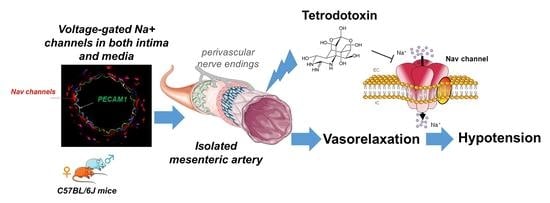
Tetrodotoxin Decreases the Contractility of Mesenteric Arteries, Revealing the Contribution of Voltage-Gated Na+ Channels in Vascular Tone Regulation
Abstract
1. Introduction
2. Results
2.1. Nav Channels Are Expressed in Both Intima and Media of Murine Arteries
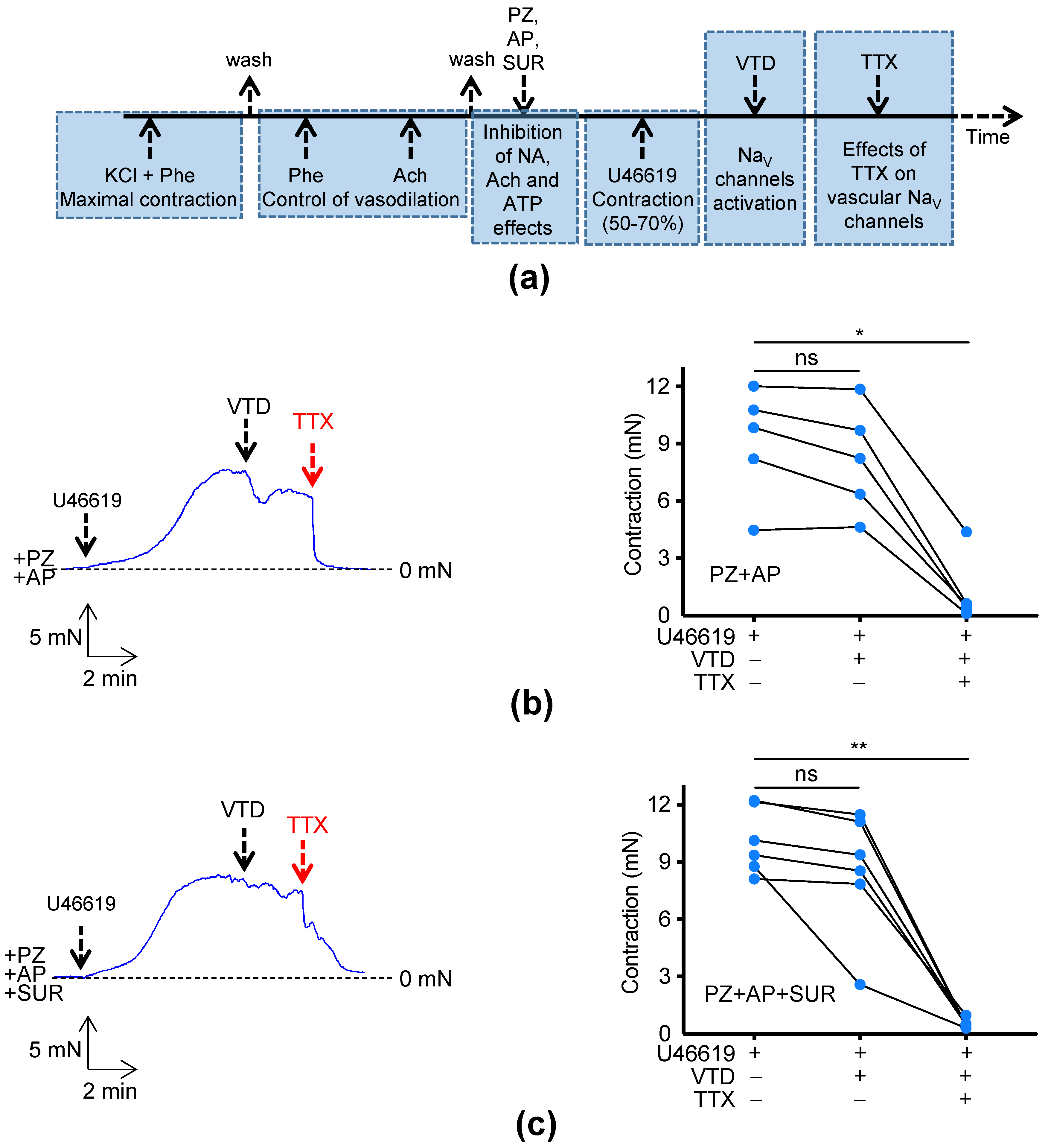
2.2. Effects of TTX on Murine Mesenteric Artery Contractility
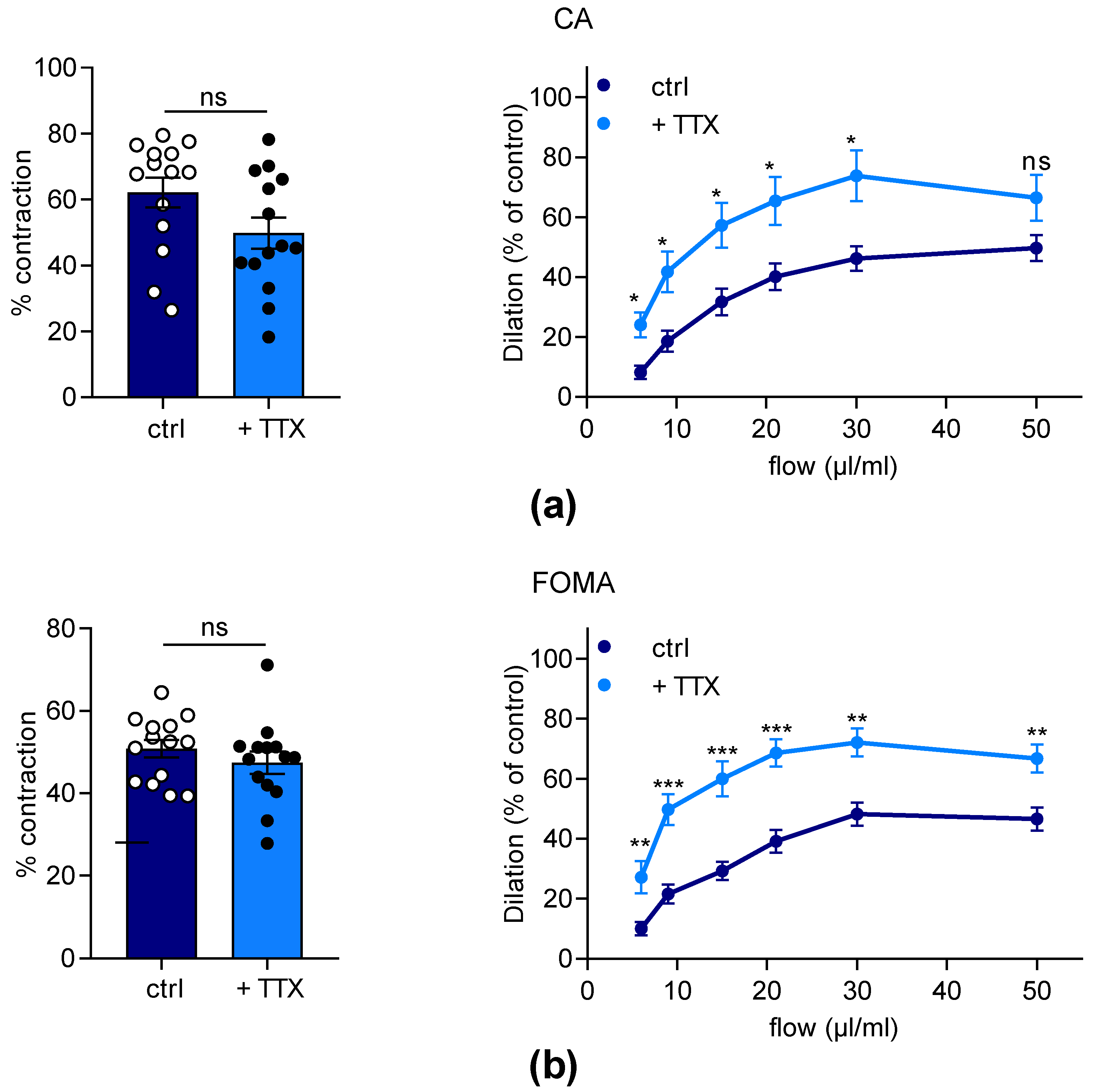
2.3. Effects of TTX on Dilation Capacity of Murine Mesenteric Arteries
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Ethical Approval, Animals and Artery Preparations
4.3. Western Blot
4.4. Immunohistochemistry
4.5. RNA Extraction and RT-qPCR
4.6. Myography
4.7. Arteriography
4.8. Data Acquisition and Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Ach | acetylcholine |
| AP | atropine |
| CA | cecocolic artery |
| EC | endothelial cell |
| eNOS | endothelial NO synthase |
| FMD | flow-mediated dilation |
| FOMA | first-order mesenteric artery |
| MA | mesenteric artery |
| NA | noradrenaline |
| NaV channel | voltage-gated Na+ channel |
| NCX | Na+/Ca2+ exchanger |
| Phe | phenylephrine |
| PSS | physiological salt solution |
| PZ | prazosin |
| SUR | suramin |
| TTX | tetrodotoxin |
| TTX-R | resistant to tetrodotoxin |
| TTX-S | sensitive to tetrodotoxin |
| VSMC | vascular smooth muscle cell |
| VTD | veratridine |
References
- Catterall, W.A.; Goldin, A.L.; Waxman, S.G. International Union of Pharmacology. XLVII. Nomenclature and Structure-Function Relationships of Voltage-Gated Sodium Channels. Pharmacol. Rev. 2005, 57, 397–409. [Google Scholar] [CrossRef]
