Inhibition Effects and Mechanisms of Marine Polysaccharide PSSD against Herpes Simplex Virus Type 2
Abstract
1. Introduction
2. Results
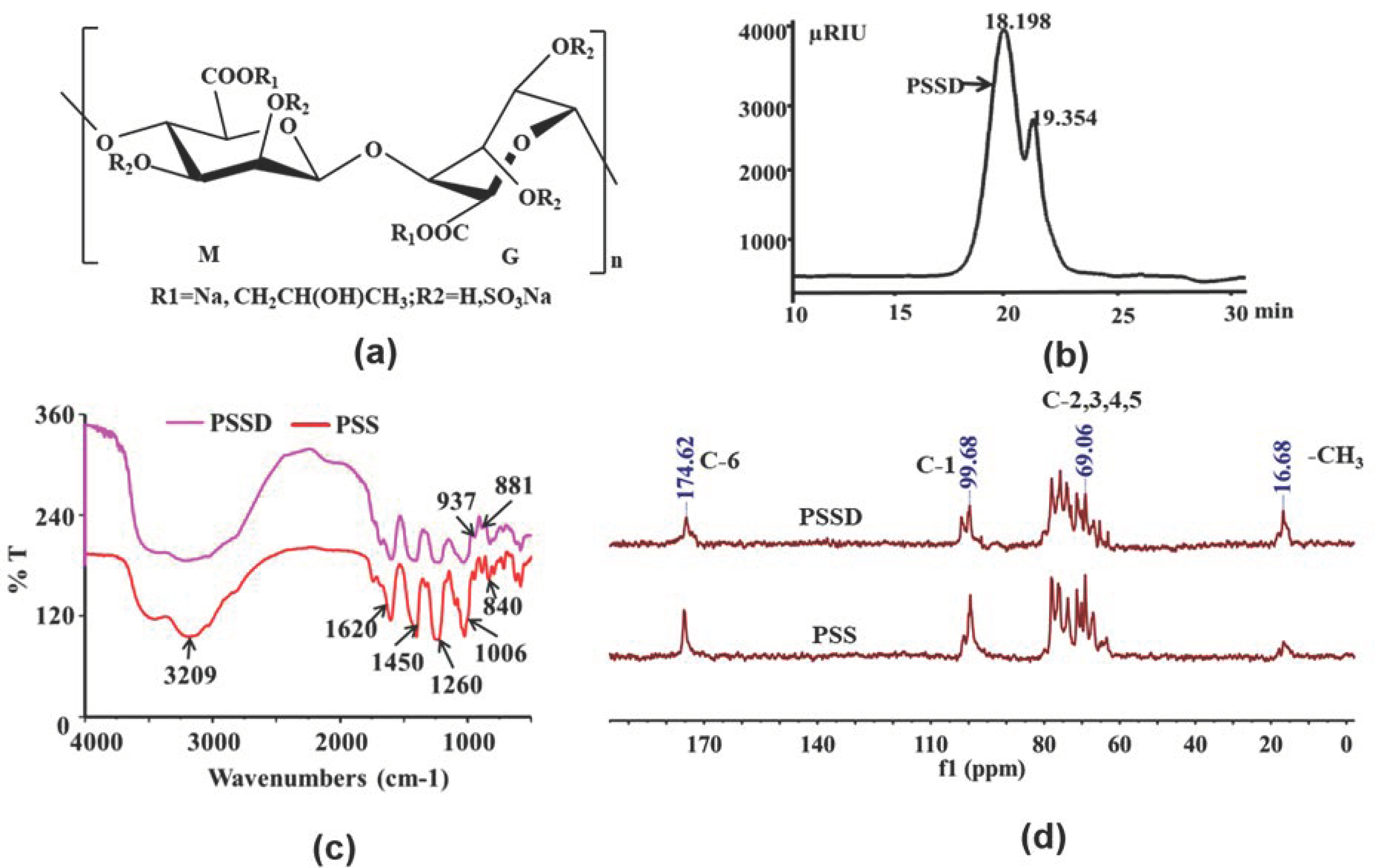
2.1. Structure Characterization of Marine Polysaccharide PSSD
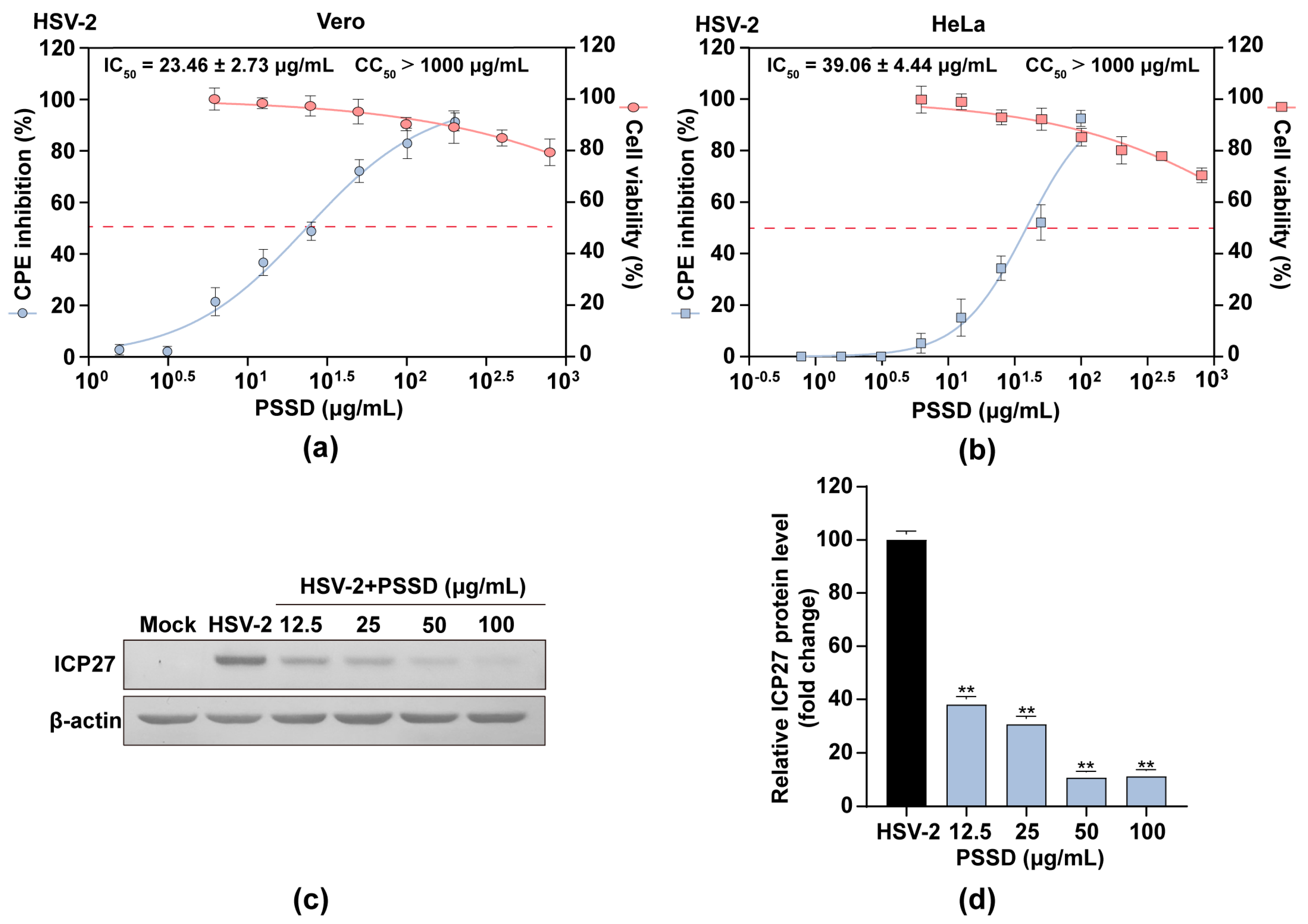
2.2. PSSD Suppresses HSV Multiplication In Vitro with Low Toxicity
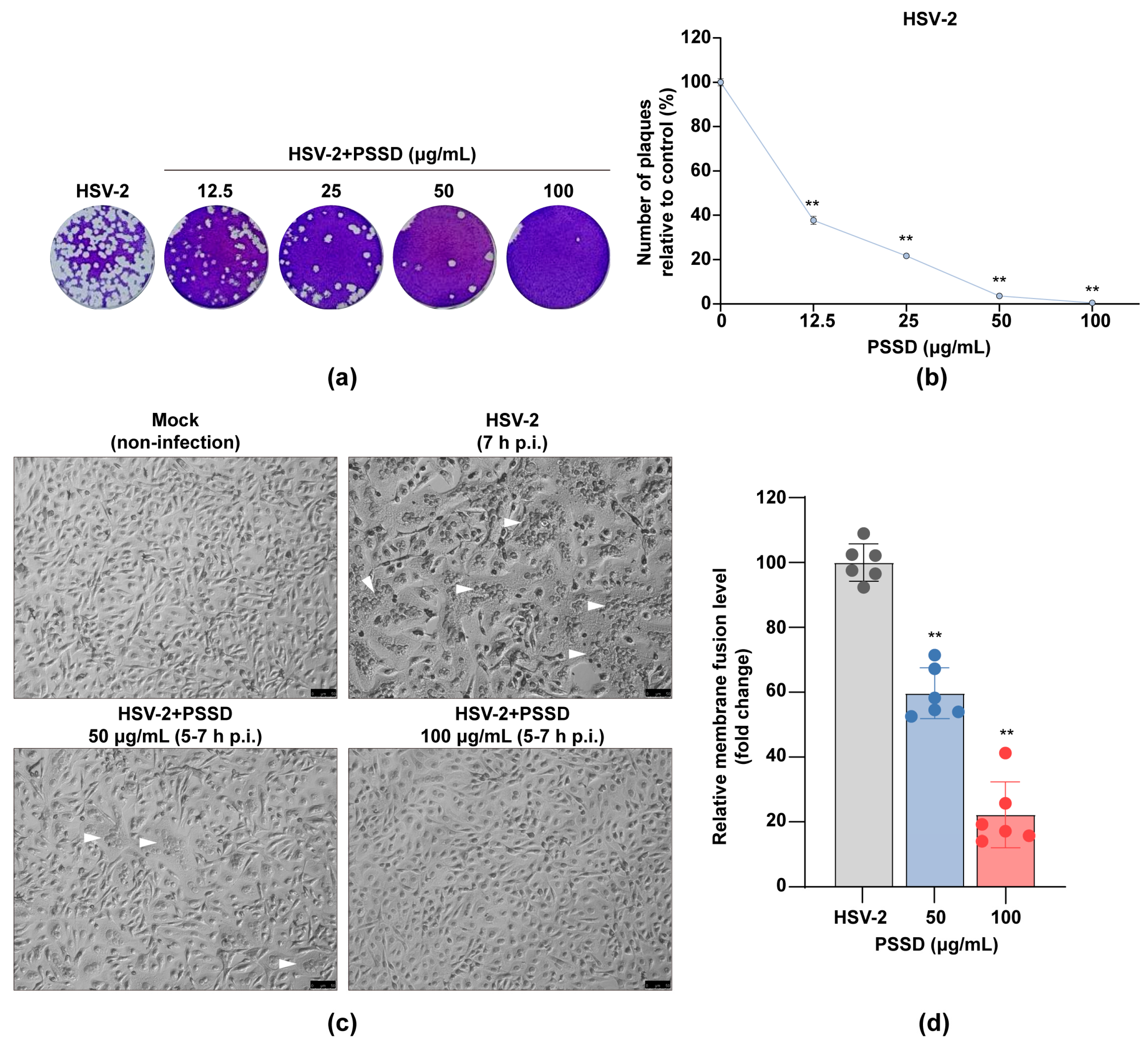
2.3. Influence of Different Treatment Conditions of PSSD on HSV-2 Infection
2.4. PSSD Possesses Direct Actions on HSV Particles to Block Virus-Induced Membrane Fusion
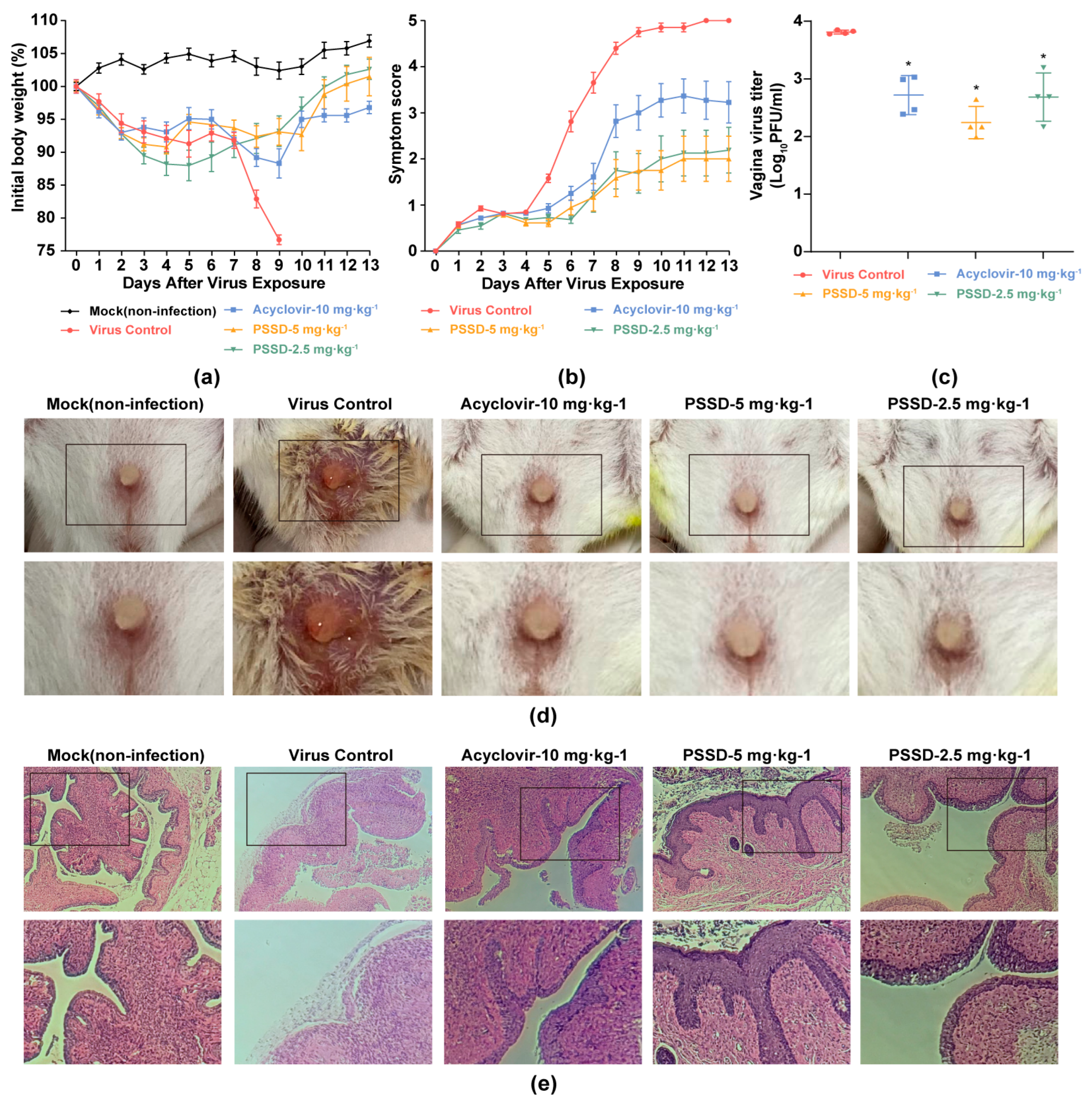
2.5. PSSD Exhibits In Vivo Antiviral Activity in HSV-2-Infected Mice
3. Discussion
4. Materials and Methods
4.1. Reagents, Cells and Viruses
4.2. Cytotoxicity Assay
4.3. Cytopathic Effect (CPE) Inhibition Assay
4.4. Time-of-Addition Assay
4.5. Plaque Reduction Assay
4.6. Western Blot Assay
4.7. Syncytium Formation Inhibition Assay
4.8. Animal Experiments
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sharma, V.; Mobeen, F.; Prakash, T. Comparative Genomics of Herpesviridae Family to Look for Potential Signatures of Human Infecting Strains. Int. J. Genom. 2016, 2016, 9543274. [Google Scholar] [CrossRef]
