterization of new halocin HA3 from fermented broth of Haloferax larsenii HA3. Extremophiles 2017, 21, 609–621. [Google Scholar] [CrossRef]
- Kaur, R.; Tiwari, S.K. Purification and characterization of a new halocin HA4 from Haloferax larsenii HA4 isolated from a Salt Lake. Probiotics Antimicrob. Proteins 2021, 13, 1458–1466. [Google Scholar] [CrossRef]
- Kumar, V.; Tiwari, S.K. Halocin Diversity Among Halophilic Archaea and Their Applications. In Microbial Diversity in Ecosystem Sustainability and Biotechnological Applications; Satyanarayana, T., Johri, B., Das, S., Eds.; Springer: Singapore, 2019. [Google Scholar] [CrossRef]
- Price, L.B.; Shand, R.F. Halocin S8: A 36-amino-acid microhalocin from the haloarchaeal strain S8a. J. Bacteriol. 2000, 182, 4951–4958. [Google Scholar] [CrossRef] [PubMed]
- Pasić, L.; Velikonja, B.H.; Ulrih, N.P. Optimization of the culture conditions for the production of a bacteriocin from halophilic archaeon Sech7a. Prep. Biochem. Biotechnol. 2008, 38, 229–245. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, P.; Bhat, S.G.; Chandrasekaran, M. Halocin SH10 production by an extreme haloarchaeon Natrinema sp. BTSH10 isolated from salt pans of South India. Saudi J. Biol. Sci. 2013, 2, 205–212. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, X.; Wang, L.; Liu, J.; Cai, L.; Xiang, H. Genome-wide analysis of gene expression in stationary phase and genetic characterization of stationary-phase-dependent halocin gene expression in the haloarchaeon Haloferax mediterranei. J. Genet. Genom. 2013, 40, 441–444. [Google Scholar] [CrossRef]
- Quadri, I.; Hassani, I.I.; l’Haridon, S.; Chalopin, M.; Hacène, H.; Jebbar, M. Characterization and antimicrobial potential of extremely halophilic archaea isolated from hypersaline environments of the Algerian Sahara. Microbiol. Res. 2016, 186–187, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Haseltine, C.; Hill, T.; Montalvo-Rodriguez, R.; Kemper, S.K.; Shand, R.F.; Blum, P. Secreted euryarchaeal microhalocins kill hyperthermophilic crenarchaea. J. Bacteriol. 2021, 183, 287–291. [Google Scholar] [CrossRef]
- Kavitha, P.; Lipton, A.P.; Sarika, A.R.; Aishwarya, M.S. Growth characteristics and halocin production by a new isolate, Haloferax volcanii KPS1 from Kovalam Solar Saltern (India). Res. J. Biol. Sci. 2011, 6, 257–262. [Google Scholar]
- Lequerica, J.L.; O’Connor, J.E.; Such, L.; Alberola, A.; Mesegue, I.; Dolz, M.; Torreblanca, M.; Moya, A.; Colom, F.; Soria, B. A halocin acting on Na+/H+ exchanger of haloarchaea as a new type of inhibitor in NHE of mammals. J. Physiol. Biochem. 2006, 62, 253–262. [Google Scholar] [CrossRef]
- Kushwaha, S.C.; Kramer, J.K.G.; Kates, M. Isolation and characterization of C50-carotenoid pigments and other polar isoprenoids from Halobacterium cutirubrum. Biochim. Biophys. Acta 1975, 398, 303–314. [Google Scholar] [CrossRef]
