Antioxidant, Antibacterial Properties of Novel Peptide CP by Enzymatic Hydrolysis of Chromis notata By-Products and Its Efficacy on Atopic Dermatitis
Abstract
1. Introduction
2. Results
2.1. Yield of Hydrolysates from Chromis notata By-Product Protein and Selection Based on ABTS Radical Scavenging
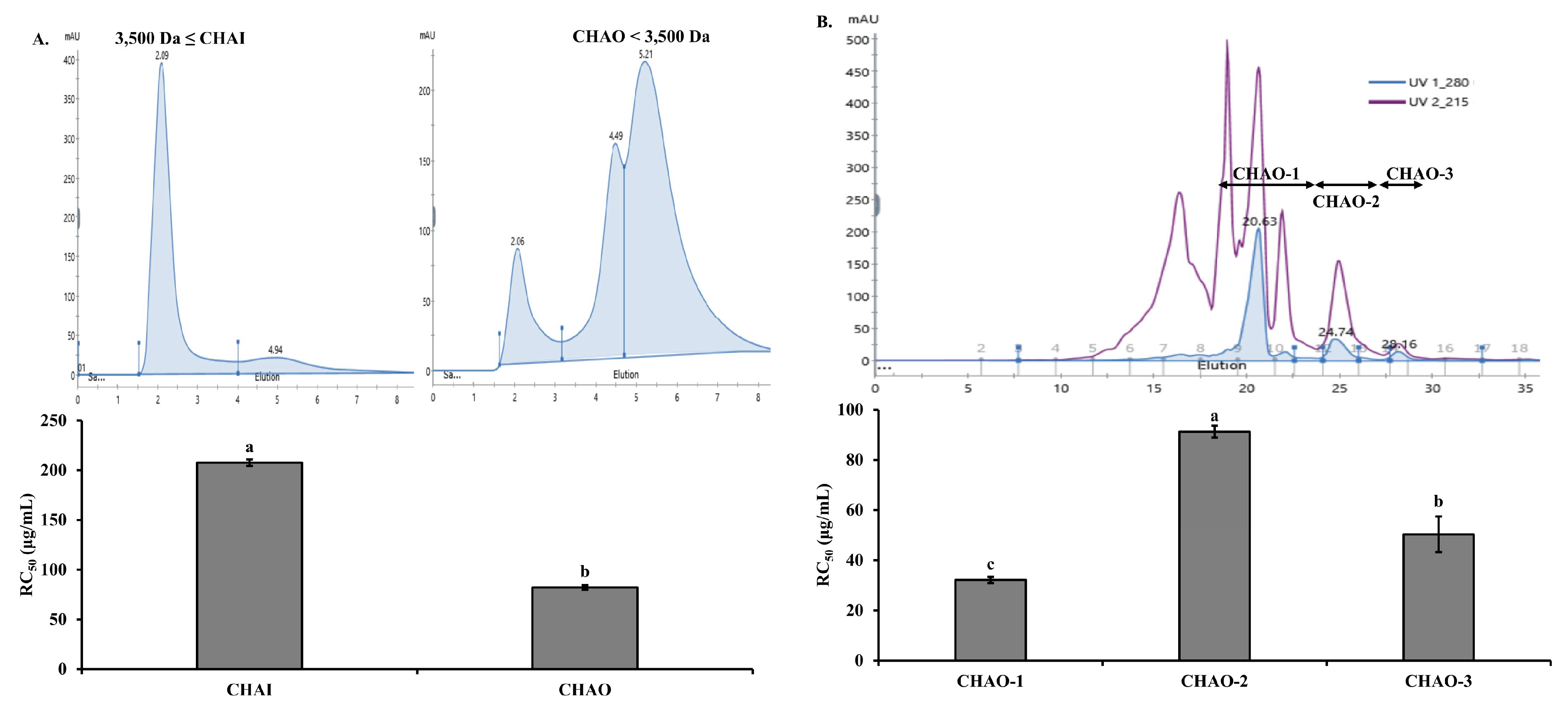
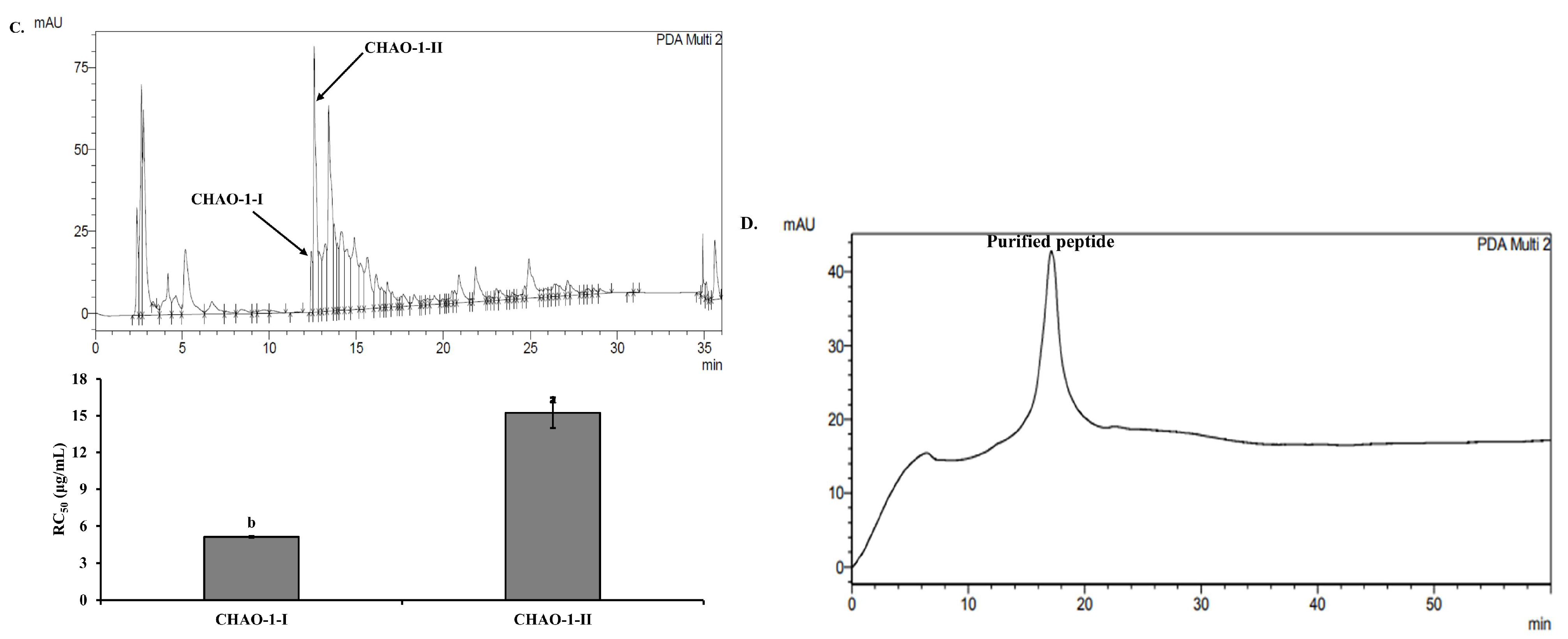
2.2. Purification of Antioxidant Peptides from C. notata By-Product Alcalase Hydrolysate
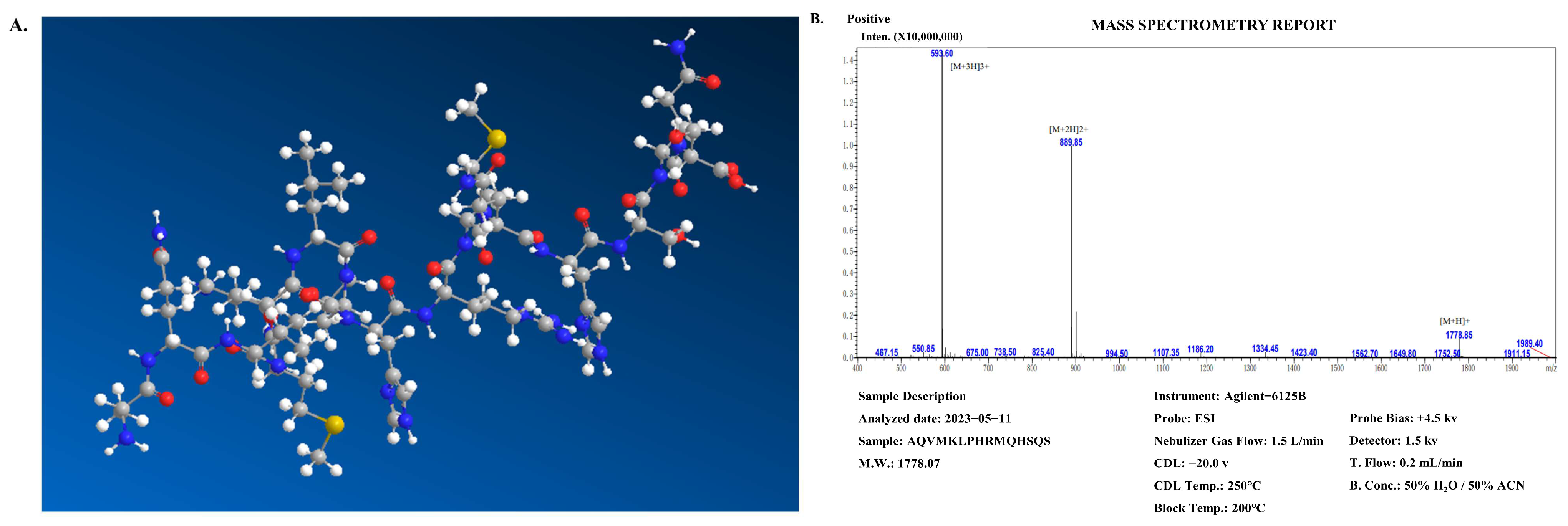
2.3. Structural Characterization of C. notata By-Product-Derived CP Peptide
2.4. Antimicrobial Activity of CP
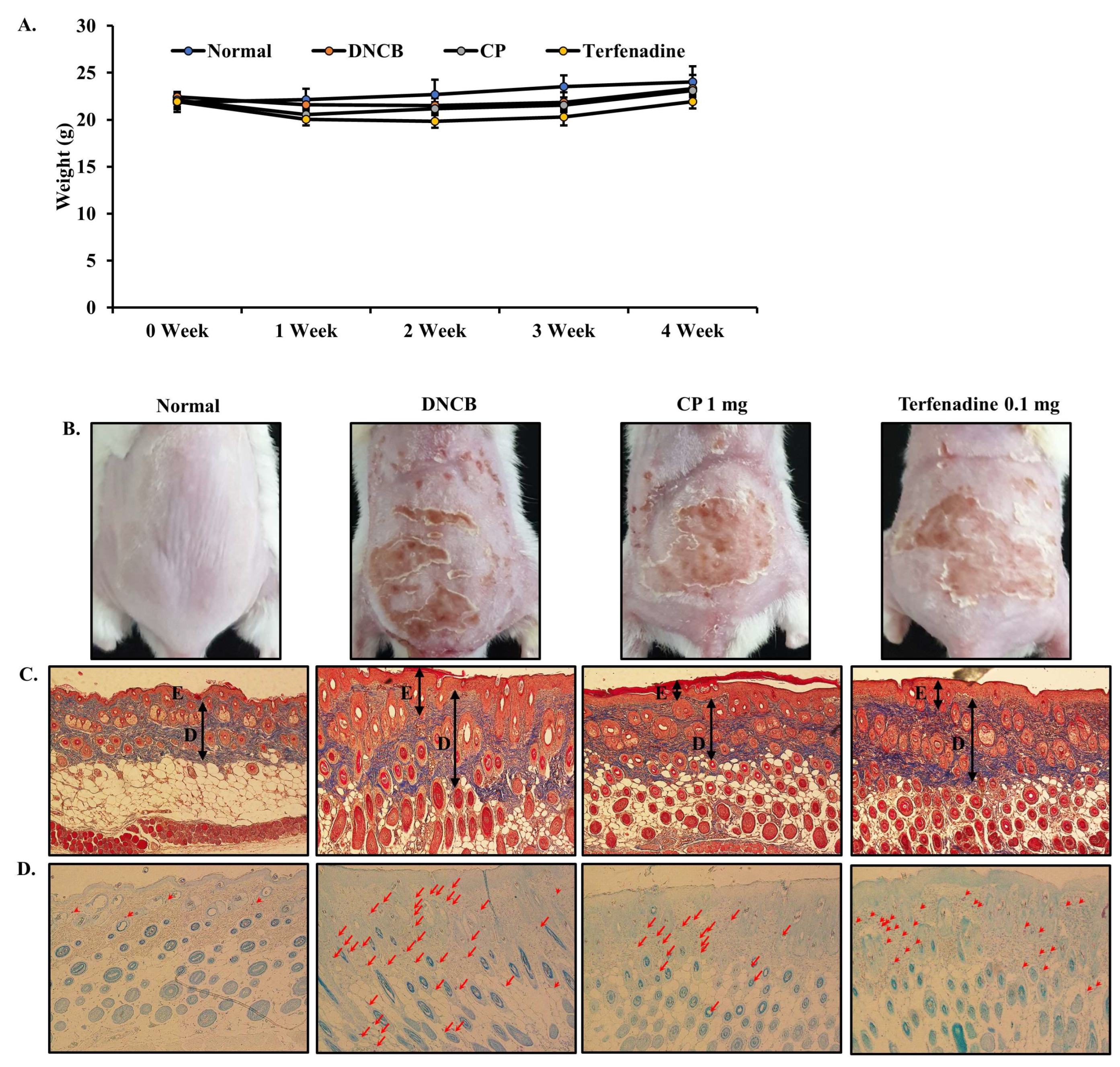
2.5. Body Weight and Histopathological Effects of CP in DNCB-Induced AD Mouse Model
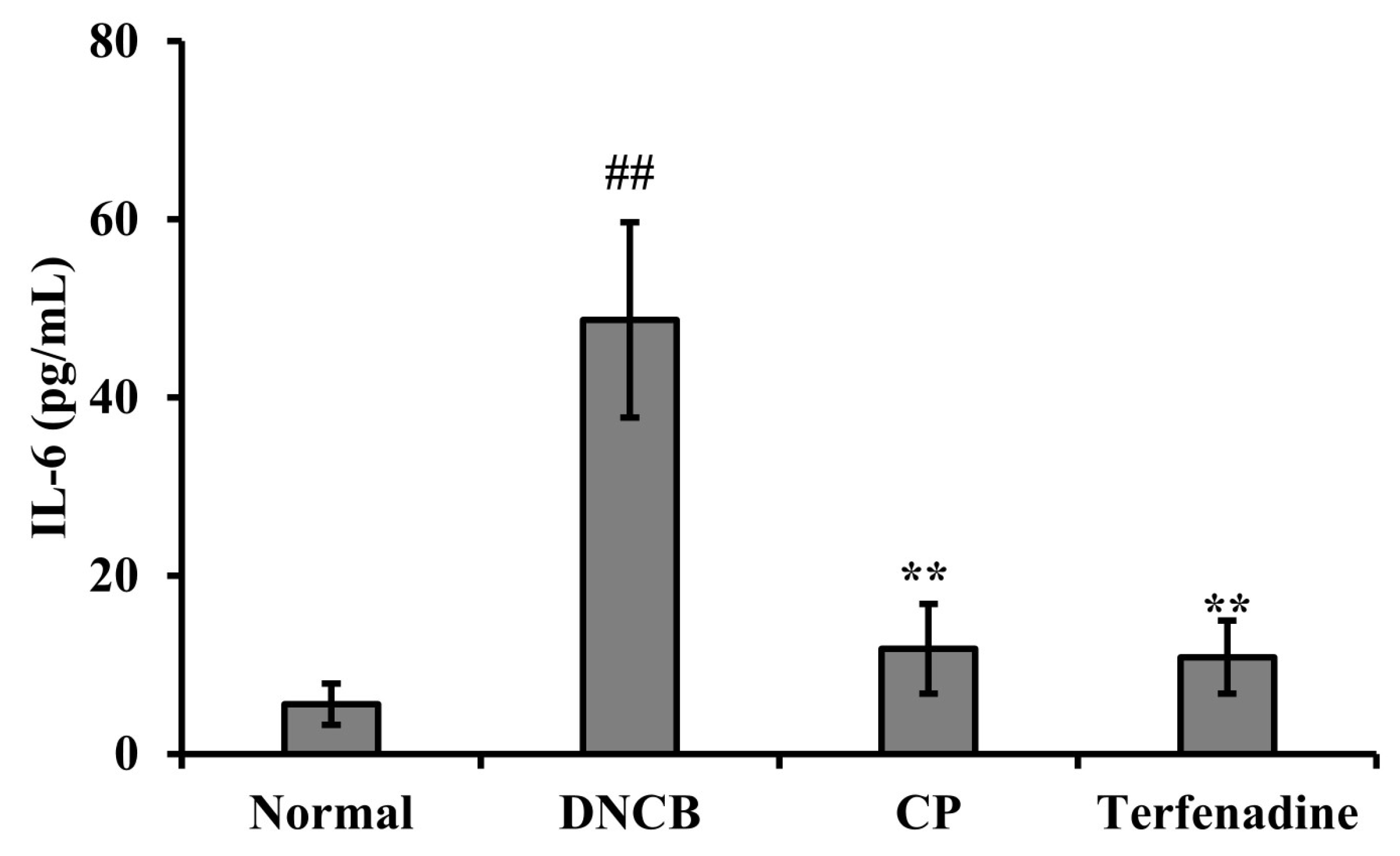
2.6. Effects of CP on Serum IL-6 Levels in the DNCB-Induced AD Mouse Model
3. Discussion
4. Materials and Methods
4.1. Experimental Materials
4.2. Preparation of Enzymatic Hydrolysate from C. notata By-Products
4.3. Measurement of ABTS Radical-Scavenging Activity in Enzymatic Hydrolysates
4.4. Peptide Isolation from Alcalase Hydrolysate of C. notata By-Product
4.5. Amino Acid Sequence Analysis and Synthesis
4.6. S. aureus Cultivation
4.7. Antimicrobial Activity Measurement of Peptides Using the Agar Paper Disc Method
4.8. Determination of Minimum Inhibitory Concentration (MIC) of Peptides
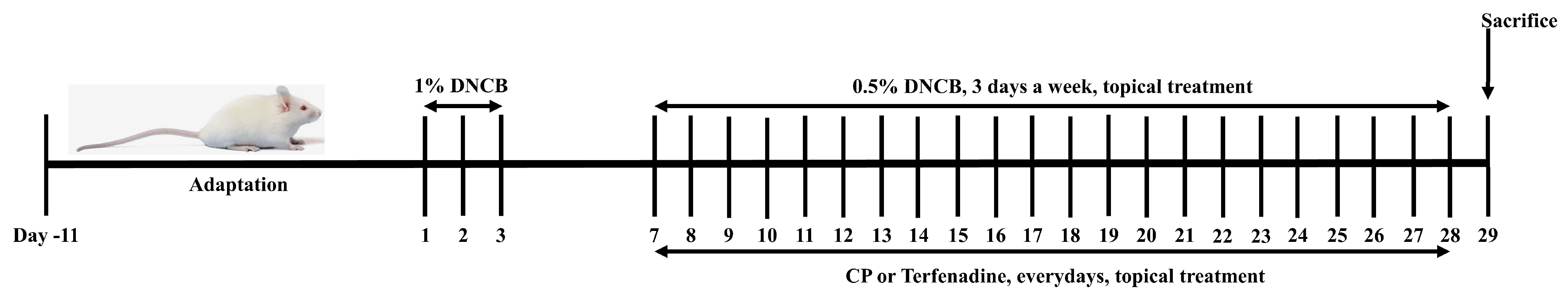
4.9. DNCB-Induced Atopic Dermatitis (AD) Mouse Model
4.10. Histological Evaluation in the AD Animal Model
4.11. Changes in Serum IL-6 Levels in the Atopic Dermatitis Animal Model
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Leung, D.Y.M. New insights into atopic dermatitis: Role of skin barrier and immune dysregulation. Allergol. Int. 2013, 62, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Elias, P.M.; Schmuth, M. Abnormal skin barrier in the etiopathogenesis of atopic dermatitis. Curr. Opin. Allergy Clin. Immunol. 2009, 9, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Salava, A.; Lauerma, A. Role of the skin microbiome in atopic dermatitis. Clin. Trans. Allergy 2014, 4, 33. [Google Scholar] [CrossRef]
- Nakatsuji, T.; Chen, T.H.; Narala, S.; Chun, K.A.; Two, A.M.; Yun, T.; Shafiq, F.; Kotol, P.F.; Bouslimani, A.; Melnik, A.V.; et al. Antimicrobials from human skin commensal bacteria protect against staphylococcus aureus and are deficient in atopic dermatitis. Sci. Transl. Med. 2017, 9, 4680. [Google Scholar] [CrossRef]
