Effects of Temperature, Light and Salt on the Production of Fucoxanthin from Conticribra weissflogii
Abstract
:1. Introduction
2. Results
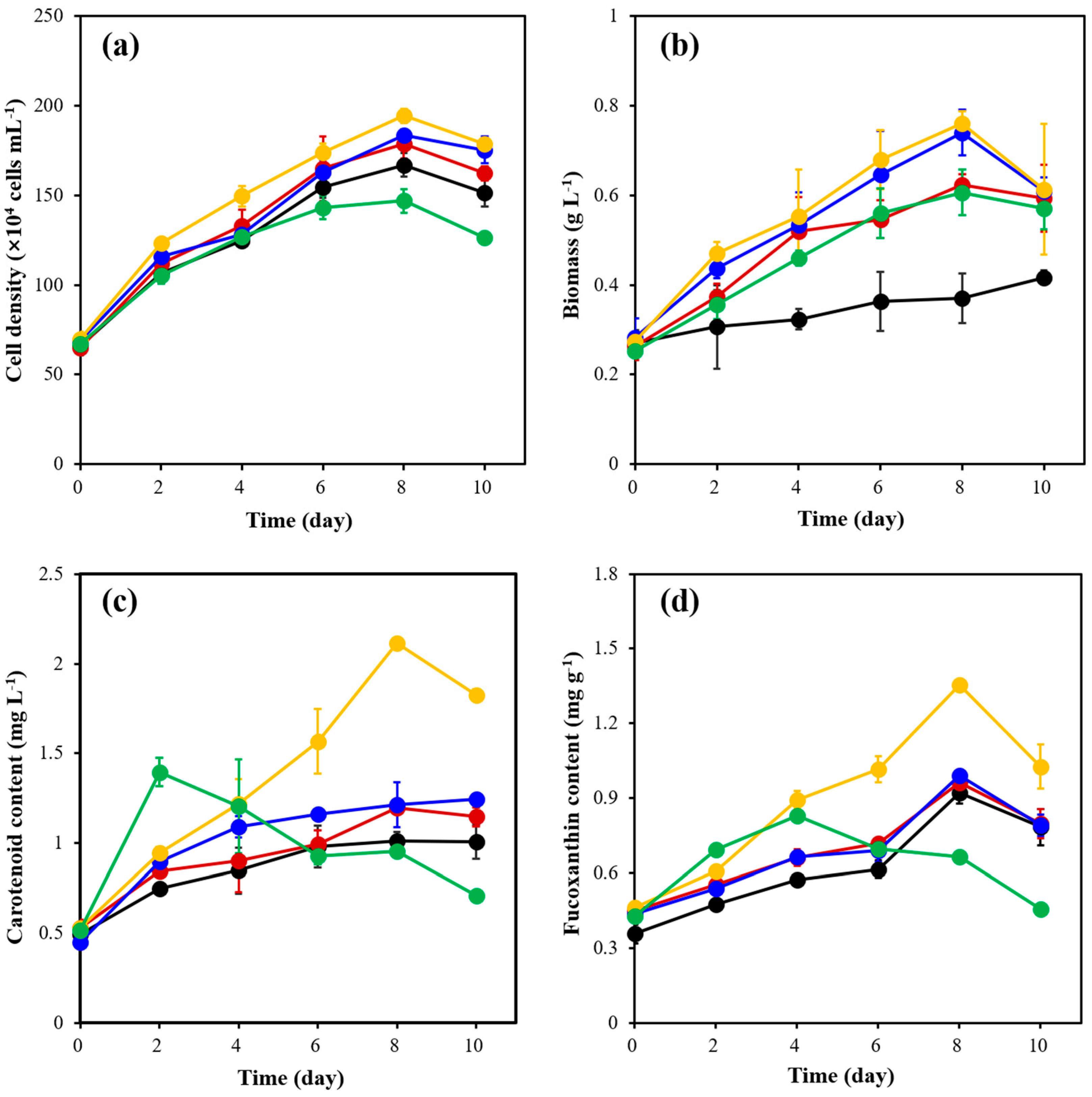
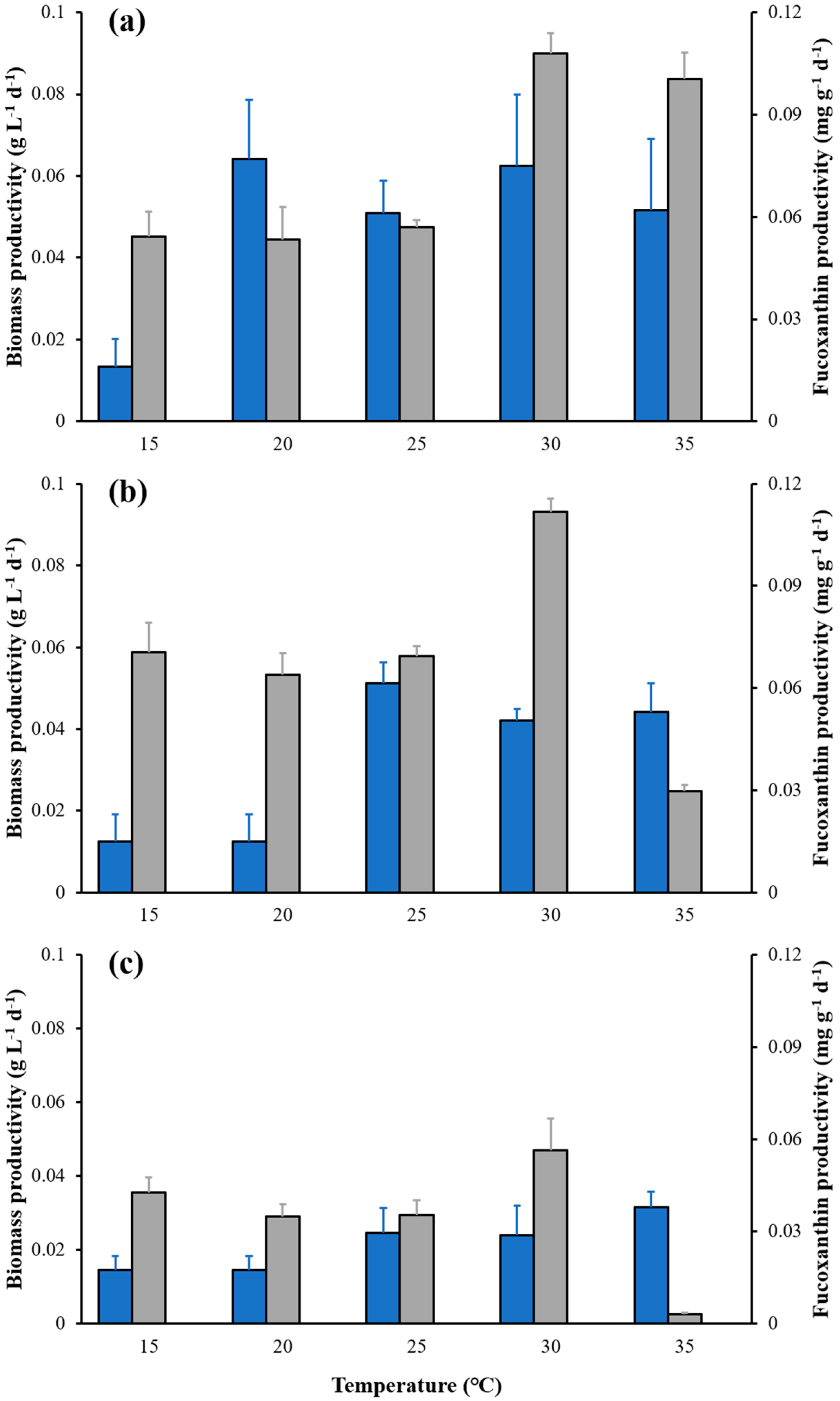
2.1. Effects of Temperature on Cell Density, Biomass, Carotenoid Content and Fucoxanthin Content of C. weissflogii
2.2. Effects of Light Intensity on Cell Density, Biomass, Carotenoid Content and Fucoxanthin Content of C. weissflogii
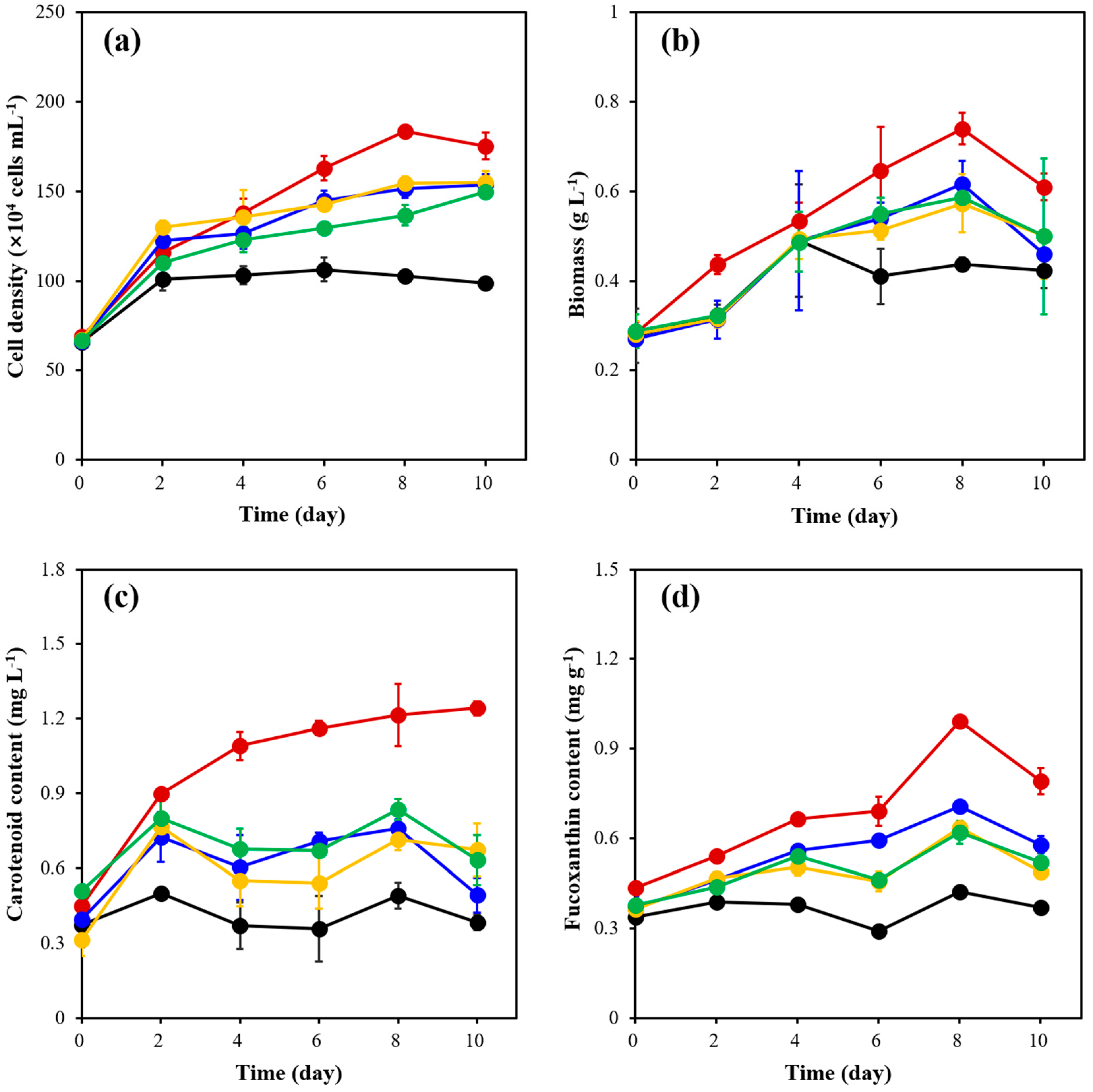
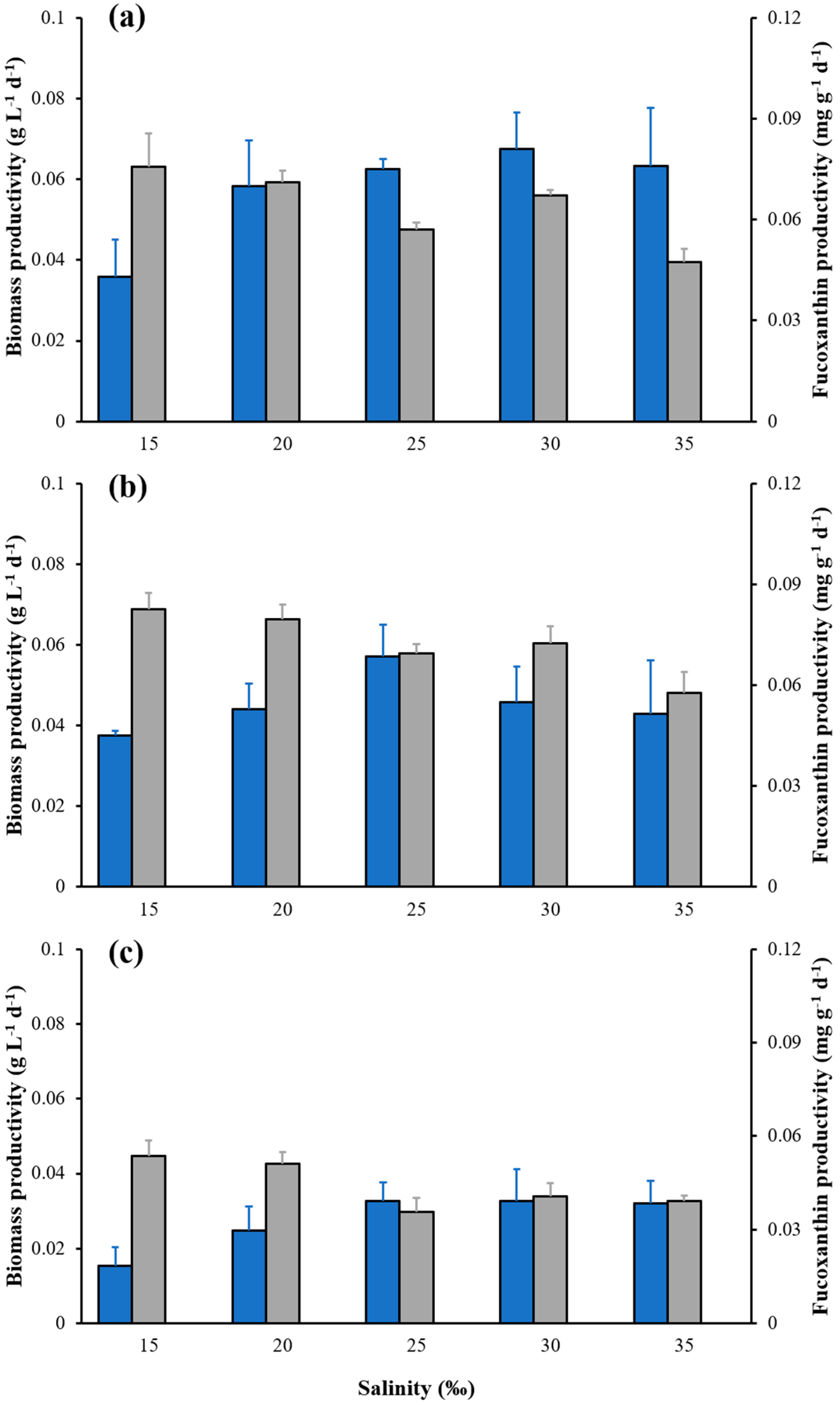
2.3. Effects of Salinity on Cell Density, Biomass, Carotenoid Content and Fucoxanthin Content of C. weissflogii
2.4. The Effect of Orthogonal Design Test on Cell Density, Biomass, Carotenoid Content and Fucoxanthin Content of C. weissflogii
3. Discussion
4. Materials and Methods
4.1. C. weissflogi Strain and Growth Conditions
4.2. Single-Factor Experiment
4.3. Orthogonal Experimental Design
4.4. Cell Density
4.5. Biomass
4.6. Pigment Content
4.7. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Arora, N.; Philippidis, G.P. Fucoxanthin production from diatoms: Current advances and challenges. In Algae; Mandotra, S.K., Upadhyay, A.K., Ahluwalia, A.S., Eds.; Springer Nature Singapore Pte Ltd.: Singapore, 2021; pp. 221–242. [Google Scholar]
- Mohamadnia, S.; Tavakoli, O.; Faramarzi, M.A.; Shamsollahi, Z. Production of fucoxanthin by the microalga Tisochrysis lutea: A review of recent developments. Aquaculture 2020, 516, 734637. [Google Scholar] [CrossRef]