- Black, J.A.; Waxman, S.G. Noncanonical Roles of Voltage-Gated Sodium Channels. Neuron 2013, 80, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Okabe, K.; Kitamura, K.; Kuriyama, H. The Existence of a Highly Tetrodotoxin Sensitive Na Channel in Freshly Dispersed Smooth Muscle Cells of the Rabbit Main Pulmonary Artery. Pflüg. Arch. 1988, 411, 423–428. [Google Scholar] [CrossRef]
- Walsh, K.B.; Wolf, M.B.; Fan, J. Voltage-Gated Sodium Channels in Cardiac Microvascular Endothelial Cells. Am. J. Physiol.-Heart Circ. Physiol. 1998, 274, H506–H512. [Google Scholar] [CrossRef] [PubMed]
- Cox, R.H.; Zhou, Z.; Tulenko, T.N. Voltage-Gated Sodium Channels in Human Aortic Smooth Muscle Cells. JVR 1998, 35, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Fort, A.; Cordaillat, M.; Thollon, C.; Salazar, G.; Mechaly, I.; Villeneuve, N.; Vilaine, J.-P.; Richard, S.; Virsolvy, A. New Insights in the Contribution of Voltage-Gated Nav Channels to Rat Aorta Contraction. PLoS ONE 2009, 4, e7360. [Google Scholar] [CrossRef] [PubMed]
- Meguro, K.; Iida, H.; Takano, H.; Morita, T.; Sata, M.; Nagai, R.; Nakajima, T. Function and Role of Voltage-Gated Sodium Channel NaV1.7 Expressed in Aortic Smooth Muscle Cells. Am. J. Physiol.-Heart Circ. Physiol. 2009, 296, H211–H219. [Google Scholar] [CrossRef]
- Quignard, J.F.; Ryckwaert, F.; Albat, B.; Nargeot, J.; Richard, S. A Novel Tetrodotoxin-Sensitive Na+ Current in Cultured Human Coronary Myocytes. Circ. Res. 1997, 80, 377–382. [Google Scholar] [CrossRef]
- Ho, W.-S.V.; Davis, A.J.; Chadha, P.S.; Greenwood, I.A. Effective Contractile Response to Voltage-Gated Na+ Channels Revealed by a Channel Activator. Am. J. Physiol.-Cell Physiol. 2013, 304, C739–C747. [Google Scholar] [CrossRef]
- Berra-Romani, R.; Blaustein, M.P.; Matteson, D.R. TTX-Sensitive Voltage-Gated Na+ Channels Are Expressed in Mesenteric Artery Smooth Muscle Cells. Am. J. Physiol.-Heart Circ. Physiol. 2005, 289, H137–H145. [Google Scholar] [CrossRef]