- Hayashida, S.; Uchi, H.; Moroi, Y.; Furue, M. Decrease in circulating Th17 cells correlates with increased levels of CCL17, IgE and eosinophils in atopic dermatitis. J. Dermatol. Sci. 2011, 61, 180–186. [Google Scholar] [CrossRef]
- De Benedetto, A.; Agnihothri, R.; McGirt, L.Y.; Bankova, L.G.; Beck, L.A. Atopic dermatitis: A disease caused by innate immune defects? J. Investig. Dermatol. 2009, 129, 14–30. [Google Scholar] [CrossRef] [PubMed]
- Saeed, K.; Marsh, P.; Ahmad, N. Cryptic resistance in staphylococcus aureus: A risk for the treatment of skin infection? Curr. Opin. Infect. Dis. 2014, 27, 130–136. [Google Scholar] [CrossRef]
- Turner, N.A.; Sharma-Kuinkel, B.K.; Maskarinec, S.A.; Eichenberger, E.M.; Shah, P.P.; Carugati, M.; Holland, T.L.; Fowler, V.G., Jr. Methicillin-resistant staphylococcus aureus: An overview of basic and clinical research. Nat. Rev. Microbiol. 2019, 17, 203–218. [Google Scholar] [CrossRef]
- Namiduru, E.S.; Namiduru, M.; Karaoğlan, İ.; Koçak, K. Oxidative and nitrosative stress in patients with meningitis. Eur. J. Clin. Exp. Med. 2022, 20, 70–74. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef]
- Galiniak, S.; Mołoń, M.; Rachel, M. Links between disease severity, bacterial infections and oxidative Stress in Cystic Fibrosis. Antioxidants 2022, 11, 887. [Google Scholar] [CrossRef] [PubMed]
- Zalewska, A.; Klimiuk, A.; Zieba, S.; Wnorowska, O.; Rusak, M.; Waszkiewicz, N.; Szarmach, I.; Dzierzanowski, K.; Maciejczyk, M. Salivary gland dysfunction and salivary redox imbalance in patients with alzheimer’s disease. Sci. Rep. 2021, 11, 23904. [Google Scholar] [CrossRef] [PubMed]
- Pleńkowska, J.; Gabig-Cimińska, M.; Mozolewski, P. Oxidative stress as an important contributor to the pathogenesis of psoriasis. Int. J. Mol. Sci. 2020, 21, 6206. [Google Scholar] [CrossRef] [PubMed]
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The chemistry of reactive oxygen species (ROS) revisited: Outlining their role in biological macromolecules (DNA, Lipids and Proteins) and induced pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef] [PubMed]
- Nettis, E.; Distaso, M.; Saitta, S.; Casciaro, M.; Cristani, M.; Saija, A.; Vacca, A.; Gangemi, S.; Minciullo, P.L. Involvement of new oxidative stress markers in chronic spontaneous urticaria. Adv. Dermatol. Allergol. 2017, 34, 448–452. [Google Scholar] [CrossRef]
- Bertino, L.; Guarneri, F.; Cannavò, S.P.; Casciaro, M.; Pioggia, G.; Gangemi, S. Oxidative stress and atopic dermatitis. Antioxidants 2020, 9, 196. [Google Scholar] [CrossRef]
