Abstract
In the food industry, manufacturers and customers have paid more attention to natural pigments instead of the synthetic counterparts for their excellent coloring ability and healthy properties. Microalgae are proven as one of the major photosynthesizers of naturally derived commercial pigments, gaining higher value in the global food pigment market. Microalgae-derived pigments, especially chlorophylls, carotenoids and phycobiliproteins, have unique colors and molecular structures, respectively, and show different physiological activities and health effects in the human body. This review provides recent updates on characteristics, application fields, stability in production and extraction processes of chlorophylls, carotenoids and phycobiliproteins to standardize and analyze their commercial production from microalgae. Potential food commodities for the pigment as eco-friendly colorants, nutraceuticals, and antioxidants are summarized for the target products. Then, recent cultivation strategies, metabolic and genomic designs are presented for high pigment productivity. Technical bottlenecks of downstream processing are discussed for improved stability and bioaccessibility during production. The production strategies of microalgal pigments have been exploited to varying degrees, with some already being applied at scale while others remain at the laboratory level. Finally, some factors affecting their global market value and future prospects are proposed. The microalgae-derived pigments have great potential in the food industry due to their high nutritional value and competitive production cost.
1. Introduction
Presently, as consumers pay more attention to healthy diets and food safety, they are inclined towards the nutritive, natural and clean-label food products [1]. Following this proclivity, food and nutraceutical manufacturers are pursuing the exploitation and industrial production of natural high-value compounds. For pigments in food and health applications, a distinct move of market interest has also been driven towards biologically synthesized natural ingredients. Up to now, most of the commercially available food pigments are obtained through chemical synthesis and are generally considered not completely safe, or even toxic, owing to their molecular configurations or the organic reactions during their processing. Compared with synthetic counterparts, natural pigments have no toxic or side effects on the human body, and even act as nutritional enhancers with various biological activities. Although some plant-derived pigments have been commercially available, it has been found that the activity of microalgal pigments increases by multiple times. As a significant food ingredient, microalgal pigments are also chased by manufacturers and customers. The market value of microalgal pigments has been predicted to be USD 452.4 million by 2025 with a 4% Compound Annual Growth Rate (CAGR), and the market of microalgae products will be a large-scale business until that time [2,3].
Microalgae represent the important sources of value-added products, including proteins, lipids, polysaccharides, minerals, vitamins, pigments, and polyunsaturated fatty acids (PUFAs), which have great trading and health value [4]. Notably, “microalgae” themselves contain eukaryotic photosynthetic microorganisms along with prokaryotic cyanobacteria, which are responsible for converting light energy to chemical energy via photosynthesis. The three major classes of pigments, carotenoids (usually 0.1–0.2% of dry weight, DW or as high as 14% within certain species), chlorophylls (0.5–1.0% of DW) and phycobiliproteins (PBPs) (8% of DW) in microalgae are responsible for their photosynthetic pathways and cell growth. Compared with additional natural sources of pigments (such as fruits, plants, or animals), microalgae-derived pigments have been proved to contain outstanding physiological activities (such as antioxidant and antibacterial) and a wide spectrum of health applications. Various options of striking shades, hues and natural tones in the foods can be exhibited by microalgal pigments, thus endowing them with strong competitiveness as edible colorants that can mimic the color of natural food [5]. Furthermore, their biggest advantage lies in the culture characteristics of the microalgae themselves.
As a stable natural source of pigments, microalgae can be cultured sustainably, in an eco-friendly manner, and are renewable on an industrial scale without the restriction of seasonal, climatic and environmental conditions. They are also outstanding for their high pigment content, fast growth rate, ability to grow in stress conditions, and the fact that they do not require arable land, thus they are becoming one of the most promising and competitive sources of natural pigments [6]. Some cultivation strategies are explored for enhancing pigment accumulation in diverse organisms. Some active pigments including PBPs (blue pigment extracted in Spirulina), astaxanthin (yellow-to-red pigment extracted in Haematococcus) and β-carotene (yellow pigment extracted in Dunaliella) have been scaled into industrial production and extensively used in food, nutraceutical, pharmaceutical, aquaculture, cosmetic and various industries. In microalgae, pigments are synthesized during vegetative growth or in stress conditions, and different biotic and abiotic factors in this process can affect the final quality of products [7]. The emergence of novel trophic modes and techniques effectively enhances pigment-producing efficiency [8]. Moreover, during the steps of extraction, purification and food processing, the structure of pigments may also be damaged or destroyed, thus affecting its coloring and nutritional function in foods. Therefore, it is essential to optimize the extraction and process methods to protect pigment stability and activity.
This paper integrates the characteristics and distribution of three commercial microalgal pigments as chlorophylls, carotenoids and PBPs. Their current application patterns and functions in the food industry are reviewed. For the large-scale culture of microalgae, the influencing mechanisms of several environmental conditions on the final pigment yield was analyzed. In addition, the features and drawbacks of several conventional methods for pigment extraction are discussed. Finally, the current market value and future prospects are analyzed.
2. Chemistry and Biochemistry of Microalgal Pigments
Microalgae represent the important natural pigment sources, which play an irreplaceable role in photosynthetic carbon fixation and cell growth [4]. In addition, the type and quantity of natural pigments in microalgae varied with the species (Table 1). The common biosynthetic pathway of chlorophyll, phycobiliprotein and carotenoid was shown in Figure 1. With unique structure and various beneficial functions, these pigments have been explored and utilized to different degrees in a wide spectrum of fields.

Table 1.
Microalgal sources, bioactivity and applications of natural pigments.
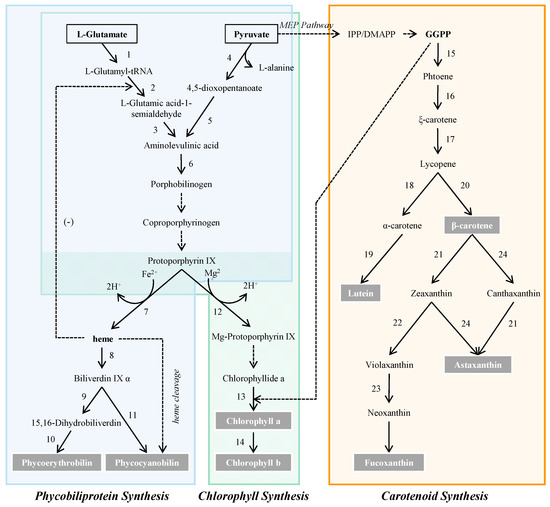
Figure 1.
