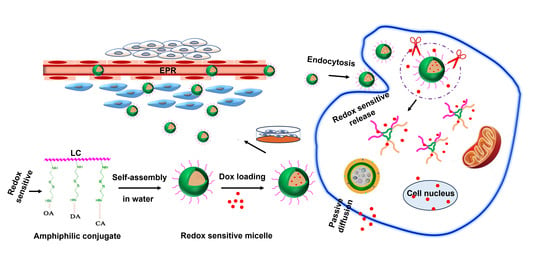
Effect of Hydrophobic Chain Length in Amphiphilic Chitosan Conjugates on Intracellular Drug Delivery and Smart Drug Release of Redox-Responsive Micelle
Abstract
:1. Introduction
2. Results and Discussion
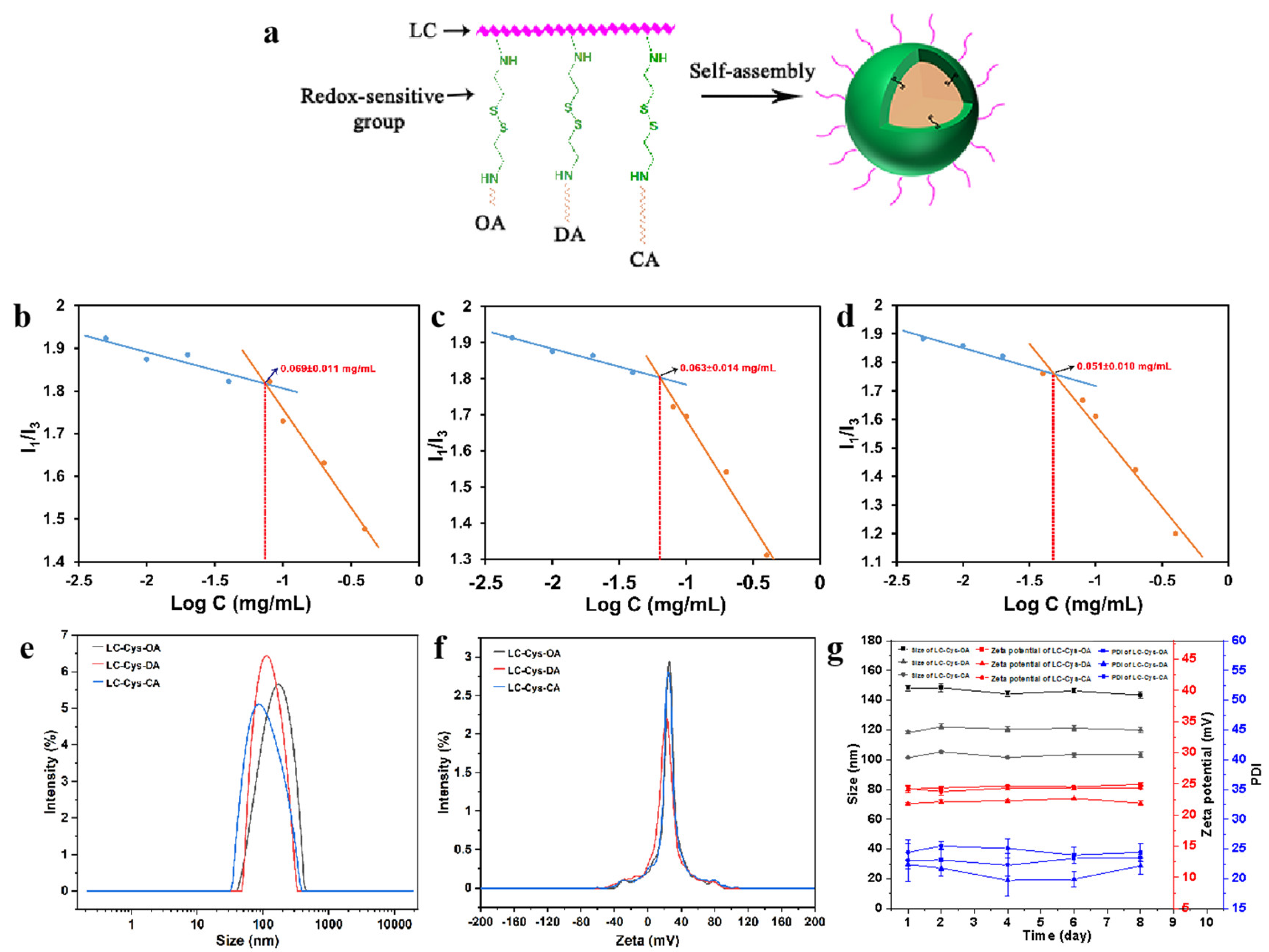
2.1. Synthesis and Structural Characterization of Amphiphilic LC-Cys-OA/LC-Cys-DA/LC-Cys-CA Conjugates
2.2. Preparation and Characterization of LC-Cys-OA/LC-Cys-DA/LC-Cys-CA Micelles
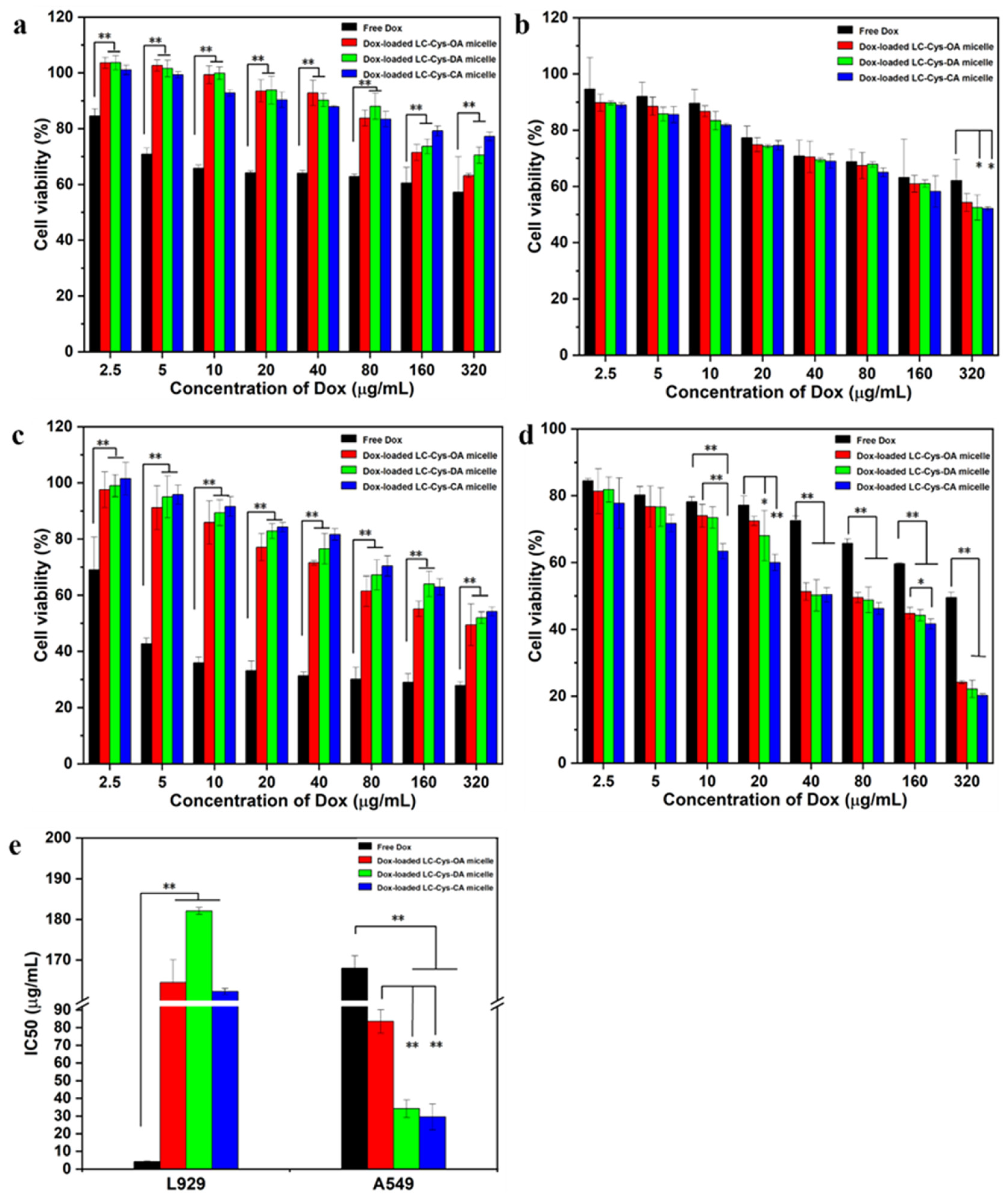
2.3. Biosafety Evaluation
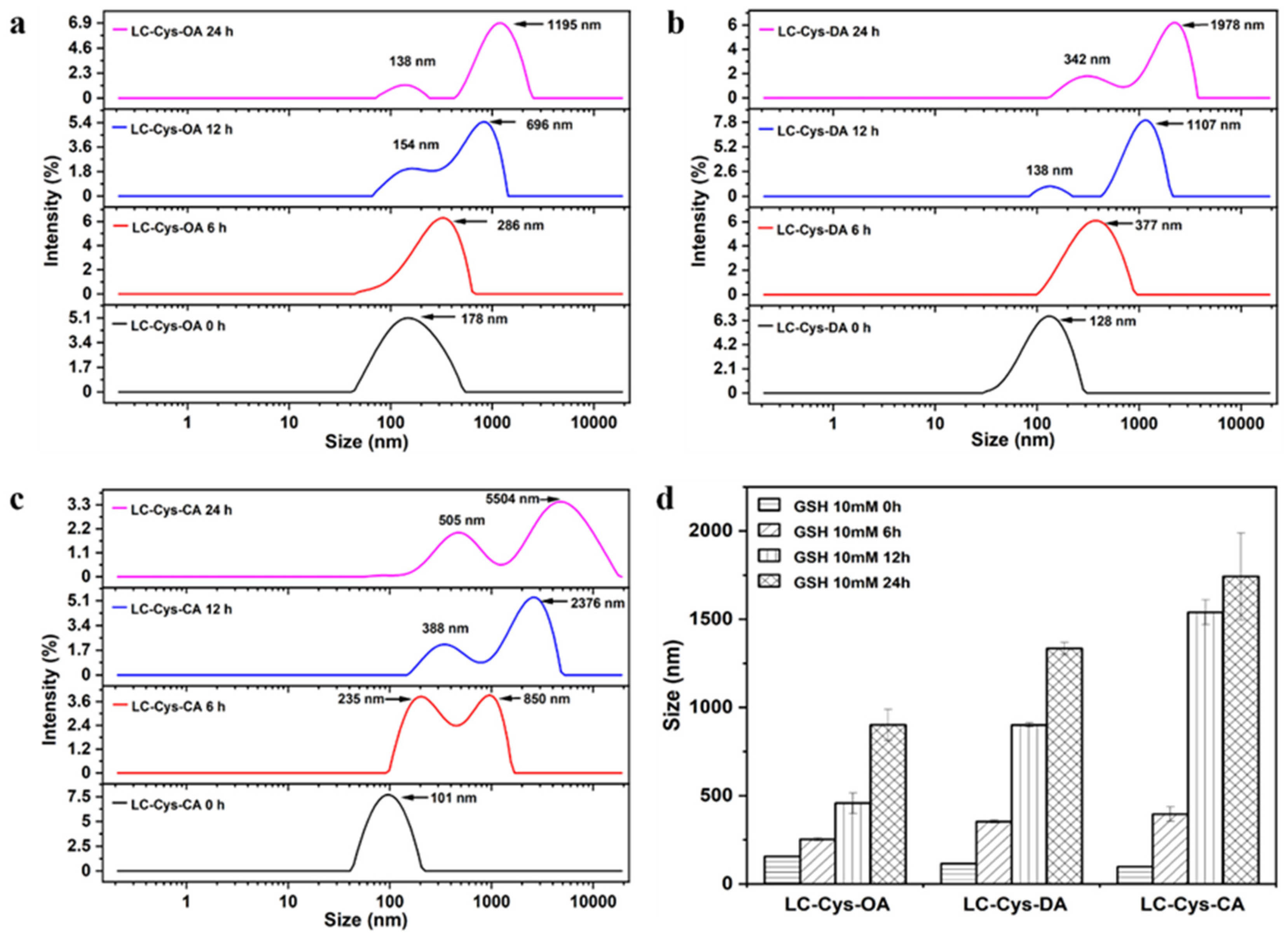
2.4. Redox-Triggered Disassembly Behavior of Three Blank Micelles
2.5. Dox-Encapsulation and Characterization of LC-Cys-OA/LC-Cys-DA/LC-Cys-OA Micelles
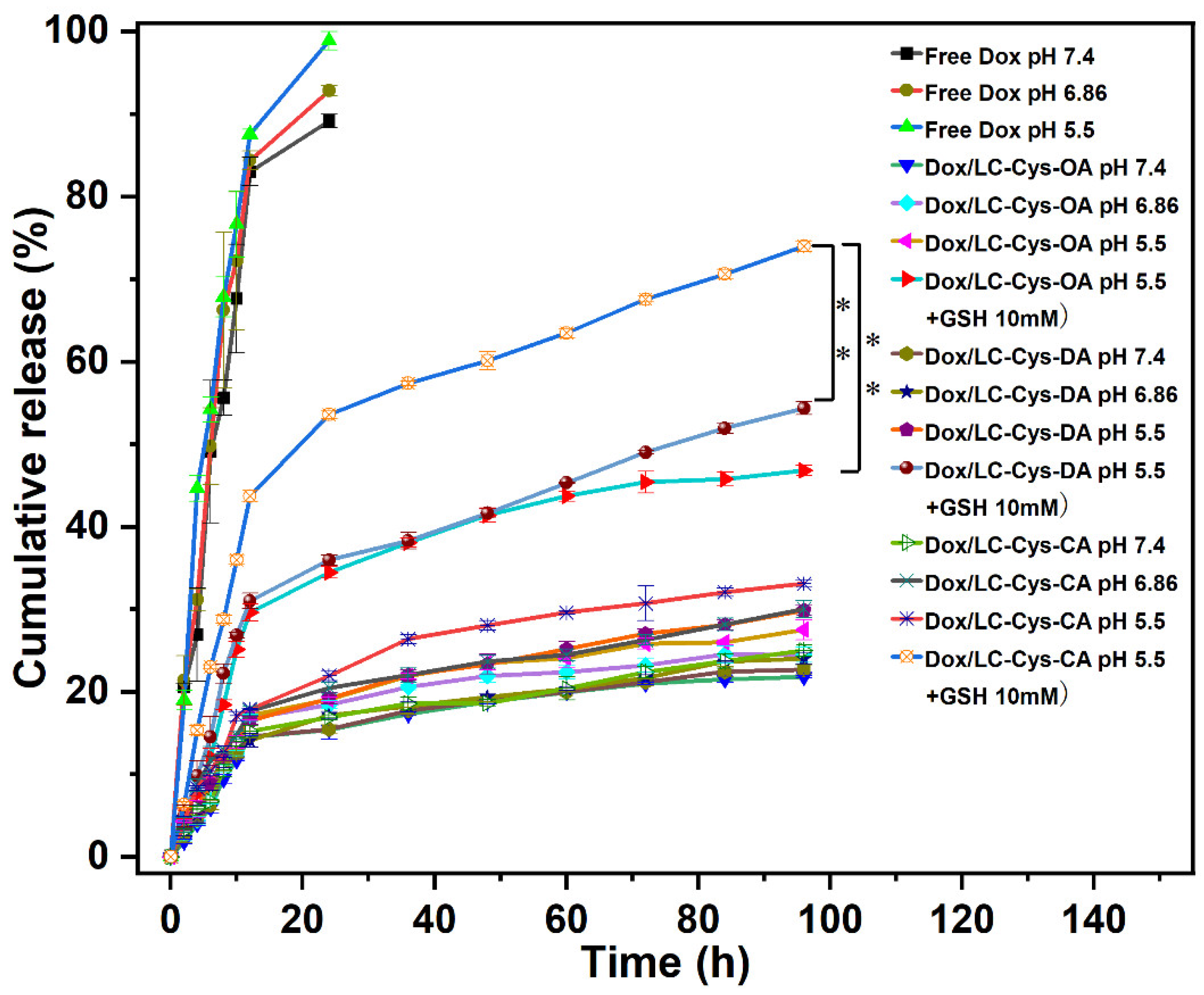
2.6. In Vitro Stimuli-Responsive Release
2.7. In Vitro Antitumor Activity of Three Dox-Loaded Micelles
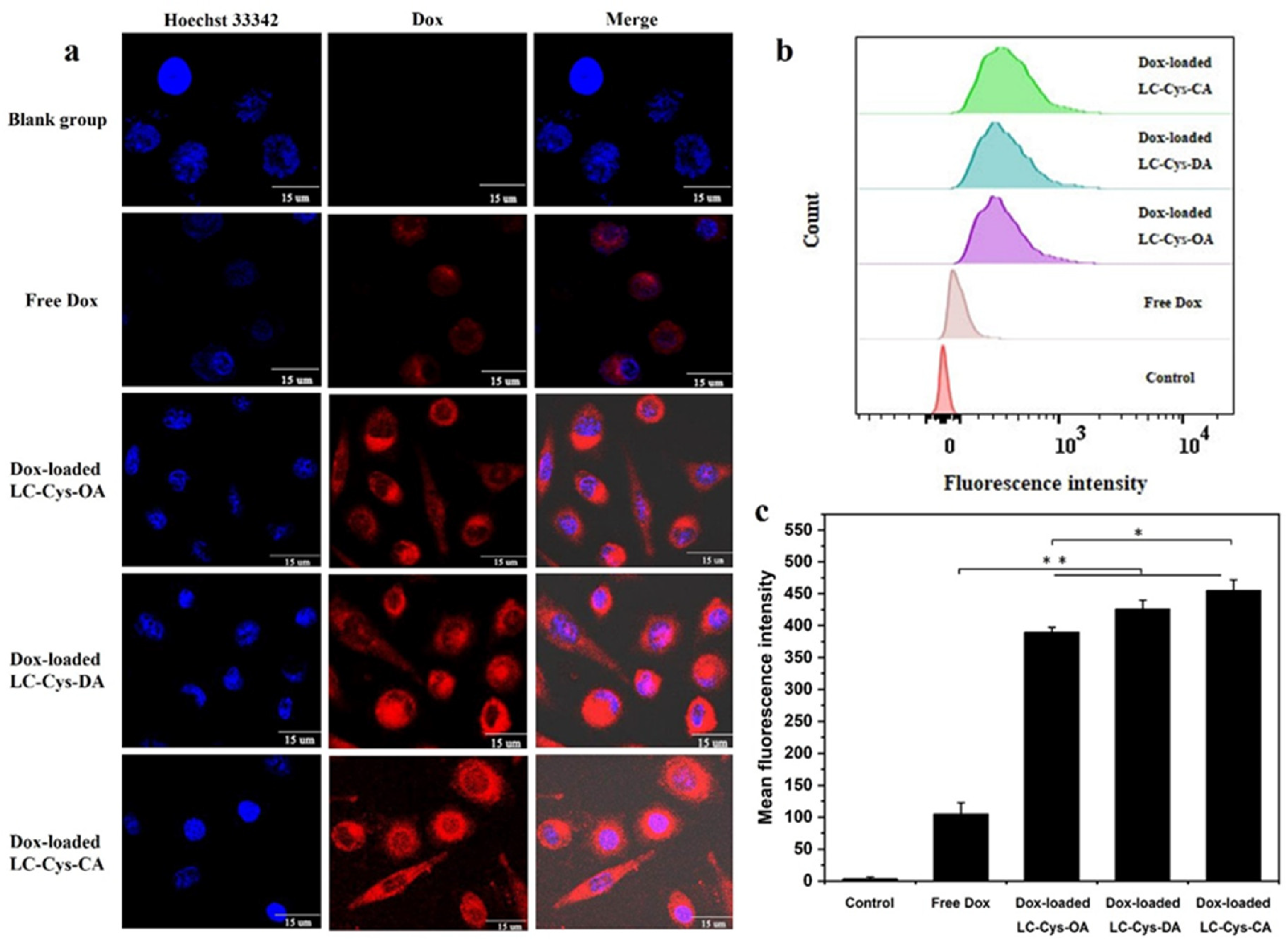
2.8. In Vitro Cellular Uptake
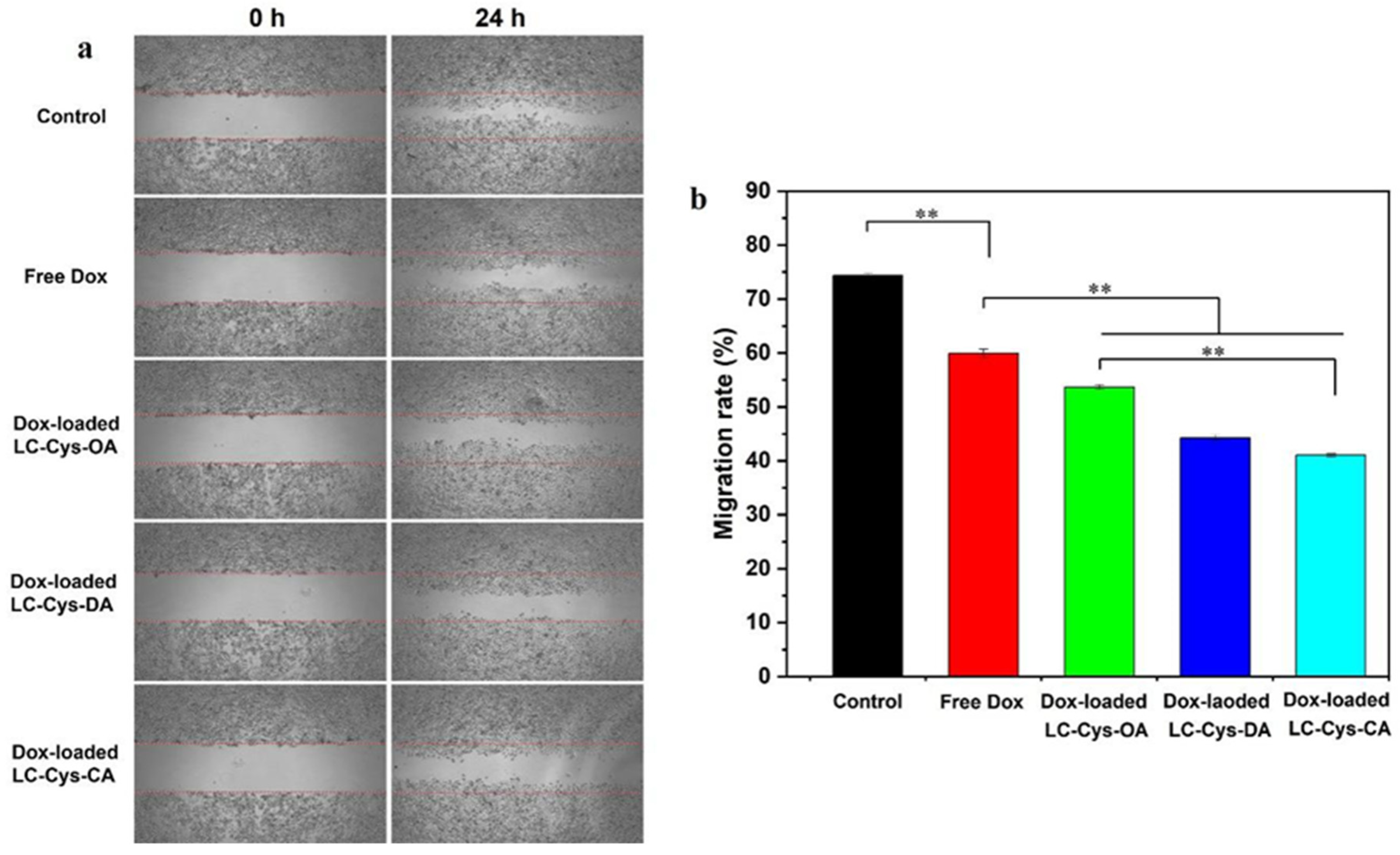
2.9. Antimigratory Activities of Three Dox-Loaded Micelles
3. Materials and Methods
3.1. Materials and Reagents
3.2. Synthesis of LC-Cys Conjugate
3.3. Synthesis of Amphiphilic LC-Cys-OA/DA/CA Conjugates
3.4. Characterization of Conjugates
3.5. Preparation of Blank Micelle and Dox Loaded Micelle
3.5.1. Preparation of Blank Micelle
3.5.2. Preparation of Dox Loaded Micelles
3.6. Characterization of Blank Micelles and Dox Loaded Micelles
3.7. Stimuli-Responsiveness Behavior of Blank Micelles Triggered by GSH
3.8. In Vitro Biosafety Evaluation
3.8.1. Hemolysis Assay
3.8.2. Cytotoxicity Assay
3.9. Redox Responsive Drug Release and Release Mechanism
3.9.1. In Vitro Drug Release Behavior
3.9.2. Drug Release Mechanism
3.10. In Vitro Antitumor Activity Assay
3.11. In Vitro Cellular Uptake
3.12. Wound Healing Assay