- Dilek, F.; Ozceker, D.; Ozkaya, E.; Guler, N.; Tamay, Z.; Kesgin, S.; Yazici, M.; Kocyigit, A. Oxidative stress in children with chronic spontaneous urticaria. Oxid. Med. Cell. Longev. 2016, 2016, e3831071. [Google Scholar] [CrossRef]
- Blanco, M.; Vazquez, J.A.; Perez-Martin, R.I.; Sotelo, C.G. Hydrolysates of fish skin collagen: An opportunity for valorizing fish industry byproducts. Mar. Drugs 2017, 15, 131. [Google Scholar] [CrossRef]
- Khong, N.M.H.; Yusoff, F.M.; Jamilah, B.; Basri, M.; Maznah, I.; Chan, K.W.; Armania, N.; Nishikawa, J. Improved collagen extraction from jellyfish (Acromitus hardenbergi) with increased physical-induced solubilization processes. Food Chem. 2018, 251, 41–50. [Google Scholar] [CrossRef]
- Ramakrishnan, S.R.; Jeong, C.R.; Park, J.W.; Cho, S.S.; Kim, S.J. A review on the processing of functional proteins or peptides derived from fish by-products and their industrial applications. Heliyon 2023, 9, e14188. [Google Scholar] [CrossRef]
- Govindharaj, M.; Roopavath, U.K.; Rath, S.N. Valorization of discarded marine eel fish skin for collagen extraction as a 3D printable blue biomaterial for tissue engineering. J. Clean. Prod. 2019, 230, 412–419. [Google Scholar] [CrossRef]
- Shepherd, C.J.; Jackson, A.J. Global fishmeal and fish-oil supply: Inputs, outputs and marketsa. J. Fish Biol. 2013, 83, 1046–1066. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Mendis, E. Bioactive compounds from marine processing byproducts—A review. Food Res. Int. 2006, 39, 383–393. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Duan, W.W.; Duan, Z.H.; Hai, Y.; Lei, X.G.; Chang, H. Extraction of chondroitin sulfate from Tilapia byproducts with ultrasonic-microwave synergistic. Adv. Mat. Res. 2013, 726–731, 4381–4385. [Google Scholar] [CrossRef]
- Ferraro, V.; Carvalho, A.P.; Piccirillo, C.; Santos, M.M.; Castro, P.M.; Pintado, M.E. Extraction of high added value biological compounds from sardine, sardine-type fish and mackerel canning residues—A review. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 3111–3120. [Google Scholar] [CrossRef]
- Abdallah, M.M.; Cardeira, M.; Matias, A.A.; Bronze, M.R.; Fernández, N. Lactic acid-based natural deep eutectic solvents to extract bioactives from marine by-products. Molecules 2022, 27, 4356. [Google Scholar] [CrossRef]
- Afonso, C.; Bandarra, N.M.; Nunes, L.; Cardoso, C. Tocopherols in seafood and aquaculture products. Crit. Rev. Food. Sci. Nutr. 2016, 56, 128–140. [Google Scholar] [CrossRef]