- Kushwaha, S.C.; Kates, M. Studies of the biosynthesis of C50 carotenoids in Halobacterium cutirubrum. Can. J. Microbiol. 1979, 25, 1292–1297. [Google Scholar] [CrossRef]
- Giani, M.; Pire, C.; Martínez-Espinosa, R.M. Bacterioruberin: Biosynthesis, Antioxidant Activity, and Therapeutic Applications in Cancer and Immune Pathologies. Mar. Drugs 2024, 22, 167. [Google Scholar] [CrossRef] [PubMed]
- Flegler, A.; Lipski, A. The C50 Carotenoid Bacterioruberin Regulates Membrane Fluidity in Pink-Pigmented Arthrobacter Species. Arch. Microbiol. 2022, 204, 70. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.R.; Tavares, R.S.N.; Canela-Garayoa, R.; Eras, J.; Rodrigues, M.V.N.; Neri-Numa, I.A.; Pastore, G.M.; Rosa, L.H.; Schultz, J.A.A.; Debonsi, H.M.; et al. Chemical Characterization and Biotechnological Applicability of Pigments Isolated from Antarctic Bacteria. Mar. Biotechnol. 2019, 21, 416–429. [Google Scholar] [CrossRef]
- Giani, M.; Martínez-Espinosa, R.M. Carotenoids as a Protection Mechanism against Oxidative Stress in Haloferax mediterranei. Antioxidants 2020, 9, 1060. [Google Scholar] [CrossRef]
- Giani, M.; Miralles-Robledillo, J.M.; Peiró, G.; Pire, C.; Martínez-Espinosa, R.M. Deciphering Pathways for Carotenogenesis in Haloarchaea. Molecules 2020, 25, 1197. [Google Scholar] [CrossRef] [PubMed]
- Calegari-Santos, R.; Diogo, R.A.; Fontana, J.D.; Bonfim, T.M.B. Carotenoid Production by Halophilic Archaea Under Different Culture Conditions. Curr. Microbiol. 2016, 72, 641–651. [Google Scholar] [CrossRef]
- Will Chen, C.; Hsu, S.; Lin, M.T.; Hsu, Y. Mass Production of C50 Carotenoids by Haloferax mediterranei in Using Extruded Rice Bran and Starch under Optimal Conductivity of Brined Medium. Bioprocess. Biosyst. Eng. 2015, 38, 2361–2367. [Google Scholar] [CrossRef]
- Zalazar, L.; Pagola, P.; Miró, M.V.; Churio, M.S.; Cerletti, M.; Martínez, C.; Iniesta-Cuerda, M.; Soler, A.J.; Cesari, A.; De Castro, R. Bacterioruberin Extracts from a Genetically Modified Hyperpigmented Haloferax volcanii Strain: Antioxidant Activity and Bioactive Properties on Sperm Cells. J. Appl. Microbiol. 2019, 126, 796–810. [Google Scholar] [CrossRef]
- Hou, J.; Cui, H.L. In Vitro Antioxidant, Antihemolytic, and Anticancer Activity of the Carotenoids from Halophilic Archaea. Curr. Microbiol. 2018, 75, 266–271. [Google Scholar] [CrossRef]
- Sahli, K.; Gomri, M.A.; Esclapez, J.; Gómez-Villegas, P.; Bonete, M.J.; León, R.; Kharroub, K. Characterization and Biological Activities of Carotenoids Produced by Three Haloarchaeal Strains Isolated from Algerian Salt Lakes. Arch. Microbiol. 2022, 204, 6. [Google Scholar] [CrossRef]
- Higa, L.H.; Schilrreff, P.; Briski, A.M.; Jerez, H.E.; De Farias, M.A.; Villares Portugal, R.; Romero, E.L.; Morilla, M.J. Bacterioruberin from Haloarchaea plus Dexamethasone in Ultra-Small Macrophage-Targeted Nanoparticles as Potential Intestinal Repairing Agent. Colloids Surf. B Biointerfaces 2020, 191, 110961. [Google Scholar] [CrossRef] [PubMed]