3.13. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sikder, A.; Vambhurkar, G.; Amulya, E.; Bagasariya, D.; Famta, P.; Shah, S.; Khatri, D.K.; Singh, S.B.; Sinha, V.R.; Srivastava, S. Advancements in redox-sensitive micelles as nanotheranostics: A new horizon in cancer management. J. Control. Release 2022, 349, 1009–1030. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zou, W.; Bian, S.; Huang, Y.; Tan, Y.; Liang, J.; Fan, Y.; Zhang, X. Bioreducible PAA-g-PEG graft micelles with high doxorubicin loading for targeted antitumor effect against mouse breast carcinoma. Biomaterials 2013, 34, 6818–6828. [Google Scholar] [CrossRef] [PubMed]
- Shymborska, Y.; Budkowski, A.; Raczkowsk, J.; Donchak, V.; Melnyk, Y.; Vasiichuk, V. Switching it Up: The Promise of Stimuli-Responsive Polymer Systems in Biomedical Science. Chem. Rec. 2023, e202300217. [Google Scholar] [CrossRef] [PubMed]
- Jeong, G.W.; Jeong, Y.I.; Nah, J.W. Triggered doxorubicin release using redox-sensitive hyaluronic acid-g-stearic acid micelles for targeted cancer therapy. Carbohydr. Polym. 2019, 209, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-S.; Huang, S.-J.; Huang, X.-S.; Wu, Y.-T.; Chen, H.-Y.; Lo, Y.-L.; Wang, L.-F. The synthesis and comparison of poly(methacrylic acid)–poly(ε-caprolactone) block copolymers with and without symmetrical disulfide linkages in the center for enhanced cellular uptake. RSC Adv. 2016, 6, 75092–75103. [Google Scholar] [CrossRef]
- Birlik Demirel, G.; Bayrak, Ş. Ultrasound/redox/pH-responsive hybrid nanoparticles for triple-triggered drug delivery. J. Drug Deliv. Sci. Technol. 2022, 71, 103267. [Google Scholar] [CrossRef]
- Lin, D.; Xiao, L.; Qin, W.; Loy, D.A.; Wu, Z.; Chen, H.; Zhang, Q. Preparation, characterization and antioxidant properties of curcumin encapsulated chitosan/lignosulfonate micelles. Carbohydr. Polym. 2022, 281, 119080. [Google Scholar] [CrossRef]
- Itoo, A.M.; Paul, M.; Ghosh, B.; Biswas, S. Oxaliplatin delivery via chitosan/vitamin E conjugate micelles for improved efficacy and MDR-reversal in breast cancer. Carbohydr. Polym. 2022, 282, 119108. [Google Scholar] [CrossRef]