- Letisse, M.; Comeau, L. Enrichment of eicosapentaenoic acid and docosahexaenoic acid from sardine by-products by supercritical fluid fractionation. J. Sep. Sci. 2008, 31, 1374–1380. [Google Scholar] [CrossRef]
- Zamora-Sillero, J.; Gharsallaoui, A.; Prentice, C. Peptides from fish by-product protein hydrolysates and its functional properties: An overview. Mar. Biotechnol. 2018, 20, 118–130. [Google Scholar] [CrossRef]
- Matsui, T.; Yukiyoshi, A.; Doi, S.; Sugimoto, H.; Yamada, H.; Matsumoto, K. Gastrointestinal enzyme production of bioactive peptides from royal jelly protein and their antihypertensive ability in SHR. J. Nutr. Biochem. 2002, 13, 80–86. [Google Scholar] [CrossRef]
- Ryan, J.T.; Ross, R.P.; Bolton, D.; Fitzgerald, G.F.; Stanton, C. Bioactive peptides from muscle sources: Meat and fish. Nutrients 2011, 3, 756–791. [Google Scholar] [CrossRef]
- Jumeri; Kim, S.M. Antioxidant and anticancer activities of enzymatic hydrolysates of solitary tunicate (Styela clava). Food Sci. Biotech. 2011, 20, 1075–1085. [Google Scholar] [CrossRef]
- Park, P.J.; Kim, E.K.; Lee, S.J.; Park, S.Y.; Kang, D.S.; Jung, B.M.; Kim, K.S.; Je, J.Y.; Ahn, C.B. Protective effects against H2O2-induced damage by enzymatic hydrolysates of an edible brown seaweed, sea tangle (Laminaria japonica). J. Med. Food. 2009, 12, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Rajapakse, N.; Mendis, E.; Byun, H.G.; Kim, S.K. Purification and in vitro antioxidative effects of giant squid muscle peptides on free radical-mediated oxidative systems. J. Nutr. Biochem. 2005, 16, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Jun, S.Y.; Park, P.J.; Jung, W.K.; Kim, S.K. Purification and characterization of an antioxidative peptide from enzymatic hydrolysate of yellowfin sole (Limanda aspera) frame protein. Eur. Food Res. Technol. 2004, 219, 20–26. [Google Scholar]
- Park, P.J.; Je, J.Y.; Kim, S.K. Free radical scavenging activity of chitooligosaccharides by electron spin resonance spectrometry. J. Agric. Food Chem. 2003, 51, 4624–4627. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.U.; Kim, J.K. Morphological study of the genus Chromis from Korea. J. Korean Fish. Soc. 1996, 30, 562–573. [Google Scholar]
- Koh, J.R.; Park, Y.C. Species identification and molecular phylogenetic position of Korean damselfishes (Pomacentridae: Chrominae) based on DNA bioinformation. Korean J. Ichthyol. 2007, 19, 274–285. [Google Scholar]
- Ochi, H. Breeding synchrony and spawning intervals in the temperate damselfish Chromis notata. Environ. Biol. Fish. 1986, 17, 117–123. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations [FAO]. The State of World Fisheries and Aquaculture 2020, Sustainability in Action; FAO: Rome, Italy, 2020. [Google Scholar]