Biosynthetic pathway of pigments in microalgae and enzymes involved in pigment biosynthesis. 1, glutamate-tRNA ligase; 2, glutamate-tRNA reductase; 3, 5-aminolevulinate synthase; 4, 5-aminolevulinas; 5, aminolevulinic acid transaminase; 6, porphobilinogen synthase; 7, ferrochelatase; 8, heme oxgenase; 9, ferrodoxin oxidoreductase (Peb A); 10, ferrodoxin oxidoreductase (Peb B); 11, phycocyanobilin synthase; 12, Mg-protoporphyrinogen IX chelatase; 13, chlorophyll synthetase; 14, chlorophyll a oxygenase (chlorophyll b synthase); 15, phytoene synthase; 16, phytoene desaturase; 17, zeta-carotene isomerase, zeta-carotene desaturase, carotenoid isomerase; 18, lycopene epsilon cyclase; 19, cytochrome P450-β hydroxylase; 20, lycopene-β cyclase; 21, β-carotene hydroxylase; 22, zeaxanthin epoxidase; 23, neoxanthin synthase; 24, β-carotene ketolase. Abbreviations: IPP, isopentenyl pyrophosphate; DMAPP, dimethylallyl pyrophosphate; GGPP, geranylgeranyl pyrophosphate.
Chlorophylls are greenish pigments discovered in oxygenic photosynthetic organisms including plants, microalgae and cyanobacteria. They are responsible for photosynthesis by absorbing and converting solar energy into chemical energy. At present, five categories of chlorophylls have been identified, including a–f, and their maximal absorption wavelengths (λAmax) are, 665, 652, 630, 696 and 707 nm, respectively. Most pigments can bind to several phyla, while chlorophyll a is the most abundant in microalgae [25]. Chlorophyll b represents the pigment with the second highest abundance within green microalgae; on the other hand, chlorophyll c has been observed within haptophytes, dinoflagellates, cryptophytes and heterokonts; while chlorophyll d and f were identified in Rhodophyta, cyanobacteria, and dinoflagellates. On average, microalgae contain 0.5–1.0% of chlorophylls per DW [26]. Many microalgae can be the source of natural chlorophylls used for commercial applications, such as Chlorella sp., Monoraphidium dybowskii, Scenedesmus dimorphus, Chlamydomonas reinhardtii, Pavlova lutheri, Ankistrodesmus falcatus, and Chlorella vulgaris [27].
Carotenoids can be dissolved in lipid, and their colors can be red, brown, yellow or orange. Carotenoids include the terpenoid pigments that can be obtained in the 40-carbon polyene chain and end with “ionone” rings. The different architectures provide carotenoids with outstanding light-absorption characteristics necessary in the photosynthetic process. On the basis of chemical structure, these hydrocarbons are classified into carotenes and xanthophylls. Carotenes, namely hydrocarbons, consist of α- and β-carotene. Xanthophylls contain oxygen as hydroxyl groups (such as zeaxanthin, lutein), keto-groups (such as echinenone, cantaxanthin), or their combination for violaxanthin and astaxanthin. Carotenoids are divided into primary or secondary ones. The primary ones act as the cellular photosynthetic apparatus’ functional components, which are linked with membranes or specific proteins in the thylakoid membrane (e.g., xanthophylls), and the secondary carotenoids are typically produced when exposed to specific environmental stimuli and exist in lipid vesicles. Carotenoids have two important effects on photosynthesis: (1) light absorption within visible spectral regions where no chlorophyll is effectively absorbed; (2) photoprotection on photosynthetic systems. Typically, the photoprotective mechanisms have been proven to weaken the energetic state change in chlorophyll induced by excess light radiation absorption. It can effectively hinder reactive oxygen species (ROS) production and endows carotenoids with superior antioxidant capacity [28]. Over 600 carotenoids have been identified, while those in microalgae are mainly lycopene, β-carotene, zeaxanthin, astaxanthin, lutein and violaxanthin, which nearly compose 90% of carotenoids within the human body and the diet. Among them, β-carotene, astaxanthin, fucoxanthin and lutein are the most studied ones.
β-carotene is the vitamin A precursor (retinol), which represents an orange-yellow color depending on the quantity of β-carotene and other pigments. Demand for natural β-carotene is increasing, for this carotenoid presents more superior bioactive properties than artificial ones. The microalgae Dunaliella contains β-carotene above 14% DW and has long been recognized as the largest natural source [26]. Scenedesmus quadricauda produces 19 mg g−1 β-carotene under autotrophic cultivation [29]. Other microalgae of Cyanobacteria, Chlorophyta, Bacillariophyta, and Euglenophyta also master the capability to produce β-carotene. Astaxanthin is a byproduct of β-carotene with a rosy color, it is converted from β-carotene under the action of β-carotene hydroxylase (CRTR-B) and β-carotene oxygenase (CRTO) under stress, followed by accumulation within lipid vesicles out of chloroplasts. In nature, astaxanthin usually exists with 1 or 2 esterified fatty acids (FAs, referred to as monoester and diester, respectively). Astaxanthin can form as different isomers according to hydroxyl group configuration: 3S and 3’S, 3R and 3’S (meso), and 3R and 3’R. Microalgae including Haematococcus pluvialis, Chlorococcum sp. and Chlorella zofingiensis have a capability to accumulate astaxanthin in vesicles when subjected to stress [30]. Lutein is a lipophilic tetraterpene, whose color ranges from yellow (low content) to red-orange (high content). The double bonds in lutein endow its high antioxidant activity to be chemically reactive with ROS. On the other hand, lutein can protect photoreceptors by filtering blue light (500 nm) [31]. One of the key commercial sources of lutein production is Muriellopsis sp., which contains lutein at 0.4–0.6% per dry biomass [32]. C. zofingiensis, Chlorella protothecoides, and Scenedesmus spp. have also been studied for lutein production. Until now, just Scenedesmus almeriensis and Muriellopsis sp. realized the mass production, which are cultured massively within the outdoor systems with continuous and feasible operation [33]. The orange pigment fucoxanthin accounts for more than 10% of total natural carotenoids and is ubiquitous among living organisms in a marine ecosystem. Fucoxanthin has a multi-olefin skeleton and specific conjugated double bonds, as well as single epoxy, carbonyl, and hydroxyl groups [34]. These unique bioactive structures in fucoxanthin are responsible for its potent health benefits to humans, such as anti-obesity, anti-cancer, and blood glucose regulatory functions. Diatoms (Bacillariophyta, especially Odontella sp.) and brown microalgae (Phaeophyceae) have abundant levels of lutein, but their application in industrialized production is still not feasible. Isochrysis galbana can generate 1.2–15-fold increased fucoxanthin compared with the conventional macroalgal source [35]. Tisochrysis lutea is an alternative source for this pigment, 79 mg g−1 of fucoxanthin can be extracted from its mixotrophic culture [36]. Some other microalgae, such as Phaeodactylum tricornutum, Cylindrotheca sp., Odontella aurita, Chaetoceros muelleri, Amphora sp., Navicula sp., and Chrysotila carterae, also showed considerable fucoxanthin production capacity [37,38].
