An Overview on Antimicrobial Potential of Edible Terrestrial Plants and Marine Macroalgae Rhodophyta and Chlorophyta Extracts
Abstract
1. Introduction
2. Causes of Antimicrobial Resistance
3. An Overview of Terrestrial Plants’ Antibacterial Activity
| Terrestrial Plant | Extract Type | Microbes | Minimum Inhibitory Concentration (MIC) | Inhibition Zone Diameter (mm) | Reference |
|---|---|---|---|---|---|
| Anacardium occidentale | Ethanolic extract | Staphylococcusaureus | 1.56 mg/mL | 16.00 | [60] |
| Bacillus subtilis | 1.56 mg/mL | 15.00 | |||
| Escherichia coli | 1.56 mg/mL | 12.00 | |||
| Pseudomonas aeruginosa | 1.56 mg/mL | 14.00 | |||
| UAE | Staphylococcusaureus | <1.56 mg/mL | 14.00 | ||
| Bacillus subtilis | <1.56 mg/mL | 16.00 | |||
| Escherichia coli | 1.56 mg/mL | 14.00 | |||
| Pseudomonas aeruginosa | 1.56 mg/mL | 15.00 | |||
| MAE | Staphylococcusaureus | 0.78 mg/mL | 17.00 | ||
| Bacillus subtilis | <1.56 mg/mL | 16.00 | |||
| Escherichia coli | 1.56 mg/mL | 15.00 | |||
| Pseudomonas aeruginosa | 1.56 mg/mL | 16.00 | |||
| Careya sphaerica | Ethanolic extract | Staphylococcusaureus | <1.56 mg/mL | 17.00 | [60] |
| Bacillus subtilis | <1.56 mg/mL | 17.00 | |||
| Escherichia coli | 3.12 mg/mL | 11.00 | |||
| Pseudomonas aeruginosa | 1.56 mg/mL | 13.00 | |||
| UAE | Staphylococcusaureus | <1.56 mg/mL | 17.00 | ||
| Bacillus subtilis | <1.56 mg/mL | 16.00 | |||
| Escherichia coli | 3.12 mg/mL | 15.00 | |||
| Pseudomonas aeruginosa | 1.56 mg/mL | 14.00 | |||
| MAE | Staphylococcusaureus | <1.56 mg/mL | 18.00 | ||
| Bacillus subtilis | <1.56 mg/mL | 17.00 | |||
| Escherichia coli | 1.56 mg/mL | 16.00 | |||
| Pseudomonas aeruginosa | 1.56 mg/mL | 16.00 | |||
| Garcinia cowa | Ethanolic extract | Staphylococcusaureus | 3.12 mg/mL | 14.00 | [60] |
| Bacillus subtilis | 1.56 mg/mL | 11.00 | |||
| Escherichia coli | 12.5 mg/mL | 11.00 | |||
| Pseudomonas aeruginosa | 12.5 mg/mL | 12.00 | |||
| UAE | Staphylococcusaureus | <1.56 mg/mL | 14.00 | ||
| Bacillus subtilis | <1.56 mg/mL | 16.00 | |||
| Escherichia coli | 12.5 mg/mL | 11.00 | |||
| Pseudomonas aeruginosa | 12.5 mg/mL | 13.00 | |||
| MAE | Staphylococcusaureus | 0.78 mg/mL | 14.00 | ||
| Bacillus subtilis | <1.56 mg/mL | 16.00 | |||
| Escherichia coli | 12.5 mg/mL | 11.00 | |||
| Pseudomonas aeruginosa | 12.5 mg/mL | 12.00 | |||
| Glochidion wallichianum | Ethanolic extract | Staphylococcusaureus | <1.56 mg/mL | 16.00 | [60] |
| Bacillus subtilis | <1.56 mg/mL | 14.00 | |||
| Escherichia coli | 6.25 mg/mL | 10.00 | |||
| Pseudomonas aeruginosa | 3.12 mg/mL | 13.00 | |||
| UAE | Staphylococcusaureus | <1.56 mg/mL | 14.00 | ||
| Bacillus subtilis | <1.56 mg/mL | 15.00 | |||
| Escherichia coli | 3.12 mg/mL | 15.00 | |||
| Pseudomonas aeruginosa | 6.25 mg/mL | 13.00 | |||
| MAE | Staphylococcusaureus | 0.78 mg/mL | 14.00 | ||
| Bacillus subtilis | <1.56 mg/mL | 15.00 | |||
| Escherichia coli | 3.12 mg/mL | 15.00 | |||
| Pseudomonas aeruginosa | 3.12 mg/mL | 13.00 | |||
| Glycyrrhiza glabra | Ethanolic extract | Mycobacteriumtuberculosis H37Ra | 500 mg/mL | - | [56] |
| Mycobacteriumtuberculosis H37Rv | 500 mg/mL | - | |||
| Glycyrrhiza glabra | Methanolic extract | Staphylococcusaureus | 6.25 mg/mL | 10 ± 1.34 | [57] |
| Bacilluscereus | 12.5 mg/mL | 7 ± 1 | |||
| Escherichiacoli | 50 mg/mL | 6 ± 1.22 | |||
| Pseudomonas aeruginosa | 100 mg/mL | - | |||
| Gnetum gnemon var. temerum | Ethanolic extract | Staphylococcusaureus | 50.00 mg/mL | 10.00 | [60] |
| Bacillus subtilis | 25.00 mg/mL | - | |||
| Escherichia coli | 50.00 mg/mL | 9.00 | |||
| Pseudomonas aeruginosa | 50.00 mg/mL | 9.00 | |||
| UAE | Staphylococcusaureus | 50 mg/mL | 10.00 | ||
| Bacillus subtilis | 12.5 mg/mL | - | |||
| Escherichia coli | 50 mg/mL | 10.00 | |||
| Pseudomonas aeruginosa | 25 mg/mL | 10.00 | |||
| MAE | Staphylococcusaureus | 12.5 mg/mL | 11.00 | ||
| Bacillus subtilis | 6.25 mg/mL | - | |||
| Escherichia coli | 50 mg/mL | 11.00 | |||
| Pseudomonas aeruginosa | 25 mg/mL | 10.00 | |||
| Hedypnois cretica | Methanolic extract | Bacilluscereus | 2GP: 0.15 mg/mL | - | [59] |
| Staphylococcusaureus | 2GP: 0.60 mg/mL | - | |||
| Listeria monocytogenes | 2GP: 0.45 mg/mL | - | |||
| Escherichiacoli | 2GP: 0.20 mg/mL | - | |||
| Enterobactercloacae | 2GP: 0.30 mg/mL | - | |||
| Salmonella typhimurium | 2GP: 0.30 mg/mL | - | |||
| Hibiscus sabdariffa | Ethanolic extract | Bacillus cereus | 5 (% w/v) | 22.2 + 0.8 | [49] |
| Water extract | 0.625 (% w/v) | 17.0 + 1.1 | |||
| Ethanolic extract | Staphylococcus aureus | 2.5 (% w/v) | 21.5 + 2.1 | ||
| Water extract | 2.5 (% w/v) | 15.7 + 1.0 | |||
| Ethanolic extract | Escherichia coli | 5 (% w/v) | 21.1 + 1.3 | ||
| Water extract | 5 (% w/v) | 15.6 + 1.2 | |||
| Ethanolic extract | Salmonella enteritidis | 5 (% w/v) | 20.2 + 1.7 | ||
| Water extract | 10 (% w/v) | 14.0 + 1.9 | |||
| Ethanolic extract | Vibrio parahaemolyticus | 2.5 (% w/v) | 20.3 + 1.8 | ||
| Water extract | 5 (% w/v) | 15.9 + 1.7 | |||
| Ethanolic extract | Pseudomonas aeruginosa | 2.5 (% w/v) | 23.4 + 1.4 | ||
| Water extract | 5 (% w/v) | 13.9 + 1.9 | |||
| Hymenonema graecum | Methanolic extract | Bacilluscereus | 1GP: 0.20 mg/mL 2GP: 0.20 mg/mL | - | [59] |
| Staphylococcusaureus | 1GP: 0.60 mg/mL 2GP: 0.60 mg/mL | - | |||
| Listeria monocytogenes | 1GP: 0.60 mg/mL 2GP: 0.60 mg/mL | - | |||
| Escherichiacoli | 1GP: 0.60 mg/mL 2GP: 0.60 mg/mL | - | |||
| Enterobactercloacae | 1GP: 0.45 mg/mL 2GP: 0.60 mg/mL | - | |||
| Salmonella typhimurium | 1GP: 0.30 mg/mL 2GP: 0.30 mg/mL | - | |||
| Morus alba | Ethanolic extract | Staphylococcusaureus | - | 10.5 ± 1.15 | [58] |
| Bacilluscereus | 2500 µg/mL | 14.75 ± 0.15 | |||
| Escherichiacoli | - | 7.5 ± 0.15 | |||
| Pasteurellamultocida | 1250 µg/mL | 15.42 ± 0.15 | |||
| Salmonella enteritidis | 625 µg/mL | 12.02 ± 0.05 | |||
| Olea europaea | Ethanolic extract | Staphylococcusaureus | 625 µg/mL | 12.02 ± 2.05 | [58] |
| Bacilluscereus | 5000 µg/mL | 16.62 ± 1.05 | |||
| Escherichiacoli | 2500 µg/mL | 16.72 ± 0.55 | |||
| Pasteurellamultocida | 625 µg/mL | 9.12 ± 0.05 | |||
| Salmonella enteritidis | 5000 µg/mL | 18.02 ± 0.05 | |||
| Picris echioides | Methanolic extract | Bacilluscereus | 1GP: 0.075 mg/mL 2GP: 0.15 mg/mL | - | [59] |
| Staphylococcusaureus | 1GP: 0.45 mg/mL 2GP: 0.30 mg/mL | - | |||
| Listeria monocytogenes | 1GP: 0.60 mg/mL 2GP: 0.30 mg/mL | - | |||
| Escherichiacoli | 1GP: 0.45 mg/mL 2GP: 0.15 mg/mL | - | |||
| Enterobactercloacae | 1GP: 0.30 mg/mL 2GP: 0.20 mg/mL | - | |||
| Salmonella typhimurium | 1GP: 0.60 mg/mL 2GP: 0.20 mg/mL | - | |||
| Psidium guajava | Ethanolic extract | Staphylococcusaureus | 1250 µg/mL | 15.62 ± 1.15 | [58] |
| Bacilluscereus | - | 10.05 ± 0.15 | |||
| Escherichiacoli | 625 µg/mL | 10.55 ± 0.15 | |||
| Pasteurellamultocida | 5000 µg/mL | 18.02 ± 0.95 | |||
| Salmonella enteritidis | 625 µg/mL | 10.12 ± 0.55 | |||
| Reichardia picroides | Methanolic extract | Bacilluscereus | 1GP: 0.15 mg/mL 2GP: 0.15 mg/mL | - | [59] |
| Staphylococcusaureus | 1GP: 0.30 mg/mL 2GP: 0.30 mg/mL | - | |||
| Listeria monocytogenes | 1GP: 0.30 mg/mL 2GP: 0.30 mg/mL | - | |||
| Escherichiacoli | 1GP: 0.15 mg/mL 2GP: 0.30 mg/mL | - | |||
