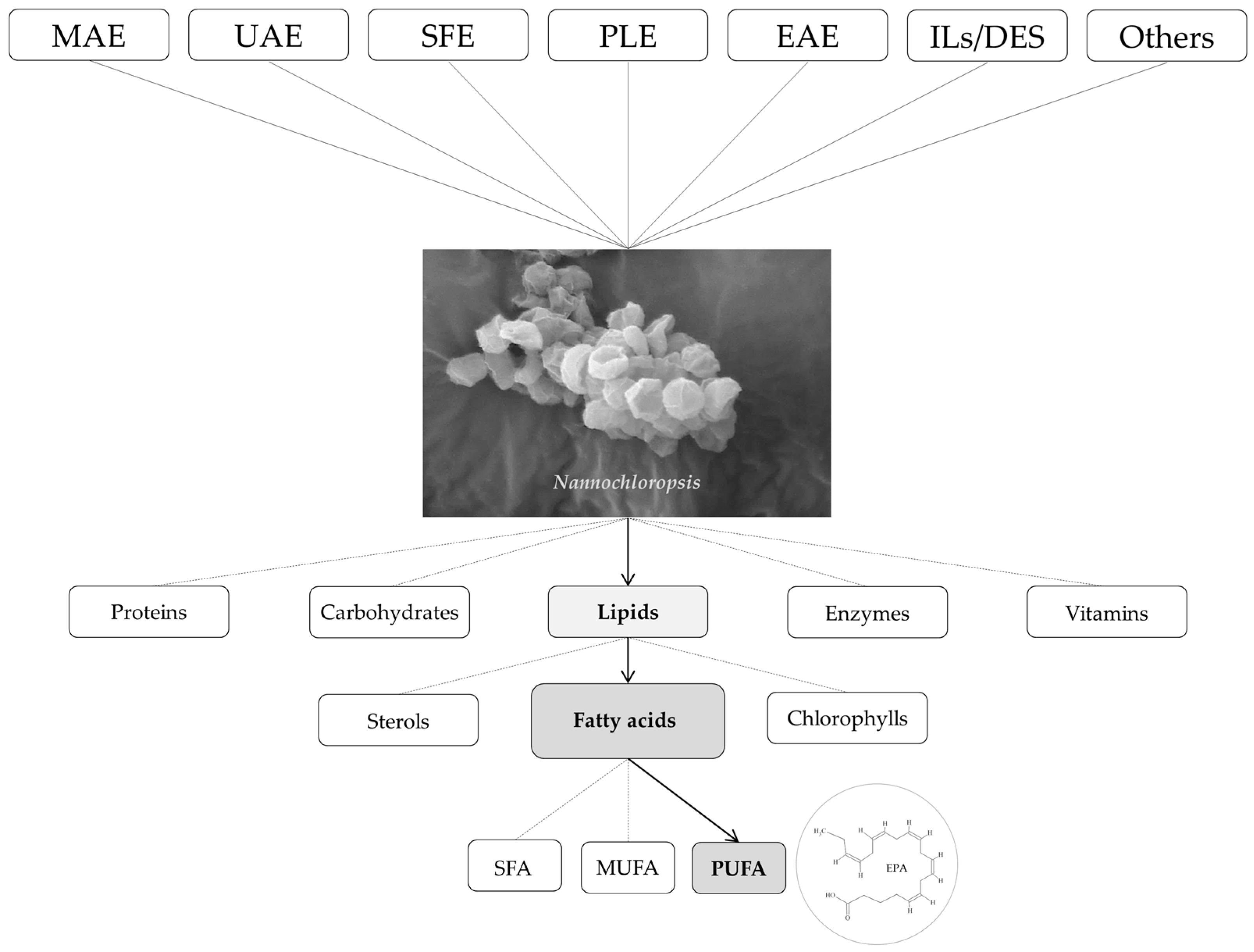
Extraction of Nannochloropsis Fatty Acids Using Different Green Technologies: The Current Path
Abstract
1. Introduction
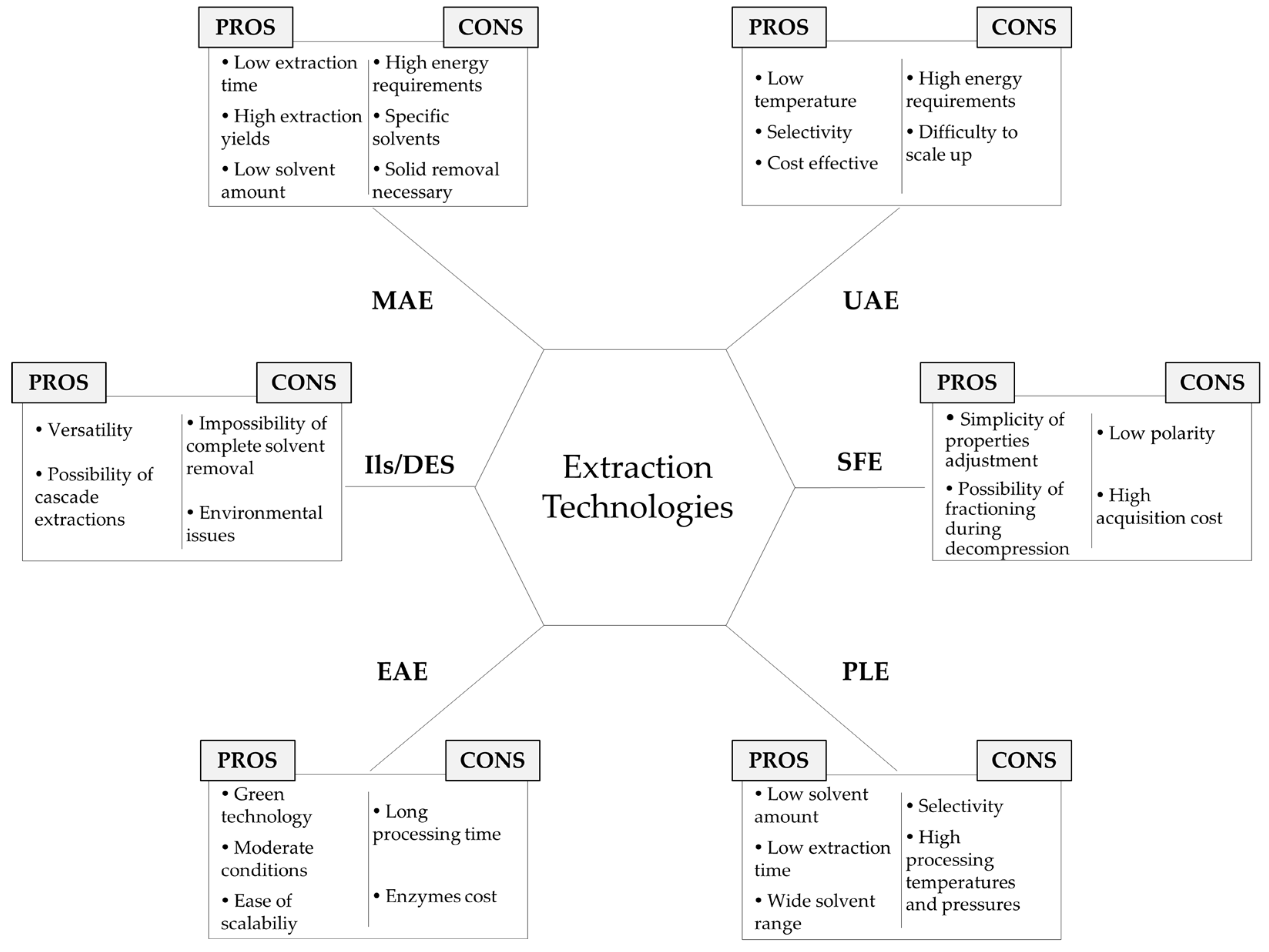
2. Extraction Technologies
2.1. Microwave-Assisted Extraction (MAE)
2.2. Ultrasound-Assisted Extraction (UAE)
| US/other | Species | Solvent | Operational Conditions | Yield | References | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lipid | Fatty Acids | |||||||||||
| Used | Tested | Optimum | SFA | MUFA | PUFA | Omega-3 | EPA | |||||
| US | N. gaditana | CHCl3:MeOH (2:1) | 130 W; 20 kHz | 50–80% amplitude; 10–30 min | 80% amplitude; 30 min | 21.7% (dw) | ― | ― | ― | ― | ― | [29] |
| N. oceanica | 45.4% (dw) | ― | ― | ― | ― | ― | ||||||
| US + Switchable hydrophilicity solvent | N. oceanica | TEPDA | 0.5 W/mL, 20 kHz, room temperature | 30–180 min | 120 min | 98.2% (wet weight) | ― | ― | ― | ― | ― | [27] |
| US + Celluclast + Viscozyme | N. gaditana | ― | 140 W; 37 kHz | 2–6 h (ultrasounds)/pH 4–8; 35–55 °C (enzymatic pretreatment) | 55 °C, pH 5.0, 6 h | 24.2% | ― | ― | ― | ― | ― | [50] |
| US + Celluclast + Viscozyme + Alcalase | 28.9% | ― | ― | ― | ― | ― | ||||||
| US | N. oceanica | EtOH | 130 W; 20 kHz; 70% amplitude; 8 min | Ultrasound probe; ultrasound bath | Ultrasound probe | 60.3% (extract dw) | 27.4 mg g−1 (dw) | 24.6 mg g−1 (dw) | 21.8 mg g−1 (dw) | ― | 15.4 mg g−1 (dw) | [8] |
| US + Low-temperature hydrothermal liquefaction | Nannochloropsis sp. | Dichloromethane | 100 W; 60 min | 30–90 s (sonication); 210–250 °C (HTL) | 90-s sonication time, 250 °C | 28.9% (dw) | ― | ― | ― | ― | ― | [51] |
| ― | N. gaditana | 2-MeTHF:EtOH (1:3) | 37 kHz; 50 °C; 30 min | EtOH; 2-MeTHF; hexane:EtOH (3:4); 2-MeTHF:EtOH (1:1, 1:2, 1:3, and 1:4) | 2-MeTHF:ethanol (1:3) | 16.3% (dw) | ― | ― | ― | ― | ― | [23] |
| US + MW | Nannochloropsis sp. | MeOH | ― | MeOH (10–30 mL); Ultrasounds and Microwaves Power (100–140 W); reaction time (3–7 min) | 30 mL MeOH, 140 W (microwaves), 140 W (ultrasounds), 7 min | 22.8% (dw) | 66.5% (TFA) | 20.7 (TFA) | 12.7% (TFA) | ― | ― | [41] |
2.3. Supercritical Fluid Extraction (SFE)
2.4. Pressurized Liquid Extraction (PLE)
2.5. Enzyme-Assisted Extraction (EAE)
2.6. Ionic Liquids (ILs) and Deep Eutectic Solvents (DES)
2.7. Others
3. Future Trends
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Jesus Raposo, M.F.; Morais, R.M.S.C.; Morais, A.M.M.B. Health Applications of Bioactive Compounds from Marine Microalgae. Life Sci. 2013, 93, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Zanella, L.; Vianello, F. Microalgae of the Genus Nannochloropsis: Chemical Composition and Functional Implications for Human Nutrition. J. Funct. Foods 2020, 68, 103919. [Google Scholar] [CrossRef]
- Raj, T.; Morya, R.; Chandrasekhar, K.; Kumar, D.; Soam, S.; Kumar, R.; Patel, A.K.; Kim, S.H. Microalgae Biomass Deconstruction Using Green Solvents: Challenges and Future Opportunities. Bioresour. Technol. 2023, 369, 128429. [Google Scholar] [CrossRef]
- Cuellar-Bermudez, S.P.; Aguilar-Hernandez, I.; Cardenas-Chavez, D.L.; Ornelas-Soto, N.; Romero-Ogawa, M.A.; Parra-Saldivar, R. Extraction and Purification of High-Value Metabolites from Microalgae: Essential Lipids, Astaxanthin and Phycobiliproteins. Microb. Biotechnol. 2015, 8, 190–209. [Google Scholar] [CrossRef] [PubMed]
- Chua, E.T.; Schenk, P.M. A Biorefinery for Nannochloropsis: Induction, Harvesting, and Extraction of EPA-Rich Oil and High-Value Protein. Bioresour. Technol. 2017, 244, 1416–1424. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Marchal, L.; Lebovka, N.; Vorobiev, E.; Grimi, N. Two-Step Procedure for Selective Recovery of Bio-Molecules from Microalga Nannochloropsis oculata Assisted by High Voltage Electrical Discharges. Bioresour. Technol. 2020, 302, 122893. [Google Scholar] [CrossRef] [PubMed]
- Chua, E.T.; Brunner, M.; Atkin, R.; Eltanahy, E.; Thomas-Hall, S.R.; Schenk, P.M. The Ionic Liquid Cholinium Arginate Is an Efficient Solvent for Extracting High-Value Nannochloropsis sp. Lipids. ACS Sustain. Chem. Eng. 2019, 7, 2538–2544. [Google Scholar] [CrossRef]