- James, C.; Harfouche, M.; Welton, N.J.; Turner, K.M.; Abu-Raddad, L.J.; Gottlieb, S.L.; Looker, K.J. Herpes simplex virus: Global infection prevalence and incidence estimates, 2016. Bull. World Health Organ. 2020, 98, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Alsweed, A.; Alsuhibani, M.; Casanova, J.L.; Al-Hajjar, S. Approach to recurrent Herpes Simplex Encephalitis in children. Int. J. Pediatr. Adolesc. Med. 2018, 5, 35–38. [Google Scholar] [CrossRef]
- Kollias, C.M.; Huneke, R.B.; Wigdahl, B.; Jennings, S.R. Animal models of herpes simplex virus immunity and pathogenesis. J. Neurovirol. 2015, 21, 8–23. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, V.C.S.; Pereira, J.G.; de Paula, V.S. Family Herpesviridae and neuroinfections: Current status and research in progress. Mem. Inst. Oswaldo Cruz 2022, 117, e220200. [Google Scholar] [CrossRef] [PubMed]
- Schelhaas, M.; Jansen, M.; Haase, I.; Knebel-Mörsdorf, D. Herpes simplex virus type 1 exhibits a tropism for basal entry in polarized epithelial cells. J. Gen. Virol. 2003, 84, 2473–2484. [Google Scholar] [CrossRef] [PubMed]
- Protto, V.; Marcocci, M.E.; Miteva, M.T.; Piacentini, R.; Li Puma, D.D.; Grassi, C.; Palamara, A.T.; De Chiara, G. Role of HSV-1 in Alzheimer’s disease pathogenesis: A challenge for novel preventive/therapeutic strategies. Curr. Opin. Pharmacol. 2022, 63, 102200. [Google Scholar] [CrossRef]
- Aoki, F.Y. Management of genital herpes in HIV-infected patients. Herpes. 2001, 8, 41–45. [Google Scholar]