- Ávila-Román, J.; Gómez-Villegas, P.; De Carvalho, C.C.C.R.; Vigara, J.; Motilva, V.; León, R.; Talero, E. Up-Regulation of the Nrf2/HO-1 Antioxidant Pathway in Macrophages by an Extract from a New Halophilic Archaea Isolated in Odiel Saltworks. Antioxidants 2023, 12, 1080. [Google Scholar] [CrossRef] [PubMed]
- Abbes, M.; Baati, H.; Guermazi, S.; Messina, C.; Santulli, A.; Gharsallah, N.; Ammar, E. Biological Properties of Carotenoids Extracted from Halobacterium halobium Isolated from a Tunisian Solar Saltern. BMC Complement. Altern. Med. 2013, 13, 255. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, G.E.; Abu-Serie, M.M.; Abo-Elela, G.M.; Ghozlan, H.; Sabry, S.A.; Soliman, N.A.; Abdel-Fattah, Y.R. In Vitro Dual (Anticancer and Antiviral) Activity of the Carotenoids Produced by Haloalkaliphilic Archaeon Natrialba sp. M6. Sci. Rep. 2020, 10, 5986. [Google Scholar] [CrossRef]
- Giani, M.; Montoyo-Pujol, Y.G.; Peiró, G.; Martínez-Espinosa, R.M. Haloarchaeal Carotenoids Exert an in Vitro Antiproliferative Effect on Human Breast Cancer Cell Lines. Sci. Rep. 2023, 13, 7148. [Google Scholar] [CrossRef]
- Shahbazi, S.; Zargar, M.; Zolfaghari, M.R.; Amoozegar, M.A. Carotenoid Pigment of Halophilic Archaeon Haloarcula sp. A15 Induces Apoptosis of Breast Cancer Cells. Cell Biochem. Funct. 2023, 41, 344–354. [Google Scholar] [CrossRef]
- Kholany, M.; Schaeffer, N.; Macário, I.P.E.; Veloso, T.; Caetano, T.; Pereira, J.L.; Dias, A.C.R.V.; Coutinho, J.A.P.; Ventura, S.P.M. Unveiling the Use of Hydrophobic Eutectic Solutions as Task-Specific Solvents To Recover Bacterioruberin from Haloferax mediterranei. ACS Sustain. Chem. Eng. 2023, 11, 13594–13605. [Google Scholar] [CrossRef]
- Baeza-Morales, A.; Medina-García, M.; Martínez-Peinado, P.; Pascual-García, S.; Pujalte-Satorre, C.; López-Jaén, A.B.; Martínez-Espinosa, R.M.; Sempere-Ortells, J.M. The Antitumour Mechanisms of Carotenoids: A Comprehensive Review. Antioxidants 2024, 13, 1060. [Google Scholar] [CrossRef]
| Type | Species/Strains | Characterization Parameters: Molecular Weight, Thermostability, Salt Dependence | References |
|---|---|---|---|
| A4 | Strain TuA4 | 7.4 kDa, >100 °C, ND | [20] |
| C8 | Natrinema sp. strain AS7092 (formerly Halobacterium sp. AS7092) | 6.3 kDa, >100 °C, ND | [16,21,22] |
| Natrinema sp. RNS21 | 7.4 kDa, ND, ND | [23] | |
| G1 | Halobacterium strain GRB | ND, ND, ND, | [20] |
| H1 | Haloferax mediterranei M2a (Xai3) | 31 kDa, <50 °C, yes | [24,25] |
| H2 | Haloarchaeon Gla2.2 | ND, ND, ND | [20] |
| H3 | Haloarchaeon Gaa12 | ND, ND, ND | [20] |
| H4 | Haloferax mediterranei R4 | 34.9 kDa, <60 °C, partially | [26,27] |
| H5 | Haloarchaeon Ma2.20 | ND, ND, ND | [20] |