| Enterobactercloacae | 1GP: 0.30 mg/mL 2GP: 0.30 mg/mL | - | |||
| Salmonella typhimurium | 1GP: 0.30 mg/mL 2GP: 0.60 mg/mL | - | |||
| Rosmarinus officinalis | Ethanolic extract | Bacillus cereus | 5 (% w/v) | 19.8 ± 0.8 | [49] |
| Water extract | 1.25 (% w/v) | 13.9 ± 1.2 | |||
| Ethanolic extract | Staphylococcus aureus | 1.25 (% w/v) | 19.8 ± 0.4 | ||
| Water extract | 20 (% w/v) | 12.7 ± 0.4 | |||
| Ethanolic extract | Escherichia coli | 5 (% w/v) | 21.1 ± 0.9 | ||
| Water extract | 20 (% w/v) | 12.5 ± 0.7 | |||
| Ethanolic extract | Salmonella enteritidis | 2.5 (% w/v) | 20.7 ± 1.2 | ||
| Ethanolic extract | Vibrio parahaemolyticus | - | - | ||
| Water extract | - | - | |||
| Ethanolic extract | Pseudomonas aeruginosa | - | - | ||
| Water extract | - | - | |||
| Salvia officinalis | Ethanolic extract | Staphylococcusaureus | 5000 µg/mL | 17.05 ± 1.05 | [58] |
| Bacilluscereus | 625 µg/mL | 16.45 ± 1.05 | |||
| Escherichiacoli | 2500 µg/mL | 19.25 ± 0.65 | |||
| Pasteurellamultocida | - | 9.05 ± 1.05 | |||
| Salmonella enteritidis | 2500 µg/mL | 16.25 ± 0.75 | |||
| Scolymus hispanicus | Methanolic extract | Bacilluscereus | 2GP: 0.10 mg/mL | - | [59] |
| Staphylococcusaureus | 2GP: 0.30 mg/mL | - | |||
| Listeria monocytogenes | 2GP: 0.20 mg/mL | - | |||
| Escherichiacoli | 2GP: 0.10 mg/mL | - | |||
| Enterobactercloacae | 2GP: 0.15 mg/mL | - | |||
| Salmonella typhimurium | 2GP: 0.15 mg/mL | - | |||
| Sonchus oleraceus | Methanolic extract | Bacilluscereus | 1GP: 0.20 mg/mL 2GP: 0.15 mg/mL | - | [59] |
| Staphylococcusaureus | 1GP: 0.45 mg/mL 2GP: 0.30 mg/mL | - | |||
| Listeria monocytogenes | 1GP: 0.45 mg/mL 2GP: 0.60 mg/mL | - | |||
| Escherichiacoli | 1GP: 0.45 mg/mL 2GP: 0.30 mg/mL | - | |||
| Enterobactercloacae | 1GP: 0.60 mg/mL 2GP: 0.30 mg/mL | - | |||
| Salmonella typhimurium | 1GP: 0.45 mg/mL 2GP: 0.30 mg/mL | - | |||
| Syzygium aromaticum | Ethanolic extract | Bacillus cereus | 2.5 (% w/v) | 18.2 ± 3.2 | [49] |
| Water extract | 0.313 (% w/v) | 15.1 ± 0.9 | |||
| Ethanolic extract | Staphylococcus aureus | 2.5 (% w/v) | 16.7 ± 1.0 | ||
| Water extract | 5 (% w/v) | 13.6 ± 1.3 | |||
| Ethanolic extract | Escherichia coli | 2.5 (% w/v) | 17.4 ± 0.8 | ||
| Water extract | 5 (% w/v) | 13.2 ± 1.6 | |||
| Ethanolic extract | Salmonella enteritidis | 5 (% w/v) | 15.1 ± 1.4 | ||
| Water extract | 5 (% w/v) | 12.2 ± 1.1 | |||
| Ethanolic extract | Vibrio parahaemolyticus | 0.625 (% w/v) | 14.7 ± 2.0 | ||
| Water extract | 2.5 (% w/v) | 13.1 ± 1.8 | |||
| Ethanolic extract | Pseudomonas aeruginosa | 5 (% w/v) | 17.0 ± 0.5 | ||
| Water extract | 10 (% w/v) | 13.2 ± 1.4 | |||
| Taraxacum officinale | Methanolic extract | Bacilluscereus | 1GP: 0.037 mg/mL 2GP: 0.20 mg/mL | - | [59] |
| Staphylococcus aureus | 1GP: 0.30 mg/mL 2GP: 0.90 mg/mL | - | |||
| Listeria monocytogenes | 1GP: 0.30 mg/mL 2GP: 0.90 mg/mL | - | |||
| Escherichiacoli | 1GP: 0.15 mg/mL 2GP: 0.30 mg/mL | - | |||
| Enterobactercloacae | 1GP: 0.15 mg/mL 2GP: 0.30 mg/mL | - | |||
| Salmonella typhimurium | 1GP: 0.15 mg/mL 2GP: 0.60 mg/mL | - | |||
| Taraxacum sp. | Methanolic extract | Bacilluscereus | 1GP: 0.075 mg/mL 2GP: 0.075 mg/mL | - | [59] |
| Staphylococcusaureus | 1GP: 0.60 mg/mL 2GP: 0.30 mg/mL | - | |||
| Enterobactermonocytogenes | 1GP: 0.45 mg/mL 2GP: 0.45 mg/mL | - | |||
| Escherichiacoli | 1GP: 0.20 mg/mL 2GP: 0.90 mg/mL | - | |||
| Enterobactercloacae | 1GP: 0.20 mg/mL 2GP: 0.20 mg/mL | - | |||
| Salmonella typhimurium | 1GP: 0.20 mg/mL 2GP: 0.30 mg/mL | - | |||
| Thymus vulgaris | Ethanolic extract | Bacillus cereus | 5 (% w/v) | 17.3 ± 0.7 | [49] |
| Water extract | 5 (% w/v) | 13.8 ± 1.1 | |||
| Ethanolic extract | Staphylococcus aureus | 5 (% w/v) | 15.9 ± 0.3 | ||
| Water extract | 2.5 (% w/v) | 12.2 ± 0.7 | |||
| Ethanolic extract | Escherichia coli | 10 (% w/v) | 15.9 ± 0.3 | ||
| Water extract | 5 (% w/v) | 12.2 ± 0.7 | |||
| Water extract | Salmonella enteritidis | 5 (% w/v) | 11.8 ± 1.4 | ||
| Water extract | Vibrio parahaemolyticus | 2.5 (% w/v) | 13.9 ± 1.3 | ||
| Ethanolic extract | 10 (% w/v) | 14.3 ± 0.1 | |||
| Water extract | Pseudomonas aeruginosa | - | - | ||
| Ethanolic extract | - | - | |||
| Urospermum picroides | Methanolic extract | Bacilluscereus | 1GP: 0.15 mg/mL 2GP: 0.15 mg/mL | - | [59] |
| Staphylococcusaureus | 1GP: 0.90 mg/mL 2GP: 0.90 mg/mL | - | |||
| Listeria monocytogenes | 1GP: 0.90 mg/mL 2GP: 0.30 mg/mL | - | |||
| Escherichiacoli | 1GP: 0. 90 mg/mL 2GP: 0.45 mg/mL | - | |||
| Enterobacter cloacae | 1GP: 0.45 mg/mL 2GP: 0.60 mg/mL | - | |||
| Salmonella typhimurium | 1GP: 0.45 mg/mL 2GP: 0.30 mg/mL | - | |||
| Ziziphusspina christi | Ethanolic extract | Staphylococcusaureus | 625 µg/mL | 11.82 ± 2.5 | [58] |
| Bacilluscereus | 625 µg/mL | 13.52 ± 2.1 | |||
| Escherichiacoli | - | 10.02 ± 0.05 | |||
| Pasteurellamultocida | - | 8.52 ± 2.5 | |||
| Salmonella enteritidis | 625 µg/mL | 12.82 ± 2.5 |
4. Antimicrobial Activity from Rhodophyta and Chlorophyta Extracts
4.1. Rhodophyta
| Rhodophyta | Extract Type | Microbes | Minimum Inhibitory Concentration (MIC) | Inhibition Zone Diameter (mm) | Reference |
|---|---|---|---|---|---|
| Asparagopsistaxiformis | Methanolic extract | Staphylococcus aureus | 0.5 mg/mL | >15 | [75] |
| Serratia sp. | 0.5 mg/mL | - | |||
| Klebseilla sp. | 0.5 mg/mL | >1 | |||
| Salmonella sp. | 0.5 mg/mL | - | |||
| Escherichia coli | 0.5 mg/mL | >10 | |||
| Klebseilla pneumonia | 0.5 mg/mL | >1 | |||
| Pseudomonas aeruginosa | 0.5 mg/mL | - | |||
| Pseudomonas fluorescens | 0.5 mg/mL | >10 | |||
| Vibrio proteolyticus | 0.5 mg/mL | >1 | |||
| Streptococcus sp. | 0.5 mg/mL | 10 | |||
| Bacillussubtilis | 0.5 mg/mL | 10 | |||
| Chondrus crispus | Bacillus subtilis | 12.5 mg/mL | - | [72] | |
| Gelidium sp. | Water extract | Salmonella enterica | 12.5 mg/mL | >10 | [76] |
| Klebsiella pneumoniae | 50 mg/mL | >11 | |||
| Listeria monocytogenes | 50 mg/mL | 11 | |||
| Enterobacter aerogenes | 25 mg/mL | >11 | |||
| Proteus mirabilis | 50 mg/mL | >11 | |||
| Vibrio parahaemolyticus | nd | >11 | |||
| Vibrio alginolyticus | nd | 13 | |||
| Bacillus licheniformis | 25 mg/mL | 11 | |||
| Bacillus cereus | 0.625 mg/mL | >11 | |||
| Bacillus subtilis | 3.125 mg/mL | >10 | |||
| Escherichia coli | 50 mg/mL | >13 | |||
| Pseudomonas putida | - | - | |||
| Pseudomonas fluorescens | - | - | |||
| Gracilaria corticata | Methanolic extract | Escherichia coli | 100 µg/mL | 7 ± 0.01 | [67] |
| Photobacterium sp. | 100 µg/mL | 6 ± 0.04 | |||
| Pseudomonas fluorescens | 100 µg/mL | 8 ± 0.1 | |||
| Staphylococcus aureus | 100 µg/mL | 4 ± 0.10 | |||
| Bacillus subtilis | 100 µg/mL | 8 ± 0.01 | |||
| Dimethyl sulfoxide (DMSO) extract | Escherichia coli | 100 µg/mL | 5 ± 0.10 | ||
| Photobacterium sp. | 100 µg/mL | 4 ± 0.30 | |||
| Pseudomonas fluorescens | 100 µg/mL | 4 ± 0.05 | |||
| Staphylococcus aureus | 100 µg/mL | 6 ± 0.05 | |||
| Bacillus subtilis | 100 µg/mL | 5 ± 0.12 | |||
| Gracilaria edulis | Methanolic extract | Escherichia coli | 100 µg/mL | 3 ± 0.01 | [67] |
| Photobacterium sp. | 100 µg/mL | 1 ± 0.00 | |||
| Pseudomonas fluorescens | 100 µg/mL | 3 ± 0.05 | |||
| Staphylococcus aureus | 100 µg/mL | 3 ± 0.05 | |||
| Bacillus subtilis | 100 µg/mL | 3 ± 0.03 | |||