- Song, W.; Sha, W.; Guan, P.Y.; Ke, H.P.; Lu, L.W.; Zhang, L.H. A review on the progress, challenges and prospects in commercializing microalgal fucoxanthin. Biotechnol. Adv. 2021, 53, 107865. [Google Scholar]
- Aslanbay, G.B.; Deniz, I.; Demirel, Z.; Yesil-Celiktas, O.; Imamoglu, E. A novel subcritical fucoxanthin extraction with a biorefinery approach. Biochem. Eng. J. 2020, 153, 107403. [Google Scholar] [CrossRef]
- Khaw, Y.S.; Yusoff, F.M.; Tan, H.T.; Noor Mazli, N.A.I.; Nazarudin, M.F.; Shaharuddin, N.A.; Omar, A.R. The Critical studies of fucoxanthin research trends from 1928 to June 2021: A bibliometric review. Mar. Drugs 2021, 19, 606. [Google Scholar] [CrossRef] [PubMed]
- Seth, K.; Kumar, A.; Rastogi, R.P.; Meena, M.; Vinayak, V.; Harish. Bioprospecting of fucoxanthin from diatoms—Challenges and perspectives. Algal Res. 2021, 60, 102475. [Google Scholar] [CrossRef]
- Guo, B.; Liu, B.; Yang, B.; Sun, P.; Lu, X.; Liu, J.; Chen, F. Screening of diatom strains and characterization of Cyclotella cryptica as a potential fucoxanthin producer. Mar. Drugs 2016, 14, 125. [Google Scholar] [CrossRef]
- Tam, L.T.; Van, C.N.; Thom, L.T.; Ha, N.C.; Hang, N.T.M.; Minh, C.V.; Vien, D.T.H.; Hong, D.D. Cultivation and biomass production of the diatom Thalassiosira weissflogii as a live feed for white-leg shrimp in hatcheries and commercial farms in Vietnam. J. Appl. Phycol. 2021, 33, 1559–1577. [Google Scholar] [CrossRef]