- Figueiredo, A.R.P.; da Costa, E.; Silva, J.; Domingues, M.R.; Domingues, P. The Effects of Different Extraction Methods of Lipids from Nannochloropsis oceanica on the Contents of Omega-3 Fatty Acids. Algal Res. 2019, 41, 101556. [Google Scholar] [CrossRef]
- Wang, X.; Fosse, H.K.; Li, K.; Chauton, M.S.; Vadstein, O.; Reitan, K.I. Influence of Nitrogen Limitation on Lipid Accumulation and EPA and DHA Content in Four Marine Microalgae for Possible Use in Aquafeed. Front. Mar. Sci. 2019, 6, 95. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kwon, Y.M.; Kim, K.W.; Kim, J.Y.H. Exploring the Potential of Nannochloropsis sp. Extract for Cosmeceutical Applications. Mar. Drugs 2021, 19, 690. [Google Scholar] [CrossRef]
- Castejón, N.; Señoráns, F.J. Simultaneous Extraction and Fractionation of Omega-3 Acylglycerols and Glycolipids from Wet Microalgal Biomass of Nannochloropsis gaditana Using Pressurized Liquids. Algal Res. 2019, 37, 74–82. [Google Scholar] [CrossRef]
- Jiménez Callejón, M.J.; Robles Medina, A.; Macías Sánchez, M.D.; González Moreno, P.A.; Navarro López, E.; Esteban Cerdán, L.; Molina Grima, E. Supercritical Fluid Extraction and Pressurized Liquid Extraction Processes Applied to Eicosapentaenoic Acid-Rich Polar Lipid Recovery from the Microalga Nannochloropsis sp. Algal Res. 2022, 61, 102586. [Google Scholar] [CrossRef]
- Brennan, B.; Regan, F. In-Situ Lipid and Fatty Acid Extraction Methods to Recover Viable Products from Nannochloropsis sp. Sci. Total Environ. 2020, 748, 142464. [Google Scholar] [CrossRef] [PubMed]
- Jiménez Callejón, M.J.; Robles Medina, A.; González Moreno, P.A.; Esteban Cerdán, L.; Orta Guillén, S.; Molina Grima, E. Simultaneous Extraction and Fractionation of Lipids from the Microalga Nannochloropsis sp. for the Production of EPA-Rich Polar Lipid Concentrates. J. Appl. Phycol. 2020, 32, 1117–1128. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, D.; Xue, S.; Wang, M.; Cong, W. The Potential of Microalgae Lipids for Edible Oil Production. Appl. Biochem. Biotechnol. 2016, 180, 438–451. [Google Scholar] [CrossRef] [PubMed]
- Servaes, K.; Maesen, M.; Prandi, B.; Sforza, S.; Elst, K. Polar Lipid Profile of Nannochloropsis oculata Determined Using a Variety of Lipid Extraction Procedures. J. Agric. Food Chem. 2015, 63, 3931–3941. [Google Scholar] [CrossRef]
- Gomes, T.A.; Zanette, C.M.; Spier, M.R. An Overview of Cell Disruption Methods for Intracellular Biomolecules Recovery. Prep. Biochem. Biotechnol. 2020, 50, 635–654. [Google Scholar] [CrossRef]
- Michalak, I.; Chojnacka, K. Algal Extracts: Technology and Advances. Eng. Life Sci. 2014, 14, 581–591. [Google Scholar] [CrossRef]
- Sales, R.; del Carmen Cerón-García, M.; Navarro-López, E.; González-López, C.V.; Tsuzuki, M.Y.; Acién-Fernández, F.G.; Alarcón-López, F.J.; Molina-Grima, E. Processing Nannochloropsis gaditana Biomass for the Extraction of High-Value Biocompounds. J. Appl. Phycol. 2020, 32, 3113–3122. [Google Scholar] [CrossRef]
- Zhao, K.; Zhang, L.; Zhang, M.; Tian, H.; He, D.; Zheng, J. Response Surface Optimization of Enzyme Pretreatment Improves Yield of Ethanol-Extracted Lipids from Nannochloropsis oceanica. Eur. J. Lipid Sci. Technol. 2022, 124, 2100200. [Google Scholar] [CrossRef]
- Jiménez Callejón, M.J.; Robles Medina, A.; Macías Sánchez, M.D.; Esteban Cerdán, L.; González Moreno, P.A.; Navarro López, E.; Hita Peña, E.; Grima, E.M. Obtaining Highly Pure EPA-Rich Lipids from Dry and Wet Nannochloropsis gaditana Microalgal Biomass Using Ethanol, Hexane and Acetone. Algal Res. 2020, 45, 101729. [Google Scholar] [CrossRef]
- Sánchez-Camargo, A.d.P.; Pleite, N.; Mendiola, J.A.; Cifuentes, A.; Herrero, M.; Gilbert-López, B.; Ibáñez, E. Development of Green Extraction Processes for Nannochloropsis gaditana Biomass Valorization. Electrophoresis 2018, 39, 1875–1883. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Llamero, C.; Señoráns, F.J. Biobased Solvents for Pressurized Liquid Extraction of Nannochloropsis gaditana Omega-3 Lipids. Mar. Drugs 2021, 19, 107. [Google Scholar] [CrossRef] [PubMed]
- Alhattab, M.; Kermanshahi-Pour, A.; Brooks, M.S.L. Microalgae Disruption Techniques for Product Recovery: Influence of Cell Wall Composition. J. Appl. Phycol. 2019, 31, 61–88. [Google Scholar] [CrossRef]