| Dimethyl sulfoxide (DMSO) extract | Escherichia coli | 100 µg/mL | 4.5 ± 0.01 | ||
| Photobacterium sp. | 100 µg/mL | 4 ± 0.01 | |||
| Pseudomonas fluorescens | 100 µg/mL | 4 ± 0.10 | |||
| Staphylococcus aureus | 100 µg/mL | 3 ± 0.00 | |||
| Gracilaria edulis | Methanolic extracts | Klebsiella oxytoca | 0.3 mg/mL | 21 | [71] |
| Escherichia coli | 0.3 mg/mL | 19 | |||
| Staphylococcus aureus | 0.3 mg/mL | 18 | |||
| Pseudomonas aeruginosa | 0.3 mg/mL | 16 | |||
| Bacillus subtilis | 0.3 mg/mL | 23 | |||
| Serratia sp. | 0.3 mg/mL | 20 | |||
| Salmonella sp. | 0.3 mg/mL | 22 | |||
| Grateloupia turuturu | Ethanolic extract | Staphylococcus aureus | 10 mg/mL | - | [70] |
| Escherichia coli | 10 mg/mL | - | |||
| Polysaccharides (carrageenan) | Staphylococcus aureus | 7.5 mg/mL | - | ||
| Escherichia coli | 7.5 mg/mL | - | |||
| Hypnea valentiae | Methanolic extract | Klebsiella oxytoca | 0.3 mg/mL | 17 | [71] |
| Escherichia coli | 0.3 mg/mL | 12 | |||
| Staphylococcus aureus | 0.3 mg/mL | 14 | |||
| Pseudomonas aeruginosa | 0.3 mg/mL | 11 | |||
| Bacillus subtilis | 0.3 mg/mL | 15 | |||
| Serratia sp. | 0.3 mg/mL | 13 | |||
| Salmonella sp. | 0.3 mg/mL | 16 | |||
| Kappaphycus alvarezii | Ethanolic extract | Escherichia coli | - | - | [73] |
| Bacillus cereus | 0.5 mg/mL | <10 | |||
| Hot water extract | Escherichia coli | - | - | ||
| Bacillus cereus | 0.5 mg/mL | <10 | |||
| Osmundea pinnatifida | Bacillus subtilis | 1.56 mg/mL | - | [72] | |
| Porphyra umbilicalis | Aqueous extract | Bacillus subtilis | 3.13 mg/mL | - | [72] |
| Pyropiaorbicularis | Methanolic extract | Staphylococcus aureus | 250 mg/mL | nd | [74] |
| Escherichia coli | 500 mg/mL |
4.2. Chlorophyta
| Chlorophyta | Extract Type | Microbes | Minimum Inhibitory Concentration (MIC) | Inhibition Zone Diameter (mm) | Reference |
|---|---|---|---|---|---|
| Caulerpa cupressoides | Benzene | Escherichia coli | nd | 6 | [81] |
| Klebsiella pneumoniae | nd | 6 | |||
| Pseudomonas aeruginosa | nd | 5 | |||
| Streptococcus pyogens | nd | 6 | |||
| Staphylococcus aureus | nd | 6 | |||
| Butanol | Escherichia coli | nd | 7 | ||
| Klebsiella pneumoniae | nd | - | |||
| Pseudomonas aeruginosa | nd | 7 | |||
| Streptococcus pyogens | nd | - | |||
| Staphylococcus aureus | nd | 6 | |||
| Propanol | Escherichia coli | nd | 7 | ||
| Klebsiella pneumoniae | nd | - | |||
| Pseudomonas aeruginosa | nd | 8 | |||
| Streptococcus pyogens | nd | 7 | |||
| Staphylococcus aureus | nd | 6 | |||
| Acetone | Escherichia coli | nd | 9 | ||
| Klebsiella pneumoniae | nd | 6 | |||
| Pseudomonas aeruginosa | nd | 5 | |||
| Streptococcus pyogens | nd | 8 | |||
| Staphylococcus aureus | nd | 7 | |||
| Water | Escherichia coli | nd | 6 | ||
| Klebsiella pneumoniae | nd | 6 | |||
| Pseudomonas aeruginosa | nd | - | |||
| Streptococcus pyogens | nd | - | |||
| Staphylococcus aureus | nd | - | |||
| Caulerpa lentillifera | Water extract | Methicillin-resistant Staphylococcus aureus, Escherichia coli | 5 µg/mL | nd | [78] |
| Caulerpa lentillifera | Methanolic extract | Escherichia coli | 136.50 ± 0.85 mg/mL | nd | [80] |
| Staphylococcus aureus | 125.25 ± 3.78 mg/mL | nd | |||
| Streptococcus sp. | 175.25 ± 0.23 mg/mL | nd | |||
| Salmonella sp. | 140.50 ± 0.55 mg/mL | nd | |||
| Caulerpa racemosa | Water extract | Methicillin-resistant Staphylococcus aureus, Escherichia coli | 5 µg/mL | nd | [78] |
| Caulerpa racemosa | Methanolic extract | Vibrio fluvialis | nd | 9 ± 0.50 | [79] |
| Caulerpa racemosa var. clavifera f. microphysa | Methanolic extract | Escherichia coli | 245.25 ± 2.11 mg/mL | nd | [80] |
| Staphylococcus aureus | 225.50 ± 0.45 mg/mL | nd | |||
| Streptococcus sp. | 450.75 ± 1.09 mg/mL | nd | |||
| Salmonella sp. | 275. 20 ± 0.66 mg/mL | nd | |||
| Caulerpa racemosa var. laetevirens | Methanolic extract | Escherichia coli | 360.50 ± 2.14 mg/mL | nd | [80] |
| Staphylococcus aureus | 375.75 ± 0.07 mg/mL | nd | |||
| Streptococcus sp. | 450. 25 ± 0.42 mg/mL | nd | |||
| Salmonella sp. | 345. 25 ± 0.35 mg/mL | nd | |||
| Caulerpa taxifolia | Chloroform/methanol extract | Escherichia coli | 640 µg/mL | 7.33–10.67 | [82] |
| Staphylococcus aureus | - | 10.00–11.17 | |||
| Chaetomorpha anteninna | Chloroform/methanol extract | Escherichia coli | 640 µg/mL | 7.33–10.67 | [82] |
| Staphylococcus aureus | 640 µg/mL | 10.00–11.17 | |||
| Chaetomorpha linum | Chloroform/methanol extract | Escherichia coli | >640 µg/mL | 7.33–10.67 | [82] |
| Staphylococcus aureus | - | 10.00–11.17 | |||
| Cladophora vagabunda | Chloroform/methanol extract | Escherichia coli | 640 µg/mL | 7.33–10.67 | [82] |
| Staphylococcus aureus | nd | 10.00–11.17 | |||
| Codium dichotomum | Methanolic extract | Staphylococcus aureus | nd | ≥20 | [90] |
| Escherichia coli | nd | <10 | |||
| Klebliella pneumoniae | nd | <10 | |||
| Enterobacter faecalis | nd | <10 | |||
| Codium fragile | Methanolic extract | Staphylococcus aureus | nd | ≥20 | [90] |
| Escherichia coli | nd | <10 | |||
| Klebliella pneumoniae | nd | <10 | |||
| Enterobacter faecalis | nd | <10 | |||
| Codium fragile | Hexane extract | Bacillus subtilis | 250 µg/mL | 6.5 | [89] |
| Bacillus cereus | 1000 µg/mL | - | |||
| Staphylococcus epidermidis | - | - | |||
| Staphylococcus aureus | - | - | |||
| Methicillin-resistant Staphylococcus aureus | - | 6.5 | |||
| Enterobacter cloacae | 1000 µg/mL | 7 | |||
| Enterobacter cloacae | - | - | |||
| Escherichia coli | 500 µg/mL | - | |||
| Escherichia coli (Hemorrhagic, O157:H7) | 500 µg/mL | - | |||
| Pseudomonas aeruginosa | <50 µg/mL | - | |||
| Proteus vulgaris | 250 µg/mL | - | |||
| Salmonella typhimurium | - | - | |||
| Candida albicans | - | ||||
| Methanol extract | Bacillus subtilis | 250 µg/mL | 6.5 | ||
| Bacillus cereus | 500 µg/mL | - | |||
| Staphylococcus epidermidis | 500 µg/mL | - | |||
| Staphylococcus aureus | 500 µg/mL | - | |||
| Methicillin-resistant Staphylococcus aureus | - | 7.5 | |||
| Enterobacter cloacae | - | 7 | |||
| Escherichia coli | - | - | |||
| Escherichia coli (Hemorrhagic, O157:H7) | - | - | |||
| Pseudomonas aeruginosa | 250 µg/mL | - | |||
| Proteus vulgaris | 250 µg/mL | - | |||
| Salmonella typhimurium | - | - | |||
| Candida albicans | - | - | |||
| Dichloromethane extract | Bacillus subtilis | - | 6.5 | ||
| Bacillus cereus | - | - | |||
| Staphylococcus epidermidis | - | - | |||
| Staphylococcus aureus | - | - | |||
| Methicillin-resistant Staphylococcus aureus | - | - | |||
| Enterobacter cloacae | - | 7 | |||
| Escherichia coli | - | 7 | |||
| Escherichia coli (Hemorrhagic, O157:H7) | - | - | |||
| Pseudomonas aeruginosa | - | - | |||
| Proteus vulgaris | - | - | |||
| Salmonella typhimurium | - | - | |||
| Candida albicans | - | - | |||
| Codium intricatum | Methanol extract | Methicillin-resistant Staphylococcus aureus | 250 µg/mL | nd | [88] |
| Bacillus cereus | 500 µg/mL | nd | |||
| Listeria monocytogenes | 500 µg/mL | nd | |||
| Streptococcus mutans | - | nd | |||
| Pseudomonas aeruginosa | - | nd | |||
| Escherichia coli | - | nd | |||
| Enterobacter cloacae | - | nd | |||
| Salmonella typhimurium | - | nd | |||
| Aeromonas hydrophila | - | nd | |||
| Codium tomentosum | Methanolic extract | Staphylococcus aureus | nd | ≥20 | [90] |