- Huervana, F.H.; Dionela, C.S.; de la Torre, E.D.S.; del Castillo, C.S.; Traifalgar, R.F.M. Utilization of marine diatom Thalassiosira weissflogii as a feed additive in seawater-tolerant Nile tilapia (Oreochromis niloticus, Linnaeus 1758) strain. Front. Sustain. Food Syst. 2022, 6, 1052951. [Google Scholar] [CrossRef]
- Khaw, Y.S.; Yusoff, F.M.; Tan, H.T.; Noor Mazli, N.A.I.; Nazarudin, M.F.; Shaharuddin, N.A.; Omar, A.R.; Takahashi, K. Fucoxanthin Production of Microalgae under Different Culture Factors: A Systematic Review. Mar. Drugs 2022, 20, 592. [Google Scholar] [CrossRef]
- Chaisutyakorn, P.; Praiboon, J.; Kaewsuralikhit, C. The effect of temperature on growth and lipid and fatty acid composition on marine microalgae used for biodiesel production. J. Appl. Phycol. 2018, 30, 37–45. [Google Scholar] [CrossRef]
- Li, T.J.W.; Zou, X.X.; Bao, S.X. Research progress of response mechanism of algae to temperature stress at home and abroad. J. Fish. Res. 2021, 2, 221–230. [Google Scholar]
- Minggat, E.; Roseli, W.; Tanaka, Y. Nutrient absorption and biomass production by the marine diatom Chaetoceros muelleri: Effects of temperature, salinity, photoperiod, and light intensity. Pol. Soc. Ecol. Eng. 2020, 22, 231–240. [Google Scholar] [CrossRef]
- Gao, X.Z.; Jiang, X.M.; Ye, L. Effects of temperature, light intensity and salinity on the growth and fatty acid composition of Skeletlnema munzelii SM-1 and SM-2. J. Biol. 2014, 31, 64–70. (In Chinese) [Google Scholar]
- Renaud, S.M.; Zhou, H.C.; Parry, D.L.; Thinh, L.V.; Woo, K.C. Effect of temperature on the growth, total lipid content and fatty acid composition of recently isolated tropical microalgae Isochrysis sp. Nitzschia closterium, Nitzschia paleacea, and commercial species Isochrysis sp. J. Appl. Phycol. 1995, 7, 595–602. [Google Scholar]