- Villamil, O.; Váquiro, H.; Solanilla, J.F. Fish viscera protein hydro-lysates: Production, potential applications and functional and bioactive properties. Food Chem. 2017, 224, 160–171. [Google Scholar] [CrossRef]
- Wang, C.H.; Doan, C.T.; Nguyen, V.B.; Nguyen, A.D.; Wang, S.L.; Wang, S.L. Reclamation of fishery processing waste: A mini-review. Molecules 2019, 24, 2234. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.R.; Newton, R.W.; Tlusty, M.; Little, D.C. The rise of aqua-culture by-products: Increasing food production, value, and sustainability through strategic utilisation. Mar. Policy 2018, 90, 115–124. [Google Scholar] [CrossRef]
- Marti-Quijal, F.J.; Remize, F.; Meca, G.; Ferrer, E.; Ruiz, M.J.; Barba, F.J. Fermentation in fish and by-products processing: An overview of current research and future prospects. Curr. Opin. Food Sci. 2020, 31, 9–16. [Google Scholar] [CrossRef]
- Ucak, I.; Afreen, M.; Montesano, D.; Carrillo, C.; Tomasevic, I.; Simal-Gandara, J.; Barba, F.J. Functional and bioactive properties of peptides derived from marine side streams. Mar. Drugs 2021, 19, 71. [Google Scholar] [CrossRef]
- Rustad, T.; Storrø, I.; Slizyte, R. Possibilities for the Utilisation of Marine By-products. Int. J. Food Sci. Technol. 2011, 46, 2001–2014. [Google Scholar] [CrossRef]
- Arvanitoyannis, I.; Kassaveti, A. Fish industry waste: Treatments, environmental impacts, current and potential uses. Int. J. Food Sci. Technol. 2008, 43, 726–745. [Google Scholar] [CrossRef]
- Moses, M.; Maria, H. Fish by-product use as biostimulants: An overview of the current state of the art, including relevant legislation and regulations within the EU and USA. Molecules 2020, 25, 1122. [Google Scholar]
- Chi, C.F.; Wang, B.; Hu, F.Y.; Wang, Y.M.; Zhang, B.; Deng, S.G.; Wu, C.W. Purification and identification of three novel antioxidant peptides from protein hydrolysate of bluefin leatherjacket (Navodon Septentrionalis) skin. Food Res. Int. 2015, 73, 124–129. [Google Scholar] [CrossRef]
- Chen, H.M.; Muramoto, K.; Yamauchi, F. Structural analysis of antioxidative peptides from soybean beta-conglycinin. J. Agric. Food Chem. 1995, 43, 574–578. [Google Scholar] [CrossRef]
- Veymar, G.T.P.; Roberto, M.S.; Siar, E.H.; Olga, T.; Ángel, B.M.; Roberto, F.L. Use of alcalase in the production of bioactive peptides: A review. Int. J. Biol. Macromol. 2020, 15, 2143–2196. [Google Scholar]
