Abstract
Cladostephus spongiosus was harvested once a month during its growing season (from May to August) from the Adriatic Sea. Algal volatile organic compounds (VOCs) were obtained by headspace solid-phase microextraction (HS-SPME) and hydrodistillation (HD) and analysed by gas chromatography and mass spectrometry (GC-MS). The effects of air drying and growing season on VOCs were determined. Two different extraction methods (ultrasound-assisted extraction (UAE) and microwave-assisted extraction (MAE)) were used to obtain ethanolic extracts of C. spongiosus. In addition, the seasonal antioxidant potential of the extracts was determined, and non-volatile compounds were identified from the most potent antioxidant extract. Aliphatic compounds (e.g., pentadecane) were predominantly found by HS-SPME/GC-MS. Hydrocarbons were more than twice as abundant in the dry samples (except in May). Aliphatic alcohols (e.g., hexan-1-ol, octan-1-ol, and oct-1-en-3-ol) were present in high percentages and were more abundant in the fresh samples. Hexanal, heptanal, nonanal, and tridecanal were also found. Aliphatic ketones (octan-3-one, 6-methylhept-5-en-2-one, and (E,Z)-octa-3,5-dien-2-one) were more abundant in the fresh samples. Benzene derivatives (e.g., benzyl alcohol and benzaldehyde) were dominant in the fresh samples from May and August. (E)-Verbenol and p-cymen-8-ol were the most abundant in dry samples in May. HD revealed aliphatic compounds (e.g., heptadecane, pentadecanal, (E)-heptadec-8-ene, (Z)-heptadec-3-ene), sesquiterpenes (germacrene D, epi-bicyclosesquiphellandrene, gleenol), diterpenes (phytol, pachydictyol A, (E)-geranyl geraniol, cembra-4,7,11,15-tetraen-3-ol), and others. Among them, terpenes were the most abundant (except for July). Seasonal variations in the antioxidant activity of the ethanolic extracts were evaluated via different assays. MAE extracts showed higher peroxyl radical inhibition activity from 55.1 to 74.2 µM TE (Trolox equivalents). The highest reducing activity (293.8 µM TE) was observed for the May sample. Therefore, the May MAE extract was analysed via high-performance liquid chromatography with high-resolution mass spectrometry and electrospray ionisation (UHPLC-ESI-HRMS). In total, 17 fatty acid derivatives, 9 pigments and derivatives, and 2 steroid derivatives were found. The highest content of pheophorbide a and fucoxanthin, as well as the presence of other pigment derivatives, could be related to the observed antioxidant activity.
1. Introduction
The genus Cladostephus currently consists of five taxonomically recognised species. These species are cosmopolitan brown algae that grow on rocks in the intertidal zone and at depths of up to six metres, mainly in temperate seas [1]. Cladostephus spongiosus occurs in the Adriatic Sea [2]. Its thalli are usually between 3 and 20 cm long, with the longest thallus present in summer, during its growing season, when the sea temperature is the highest. In winter, when the sea temperature is at its lowest, only small specimens 3 cm in length are observed [3].
Several studies have confirmed the bioactivity of compounds obtained from this species. The extracts from C. spongiosus have been reported to exhibit antioxidant, antibacterial, anticandidal, cytoprotective, insecticidal, and amoebicidal activities [4,5,6,7,8]. It has been previously reported that the bioactivity of the extracts could be affected by the season of algal harvest and extraction method [9,10,11]. However, to our knowledge, no studies on these effects have been performed for C. spongiosus. Air-dried samples of C. spongiosus harvested from the Algerian coast were extracted and the extracts were analysed by HPLC and GC-MS. Fucoxanthin, apo-9′-fucoxanthinone, apo-13′-fucoxanthinone, and loliolid were identified in the samples [12]. The major compounds identified in the acetone extracts of C. spongiosus were 4-hydroxy-4-methylpentan-2-one, n-hexadecanoic acid, and propenylguaiacol, while octadeca-9,12,15-trienoic acid ethyl ester, hexadecanoic acid ethyl ester, and 3,7,11,15-tetramethylhexadec-2-en-1-ol were the major compounds in the ethanol extracts [8].
Whitfield et al. [13] isolated volatile compounds from C. spongiosus harvested in Australia using combined steam distillation solvent extraction with pentane/diethyl ether. Overall, 2,6-dibromophenol, 2-bromophenol, 2,4,6-tribromophenol, 4-bromophenol, and 2,4-dibromophenol were identified in the samples using gas chromatography and mass spectrometry (GC-MS). In addition, the lipid phase of freeze-dried samples of C. spongiosus collected off the Algarve coast in Portugal was extracted and analysed by GC-MS. The authors reported 31.74% of saturated fatty acids, 12.15% of monounsaturated fatty acids, and 56.11% of polyunsaturated fatty acids [14]. Rubiño et al. [15] determined the profile of volatile compounds in C. spongiosus harvested in Spain using headspace solid-phase microextraction (HS-SPME). The authors tentatively identified pent-1-en-3-one, pent-2-en-1-ol, hexanal, hexan-1-ol, tribromomethane, 1-iodo-3-methylbutane, and oct-1-en-3-one. To our knowledge, there are no studies on the changes of volatile organic compounds (VOCs) during the growth period of C. spongiosus. In the present study, VOCs were separated and analysed from air-dried and fresh C. spongiosus samples from the Adriatic Sea using HS-SPME and hydrodistillation (HD). These techniques were chosen because they allow the isolation headspace, volatile and semi-volatile compounds. Algal samples were collected during the growing season from May to August. The study objectives were: (1) to isolate VOCs from C. spongiosus, (2) to determine the chemical composition of obtained VOCs, (3) to determine the effects of air drying and growing season on VOCs composition, (4) to determine the seasonal antioxidant potential of C. spongiosus ethanolic extracts using different assays, (5) to compare the ultrasound-assisted extraction (UAE) and microwave-assisted extraction (MAE) to produce the extracts with higher antioxidant activity, and (6) to identify the non-volatile compounds in the most potent ethanolic extract.