| Escherichia coli | nd | <10 | |||
| Klebliella pneumoniae | nd | <10 | |||
| Enterobacter faecalis | nd | <10 | |||
| Enteromorpha compressa | Ethanolic extract | Salmonella sp. | nd | 15 | [86] |
| Klebsiella sp. | nd | 10 | |||
| Proteus sp. | nd | 5 | |||
| Staphylococcus aureus | nd | 5 | |||
| Enteromorpha sp. | Methanol:acetone extract | Pseudomonas aeruginosa | 150 g/mL | 11 ± 0.2 | [85] |
| Staphylococcus aureus | 100 g/mL | 10 ± 0.2 | |||
| Escherichia coli | 100 g/mL | 11 ± 0.2 | |||
| Ulva fasciata | Chloroform/methanol extract | Escherichia coli | 640 µg/mL | 7.33–10.67 | [82] |
| Staphylococcus aureus | >640 µg/mL | 10.00–11.17 | |||
| Ulva intestinalis | Methanolic extract | Vibrio fluvialis | nd | 7 ± 0.56 | [79] |
| Ulva intestinalis | Benzene | Escherichia coli | nd | 6 | [81] |
| Klebsiella pneumoniae | nd | - | |||
| Pseudomonas aeruginosa | nd | 6 | |||
| Streptococcus pyogens | nd | 6 | |||
| Staphylococcus aureus | nd | 6 | |||
| Butanol | Escherichia coli | nd | 7 | ||
| Klebsiella pneumoniae | nd | 7 | |||
| Pseudomonas aeruginosa | nd | 6 | |||
| Streptococcus pyogens | nd | 7 | |||
| Staphylococcus aureus | nd | 6 | |||
| Propanol | Escherichia coli | nd | 6 | ||
| Klebsiella pneumoniae | nd | - | |||
| Pseudomonas aeruginosa | nd | 7 | |||
| Streptococcus pyogens | nd | 7 | |||
| Staphylococcus aureus | nd | 7 | |||
| Acetone | Escherichia coli | nd | - | ||
| Klebsiella pneumoniae | nd | - | |||
| Pseudomonas aeruginosa | nd | - | |||
| Streptococcus pyogens | nd | - | |||
| Staphylococcus aureus | nd | - | |||
| Water | Escherichia coli | nd | 6 | ||
| Klebsiella pneumoniae | nd | 10 | |||
| Pseudomonas aeruginosa | nd | - | |||
| Streptococcus pyogens | nd | - | |||
| Staphylococcus aureus | nd | - | |||
| Ulva intestinalis | Methanolic extract | Escherichia coli | nd | - | [84] |
| Klebsiella pneumoniae | nd | - | |||
| Proteus mirabilis | nd | - | |||
| Pseudomonas aeruginosa | nd | - | |||
| Salmonella typhi | nd | - | |||
| Vibrio alginolyticus | nd | - | |||
| Vibrio harveyi | nd | - | |||
| Vibrio parahaemolyticus | nd | - | |||
| Bacillus cereus | 1024 µg/mL | 6.85 ± 0.17 | |||
| Enterobacter faecalis | nd | - | |||
| Listeria monocytogenes | - | ||||
| Methicillin-resistant Staphylococcus aureus | >1024 µg/mL | 12.71 ± 0.98 | |||
| Staphylococcus aureus | >1024 µg/mL | 8.41 ± 0.56 | |||
| Ethanolic extract | Escherichia coli | nd | - | ||
| Klebsiella pneumoniae | nd | - | |||
| Proteus mirabilis | nd | - | |||
| Pseudomonas aeruginosa | nd | - | |||
| Salmonella typhi | nd | - | |||
| Vibrio alginolyticus | nd | - | |||
| Vibrio harveyi | nd | - | |||
| Vibrio parahaemolyticus | nd | - | |||
| Bacillus cereus | nd | - | |||
| Enterobacter faecalis | nd | - | |||
| Listeria monocytogenes | >1024 µg/mL | 7.96 ± 0.38 | |||
| Methicillin-resistant Staphylococcus aureus | nd | - | |||
| Staphylococcus aureus | nd | - | |||
| Dichloromethane extract | Escherichia coli | nd | - | ||
| Klebsiella pneumoniae | nd | - | |||
| Proteus mirabilis | nd | - | |||
| Pseudomonas aeruginosa | nd | - | |||
| Salmonella typhi | nd | - | |||
| Vibrio alginolyticus | nd | - | |||
| Vibrio harveyi | nd | - | |||
| Vibrio parahaemolyticus | nd | - | |||
| Bacillus cereus | nd | - | |||
| Enterobacter faecalis | 1024 µg/mL | - | |||
| Listeria monocytogenes | nd | 9.89 ± 0.24 | |||
| Methicillin-resistant Staphylococcus aureus | nd | - | |||
| Staphylococcus aureus | nd | - | |||
| Hexane extract | Escherichia coli | nd | - | ||
| Klebsiella pneumoniae | nd | - | |||
| Proteus mirabilis | nd | - | |||
| Pseudomonas aeruginosa | nd | - | |||
| Salmonella typhi | nd | - | |||
| Vibrio alginolyticus | nd | - | |||
| Vibrio harveyi | nd | - | |||
| Vibrio parahaemolyticus | nd | - | |||
| Bacillus cereus | 256 µg/mL | 7.28 ± 0.02 | |||
| Enterobacter faecalis | nd | - | |||
| Listeria monocytogenes | 1024 µg/mL | 10.55 ± 0.29 | |||
| Methicillin-resistant Staphylococcus aureus | 256 µg/mL | 16.4 ± 2.4 | |||
| Staphylococcus aureus | 256 µg/mL | 12.13 ± 0.16 | |||
| Ulva lactuca | Benzene | Escherichia coli | nd | 6 | [81] |
| Klebsiella pneumoniae | nd | 6 | |||
| Pseudomonas aeruginosa | nd | 6 | |||
| Streptococcus pyogens | nd | 6 | |||
| Staphylococcus aureus | nd | 6 | |||
| Butanol | Escherichia coli | nd | 6 | ||
| Klebsiella pneumoniae | nd | 7 | |||
| Pseudomonas aeruginosa | nd | - | |||
| Streptococcus pyogens | nd | - | |||
| Staphylococcus aureus | nd | 8 | |||
| Propanol | Escherichia coli | nd | 6 | ||
| Klebsiella pneumoniae | nd | 6 | |||
| Pseudomonas aeruginosa | nd | 6 | |||
| Streptococcus pyogens | nd | - | |||
| Staphylococcus aureus | nd | 7 | |||
| Acetone | Escherichia coli | nd | - | ||
| Klebsiella pneumoniae | nd | - | |||
| Pseudomonas aeruginosa | nd | 8 | |||
| Streptococcus pyogens | nd | - | |||
| Staphylococcus aureus | nd | - | |||
| Water | Escherichia coli | nd | - | ||
| Klebsiella pneumoniae | nd | - | |||
| Pseudomonas aeruginosa | nd | - | |||
| Streptococcus pyogens | nd | - | |||
| Staphylococcus aureus | nd | - | |||
| Ulva lactuca | Chloroform/methanol extract | Escherichia coli | >640 µg/mL | 7.33–10.67 | [82] |
| Staphylococcus aureus | 640 µg/mL | 10.00–11.17 | |||
| Ulva lactuca | N–hexane extract | Staphylococcus aureus | nd | 10 | [87] |
| Staphylococcus epidermidis | nd | 12 | |||
| Escherichia coli | nd | 11 | |||
| Pseudomonas aeruginosa | nd | 12 | |||
| Chloroform extract | Staphylococcus aureus | nd | 11 | ||
| Staphylococcus epidermidis | nd | 11 | |||
| Escherichia coli | nd | 11 | |||
| Pseudomonas aeruginosa | nd | 10 | |||
| ethanol: water (1:1) extract | Staphylococcus aureus | nd | 9 | ||
| Staphylococcus epidermidis | nd | 10 | |||
| Escherichia coli | nd | 9 | |||
| Pseudomonas aeruginosa | nd | 9 | |||
| Ulva lactuca | Polysaccharide (ulvan) | Staphylococcus aureus | - | - | [102] |
| Enterobacter faecalis | - | - | |||
| Bacillus subtilis | 12.50 ± 0.0 mg/mL | 15 ± 0.50 | |||
| Listeria monocytogenes | - | - | |||
| Pseudomonas aeruginosa | 25.00 ± 0.0 mg/mL | 12 ± 0.10 | |||
| Escherichia coli | 6.25 ± 0.0 mg/mL | 11 ± 0.21 | |||
| Klebsiella pneumoniae | 6.25 ± 0.0 mg/mL | 12 ± 0.00 | |||
| Bordetella pertussis | - | - | |||
| Ulva reticulata | Polysaccharide (ulvan) | Bacillus cereus | nd | - | [101] |
| Enterobacter faecalis | nd | - | |||
| Enterobacter cloacae | nd | 20.00 ± 1.00 | |||
| Staphylococcus aureus | nd | - | |||
| Escherichia coli | nd | 18 ± 0.5 | |||
| Pseudomonas aeruginosa | nd | <18 ± 0.5 | |||
| Vibrio harveyi | nd | - | |||
| Ulva sp. | Methanolic extract | Staphylococcus saprophyticus | 16 µg/mL | 29 ± 0.592 | [83] |
| Staphylococcus epidermidis | 4 µg/mL | 26 ± 0.548 | |||
| Streptococcus agalactiae (group B) | 0.5 µg/mL | 14 ± 0.592 | |||
| Enterobacter faecalis | 2 µg/mL | 21 ± 0.592 | |||
| Stenotrophomonas maltophilia | 1 µg/mL | 15 ± 0.592 | |||
| Salmonella enterica | 2 µg/mL | 11 ± 0.592 | |||
| Shigella sonnei | 2 µg/mL | 12 ± 0.592 | |||
| Pproteus vulgaris | 2 µg/mL | 20 ± 0.592 | |||
| Pproteus mirabilis | - | - | |||
| Enterobacter cloacae | - | - | |||
| Haemophilus influenzae | - | - |
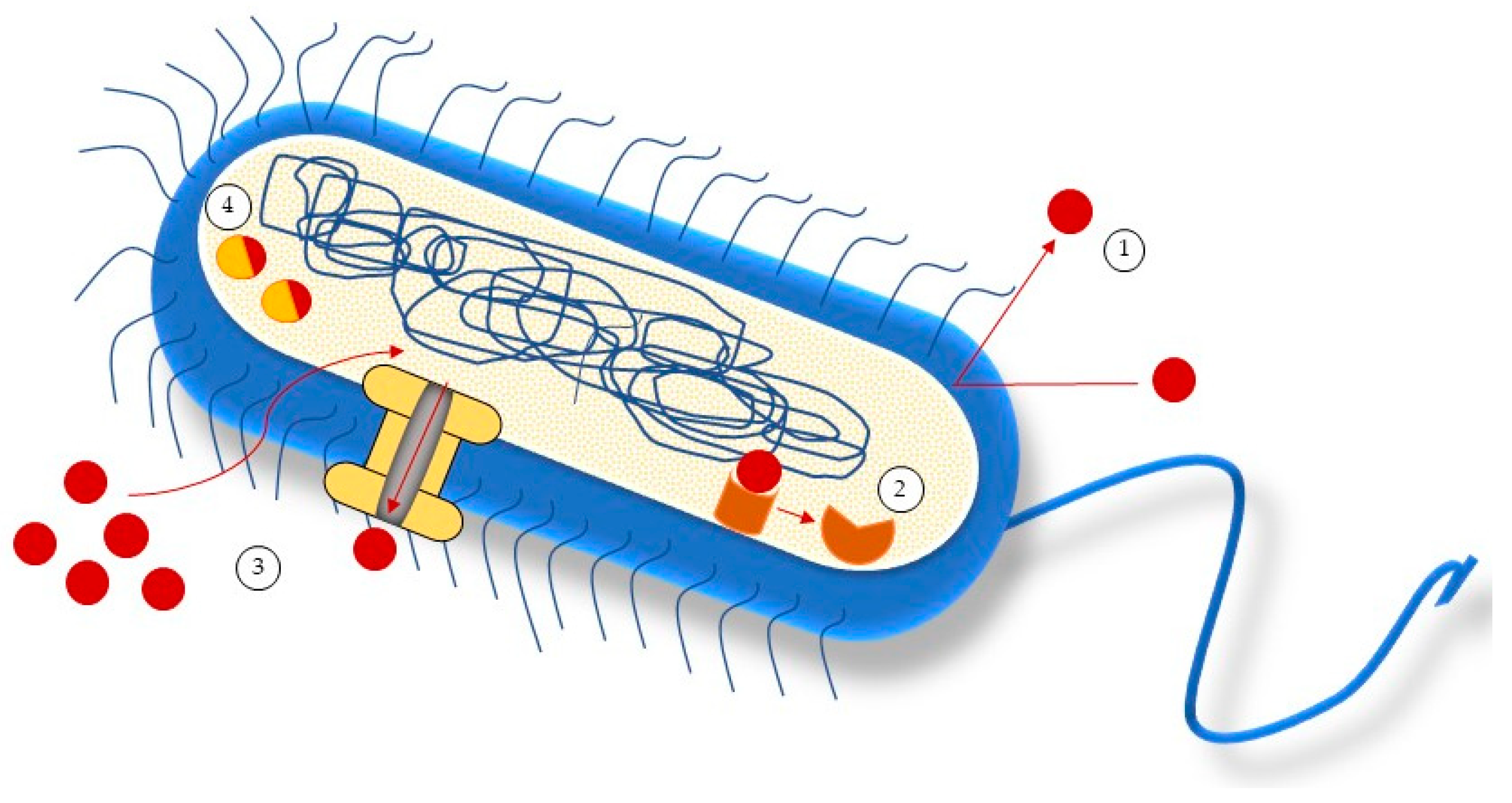
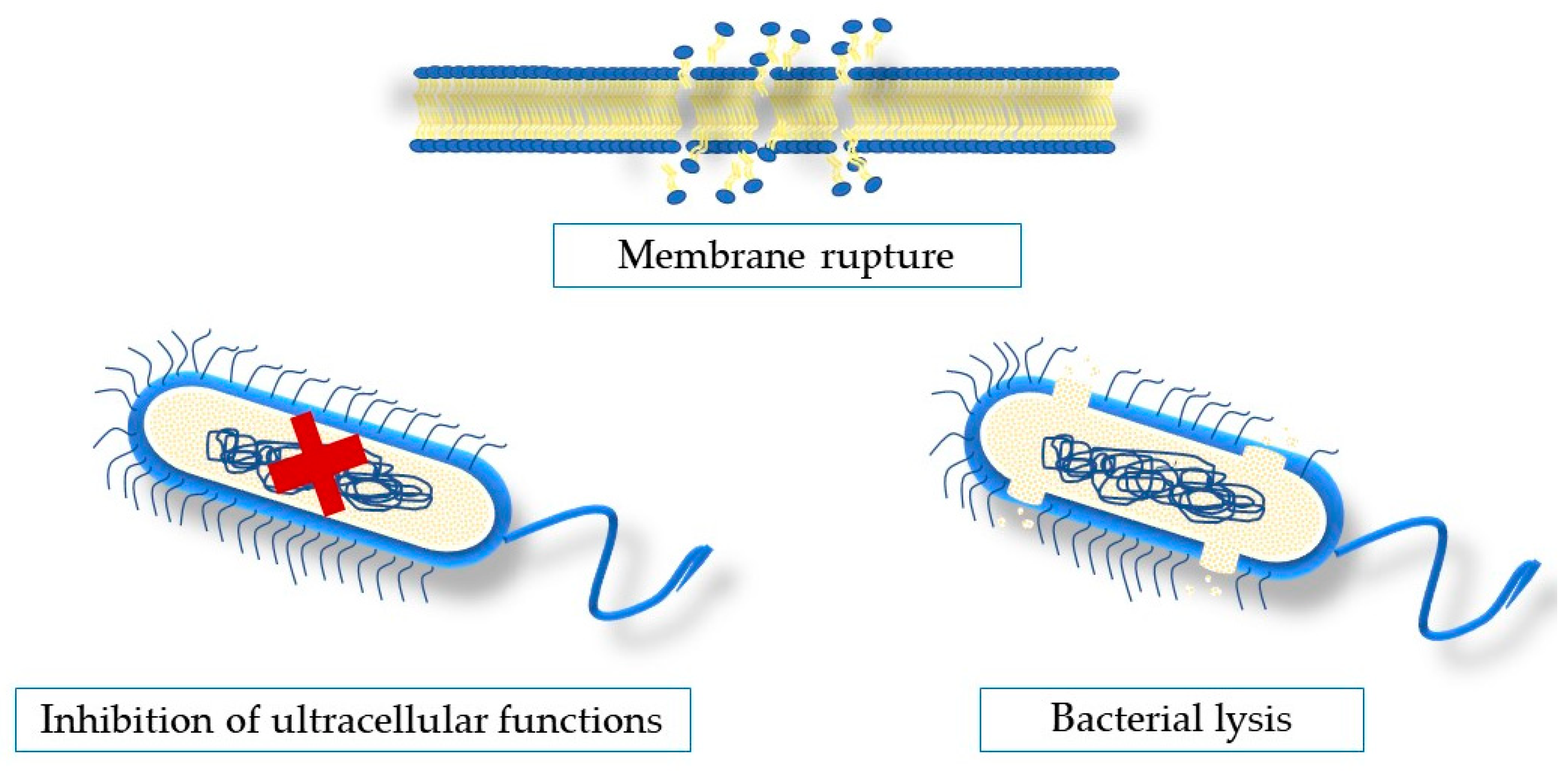