- Boccara, G.; Choby, C.; Frapier, J.M.; Quignard, J.F.; Nargeot, J.; Dayanithi, G.; Richard, S. Regulation of Ca2+ Homeostasis by Atypical Na+ Currents in Cultured Human Coronary Myocytes. Circ. Res. 1999, 85, 606–613. [Google Scholar] [CrossRef]
- Platoshyn, O.; Remillard, C.V.; Fantozzi, I.; Sison, T.; Yuan, J.X.-J. Identification of Functional Voltage-Gated Na(+) Channels in Cultured Human Pulmonary Artery Smooth Muscle Cells. Pflüg. Arch. 2005, 451, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, X.F.; Chen, C.-C.; Campbell, K.P.; Damon, D.N.; Day, K.H.; Ramos, S.; Duling, B.R. Are Voltage-Dependent Ion Channels Involved in the Endothelial Cell Control of Vasomotor Tone? Am. J. Physiol.-Heart Circ. Physiol. 2007, 293, H1371–H1383. [Google Scholar] [CrossRef] [PubMed]
- Andrikopoulos, P.; Fraser, S.P.; Patterson, L.; Ahmad, Z.; Burcu, H.; Ottaviani, D.; Diss, J.K.J.; Box, C.; Eccles, S.A.; Djamgoz, M.B.A. Angiogenic Functions of Voltage-Gated Na+ Channels in Human Endothelial Cells. J. Biol. Chem. 2011, 286, 16846–16860. [Google Scholar] [CrossRef] [PubMed]
- Virsolvy, A.; Fort, A.; Erceau, L.; Charrabi, A.; Hayot, M.; Aimond, F.; Richard, S. Hypoxic Conditions Promote Rhythmic Contractile Oscillations Mediated by Voltage-Gated Sodium Channels Activation in Human Arteries. Int. J. Mol. Sci. 2021, 22, 2570. [Google Scholar] [CrossRef]
- Traub, O.; Ishida, T.; Ishida, M.; Tupper, J.C.; Berk, B.C. Shear Stress-Mediated Extracellular Signal-Regulated Kinase Activation Is Regulated by Sodium in Endothelial Cells. J. Biol. Chem. 1999, 274, 20144–20150. [Google Scholar] [CrossRef]
- Duran-Riveroll, L.M.; Cembella, A.D. Guanidinium Toxins and Their Interactions with Voltage-Gated Sodium Ion Channels. Mar. Drugs 2017, 15, 303. [Google Scholar] [CrossRef]
