Rare Ophiuroid-Type Steroid 3β,21-, 3β,22-, and 3α,22-Disulfates from the Slime Sea Star Pteraster marsippus and Their Colony-Inhibiting Effects against Human Breast Cancer Cells
Abstract
1. Introduction
2. Results and Discussion
2.1. Structure Determination of Compounds 1–5
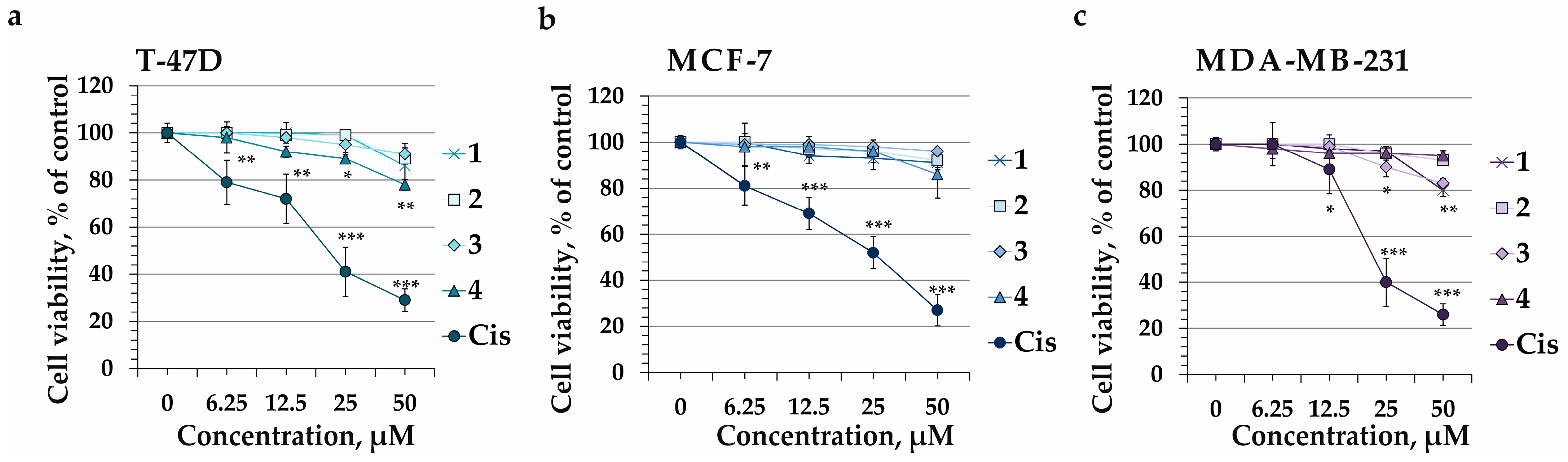
2.2. The Effect of Compounds 1–4 on Cell Viability of Human Breast Cancer Cells
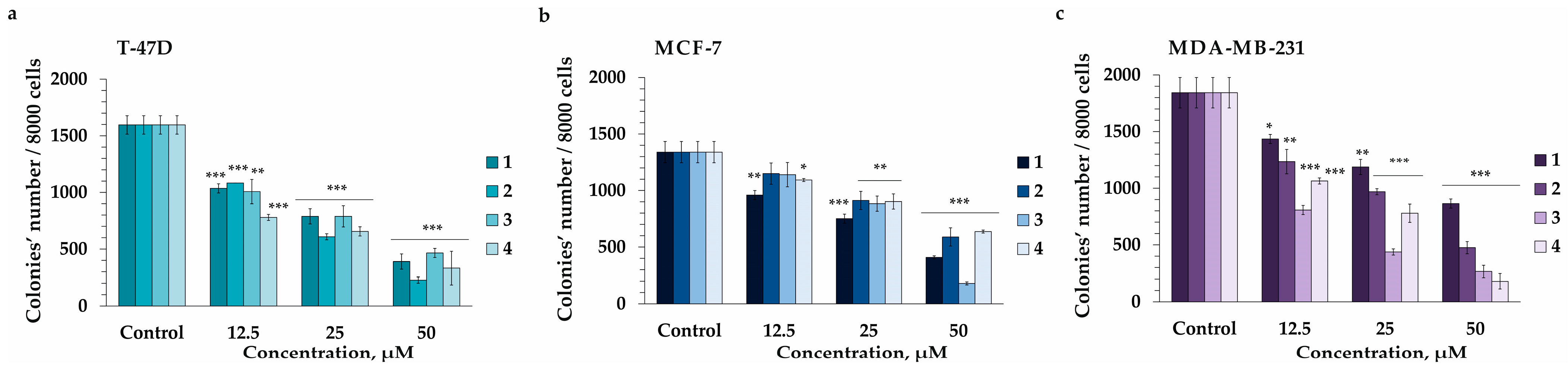
2.3. The Effect of Compounds 1–4 on Colony Formation and Growth of Human Breast Cancer Cells
3. Materials and Methods
3.1. General Procedures
3.2. Animal Material
3.3. Extraction and Isolation
3.4. Compound Characterization Data
3.5. Reagents
3.6. Cell Lines
3.7. Cell Culture Assay
3.8. Cytotoxicity Assay
3.9. Colony-Formation Assay (Soft Agar Assay)
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Minale, L.; Riccio, R.; Zollo, F. Steroid oligoglycosides and polyhydroxysteroids from Echinoderms. Fortschr. Chem. Org. Naturst. 1993, 62, 75–308. [Google Scholar] [CrossRef]
- Iorizzi, M.; De Marino, S.; Zollo, F. Steroidal oligoglycosides from the Asteroidea. Curr. Org. Chem. 2001, 5, 951–973. [Google Scholar] [CrossRef]
- Dong, G.; Xu, T.H.; Yang, B.; Lin, X.P.; Zhou, X.F.; Yang, X.W.; Liu, Y.H. Chemical constituents and bioactivities of starfish. Chem. Biodivers. 2011, 8, 740–791. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.M.; Miao, Z.; Xie, C.L.; Zhang, J.W.; Yang, X.W. Chemical constituents and bioactivities of starfishes: An update. Chem. Biodivers. 2020, 17, e1900638. [Google Scholar] [CrossRef]
- Gomes, A.R.; Freitas, A.C.; Duarte, A.C.; Rocha-Santos, T.A.P. Chapter 1—Echinoderms: A Review of Bioactive Compounds with Potential Health Effects. In Studies in Natural Products Chemistry; Atta-ur-Rahman, Ed.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 49, pp. 1–54. [Google Scholar] [CrossRef]
- Carvalhal, F.; Correia-da-Silva, M.; Sousa, E.; Pinto, M.; Kijjoa, A. Sources and biological activities of marine sulfated steroids. J. Mol. Endocrinol. 2018, 61, T211–T231. [Google Scholar] [CrossRef] [PubMed]
- Pounina, T.A.; Gloriozova, T.A.; Savidov, N.; Dembitsky, V.M. Sulfated and sulfur-containing steroids and their pharmacological profile. Mar. Drugs 2021, 19, 240. [Google Scholar] [CrossRef]
- Ghelani, H.; Khursheed, M.; Adrian, T.E.; Jan, R.K. Anti-inflammatory effects of compounds from echinoderms. Mar. Drugs 2022, 20, 693. [Google Scholar] [CrossRef]