- Looker, K.J.; Elmes, J.A.R.; Gottlieb, S.L.; Schiffer, J.T.; Vickerman, P.; Turner, K.M.E.; Boily, M.C. Effect of HSV-2 infection on subsequent HIV acquisition: An updated systematic review and meta-analysis. Lancet Infect. Dis. 2017, 17, 1303–1316. [Google Scholar] [CrossRef]
- Van Wagoner, N.; Qushair, F.; Johnston, C. Genital Herpes Infection: Progress and Problems. Infect. Dis. Clin. N. Am. 2023, 37, 351–367. [Google Scholar] [CrossRef]
- van Velzen, M.; van de Vijver, D.A.; van Loenen, F.B.; Osterhaus, A.D.; Remeijer, L.; Verjans, G.M. Acyclovir prophylaxis predisposes to antiviral-resistant recurrent herpetic keratitis. J. Infect. Dis. 2013, 208, 1359–1365. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, S.X.; Guan, H.S. The Antiviral Activities and Mechanisms of Marine Polysaccharides: An Overview. Mar. Drugs 2012, 10, 2795–2816. [Google Scholar] [CrossRef] [PubMed]
- Thuy, T.T.; Ly, B.M.; Van, T.T.; Quang, N.V.; Tu, H.C.; Zheng, Y.; Seguin-Devaux, C.; Mi, B.; Ai, U. Anti-HIV activity of fucoidans from three brown seaweed species. Carbohydr. Polym. 2015, 115, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Astani, A.; Ghosh, T.; Schnitzler, P.; Ray, B. Polysaccharides from Sargassum tenerrimum: Structural features, chemical modification and anti-viral activity. Phytochemistry 2010, 71, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.X.; Lu, Z.; Wang, S.Y.; Liu, W.; Gao, J.M.; Tian, L.N.; Wang, L.; Zhang, X.S.; Zhao, X.; Wang, W.; et al. The inhibitory effects and mechanisms of polymannuroguluronate sulfate against human papillomavirus infection in vitro and in vivo. Carbohydr. Polym. 2020, 241, 116365. [Google Scholar] [CrossRef]
- Xue, Y.T.; Ren, L.; Li, S.; Wang, L.L.; He, X.X.; Zhao, X.; Yu, G.L.; Guan, H.S.; Li, C.X. Study on quality control of sulfated polysaccharide drug, propylene glycol alginate sodium sulfate (PSS). Carbohydr. Polym. 2016, 144, 330–337. [Google Scholar] [CrossRef]
- Xin, M.; Ren, L.; Sun, Y.; Li, H.H.; Guan, H.S.; He, X.X.; Li, C.X. Anticoagulant and antithrombotic activities of low-molecular-weight propylene glycol alginate sodium sulfate (PSS). Eur. J. Med. Chem. 2016, 114, 33–40. [Google Scholar] [CrossRef]
- Li, M.K.; Liu, Y.Y.; Wei, F.; Shen, M.X.; Zhong, Y.; Li, S.; Chen, L.J.; Ma, N.; Liu, B.Y.; Mao, Y.D.; et al. Antiviral activity of arbidol hydrochloride against herpes simplex virus I in vitro and in vivo. Int. J. Antimicrob. Agents 2018, 51, 98–106. [Google Scholar] [CrossRef]
- Li, W.M.; Xu, C.J.; Hao, C.; Zhang, Y.; Wang, Z.Q.; Wang, S.Y.; Wang, W. Inhibition of herpes simplex virus by myricetin through targeting viral gD protein and cellular EGFR/PI3K/Akt pathway. Antivir. Res. 2020, 177, 104714. [Google Scholar] [CrossRef]