PBPs are soluble in water and are relatively easy to isolate and purify. They can generally be divided into phycoerythrin (PE, red, absorption detected at 565–575 nm), phycocyanin (PC, blue, absorption detected at 651–655 nm), as well as allophycocyanin (APC, green-blue, absorption detected at 650–655 nm) in line with spectroscopy characteristics and amino acid sequences [39]. PBPs, as oligomeric proteins, are comprised by chromophore-bearing polypeptides. They can be assembled into phycobilisomes or adhered onto thylakoid membrane for photosynthesis. In some microalgae that are lacking chlorophyll b, PBPs are synthesized for compensating for the huge chlorophyll a absorption gap while optimizing light energy collection. In the photosynthetic process, such PBPs can capture light energy, subsequently passing it into the chlorophylls. PBPs have been widely used as food additives and/or excellent nutrient supplements for the human diet, demonstrating a number of healthy functions. In most cyanobacteria, cryptophytes, glaucophytes and red algae, the common PBP is PC, which is especially rich in numerous cyanobacterial strains under the natural environments. Nowadays, PC can be generated from autotrophic cyanobacteria Spirulina platensis because it is ubiquitous. A strain of Geitlerinema can also achieve a yield of 172 mg g−1 [40]. PE is a kind of PBP with the highest abundance level within numerous red algae as well as certain cyanobacteria, it can now be produced from Porphyridium sp. (such as P. purpureum and P. cruentum), and its content is 15% DW (as high as 30% in the ideal environment), while its yield is 200 mg L−1 [41]. In comparison to PC and PE, the massive commercial APC manufacturing process has not been established so far because it is limited by the poor intracellular pigment content and biomass generation.
Several unusual minor pigments exist in microalgae, including prasinoxanthin, loroxanthin, siphonaxanthin, peridinin, flavonoids, and tannin [27]. Their structural variety and beneficial activities are attractive in the food and pharmaceutical industries. The pigment analysis of seven prasinophyceans (Chlorophyta) distinguished three groups that contained prasinoxanthin, loroxanthin ester and siphonaxanthin, respectively [42]. The bioactive compound analysis revealed that Nannochloropsis sp. contained 7.25% β-sitosterol, and 10.3% stigmasterol [43]. As little information is available for the biotransformation of such unusual pigments during the metabolic process, further studies are required to reveal the bioavailability and biodigestibility of these pigments.
3. Potential Application of Microalgal Pigments and Target Foods
With unique molecular structure and various beneficial activities, microalgal pigments have been reckoned as promising eco-friendly colorants, nutraceuticals and antioxidants with great commercial value in food and dairy, alternative foods, dietary supplements, pharmaceuticals, cosmetics, aquaculture, textiles, and other light manufacturing fields [44,45]. At present, these pigments have been developed and utilized to different degrees, some of them have achieved efficient industrial production and high economic value. This section reviewed the popular application examples and acting modes of microalgal pigments in food and other industries (Figure 2).
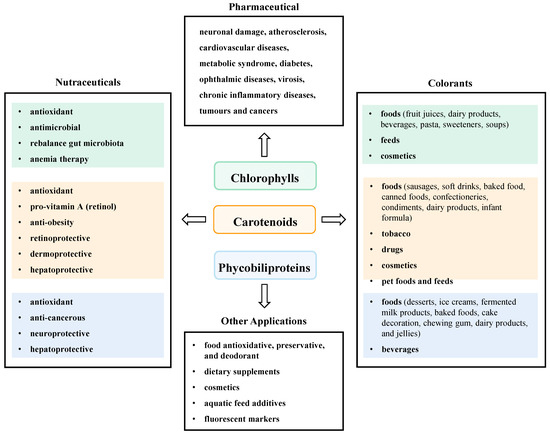
Figure 2.
Application of microalgal pigment in various fields.
3.1. Chlorophylls
Similar to most natural pigments, chlorophylls have numerous functions in food additive industry, within which, the most extensive application is colorants. Out of the antipathy to synthesized pigments with potential side effects, food industries now tend to use natural green colorants to endow, intensify, or uniformize color of foods, thus presenting best color intensity and hue to satisfy consumers’ expectations, requirements and needs [46]. At present, microalgae have been identified to be the sustainable source to produce natural food-grade colorants with low allergenicity, toxicity and carcinogenicity. The common application of microalgal pigments including chlorophylls and their target food products as colorants is listed in Table 2. As the most abundant natural pigment with green color that are rare in foods, chlorophylls are currently gaining immense attention as food, feed, cosmetic and pharmaceutical colorants and functional food supplements [47]. Addition to colorants, such as microalgal pigments can display auxiliary effects of preservation and antisepsis in food storage, as the consequence of their physiological activities. Chlorophylls were proven to serve as an excellent deodorant of foods [48].

Table 2.
Common application of microalgal pigments and their target food products as colorants.
Microalgal pigments can also be utilized in nutraceuticals and as dietary supplements. Due to their excellent bioactive properties, chlorophylls have been consumed as nutraceutical agents and antioxidants. They can also contribute to rebalancing the gut microbiota and decreasing colorectal cancer risk. Since chlorophylls regenerate or act as substitutions for hemoglobin in deficiency conditions, the utilization of chlorophylls supplement has been recommended for thalassemia and hemolytic anemia adjuvant therapy [49]. Chlorophyll compounds are also suggested to have medicinal application with wound-healing, antimicrobial, anticancer, antimutagenic, antitumor and anti-inflammatory properties [50,51]. Furthermore, they may be applied to be components of dentifrice or in producing cosmetics for skin care. Chlorophyllin represents the chlorophyll derivative, where copper or sodium replaces magnesium, with the loss of phytol chains. It is employed as a dietary supplement and was found to exhibit antimutagenic and anticarcinogenic action.