- Ho, B.C.H.; Kamal, S.M.M.; Danquah, M.K.; Harun, R. Optimization of Subcritical Water Extraction (SWE) of Lipid and Eicosapentaenoic Acid (EPA) from Nannochloropsis gaditana. BioMed Res. Int. 2018, 2018, 8273581. [Google Scholar] [CrossRef]
- Kröger, M.; Klemm, M.; Nelles, M. Hydrothermal Disintegration and Extraction of Different Microalgae Species. Energies 2018, 11, 450. [Google Scholar] [CrossRef]
- Guo, H.; Cheng, J.; Mao, Y.; Qian, L.; Yang, W.; Park, J.Y. Synergistic Effect of Ultrasound and Switchable Hydrophilicity Solvent Promotes Microalgal Cell Disruption and Lipid Extraction for Biodiesel Production. Bioresour. Technol. 2022, 343, 126087. [Google Scholar] [CrossRef] [PubMed]
- Rezaei Motlagh, S.; Harun, R.; Awang Biak, D.R.; Hussain, S.A.; Omar, R.; Khezri, R.; Elgharbawy, A.A. Ionic Liquid-Based Microwave-Assisted Extraction of Lipid and Eicosapentaenoic Acid from Nannochloropsis oceanica Biomass: Experimental Optimization Approach. J. Appl. Phycol. 2021, 33, 2015–2029. [Google Scholar] [CrossRef]
- Quesada-Salas, M.C.; Delfau--bonnet, G.; Willig, G.; Préat, N.; Allais, F.; Ioannou, I. Optimization and Comparison of Three Cell Disruption Processes on Lipid Extraction from Microalgae. Processes 2021, 9, 369. [Google Scholar] [CrossRef]
- Askari, M.; Jafari, A.; Esmaeilzadeh, F.; Khorram, M.; Mohammadi, A.H. Kinetic Study on Nannochloropsis oculata’s Lipid Extraction Using Supercritical CO2 and n-Hexane for Biodiesel Production. ACS Omega 2022, 7, 23027–23040. [Google Scholar] [CrossRef]
- Eikani, M.H.; Khandan, N.; Feyzi, E. Increased Bio-Oil Yield from Nannochloropsis salina through Tuning the Polarity of Subcritical Water. J. Mol. Liq. 2019, 277, 163–169. [Google Scholar] [CrossRef]
- Gallego, R.; Bueno, M.; Chourio, A.M.; Ibáñez, E.; Saldaña, M.D.A.; Herrero, M. Use of High and Ultra-High Pressure Based-Processes for the Effective Recovery of Bioactive Compounds from Nannochloropsis oceanica Microalgae. J. Supercrit. Fluids 2021, 167, 105039. [Google Scholar] [CrossRef]
- He, Y.; Wang, X.; Wei, H.; Zhang, J.; Chen, B.; Chen, F. Direct Enzymatic Ethanolysis of Potential Nannochloropsis Biomass for Co-Production of Sustainable Biodiesel and Nutraceutical Eicosapentaenoic Acid. Biotechnol. Biofuels 2019, 12, 78. [Google Scholar] [CrossRef] [PubMed]
- Angles, E.; Jaouen, P.; Pruvost, J.; Marchal, L. Wet Lipid Extraction from the Microalga Nannochloropsis sp.: Disruption, Physiological Effects and Solvent Screening. Algal Res. 2017, 21, 27–34. [Google Scholar] [CrossRef]
- Qiu, Y.; Frear, C.; Chen, S.; Ndegwa, P.; Harrison, J.; Yao, Y.; Ma, J. Accumulation of Long-Chain Fatty Acids from Nannochloropsis salina Enhanced by Breaking Microalgae Cell Wall under Alkaline Digestion. Renew. Energy 2020, 149, 691–700. [Google Scholar] [CrossRef]
- Gkioni, M.D.; Andriopoulos, V.; Koutra, E.; Hatziantoniou, S.; Kornaros, M.; Lamari, F.N. Ultrasound-Assisted Extraction of Nannochloropsis oculata with Ethanol and Betaine: 1,2-Propanediol Eutectic Solvent for Antioxidant Pigment-Rich Extracts Retaining Nutritious the Residual Biomass. Antioxidants 2022, 11, 1103. [Google Scholar] [CrossRef]
- Pan, J.; Muppaneni, T.; Sun, Y.; Reddy, H.K.; Fu, J.; Lu, X.; Deng, S. Microwave-Assisted Extraction of Lipids from Microalgae Using an Ionic Liquid Solvent [BMIM][HSO4]. Fuel 2016, 178, 49–55. [Google Scholar] [CrossRef]
- Teo, C.L.; Idris, A. Enhancing the Various Solvent Extraction Method via Microwave Irradiation for Extraction of Lipids from Marine Microalgae in Biodiesel Production. Bioresour. Technol. 2014, 171, 477–481. [Google Scholar] [CrossRef]
- Mubarak, M.; Shaija, A.; Suchithra, T.V. A Review on the Extraction of Lipid from Microalgae for Biodiesel Production. Algal Res. 2015, 7, 117–123. [Google Scholar] [CrossRef]
- Zghaibi, N.; Omar, R.; Kamal, S.M.M.; Biak, D.R.A.; Harun, R. Kinetics Study of Microwave-Assisted Brine Extraction of Lipid from the Microalgae Nannochloropsis sp. Molecules 2020, 25, 784. [Google Scholar] [CrossRef]