- Shirani, S.; Varshosaz, J.; Rostami, M.; Mirian, M. Redox responsive polymeric micelles of gellan gum/abietic acid for targeted delivery of ribociclib. Int. J. Biol. Macromol. 2022, 215, 334–345. [Google Scholar] [CrossRef]
- Liu, M.; Du, H.; Zhai, G. Self-assembled nanoparticles based on chondroitin sulfate-deoxycholic acid conjugates for docetaxel delivery: Effect of degree of substitution of deoxycholic acid. Colloids Surf. B Biointerfaces 2016, 146, 235–244. [Google Scholar] [CrossRef]
- Lu, A.; Petit, E.; Jelonek, K.; Orchel, A.; Kasperczyk, J.; Wang, Y.; Su, F.; Li, S. Self-assembled micelles prepared from bio-based hydroxypropyl methyl cellulose and polylactide amphiphilic block copolymers for anti-tumor drug release. Int. J. Biol. Macromol. 2020, 154, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhao, Y.; Sun, J.; Chen, H.; Liu, Z.; Lin, K.; Ma, P.; Zhang, W.; Zhen, Y.; Zhang, S.; et al. pH/reduction dual-responsive hyaluronic acid-podophyllotoxin prodrug micelles for tumor targeted delivery. Carbohydr. Polym. 2022, 288, 119402. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Han, Q.; Chen, L.; Fan, X.; Wang, Y.; Fei, Z.; Zhang, H.; Wang, Y. Redox response, antibacterial and drug package capacities of chitosan-alpha-lipoic acid conjugates. Int. J. Biol. Macromol. 2020, 154, 1166–1174. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.Y.; Jang, M.-S.; Gao, G.H.; Lee, J.H.; Lee, D.S. Construction of redox/pH dual stimuli-responsive PEGylated polymeric micelles for intracellular doxorubicin delivery in liver cancer. Polym. Chem. 2016, 7, 1813–1825. [Google Scholar] [CrossRef]
- Liu, M.; Du, H.; Khan, A.R.; Ji, J.; Yu, A.; Zhai, G. Redox/enzyme sensitive chondroitin sulfate-based self-assembled nanoparticles loading docetaxel for the inhibition of metastasis and growth of melanoma. Carbohydr. Polym. 2018, 184, 82–93. [Google Scholar] [CrossRef] [PubMed]