2. Results and Discussion
VOCs were obtained using two methods (HS-SPME and HD) followed by GC-MS analysis. A wide range of VOCs were identified (headspace, as well as low to medium volatile compounds). Antioxidant assays were performed to analyse the seasonal potential of the extracts. High-performance liquid chromatography with high-resolution mass spectrometry and electrospray ionisation (UHPLC-ESI-HRMS) analysis was performed on the most potent antioxidant extract to identify present non-volatile compounds.
2.1. Headspace Volatilome Variations of C. spongiosus
Two fibres of different polarity, divinylbenzene/carboxene/polydimethylsiloxane (DVB/CAR/PDMS, f1) with medium polarity and polydimethylsiloxane/divinylbenzene (PDMS/DVB, f2) with high polarity, were used to study the headspace composition.
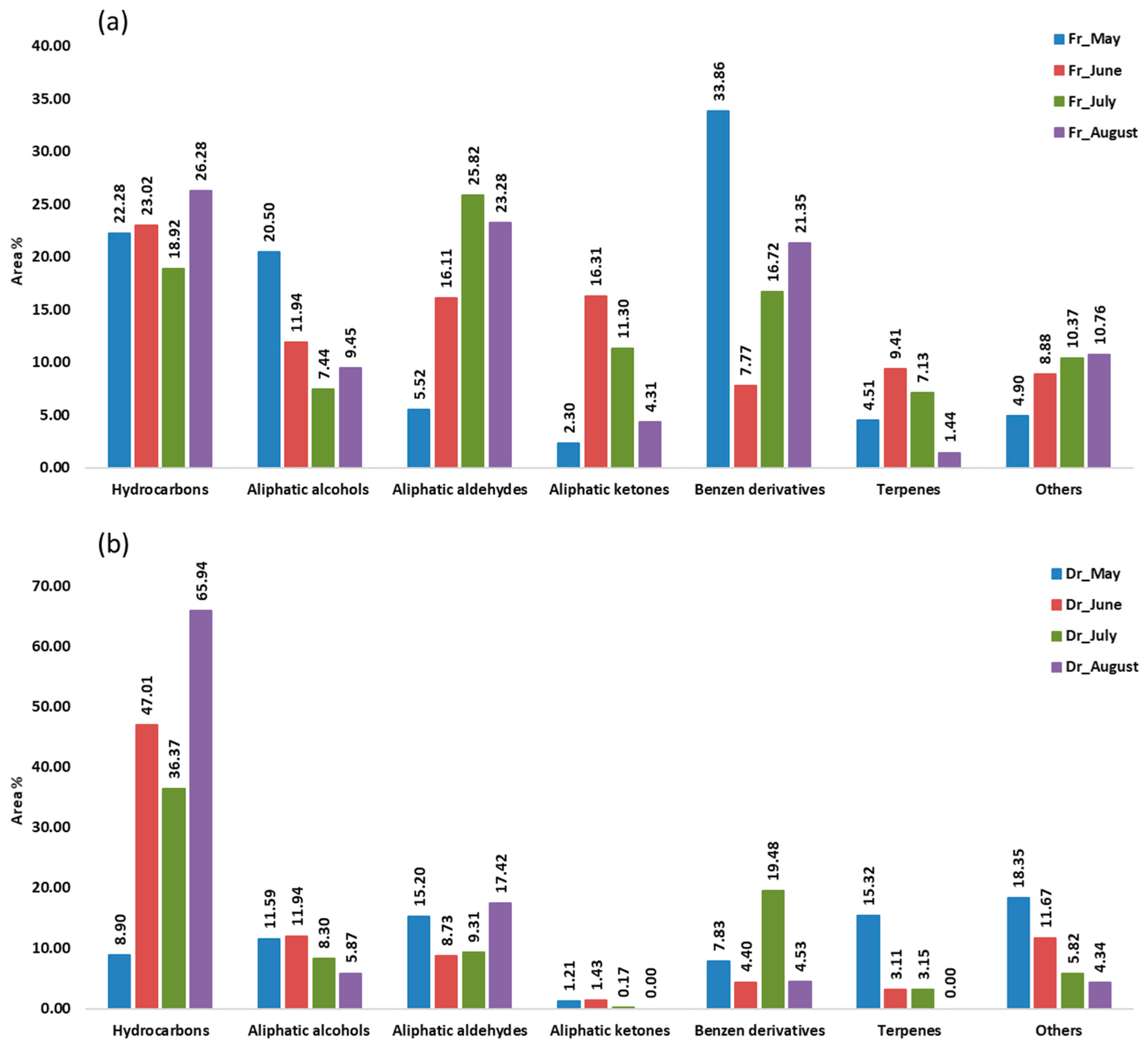
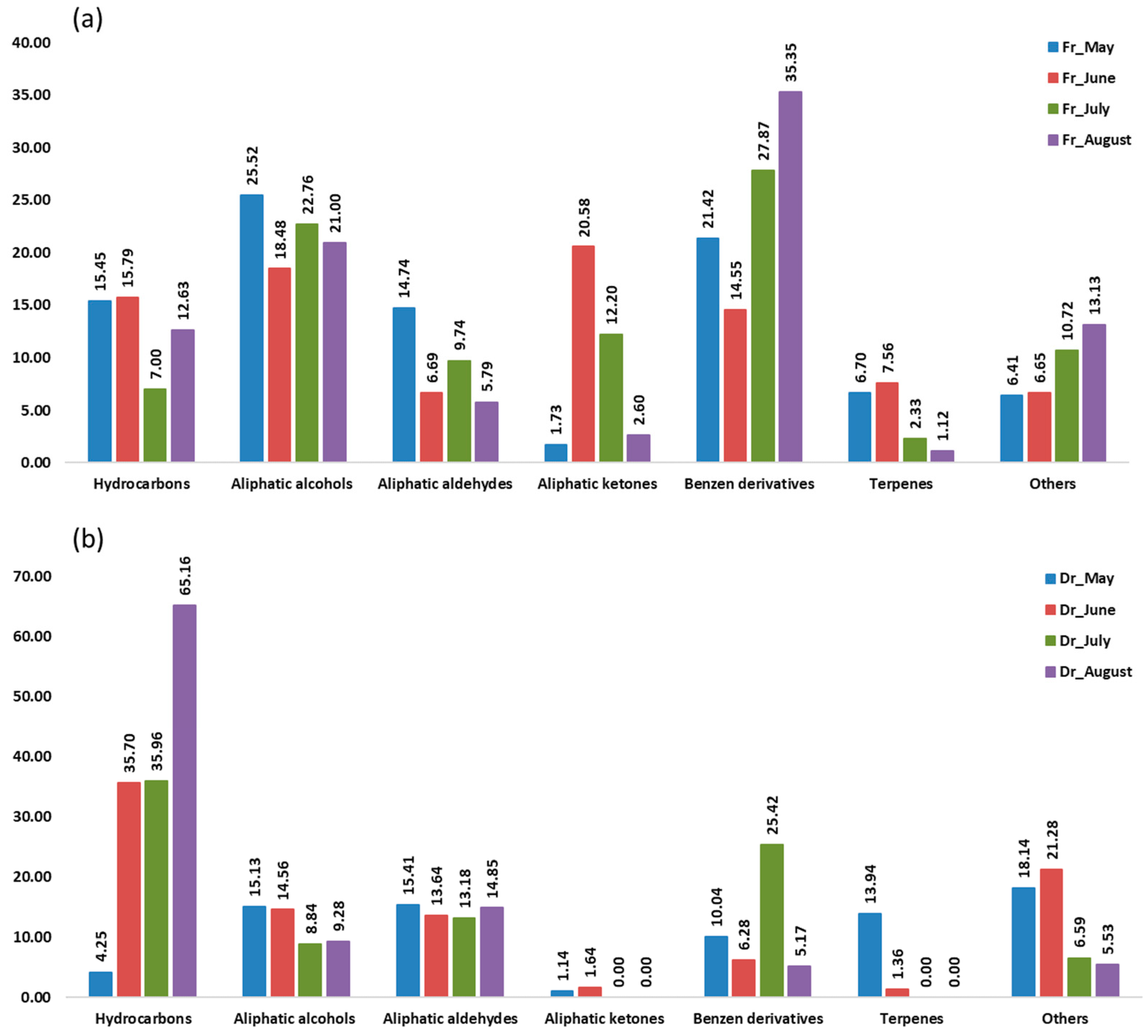
Aliphatic compounds accounted for the majority of compounds identified in all samples from four consecutive months. Their lowest percentage was in the air-dried sample in May (36.90%, f1; 35.93%, f2) and the highest in the air-dried sample in August (91.14%, f1; 89.30%, f2). When extracted with f1 (fibre of medium polarity), hydrocarbons were dominant in both the fresh and dry samples from June (23.02%; 47.01%) and from August (26.28%; 65.94%) and in the dry sample from July (47.01%) (Figure 1). Extraction with the more polar f2 fibre resulted in a dominance of hydrocarbons only in the dry samples from June (35.70%), July (35.96%), and August (65.16%) (Figure 2). In the dry samples, hydrocarbons were more than twice as high as in the fresh samples, except in May, when they were more abundant in the fresh samples. Pentadecane dominated with the highest abundance in August samples (43.88%, f1; 35.68%, f2) (Table 1 and Table 2). This result is consistent with previous findings on the predominance of pentadecane in brown algae [16]. Other abundant hydrocarbons were pentadec-1-ene and heptadecane. Hydrocarbons are likely formed as oxidative degradation products of lipids [17].
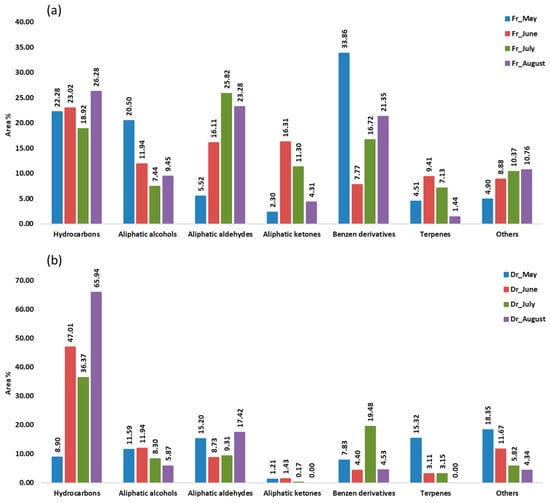
Figure 1.
The volatile organic compounds (VOCs) from Cladostephus spongiosus sorted by structural groups obtained by headspace solid-phase microextraction (HS-SPME) with divinylbenzene/carboxene/polydimethylsiloxane fibre (f1) and analysed by gas chromatography–mass spectrometry (GC-MS): (a) fresh (Fr) samples; (b) dry (Dr) samples.
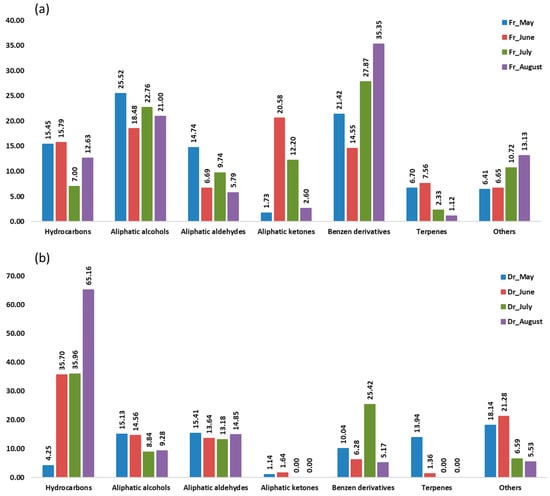
Figure 2.
The volatile organic compounds (VOCs) of Cladostephus spongiosus sorted by structural groups obtained via headspace solid-phase microextraction (HS-SPME) with polydimethylsiloxane/divinylbenzene fibre (f2) and analysed via gas chromatography–mass spectrometry (GC-MS): (a) fresh (Fr) samples; (b) dry (Dr) samples.

Table 1.
The volatile organic compounds (VOCs) of Cladostephus spongiosus obtained via headspace solid-phase microextraction (HS-SPME) with divinylbenzene/carboxen/polydimethylsiloxane fibre (f1) and analysed via gas chromatography–mass spectrometry (GC-MS): fresh (Fr) samples, dry (Dr) samples.

Table 2.
The volatile organic compounds (VOCs) from Cladostephus spongiosus obtained via headspace solid-phase microextraction (HS-SPME) with polydimethylsiloxane/divinylbenzene fibre (f2) and analysed via gas chromatography–mass spectrometry (GC-MS): fresh (Fr) samples; dry (Dr) samples.
Aliphatic alcohols were present with high percentages in all months studied, especially when extracted with the fibre f2 (Figure 2), and they were more abundant in the fresh samples. The short-chain alcohols ethanol, hexan-1-ol, octan-1-ol, and oct-1-en-3-ol were the most abundant (Table 1 and Table 2). Previous studies showed that the oxylipin oct-1-en-3-ol is a defence compound in marine algae [18,19,20].
Hexanal, heptanal, nonanal, and tridecanal constituted the majority of aldehydes (Table 1 and Table 2). The algal aldehydes are formed through degradation of fatty acids, which can occur either via oxidation or via the enzymatic action of lipoxygenases [21]. Hexanal and heptanal are mainly derived from linoleic acid [22,23]. Nonanal could originate from ω9 monounsaturated fatty acids (MUFAs) and ω6 polyunsaturated fatty acids (PUFAs), such as linoleic acid [24]. The fatty aldehyde tridecanal could be derived from long-chain fatty acids. Aldehydes obtained with the polar fibre f2 were more abundant in the air-dried samples in all months.
Aliphatic ketones, such as octan-3-one, 6-methylhept-5-en-2-one, and (E,Z)-octa-3,5-dien-2-one, were more abundant in fresh samples (f1, f2). The fresh sample from June contained the highest percentage of aliphatic ketones (16.31%, f1; 20.58%, f2) (Table 1 and Table 2), which play the role of pheromones in marine algae [12].
Benzene derivatives group dominated the fresh samples from May (33.86%, f1; 21.42%, f2), July (16.72%, f1; 27.87%, f2), and August (21.35%, f1; 35.34%, f2), as can be seen in Figure 1 and Figure 2. Benzyl alcohol and benzaldehyde formed the majority of benzene derivatives. Benzaldehyde was the predominant compound in all fresh samples obtained by f2 (Table 2) and in all fresh samples (except June) obtained by f1 (Table 1). In contrast, benzyl alcohol was more abundant in the dry samples. The volatile benzene derivatives can be formed from phenyalanine when the side chain of a carbon skeleton shortens by C2-unit. This reaction most commonly occurs via the β-oxidative pathway [25]. The loss of benzaldehyde during air-drying could be due to its high volatility [26,27,28].
Terpenes, monoterpenes, and sesquiterpenes showed the largest proportion (Figure 1 and Figure 2) in May dry sample (15.32%, f1; 13.94%, f2), mainly with the high abundance of monoterpene alcohols (E)-verbenol and p-cymen-8-ol (Table 1 and Table 2).
In the group of other compounds carboxylic acids, dictyopterenes, and C13-norisoprenoides were abundant. Carboxylic acids such as hexanoic and heptanoic acids contributed strongly to the increase in other compounds abundance, especially in the dry May sample.