- Sözmen, A.B.; Ata, A.; Ovez, B. Optimization of the algal species Chlorella miniata growth: Mathematical modelling and evaluation of temperature and light intensity effects. Biocatal. Agric. Biotechnol. 2022, 39, 102239. [Google Scholar] [CrossRef]
- Li, T.T.; Wang, Z.W.; Ren, P.; Lan, T.Y.; Shao, Y.Q.; Fang, J. Effect of temperature and light intensity on growth of four species of microalgae. J. Zhejiang Agric. Sci. 2021, 62, 2074–2078. [Google Scholar]
- Singh, S.P.; Singh, P. Effect of temperature and light on the growth of algae species: A review. Renew. Sustain. Energy Rev. 2015, 50, 431–444. [Google Scholar] [CrossRef]
- Wang, S.K.; Stiles, A.R.; Guo, C.; Liu, C.Z. Microalgae cultivation in photobioreactors: An overview of light characteristics. Eng. Life Sci. 2015, 14, 550–559. [Google Scholar] [CrossRef]
- Fakhri, M.; Arifin, N.B.; Hariati, A.M.; Yuniarti, A. Growth, biomass, and chlorophyll-a and carotenoid content of Nannochloropsis sp. strain BJ17 under different light intensities. J. Akuakultur Indones. 2017, 16, 15–21. [Google Scholar] [CrossRef]
- Holt, M.G.; Smayda, T.J. The effect of daylength and light intensity on the growth rate of the marine diatom detonula confervacea (cleve) gran. J. Phycol. 2010, 10, 231–237. [Google Scholar] [CrossRef]