- He, X.-H.; Zhao, M.; Tian, X.-Y.; Lu, Y.-J.; Yang, S.-Y.; Peng, Q.-R.; Yang, M.; Jiang, W.-W. Redox-responsive nano-micelles containing trisulfide bonds to enhance photodynamic efficacy of zinc naphthalocyanine. Chem. Phys. Lett. 2022, 803, 139785. [Google Scholar] [CrossRef]
- Yadav, S.; Kumar, P.; Jo, S.-H.; Park, S.-H.; Lee, W.-K.; Yoo, S., II; Lim, K.T. Redox-responsive properties of core-cross-linked micelles of poly(ethylene oxide)-b-poly(furfuryl methacrylate) for anticancer drug delivery application. React. Funct. Polym. 2022, 175, 105271. [Google Scholar] [CrossRef]
- Li, J.; Liu, P. Facile Synthesis of a Redox-Responsive Hyperbranched Polymer Prodrug as a Unimolecular Micelle for the Tumor-Selective Drug Delivery. Bioconjug. Chem. 2022, 33, 411–417. [Google Scholar] [CrossRef]
- Sang, X.; Yang, Q.; Shi, G.; Zhang, L.; Wang, D.; Ni, C. Preparation of pH/redox dual responsive polymeric micelles with enhanced stability and drug controlled release. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 91, 727–733. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, X.; Xu, K.; Du, B.; Zhu, C.; Li, Y. Biodegradable polyurethane PMeOx-PU(SS)-PMeOx micelles with redox and pH-sensitivity for efficient delivery of doxorubicin. Eur. Polym. J. 2020, 140, 110054. [Google Scholar] [CrossRef]
- Vakilzadeh, H.; Varshosaz, J.; Dinari, M.; Mirian, M.; Hajhashemi, V.; Shamaeizadeh, N.; Sadeghi, H.M. Smart redox-sensitive micelles based on chitosan for dasatinib delivery in suppressing inflammatory diseases. Int. J. Biol. Macromol. 2023, 229, 696–712. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Wang, J.; Pan, H.; Sang, Y.; Wang, D.; Wang, Z.; Ai, J.; Lin, B.; Chen, L. pH-redox responsive polymer-doxorubicin prodrug micelles studied by molecular dynamics, dissipative particle dynamics simulations and experiments. J. Drug Deliv. Sci. Technol. 2022, 69, 103136. [Google Scholar] [CrossRef]