3.2. Carotenoids
With striking color characteristics and bioactivity, natural carotenoids are the best colorant option for food producers, particularly for cooked sausages, soft drinks, and baked goods. Apart from that, its excellent antioxidative and preservative attributes help food retain original color and taste during storage, and preventing FAs and other substances from oxidizing and deteriorating [52,53]. Thus, they have recognizable impact and demand in foods with increased FAs (such as butter, margarine, milk products, soft drinks and cakes).
Carotenoids also have the effects of antioxidation, anti-inflammation, neuroprotection, which can also improve age-related macular degeneration and cardiac dysfunction. Carotenoids can effectively hinder the formation of intracellular ROS and play a strong physiological antioxidant effect [28]. Microalgal carotenoids contain trans- and cis-enantiomers that further enhance the bio-efficacy and bioavailability. They are also bioactive and can resist cancer because they contain xanthophylls. Thus, these biomolecules have been applicated into numerous fields, including cosmetics, pharmaceutical, functional foods, nutraceuticals, agriculture and aquatic feeds [54].
β-carotene represents the red pigment, which is currently most popular and has been widely applied to many food products (e.g., cakes, margarine, butter, cheese, canned foods, baked products, health condiments, confectioneries, dairy products and soft drinks) and pet foods to enhance their attractiveness. Since vitamin A enhances body immunity while preventing cataracts, cutaneous disorders and night blindness, it is reckoned as being a necessary nutrient for the human body. β-carotene can be adopted to be the pro-vitamin A (retinol) in multi-vitamin preparations, which is also used to formulate cosmetics (such as sunscreen formulations) or healthy foods. β-carotene can also act as a strong antioxidative agent (healthy food or cosmetics additives), a hepatoprotective agent, a cosmetic or a multi-vitamin preparation additive, and as a functional food component [55,56]. As a health care product, it offers medical effects that can be described as being retinoprotective, dermoprotective, anti-inflammatory, antihypertensive, antitumor, and antidiabetic and these effects cannot be achieved by a synthetic version [57]. Additionally, it can be applied in animal feed for improving fish (such as salmon), crustaceans, and a bird’s appearance (color).
Astaxanthin is also a carotenoid with important commercial value, given it is a natural colorant. Astaxanthin extracted from H. pluvialis is approved in Japan, the USA as well as some European countries to be used in human dietary supplements and salmonid feeds. Moreover, bread that contains H. pluvialis-extracted astaxanthin is also developed by researchers, which is under favorable preservation against degradation in the process of baking [58]. Astaxanthin is a superb antioxidant with a 12-fold increased activity compared with vitamin E by sequestering free radicals and quenching ROS. There is also an increasing market interest of its anti-obese activity, which now has applications in nutraceuticals, dietary supplements, cosmetics, pharmaceuticals, and the healthcare area [59]. An astaxanthin-containing oral preparation was already developed to treat Helicobacter infections in the gastrointestinal tract (GIT). The experiments revealed the great capability of natural astaxanthin to act as hepatoprotector, anticancerogen and anti-aging compound [60]. It can be used in the adjuvant treatment of neuronal damage, central nervous system injuries, heart disease, atherosclerosis, diabetes, chronic inflammatory disorders, cutaneous disorders and in functional foods.
Lutein has been considered as a significant nutraceutical, which can be applied in food, cosmetics and drug coloration. It is now commercially added to flavorings, tobacco, pastries, confectionery, infant formula and a variety of feed products to provide them with a distinctive and attractive color. When used as a nutraceutical, lutein generally has effects on eye structure and vision-related conditions (macular degeneration, retinopathies or cataracts associated with age). Since blue light radiation has unfavorable effects on human eye macula, lutein protects retina through filtering blue light (500 nm) and reducing 40% of light incidence [31]. Moreover, investigations of lutein for its anti-inflammation and antioxidation effects go beyond these eye diseases. Lutein has been successfully applied in nutraceuticals for the prevention of other chronic disorders such as cardiovascular diseases (CVDs), diabetes, atherosclerosis, and together with some cancers, have gained widespread attention associated with human health [61]. As revealed by some studies, lutein protects against solar UV radiation-induced skin damage, thus displaying its potential application in skin care products such as sunscreens.
Microalgal fucoxanthin production has not achieved industrialization on a large scale, and its application as a food colorant is limited. It can be applied to egg yolk, butter, pie, green tea cakes, baked foods and dairy products for a better color and functional role [62]. Fucoxanthin from P. tricornutum added to dairy products was proven to improve its stability and bioavailability. Natural fucoxanthin from diatoms obtains a great potential for application on cosmetics, pharmaceuticals, food, poultry feed, and aquaculture industries, due to its unique bioactive structures and potent health benefits to humans. Studies have demonstrated that consuming fucoxanthin in dietary supplements and beauty products can serve as an antioxidant and scavenger for ROS. For obese people, fucoxanthin has been proven to have outstanding weight loss benefits and can be used in weight loss supplements in diets. In the medical field, it also helps to cur many chronic diseases such as type 2 diabetes (T2DM), heart disease, high cholesterol, osteoporosis, hypertension, metabolic syndrome, liver disease, and some specified cancer (e.g., skin, colon, prostate, liver cancer and other cancers). Notably, the combination with edible lipids can increase the rate of fucoxanthin absorption when utilized as a nutritional supplement [57]. Fucoxanthinol, a deacetylated fucoxanthin, is extracted in diatom Nitzschia laevis, which shows neuroprotection; therefore, it can be used in food supplements and pharmaceutical products [63].
3.3. Phycobiliproteins
PBPs are widely used in the commercial sector as natural colorants. The red or pinkish-red PE as a food colorant has received several patents in foodstuffs, including confectioneries, syrups, dairy products, baked foods, gelatin desserts, dried foods, fermented milk products, ice cream and milk shakes [41,64]. In addition, PE has yellow fluorescence properties and can be added in cake decoration, soft drinks, and alcoholic beverages to endow them with fluorescence properties and improve their appeal to consumers [41]. As to its natural blue color, PC from cyanobacteria is being adopted to color ice cream, soft drinks and yogurt in Japan. It can also be added in chewing gum, candies, popsicles, jellies, dairy products and soft drinks. For example, a PC-enriched yogurt showed positive effects in coloring stability during storage [65]. The natural protein pigment C-phycocyanin (C-PC) in Spirulina is verified with high safety, which can be used in beverages and foods as a colorant [66].