- Martinez-Guerra, E.; Howlader, M.S.; Shields-Menard, S.; French, W.T.; Gude, V.G. Optimization of Wet Microalgal FAME Production from Nannochloropsis sp. under the Synergistic Microwave and Ultrasound Effect. Int. J. Energy Res. 2018, 42, 1934–1949. [Google Scholar] [CrossRef]
- Wahidin, S.; Idris, A.; Yusof, N.M.; Kamis, N.H.H.; Shaleh, S.R.M. Optimization of the Ionic Liquid-Microwave Assisted One-Step Biodiesel Production Process from Wet Microalgal Biomass. Energy Convers. Manag. 2018, 171, 1397–1404. [Google Scholar] [CrossRef]
- Motlagh, S.R.; Khezri, R.; Harun, R.; Radiah, D.; Biak, A.; Hussain, S.A.; Chee, C.Y.; Kheawhom, S. Kinetic and Thermodynamic Studies of Eicosapentaenoic Acid Extraction from Nannochloropsis oceanica Using Tetramethyl Ammonium Chloride and Microwave Irradiation. PLoS ONE 2022, 17, e0267626. [Google Scholar] [CrossRef] [PubMed]
- Zghaibi, N.; Omar, R.; Kamal, S.M.M.; Biak, D.R.A.; Harun, R. Microwave-Assisted Brine Extraction for Enhancement of the Quantity and Quality of Lipid Production from Microalgae Nannochloropsis sp. Molecules 2019, 24, 3581. [Google Scholar] [CrossRef]
- De Moura, R.R.; Etges, B.J.; dos Santos, E.O.; Martins, T.G.; Roselet, F.; Abreu, P.C.; Primel, E.G.; D’Oca, M.G.M. Microwave-Assisted Extraction of Lipids from Wet Microalgae Paste: A Quick and Efficient Method. Eur. J. Lipid Sci. Technol. 2018, 120, 1700419. [Google Scholar] [CrossRef]
- Han, F.; Pei, H.; Hu, W.; Zhang, S.; Han, L.; Ma, G. The Feasibility of Ultrasonic Stimulation on Microalgae for Efficient Lipid Accumulation at the End of the Logarithmic Phase. Algal Res. 2016, 16, 189–194. [Google Scholar] [CrossRef]
- Adam, F.; Abert-Vian, M.; Peltier, G.; Chemat, F. “Solvent-Free” Ultrasound-Assisted Extraction of Lipids from Fresh Microalgae Cells: A Green, Clean and Scalable Process. Bioresour. Technol. 2012, 114, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Parniakov, O.; Apicella, E.; Koubaa, M.; Barba, F.J.; Grimi, N.; Lebovka, N.; Pataro, G.; Ferrari, G.; Vorobiev, E. Ultrasound-Assisted Green Solvent Extraction of High-Added Value Compounds from Microalgae Nannochloropsis spp. Bioresour. Technol. 2015, 198, 262–267. [Google Scholar] [CrossRef]
- Bligh, E.; Dyer, W. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Llamero, C.; García-García, P.; Señoráns, F.J. Combination of Synergic Enzymes and Ultrasounds as an Effective Pretreatment Process to Break Microalgal Cell Wall and Enhance Algal Oil Extraction. Foods 2021, 10, 1928. [Google Scholar] [CrossRef] [PubMed]
- Saber, M.; Golzary, A.; Wu, H.; Takahashi, F.; Yoshikawa, K. Ultrasonic Pretreatment for Low-Temperature Hydrothermal Liquefaction of Microalgae: Enhancing the Bio-Oil Yield and Heating Value. Biomass Convers. Biorefin. 2018, 8, 509–519. [Google Scholar] [CrossRef]
- Herrero, M.; Sánchez-Camargo, A.d.P.; Cifuentes, A.; Ibáñez, E. Plants, Seaweeds, Microalgae and Food by-Products as Natural Sources of Functional Ingredients Obtained Using Pressurized Liquid Extraction and Supercritical Fluid Extraction. TrAC Trends Anal. Chem. 2015, 71, 26–38. [Google Scholar] [CrossRef]
- Leone, G.P.; Balducchi, R.; Mehariya, S.; Martino, M.; Larocca, V.; Di Sanzo, G.; Iovine, A.; Casella, P.; Marino, T.; Karatza, D.; et al. Selective Extraction of ω-3 Fatty Acids from Nannochloropsis sp. Using Supercritical CO2 Extraction. Molecules 2019, 24, 2406. [Google Scholar] [CrossRef] [PubMed]
- Jafari, A.; Esmaeilzadeh, F.; Mowla, D.; Sadatshojaei, E.; Heidari, S.; Wood, D.A. New Insights to Direct Conversion of Wet Microalgae Impregnated with Ethanol to Biodiesel Exploiting Extraction with Supercritical Carbon Dioxide. Fuel 2021, 285, 119199. [Google Scholar] [CrossRef]
- Mouahid, A.; Seengeon, K.; Martino, M.; Crampon, C.; Kramer, A.; Badens, E. Selective Extraction of Neutral Lipids and Pigments from Nannochloropsis salina and Nannochloropsis maritima Using Supercritical CO2 Extraction: Effects of Process Parameters and Pre-Treatment. J. Supercrit. Fluids 2020, 165, 104934. [Google Scholar] [CrossRef]
- Molino, A.; Martino, M.; Larocca, V.; Di Sanzo, G.; Spagnoletta, A.; Marino, T.; Karatza, D.; Iovine, A.; Mehariya, S.; Musmarra, D. Eicosapentaenoic Acid Extraction from Nannochloropsis gaditana Using Carbon Dioxide at Supercritical Conditions. Mar. Drugs 2019, 17, 132. [Google Scholar] [CrossRef] [PubMed]