2.2. Statistical Analysis of the Headspace VOCs
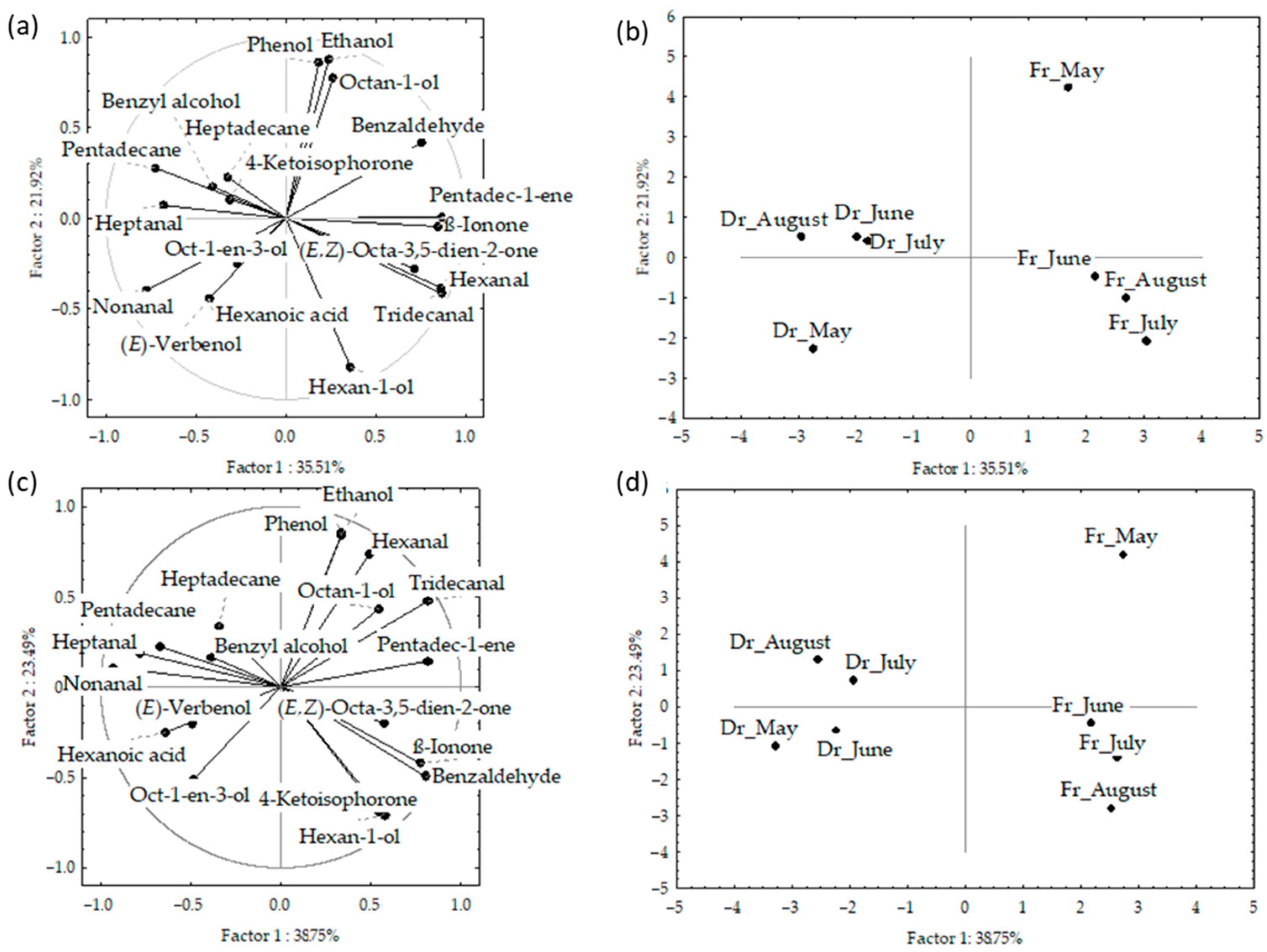
The results of the principal component analyses (PCA) are shown in Figure 3a–d and Figure 4. PCA was used to describe the variations among the dominant volatiles (>2%) in relation to the sampling month, material preparation (fresh or dry), and fibre.
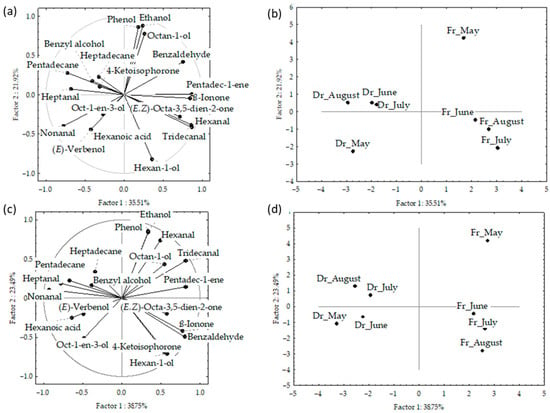
Figure 3.
Correlation loadings (a,c) and score plots (b,d) of the dominant compounds from the headspace volatiles obtained via headspace solid-phase microextraction (HS-SPME) with two fibres divinylbenzene/carboxene/polydimethylsiloxane (f1) and polydimethylsiloxane/divinylbenzene (f2) and analysed via gas chromatography–mass spectrometry (GC-MS): fresh (Fr) and dried (Dr) Cladostephus spongiosus samples collected from May to August.
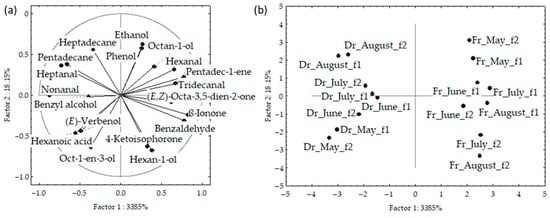
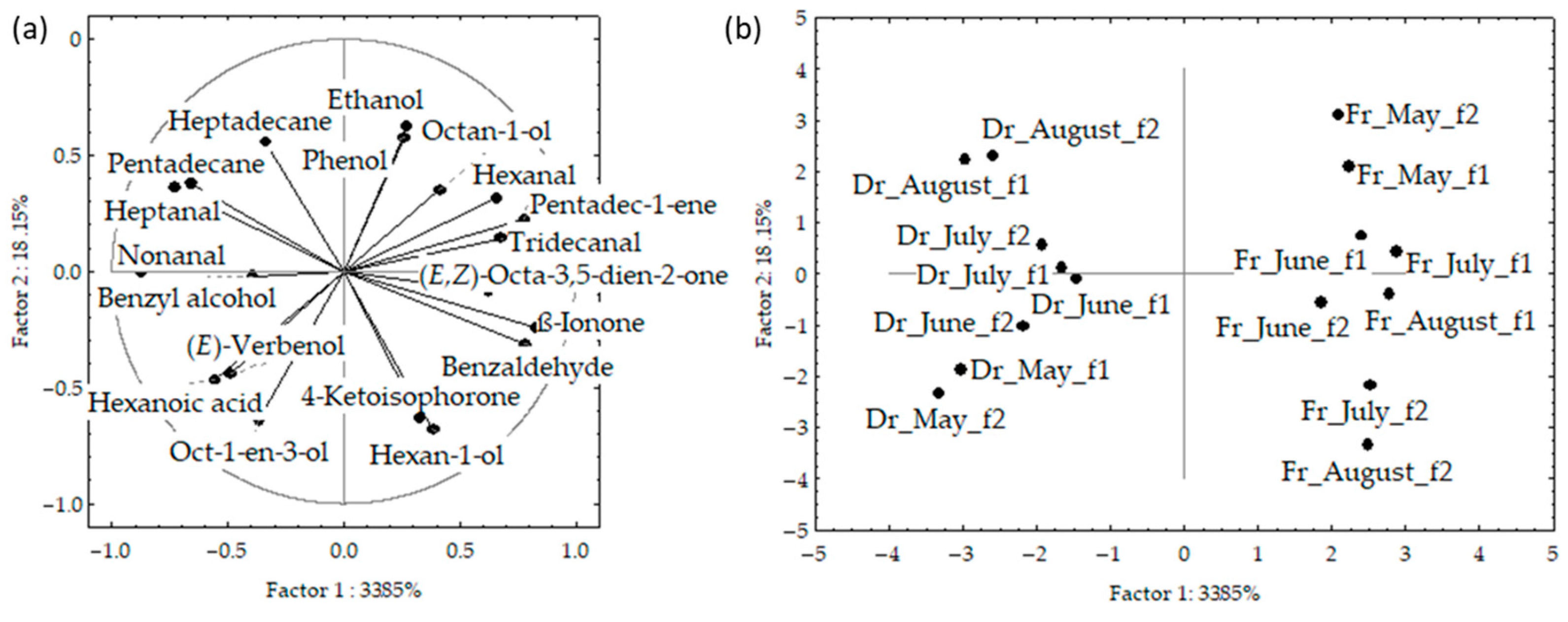
Figure 4.
Correlation (a) and score plots (b) of the dominant compounds from headspace volatiles (two different fibres obtained via headspace solid-phase microextraction (HS-SPME) with two fibres divinylbenzene/carboxenene/polydimethylsiloxane (f1) and polydimethylsiloxane/divinylbenzene (f2)) and analysed via gas chromatography–mass spectrometry (GC-MS): fresh (Fr) and dried (Dr) Cladostephus spongiosus samples analysed together.
The correlation plot and score plot of the dominant components from data obtained by f1 and f2 fibres are shown in Figure 3a–d, respectively. The first two PCs of the data obtained by f1 fibre described 53.4% of the initial data variability. The highest variable contribution to PC1 was observed for tridecanal, hexanal, pentadec-1-ene, and ß-ionone, while ethanol, hexan-1-ol, octan-1-ol, and phenol contributed to PC2.
The first two PCs in the data obtained by f2 fibre described 62.2% of the initial data variability. The abundance of two aldehydes (nonanal and tridecanal) contributed to PC1, while alcohols (ethanol and hexan-1-ol) and phenol had the highest contribution to the PC2. The distribution of the samples in the multivariate space showed no significant difference between the fibres. However, a clear separation between the fresh and dried samples was obtained (Figure 3b,d). For both f1 and f2, the dry samples were positioned on the left part of the multivariate space and the fresh samples, taken in May, were separated in the bottom right part of the score plot. Interestingly, the sampling month showed no effect on the dry samples, while in fresh samples there are similarities between June, July, and August, while May was clearly separated in the upper right.
Figure 4a,b shows the PCA of the data for f1 and f2 analysed together. The initial data variability of the first two PCs described 52%; however, the fresh and dried samples were clearly separated based on the higher content of (E,Z)-octa-3,5-dien-2-one and benzyl alcohol, as well as low content of pentadecane in the fresh samples. The samples from both fibres were segregated according to the sampling month, with clear vertical distribution from May to August.