PBPs have antioxidation, anti-inflammation and liver protection effects, thus giving them outstanding potential in human health. PC is reckoned as a strong in vivo antioxidant with radical scavenging properties. In experiments, PC can act as a functional food with excellent anti-inflammatory, anti-cancerous, auxiliary hepatoprotective and neuroprotective effects. APC is reported to hinder virus-induced entero cytopathic impacts, viral plaque formation as well as viral-induced apoptosis, while C-PC shows strong hypocholesterolemic activity by adjusting serum cholesterol concentrations. Some studies have shown that PE is a novel photosensitive drug with high efficiency and no side effects, which can be applied to kill tumor cells.
Due to their bioactive properties, PBPs are also extensively utilized in immunological laboratories and industries. Generally, they have been usually used as the fluorescent markers in molecular biology and as fluorescent dyes in microscopy and immunoassays [26]. Over 297 patent records are available for PBPs such as fluorescent markers. For example, PC and PE collected in the cyanobacterial species have been adopted to be fluorescence probes in gel electrophoresis as the protein markers.
4. Factors Affecting the Microalgal Pigment Production
Nowadays, fermentation of microalgae has become one of the effective strategies of natural pigment production. Compared with those from plants and aquatic animals, pigments obtained from the industrial production of microalgae have a multitude of advantages, including controllable production, easy extraction, high yields, no raw materials scarcity, and no seasonal variations. Within the culture period, any slight change of environmental conditions can cause an alteration of the pigment productivity and molecular structure, ultimately affecting the market acceptance, and bioaccessibility of final products.
4.1. Light
Light is the most affecting factor of converting inorganic carbon into organic molecules in phototrophic organisms. Light utilization can proportionally promote microalgal growth, which is under the regulation of accurate light intensity and photoperiod [67]. Light intensity represents the most obvious and easily regulated factor that affects cell photosynthesis and pigment biosynthesis. Light has a negative effect on photosynthesis under extreme light conditions out of its tolerance limit through the destruction of photosynthetic apparatus. Phycobiliprotein and chlorophyll production is the light collection-related adaptive response. Low light intensity leads to the decreased specific maintenance energy ratio in cyanobacteria, which also stimulates its phycobiliprotein production.
Experiments showed that 25–50 μmol photons/m2/s was the highest phycobiliprotein generation intensity in some blue-green microalgae, while that of Spirulina increased with the increases in light intensity (135 μmol photons/m2/s) [68]. The chlorophyll content in several microalgae (including Chlorella sp., Dunaliella salina, C. reinhardtii, and S. platensis) displays an inverse relationship to light intensity [69]. In contrast, elevating light intensity shows a positive effect on β-carotene synthesis and accumulation of microalgal cells. D. salina was validated to have an enhanced β-carotene productivity at higher irradiation [70]. Large-scale cultivation of microalgal carotenoid (such as lutein and astaxanthin) mostly operates in two steps, cultivating microalgae in optimum conditions for fast growth and then improving desired carotenoid content in stress conditions [71]. Astaxanthin accumulation in H. pluvialis was reported to be enhanced (30 mg/g) as the result of enhanced oxidative stress under intense illumination (>500 μmol photons/m2/s) [72]. For lutein production, Tetraselmis sp., Scenedesmus sp., and Chlamydomonas sp., respectively, gain highest productivity at 170, 300 and 625 μmol photons/m2/sec light intensities [73,74,75].
The significance of the light quality on photosynthetic pigments goes beyond that of the light intensity under certain circumstances, and even influences cell maturity, culture density, light path and the medium nutrients profile. Discontinuous illuminating strategies such as the light/dark photoperiod cycle and the flashing light effect have been used to achieve higher light availability. The photoperiod effectively regulates microalgal chlorophyll levels [76], and usage of the flashing light effect in industrial culture can enhance the astaxanthin generation rate 4-fold within H. pluvialis per photon relative to continuous light sources [77].
Light color is also related to pigment generation within diverse microalgae. The photoreception systems’ absorption and utilization of light depend on wavelength of the incident light to a great extent. In order to ensure the highest light availability, the most suitable light wavelength should be chosen for target pigment production. In some studies, red light is found to favorably enhance PBPs generation within most blue-green microalgae, whereas blue light stimulated that of Spirulina sp. and enhanced its chlorophyll production. Green light increases the C-phycoerythrin (C-PE) level in nitrogen-fixation cyanobacteria, and red light irradiation increases the C-PC level [78]. The intensity of white light is helpful for synthesizing chlorophyll as well as accumulating C-PC in Spirulina sp., whereas green light positively affects its PC generation [68]. The optimal lutein production of marine microalga Chlamydomonas sp. can be achieved in blue light at 20–25 °C [74].
Light spectrum is the photomorphogenic signal in microalgae cultures. Various lengths of spectrum, including blue: red, red: far red, green: red, and blue: green have been often used at different proportions to achieve the best induction effect. Alteration of spectrum proportions influence the relative pigment composition. The lutein production from Scenedesmus obliquus could obtain the maximal productivity (1.43 mg L−1 day−1) with 4-day blue followed by 4-day red exposure [79]. Chlorophyll, a content of Chlorella pyrenoidosa, declines after blue light irradiation, while it greatly elevates after blue: red irradiation [80]. Blue: green lights are helpful for high Pyropia haitanensis effectiveness and increased pigment levels [81]. G. membranacea and C. vulgaris attain highest chlorophyll a levels after red: green light irradiation [82].
4.2. Temperature
Temperature is the elementary factor that can govern the rate of all metabolic processes and cellular component structure by influencing membrane fluidity, enzymatic activities, as well as electron transport chain efficiency. The suitable growth temperature and extreme value tolerance are usually different among diverse microalgal strains.