- Obeid, S.; Beaufils, N.; Camy, S.; Takache, H.; Ismail, A.; Pontalier, P.Y. Supercritical Carbon Dioxide Extraction and Fractionation of Lipids from Freeze-Dried Microalgae Nannochloropsis oculata and Chlorella vulgaris. Algal Res. 2018, 34, 49–56. [Google Scholar] [CrossRef]
- Eikani, M.H.; Khandan, N.; Feyzi, E.; Ebrahimi, I.M. A Shrinking Core Model for Nannochloropsis salina Oil Extraction Using Subcritical Water. Renew. Energy 2019, 131, 660–666. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A Simple Method for the Isolation and Purification of Total Lipides from Animal Tissues. J. Biol. Chem. 1957, 55, 497–509. [Google Scholar] [CrossRef]
- Maffei, G.; Bracciale, M.P.; Broggi, A.; Zuorro, A.; Santarelli, M.L.; Lavecchia, R. Effect of an Enzymatic Treatment with Cellulase and Mannanase on the Structural Properties of Nannochloropsis Microalgae. Bioresour. Technol. 2018, 249, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Zuorro, A.; Maffei, G.; Lavecchia, R. Optimization of Enzyme-Assisted Lipid Extraction from Nannochloropsis Microalgae. J. Taiwan Inst. Chem. Eng. 2016, 67, 106–114. [Google Scholar] [CrossRef]
- Kumar, R.R.; Rao, P.H.; Arumugam, M. Lipid Extraction Methods from Microalgae: A Comprehensive Review. Front. Energy Res. 2015, 3, 61. [Google Scholar] [CrossRef]
- Qiu, C.; He, Y.; Huang, Z.; Li, S.; Huang, J.; Wang, M.; Chen, B. Lipid Extraction from Wet Nannochloropsis Biomass via Enzyme-Assisted Three Phase Partitioning. Bioresour. Technol. 2019, 284, 381–390. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhang, B.; Guo, S.; Guo, Z.; Chen, B.; Wang, M. Sustainable Biodiesel Production from the Green Microalgae Nannochloropsis: Novel Integrated Processes from Cultivation to Enzyme-Assisted Extraction and Ethanolysis of Lipids. Energy Convers. Manag. 2020, 209, 112618. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, M.; Saraiva, J.A.; Martins, A.P.; Pinto, C.A.; Prieto, M.A.; Simal-Gandara, J.; Cao, H.; Xiao, J.; Barba, F.J. Extraction of Lipids from Microalgae Using Classical and Innovative Approaches. Food Chem. 2022, 384, 132236. [Google Scholar] [CrossRef]
- Imbimbo, P.; D’Elia, L.; Liberti, D.; Olivieri, G.; Monti, D.M. Towards Green Extraction Methods from Microalgae Learning from the Classics. Appl. Microbiol. Biotechnol. 2020, 104, 9067–9077. [Google Scholar] [CrossRef] [PubMed]
- Orr, V.C.A.; Rehmann, L. Ionic Liquids for the Fractionation of Microalgae Biomass. Curr. Opin. Green Sustain. Chem. 2016, 2, 22–27. [Google Scholar] [CrossRef]
- Tripathy, D.B.; Gupta, A.; Mishra, A.; Quraishi, M.A.; Luqman, M.; Farhan Khan, M. Chapter 6—Switchable Solvents as Alternative Solvents for Green Chemistry. In Green Sustainable Process for Chemical and Environmental Engineering and Science; Inamuddin, Boddula, R., Asiri, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 109–131. ISBN 978-0-12-819850-6. [Google Scholar]
- Shankar, M.; Chhotaray, P.K.; Gardas, R.L.; Tamilarasan, K.; Rajesh, M. Application of Carboxylate Protic Ionic Liquids in Simultaneous Microalgal Pretreatment and Lipid Recovery from Marine Nannochloropsis sp. and Chlorella sp. Biomass Bioenergy 2019, 123, 14–24. [Google Scholar] [CrossRef]
- Mehariya, S.; Fratini, F.; Lavecchia, R.; Zuorro, A. Green Extraction of Value-Added Compounds Form Microalgae: A Short Review on Natural Deep Eutectic Solvents (NaDES) and Related Pre-Treatments. J. Environ. Chem. Eng. 2021, 9, 105989. [Google Scholar] [CrossRef]
- Cai, C.; Chen, X.; Li, F.; Tan, Z. Three-Phase Partitioning Based on CO2-Responsive Deep Eutectic Solvents for the Green and Sustainable Extraction of Lipid from Nannochloropsis sp. Sep. Purif. Technol. 2021, 279, 119685. [Google Scholar] [CrossRef]
- Rezaei Motlagh, S.; Harun, R.; Biak, D.R.A.; Hussain, S.A. Microwave Assisted Extraction of Lipid from Nannochloropsis gaditana Microalgae Using [EMIM]Cl. IOP Conf. Ser. Mater. Sci. Eng. 2020, 778, 012164. [Google Scholar] [CrossRef]
- Wang, M.; Cheng, H.; Chen, S.; Wen, S.; Wu, X.; Zhang, D.; Yuan, Q.; Cong, W. Microalgal Cell Disruption via Extrusion for the Production of Intracellular Valuables. Energy 2018, 142, 339–345. [Google Scholar] [CrossRef]