2.3. Hydrodistillation Obtained Volatilome Variations of C. spongiosus
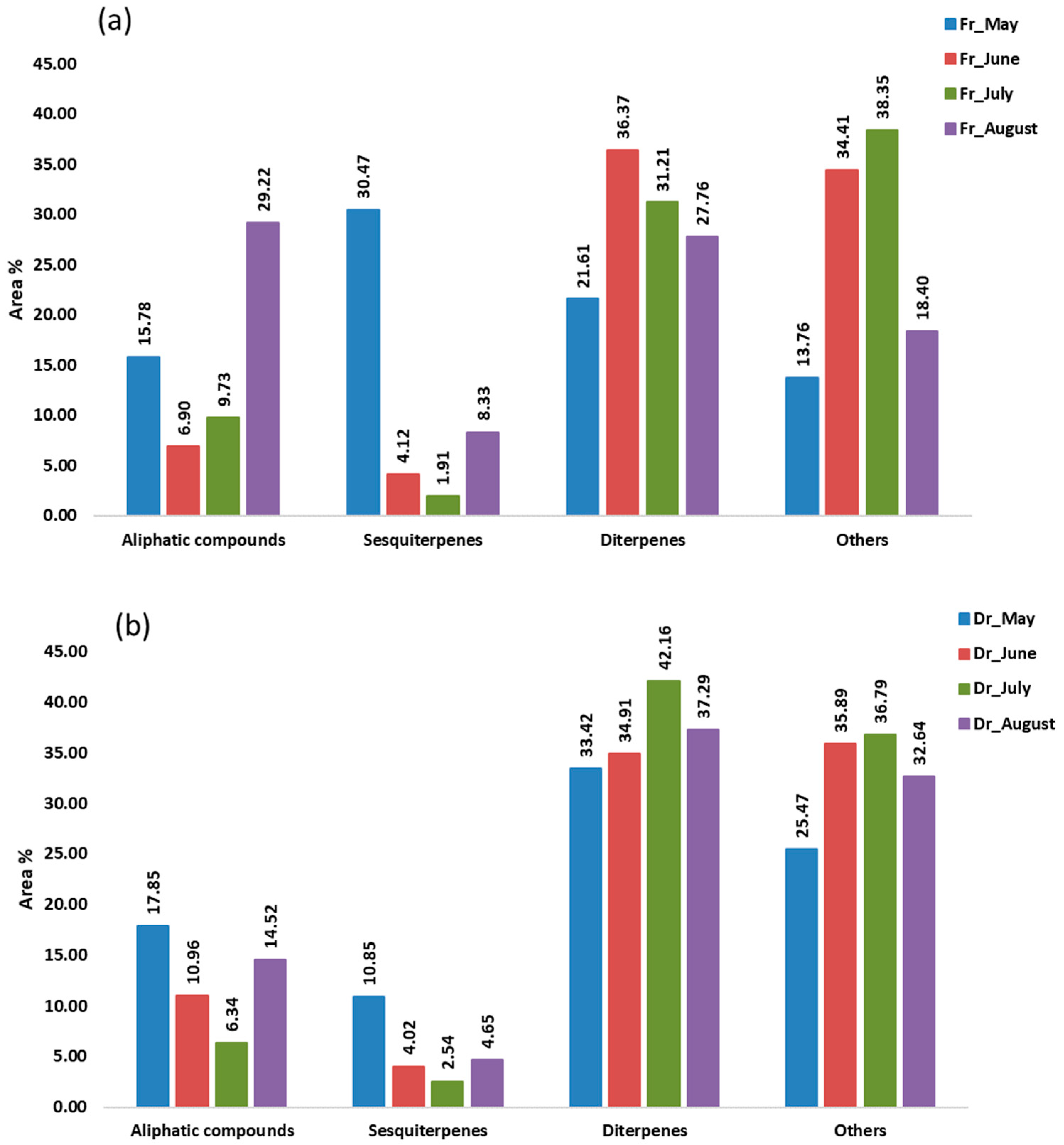
In the hydrodistillate, the percentages of the identified compounds ranged from 81.21% (Fr_July) to 89.11% (Dr_August) of the total compounds detected. These compounds were classified into four different groups: aliphatic compounds, sesquiterpenes, diterpenes, and others (Figure 5). Terpenes, especially sesquiterpenes and diterpenes, were the most abundant in all samples (36.09%, Fr_August—52.08%, Fr_May), except in the fresh sample in July (33.13%). Sesquiterpenes were more abundant only in May’s fresh sample (30.47%), mainly because of the high proportion of germacrene D (9.63%) and epi-bicyclosesquiphellandrene (4.49%) and gleenol (11.58%) (Table 3). All three detected sesquiterpenes were the predominant sesquiterpenes in the hydrodistillate of brown alga T. atomaria which belongs to the same subclass Dictyotophycidae [29]. When observing the seasonal changes in H. scoparia, which belongs to the same order of Sphacelariales, the highest proportion of germacrene D and gleenol in the hydrodistillate of the fresh sample was also found in May and later decreased each month until September [30]. Diterpenes were more abundant in the remaining samples (Figure 5). Diterpene alcohols phytol (4.42%, Fr_July—18.73%, Dr_August), pachydictyol A (3.18%, Fr_August—12.44%, Dr_May), (E)-geranyl geraniol (1.40%, Fr_May—6.77%, Dr_July), and cembra-4,7,11,15-tetraen-3-ol (4.92%, Fr_May—16.04%, Dr_July) dominated. The lowest percentage of detected diterpenes was always found in the fresh samples and the highest in dry samples. Phytol content was the highest in August. A similar behaviour was observed in H. scoparia where phytol was the predominant compound [30]. Phytol and its derivatives have a wide range of bioactive functions such as antioxidant, cytotoxic, anti-inflammatory, antimicrobial, autophagy- and apoptosis-inducing, immune- and metabolism-modulating, and anxiety-relieving activities [31]. Cembra-4,7,11,15-tetraen-3-ol was also found to be a very dominant terpene in D. dichotoma and H. scoparia, as was pachydictyol A in D. dichotoma [30,32]. Cembranoid-type diterpenes have good antitumor, antimicrobial, and neuroprotective activity [33], and pachydictyol A can be used as an antifouling paint, among other applications [34]. Monoterpenes were present only in the dry sample in May (< 1%) and in both the fresh and dry samples in June (<0.1%). In general, terpenes are bioactive compounds and, as such, play an important role in human health, as well as food preservatives [35].
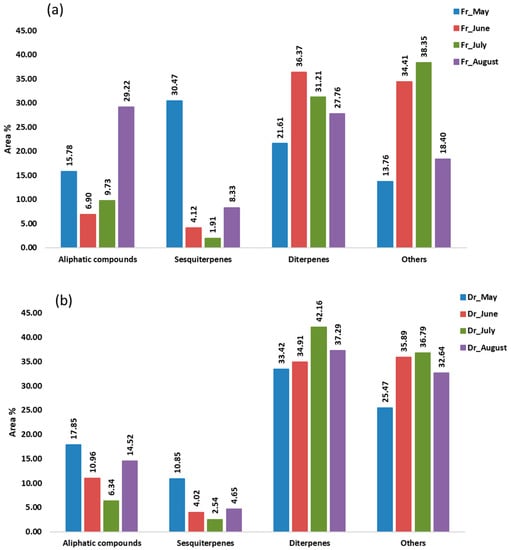
Figure 5.
The volatile organic compounds (VOCs) from Cladostephus spongiosus sorted by structural groups isolated via hydrodistillation (HD) and analysed via gas chromatography–mass spectrometry (GC-MS): (a) fresh (Fr) samples; (b) dry (Dr) samples.

Table 3.
The volatile organic compounds (VOCs) from Cladostephus spongiosus isolated via hydrodistillation (HD) and analysed via gas chromatography–mass spectrometry (GC-MS): Fr (fresh) samples; Dr (dry) samples.
Fatty acid ethyl ester (FAEE) ethyl icosanoate, assigned to the group of others, was the dominant compound in all air-dried samples (12.85%, Dr_May—30.74%, Dr_July) and in the fresh samples in June (30.84%) and July (34.51%). Naturally occurring FAEEs as well as fatty acid methyl esters (FAMEs) could potentially be used as biofuels [36].
The saturated hydrocarbon heptadecane and aldehyde pentadecanal, as well as the unsaturated hydrocarbons (E)-heptadec-8-ene and (Z)-heptadec-3-ene, were the major aliphatic compounds with the highest percentage in August, contributing to the representation of the aliphatic compounds (Figure 5). All were most abundant in the fresh sample in August except heptadecane which was not detected but was most abundant in the dry sample in the same month (Table 3).
2.4. Statistical Analysis of the VOCs Obtained via Hydrodistillation
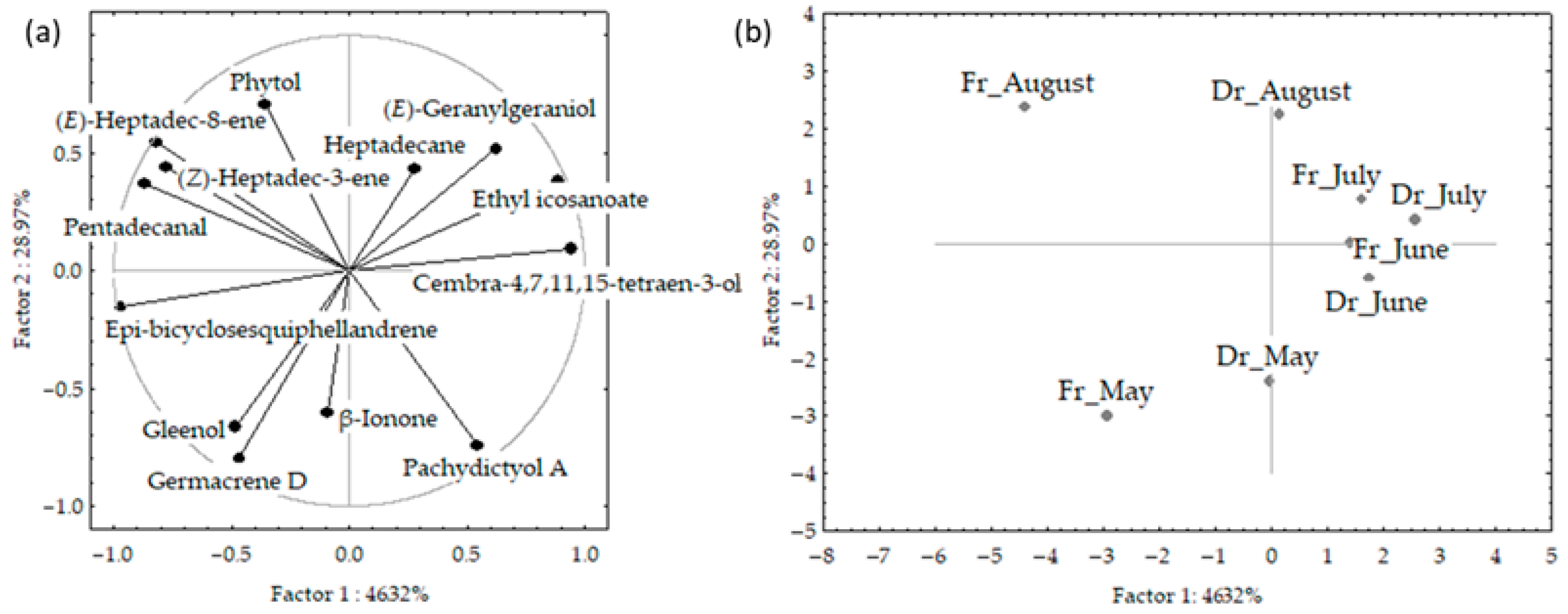
The PCA results for VOCs of fresh and air-dried C. spongiosus obtained via hydrodistillation are shown in Figure 6a,b. The first two PCs described 76.3% of the initial data variability. The correlation loadings of the first two PCs (Figure 6a) showed correlations between germacrene D and gleenol, and between aliphatic compounds ((E)-heptadec-8-ene, (Z)-heptadec-3-ene and pentadecanal). Epi-bicyclosesquiphellandrene and cembra-4,7,11,15-tetraen-3-ol were the variables with the highest variable contributions, based on the correlations. The PC2 was associated with germacrene D, phytol, and pachydictyol A abundance in the samples. The score plot (Figure 6b) showed the position samples in the multivariate space of the first two PCs. There was a clear separation between June and July samples in the centre right part of the plot, and segregation between May and August samples.
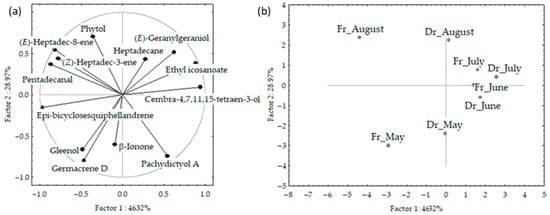
Figure 6.
Correlation loadings (a) and score plot (b) of the dominant volatile organic compounds (VOCs) of fresh and air-dried Cladostephus spongiosus samples obtained via hydrodistillation and analysed via gas chromatography-mass spectrometry (GC-MS).