- Yun, C.W.; Kim, H.J.; Lee, S.H. Therapeutic application of diverse marine-derived natural products in cancer therapy. Anticancer. Res. 2019, 39, 5261–5284. [Google Scholar] [CrossRef]
- Lazzara, V.; Arizza, V.; Luparello, C.; Mauro, M.; Vazzana, M. Bright spots in the darkness of cancer: A review of starfishes-derived compounds and their anti-tumor action. Mar. Drugs 2019, 17, 617. [Google Scholar] [CrossRef]
- D’Auria, M.V.; Paloma, L.G.; Minale, L.; Riccio, R.; Zampella, A. On the composition of sulfated polyhydroxysteroids in some ophiuroids and the structure determination of six new constituents. J. Nat. Prod. 1995, 58, 189–196. [Google Scholar] [CrossRef]
- Roccatagliata, A.J.; Maier, M.S.; Seldes, A.M.; Pujol, C.A.; Damonte, E.B. Antiviral sulfated steroids from the ophiuroid Ophioplocus januarii. J. Nat. Prod. 1996, 59, 887–889. [Google Scholar] [CrossRef]
- Roccatagliata, A.J.; Maier, M.S.; Seldes, A.M. New sulfated polyhydroxysteroids from the Antarctic ophiuroid Astrotoma agassizii. J. Nat. Prod. 1998, 61, 370–374. [Google Scholar] [CrossRef] [PubMed]
- Fedorov, S.N.; Levina, E.V.; Kalinovsky, A.I.; Dmitrenok, P.S.; Stonik, V.A. Sulfated steroids from Pacific brittle stars Ophiopholis aculeata, Ophiura sarsi, and Stegophiura brachiactis. J. Nat. Prod. 1994, 57, 1631–1637. [Google Scholar] [CrossRef]
- Kicha, A.A.; Kalinovsky, A.I.; Malyarenko, T.V.; Malyarenko, O.S.; Ermakova, S.P.; Popov, R.S.; Stonik, V.A.; Ivanchina, N.V. Disulfated ophiuroid type steroids from the Far Eastern starfish Pteraster marsippus and their cytotoxic activity on the models of 2D and 3D cultures. Mar. Drugs 2022, 20, 164. [Google Scholar] [CrossRef] [PubMed]
- Ivanchina, N.V.; Kicha, A.A.; Kalinovsky, A.I.; Dmitrenok, P.S.; Stonik, V.A. Hemolytic steroid disulfates from the Far Eastern starfish Pteraster pulvillus. J. Nat. Prod. 2003, 66, 298–301. [Google Scholar] [CrossRef] [PubMed]
- Kicha, A.A.; Kalinovsky, A.I.; Antonov, A.S.; Radchenko, O.S.; Ivanchina, N.V.; Malyarenko, T.V.; Savchenko, A.M.; Stonik, V.A. Determination of C-23 configuration in (20R)-23-hydroxycholestane side chain of steroid compounds by 1H and 13C NMR spectroscopy. Nat. Prod. Commun. 2013, 8, 1219–1222. [Google Scholar] [CrossRef]