- Park, J.Y.; Kim, M.C.; Cheng, J.; Yang, W.; Kim, D.K. Extraction of Microalgal Oil from Nannochloropsis oceanica by Potassium Hydroxide-Assisted Solvent Extraction for Heterogeneous Transesterification. Renew. Energy 2020, 162, 2056–2065. [Google Scholar] [CrossRef]
- Halim, R.; Papachristou, I.; Kubisch, C.; Nazarova, N.; Wüstner, R.; Steinbach, D.; Chen, G.Q.; Deng, H.; Frey, W.; Posten, C.; et al. Hypotonic Osmotic Shock Treatment to Enhance Lipid and Protein Recoveries from Concentrated Saltwater Nannochloropsis slurries. Fuel 2021, 287, 119442. [Google Scholar] [CrossRef]
- Lorente, E.; Hapońska, M.; Clavero, E.; Torras, C.; Salvadó, J. Steam Explosion and Vibrating Membrane Filtration to Improve the Processing Cost of Microalgae Cell Disruption and Fractionation. Processes 2018, 6, 28. [Google Scholar] [CrossRef]
- Matos, Â.P.; Teixeira, M.S.; Corrêa, F.M.P.S.; Machado, M.M.; Werner, R.I.S.; Aguiar, A.C.; Cubas, A.L.V.; Sant’Anna, E.S.; Moecke, E.H.S. Disruption of Nannochloropsis gaditana (Eustigmatophyceae) Rigid Cell Wall by Non-Thermal Plasma Prior to Lipid Extraction and Its Effect on Fatty Acid Composition. Braz. J. Chem. Eng. 2019, 36, 1419–1428. [Google Scholar] [CrossRef]
- Setyawan, M.; Budiman, A.; Mulyono, P. Sutijan Optimum Extraction of Algae-Oil from Microalgae Using Hydrodynamic Cavitation. Int. J. Renew. Energy Res. 2018, 8, 451–458. [Google Scholar] [CrossRef]
- Teymouri, A.; Adams, K.J.; Dong, T.; Kumar, S. Evaluation of Lipid Extractability after Flash Hydrolysis of Algae. Fuel 2018, 224, 23–31. [Google Scholar] [CrossRef]
- Kwak, M.; Kim, D.; Kim, S.; Lee, H.; Chang, Y.K. Solvent Screening and Process Optimization for High Shear-Assisted Lipid Extraction from Wet Cake of Nannochloropsis sp. Renew. Energy 2020, 149, 1395–1405. [Google Scholar] [CrossRef]
- Bensalem, S.; Lopes, F.; Bodénès, P.; Pareau, D.; Français, O.; Le Pioufle, B. Understanding the Mechanisms of Lipid Extraction from Microalga Chlamydomonas reinhardtii after Electrical Field Solicitations and Mechanical Stress within a Microfluidic Device. Bioresour. Technol. 2018, 257, 129–136. [Google Scholar] [CrossRef]
- Silve, A.; Papachristou, I.; Wüstner, R.; Sträßner, R.; Schirmer, M.; Leber, K.; Guo, B.; Interrante, L.; Posten, C.; Frey, W. Extraction of Lipids from Wet Microalga Auxenochlorella protothecoides Using Pulsed Electric Field Treatment and Ethanol-Hexane Blends. Algal Res. 2018, 29, 212–222. [Google Scholar] [CrossRef]
- Zbinden, M.D.A.; Sturm, B.S.M.; Nord, R.D.; Carey, W.J.; Moore, D.; Shinogle, H.; Stagg-Williams, S.M. Pulsed Electric Field (PEF) as an Intensification Pretreatment for Greener Solvent Lipid Extraction from Microalgae. Biotechnol. Bioeng. 2013, 110, 1605–1615. [Google Scholar] [CrossRef]
- Pagels, F.; Pereira, R.N.; Amaro, H.M.; Vasconcelos, V.; Guedes, A.C.; Vicente, A.A. Continuous Pressurized Extraction versus Electric Fields-Assisted Extraction of Cyanobacterial Pigments. J. Biotechnol. 2021, 334, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Parniakov, O.; Barba, F.J.; Grimi, N.; Marchal, L.; Jubeau, S.; Lebovka, N.; Vorobiev, E. Pulsed Electric Field and PH Assisted Selective Extraction of Intracellular Components from Microalgae Nannochloropsis. Algal Res. 2015, 8, 128–134. [Google Scholar] [CrossRef]
- Grimi, N.; Dubois, A.; Marchal, L.; Jubeau, S.; Lebovka, N.I.; Vorobiev, E. Selective Extraction from Microalgae Nannochloropsis sp. Using Different Methods of Cell Disruption. Bioresour. Technol. 2014, 153, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Kojima, Y.; Shimizu, A. Effect of High Hydrostatic Pressure Treatment with Room-Temperature Ionic Liquid 1-Ethyl-3-Methylimidazolium Acetate-Dimethyl Sulfoxide Mixture on Lipid Extraction from Chlorella vulgaris. High Press. Res. 2022, 42, 105–120. [Google Scholar] [CrossRef]
- Xu, J.; Zhao, F.; Su, X. Direct Extraction of Lipids from Wet Microalgae Slurries by Super-High Hydrostatic Pressure. Algal Res. 2021, 58, 102412. [Google Scholar] [CrossRef]