- Wang, Y.; Khan, A.; Liu, Y.; Feng, J.; Dai, L.; Wang, G.; Alam, N.; Tong, L.; Ni, Y. Chitosan oligosaccharide-based dual pH responsive nano-micelles for targeted delivery of hydrophobic drugs. Carbohydr. Polym. 2019, 223, 115061. [Google Scholar] [CrossRef] [PubMed]
- Jelonek, K.; Li, S.; Wu, X.; Kasperczyk, J.; Marcinkowski, A. Self-assembled filomicelles prepared from polylactide/poly(ethylene glycol) block copolymers for anticancer drug delivery. Int. J. Pharm. 2015, 485, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, G.; Guo, X.; Tang, Z.; Zhong, Z.; Zhou, S. Redox-responsive polyanhydride micelles for cancer therapy. Biomaterials 2014, 35, 3080–3090. [Google Scholar] [CrossRef] [PubMed]
- Gebrie, H.T.; Addisu, K.D.; Darge, H.F.; Birhan, Y.S.; Thankachan, D.; Tsai, H.C.; Wu, S.Y. pH/redox-responsive core cross-linked based prodrug micelle for enhancing micellar stability and controlling delivery of chemo drugs: An effective combination drug delivery platform for cancer therapy. Biomater. Adv. 2022, 139, 213015. [Google Scholar] [CrossRef]
- Han, L.; Hu, L.; Liu, F.; Wang, X.; Huang, X.; Liu, B.; Feng, F.; Liu, W.; Qu, W. Redox-sensitive micelles for targeted intracellular delivery and combination chemotherapy of paclitaxel and all-trans-retinoid acid. Asian J. Pharm. Sci. 2019, 14, 531–542. [Google Scholar] [CrossRef]
- Kaur, J.; Singla, P.; Kaur, I. Labrasol mediated enhanced solubilization of natural hydrophobic drugs in Pluronic micelles: Physicochemical and in vitro release studies. J. Mol. Liq. 2022, 361, 119596. [Google Scholar] [CrossRef]
- Zhao, D.; Wu, J.; Li, C.; Zhang, H.; Li, Z.; Luan, Y. Precise ratiometric loading of PTX and DOX based on redox-sensitive mixed micelles for cancer therapy. Colloids Surf. B Biointerfaces 2017, 155, 51–60. [Google Scholar] [CrossRef]
- Jin, R.; Liu, Z.; Liu, T.; Yuan, P.; Bai, Y.; Chen, X. Redox-responsive micelles integrating catalytic nanomedicine and selective chemotherapy for effective tumor treatment. Chin. Chem. Lett. 2021, 32, 3076–3082. [Google Scholar] [CrossRef]