- Ivanchina, N.V.; Kicha, A.A.; Malyarenko, T.V.; Kalinovsky, A.I.; Dmitrenok, P.S.; Stonik, V.A. Biosynthesis of polar steroids from the Far Eastern starfish Patiria (=Asterina) pectinifera. Cholesterol and cholesterol sulfate are converted into polyhydroxylated sterols and monoglycoside asterosaponin P1 in feeding experiments. Steroids 2013, 78, 1183–1191. [Google Scholar] [CrossRef]
- Nes, W.R.; Varkey, T.E.; Krevitz, K. The stereochemistry of sterols at C-20 and its biosynthetic implications. J. Am. Chem. Soc. 1977, 99, 260–262. [Google Scholar] [CrossRef]
- Vanderach, D.J.; Djerassi, C. Marine natural products—Synthesis of four naturally occurring 20-β-H cholanic acid-derivatives. J. Org. Chem. 1978, 43, 1442–1448. [Google Scholar] [CrossRef]
- Hamdy, A.-H.A.; Aboutabl, E.A.; Sameer, S.; Hussein, A.A.; Díaz-Marrero, A.R.; Darias, J.; Cueto, M. 3-Keto-22-epi-28-nor-cathasterone, a brassinosteroid-related metabolite from Cystoseira myrica. Steroids 2009, 74, 927–930. [Google Scholar] [CrossRef]
- Amann, A.; Ourisson, G.; Luu, B. Stereospecific synthesis of the 4 epimers of 7,22-dihydroxycholesterol. Synthesis 1988, 1987, 1002–1005. [Google Scholar] [CrossRef]
- Kurek-Tyrlik, A.; Wicha, J.; Zarecki, A.; Snatzke, G. Methylation and hydroxymethylation of allylic alcohols via radical cyclization. Methodology for stereoselective construction of an aliphatic chain in application to sterol synthesis. J. Org. Chem. 1990, 55, 3484–3492. [Google Scholar] [CrossRef]
- Da Cunha, U.S.; Vendramim, J.D.; Rocha, W.C.; Vieira, P.C. Bioatividade de moléculas isoladas de Trichilia pallida Swartz (Meliaceae) sobre Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) [Bioactivity of Trichilia pallida Swartz (Meliaceae) derived molecules on Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae)]. Neotrop. Entomol. 2008, 37, 709–715. [Google Scholar] [CrossRef][Green Version]
- Kicha, A.A.; Ivanchina, N.V.; Kalinovsky, A.I.; Dmitrenok, P.S.; Stonik, V.A. Alkaloidosteroids from the starfish Lethasterias nanimensis chelifera. Tetrahedron Lett. 2003, 44, 1935–1937. [Google Scholar] [CrossRef]
- Pathirana, C.; Andersen, R.J. Imbricatine, an unusual benzyltetrahydroisoquinoline alkaloid isolated from the starfish Dermasterias imbricata. J. Am. Chem. Soc. 1986, 108, 8288–8289. [Google Scholar] [CrossRef]
- Turner, E.; Klevit, R.; Hager, L.J.; Shapiro, B.M. Ovothiols, a family of redox-active mercaptohistidine compounds from marine invertebrate eggs. Biochemistry 1987, 26, 4028–4036. [Google Scholar] [CrossRef]