- Bueno, M.; Gallego, R.; Chourio, A.M.; Ibáñez, E.; Herrero, M.; Saldaña, M.D.A. Green Ultra-High Pressure Extraction of Bioactive Compounds from Haematococcus pluvialis and Porphyridium cruentum Microalgae. Innov. Food Sci. Emerg. Technol. 2020, 66, 102532. [Google Scholar] [CrossRef]
- Halim, R.; Hill, D.R.A.; Hanssen, E.; Webley, P.A.; Blackburn, S.; Grossman, A.R.; Posten, C.; Martin, G.J.O. Towards Sustainable Microalgal Biomass Processing: Anaerobic Induction of Autolytic Cell-Wall Self-Ingestion in Lipid-Rich: Nannochloropsis slurries. Green Chem. 2019, 21, 2967–2982. [Google Scholar] [CrossRef]
- United Nations Development Programme. Transforming Our World: The 2030 Agenda for Sustainable Development 2015; United Nations Development Programme: New York, NY, USA, 2015. [Google Scholar]
| MW/IL/US | Species | Solvent | Operational Conditions | Yield | References | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lipid | Fatty Acids | |||||||||||
| Used | Tested | Optimum | SFA | MUFA | PUFA | Omega-3 | EPA | |||||
| MW + IL | N. oceanica | [TMam] [Cl] | 1.65 g IL 2.45 GHz 700 W | 60–90 °C; 1–25 min | 90 °C; 25 min | ― | ― | ― | ― | ― | 37.92 mg g−1 FAME | [43] |
| MW + IL | N. oceanica | [TMam] [Cl] | 2.45 GHz 700 W | 0.5–2.5 g IL; 60–100 °C; 5–30 min | 1.65 g IL; 88.2 °C; 24.7 min | 19.6% (dw) | 35.8 mg g−1 FAME | 35.2 mg g−1 FAME | 51.1 mg g−1 FAME | 39.4 mg g−1 FAME | 19.6 g g−1 biomass; 37.9 mg g−1 FAME | [28] |
| MW | N. gaditana | CHCl3:MeOH (2:1) | ≤100 W | 50–100 °C 5–25 min | 100 °C; 5 min | 17.7% (dw) | ― | ― | ― | ― | ― | [29] |
| N. oceanica | 91 °C; 25 min | 49% (dw) | ― | ― | ― | ― | ― | |||||
| MW | Nannochloropsis sp. | Brine (NaCl) solution | 2.45 GHz ≤800 W | 60–100 °C 1–30 min | 100 °C; 30 min | 16.1% (dw) | ~26% (TFA) | ~23% (TFA) | 44.1% (TFA); 25.2 mg g−1 (dw) | 41.4% (TFA); 23.6 mg g−1 (dw) | 31.5% (TFA); 17.9 mg g−1 (dw) | [40] |
| MW | Nannochloropsis sp. | Brine (NaCl) solution | 2.45 GHz ≤ 800 W | 1–35% (w/v) NaCl; 5–25% solid loading; 60–100 °C; 5–30 min | 10% (w/v) NaCl; 5% solid loading; 100 °C; 30 min | 16.1% (dw) | ~27% (TFA) | ~24% (TFA) | 44.5% (TFA) | 43% (TFA) | 43% (TFA) | [44] |
| MW + IL | Nannochloropsis sp. | MeOH:[EMIM] [MeSO4] | 2.45 GHz 700 W | 4–12 (Alga:MeOH ratio); 0.5–1 (MeOH:IL ratio); 5–25 min | 1:4 (Alga:MeOH ratio); 1:0.5 (MeOH:IL ratio); 25 min | 42.2% (dw) | 10.2% (ww) | 18.7% (ww) | 9.3% (ww) | ― | ― | [42] |
| MW | N. oculata | CHCl3:MeOH (2:1) | 300 W | 40–80 °C; 1–10 min | 300 W; 80 °C; 1 min | 33.6% | 30.9% (TFA) | 32.1% (TFA) | 36.6% (TFA) | 29.8% (TFA) | 29.8% (TFA) | [45] |
| MW + US | Nannochloropsis sp. | MeOH | ― | 2:1–2:3 (Alga:MeOH) ratio; 100–140 W (US and MW); 3–7 min | 2:3 (Alga:MeOH) ratio; 140 W; 7 min | FAME–48.2% | ― | ― | ― | ― | ― | [41] |
| SF/PL/DEG | Species | Co-Solvent | Operational Conditions | Yield | References | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lipid | Fatty Acids | |||||||||||
| Used | Tested | Optimum | SFA | MUFA | PUFA | Omega-3 | EPA | |||||
| SF | N. oculata | n-hexane | 200 mL min−1 (fr) | 150–550 bar; 35–75 °C; 0–300 min; 0–3% co-solvent | 550 bar; 75 °C; 150 min; 3% co-solvent | 0.262 g g−1 (dw) | 34.4% (TFA) | 52.5% (TFA) | 13.7% (TFA) | ― | 7.4% (TFA) | [30] |
| SF + PL | Nannochloropsis sp. | Ethanol | 35 MPa; 50 °C; 10% co-solvent; 0.15 kg h−1 (fr) | ― | ― | ― | ― | ― | ― | ― | 61.9% | [12] |
| SF | N. oculata | Ethanol | ― | 0–80% water content; 100–200 bar; 100–150 °C; 80–160 min | 40% water content; 150 °C; 150 bar; 120 min | 0.253 g g−1 (dw) | 59% (TFA) | 35.6% (TFA) | 5% (TFA) | ― | ― | [54] |
| SF | N. maritima | Ethanol | 80 min; 0.02 kg h−1 (fr) | 100–300 bar; 40–60 °C | 300 bar; 40 °C | ― | ― | ― | ― | ― | 8.5% (total lipids) | [55] |
| SF + DEG | Nannochloropsis sp. | ― | 100 min | 100–550 bar; 50–75 °C; 7.24–14.48 g min−1 (fr) | 550 bar; 75 °C; 14.48 g min−1 (fr)–EPA 400 bar; 50 °C; 14.48 g min−1 (fr)–DHA | 18.39 mg g−1 (dw) | 4.74 mg g−1 (dw) | 5.89 mg g−1 (dw) | 6.92 mg g−1 (dw) | ― | 5.69 mg g−1 (dw) | [53] |
| SF + DEG | N. gaditana | ― | 100 min; 50 or 65 °C | 250–550 bar; 7.24–14.48 g min−1 (fr) | 250 bar; 65 °C; 7.24 g min−1 (fr) | 34.61 mg g−1 (dw) | ― | ― | ― | ― | 11.50 mg g−1 (dw) | [56] |
| SF | N. oculata | Ethanol | 50 °C; 25 g min−1 (fr) | 250–750 bar; 0–240 min | 450 bar; 240 min | 20% | ― | ― | ― | ― | ― | [57] |