- Katikou, P.; Gokbulut, C.; Kosker, A.R.; Campàs, M.; Ozogul, F. An Updated Review of Tetrodotoxin and Its Peculiarities. Mar. Drugs 2022, 20, 47. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, T. Effects of Tetrodotoxin on the Mammalian Cardiovascular System. Mar. Drugs 2010, 8, 741–762. [Google Scholar] [CrossRef] [PubMed]
- Flachsenberger, W.A. Respiratory Failure and Lethal Hypotension Due to Blue-Ringed Octopus and Tetrodotoxin Envenomation Observed and Counteracted in Animal Models. J. Toxicol. Clin. Toxicol. 1986, 24, 485–502. [Google Scholar] [CrossRef] [PubMed]
- Rice, F.L.; Albrecht, P.J.; Wymer, J.P.; Black, J.A.; Merkies, I.S.; Faber, C.G.; Waxman, S.G. Sodium Channel Nav1.7 in Vascular Myocytes, Endothelium, and Innervating Axons in Human Skin. Mol. Pain 2015, 11, 26. [Google Scholar] [CrossRef]
- Shinjoh, M.; Nakaki, T.; Otsuka, Y.; Sasakawa, N.; Kato, R. Vascular Smooth Muscle Contraction Induced by Na+ Channel Activators, Veratridine and Batrachotoxin. Eur. J. Pharmacol. 1991, 205, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Aalkjær, C.; Nilsson, H.; De Mey, J.G.R. Sympathetic and Sensory-Motor Nerves in Peripheral Small Arteries. Physiol. Rev. 2021, 101, 495–544. [Google Scholar] [CrossRef] [PubMed]
- Morris, C.E. Voltage-Gated Channel Mechanosensitivity: Fact or Friction? Front. Physiol. 2011, 2, 25. [Google Scholar] [CrossRef] [PubMed]
- Strege, P.R.; Mercado-Perez, A.; Mazzone, A.; Saito, Y.A.; Bernard, C.E.; Farrugia, G.; Beyder, A. SCN5A Mutation G615E Results in Na V 1.5 Voltage-Gated Sodium Channels with Normal Voltage-Dependent Function yet Loss of Mechanosensitivity. Channels 2019, 13, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Körner, J.; Meents, J.; Machtens, J.-P.; Lampert, A. Β1 Subunit Stabilises Sodium Channel Nav1.7 against Mechanical Stress: Nav Β1 Subunit as Mechanical Stabiliser. J. Physiol. 2018, 596, 2433–2445. [Google Scholar] [CrossRef]