| H6/H7 | Haloferax gibbonsii Ma2.39 | 32 kDa, <90 °C, no | [28] |
| HA1 | Haloferax larsenii KPS1 | ∼14 kDa, <121 °C, ND pH 4.0–12.0 | [29] |
| HA3 | Haloferax larsenii NCIM5678 | 13 kDa, ND, ND | [30] |
| HA4 | Haloferax larsenii (HA4) | ~14 kDa, <100 °C, ND pH 2.0–10.0, | [31] |
| R1 | Halobacterium strain GN101 | 3.8 kDa, <93 °C, no | [32] |
| S8 | Strain S8a | 3.6 kDa, >100 °C, no | [33] |
| Sech7a | Haloferax mediterranei Sech7a | 10.7 kDa, <80 °C, yes | [34] |
| SH10 | Natrinema sp. BTSH10 | 20 kDa, <50 °C, ND | [35] |
| Entry | Gene Name | Organism | Nº Amino Acids |
|---|---|---|---|
| A0A1W6ALE4 | halC8 | Haloterrigena thermotolerans | 283 |
| L9ZDM4 | C485_14015 | Natrinema altunense JCM 12890 | 248 |
| A0A1W6ALE2 | halC8 | Natrinema versiforme | 217 |
| A0A0K0KG39 | proC8 | Natrinema sp. SSI3 | 283 |
| A0A0K0KFP1 | proC8 | Natrinema sp. SI14 | 283 |
| A0A1W6ALD3 | halC8 | Haloterrigena turkmenica | 283 |
| A0A1W6ALD1 | halC8 | Natrinema ejinorense | 283 |
| A0A1W6ALB1 | halC8 | Haloterrigena jeotgali | 283 |
| A0A1W6ALC8 | halC8 | Natrinema altunense | 283 |
| A0A0K0KFY8 | proC8 | Natrinema sp. SI4 | 283 |
| A0A0K0KGM5 | proC8 | Natrinema sp. SWI6 | 283 |
| A0A0K0KFP9 | proC8 | Natrinema sp. SWI15 | 283 |
| A0A1W6ALC4 | halC8 | Natrinema salaciae | 283 |
| Common Name and Chemical Formula | Chemical Structure (Stereoisomers) |
|---|---|
| Bacterioruberin C50H76O4 |  (2S,2′S)-2,2′-bis(3-hydroxy-3-methylbutyl)-3,4,3′,4′-tetradehydro-1,2,1′,2′-tetrahydro-γ,γ-carotene-1,1′-diol |
| Monoanhydrobacterioruberin C50H74O3 | 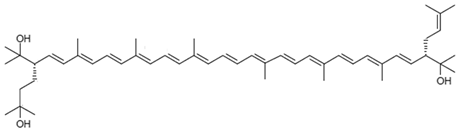 (3S,4E,6E,8E,10E,12E,14E,16E,18E,20E,22E,24E,26E,28E,30S)-30-(2-hydroxypropan-2-yl)-2,6,10,14,19,23,27,33-octamethyl-3-(3-methylbut-2-en-1-yl)tetratriaconta-4,6,8,10,12,14,16,18,20,22,24,26,28-tridecaene-2,33-diol |
| Bisanhydrobacterioruberin C50H72O2 | 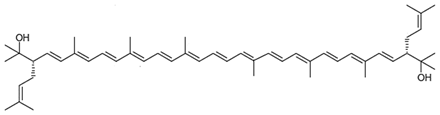 (3S,4E,6E,8E,10E,12E,14E,16E,18E,20E,22E,24E,26E,28E,30S)-2,6,10,14,19,23,27,31-octamethyl-3,30-bis(3-methylbut-2-en-1-yl)dotriaconta-4,6,8,10,12,14,16,18,20,22,24,26,28-tridecaene-2,31-diol |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Espinosa, R.M. Halocins and C50 Carotenoids from Haloarchaea: Potential Natural Tools against Cancer. Mar. Drugs 2024, 22, 448. https://doi.org/10.3390/md22100448
Martínez-Espinosa RM. Halocins and C50 Carotenoids from Haloarchaea: Potential Natural Tools against Cancer. Marine Drugs. 2024; 22(10):448. https://doi.org/10.3390/md22100448
Chicago/Turabian StyleMartínez-Espinosa, Rosa María. 2024. "Halocins and C50 Carotenoids from Haloarchaea: Potential Natural Tools against Cancer" Marine Drugs 22, no. 10: 448. https://doi.org/10.3390/md22100448
APA StyleMartínez-Espinosa, R. M. (2024). Halocins and C50 Carotenoids from Haloarchaea: Potential Natural Tools against Cancer. Marine Drugs, 22(10), 448. https://doi.org/10.3390/md22100448