Typically, high temperatures can promote microalgal pigment production. A range of 25–28 °C was reported as optimum in terms of chlorophylls accumulation temperatures, for higher temperatures may stimulate damaging of cells by osmotic pressure [69]. Likewise, 28 °C is the best temperature of astaxanthin generation in H. pluvialis [83], and 30 °C is optimum for C. zofingiensis [84]. Blue-green microalgae can generate numerous carotenoids at high temperature, in particular β-carotene [27]. Total carotenoid production from microalgae H. pluvialis and Phormidium autumnale have demonstrated the maximum yield, respectively, at 23 °C and 26 °C [85,86]. For D. salina, a temperature at 30 °C leads to its highest β-carotene production [87]. Muriellopsis sp., C. protothecoides, C. zofingiensis, and Neospongiococcus gelatinosum display best lutein productivity when cultured at 28 °C [88]. In case of PBP, the optimum temperature for its production is at 25 °C, 30 °C, 35 °C and 36 °C for S. platensis, Anabaena sp., Nostoc sp., as well as Synechococcus sp. [78,89].
4.3. Culture Media
4.3.1. Nitrogen
Nitrogen is the principal nutrient requirement for microalgae growth and protein/chlorophyll molecule/nucleic acid production. Nitrogen deficiency has been identified for inducing various cell responses within microalgae, such as stimulating excessive free radical formation. Since carotenoids production has been recognized to be the protective response to photo-oxidative stress resulting from the excessively reduced photosynthetic electron transport chain, nitrogen deficiency can cause a marked increase of their contents. On the other hand, four nitrogen atoms are required to compose four pyrrole groups for the synthesis of each chlorophyll molecule and nitrogen stress displays an adverse effect on its synthesis. C. vulgaris, Scenedesmus subspicatus, Chlorella fusca, S. platensis and C. reinhardtii have a reduced chlorophyll level and growth dose-dependently as a consequence of nitrogen deficiency [90]. Cyanobacteria shows particular requirements for nitrogen sources, many blue-green microalgae (such as Anabaena sp.) were proven to produce a high amount of PBPs with nitrogen-free condition, while Fischerella sp. showed the opposite tendency [78].
4.3.2. PH and Salinity
Little research is conducted to investigate how pH affects pigment production in microalga, but its alteration can effectively influence nutrient bioavailability and solubility in a culture system. pH levels < 5.0 and >8.5 are proven to suppress microalgae growth [67]. pH confines the substance dissolution and cell nutrient absorption. S. platensis generated the highest levels of C-PC (91 mg/g DW), carotenoids (2.4 mg/g DW) and chlorophyll a (10.6 mg/g DW) at pH = 8.5, whereas PC (159 mg/g) at pH = 9.0 [2]. A study of cyanobacterium Nostoc sp. showed that an increase in pH could result in elevated content of PC, PE and APC, and optimum total PBP production was found at pH 9. The maximum PBPs and chlorophylls production in blue-green microalgae (such as S. platensis) were respectively obtained at pH 8 and pH 9 [69,78]. As to the carotenoid, pH 7–8 is identified as the optimal pH value to generate carotenoids within most green microalgae, typically, the best pH to produce β-carotene is 7.5 for D. salina. However, such pH alterations within some microalgal culture mediums can suppress carotenoid and chlorophyll synthesis.
Salinity plays a vital role in industrial pigment production of both marine and limnetic microalgae, and osmosis shows a great impact on pigment accumulation. Rapid entry of sodium ions into cells results in phycobilisome detachment from the thylakoid membrane, leading to a significant reduction of photosynthesis and a restriction of pigment production. Thus, excessive salinity is usually not expected, for it produces hypertonic solution in the culture liquid and causes cell shrinkage. The chlorophylls and total carotenoids content decreases with the increase in salinity; their optimum productivities were found at low salinity (2–3 ppt) for most microalgae [70]. On the other hand, β-carotene generation increased within blue-green microalgae as salinity levels elevated. As a result, 10–15 ppt of salinity was proven to maximize the PBPs generation of blue-green microalgae Anabaena sp. (135.73 mg/g) and Oscillatoria sp. (66.7 mg/g) [78].
4.3.3. Micronutrients
Micronutrients (such as manganese, iron and zinc) have critical effects for pigment metabolic pathways and generally low or even trace amounts are needed. Iron is involved in the tricarboxylic acid cycle and other metabolic pathways within C. pyrenoidosa, chlorophyll level decreased synchronizing with iron restriction. Iron electrical valency as well as counter ions affect astaxanthin accumulation [27]. Astaxanthin production can be enhanced after iron supplementation into the medium [85]. By adding 18 mM Fe2+-EDTA, astaxanthin synthesis can be effectively stimulated. β-carotene content also elevated significantly when supplied with 450 mM FeSO4 [91].
Copper serves as an essential cofactor for metalloenzymes in several metabolic pathways but is toxic for microalgae growth at high concentrations. A 1 mg/L copper cultures has been proved to hinder the growth of I. galbana, Pavlova viridis, and P. tricornutum, and meanwhile reduce their chlorophyll content. High zinc and copper contents are associated with chloroplast membrane peroxidation caused by free radical production, thus leading to reduction of chlorophyll content.
Magnesium ions, the pivotal chlorophyll ions, participate in pigment biosynthesis; moreover, they are also related to pigment metabolic pathway as the cofactor for critical enzymes [92]. Chlorophyll levels in cells of Chlorella sp. show a gradual decrease with and without magnesium restriction.
Sulfur deprivation represents another modification within the microalgae culture environment. Microalgae require sulfur for producing numerous essential metabolites, such as sulfur-containing amino acids cysteine and methionine. Sulfur deprivation reduces oxygenic photosynthesis, promotes the activation of hydrogenase and has a negative effect on microalgal chlorophyll accumulation in C. reinhardtii and C. fusca [93,94]. On the contrary, sulfur deprivation displays a promoting effect for carotenoids biosynthesis. It is a more efficient approach to induce astaxanthin and lipids accumulation in H. pluvialis compared with nitrogen restriction, which was also verified in C. reinhardtii and Parachlorella kessleri [93,95].
5. Metabolic and Genomic Design for Pigment Production
The recent few years has seen an increased interest in the exploitation of microalgae as recombinant platforms for pigment production [96], requiring easy and efficient genetic engineering methods targeting nuclear and chloroplast genomes.
Compared to plants, the research of microalgae transgenic system started late. Transformation of microalgae was first accomplished within C. reinhardtii by using the high-speed microprojectiles in 1988 and it was also the first report of chloroplast conversion [97]. Ever since then, great progress has been made on the transgenic approach of this species, and achievements has been applied to other algae species [98]. Apart from C. reinhardtii, tremendous advances are attained in genetically engineering other several microalgal species, such as Nannochloropsis sp. [99], P. tricornutum [100], Chlorella spp. [101] together with C. vulgaris [102]. The most frequently used method of transformation includes glass bead method, electroporation, agrobacterium-based transformation, PEG-mediated method and biolistic delivery. Apart from transformation methods, regulatory elements also affect the transformation efficiency and stability such as vector backbones, promoters, UTR, selectable and screenable markers [103]. Since carotenoid is primarily synthesized in the chloroplasts of microalgae, the transformation of either nuclear or chloroplast is available to improve the production.