- Shi, P.L.; Shen, H.; Wang, W.J.; Chen, W.; Xie, P. The relationship between light intensity and nutrient uptake kinetics in six freshwater diatoms. J. Environ. Sci. 2015, 34, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Che, C.A.; Kim, S.H.; Hong, H.J.; Kityo, M.K.; Sunwoo, I.Y.; Jeong, G.T.; Kim, S.K. Optimization of light intensity and photoperiod for Isochrysis galbana culture to improve the biomass and lipid production using 14-L photobioreactors with mixed light emitting diodes (LEDs) wavelength under two-phase culture system. Bioresour. Technol. 2019, 285, 121323. [Google Scholar] [CrossRef]
- Shah, M.M.R.; Liang, Y.M.; Chen, J.J.; Daroch, M. Astaxanthin-producing green microalga Haematococcus pluvialis: From single cell to high value commercial products. Front. Plant Sci. 2016, 7, 531. [Google Scholar] [CrossRef]
- Mao, X.; Chen, S.H.Y.; Lu, X.; Yu, J.; Liu, B. High silicate concentration facilitates fucoxanthin and eicosapentaenoic acid (EPA) production under heterotrophic condition in the marine diatom Nitzschia laevis. Algal Res. 2020, 52, 102086. [Google Scholar] [CrossRef]
- Rahimisuruee, M.; Ghadikolaei, K.R.; Mohammadizade, F. Study on the effect of salinity changes on growth, biomass, and chlorophyll a and carotenoid pigments of microalgae Nannochloropsis oculata. Iran. Sci. Fish. J. 2016, 25, 263–272. [Google Scholar]
- Peng, J.J.; Cui, L.B.; Ma, K. Effects of two kinds of nitrogen source with different salinity on Dunaliella Salina. J. Salt Sci. Chem. Ind. 2020, 49, 20–23. [Google Scholar]
- Mousavi Nadushan, R.; Hosseinzade, I. Optimization of Production and Antioxidant Activity of Fucoxanthin from Marine Haptophyte Algae, Isochrysis galbana. Iran. J. Fish. Sci. 2020, 19, 2901–2908. [Google Scholar]