- Li, T.; Liu, L.B.; Wu, H.L.; Chen, S.D.; Zhu, Q.C.; Gao, H.; Yu, X.T.; Wang, Y.; Su, W.H.; Yao, X.S.; et al. Anti-herpes simplex virus type 1 activity of Houttuynoid A, a flavonoid from Houttuynia cordata Thunb. Antivir. Res. 2017, 144, 273–280. [Google Scholar] [CrossRef]
- Hook, L.M.; Friedman, H.M.; Awasthi, S. Guinea Pig and Mouse Models for Genital Herpes Infection. Curr. Protoc. 2021, 1, e332. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.; Bruun, B.; Minson, T.; Browne, H. Glycoproteins gB, gD, and gHgL of herpes simplex virus type 1 are necessary and sufficient to mediate membrane fusion in a Cos cell transfection system. J. Virol. 1998, 72, 873–875. [Google Scholar] [CrossRef] [PubMed]
- Du, R.K.; Wang, L.L.; Xu, H.; Wang, Z.Y.; Zhang, T.; Wang, M.L.; Ning, Y.J.; Deng, F.; Hu, Z.H.; Wang, H.L.; et al. A novel glycoprotein D-specific monoclonal antibody neutralizes herpes simplex virus. Antivir. Res. 2017, 147, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.F.; Brendle, S.A.; Li, J.J.; Walter, V.; Cladel, N.M.; Cooper, T.; Shearer, D.A.; Balogh, K.K.; Christensen, N.D. Depo Medroxyprogesterone (DMPA) Promotes Papillomavirus Infections but Does Not Accelerate Disease Progression in the Anogenital Tract of a Mouse Model. Viruses 2022, 14, 980. [Google Scholar] [CrossRef]