- Maroni, M.; Körner, J.; Schüttler, J.; Winner, B.; Lampert, A.; Eberhardt, E. Β1 and Β3 Subunits Amplify Mechanosensitivity Ofthe Cardiac Voltage-Gated Sodium Channel Nav1.5. Pflüg. Arch.-Eur. J. Physiol. 2019, 471, 1481–1492. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Wen, J.; Wang, N.; Wang, C.; Xu, Q.; Yang, Y. Ion Channels and Vascular Diseases. ATVB 2019, 39, e146–e156. [Google Scholar] [CrossRef]
- Förstermann, U.; Sessa, W.C. Nitric Oxide Synthases: Regulation and Function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Rafikov, R.; Fonseca, F.V.; Kumar, S.; Pardo, D.; Darragh, C.; Elms, S.; Fulton, D.; Black, S.M. ENOS Activation and NO Function: Structural Motifs Responsible for the Posttranslational Control of Endothelial Nitric Oxide Synthase Activity. J. Endocrinol. 2011, 210, 271–284. [Google Scholar] [CrossRef]
- Cale, J.M.; Bird, I.M. Inhibition of MEK/ERK1/2 Signalling Alters Endothelial Nitric Oxide Synthase Activity in an Agonist-Dependent Manner. Biochem. J. 2006, 398, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Ulyanova, A.V.; Shirokov, R.E. Voltage-Dependent Inward Currents in Smooth Muscle Cells of Skeletal Muscle Arterioles. PLoS ONE 2018, 13, e0194980. [Google Scholar] [CrossRef] [PubMed]
- Kilpatrick, D.L.; Slepetis, R.; Kirshner, N. Inhibition of Catecholamine Secretion from Adrenal Medulla Cells by Neurotoxins and Cholinergic Antagonists. J. Neurochem. 1981, 37, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Dahlöf, C.; Linton-Dahlöf, P.; Lundberg, J.M. Tetrodotoxin-Sensitive Release of Adrenaline, Noradrenaline and Neuropeptide Y-like Immunoreactivity in the Pithed Guinea-Pig in the Absence of Electrical Preganglionic Nerve Stimulation. J. Neurosci. Methods 1994, 52, 215–218. [Google Scholar] [CrossRef]