- Wang, H.; Zhang, Y.; Chen, L.; Cheng, W.; Liu, T. Combined production of fucoxanthin and EPA from two diatom strains Phaeodactylum tricornutum and Cylindrotheca fusiformis cultures. Bioprocess Biosyst. Eng. 2018, 41, 1061–1071. [Google Scholar] [CrossRef]
- Chen, S.X.; Luo, S.S.; Zhang, J.A.; Li, C.L.; Huang, X.H. Effects of salinity on growth and biochemical components of microalga Thalassiosira weissflogii. J. Guangdong Ocean Univ. 2021, 41, 53–59. [Google Scholar]
- Rui, X.; Amenorfenyo, D.K.; Peng, K.; Li, H.; Wang, L.; Huang, X.; Li, C.; Li, F. Effects of different nitrogen concentrations on co-production of fucoxanthin and fatty acids in Conticribra weissflogii. Mar. Drugs 2023, 21, 106. [Google Scholar] [CrossRef]
- Li, F.; Cai, M.; Lin, M.; Huang, X.; Wang, J.; Ke, H.; Zheng, X.; Chen, D.; Wang, C.; Wu, S.; et al. Differences between Motile and Nonmotile Cells of Haematococcus pluvialis in the Production of Astaxanthin at Different Light Intensities. Mar. Drugs 2019, 17, 39. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yang, R.Q.; Song, P.Q.; Wei, D. Improving production of biomass and fucoxanthin in mixotrophic Phaeodactylum tricornutum by optimization of carbon and nitrogen sources. J. Food Sci. Biotechnol. 2021, 40, 82–90. [Google Scholar]
- Parsons, T.R.; Maita, Y.; Lalli, C.M. Determination of chlorophylls and total carotenoids: Spectrophotometric method—scienceDirect. In A Manual of Chemical & Biological Methods for Seawater Analysis; Pergamon Press: Oxford, UK, 1984; pp. 101–104. [Google Scholar]