- Qin, L.; Zhu, B.W.; Zhou, D.Y.; Wu, H.T.; Tan, H.; Yang, J.F.; Li, D.M.; Dong, X.P.; Murata, Y. Preparation and antioxidant activity of enzymatic hydrolysates from purple sea urchin (Strongylocentrotus nudus) gonad. LWT—Food Sci. Technol. 2011, 44, 1113–1118. [Google Scholar] [CrossRef]
- Shimizu, M. Food-derived peptides and intestinal functions. Biofactors 2004, 21, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Gonzalez de Mejia, E. A new frontier in soy bioactive peptides that may prevent age-related chronic diseases. Comp. Rev. Food Sci. Food Saf. 2005, 4, 63–78. [Google Scholar] [CrossRef]
- Castro, M.S.; Cilli, E.M.; Fontes, W. Combinatorial synthesis and directed evolution applied to the production of alpha-helix forming antimicrobial peptides analogues. Curr. Protein Pept. Sci. 2006, 7, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Schiffer, M.; Edmundson, A.B. Use of helical wheels to represent the structures of proteins and to identify segments with helical potential. Biophys. J. 1967, 7, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Sarmadi, B.; Ismail, A. Antioxidative peptides from food proteins: A review. Peptides 2010, 31, 1949–1956. [Google Scholar] [CrossRef] [PubMed]
- Abbas, E.; Osman, A.; Sitohy, M. Biochemical control of Alternaria tenuissima infecting post-harvest fig fruit by chickpea vicilin. J. Sci. Food Agric. 2020, 100, 2889–2897. [Google Scholar] [CrossRef]
- Sitohy, M.; Osman, A. Antimicrobial activity of native and esterified legume proteins against Gram-negative and Gram-positive bacteria. Food Chem. 2010, 120, 66–73. [Google Scholar] [CrossRef]
- Cheng, A.C.; Lin, H.L.; Shiu, Y.L.; Tyan, Y.C.; Liu, C.H. Isolation and characterization of antimicrobial peptides derived from Bacillus subtilis E20-fermented soybean meal and its use for preventing Vibrio infection in shrimp aquaculture. Fish Shellfish. Immunol. 2017, 67, 270–279. [Google Scholar] [CrossRef]
- Abdel-Hamid, M.; Romeih, E.; Saporito, P.; Osman, A.; Mateiu, R.V.; Mojsoska, B.; Jenssen, H. Camel milk whey hydrolysate inhibits growth and biofilm formation of Pseudomonas aeruginosa PAO1 and methicillin-resistant Staphylococcus aureus. Food Control 2020, 111, 107056. [Google Scholar] [CrossRef]
- Naima, N.; Karima, H.; Gabrielle, C.; Rafik, B.; Moncef, N.; Pascal, D.; Ali, B. Antibacterial peptides from barbel muscle protein hydrolysates: Activity against some pathogenic bacteria. LWT—Food Sci. Technol. 2014, 55, 183–188. [Google Scholar]