- Liu, T.; Shao, Q.; Wang, W.; Ma, Y.; Liu, T.; Jin, X.; Fang, J.; Huang, G.; Chen, Z. Integrating network pharmacology and experimental validation to decipher the mechanism of the Chinese herbal prescription JieZe-1 in protecting against HSV-2 infection. Pharm. Biol. 2022, 60, 451–466. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, H.; Wang, J.; Yang, J.; Xu, Z.; Li, C.; Hao, C.; Wang, S.; Wang, W. Inhibition Effects and Mechanisms of Marine Polysaccharide PSSD against Herpes Simplex Virus Type 2. Mar. Drugs 2023, 21, 364. https://doi.org/10.3390/md21060364
Yan H, Wang J, Yang J, Xu Z, Li C, Hao C, Wang S, Wang W. Inhibition Effects and Mechanisms of Marine Polysaccharide PSSD against Herpes Simplex Virus Type 2. Marine Drugs. 2023; 21(6):364. https://doi.org/10.3390/md21060364
Chicago/Turabian StyleYan, Han, Jie Wang, Jiayi Yang, Zhongqiu Xu, Chunxia Li, Cui Hao, Shixin Wang, and Wei Wang. 2023. "Inhibition Effects and Mechanisms of Marine Polysaccharide PSSD against Herpes Simplex Virus Type 2" Marine Drugs 21, no. 6: 364. https://doi.org/10.3390/md21060364
APA StyleYan, H., Wang, J., Yang, J., Xu, Z., Li, C., Hao, C., Wang, S., & Wang, W. (2023). Inhibition Effects and Mechanisms of Marine Polysaccharide PSSD against Herpes Simplex Virus Type 2. Marine Drugs, 21(6), 364. https://doi.org/10.3390/md21060364