June and July samples, characterized with higher content of cembra-4,7,11,15-tetraen-3-ol and ethyl icosanoate, and lower percentage of germacrene D and gleenol were positioned in the right part of the plot. The vertical distribution between May and August samples was related to differences in germacrene D, gleenol, pachydictyol A, and phytol abundance. There were more differences in seasonal variation between fresh samples. The distribution was along the PC1 axis and cannot be related to one group of compounds but was in the relation to the abundance of epi-bicyclosesquiphellandrene, cembra-4,7,11,15-tetraen-3-ol, ethyl icosanoate and pentadecanal, while the distribution along the PC2 axis was related to terpenes (germacrene D, phytol and pachydictyol A) abundance in the samples. No correlation was found between the compounds’ content and temperature change.
2.5. Antioxidant Activity of Ethanol Extracts In Vitro
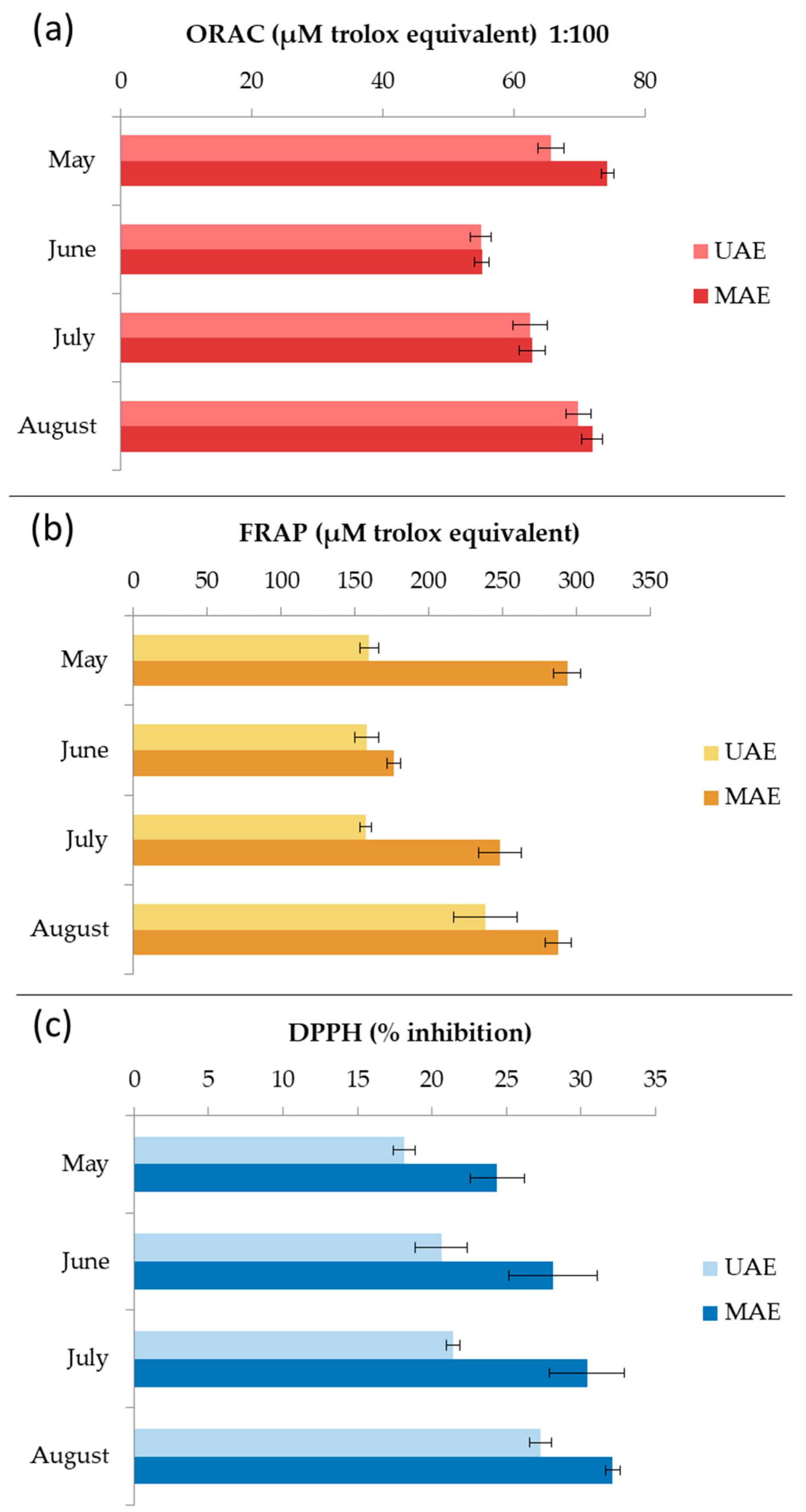
Seasonal variations in antioxidant activity of C. spongiosus extracts produced by MAE and UAE were evaluated by ORAC, FRAP, and DPPH assays (Figure 7). All extracts from MAE showed higher activity. The ORAC results ranged from 54.9 ± 1.5 to 69.8 ± 1.9 µM TE for UAE extracts and from 55.1 ± 1.1 to 74.2 ± 1.0 µM TE for MAE extracts. The highest peroxyl radical inhibition activity was observed for the samples harvested in May. The FRAP results ranged from 157.4 ± 3.9 to 238.5 ± 21.4 µM TE for UAE extracts, and from 176.4 ± 4.7 to 293.8 ± 9.2 µM TE for MAE extracts. The highest reducing activity was also observed for the samples harvested in May. The scavenging ability of DPPH radical ranged from 18.2 ± 0.7 to 27.3 ± 0.7% for UAE extracts, and from 24.4 ± 1.8 to 32.1 ± 0.5% for MAE extracts. The highest inhibition was recorded for August samples. Chiboub et al. [4] reported DPPH inhibition of C. spongiosus macerated with hexane, ethyl acetate, and methanol for 48 h at 15.6 ± 0.5%, 52.2 ± 0.0%, and 41.5 ± 0.1%, respectively, which is comparable to our results. Moreover, Yalçın et al. [37] extracted C. spongiosus f. verticillatum with methanol and ethanol solutions (100%, 80%, and 70%) for 120 min using UAE. The extracts showed antioxidant potential through 2,2-azino-bis(3-ethylbenzothiazoline-6-sulfonate) (ABTS) radical scavenging and cupric ion reduction. Pinteus et al. [6] showed that C. spongiosus harvested from the Peniche coast, and extracted with dichloromethane, has the ability to scavenge peroxyl and DPPH radicals. The antioxidant activity results mentioned above cannot be compared with our results because the authors used a different methodology or expressed their results in different units [38].
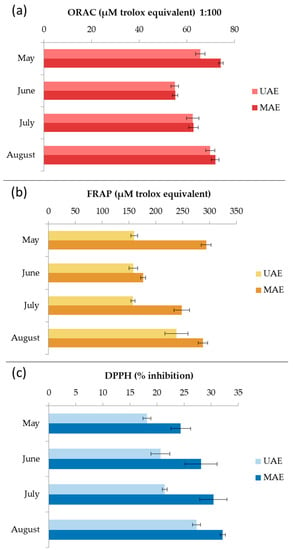
Figure 7.
Seasonal variation of the oxygen radical absorbance capacity (ORAC) (a), ferric reducing/antioxidant power (FRAP) (b), and 2,2-diphenyl-1-picrylhydrazyl radical scavenging ability (DPPH) (c) results for Cladostephus spongiosus extracts obtained with ultrasound-assisted extraction (UAE) and microwave-assisted extraction (MAE).
2.6. Non-Target Screening of Non-Volatile Compounds in Ethanol Extract
The ethanolic extract of the freeze-dried algal sample with the best antioxidant potential according to ORAC and FRAP assay (May, MAE) was analysed using UHPLC-ESI–HRMS. The major compounds were selected according to signal intensity (peak area in counts). From the extracted ion chromatograms (XICs) in positive ion mode, these compounds were identified based on the given elemental composition and MS/MS spectra with confidence level 2 (probable structure) and 3 (possible structure) [39]. Seventeen fatty acid derivatives, nine pigments and derivatives, and two steroid derivatives were found (Table 4).

Table 4.
Major non-volatile compounds in Cladostephus spongiosus ethanol extract identified using high performance liquid chromatography–high-resolution mass spectrometry with electrospray ionisation (UHPLC-ESI–HRMS).
Of the seventeen fatty acid derivatives, eight were fatty acid amides (FAAs) (compounds 7–10, 13, 19, 24), eight were glycerolipids (GLs) (compounds 3–6, 11, 12, 14, 18), and one was a fatty acid (FA) (compound 9) (Table 4). The FAAs were dominant.
Three C18 FAAs, derivatives of linoleic acid (linoleamide, no. 9), oleic acid (oleamide, no. 13), and stearic acid (stearamide, no. 17), two C16 FAAs, derivatives of palmitic acid (palmitamide, no. 10) and palmitoleic acid (palmitoleamide, no. 8), and one C14, derivative of myristic acid (myristamide, no. 7), C20 derivative of (11Z)-icos-11-enoic acid (gondamide, no. 19), and C22 FAA derivative of erucic acid (erucamide, no. 24) were detected. In our previous studies, we detected oleamide, erucamide, and palmitamide in the green alga Dasycladus vermicularis [40] and two others, palmitoleamide and linoleamide, in the brown algae Ericaria crinita and Ericaria amentacea [28]. The FAAs detected and identified in C. spongiosus belong to the group of primary fatty acid amides (PFAMs) with the structure R-CO-NH2, where R represents a long-chain FA [41], and are bioactive signalling molecules. In general, PFFAs have a broad therapeutic spectrum including anticancer, antibiotic, anthelmintic and antidiabetic [42]. They play an important role in the nervous system of the mammals because they have the potential to bind to the receptors of many drugs, indicating effects on locomotion, angiogenesis, and sleep [43]. It has been reported that PFAMs have a great anticancer activity as they effect cell proliferation [44]. Oleamide is the most studied PFFA and has great potential against Alzheimer disease [45]. Certain plant essential oils are rich in PFFAs because they act as self-defence agents in plants [42], but data on the occurrence of PFAAs in marine algae are lacking.
Among the GLs were four glycoglycerolipids, more specifically monogalactosylmonoacylglycerols (MGMGs) (compounds 3–6) and four monoacylglycerols (MG) (compounds 11, 12, 14, 18). All eukaryotes and prokaryotes can synthesise GLs which form membrane lipids [46]. Marine algae are known to be a great source of lipid molecules with wide-ranging bioactive properties [47]. Glycoglycerolipids are known for their antiviral, antitumor, antimicrobial, anti-inflammatory, and antioxidant properties [48]. They can be classified into three groups based on the nature of the glycosyl and acyl structures: monogalactosyl diacylglycerols (MGDG), digalactosyl diacylglycerols (DGDG), and sulfoquinovosyldiacyl glycerols (SQDG) [49]. MGMG is a byproduct of the degradation of accumulated MGDG in the outer membrane of chloroplasts [50].
In the group of pigments, one chlorophyll a derivative (pheophorbide a, compound 21), one demetallized chlorophyll b (pheophytin b, compound 27), three pheophorbide derivatives (compounds 22, 23, 25), one xanthophyll and two derivatives (compounds 15, 16, 28), and one monoterpene lactone (loliolide, compound 1) were detected (Table 4). The pigments are known antioxidants [51,52]. Pheophorbide a, a derivative of chlorophyll a, has been extracted from marine algae and has shown great antitumor activity [53]. Among the pigments, it was the most abundant in the extract (Table 4), which contributes to the antioxidant activity of the extract. Cho et al. [54] studied the green alga Ulva prolifera (formerly Enteromorpha prolifera) and its ethanolic extract, which showed high antioxidant activities. The later subfraction with chloroform showed the strongest antioxidant activity of all subfractions, and further spectroscopic analysis revealed the pheophorbide a as the major compound.