- Osman, A.; Goda, H.A.; Abdel-Hamid, M.; Badran, S.M.; Otte, J. Antibacterial peptides generated by Alcalase hydrolysis of goat whey. LWT—Food Sci. Technol. 2016, 65, 480–486. [Google Scholar] [CrossRef]
- Cumby, N.; Zhong, Y.; Naczk, M.; Shahidi, F. Antioxidant activity and water-holding capacity of canola protein hydrolysates. Food Chem. 2008, 109, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Chalamaiah, M.; Rao, G.N.; Rao, D.G.; Jyothirmay, T. Protein hydrolysates from meriga (Cirrhinusmrigala) egg and evaluation of their functional properties. Food Chem. 2010, 120, 652–657. [Google Scholar] [CrossRef]
- Al-Mohammadi, A.R.; Osman, A.; Enan, G.; Abdel-Shafi, S.; El-Nemer, M.; Sitohy, M.; Taha, M. Powerful antibacterial peptides from egg albumin hydrolysates. Antibiotics 2020, 9, 901. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, A.; Pirri, G.; Rinaldi, A.C. Antimicrobial Peptides: The LPS Connection. Methods Mol. Biol. 2010, 618, 137–154. [Google Scholar]
- Abdel-Shafi, S.; Osman, A.; Al-Mohammadi, A.R.; Enan, G.; Kamal, N.; Sitohy, M. Biochemical, biological characteristics and antibacterial activity of glycoprotein extracted from the epidermal mucus of African catfish (Clariasgariepinus). Int. J. Biol. Macromol. 2019, 138, 773–780. [Google Scholar] [CrossRef]
- Wang, G.; Li, X.; Wang, Z. APD3: The antimicrobial peptide database as a tool for research and education. Nucleic Acids Res. 2016, 44, 1087–1093. [Google Scholar] [CrossRef]
- Elias, P.M.; Steinhoff, M. “Outside-to-inside” (and now back to “outside”) pathogenic mechanisms in atopic dermatitis. J. Investig. Dermatol. 2008, 128, 1067–1070. [Google Scholar] [CrossRef]
- Wang, Q.; Gao, S.; Wu, G.Z.; Yang, N.; Zu, X.P.; Li, W.C.; Xie, N.; Zhang, R.R.; Li, C.W.; Hu, Z.L.; et al. Total sesquiterpene lactones isolated from Inula helenium L. attenuates 2, 4-dinitrochlorobenzene-induced atopic dermatitis-like skin lesions in mice. Phytomedicine 2018, 46, 78–84. [Google Scholar] [CrossRef]
- Kim, J.E.; Kim, J.S.; Cho, D.H.; Park, H.J. Molecular mechanisms of cutaneous inflammatory disorder: Atopic dermatitis. Int. J. Mol. Sci. 2016, 17, 1234. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evansa, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Soares, T.; Ferreiraa, F.R.B.; Gomes, F.S.; Coelhoa, L.C.B.B.; Torquato, R.J.S.; Napoleãoa, T.H.; Cavalcanti, M.S.M.; Tanaka, A.S.; Paivaa, P.M.G. The first serine protease inhibitor from Lasiodora sp. (Araneae: Theraphosidae) hemocytes. Process Biochem. 2011, 46, 2317–2321. [Google Scholar] [CrossRef]