- Luo, T.; Han, J.; Zhao, F.; Pan, X.; Tian, B.; Ding, X.; Zhang, J. Redox-sensitive micelles based on retinoic acid modified chitosan conjugate for intracellular drug delivery and smart drug release in cancer therapy. Carbohydr. Polym. 2019, 215, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Y.T.; Yen, Y.W.; Lo, C.L. Reactive oxygen species and glutathione dual redox-responsive micelles for selective cytotoxicity of cancer. Biomaterials 2015, 61, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, J.; Song, M.; Tian, B.; Li, K.; Liang, Y.; Han, J.; Wu, Z. A shell-crosslinked polymeric micelle system for pH/redox dual stimuli-triggered DOX on-demand release and enhanced antitumor activity. Colloids Surf. B Biointerfaces 2017, 152, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Guo, X.; Qu, Q.; Tang, Z.; Wang, Y.; Zhou, S. Actively targeted delivery of anticancer drug to tumor cells by redox-responsive star-shaped micelles. Biomaterials 2014, 35, 8711–8722. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Guan, H.; Li, Z.; Wang, F.; Wu, J.; Zhang, B. Study on different particle sizes of DOX-loaded mixed micelles for cancer therapy. Colloids Surf. B Biointerfaces 2020, 196, 111303. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hu, D.; Pan, M.; Qu, Y.; Chu, B.; Liao, J.; Zhou, X.; Liu, Q.; Cheng, S.; Chen, Y.; et al. Near-infrared light and redox dual-activatable nanosystems for synergistically cascaded cancer phototherapy with reduced skin photosensitization. Biomaterials 2022, 288, 121700. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Liu, H.; Li, Y.; Li, C.; Wan, G.; Chen, B.; Li, C.; Wang, Y. A pH-sensitive nanotherapeutic system based on a marine sulfated polysaccharide for the treatment of metastatic breast cancer through combining chemotherapy and COX-2 inhibition. Acta Biomater. 2019, 99, 412–425. [Google Scholar] [CrossRef]