Fucoxanthin, the major xanthophyll in brown algae [55,56], and xantophyll derivatives, halocynthiaxanthin acetate and loroxanthin decenoate, were detected. El Hattab et al. [57] reported fucoxanthin in C. spongiosus f. verticillatus. Halocynthiaxanthin acetate and loroxanthin decenoate are more abundant in green algae [58]. Xanthophylls exhibit diverse bioactive properties, such as antioxidant, antitumor, and anti-inflammatory activities [55,56,59]. The antioxidant activity of the extract might be related to the high amounts of fucoxanthin (Table 4). Ibrahim et al. [60] evaluated the potent antimicrobial activity against Gram-positive and Gram-negative bacteria and fungi and the strong antioxidant activity of fucoxanthin extracted from Dictyota fasciola (formerly Dilophys fasciola). Fucoxanthin is one of the most studied xanthophylls against cancer cells because of its antiproliferative effect [59]. Halocynthiaxanthin is a metabolite of fucoxanthin and has shown even stronger cytotoxic results than fucoxanthin when tested on human neuroblastoma cells [61]. Sansone et al. [62] studied the biological activity of the marine green alga Tetraselmis suecica, whose ethanolic extract contained a high proportion of xhantophylls, among which loraxanthin esters were present. The extract showed potent antioxidant activity and antitumor activity against human lung cancer line (A549).
Loliolide, the only monoterpernoid lactone detected in our study, was previously detected in C. spongiosus f. verticillatus by El Hattab et al. [57]. It has also been detected in the genus Ericaria [28], D. vermicularis [40], and the microalga Tetradesmus obliquus [63]. Silva et al. [64] demonstrated the neuroprotective, anti-inflammatory, and antioxidant effects of loliolide in an extract of Codium tomentosum.
3. Materials and Methods
3.1. Macroalga Samples
Between May and August 2021, the samples of the brown alga C. spongiosus (Hudson) C. Agardh, 1817 were collected. The sampling took place in the Adriatic Sea, off the coast of the island of Čiovo (43.493373° N, 16.272519° E). Each sample was collected at a depth of 20 to 120 cm from the same lagoon. The sea temperature was measured during each sampling with a YSI Pro2030 probe (Yellow Springs, OH, USA) and increased from 20.1 °C in May to 28.1 °C in August. Alga species was determined according to its morphological attributes by marine botanist. For the determination of VOCs, the samples were dried in the shade at room temperature for 10 days, while samples for the determination of non-volatile compounds were freeze-dried (FreeZone 2.5, Labconco, Kansas City, MO, USA) prior the extraction. Algal samples were extracted in 50% ethanol using MAE in the advanced microwave extraction system (ETHOS X, Milestone Srl, Sorisole, Italy) and UAE based on the prior research [9]. The samples were pulverised and mixed with 50% ethanol at a 1:10 (w/v) algae to solvent ratio and extracted for: (1) 15 min at 200 W and 60 °C for MAE, and (2) 60 min at 40 kHz frequency and 60 °C in an ultrasonic bath for UAE.
3.2. Headspace Solid-Phase Microextraction (HS-SPME)
HS-SPME was performed on the PAL Auto Sampler System (PAL RSI 85, CTC Analytics AG, Schlieren, Switzerland) using two SPME fibres of different polarity: PDMS/DVB (polydimethylsiloxane/divinylbenzene) or VB/CAR/PDMS (divinylbenzene/carboxene/polydimethylsiloxane) (Agilent Technologies, Palo Alto, Santa Clara, CA, USA). Both fibres were conditioned before analysis according to the manufacturer’s instructions. Samples (1 g) were placed in 20 mL glass vials sealed with a stainless steel lid with polytetrafluorethylene (PTFE)/silicon septum. First, the sample was equilibrated at a temperature of 60 °C for 15 min, after which the extraction was continued for 45 min. Subsequent thermal desorption lasted 6 min in the inlet set at 250 °C, from where the compounds were passed directly into the GC column.
3.3. Hydrodistillation (HD)
Approximately 50 g of fresh and 30 g of air-dried algal material were hydrodistilled in a modified Clevenger apparatus containing 3 mL of organic solvent trap (pentane and diethyl ether, 1:2 v/v, both from Kemika, Zagreb, Croatia). After 2.5 h of hydrodistillation, the VOC-containing organic solvent trap was collected and concentrated to a final volume of 0.2 mL under slow nitrogen flow. GC-MS analysis was performed using 2 µL of the sample.
3.4. Gas Chromatography–Mass Spectrometry Analysis (GC–MS)
GC-MS analysis was performed using an Agilent Technologies (Palo Alto, CA, USA) model 8890A gas chromatograph, coupled to a model 5977E mass selective detector. The compounds were separated on a HP -5MS column (Agilent Technologies, Santa Clara, CA, USA) 30 m × 0.25 mm with a nonpolar stationary phase (5% diphenyl/95% dimethylpolysiloxane) and a film thickness of 0.25 μm. The following operating conditions were used for the gas chromatograph: 250 °C injector temperature; 300 °C detector temperature; column temperature program: 2 min isothermal at 70 °C, followed by a temperature gradient from 70 °C to 200 °C at 3 °C/min and a further retention of 15 min. The split ratio was 1:50; the carrier gas was helium with a flow rate of 1.0 mL/min; the MSD (EI mode) was operated at 70 eV; the mass range was set to 30 to 300 amu. The compounds were identified by comparing their retention indices (RI), which were based on the retention times of n-alkanes (C9–C25), with those reported in the literature (National Institute of Standards and Technology) and their mass spectra with those from the Wiley 9 (Wiley, New York, NY, USA) and NIST 17 (D-Gaithersburg) mass spectral libraries. Percent composition was calculated using the normalisation method (without correction factors). Analyses were performed in duplicate and expressed as the average percentage of peak area.
3.5. Ultra-High Performance Liquid Chromatography-High-Resolution Mass Spectrometry (UHPLC-ESI-HRMS) of Ethanol Extract
The UHPLC-ESI-HRMS analyses were performed using the ExionLC AD UHPLC system (AB Sciex, Concord, ON, Canada) equipped with the following ExionLC modules: AD Controller, solvent delivery system (AD Pump and AD Degasser), AD Autosampler and AD Column oven, connected to quadrupole time-of-flight (Q-TOF) mass spectrometer TripleTOF 6600+ (AB Sciex, Concord, ON, Canada) with duospray ion source. Chromatographic separations of the compounds were performed using the Acquity UPLC BEH Phenyl-Hexyl analytical column (Waters, Milford, MA, USA) 2.1 mm × 100 mm with a particle size of 1.7 µm. A continuous flow rate of 0.4 mL/min was set to pump the mobile phases, water (A) and acetonitrile (B), both containing 0.1% formic acid. The oven temperature was set at 30 °C. Elution started isocratically with 2% B (0.6 min), followed by a gradient program: 0.6–18.5 min (linear B gradient to 100%), 18.5–25 min (100% B). Electrospray ionisation was set in positive mode (ESI+) with collision-induced dissociation (CID) in information-dependent acquisition mode (IDA) for MS/MS mass spectra acquisition. In our previous work, description of the parameters in details can be found [40]. ACD/Spectrus Processor 2021.1.0 software (ACD/Labs, Toronto, ON, Canada) was used to process the mass spectrometer data. Based on the mass spectra and the given elemental compositions of the compounds, and in conjunction with the results of the search in the ChEBI, Lipid Maps, and MassBank databases, the identification of the compounds was proposed.
3.6. Antioxidant Activity of Extracts
Three different methods were used to assess in vitro antioxidant activity of crude algal extracts. The oxygen radical absorbance capacity (ORAC) and 2,2-diphenyl-1-picrylhydrazyl radical scavenging ability (DPPH) methods are based on hydrogen atom transfer, while ferric reducing/antioxidant power (FRAP) method is based on electron transfer [11,65,66].
Previously reported methods [11,65] were used to measure the reducing activity as FRAP. The results were expressed using the Trolox standard calibration curve (y = 0.0013x, R2 = 0.99) as micromoles of Trolox equivalents (µM TE). The inhibition of the free peroxyl radicals, measured as ORAC [11], was measured for the samples in 1:100 dilution, and the results were expressed using the Trolox standard calibration curve (y = 22.842x + 26.473, R2 = 0.99) as µM TE. DPPH radical inhibition was expressed as a percentage of inhibition and measured according to the previously reported methods [11,67].
3.7. Statistical Analyses
The relationship between the dominant volatiles (>2%) of fresh and dried C. spongiosus samples was determined via principal component analysis (PCA) using the software STATISTICA® (version 13, StatSoft Inc., Tulsa, OK, USA). Before analyses, the data (average percentage of peak areas of the dominant volatiles) were log-transformed [9].
4. Conclusions
HS-SPME showed that aliphatic compounds dominated in all samples. Hydrocarbons were more than twice as high in the dry samples (except in May). Aliphatic alcohols were present in large amounts and were more abundant in the fresh samples. Hexanal, heptanal, nonanal, and tridecanal formed the majority of aldehydes. Aliphatic ketones were more abundant in the fresh samples. Benzene derivatives were predominant in the fresh samples of May and August. Mono- and sesquiterpenes (mainly (E)-verbenol and p-cymen-8-ol) were the most abundant in the May dry sample.
HD obtained four groups of compounds: aliphatic compounds (e.g., heptadecane, pentadecanal, (E)-heptadec-8-ene and (Z)-heptadec-3-ene with the highest percentage in August), sesquiterpenes (germacrene D, epi-bicyclosesquiphellandrene, gleenol), diterpenes (phytol, pachydictyol A, (E)-geranyl geraniol, cembra-4,7,11,15-tetraen-3-ol) and others. Except for the July fresh sample, terpenes were the most abundant. The lowest percentage of diterpenes was always in fresh samples and the highest in dry samples.
Seasonal variations in antioxidant activity of C. spongiosus extracts obtained with MAE and UAE were confirmed. All MAE extracts showed higher activity. The highest peroxyl radical inhibition and reducing activity was observed in the May samples. Therefore, the ethanolic extract from May was analysed by UHPLC-ESI-HRMS. A total of 17 fatty acid derivatives, 9 pigments and derivatives, and 2 steroid derivatives were found. The highest content of pheophorbide a and fucoxanthin and the presence of other pigment derivatives could be related to the observed antioxidant activity.
Further research should focus on exploring the seasonal variations of the non-volatile compounds of this alga and the potential use of the extracts obtained.
Author Contributions
Conceptualisation, I.J. and V.Š.; methodology, S.R., I.J., M.Č. and V.Š.; formal analysis, S.R. and M.Č.; investigation, S.R., M.Č., V.Š. and I.J.; resources, I.J. and V.Š.; data curation, S.R. and M.Č.; writing—original draft preparation, S.R., I.J., V.Š. and M.Č.; writing—review and editing, S.R., I.J., V.Š. and M.Č.; supervision, I.J. and V.Š.; funding acquisition, I.J. and V.Š. All authors have read and agreed to the published version of the manuscript. S.R. and M.Č. have shared co-first authorship.