- Gordienko, D.V.; Tsukahara, H. Tetrodotoxin-Blockable Depolarization-Activated Na+ Currents in a Cultured Endothelial Cell Line Derived from Rat Interlobar Arter and Human Umbilical Vein. Pflüg. Arch. 1994, 428, 91–93. [Google Scholar] [CrossRef]
- Aziz, Q.; Li, Y.; Tinker, A. Endothelial Biology and ATP-Sensitive Potassium Channels. Channels 2018, 12, 45–46. [Google Scholar] [CrossRef]
- Vessieres, E.; Guihot, A.-L.; Grimaud, L.; Rivron, J.; Arnal, J.-F.; Loufrani, L.; Henrion, D. Estrogens and the Angiotensin II Type 2 Receptor Control Flow-Mediated Outward Remodeling in the Female Mouse Mesenteric Artery. J. Vasc. Res. 2021, 58, 16–26. [Google Scholar] [CrossRef]
- Morrison, T.B.; Weis, J.J.; Wittwer, C.T. Quantification of Low-Copy Transcripts by Continuous SYBR Green I Monitoring during Amplification. Biotechniques 1998, 960, 962. [Google Scholar]
- Lee, C.; Kim, J.; Shin, S.G.; Hwang, S. Absolute and Relative QPCR Quantification of Plasmid Copy Number in Escherichia Coli. J. Biotechnol. 2006, 123, 273–280. [Google Scholar] [CrossRef]
- Henrion, D.; Terzi, F.; Matrougui, K.; Duriez, M.; Boulanger, C.M.; Colucci-Guyon, E.; Babinet, C.; Briand, P.; Friedlander, G.; Poitevin, P.; et al. Impaired Flow-Induced Dilation in Mesenteric Resistance Arteries from Mice Lacking Vimentin. J. Clin. Investig. 1997, 100, 2909–2914. [Google Scholar] [CrossRef]
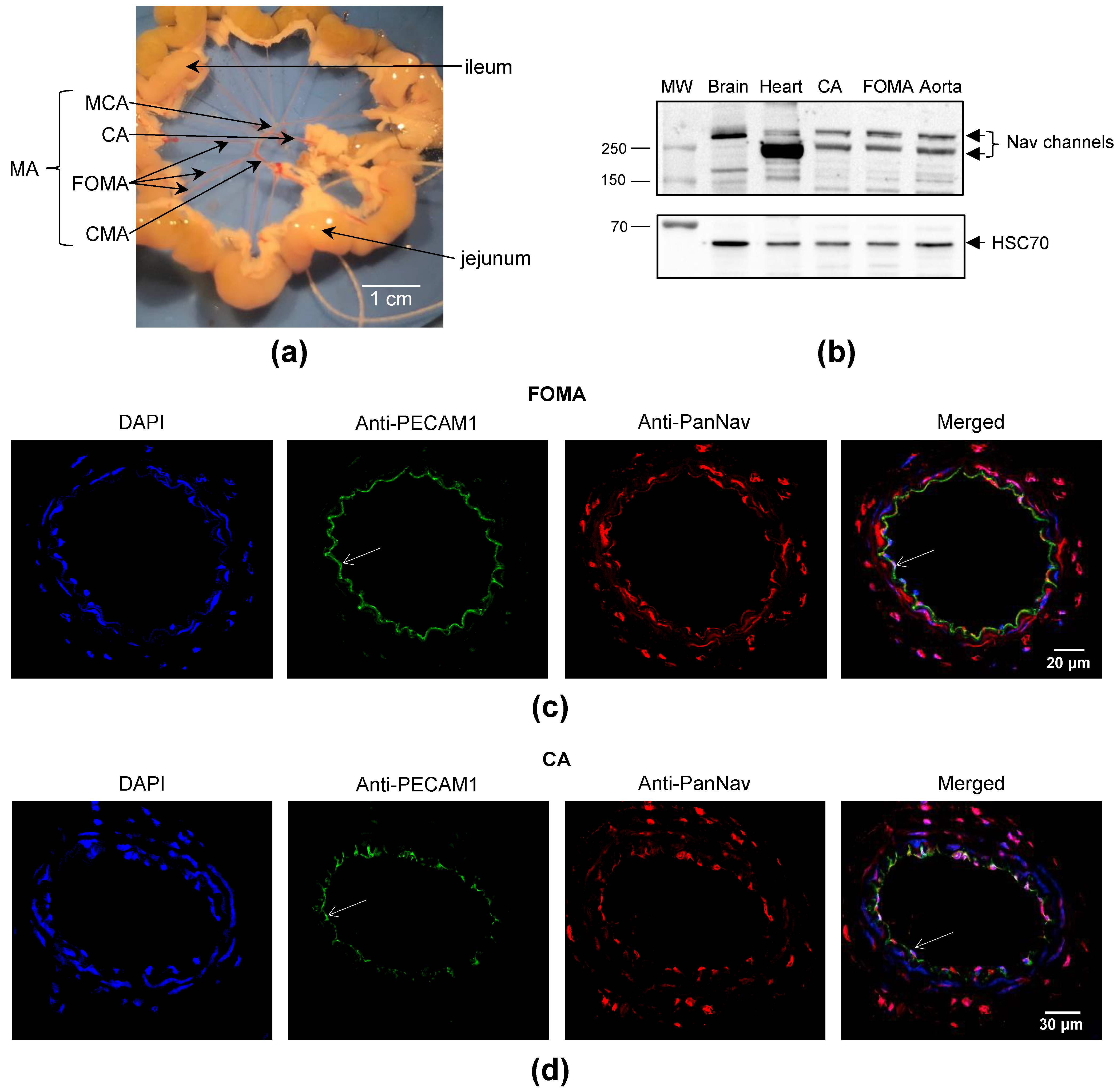
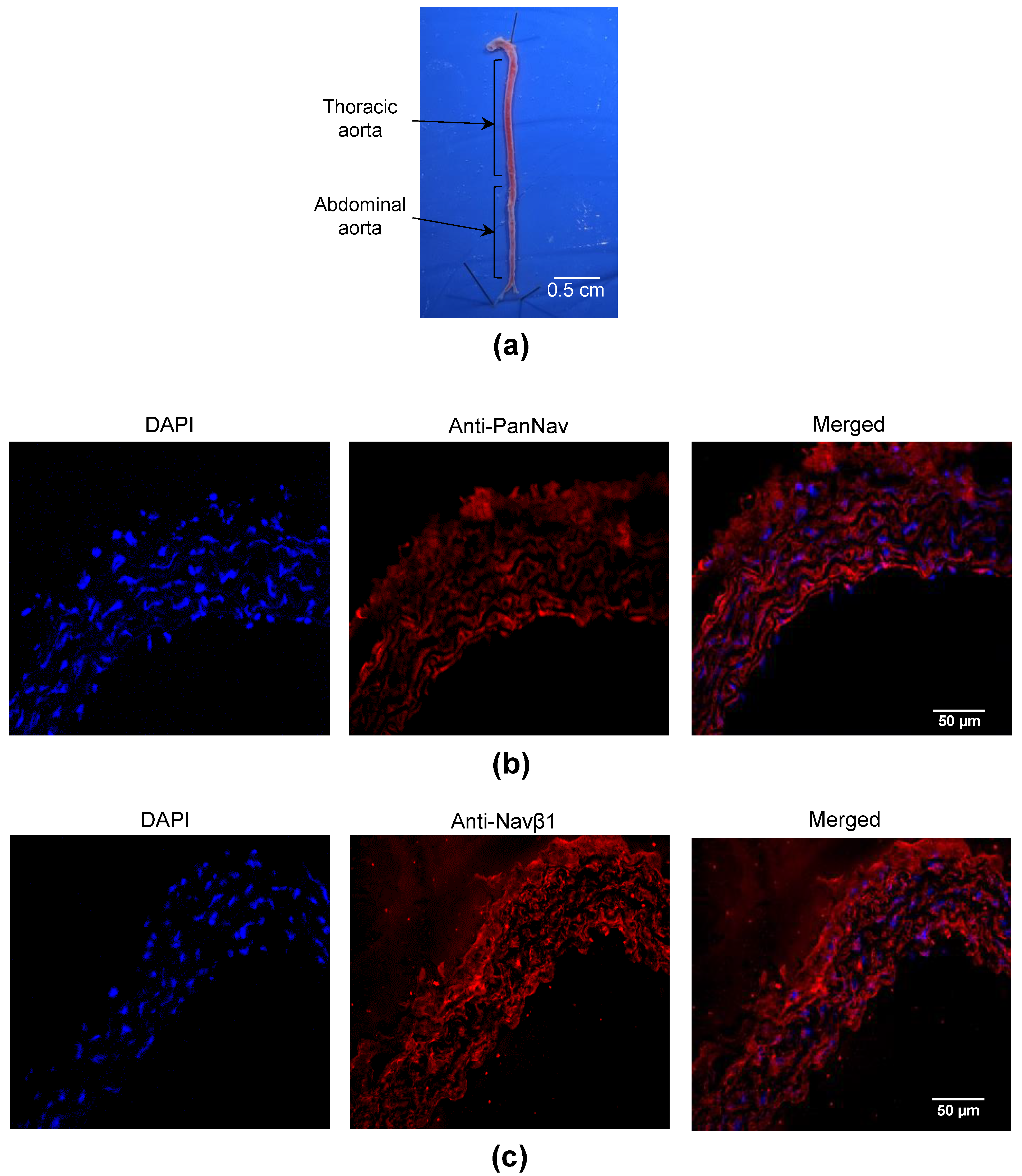
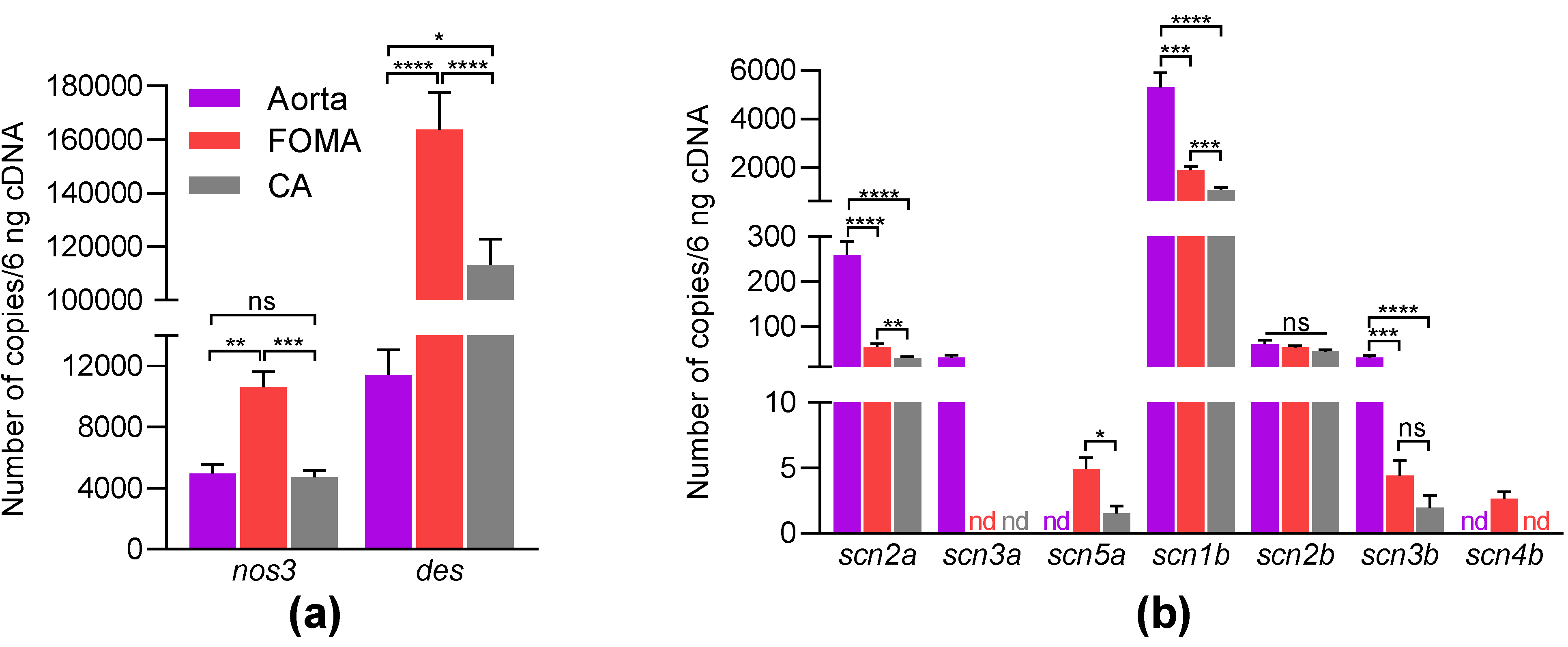
| Gene Name | Protein Name | Arteries | Number of Genes (Mean ± SEM, n = 11–12) | Pairwise Comparison | p-Value |
|---|---|---|---|---|---|
| scn2a | aorta | 258.58 ± 30.51 | aorta vs. FOMA | <0.0001 | |
| NaV1.2 | FOMA | 54.5 ± 6.9 | aorta vs. CA | <0.0001 | |
| CA | 30.7 ± 2.2 | FOMA vs. CA | 0.0051 | ||