5. Antimicrobial Mechanisms of Action of Seaweeds Compounds
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hoffman, P.S. Antibacterial Discovery: 21st Century Challenges. Antibiotics 2020, 9, 213. [Google Scholar] [CrossRef]
- Breijyeh, Z.; Jubeh, B.; Karaman, R. Resistance of Gram-Negative Bacteria to Current Antibacterial Agents and Overcoming Approaches to Resolve It. Molecules 2020, 25, 1340. [Google Scholar] [CrossRef] [PubMed]
- Silhavy, T.J.; Kahne, D.; Walker, S. The Bacterial Cell Envelope. Cold Spring Harb. Perspect. Biol. 2010, 2, 1–17. [Google Scholar] [CrossRef]
- Yuan, R.; Hou, Y.; Sun, W.; Yu, J.; Liu, X.; Niu, Y.; Lu, J.J.; Chen, X. Natural Products to Prevent Drug Resistance in Cancer Chemotherapy: A Review. Ann. N. Y. Acad. Sci. 2017, 1401, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; El-Hossary, E.M.; Oelschlaeger, T.A.; Donia, M.S.; Quinn, R.J.; Abdelmohsen, U.R. Potential of Marine Natural Products against Drug-Resistant Bacterial Infections. Lancet Infect. Dis. 2019, 19, e237–e245. [Google Scholar] [CrossRef]
- Qin, Y. Health Benefits of Bioactive Seaweed Substances; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128133125. [Google Scholar]
- Gerwick, W.H.; Moore, B.S. Lessons from the Past and Charting the Future of Marine Natural Products Drug Discovery and Chemical Biology. Chem. Biol. 2012, 19, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Weinheimer, A.J.; Spraggins, R.L. The Occurrence of Two New Prostaglandin Derivatives (15-Epi-PGA2 and Its Acetate, Methyl Ester) in the Gorgonian Plexaura homomalla Chemistry of Coelenterates. XV. Tetrahedron Lett. 1969, 10, 5185–5188. [Google Scholar] [CrossRef]
- Masuda, M.; Abe, T.; Suzuki, T.; Suzuki, M. Morphological and Chemotaxonomic Studies on Laurencia Composita and L. okamurae (Ceramiales, Rhodophyta). Phycologia 1996, 35, 550–562. [Google Scholar] [CrossRef]
- Taylor, R.E. Tedanolide and the Evolution of Polyketide Inhibitors of Eukaryotic Protein Synthesis. Nat. Prod. Rep. 2008, 25, 854–861. [Google Scholar] [CrossRef]
- Santos, J.D.; Vitorino, I.; Reyes, F.; Vicente, F.; Lage, O.M. From Ocean to Medicine: Pharmaceutical Applications of Metabolites from Marine Bacteria. Antibiotics 2020, 9, 455. [Google Scholar] [CrossRef]
- Liang, X.; Luo, D.; Luesch, H. Advances in Exploring the Therapeutic Potential of Marine Natural Products. Pharmacol. Res. 2019, 147, 104373. [Google Scholar] [CrossRef]
- Singh, S.B.; Barrett, J.F. Empirical Antibacterial Drug Discovery-Foundation in Natural Products. Biochem. Pharmacol. 2006, 71, 1006–1015. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, A.A.; Al-Askar, A.A.; Almaary, K.S.; Dawoud, T.M.; Sholkamy, E.N.; Bakri, M.M. Antimicrobial Activity of Some Plant Extracts against Bacterial Strains Causing Food Poisoning Diseases. Saudi J. Biol. Sci. 2018, 25, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Takó, M.; Beáta Kerekes, E.; Zambrano, C.; Kotogán, A.; Papp, T.; Krisch, J.; Vágvölgyi, C. Plant Phenolics and Phenolic-Enriched Extracts as Antimicrobial Agents against Food-Contaminating Microorganisms. Antioxidants 2020, 9, 165. [Google Scholar] [CrossRef]
- Manandhar, S.; Luitel, S.; Dahal, R.K. In Vitro Antimicrobial Activity of Some Medicinal Plants against Human Pathogenic Bacteria. J. Trop. Med. 2019, 2019, 1895340. [Google Scholar] [CrossRef]
- Alzoreky, N.S.; Nakahara, K. Antibacterial Activity of Extracts from Some Edible Plants Commonly Consumed in Asia. Int. J. Food Microbiol. 2003, 80, 223–230. [Google Scholar] [CrossRef]
- Fankam, A.G.; Kuete, V.; Voukeng, I.K.; Kuiate, J.R.; Pages, J. Antibacterial Activities of Selected Cameroonian Spices and Their Synergistic Effects with Antibiotics against Multidrug-Resistant Phenotypes. Complement. Altern. Med. 2011, 11, 104. [Google Scholar] [CrossRef]
- Nascimento, G.G.F.; Locatelli, J.; Freitas, P.C.; Silva, G.L. Antibacterial Activity of Plant Extracts and Phytochemicals on Antibiotic-Resistant Bacteria. Brazilian J. Microbiol. 2000, 31, 247–256. [Google Scholar] [CrossRef]
- Pereira, L. Macroalgae. Encyclopedia 2021, 1, 17. [Google Scholar] [CrossRef]
- Bhuyar, P.; Rahim, M.H.A.; Sundararaju, S.; Ramaraj, R.; Maniam, G.P.; Govindan, N. Synthesis of Silver Nanoparticles Using Marine Macroalgae Padina sp. and Its Antibacterial Activity towards Pathogenic Bacteria. Beni-Suef Univ. J. Basic Appl. Sci. 2020, 9, 1–15. [Google Scholar] [CrossRef]
- Silva, A.; Silva, S.A.; Lourenço-Lopes, C.; Jimenez-Lopez, C.; Carpena, M.; Gullón, P.; Fraga-Corral, M.; Domingues, V.F.; Fátima Barroso, M.; Simal-Gandara, J.; et al. Antibacterial Use of Macroalgae Compounds against Foodborne Pathogens. Antibiotics 2020, 9, 712. [Google Scholar] [CrossRef]
- Kizhakkekalam, V.K.; Chakraborty, K. Marine Macroalgae-Associated Heterotrophic Firmicutes and Gamma-Proteobacteria: Prospective Anti-Infective Agents against Multidrug Resistant Pathogens. Arch. Microbiol. 2020, 202, 905–920. [Google Scholar] [CrossRef]
- Arguelles, E.D.L.R.; Monsalud, R.G.; Sapin, A.B. Chemical Composition and in Vitro Antioxidant and Antibacterial Activities of Sargassum Vulgare c. Agardh from Lobo, Batangas, Philippines. J. Int. Soc. Southeast Asian Agric. Sci. 2019, 25, 112–122. [Google Scholar]
- Salvador, N.; Gómez Garreta, A.; Lavelli, L.; Ribera, M.A. Antimicrobial Activity of Iberian Macroalgae. Sci. Mar. 2007, 71, 101–113. [Google Scholar] [CrossRef]
- Maschek, J.A.; Baker, B.J. The Chemistry of Algal Secondary Metabolism. In Algal Chemical Ecology; Springer: Berlin/Heidelberg, Germany, 2008; pp. 1–20. ISBN 9783540741800. [Google Scholar]
- Ayyad, S.-E.N.; Al-Footy, K.O.; Alarif, W.M.; Sobahi, T.R.; Bassaif, S.A.; Makki, M.S.; Asiri, A.M.; Al Halawani, A.Y.; Bandria, A.F.; Bandria, F.A.A. Bioactive C15 Acetogenins from the Red Alga Laurencia obtusa. Chem. Pharm. Bull. 2011, 59, 1294–1298. [Google Scholar] [CrossRef] [PubMed]
- OECD, (Organisation for Economic Co-operation and Development). Stemming the Superbug Tide: Just A Few Dollars More. In OECD Health Policy Studies; OECD Publishing: Paris, France, 2018; pp. 1–12. ISBN 9789264307582. [Google Scholar]
- Thompson, T. The Staggering Death Toll of Drug-Resistant Bacteria. Nature, 2022; Epub ahead print. [Google Scholar] [CrossRef]
- Davies, J.; Davies, D. Origins and Evolution of Antibiotic Resistance. Microbiol. Mol. Biol. Rev. 2010, 74, 417–433. [Google Scholar] [CrossRef] [PubMed]
- Holmes, A.H.; Moore, L.S.P.; Steinbakk, M.; Regmi, S.; Karkey, A.; Guerin, P.J.; Piddock, L.J. V Understanding the Mechanisms and Drivers of Antimicrobial Resistance. Lancet Infect. Dis. 2016, 387, 176–187. [Google Scholar] [CrossRef]
- Ventola, C.L. The Antibiotic Resistance Crisis Part 1: Causes and Threats. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Read, A.F.; Woods, R.J. Antibiotic Resistance Management. Evol. Med. Public Heal. 2020, 1, 147. [Google Scholar] [CrossRef]
- Lushniak, B.D. Antibiotic Resistance: A Public Health Crisis. Surg. Gen. Perspect. 2014, 129, 314–316. [Google Scholar] [CrossRef]
- World Health Orgnization Antimicrobial Resistance: Global Report on Surveillance. 2014. Available online: https://apps.who.int/iris/handle/10665/112642 (accessed on 8 February 2023).