- Palagiano, E.; De Marino, S.; Minale, L.; Riccio, R.; Iorizzi, M.; Zollo, F.; Carre, J.B.; Provost, J. Ptilomycalin A, crambescidin 800 and related new highly cytotoxic guanidine alkaloids from starfishes Fromia monilis and Celerina heffernani. Tetrahedron 1995, 51, 3675–3682. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Du, F.; Zhao, X.; Fan, D. Soft agar colony formation assay as a hallmark of carcinogenesis. Bio Protoc. 2017, 7, e2351. [Google Scholar] [CrossRef]



| Position | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| 1β α | 1.89 m 1.10 m | 1.75 dt (13.4, 3.5) 1.01 m | 1.89 m 1.10 m | 1.75 m 1.36 m |
| 2α β | 2.04 m 1.62 m | 2.00 m 1.52 m | 2.05 m 1.61 m | 4.07 br d (2.5) |
| 3 | 4.13 m (1/2 ΔW = 19.8 Hz) | 4.25 m | 4.13 m | 4.39 br d (2.5) |
| 4α β | 2.53 ddd (13.4, 4.8, 2.2) 2.33 m | 1.80 m 1.40 m | 2.53 ddd (13.3, 4.9, 2.2) 2.34 m | 1.81 m 1.59 m |
| 5 | – | 1.14 m | – | 1.58 m |
| 6 | 5.38 m | 1.28 m | 5.38 m | 1.24 m |
| 7β α | 1.96 m 1.54 m | 1.67 m 0.90 m | 1.96 m 1.54 m | 1.67 m 0.93 m |
| 8 | 1.47 m | 1.38 m | 1.46 m | 1.38 m |
| 9 | 0.95 m | 0.67 m | 0.95 m | 0.70 m |
| 10 | – | – | – | – |
| 11 | 1.52 m 1.02 m | 1.53 m 1.32 m | 1.53 m 1.04 m | 1.52 m 1.31 m |
| 12β α | 1.99 m 1.20 m | 1.95 dt (12.6, 3.0) 1.17 m | 2.01 m 1.24 m | 1.96 m 1.18 m |
| 13 | – | – | – | – |
| 14 | 1.05 m | 1.04 m | 1.08 m | 1.09 m |
| 15α β | 1.60 m 1.08 m | 1.58 m 1.07 m | 1.62 m 1.09 m | 1.59 m 1.07 m |
| 16α β | 1.66 m 1.37 m | 1.64 m 1.32 m | 2.25 m 1.15 m | 2.22 m 1.14 m |
| 17 | 1.44 m | 1.44 m | 1.64 m | 1.64 m |
| 18 | 0.75 s | 0.71 s | 0.67 s | 0.64 s |
| 19 | 1.03 s | 0.84 s | 1.02 s | 0.98 s |
| 20 | 2.31 m | 2.30 m | 1.63 m | 1.64 m |
| 21 | 4.13 dd (9.8, 4.4) 3.83 dd (9.8, 7.4) | 4.13 dd (9.4, 4.4) 3.81 dd (9.4, 7.6) | 0.96 d (6.2) | 0.95 d (6.0) |
| 22 | 5.24 dd (15.4, 9.3) | 5.24 dd (15.3, 9.8) | 4.58 dd (11.0, 4.0) | 4.57 dd (11.0, 4.2) |
| 23 | 5.39 dd (15.4, 6.8) | 5.38 dd (15.3, 6.8) | 2.85 dd (13.7, 4.0) 2.35 dd (13.7, 11.0) | 2.85 dd (13.7, 3.8) 2.35 dd (13.7, 11.0) |
| 24 | – | – | – | – |
| 25 | 2.22 m | 2.22 m | 2.25 m | 2.26 m |
| 26 | 0.96 d (6.7) | 0.96 d (6.9) | 1.06 d (6.7) | 1.05 d (6.8) |
| 27 | 0.96 d (6.7) | 0.96 d (6.9) | 1.05 d (6.7) | 1.04 d (6.8) |
| 28 | 4.84 s 4.75 s | 4.84 s 4.74 s |
| Position | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| 1 | 38.5 | 38.2 | 38.3 | 41.0 |
| 2 | 30.0 | 29.8 | 29.9 | 70.0 |
| 3 | 79.9 | 79.7 | 79.8 | 78.5 |
| 4 | 40.4 | 36.4 | 40.3 | 30.3 |