Sufficient isoprenoid precursor is the prerequisite for the massive carotenoid accumulation. Overexpressing the key enzymes involved in the synthetic pathway is the efficient strategy to increase carotenoid content. The phytoene synthase (PSY), phytoene desaturase (PDS) and beta-carotene ketolase (BKT) are reported as the key enzymes during carotenoid biosynthesis. The overexpression of PSY gene from D. salina in C. reinhardtii resulted in an improved carotenoid content, of which violaxanthin, lutein, neoxanthin and β-carotene showed a 2, 2.6, 1.8 and 1.25-fold increase compared with the wild type [104]. Introducing PSY into P. tricornutum at the exponential phase increased fucoxanthin content by about 1.45-time [105]. The internal PDS was codon optimized and overexpressed in H. pluvialis, and astaxanthin content was up to 67% higher than the wild-type (WT) [106]. The BKT and beta-carotene hydroxylase (CRTR-B) in H. pluvialis were introduced into Dunaliella viridis to produce astaxanthin and canthaxanthin content at 77.5 and 50.1 μg/g, respectively [107]. Alternatively, microRNA-mediated silencing was applied to knock down the autophagy-related genes ATG1 and ATG8, which increased the β-carotene content at 2.34-fold of the wild type [108].
The carbon flux and available carbon molecule are anticipated to guide pigment biosynthesis for high carbon efficiency [109]. The carbon skeleton and energy for pigment biosynthesis are mainly from Calvin-Benson-Bassham (CBB) cycle as autotrophic mode or Embden-Meyerhof-Parnas (EMP) pathway as heterotrophic mode [110]. As the active photosystem was conducive to pigment accumulation, the low light and blue light was applied to elevate acetyl-CoA and pyruvate for improving fucoxanthin content in I. zhangjiangensis and N. laevis at 22 and 11.1 mg/g, respectively [111,112]. The red light was beneficial for cell division with high chlorophyll but the blue light was favorable for astaxanthin accumulation in H. pluvialis at 91.8 mg/L yield [113]. As the secondary metabolite, astaxanthin usually competes the carbon availability with protein accumulation for cell growth. The addition of glucose could vastly promote carbon availability in microalgae, but it restrains the photosystem, thus limiting the pigment biosynthesis. As nitrogen deficiency was an effective condition to up-regulate the genes involved in carotenoid biosynthesis several times, it was combined with carbon-based fed-batch culture to guide the elevated acetyl-CoA and pyruvate into astaxanthin biosynthesis in C. zofingiensis at 2.0 mg/L/d productivity [114]. The metabolic and genetic engineering approaches are efficient to improve pigment production from microalgae, but the food safety concerns and consumers’ acceptance are still required to be overcome.
6. Downstream Processing for the Stability of Microalgal Pigments
6.1. Pigment Stability by Extraction Technology
Pigments produced by microalgae fermentation demand effective technologies for cell (membranes and/or cell wall) disruption and extraction. The stability of target pigments is one of the most fundamental requirements during extraction, but is also easily destroyed by extreme conditions, impacting the final quality and bioaccessibility of products. For the extraction of microalgal pigments, appropriate methods should be chosen to protect their structure. Common extraction methods can be divided into mechanical (e.g., bead milling, pressure, ultrasonication, microwave, and electric fields), chemical (supercritical extraction) and biological (enzymatic) methods. Table 3 presents a summary of techniques mentioned in this section to obtain microalgal pigments.

Table 3.
The reported extraction methods of microalgal pigments a.
6.1.1. Classic Methods
Solvent extraction is a classic method for microalgal pigments [144]. A solvent can be absorbed within the cell wall, causing perforation or dissolution of the membrane and/or wall, and acting as the extractant of intracellular compounds. The selection of the most effective solvent relies on their chemical affinities for target pigments and the composition of a microalgal membrane or cell wall. Carotenoid extraction can be performed with non-polar solvents due to their high hydrophobicity. For instance, lutein extraction from wet C. vulgaris was investigated, with ethanol/hexane 3:1 (v/v) being proven to be the most suitable solvent [145]. Fucoxanthin from I. galbana can be intactly obtained via ethanol extraction [35], and astaxanthin extraction from H. pluvialis is performed using chloroform/methanol or acetone/ethyl acetate/ethanol [115]. Since natural pigments are thermally sensitive compounds, their solvent extraction should be operated at appropriate temperatures. While the application of thermal treatment can improve the extraction rate, excessive exposure to high temperatures (usually over 65 °C) leads to structure degradation, which has been verified on carotenoids and chlorophylls [146]. For some extractants that are short of cell destructive effects, they can be combined to other chelating agents, antibiotics, hypochlorite, detergents, chaotropes, bases and acids with their own mechanism of action on cell disruption. For instance, bases contribute to membrane lipid saponification, while acids cause membrane pore formation. Solvent extraction has obtained high degrees of efficiency and purity in large-scale production of microalgal pigments. As the basic extraction method, it has been widely combined with other cell disruption methods. However, considering the possible applications in the food industry, the scale application of this technique is limited by toxicity and a residual of solvents, which may adversely affect the edible safety of pigments.
Bead milling (BM) uses high-speed beads made of steel, glass or ceramic by microbiological cell collision. Cell disruption occurs because of shear forces produced due to friction and collision. This method is a simple, fast, and low energy input disruption approach, which is suitable for production in the industry. It causes relatively less damage to intracellular substances, and shows a high disruption efficiency. It has been adopted for determining overall C-PC content in cyanobacteria. For extraction, BM is lack of selectivity, and usually operated with additional solvent. When used for extracting pigments from S. almeriensis or Chlorella sp., it has been proved to be effective and repeatable [147]. The addition of an appropriate solvent can also facilitate the timely release of thermal energy generated from mechanical action, so as to avoid the damage of pigment structures caused by the temperature rise.