- Xu, R.R.; Gong, Y.F.; Chen, W.T.; Li, S.R.; Chen, R.Y.; Zheng, X.Y.; Chen, X.M.Z.; Wang, H.Y. Effects of LED monochromatic light quality of different colors on fucoxanthin content and expression levels of related genes in Phaeodactylum tricornutum. Acta Opt. Sin. 2019, 9, 299–307. [Google Scholar]


| Group | Temperature (°C) | Light Intensity (μmol m−2 s−1) | Salinity (‰) |
|---|---|---|---|
| 1 | 20 | 30 | 15 |
| 2 | 20 | 60 | 25 |
| 3 | 20 | 90 | 20 |
| 4 | 25 | 30 | 20 |
| 5 | 25 | 60 | 15 |
| 6 | 25 | 90 | 25 |
| 7 | 30 | 30 | 25 |
| 8 | 30 | 60 | 20 |
| 9 | 30 | 90 | 15 |
| Group | Cell Density (×104 Cell mL−1) | Biomass (g L−1) | Carotenoid (mg L−1) | Fucoxanthin (mg g−1) |
|---|---|---|---|---|
| 1 | 143 ± 6.42 | 0.53 ± 0.02 | 0.814 ± 0.03 | 0.629 ± 0.04 |
| 2 | 153 ± 2.89 | 0.52 ± 0.02 | 0.956 ± 0.03 | 0.619 ± 0.04 |
| 3 | 140 ± 1.53 | 0.56 ± 0.03 | 0.766 ± 0.03 | 0.602 ± 0.06 |
| 4 | 136 ± 6.56 | 0.54 ± 0.03 | 0.929 ± 0.01 | 0.710 ± 0.05 |
| 5 | 150 ± 2.52 | 0.53 ± 0.04 | 0.708 ± 0.02 | 0.690 ± 0.01 |
| 6 | 170 ± 2.89 | 0.53 ± 0.05 | 0.732 ± 0.01 | 0.683 ± 0.03 |
| 7 | 197 ± 5.77 | 0.76 ± 0.07 | 2.209 ± 0.07 | 1.372 ± 0.04 |
| 8 | 160 ± 2.52 | 0.67 ± 0.07 | 1.430 ± 0.06 | 1.160 ± 0.05 |
| 9 | 167 ± 1.73 | 0.57 ± 0.04 | 1.398 ± 0.10 | 1.134 ± 0.07 |
| Item | Level | Temperature | Light Intensity | Salinity |
|---|---|---|---|---|
| K-value | 1 | 1.61 | 1.83 | 1.63 |
| 2 | 1.60 | 1.72 | 1.77 | |
| 3 | 2.00 | 1.66 | 1.81 | |