| scn3a | aorta | 30.9 ± 5.8 | |||
| NaV1.3 | FOMA | nd | |||
| CA | nd | ||||
| scn5a | aorta | nd | 0.0005 | ||
| NaV1.5 | FOMA | 4.9 ± 0.9 | FOMA vs. CA | 0.022 | |
| CA | 1.5 ± 0.6 | 0.0122 | |||
| scn1b | aorta | 5300.9 ± 609.6 | aorta vs. FOMA | 0.0003 | |
| β1-subunit | FOMA | 1879.4 ± 157.7 | aorta vs. CA | <0.0001 | |
| CA | 1069.5 ± 93.2 | FOMA vs. CA | 0.0008 | ||
| scn2b | aorta | 60.8 ± 9.2 | aorta vs. FOMA | 0.5517 | |
| β2-subunit | FOMA | 54.2 ± 3.2 | aorta vs. CA | 0.2275 | |
| CA | 45.3 ± 3.1 | FOMA vs. CA | 0.1176 | ||
| scn3b | aorta | 31.8 ± 4.1 | aorta vs. FOMA | 0.0001 | |
| β3-subunit | FOMA | 4.4 ± 1.2 | aorta vs. CA | <0.0001 | |
| CA | 2.0 ± 0.9 | FOMA vs. CA | 0.1134 | ||
| Scn4b | aorta | nd | |||
| β4-subunit | FOMA | 2.7 ± 1.2 | |||
| CA | nd | ||||
| nos3 | aorta | 4961.4 ± 585.6 | aorta vs. FOMA | 0.0017 | |
| eNOS | FOMA | 10,619.8 ± 1007.8 | aorta vs. CA | 0.7544 | |
| CA | 4721.6 ± 449.2 | FOMA vs. CA | 0.0008 | ||
| des | aorta | 11,414.8 ± 1644.2 | aorta vs. FOMA | <0.0001 | |
| desmin | FOMA | 163,847.8 ± 13,848.2 | Aorta vs. CA | <0.0001 | |
| CA | 113,193.8 ± 9609.1 | FOMA vs. CA | 0.0156 |
| Gene Name | GenBank Accession Number | Forward Primer (5′−3′) | Reverse Primer (5′−3′) |
|---|---|---|---|
| scn1a | NM_018733.2 | ttgcaaggggcttctgttta | aggtccacaaactccgtcac |
| scn2a | NM_001099298.2 | gggttgcatatggtttccaa | cccaaggcatttgcagtta |
| scn3a | NM_018732.3 | tcctcagtagtggtgctttgg | gatgtaagtgaagactttgtcagca |
| scn4a | NM_133199.2 | gaaaaccatcacggtcatcc | tccgagagctttttcacagac |
| scn5a | NM_021544.4 | gccagatctctatggcaacc | ttgcccttattcagcacgat |
| scn8a | NM_001077499.2 | ctggtgctggttggacttc | gcccagggcattagctataa |
| scn9a | NM_001290674.1 | gctgagcctatcaatgcaga | acttggcagcatggaaatct |
| scn10a | NM_001205321.1 | tgggtagcttatggcttcaaa | ctatgaggcttgtgagggaga |