- Okeke, I.N.; Lamikanra, A.; Edelman, R. Socioeconomic and Behavioral Factors Leading to Acquired Bacterial Resistance to Antibiotics in Developing Countries. Emerg. Infect. Dis. 1999, 5, 18–27. [Google Scholar] [CrossRef]
- Chokshi, A.; Sifri, Z.; Cennimo, D.; Horng, H. Global Contributors to Antibiotic Resistance. J. Glob. Infect. Dis. 2019, 11, 36–42. [Google Scholar] [CrossRef]
- Malmir, S.; Bahreinian, M.; Zahiri Yeganeh, S.; Mirnejad, R.; Moosazadeh Moghaddam, M.; Saberi, F. Molecular Mechanisms of Resistance to Conventional Antibiotics in Bacteria. Int. J. Med. Rev. 2018, 5, 118–129. [Google Scholar] [CrossRef]
- Kapoor, G.; Saigal, S.; Elongavan, A. Action and Resistance Mechanisms of Antibiotics: A Guide for Clinicians. J. Anaesthesiol. Clin. Pharmacol. 2017, 33, 300–305. [Google Scholar] [CrossRef]
- Lambert, T. Antibiotics That Affect the Ribosome. OIE Rev. Sci. Technol. 2012, 31, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Sales, A.J.; Shadbad, N.N.; Kaleybar, V.P. The Investigation of the Antibacterial Effects of Ethanol Extract of Cichorium The Investigation of the Antibacterial Effects of Ethanol Extract of Cichorium intybus L. on Antibiotic-Resistant Staphylococcus aureus Strains. Bull. Environ. Pharmacol. Life Sci. 2018, 4, 161–164. [Google Scholar]
- Cowan, M.M. Plant Products as Antimicrobial Agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef] [PubMed]
- Porras, G.; Chassagne, F.; Lyles, J.T.; Marquez, L.; Dettweiler, M.; Salam, A.M.; Samarakoon, T.; Shabih, S.; Farrokhi, D.R.; Quave, C.L. Ethnobotany and the Role of Plant Natural Products in Antibiotic Drug Discovery. Chem. Rev. 2021, 121, 3495–3560. [Google Scholar] [CrossRef]
- Anand, U.; Jacobo-Herrera, N.; Altemimi, A.; Lakhssassi, N. A Comprehensive Review on Medicinal Plants as Antimicrobial Therapeutics: Potential Avenues of Biocompatible Drug Discovery. Metabolites 2019, 9, 258. [Google Scholar] [CrossRef]
- Bereksi, M.S.; Hassaïne, H.; Bekhechi, C.; Abdelouahid, D.E. Evaluation of Antibacterial Activity of Some Medicinal Plants Extracts Commonly Used in Algerian Traditional Medicine against Some Pathogenic Bacteria. Pharmacogn. J. 2018, 10, 507–512. [Google Scholar] [CrossRef]
- Zaynab, M.; Fatima, M.; Sharif, Y.; Zafar, M.H.; Ali, H.; Ali, K. Microbial Pathogenesis Role of Primary Metabolites in Plant Defense against Pathogens. Microb. Pathog. 2019, 137, 103728. [Google Scholar] [CrossRef]
- Gonelimali, F.D.; Lin, J.; Miao, W.; Xuan, J.; Charles, F.; Chen, M.; Hatab, S.R. Antimicrobial Properties and Mechanism of Action of Some Plant Extracts Against Food Pathogens and Spoilage Microorganisms. Front. Microbiol. 2018, 9, 1639. [Google Scholar] [CrossRef]
- Burt, S. Essential Oils: Their Antibacterial Properties and Potential Applications in Foods—A Review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Saxena, S. Glycyrrhiza Glabra: Medicine over the Millennium. Nat. Prod. Radiance 2005, 4, 358–367. [Google Scholar]
- Ikeda, K.; Murashima, N.; Chayama, K.; Tsubota, A.; Koida, I.; Suzuki, Y.; Kobayashi, M.; Kumada, H. The Long Term Efficacy of Glycyrrhizin in Chronic Hepatitis C Patients. Am. Cancer Soc. 1997, 79, 1494–1500. [Google Scholar]
- Baba, M.; Shigeta, S. Antiviral Activity of Glycyrrhizin against Varicella-Zoster Virus in Vitro. Antiviral Res. 1987, 7, 99–107. [Google Scholar] [CrossRef]
- Thirugnanam, S.; Xu, L.; Ramaswamy, K.; Gnanasekar, M. Glycyrrhizin Induces Apoptosis in Prostate Cancer Cell Lines DU-145 and LNCaP. Oncol. Rep. 2008, 20, 1387–1392. [Google Scholar] [CrossRef]
- Lin, D.; Zhong, W.; Li, J.; Zhang, B.; Song, G.; Hu, T.; Lin, D.; Zhong, W.; Li, J.; Zhang, B. Involvement of BID Translocation in Glycyrrhetinic Acid and 11-Deoxy Glycyrrhetinic Acid-Induced Attenuation of Gastric Cancer Growth Involvement of BID Translocation in Glycyrrhetinic Acid and 11-Deoxy Glycyrrhetinic Acid-Induced Attenuation of Gastric C. Nutr. Cancer 2014, 66, 463–473. [Google Scholar] [CrossRef]
- Gupta, V.K.; Fatima, A.; Faridi, U.; Negi, A.S.; Shanker, K.; Kumar, J.K.; Rahuja, N.; Luqman, S.; Sisodia, B.S.; Saikia, D.; et al. Antimicrobial Potential of Glycyrrhiza glabra Roots. J. Ethnopharmacol. 2008, 116, 377–380. [Google Scholar] [CrossRef] [PubMed]
- Jafari-sales, A.; Bolouri, P. Evaluation of the Antimicrobial Effects of Glycyrrhiza glabra L. on Some Gram Positive and Gram Negative Pathogenic Bacteria in Laboratory. Jorjani Biomed. J. 2018, 6, 78–84. [Google Scholar] [CrossRef]
- Hemeg, H.A.; Moussa, I.M.; Ibrahim, S.; Dawoud, T.M.; Alhaji, J.H.; Mubarak, A.S.; Kabli, S.A.; Alsubki, R.A.; Tawfik, A.M.; Marouf, S.A. Saudi Journal of Biological Sciences Antimicrobial Effect of Different Herbal Plant Extracts against Different Microbial Population. Saudi J. Biol. Sci. 2020, 27, 3221–3227. [Google Scholar] [CrossRef] [PubMed]
- Petropoulos, S.A.; Fernandes, Â.; Tzortzakis, N.; Sokovic, M.; Ciric, A.; Barros, L.; Ferreira, I.C.F.R. Bioactive Compounds Content and Antimicrobial Activities of Wild Edible Asteraceae Species of the Mediterranean Flora under Commercial Cultivation Conditions. Food Res. Int. 2019, 119, 859–868. [Google Scholar] [CrossRef]
- Junsathian, P.; Nakamura, S.; Katayama, S.; Rawdkuen, S. Antioxidant and Antimicrobial Activities of Thai Edible Plant Extracts Prepared Using Different Extraction Techniques. Molecules 2022, 27, 6489. [Google Scholar] [CrossRef]
- Zazharskyi, V.V.; Davydenko, P.; Kulishenko, O.; Borovik, I.V.; Brygadyrenko, V.V. Antimicrobial Activity of 50 Plant Extracts. Biosyst. Divers. 2019, 27, 163–169. [Google Scholar] [CrossRef]
- Kumar, S.; Kishore, Y.; Padhi, L.; Luyten, W. Antimicrobial Activity of Select Edible Plants from Odisha, India against Food-Borne Pathogens. LWT Food Sci. Technol. 2019, 113, 108246. [Google Scholar] [CrossRef]
- Hochma, E.; Yarmolinsky, L.; Khalfin, B.; Nisnevitch, M.; Ben-shabat, S.; Nakonechny, F. Antimicrobial Effect of Phytochemicals from Edible Plants. Processes 2021, 9, 2089. [Google Scholar] [CrossRef]
- Chibane, L.B.; Degraeve, P.; Ferhout, H.; Bouajila, J.; Oulahal, N. Plant Antimicrobial Polyphenols as Potential Natural Food Preservatives. J. Sci. Food Agric. 2018, 99, 1457–1474. [Google Scholar] [CrossRef]
- Rosaline, X.D.; Sakthivelkumar, S.; Rajendran, K.; Janarthanan, S. Screening of Selected Marine Algae from the Coastal Tamil Nadu, South India for Antibacterial Activity. Asian Pac. J. Trop. Biomed. 2012, 2, S140–S146. [Google Scholar] [CrossRef]
- Vallinayagam, K.; Arumugam, R.; Kannan, R.R.R.; Thirumaran, G.; Anantharaman, P. Antibacterial Activity of Seaweeds of Pudumadam Coast Antibacterial Activity of Some Selected Seaweeds from Pudumadam Coastal Regions. Glob. J. Pharmacol. 2016, 3, 50–52. [Google Scholar]
- Arulkumar, A.; Rosemary, T.; Paramasivam, S. Phytochemical Composition, In Vitro Antioxidant, Antibacterial Potential and GC-MS Analysis of Red Seaweeds (Gracilaria corticata and Gracilaria edulis) from Palk Bay, India. Biocatal. Agric. Biotechnol. 2018, 15, 63–71. [Google Scholar] [CrossRef]
- Ivanovic, J.; Dimitrijevic-brankovic, S.; Misic, D.; Ristic, M.; Zizovic, I. Evaluation and Improvement of Antioxidant and Antibacterial Activities of Supercritical Extracts from Clove Buds. J. Funct. Foods 2012, 5, 416–423. [Google Scholar] [CrossRef]
- Devi, K.P.; Nisha, S.A.; Sakthivel, R.; Pandian, S.K. Eugenol (an Essential Oil of Clove) Acts as an Antibacterial Agent against Salmonella typhi by Disrupting the Cellular Membrane. J. Ethnopharmacol. 2010, 130, 107–115. [Google Scholar] [CrossRef]
- Cardoso, I.; Cotas, J.; Rodrigues, A.; Ferreira, D.; Osório, N.; Pereira, L. Extraction and Analysis of Compounds with Antibacterial Potential from the Red Alga Grateloupia turuturu. J. Mar. Sci. Eng. 2019, 7, 220. [Google Scholar] [CrossRef]
- Mahendran, S.; Maheswari, P.; Sasikala, V.; Rubika, J.J.; Pandiarajan, J. In Vitro Antioxidant Study of Polyphenol from Red Seaweeds Dichotomously Branched Gracilaria edulis and Robust Sea Moss Hypnea valentiae. Toxicol. Rep. 2021, 8, 1404–1411. [Google Scholar] [CrossRef] [PubMed]
- Freitas, M.V.; Inácio, L.G.; Ruas, A.; Silva, I.A.; Mouga, T.; Pereira, L.; Afonso, C. Antioxidant and Antimicrobial Properties of Selected Red Seaweeds from Central Portugal. Appl. Sci. 2022, 13, 157. [Google Scholar] [CrossRef]
- Bhuyar, P.; Rahim, M.H.; Sundararaju, S.; Maniam, G.P.; Govindan, N. Antioxidant and Antibacterial Activity of Red Seaweed: Kappaphycus alvarezii against Pathogenic Bacteria. Glob. J. Environ. Sci. Manag. 2020, 6, 47–58. [Google Scholar]