| K-average | 1 | 0.54 | 0.61 | 0.54 |
| 2 | 0.53 | 0.57 | 0.59 | |
| 3 | 0.67 | 0.55 | 0.60 | |
| R | 0.14 | 0.06 | 0.06 | |
| Extreme order | 1 | 2 | 3 |
| Item | Level | Temperature | Light Intensity | Salinity |
|---|---|---|---|---|
| K-value | 1 | 1.848 | 2.711 | 1.832 |
| 2 | 2.030 | 2.469 | 2.472 | |
| 3 | 3.666 | 2.419 | 2.672 | |
| K-average | 1 | 0.616 | 0.904 | 0.611 |
| 2 | 0.694 | 0.823 | 0.824 | |
| 3 | 1.222 | 0.806 | 0.891 | |
| R | 0.606 | 0.098 | 0.280 | |
| Extreme order | 1 | 3 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, F.; Rui, X.; Amenorfenyo, D.K.; Pan, Y.; Huang, X.; Li, C. Effects of Temperature, Light and Salt on the Production of Fucoxanthin from Conticribra weissflogii. Mar. Drugs 2023, 21, 495. https://doi.org/10.3390/md21090495
Li F, Rui X, Amenorfenyo DK, Pan Y, Huang X, Li C. Effects of Temperature, Light and Salt on the Production of Fucoxanthin from Conticribra weissflogii. Marine Drugs. 2023; 21(9):495. https://doi.org/10.3390/md21090495
Chicago/Turabian StyleLi, Feng, Xiangyu Rui, David Kwame Amenorfenyo, Yao Pan, Xianghu Huang, and Changling Li. 2023. "Effects of Temperature, Light and Salt on the Production of Fucoxanthin from Conticribra weissflogii" Marine Drugs 21, no. 9: 495. https://doi.org/10.3390/md21090495
APA StyleLi, F., Rui, X., Amenorfenyo, D. K., Pan, Y., Huang, X., & Li, C. (2023). Effects of Temperature, Light and Salt on the Production of Fucoxanthin from Conticribra weissflogii. Marine Drugs, 21(9), 495. https://doi.org/10.3390/md21090495