| Species | Co-Solvent | Operational Conditions | Yield | References | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Lipid | Fatty Acids | ||||||||||
| Used | Tested | Optimum | SFA | MUFA | PUFA | Omega-3 | EPA | ||||
| N. salina | Ethanol | 20 bar; 120 min | 90–150 °C; 25–75% (water ethanol); 1–4 mL min−1 (fr) | 90 °C; 75% (water ethanol); 4 mL min−1 (fr) | 33.9% (dw) | 44.26% (TFA) | 41.35% (TFA) | 14.39% (TFA) | ― | ― | [31] |
| N. salina | ― | 20 bar; 120 min | 150–200 °C; 1–4 mL min−1 (fr); 1–4 gsample | 175 °C; 4 mL min−1 (fr); 1 gsample | 19.52% (dw) | 35.48% (TFA) | 53.11% (TFA) | 11.41% (TFA) | ― | ― | [58] |
| N. gaditana | ― | ― | 156.1–273.9 °C; 6.6–23.4 min; 33–117 gsample L−1 | 236.54 °C; 13.95 min; 60.5 gsample L−1 | 13.4% (dw) | ― | ― | ― | ― | 15.04% (FAME) | [25] |
| N. gaditana | ― | 10 min | hexane or ethanol | hexane; 120 °C | 17.6% (dw) | 13.4% (TFA) | 20.0% (TFA) | 66.5% (TFA) | 55.9% (FAME) | 53% (FAME) | [11] |
| N. gaditana | ― | 120 °C; 15 min | Solvents | 2-MeTHF:ethanol (1:3) | 46.1% (dw) | 20.17% (TFA) | 27.86% (TFA) | 51.94% (TFA) | 39.73% (TFA) | 38.54% | [23] |
| Enzymes | Species | Solvent (Post-Enzymes) | Operational Conditions | Yield | References | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lipid | Fatty Acids | |||||||||||
| Used | Tested | Optimum | SFA | MUFA | PUFA | Omega-3 | EPA | |||||
| Laccase and cellulase | N. oceanica | EtOH | ― | Laccase:cellulase (4:1, 2:1, 1:1,1:2, 1:4); pH (4.2–5.8); T (40–60 °C); time (1.5–24 h) | 1:2.5 (laccase:cellulase); pH 5; 45 °C; 6 h | 26.9% | ― | ― | ― | ― | 20.7 g 100 g−1 | [20] |
| Cellulase, hemicellulase, papain and pectinase | N. oculata | ― | 200 U enzyme; pH 5.5; 45 °C; 12 h | Combinations | Mixture of all four | 221.4 mg g−1 (dw) | ― | ― | ― | ― | ― | [64] |
| Cellulase, hemicellulase, papain and pectinase | Nannochloropsis sp. | tert-butanol | 200 U enzyme; pH 5.0; 50 °C; 4 h | Cellulase; hemicellulase; papain; pectinase | cellulase | 88.70% (ww) | 53% (TFA) | 25.69% (TFA) | 19.91% (TFA) | 13.98% (TFA) | 13.98% (TFA) | [35] |
| Cellulase and mannase | Nannochloropsis sp. | n-hexane:2-propanol (3:2) | 13.8 mg g−1 (cellulase), 1.5 mg g−1 (mannase); pH 4.4; 53 °C; 24 h | Cellulase; mannase; cellulase/mannase | cellulase/mannase | 73% (total lipids) | ― | ― | ― | ― | ― | [60] |
| Species | IL or DES | Parameters | Lipid Yield | References | |
|---|---|---|---|---|---|
| Tested | Optimum | ||||
| Nannochloropsis sp. | Tetramethylguanidine:menthol | 1:1–5:1 (HBA:HBD molar ratio); 50–90 °C; 30–110 min; 5–40% (ammonium sulfate); 20:1–40:1 (liquid:solid ratio) | 3:1 (HBA:HBD molar ratio); 80 °C; 90 min; 20% (ammonium sulfate); 35:1 (liquid:solid ratio) | 127 mg g−1 (dw) | [71] |
| N. oceanica | [EMIM]Cl | 0.2–3 g IL | 2 g IL | 13.9% | [72] |
| N. oculata | Lactam- or ammonium carboxylate-based | ILs | butyrolactam hexanoate | 13.5% (dw) | [69] |
| Nannochloropsis sp. | Cholinium amino acid-based | ILs | cholinium arginate | 98.6% (total lipids) | [7] |
| N. gaditana | [EMIM] [MeSO4] | 65–95 °C; 5–25 min; 1:4–1:12 (wet alga:MeOH); 1:0.5–1:1 (MeOH:IL) | 14 min; 1:4 (wet alga:MeOH); 1:0.5 (MeOH:IL) | 41.2% (dw) | [42] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sousa, S.C.; Freitas, A.C.; Gomes, A.M.; Carvalho, A.P. Extraction of Nannochloropsis Fatty Acids Using Different Green Technologies: The Current Path. Mar. Drugs 2023, 21, 365. https://doi.org/10.3390/md21060365
Sousa SC, Freitas AC, Gomes AM, Carvalho AP. Extraction of Nannochloropsis Fatty Acids Using Different Green Technologies: The Current Path. Marine Drugs. 2023; 21(6):365. https://doi.org/10.3390/md21060365
Chicago/Turabian StyleSousa, Sérgio Cruz, Ana Cristina Freitas, Ana Maria Gomes, and Ana P. Carvalho. 2023. "Extraction of Nannochloropsis Fatty Acids Using Different Green Technologies: The Current Path" Marine Drugs 21, no. 6: 365. https://doi.org/10.3390/md21060365
APA StyleSousa, S. C., Freitas, A. C., Gomes, A. M., & Carvalho, A. P. (2023). Extraction of Nannochloropsis Fatty Acids Using Different Green Technologies: The Current Path. Marine Drugs, 21(6), 365. https://doi.org/10.3390/md21060365