Funding
This research was funded by the Croatian Government and the European Union (European Regional Development Fund—the Competitiveness and Cohesion Operational Programme—KK.01.1.1.01) through project Bioprospecting of the Adriatic Sea (KK.01.1.1.01.0002) granted to The Scientific Centre of Excellence for Marine Bioprospecting-BioProCro and supported by the PRIMA program under project BioProMedFood (Project ID 1467). The PRIMA programme is supported by the European Union.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
We would like to thank the Croatian Government and the European Union (European Regional Development Fund—the Competitiveness and Cohesion Operational Programme—KK.01.1.1.01) through project Bioprospecting of the Adriatic Sea (KK.01.1.1.01.0002) granted to The Scientific Centre of Excellence for Marine Bioprospecting-BioProCro and to the PRIMA program under the project BioProMedFood (Project ID 1467), which is also supported by the European Union).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Heesch, S.; Rindi, F.; Guiry, M.D.; Nelson, W.A. Molecular Phylogeny and Taxonomic Reassessment of the Genus Cladostephus (Sphacelariales, Phaeophyceae). Eur. J. Phycol. 2020, 55, 426–443. [Google Scholar] [CrossRef]
- Antolić, B.; Špan, A.; Žuljević, A.; Nikolić, V.; Grubelić, I.; Despalatović, M.; Cvitković, I. A Checklist of the Benthic Marine Macroalgae from the Eastern Adriatic Coast: II. Heterokontophyta: Phaeophyceae. Acta Adriat. 2010, 51, 9–33. [Google Scholar]
- Mazariegos-Villareal, A.; Riosmena-Rodríguez, R.; Rosa Rivera-Camacho, A.; Serviere-Zaragoza, E. First Report of Cladostephus Spongiosus (Sphacelariales: Phaeophyta) from the Pacific Coast of Mexico. Bot. Mar. 2010, 53, 153–157. [Google Scholar] [CrossRef]
- Chiboub, O.; Ktari, L.; Sifaoui, I.; López-Arencibia, A.; Reyes-Batlle, M.; Mejri, M.; Valladares, B.; Abderrabba, M.; Piñero, J.E.; Lorenzo-Morales, J. In Vitro Amoebicidal and Antioxidant Activities of Some Tunisian Seaweeds. Exp. Parasitol. 2017, 183, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Zbakh, H.; Chiheb, H.; Bouziane, H.; Sánchez, V.M.; Riadi, H. Antibacterial Activity of Benthic Marine Algae Extracts from the Mediterranean Coast of Morocco. J. Microbiol. Biotechnol. Food Sci. 2012, 2, 219–228. [Google Scholar]
- Pinteus, S.; Silva, J.; Alves, C.; Horta, A.; Fino, N.; Rodrigues, A.I.; Mendes, S.; Pedrosa, R. Cytoprotective Effect of Seaweeds with High Antioxidant Activity from the Peniche Coast (Portugal). Food Chem. 2017, 218, 591–599. [Google Scholar] [CrossRef]
- Kilic, M.; Orhan, I.E.; Eren, G.; Okudan, E.S.; Estep, A.S.; Bencel, J.J.; Tabanca, N. Insecticidal Activity of Forty-Seven Marine Algae Species from the Mediterranean, Aegean, and Sea of Marmara in Connection with Their Cholinesterase and Tyrosinase Inhibitory Activity. S. Afr. J. Bot. 2021, 143, 435–442. [Google Scholar] [CrossRef]
- El Zawawy, N.A.; El-Shenody, R.A.; Ali, S.S.; El-Shetehy, M. A Novel Study on the Inhibitory Effect of Marine Macroalgal Extracts on Hyphal Growth and Biofilm Formation of Candidemia Isolates. Sci. Rep. 2020, 10, 9339. [Google Scholar] [CrossRef]
- Čagalj, M.; Skroza, D.; Tabanelli, G.; Özogul, F.; Šimat, V. Maximizing the Antioxidant Capacity of Padina pavonica by Choosing the Right Drying and Extraction Methods. Processes 2021, 9, 587. [Google Scholar] [CrossRef]
- Čagalj, M.; Fras Zemljič, L.; Kraševac Glaser, T.; Mežnar, E.; Sterniša, M.; Smole Možina, S.; Razola-Díaz, M.d.C.; Šimat, V. Seasonal Changes in Chemical Profile and Antioxidant Activity of Padina pavonica Extracts and Their Application in the Development of Bioactive Chitosan/PLA Bilayer Film. Foods 2022, 11, 3847. [Google Scholar] [CrossRef]
- Čagalj, M.; Skroza, D.; Razola-Díaz, M.D.C.; Verardo, V.; Bassi, D.; Frleta, R.; Mekinić, I.G.; Tabanelli, G.; Šimat, V. Variations in the Composition, Antioxidant and Antimicrobial Activities of Cystoseira compressa during Seasonal Growth. Mar. Drugs 2022, 20, 64. [Google Scholar] [CrossRef]
- El Hattab, M. Algae Essential Oils: Chemistry, Ecology, and Biological Activities. In Essential Oils-Bioactive Compounds, New Perspectives and Applications; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Whitfield, F.B.; Helidoniotis, F.; Shaw, K.J.; Svoronos, D. Distribution of Bromophenols in Species of Marine Algae from Eastern Australia. J. Agric. Food Chem. 1999, 47, 2367–2373. [Google Scholar] [CrossRef]
- Pereira, H.; Barreira, L.; Figueiredo, F.; Custódio, L.; Vizetto-Duarte, C.; Polo, C.; Rešek, E.; Aschwin, E.; Varela, J. Polyunsaturated Fatty Acids of Marine Macroalgae: Potential for Nutritional and Pharmaceutical Applications. Mar. Drugs 2012, 10, 1920–1935. [Google Scholar] [CrossRef] [PubMed]
- Rubiño, S.; Peteiro, C.; Aymerich, T.; Hortós, M. Brown Macroalgae (Phaeophyceae): A Valuable Reservoir of Antimicrobial Compounds on Northern Coast of Spain. Mar. Drugs 2022, 20, 775. [Google Scholar] [CrossRef] [PubMed]
- Youngblood, W.W.; Blumer, M. Alkanes and Alkenes in Marine Benthic Algae. Mar. Biol. 1973, 21, 163–172. [Google Scholar] [CrossRef]
- Zhang, J.; Wei, Z.; Zhang, H.; Xie, L.; Vincenzetti, S.; Polidori, P.; Li, L.; Liu, G. Changes in the Physical–Chemical Properties and Volatile Flavor Components of Dry-Cured Donkey Leg during Processing. Foods 2022, 11, 3542. [Google Scholar] [CrossRef]
- Herrero-Garcia, E.; Garzia, A.; Cordobés, S.; Espeso, E.A.; Ugalde, U. 8-Carbon Oxylipins Inhibit Germination and Growth, and Stimulate Aerial Conidiation in Aspergillus nidulans. Fungal Biol. 2011, 115, 393–400. [Google Scholar] [CrossRef]
- Jian, Q.; Zhu, X.; Chen, J.; Zhu, Z.; Yang, R.; Luo, Q.; Chen, H.; Yan, X. Analysis of Global Metabolome by Gas Chromatography-Mass Spectrometry of Pyropia haitanensis Stimulated with 1-Octen-3-Ol. J. Appl. Phycol. 2017, 29, 2049–2059. [Google Scholar] [CrossRef]
- Jerković, I.; Radman, S.; Jokić, S. Distribution and Role of Oct-1-en-3-ol in Marine Algae. Compounds 2021, 1, 125–133. [Google Scholar] [CrossRef]
- Le Pape, M.A.; Grua-Priol, J.; Prost, C.; Demaimay, M. Optimization of Dynamic Headspace Extraction of the Edible Red Algae Palmaria palmata and Identification of the Volatile Components. J. Agric. Food Chem. 2004, 52, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Jerković, I.; Marijanović, Z.; Roje, M.; Kus, P.M.; Jokić, S.; Čož-Rakovac, R. Phytochemical Study of the Headspace Volatile Organic Compounds of Fresh Algae and Seagrass from the Adriatic Sea (Single Point Collection). PLoS ONE 2018, 13, 6462. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Deng, L.; Zhu, X.M.; Fan, Y.; Hu, J.N.; Li, J.; Deng, Z.Y. Novel Approach to Evaluate the Oxidation State of Vegetable Oils Using Characteristic Oxidation Indicators. J. Agric. Food Chem. 2014, 62, 12545–12552. [Google Scholar] [CrossRef]
- Enoiu, M.; Wellman, M.; Leroy, P.; Ziegler, J.; Mitrea, N.; Siest, G.; Ea, U.; Henri, U. S Gas and Liquid Chromatography-Mass Spectrometry of Aldehydic Products from Lipid Peroxidation. Analusis 2000, 28, 285–290. [Google Scholar] [CrossRef]
- Boatright, J.; Negre, F.; Chen, X.; Kish, C.M.; Wood, B.; Peel, G.; Orlova, I.; Gang, D.; Rhodes, D.; Dudareva, N. Understanding in Vivo Benzenoid Metabolism in Petunia petal Tissue 1. Plant Physiol. 2011, 135, 1993–2011. [Google Scholar] [CrossRef]
- Jerković, I.; Cikoš, A.M.; Babić, S.; Čižmek, L.; Bojanić, K.; Aladić, K.; Ul’yanovskii, N.V.; Kosyakov, D.S.; Lebedev, A.T.; Čož-Rakovac, R.; et al. Bioprospecting of Less-Polar Constituents from Endemic Brown Macroalga Fucus virsoides J. Agardh from the Adriatic Sea and Targeted Antioxidant Effects in Vitro and in Vivo (Zebrafish Model). Mar. Drugs 2021, 19, 235. [Google Scholar] [CrossRef] [PubMed]
- Radman, S.; Cikoš, A.M.; Flanjak, I.; Babić, S.; Čižmek, L.; Šubarić, D.; Čož-Rakovac, R.; Jokić, S.; Jerković, I. Less Polar Compounds and Targeted Antioxidant Potential (In Vitro and in Vivo) of Codium adhaerens c. Agardh 1822. Pharmaceuticals 2021, 14, 944. [Google Scholar] [CrossRef]
- Radman, S.; Čižmek, L.; Babić, S.; Cikoš, A.M.; Čož-Rakovac, R.; Jokić, S.; Jerković, I. Bioprospecting of Less-Polar Fractions of Ericaria crinita and Ericaria amentacea: Developmental Toxicity and Antioxidant Activity. Mar. Drugs 2022, 20, 57. [Google Scholar] [CrossRef]
- Jerković, I.; Kranjac, M.; Marijanović, Z.; Roje, M.; Jokić, S. Chemical Diversity of Headspace and Volatile Oil Composition of Two Brown Algae (Taonia atomaria and Padina pavonica) from the Adriatic Sea. Molecules 2019, 24, 495. [Google Scholar] [CrossRef] [PubMed]
- Čagalj, M.; Radman, S.; Šimat, V.; Jerković, I. Detailed Chemical Prospecting of Volatile Organic Compounds Variations from Adriatic Macroalga Halopteris scoparia. Molecules 2022, 27, 4997. [Google Scholar] [CrossRef]
- Islam, M.T.; Ali, E.S.; Uddin, S.J.; Shaw, S.; Islam, M.A.; Ahmed, M.I.; Chandra Shill, M.; Karmakar, U.K.; Yarla, N.S.; Khan, I.N.; et al. Phytol: A Review of Biomedical Activities. Food Chem. Toxicol. 2018, 121, 82–94. [Google Scholar] [CrossRef]