- García, V.; Uribe, E.; Vega-Gálvez, A.; Delporte, C.; Valenzuela-Barra, G.; López, J.; Pastén, A. Health-Promoting Activities of Edible Seaweed Extracts from Chilean Coasts: Assessment of Antioxidant, Anti-Diabetic, Anti-Inflammatory and Antimicrobial Potential. Rev. Chil. Nutr. 2020, 47, 792–800. [Google Scholar] [CrossRef]
- Saim, S.; Sahnouni, F.; Bouhadi, D.; Kharbouche, S. The Antimicrobial Activity of Two Marine Red Algae Collected from Algerian West Coast. Trends Pharmacol. Sci. 2021, 7, 233–242. [Google Scholar] [CrossRef]
- Miranda, J.M.; Trigo, M.; Barros-Velázquez, J.; Aubourg, S.P. Antimicrobial Activity of Red Alga Flour (Gelidium sp.) and Its Effect on Quality Retention of Scomber scombrus during Refrigerated Storage. Foods 2022, 11, 904. [Google Scholar] [CrossRef] [PubMed]
- dos Santos Oliviera, A.; Veiga-santos, P.; Prado, B.; Filho, D.; Sudatti, D.B.; Pereira, R.C.; Nakamura, C.V.; De Londrina, U.E.; Celso, R.; Cid, G.; et al. Effect of Elatol, Isolated from Red Seaweed Laurencia dendroidea, on Leishmania amazonensis. Mar. Drugs 2010, 8, 2733–2743. [Google Scholar] [CrossRef]
- Yap, W.-F.; Tay, V.; Tan, S.-H.; Yow, Y.-Y.; Chew, J. Decoding Antioxidant and Antibacterial Potentials of Malaysian Green Seaweeds: Caulerpa racemosa and Caulerpa lentillifera. Antibiotics 2019, 8, 152. [Google Scholar] [CrossRef]
- Aftabuddin, S.; Akter, S.; Hossen, S.; Rahman, M.A. Antioxidant, Antibacterial and Cytotoxic Activity of Caulerpa racemosa (Forsskål) J. Agardh and Ulva (Enteromorpha) intestinalis L. Bangladesh J. Sci. Ind. Res. 2020, 55, 237–244. [Google Scholar]
- Nagappan, T.; Vairappan, C.S. Nutritional and Bioactive Properties of Three Edible Species of Green Algae, Genus Caulerpa (Caulerpaceae). J. Appl. Phycol. 2014, 26, 1019–1027. [Google Scholar] [CrossRef]
- Ravikumar, S.; Anburajan, L.; Ramanathan, G.; Kaliaperumal, N. Screening of Seaweed Extracts against Antibiotic Resistant Post Operative Infectious Pathogens. Seaweed Res. Util. 2002, 24, 95–99. [Google Scholar]
- Agbaje-Daniels, F.; Adeleye, A.; Nwankwo, D.; Adeniyi, B.; Seku, F.; Beukes, D. Antibacterial Activities of Selected Green Seaweeds from West African Coast. EC Pharmacol. Toxicol. 2020, 4, 84–92. [Google Scholar]
- Al-Zahrani, A.; Al-Haj, N.; Omer, H.; Al-Judaibi, A. Impact of Extracts of Marine Macroalgae on Multidrug-Resistant Bacteria. J. Microbiol. Res. 2014, 4, 18–24. [Google Scholar] [CrossRef]
- Srikong, W.; Bovornreungroj, N.; Mittraparparthorn, P.; Bovornreungroj, P. Antibacterial and Antioxidant Activities of Differential Solvent Extractions from the Green Seaweed Ulva intestinalis. ScienceAsia 2017, 43, 88–95. [Google Scholar] [CrossRef]
- Swathi, N.; Kumar, A.G.; Parthasarathy, V.; Sankarganesh, P. Isolation of Enteromorpha Species and Analyzing Its Crude Extract for the Determination of in Vitro Antioxidant and Antibacterial Activities. Biomass Convers. Biorefinery 2022, 3, 1–10. [Google Scholar] [CrossRef]
- Priya, N.; Kokila, M.; Janani, J. Comparative Phytochemical Studies and Antibacterial Activity of Green and Brown Seaweeds Extract of Enteromorpha compressa and Padina pavonica. Int. J. Res. Eng. Sci. Manag. 2021, 4, 136–139. [Google Scholar]
- Cadar, E.; Negreanu-Pirjol, T.; Negreanu-Pirjo, B.-S. Antioxidant and Antibacterial Potential of Ulva lactuca Species from Romanian Black Sea Coast. Eur. J. Nat. Sci. Med. 2022, 8705, 26–38. [Google Scholar] [CrossRef]
- Arguelles, E.D.L.R. Evaluation of Nutritional Composition and In Vitro Antioxidant and Antibacterial Activities of Codium intricatum Okamura from Ilocos Norte (Philippines). Jordan J. Biol. Sci. 2020, 13, 375–382. [Google Scholar]
- Koz, F.F.Y.; Yavasoglu, N.U.; Demirel, Z.; Sukatar, A.; Ozdemir, G. Antimicrobial Activities of Some Macroalgal Essential Oil and Extracts from Antioxidant and Antimicrobial Activities of Codium fragile (Suringar) Hariot (Chlorophyta) Essential Oil and Extracts. Asian J. Chem. 2009, 21, 1197–1209. [Google Scholar]
- Ibtissam, C.; Hassane, R.; Jose, M.; Francisco, D.S.; Antonio, G.V.; Hassan, B.; Mohamed, K. Screening of Antibacterial Activity in Marine Green and Brown Macroalgae from the Coast of Morocco. Afr. J. Biotechnol. 2009, 8, 1258–1262. [Google Scholar] [CrossRef]
- Kaeffer, B.; Benard, C.; Lahaye, M.; Herve, M.B.; Cherbut, C. Biological Properties of Ulvan, a New Source of Green Seaweed Sulfated Polysaccharides, on Cultured Normal and Cancerous Colonic Epithelial Cells. Planta Med. 1999, 65, 527–531. [Google Scholar] [CrossRef]
- Mao, W.; Zang, X.; Li, Y.; Zhang, H. Sulfated Polysaccharides from Marine Green Algae Ulva conglobata and Their Anticoagulant Activity. J. Appl. Phycol. 2006, 18, 9–14. [Google Scholar] [CrossRef]
- Jiao, G.; Yu, G.; Zhang, J.; Ewart, H. Chemical Structures and Bioactivities of Sulfated Polysaccharides from Marine Algae. Mar. Drugs 2011, 9, 196–223. [Google Scholar] [CrossRef]
- Alves, A.; Sousa, R.A.; Reis, R.L. In Vitro Cytotoxicity Assessment of Ulvan, a Polysaccharide Extracted from Green Algae. Phyther. Res. 2013, 27, 1143–1148. [Google Scholar] [CrossRef]
- Quemener, B.; Lahaye, M.; Bobin-Dubigeon, C. Sugar Determination in Ulvans by a Chemical-Enzymatic Method Coupled to High Performance Anion Exchange Chromatography. J. Appl. Phycol. 1997, 9, 179–188. [Google Scholar] [CrossRef]
- Alves, A.; Sousa, R.A.; Reis, R.L. A Practical Perspective on Ulvan Extracted from Green Algae. J. Appl. Phycol. 2013, 25, 407–424. [Google Scholar] [CrossRef]
- Ray, B.; Lahaye, M. Cell-Wall Polysaccharides from the Marine Green Alga Ulva “rigida” (Ulvales, Chlorophyta). Extraction and Chemical Composition. Carbohydr. Res. 1995, 274, 251–261. [Google Scholar] [CrossRef]
- Lahaye, M.; Inizan, F.; Vigouroux, J. Carbohydrate Polymers NMR Analysis of the Chemical Structure of Ulvan and of Ulvan-Boron Complex Formation. Carbohydr. Polym. 1998, 36, 239–249. [Google Scholar] [CrossRef]
- Yaich, H.; Garna, H.; Besbes, S.; Paquot, M.; Blecker, C.; Attia, H. Food Hydrocolloids Effect of Extraction Conditions on the Yield and Purity of Ulvan Extracted from Ulva lactuca. Food Hydrocoll. 2020, 31, 375–382. [Google Scholar] [CrossRef]
- Thanh, T.T.T.; Quach, T.M.T.; Nguyen, T.N.; Vu Luong, D.; Bui, M.L.; Tran, T.T. Van Structure and Cytotoxic Activity of Ulvan Extracted from Green Seaweed Ulva lactuca. Int. J. Biol. Macromol. 2016, 93, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Van Tran, T.T.; Truong, H.B.; Ha, N.; Tran, V.; Thu, T.M. Structure, Conformation in Aqueous Solution and Antimicrobial Activity of Ulvan Extracted from Green Seaweed Ulva reticulata. Nat. Prod. Res. 2018, 32, 2291–2296. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.I.A.; Amer, M.S.; Ibrahim, H.A.H.; Zaghloul, E.H. Considerable Production of Ulvan from Ulva lactuca with Special Emphasis on Its Antimicrobial and Anti-Fouling. Appl. Biochem. Biotechnol. 2022, 194, 3097–3118. [Google Scholar] [CrossRef]
- García-Poza, S.; Leandro, A.; Cotas, C.; Cotas, J.; Marques, J.C.; Pereira, L.; Gonçalves, A.M.M. The Evolution Road of Seaweed Aquaculture: Cultivation Technologies and the Industry 4.0. Int. J. Environ. Res. Public Health 2020, 17, 6528. [Google Scholar] [CrossRef]
- Kraan, S. Seaweed Resources, Collection, and Cultivation with Respect to Sustainability; Elsevier Inc.: Amsterdam, The Netherlands, 2020; ISBN 9780128179437. [Google Scholar]
- Hayashi, L.; Cantarino, S.D.J.; Critchley, A.T. Challenges to the Future Domestication of Seaweeds as Cultivated Species: Understanding Their Physiological Processes for Large-Scale Production; Elsevier Ltd.: Amsterdam, The Netherlands, 2020; Volume 95, ISBN 9780081027103. [Google Scholar]
- Freile-Pelegŕn, Y.; Morales, J.L. Antibacterial Activity in Marine Algae from the Coast of Yucatan, Mexico. Bot. Mar. 2004, 47, 140–146. [Google Scholar] [CrossRef]
- Trigui, M.; Gasmi, L.; Zouari, I.; Tounsi, S. Seasonal Variation in Phenolic Composition, Antibacterial and Antioxidant Activities of Ulva rigida (Chlorophyta) and Assessment of Antiacetylcholinesterase Potential. J. Appl. Phycol. 2013, 25, 319–328. [Google Scholar] [CrossRef]
- Kandhasamy, M.; Arunachalam, K.D. Evaluation of in Vitro Antibacterial Property of Seaweeds of Southeast Coast of India. Afr. J. Biotechnol. 2008, 7, 1958–1961. [Google Scholar]
- Garcia-Vaquero, M.; Hayes, M. Red and Green Macroalgae for Fish and Animal Feed and Human Functional Food Development. Food Rev. Int. 2016, 32, 15–45. [Google Scholar] [CrossRef]
- Holdt, S.L.; Kraan, S. Bioactive Compounds in Seaweed: Functional Food Applications and Legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Pereira, R.C.; Costa, E.d.S.; Sudatti, D.B.; da Gama, B.A.P. Inducible Defenses against Herbivory and Fouling in Seaweeds. J. Sea Res. 2017, 122, 25–33. [Google Scholar] [CrossRef]