- Zhou, L.; Liang, Q.; Li, Y.; Cao, Y.; Li, J.; Yang, J.; Liu, J.; Bi, J.; Liu, Y. Collagenase-I decorated co-delivery micelles potentiate extracellular matrix degradation and hepatic stellate cell targeting for liver fibrosis therapy. Acta Biomater. 2022, 152, 235–254. [Google Scholar] [CrossRef]
- Sun, C.; Li, X.; Du, X.; Wang, T. Redox-responsive micelles for triggered drug delivery and effective laryngopharyngeal cancer therapy. Int. J. Biol. Macromol. 2018, 112, 65–73. [Google Scholar] [CrossRef]
- Wu, C.; Yang, J.; Xu, X.; Gao, C.; Lü, S.; Liu, M. Redox-responsive core-cross-linked mPEGylated starch micelles as nanocarriers for intracellular anticancer drug release. Eur. Polym. J. 2016, 83, 230–243. [Google Scholar] [CrossRef]
- Chai, Z.; Teng, C.; Yang, L.; Ren, L.; Yuan, Z.; Xu, S.; Cheng, M.; Wang, Y.; Yan, Z.; Qin, C.; et al. Doxorubicin delivered by redox-responsive Hyaluronic Acid-Ibuprofen prodrug micelles for treatment of metastatic breast cancer. Carbohydr. Polym. 2020, 245, 116527. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, H.; Xu, J.; Gao, Z.; Wu, J.; Zhu, L.; Zhan, X. Novel amphiphilic carboxymethyl curdlan-based pH responsive micelles for curcumin delivery. LWT 2022, 153, 112419. [Google Scholar] [CrossRef]
- Yang, F.; Wei, P.; Yang, M.; Chen, W.; Zhao, B.; Li, W.; Wang, J.; Qiu, L.; Chen, J. Redox-sensitive hyaluronic acid-ferrocene micelles delivering doxorubicin for enhanced tumor treatment by synergistic chemo/chemodynamic therepay. J. Drug Deliv. Sci. Technol. 2022, 77, 103851. [Google Scholar] [CrossRef]
- Mahani, M.; Bahmanpouri, M.; Khakbaz, F.; Divsar, F. Doxorubicin-loaded polymeric micelles decorated with nitrogen-doped carbon dots for targeted breast cancer therapy. J. Drug Deliv. Sci. Technol. 2023, 79, 104055. [Google Scholar] [CrossRef]
- Unagolla, J.M.; Jayasuriya, A.C. Drug transport mechanisms and in vitro release kinetics of vancomycin encapsulated chitosan-alginate polyelectrolyte microparticles as a controlled drug delivery system. Eur. J. Pharm. Sci. 2018, 114, 199–209. [Google Scholar] [CrossRef]