- Radman, S.; Čagalj, M.; Šimat, V.; Jerković, I. Seasonal Variability of Volatilome from Dictyota dichotoma. Molecules 2022, 27, 3012. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.L.; Mao, X.X.; Du, Y.M.; Yan, P.Z.; Hou, X.D.; Zhang, Z.F. Anti-Tumor Activity of Cembranoid-Type Diterpenes Isolated from Nicotiana Tabacum L. Biomolecules 2019, 9, 45. [Google Scholar] [CrossRef] [PubMed]
- Siless, G.E.; García, M.; Pérez, M.; Blustein, G.; Palermo, J.A. Large-Scale Purification of Pachydictyol A from the Brown Alga Dictyota dichotoma Obtained from Algal Wash and Evaluation of Its Antifouling Activity against the Freshwater Mollusk Limnoperna Fortunei. J. Appl. Phycol. 2018, 30, 629–636. [Google Scholar] [CrossRef]
- Masyita, A.; Mustika Sari, R.; Dwi Astuti, A.; Yasir, B.; Rahma Rumata, N.; Bin Emran, T.; Nainu, F.; Simal-Gandara, J. Terpenes and Terpenoids as Main Bioactive Compounds of Essential Oils, Their Roles in Human Health and Potential Application as Natural Food Preservatives. Food Chem. X 2022, 13, 100217. [Google Scholar] [CrossRef]
- Herrera-Valencia, V.A.; Us-Vázquez, R.A.; Larqué-Saavedra, F.A.; Barahona-Pérez, L.F. Naturally Occurring Fatty Acid Methyl Esters and Ethyl Esters in the Green Microalga Chlamydomonas reinhardtii. Ann. Microbiol. 2012, 62, 865–870. [Google Scholar] [CrossRef]
- Yalçın, S.; Karakaş, Ö.; Okudan, E.Ş.; Kocaoba, S.; Apak, M.R. Comparative Spectrophotometric and Chromatographic Assessment of Antioxidant Capacity in Different Marine Algae. J. Aquat. Food Prod. Technol. 2023, 32, 81–94. [Google Scholar] [CrossRef]
- Mekinić, I.G.; Skroza, D.; Šimat, V.; Hamed, I.; Čagalj, M.; Perković, Z.P. Phenolic Content of Brown Algae (Pheophyceae) Species: Extraction, Identification, and Quantification. Biomolecules 2019, 9, 244. [Google Scholar] [CrossRef]
- Blaženović, I.; Kind, T.; Ji, J.; Fiehn, O. Software Tools and Approaches for Compound Identification of LC-MS/MS Data in Metabolomics. Metabolites 2018, 8, 31. [Google Scholar] [CrossRef]
- Radman, S.; Cikoš, A.M.; Babić, S.; Čižmek, L.; Čož-Rakovac, R.; Jokić, S.; Jerković, I. In Vivo and In Vitro Antioxidant Activity of Less Polar Fractions of Dasycladus vermicularis (Scopoli) Krasser 1898 and the Chemical Composition of Fractions and Macroalga Volatilome. Pharmaceuticals 2022, 15, 743. [Google Scholar] [CrossRef]
- Arafat, E.S.S.; Trimble, J.W.; Andersen, R.N.; Dass, C.; Desiderio, D.M. Identification of Fatty Acid Amides in Human Plasma. Life Sci. 1989, 45, 1679–1687. [Google Scholar] [CrossRef]
- Tanvir, R.; Javeed, A.; Rehman, Y. Fatty Acids and Their Amide Derivatives from Endophytes: New Therapeutic Possibilities from a Hidden Source. FEMS Microbiol. Lett. 2018, 365, fny114. [Google Scholar] [CrossRef]
- Farrell, E.K.; Chen, Y.; Barazanji, M.; Jeffries, K.A.; Cameroamortegui, F.; Merkler, D.J. Primary Fatty Acid Amide Metabolism: Conversion of Fatty Acids and an Ethanolamine in N 18TG 2 and SCP Cells. J. Lipid Res. 2012, 53, 247–256. [Google Scholar] [CrossRef]
- D’Oca, C.D.R.M.; Coelho, T.; Marinho, T.G.; Hack, C.R.L.; Da Costa Duarte, R.; Da Silva, P.A.; D’Oca, M.G.M. Synthesis and Antituberculosis Activity of New Fatty Acid Amides. Bioorganic Med. Chem. Lett. 2010, 20, 5255–5257. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Snowden, S.; Suvitaival, T.; Ali, A.; Merkler, D.J.; Ahmad, T.; Westwood, S.; Baird, A.; Proitsi, P.; Nevado-Holgado, A.; et al. Primary Fatty Amides in Plasma Associated with Brain Amyloid Burden, Hippocampal Volume, and Memory in the European Medical Information Framework for Alzheimer’s Disease Biomarker Discovery Cohort. Alzheimer’s Dement. 2019, 15, 817–827. [Google Scholar] [CrossRef] [PubMed]
- Lopes, G.; Daletos, G.; Proksch, P.; Andrade, P.B.; Valentão, P. Anti-Inflammatory Potential of Monogalactosyl Diacylglycerols and a Monoacylglycerol from the Edible Brown Seaweed Fucus spiralis Linnaeus. Mar. Drugs 2014, 12, 1406–1418. [Google Scholar] [CrossRef]
- Rosário Domingues, M.; Calado, R. Lipids of Marine Algae—Biomolecules with High Nutritional Value and Important Bioactive Properties. Biomolecules 2022, 12, 10–13. [Google Scholar] [CrossRef]
- Cepas, V.; Gutiérrez-Del-río, I.; López, Y.; Redondo-Blanco, S.; Gabasa, Y.; Iglesias, M.J.; Soengas, R.; Fernández-Lorenzo, A.; López-Ibáñez, S.; Villar, C.J.; et al. Microalgae and Cyanobacteria Strains as Producers of Lipids with Antibacterial and Antibiofilm Activity. Mar. Drugs 2021, 19, 675. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.S.; Wang, Z.G. Glyceroglycolipids in Marine Algae: A Review of Their Pharmacological Activity. Front. Pharmacol. 2022, 13, 8797. [Google Scholar] [CrossRef]
- Song, Y.; Zoong Lwe, Z.S.; Wickramasinghe, P.A.D.B.V.; Welti, R. Head-Group Acylation of Chloroplast Membrane Lipids. Molecules 2021, 26, 1273. [Google Scholar] [CrossRef]
- Imchen, T.; Singh, K.S. Marine Algae Colorants: Antioxidant, Anti-Diabetic Properties and Applications in Food Industry. Algal Res. 2023, 69, 102898. [Google Scholar] [CrossRef]
- Sanger, G.; Wonggo, D.; Montolalu, L.A.D.Y.; Dotulong, V. Pigments Constituents, Phenolic Content and Antioxidant Activity of Brown Seaweed Sargassum sp. IOP Conf. Ser. Earth Environ. Sci. 2022, 1033, 2057. [Google Scholar] [CrossRef]
- Saide, A.; Lauritano, C.; Ianora, A. Pheophorbide A: State of the Art. Mar. Drugs 2020, 18, 257. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.; Lee, H.S.; Kang, I.J.; Won, M.H.; You, S. Antioxidant Properties of Extract and Fractions from Enteromorpha prolifera, a Type of Green Seaweed. Food Chem. 2011, 127, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.G.; Otero, P.; Echave, J.; Carreira-Casais, A.; Chamorro, F.; Collazo, N.; Jaboui, A.; Lourenço-Lopes, C.; Simal-Gandara, J.; Prieto, M.A. Xanthophylls from the Sea: Algae as Source of Bioactive Carotenoids. Mar. Drugs 2021, 19, 188. [Google Scholar] [CrossRef]
- Muñoz-Miranda, L.A.; Iñiguez-Moreno, M. An Extensive Review of Marine Pigments: Sources, Biotechnological Applications, and Sustainability. Aquat. Sci. 2023, 85, 68. [Google Scholar] [CrossRef]
- El Hattab, M.; Culioli, G.; Valls, R.; Richou, M.; Piovetti, L. Apo-Fucoxanthinoids and Loliolide from the Brown Alga Cladostephus spongiosus f. verticillatus (Heterokonta, Sphacelariales). Biochem. Syst. Ecol. 2008, 36, 447–451. [Google Scholar] [CrossRef]
- van den Berg, T.E.; Croce, R. The Loroxanthin Cycle: A New Type of Xanthophyll Cycle in Green Algae (Chlorophyta). Front. Plant Sci. 2022, 13, 7294. [Google Scholar] [CrossRef]
- Galasso, C.; Corinaldesi, C.; Sansone, C. Carotenoids from Marine Organisms: Biological Functions and Industrial Applications. Antioxidants 2017, 6, 96. [Google Scholar] [CrossRef]
- Ibrahim, E.A.; El-Sayed, S.M.; Murad, S.A.; Abdallah, W.E.; El-Sayed, H.S. Evaluation of the Antioxidant and Antimicrobial Activities of Fucoxanthin from Dilophys fasciola and as a Food Additive in Stirred Yoghurt. S. Afr. J. Sci. 2023, 119, 13722. [Google Scholar] [CrossRef]
- Nishino, H.; Tsushima, M.; Matsuno, T.; Tanaka, Y.; Okuzumi, J.; Murakoshi, M.; Satomi, Y.; Takayasu, J.; Tokuda, H.; Nishino, A.; et al. Anti-Neoplastic Effect of Halocynthiaxanthin, a Metabolite of Fucoxanthin. Anticancer. Drugs 1992, 3, 493–498. [Google Scholar] [CrossRef]
- Sansone, C.; Galasso, C.; Orefice, I.; Nuzzo, G.; Luongo, E.; Cutignano, A.; Romano, G.; Brunet, C.; Fontana, A.; Esposito, F.; et al. The Green Microalga Tetraselmis suecica Reduces Oxidative Stress and Induces Repairing Mechanisms in Human Cells. Sci. Rep. 2017, 7, 1215. [Google Scholar] [CrossRef]
- Vladić, J.; Jerković, I.; Radman, S.; Jazić, J.M.; Ferreira, A.; Maletić, S.; Gouveia, L. Supercritical CO2 Extract from Microalga Tetradesmus obliquus: The Effect of High-Pressure Pre-Treatment. Molecules 2022, 27, 3883. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.; Alves, C.; Martins, A.; Susano, P.; Simões, M.; Guedes, M.; Rehfeldt, S.; Pinteus, S.; Gaspar, H.; Rodrigues, A.; et al. Loliolide, a New Therapeutic Option for Neurological Diseases? In Vitro Neuroprotective and Anti-Inflammatory Activities of a Monoterpenoid Lactone Isolated from Codium tomentosum. Int. J. Mol. Sci. 2021, 22, 1888. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Granato, D.; Shahidi, F.; Wrolstad, R.; Kilmartin, P.; Melton, L.D.; Hidalgo, F.J.; Miyashita, K.; van Camp, J.; Alasalvar, C.; Ismail, A.B.; et al. Antioxidant Activity, Total Phenolics and Flavonoids Contents: Should We Ban in Vitro Screening Methods? Food Chem. 2018, 264, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Milat, A.M.; Boban, M.; Teissedre, P.L.; Šešelja-Perišin, A.; Jurić, D.; Skroza, D.; Generalić-Mekinić, I.; Ljubenkov, I.; Volarević, J.; Rasines-Perea, Z.; et al. Effects of Oxidation and Browning of Macerated White Wine on Its Antioxidant and Direct Vasodilatory Activity. J. Funct. Foods 2019, 59, 138–147. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).