- Besednova, N.N.; Andryukov, B.G.; Zaporozhets, T.S.; Kryzhanovsky, S.P.; Kuznetsova, T.A.; Fedyanina, L.N.; Makarenkova, I.D.; Zvyagintseva, T.N. Algae Polyphenolic Compounds and Modern Antibacterial Strategies: Current Achievements and Immediate Prospects. Biomedicines 2020, 8, 342. [Google Scholar] [CrossRef]
- Cherian, C.; Jannet Vennila, J.; Sharan, L. Marine Bromophenols as an Effective Inhibitor of Virulent Proteins (Peptidyl Arginine Deiminase, Gingipain R and Hemagglutinin A) in Porphyromas Gingivalis. Arch. Oral Biol. 2019, 100, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Lopes, G.; Pinto, E.; Andrade, P.B.; Valentão, P. Antifungal Activity of Phlorotannins against Dermatophytes and Yeasts: Approaches to the Mechanism of Action and Influence on Candida albicans Virulence Factor. PLoS ONE 2013, 8, e72203. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.; Silva, S.A.; Carpena, M.; Garcia-Oliveira, P.; Gullón, P.; Barroso, M.F.; Prieto, M.A.; Simal-Gandara, J. Macroalgae as a Source of Valuable Antimicrobial Compounds: Extraction and Applications. Antibiotics 2020, 9, 642. [Google Scholar] [CrossRef] [PubMed]
- Hierholtzer, A.; Chatellard, L.; Kierans, M.; Akunna, J.C.; Collier, P.J. The Impact and Mode of Action of Phenolic Compounds Extracted from Brown Seaweed on Mixed Anaerobic Microbial Cultures. J. Appl. Microbiol. 2012, 114, 964–973. [Google Scholar] [CrossRef]
- Bhowmick, S.; Mazumdar, A.; Moulick, A.; Adam, V. Algal Metabolites: An Inevitable Substitute for Antibiotics. Biotechnol. Adv. 2020, 43, 107571. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Z.; Bach, S.J.; McAllister, T.A. Sensitivity of Escherichia Coli to Seaweed (Ascophyllum nodosum) Phlorotannins and Terrestrial Tannins. Asian-Australasian J. Anim. Sci. 2009, 22, 238–245. [Google Scholar] [CrossRef]
- Wei, Y.; Liu, Q.; Xu, C.; Yu, J.; Zhao, L.; Guo, Q. Damage to the Membrane Permeability and Cell Death of Vibrio parahaemolyticus Caused by Phlorotannins with Low Molecular Weight from Sargassum thunbergii. J. Aquat. Food Prod. Technol. 2016, 25, 323–333. [Google Scholar] [CrossRef]
- Bogolitsyn, K.; Dobrodeeva, L.; Druzhinina, A.; Ovchinnikov, D.; Parshina, A.; Shulgina, E. Biological Activity of a Polyphenolic Complex of Arctic Brown Algae. J. Appl. Phycol. 2019, 31, 3341–3348. [Google Scholar] [CrossRef]
- Cabral, E.M.; Oliveira, M.; Mondala, J.R.M.; Curtin, J.; Tiwari, B.K.; Garcia-Vaquero, M. Antimicrobials from Seaweeds for Food Applications. Mar. Drugs 2021, 19, 211. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.S.; Walia, A.K. Lectins from Red Algae and Their Biomedical Potential. J. Appl. Phycol. 2018, 30, 1833–1858. [Google Scholar] [CrossRef]
- Yoon, B.K.; Jackman, J.A.; Valle-González, E.R.; Cho, N.J. Antibacterial Free Fatty Acids and Monoglycerides: Biological Activities, Experimental Testing, and Therapeutic Applications. Int. J. Mol. Sci. 2018, 19, 1114. [Google Scholar] [CrossRef]
- Ahmed, G.; Shoubaky, E. Active Ingredients Fatty Acids as Antibacterial Agent from the Brown Algae Padina pavonica and Hormophysa triquetra. J. Coast. Life Med. 2014, 2, 535–542. [Google Scholar] [CrossRef]
- Da Costa, E.; Melo, T.; Moreira, A.S.P.; Bernardo, C.; Helguero, L.; Ferreira, I.; Cruz, M.T.; Rego, A.M.; Domingues, P.; Calado, R.; et al. Valorization of Lipids from Gracilaria sp. through Lipidomics and Decoding of Antiproliferative and Anti-Inflammatory Activity. Mar. Drugs 2017, 15, 62. [Google Scholar] [CrossRef] [PubMed]
- Stabili, L.; Acquaviva, M.I.; Biandolino, F.; Cavallo, R.A.; De Pascali, S.A.; Fanizzi, F.P.; Narracci, M.; Cecere, E.; Petrocelli, A. Biotechnological Potential of the Seaweed Cladophora rupestris (Chlorophyta, Cladophorales) Lipidic Extract. N. Biotechnol. 2014, 31, 436–444. [Google Scholar] [CrossRef]
- Patra, J.K.; Kim, S.H.; Baek, K.H. Antioxidant and Free Radical-Scavenging Potential of Essential Oil from Enteromorpha linza L. Prepared by Microwave-Assisted Hydrodistillation. J. Food Biochem. 2015, 39, 80–90. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Baek, K.H. Chemical Composition and Antioxidant and Antibacterial Activities of an Essential Oil Extracted from an Edible Seaweed, Laminaria japonica L. Molecules 2015, 20, 12093–12113. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Lee, S.W.; Park, J.G.; Baek, K.H. Antioxidant and Antibacterial Properties of Essential Oil Extracted from an Edible Seaweed Undaria pinnatifida. J. Food Biochem. 2017, 41, e12278. [Google Scholar] [CrossRef]
- Tiwari, B.K.; Valdramidis, V.P.; O’Donnell, C.P.; Muthukumarappan, K.; Bourke, P.; Cullen, P.J. Application of Natural Antimicrobials for Food Preservation. J. Agric. Food Chem. 2009, 57, 5987–6000. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zheng, Y.; Wang, J.; Ma, S.; Yu, Y.; White, W.L.; Yang, S.; Yang, F.; Lu, J. Fucoidan Extracted from Undaria pinnatifida: Source for Nutraceuticals/Functional Foods. Mar. Drugs 2018, 16, 321. [Google Scholar] [CrossRef]
- He, F.; Yang, Y.; Yang, G.; Yu, L. Studies on Antibacterial Activity and Antibacterial Mechanism of a Novel Polysaccharide from Streptomyces virginia H03. Food Control 2010, 21, 1257–1262. [Google Scholar] [CrossRef]
- Kavita, K.; Singh, V.K.; Jha, B. 24-Branched Δ5 Sterols from Laurencia papillosa Red Seaweed with Antibacterial Activity against Human Pathogenic Bacteria. Microbiol. Res. 2014, 169, 301–306. [Google Scholar] [CrossRef]
- Ikekawa, N.; Morisaki, N.; Tsuda, K.; Yoshida, T. Sterol Compositions in Some Green Algae and Brown Algae. Steroids 1968, 12, 41–48. [Google Scholar] [CrossRef]
- Prakash, S.; Sasikala, S.L.; Aldous, V.H.J. Isolation and Identification of MDR-Mycobacterium tuberculosis and Screening of Partially Characterised Antimycobacterial Compounds from Chosen Marine Micro Algae. Asian Pac. J. Trop. Med. 2010, 3, 655–661. [Google Scholar] [CrossRef]
- Wächter, G.A.; Franzblau, S.G.; Montenegro, G.; Hoffmann, J.J.; Maiese, W.M.; Timmermann, B.N. Inhibition of Mycobacterium tuberculosis Growth by Saringosterol from Lessonia nigrescens. J. Nat. Prod. 2001, 64, 1463–1464. [Google Scholar] [CrossRef]
- Kawakami, L.M.; Yoon, B.K.; Jackman, J.A.; Knoll, W.; Weiss, P.S.; Cho, N.J. Understanding How Sterols Regulate Membrane Remodeling in Supported Lipid Bilayers. Langmuir 2017, 33, 14756–14765. [Google Scholar] [CrossRef]
- Eng, R.H.K.; Padberg, F.T.; Smith, S.M.; Tan, E.N.; Cherubin, C.E. Bactericidal Effects of Antibiotics on Slowly Growing and Nongrowing Bacteria. Antimicrob. Agents Chemother. 1991, 35, 1824–1828. [Google Scholar] [CrossRef] [PubMed]
- Kanauchi, O.; Mitsuyama, K.; Araki, Y.; Andoh, A. Modification of Intestinal Flora in the Treatment of Inflammatory Bowel Disease. Curr. Pharm. Des. 2003, 9, 333–346. [Google Scholar] [CrossRef] [PubMed]
- Takesue, Y.; Yokoyama, T.; Akagi, S.; Ohge, H.; Imamura, Y.; Murakami, Y.; Seuda, T. Changes in the Intestinal Flora After the Administration of Prophylactic Antibiotics to Patients Undergoing a Gastrectomy. Surg. Today 2002, 35, 581–586. [Google Scholar] [CrossRef]
- Basappa, K.; Gopal, J.V. Natural Alternatives to Antibiotic Agents. Asian J. Biomed. Pharm. Sci. 2013, 25, 1–4. [Google Scholar]
- Setty, A.R.; Sigal, L.H. Herbal Medications Commonly Used in the Practice of Rheumatology: Mechanisms of Action, Efficacy, and Side Effects. Semin. Arthritis Rheum. 2005, 34, 773–784. [Google Scholar] [CrossRef] [PubMed]
- Niggemann, B.; Gruber, C. Side-Effects of Complementary and Alternative Medicine. Allergy 2003, 58, 707–716. [Google Scholar] [CrossRef]
- Tattelman, E. Health Effects of Garlic. Complement. Altern. Med. 2005, 72, 103–106. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lomartire, S.; Gonçalves, A.M.M. An Overview on Antimicrobial Potential of Edible Terrestrial Plants and Marine Macroalgae Rhodophyta and Chlorophyta Extracts. Mar. Drugs 2023, 21, 163. https://doi.org/10.3390/md21030163
Lomartire S, Gonçalves AMM. An Overview on Antimicrobial Potential of Edible Terrestrial Plants and Marine Macroalgae Rhodophyta and Chlorophyta Extracts. Marine Drugs. 2023; 21(3):163. https://doi.org/10.3390/md21030163
Chicago/Turabian StyleLomartire, Silvia, and Ana M. M. Gonçalves. 2023. "An Overview on Antimicrobial Potential of Edible Terrestrial Plants and Marine Macroalgae Rhodophyta and Chlorophyta Extracts" Marine Drugs 21, no. 3: 163. https://doi.org/10.3390/md21030163
APA StyleLomartire, S., & Gonçalves, A. M. M. (2023). An Overview on Antimicrobial Potential of Edible Terrestrial Plants and Marine Macroalgae Rhodophyta and Chlorophyta Extracts. Marine Drugs, 21(3), 163. https://doi.org/10.3390/md21030163