- Kim, S.W.; Hong, S.Y.; Kwon, B.G.; Kim, M.G.; Kim, S.B.; Jin, D.H.; Choi, W.C.; Sohn, Y.G.; Jung, H.S. Effect of persicae semen for atopic dermatitis skin tissue and regulate to inflammation mediator in serum. Korea J. Herbol. 2020, 35, 51–60. [Google Scholar]
- Lee, J.S.; Woo, H.J. A study on natural dye having the effects on the atopic dermatitis (Part II): Pine needles extract. Text. Color. Finish. 2012, 24, 196–203. [Google Scholar] [CrossRef]
- Jeon, H.B.; Veerappan, K.; Moon, H.H.; Lee, T.H.; Lee, K.W.; Park, J.H.; Chung, H.Y. Parnassin, a novel therapeutic peptide, alleviates skin lesions in a DNCB-induced atopic dermatitis mouse model. Biomedicines 2023, 11, 1389. [Google Scholar]
- Zhang, Q.; Wang, H.; Ran, C.; Lyu, Y.; Li, F.; Yao, Y.; Xing, S.; Wang, L.; Chen, S. Anti-inflammatory effects of amarogentin on 2,4-dinitrochlorobenzene-induced atopic dermatitis–like mice and in HaCat cells. Anim. Model. Exp. Med. 2023, 6, 255–265. [Google Scholar] [CrossRef]



| Enzymes | Alcalase | Flavourzyme | Neutrase | Protamex | Vit.C |
|---|---|---|---|---|---|
| Yield (%) | 68.72 | 34.14 | 49.71 | 61.31 | - |
| RC50 (μg/mL) | 102.62 ± 5.63 d | 260.48 ± 3.40 b | 605.61 ± 220.58 a | 175.64 ± 6.98 c | 7.17 ± 2.02 e |
| Enzymes | pH | Temperature (°C) | Substrate:Enzyme |
|---|---|---|---|
| Alcalase | 7.0 | 50.0 | 50:1 |
| Flavourzyme | 7.0 | 50.0 | 50:1 |
| Neutrase | 7.0 | 50.0 | 50:1 |
| Protamex | 7.0 | 50.0 | 50:1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, J.-W.; Lee, S.-G.; Kang, H. Antioxidant, Antibacterial Properties of Novel Peptide CP by Enzymatic Hydrolysis of Chromis notata By-Products and Its Efficacy on Atopic Dermatitis. Mar. Drugs 2024, 22, 44. https://doi.org/10.3390/md22010044
Hwang J-W, Lee S-G, Kang H. Antioxidant, Antibacterial Properties of Novel Peptide CP by Enzymatic Hydrolysis of Chromis notata By-Products and Its Efficacy on Atopic Dermatitis. Marine Drugs. 2024; 22(1):44. https://doi.org/10.3390/md22010044
Chicago/Turabian StyleHwang, Jin-Woo, Sung-Gyu Lee, and Hyun Kang. 2024. "Antioxidant, Antibacterial Properties of Novel Peptide CP by Enzymatic Hydrolysis of Chromis notata By-Products and Its Efficacy on Atopic Dermatitis" Marine Drugs 22, no. 1: 44. https://doi.org/10.3390/md22010044
APA StyleHwang, J.-W., Lee, S.-G., & Kang, H. (2024). Antioxidant, Antibacterial Properties of Novel Peptide CP by Enzymatic Hydrolysis of Chromis notata By-Products and Its Efficacy on Atopic Dermatitis. Marine Drugs, 22(1), 44. https://doi.org/10.3390/md22010044