| scn11a | NM_011887.3 | ttcataatgtgtggcaactgg | ttattgcacgtggaaccatc |
| nos3 | NM_008713.4 | ccagtgccctgcttcatc | gcagggcaagttaggatcag |
| pecam1 | NM_008816.3 | ccagtgccctgcttcatc | gcagggcaagttaggatcag |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.; Proux, C.; Ehanno, W.; Réthoré, L.; Vessières, E.; Bourreau, J.; Favre, J.; Kauffenstein, G.; Mattei, C.; Tricoire-Leignel, H.; et al. Tetrodotoxin Decreases the Contractility of Mesenteric Arteries, Revealing the Contribution of Voltage-Gated Na+ Channels in Vascular Tone Regulation. Mar. Drugs 2023, 21, 196. https://doi.org/10.3390/md21030196
Park J, Proux C, Ehanno W, Réthoré L, Vessières E, Bourreau J, Favre J, Kauffenstein G, Mattei C, Tricoire-Leignel H, et al. Tetrodotoxin Decreases the Contractility of Mesenteric Arteries, Revealing the Contribution of Voltage-Gated Na+ Channels in Vascular Tone Regulation. Marine Drugs. 2023; 21(3):196. https://doi.org/10.3390/md21030196
Chicago/Turabian StylePark, Joohee, Coralyne Proux, William Ehanno, Léa Réthoré, Emilie Vessières, Jennifer Bourreau, Julie Favre, Gilles Kauffenstein, César Mattei, Hélène Tricoire-Leignel, and et al. 2023. "Tetrodotoxin Decreases the Contractility of Mesenteric Arteries, Revealing the Contribution of Voltage-Gated Na+ Channels in Vascular Tone Regulation" Marine Drugs 21, no. 3: 196. https://doi.org/10.3390/md21030196
APA StylePark, J., Proux, C., Ehanno, W., Réthoré, L., Vessières, E., Bourreau, J., Favre, J., Kauffenstein, G., Mattei, C., Tricoire-Leignel, H., Henrion, D., Legendre, C., & Legros, C. (2023). Tetrodotoxin Decreases the Contractility of Mesenteric Arteries, Revealing the Contribution of Voltage-Gated Na+ Channels in Vascular Tone Regulation. Marine Drugs, 21(3), 196. https://doi.org/10.3390/md21030196