| 5 | 141.6 | 46.3 | 141.5 | 40.6 |
| 6 | 123.2 | 29.9 | 123.2 | 29.3 |
| 7 | 33.0 | 33.2 | 32.9 | 33.2 |
| 8 | 33.2 | 36.9 | 33.2 | 36.4 |
| 9 | 51.9 | 55.8 | 51.5 | 56.5 |
| 10 | 37.9 | 36.5 | 37.6 | 36.4 |
| 11 | 22.1 | 22.2 | 22.1 | 22.0 |
| 12 | 40.2 | 40.4 | 40.9 | 41.3 |
| 13 | 43.3 | 43.6 | 43.3 | 43.7 |
| 14 | 58.1 | 57.8 | 57.9 | 57.7 |
| 15 | 25.1 | 25.0 | 25.3 | 25.3 |
| 16 | 28.6 | 28.6 | 29.1 | 29.1 |
| 17 | 51.9 | 52.1 | 53.1 | 53.3 |
| 18 | 12.9 | 12.6 | 12.0 | 12.3 |
| 19 | 19.7 | 13.1 | 19.6 | 14.6 |
| 20 | 46.1 | 46.1 | 38.5 | 38.6 |
| 21 | 71.5 | 71.5 | 12.4 | 12.5 |
| 22 | 129.5 | 129.6 | 81.2 | 81.3 |
| 23 | 139.6 | 139.5 | 38.2 | 38.3 |
| 24 | – | – | 154.3 | 154.3 |
| 25 | 32.4 | 32.4 | 34.4 | 34.4 |
| 26 | 23.1 | 23.1 | 22.6 | 22.7 |
| 27 | 22.9 | 22.9 | 21.8 | 21.9 |
| 28 | 110.1 | 110.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kicha, A.A.; Malyarenko, T.V.; Kuzmich, A.S.; Malyarenko, O.S.; Kalinovsky, A.I.; Popov, R.S.; Tolkanov, D.K.; Ivanchina, N.V. Rare Ophiuroid-Type Steroid 3β,21-, 3β,22-, and 3α,22-Disulfates from the Slime Sea Star Pteraster marsippus and Their Colony-Inhibiting Effects against Human Breast Cancer Cells. Mar. Drugs 2024, 22, 43. https://doi.org/10.3390/md22010043
Kicha AA, Malyarenko TV, Kuzmich AS, Malyarenko OS, Kalinovsky AI, Popov RS, Tolkanov DK, Ivanchina NV. Rare Ophiuroid-Type Steroid 3β,21-, 3β,22-, and 3α,22-Disulfates from the Slime Sea Star Pteraster marsippus and Their Colony-Inhibiting Effects against Human Breast Cancer Cells. Marine Drugs. 2024; 22(1):43. https://doi.org/10.3390/md22010043
Chicago/Turabian StyleKicha, Alla A., Timofey V. Malyarenko, Alexandra S. Kuzmich, Olesya S. Malyarenko, Anatoly I. Kalinovsky, Roman S. Popov, Dmitriy K. Tolkanov, and Natalia V. Ivanchina. 2024. "Rare Ophiuroid-Type Steroid 3β,21-, 3β,22-, and 3α,22-Disulfates from the Slime Sea Star Pteraster marsippus and Their Colony-Inhibiting Effects against Human Breast Cancer Cells" Marine Drugs 22, no. 1: 43. https://doi.org/10.3390/md22010043
APA StyleKicha, A. A., Malyarenko, T. V., Kuzmich, A. S., Malyarenko, O. S., Kalinovsky, A. I., Popov, R. S., Tolkanov, D. K., & Ivanchina, N. V. (2024). Rare Ophiuroid-Type Steroid 3β,21-, 3β,22-, and 3α,22-Disulfates from the Slime Sea Star Pteraster marsippus and Their Colony-Inhibiting Effects against Human Breast Cancer Cells. Marine Drugs, 22(1), 43. https://doi.org/10.3390/md22010043