- Guo, Z.; Liang, E.; Sui, J.; Ma, M.; Yang, L.; Wang, J.; Hu, J.; Sun, Y.; Fan, Y. Lapatinib-loaded acidity-triggered charge switchable polycarbonate-doxorubicin conjugate micelles for synergistic breast cancer chemotherapy. Acta Biomater. 2020, 118, 182–195. [Google Scholar] [CrossRef]
- Qiu, X.; Ma, S.; Wang, D.; Fan, Z.; Qiu, P.; Wang, S.; Li, C. The development of multifunctional sulfated polyguluronic acid-based polymeric micelles for anticancer drug delivery. Carbohydr. Polym. 2023, 303, 120451. [Google Scholar] [CrossRef]
- Nezhadi, S.; Norouzi, P.; Rasouli, A.; Akbari Javar, H.; Ostad, S.N.; Dorkoosh, F. Co-delivery of paclitaxel and regorafenib by F127/TPGS mixed micelles for triple negative breast cancer treatment. J. Drug Deliv. Sci. Technol. 2023, 86, 104673. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, Y.; Tan, W.; Mi, Y.; Wang, L.; Qi, Z.; Guo, Z. Effect of Hydrophobic Chain Length in Amphiphilic Chitosan Conjugates on Intracellular Drug Delivery and Smart Drug Release of Redox-Responsive Micelle. Mar. Drugs 2024, 22, 18. https://doi.org/10.3390/md22010018
Yuan Y, Tan W, Mi Y, Wang L, Qi Z, Guo Z. Effect of Hydrophobic Chain Length in Amphiphilic Chitosan Conjugates on Intracellular Drug Delivery and Smart Drug Release of Redox-Responsive Micelle. Marine Drugs. 2024; 22(1):18. https://doi.org/10.3390/md22010018
Chicago/Turabian StyleYuan, Yuting, Wenqiang Tan, Yingqi Mi, Linqing Wang, Zhen Qi, and Zhanyong Guo. 2024. "Effect of Hydrophobic Chain Length in Amphiphilic Chitosan Conjugates on Intracellular Drug Delivery and Smart Drug Release of Redox-Responsive Micelle" Marine Drugs 22, no. 1: 18. https://doi.org/10.3390/md22010018
APA StyleYuan, Y., Tan, W., Mi, Y., Wang, L., Qi, Z., & Guo, Z. (2024). Effect of Hydrophobic Chain Length in Amphiphilic Chitosan Conjugates on Intracellular Drug Delivery and Smart Drug Release of Redox-Responsive Micelle. Marine Drugs, 22(1), 18. https://doi.org/10.3390/md22010018