Freeze-thaw (FT) is usually utilized in the laboratories for disrupting cyanobacteria cells and extract PC, C-PC, or PE, for PBPs are preferable to be handled and preserved at low temperatures [148]. As protein pigments, their denaturation is mainly due to high temperatures, which lead to decreasing amounts of alpha helix. The freezing/thawing for three or four cycles were reported to provide best C-PC extraction purity and yield, and the optimum temperature was around 4 °C [148]. Although FT provides high-purity extracts (0.66–0.87), the repeated cycles consume a lot of energy and time. As a result, this approach is only appropriate in laboratories [148].
6.1.2. Pressurized Systems
High pressure homogenization (HPH) can be regarded as a promising and scalable method, it induces cell disruption by creating mechanical impacts of dramatical turbulence, shear stress, as well as cavitation. When combined with solvent extraction, it has been used on high-quality pigments, especially lipid-soluble ones, from different microalgae. For Nannochloropsis sp., the 1000 bar HPH can enhance the bioaccessibility of extracted violoxanthin, antheraxanthin, zeaxanthin, and β-carotene for food products, but decreases their contents due to the sharp raise of temperature [119]. To avoid pigment degradation, an additional cooling system is required.
Pressurized liquid extraction (PLE) has been used in food processing and was lately evaluated as an extraction method. PLE is usually operated at high temperatures (50–200 °C) and pressures (100–200 bar), which is generally believed to destroy the molecular structure of pigments [149]. However, some studies have successfully extracted carotenoids and chlorophylls with acceptable stability via this method. Its protection of stability and recovery can be verified on C. vulgaris for lutein, β-carotene, chlorophyll a/b extraction [120], D. salina for β-carotene extraction [121], and Phormidium spp. for carotenoids extraction [122]. Deeper investigations are required to establish specific PLE strategies for pigments with different structural characteristics.
Furthermore, a method called continuous pressurized solvent extraction (CPSE) was established with more moderate conditions (generally room temperature at 70 °C) to protect pigments from degradation and has been applied in carotenoids extraction from Gloeothece sp. at 60 °C [123]. It also reduces solvent consumption via more effective recirculation.
While a pressurized system does not need cell drying and is easy to achieve scale expansion, it can only be conducted at the low cell concentration (usually 0.01–0.85% w/w), leading to increased energy consumption. Meanwhile, these methods are not specific enough, because cell debris and additional compounds will be produced into the system, which lead to higher requirements for downstream purification.
6.1.3. Wave-Energy Treatment
Microwave-assisted extraction (MAE) and ultrasound-assisted extraction (UAE) are already investigated for optimization in cell disruption and extraction of high-value compounds from microalgae. Acoustic cavitation is a main driving force of UAE, which increases local pressure and induces thinned cell membrane as well as cell disruption, allowing the penetration of solvent. This method is advantageous for the integral extraction of pigments at low temperature ranges (<70 °C) and has been practiced in β-carotene extraction from S. platensis and astaxanthin extraction from H. pluvialis [115,126]. It can perform outstanding effects on elevating extraction yield of PBP from Spirulina sp., but was also claimed to have adverse effects on PC purity, color and antioxidant activity.
Regarding efficient disruption of microalgae cells, radiation in MAE can cause heat shock and cell wall degradation. This method requires a much shorter time for thermal treatment, thus lessening the restriction of pigment thermostability [149]. It further achieved a lower temperature when performed under vacuum conditions in a recent study [124]. This method has been practiced on the stable extraction of carotenoids, chlorophylls, PE, PC and APC in low treatment temperatures (around 55 °C) from various microalgae, such as Scenedesmus sp. and Spirulina sp. [150]. So far, the exploration of MAE for microalgal pigment extraction is still not comprehensive enough.
6.1.4. Enzymatic Extraction
Glucanase, glycosidase, lipase and peptidase have been extensively applied to lyse cells with high specificity [144,151]. These enzymes can disrupt the cell wall and/or membrane, thus leading to the exposure of the intracellular contents to solvent. Enzymatic extraction demands mild reaction conditions and no downstream drying steps and displays a higher extraction rate than any other methods. Specifically, lysozyme can be used for APC obtention from S. platensis and guarantee its high quality and purity [117]. The large-scale application of this technology requires the search for enzymes with catalyzing ability to cell disruption and the selection of processing conditions that ensures enzyme activity and pigment stability.
6.1.5. Pulsed Electric Field (PEF)
Electric field technology ash been applied in food processing since the 1970s. Pulsed electric fields (PEF) are utilized to be the appropriate extraction approaches of multiple microalgal pigments by employing a pulse charge onto cell membranes to trigger an electroporation effect [152]. In a study about C. vulgaris, PEF displayed an excellent effect on cell disruption without massive carotenoid and chlorophyll inactivation. The process can be refined to perform at 25–30 °C to further increase the extraction effect of temperature-sensitive pigments, especially lutein. In the case of PBP, PEF have been recommended for the extraction of PC from S. platensis, Nostoc commune, and Porphyridium cruentum [128,129,130]. However, it was suggested to be unsuitable for microalgal organisms with a resistant cell wall, such as Oscillatoria okeni. PEF can also serve as the pre-treatment of other methods, which has been applied on the first stage of solvent extraction of pigments from Nannochloropsis spp. and H. pluvialis [131,132].
PEF requires low amounts of solvent and has been widely accepted as a “green”, scalable and continuable method for industrial extraction. The parameters such as electric field strength, pulse number and duration should be optimized for different microalgal organisms and desired pigments. For instance, cyanobacteria disruption is energy-consuming for electroporation compared with microalgae because of the membrane components and small size [130].
6.1.6. Novel Methodologies
Some novel methodologies suggested for pigment extraction are mostly adaptations for existing technologies, including laser, flotation, high-voltage electrostatic fields as well as ohmic heating.
Laser has been used for cell disruption in Nannochloropsis oculata without destroying pigment structures, but it is difficult for scaling-up into large-scale production [142]. Liquid biphasic flotation is used in combination with the liquid biphasic system as well as solvent sublation to protect pigment stability during extraction. It has been recommended for astaxanthin extraction from H. pluvialis [153]. Ohmic heating (OH) and high voltage electrical discharge (HVED) may become options with the highest scalability and economic viability. A continuous electric field with high intensity in HVED can preserve pigment stability and quality. In the extraction of chlorophyll and carotenoids from N. oculata, HVED acts as the pre-treatment [143]. OH causes Joule effect via an alternate current, which can accelerate cell breakage and electroporation, while its destruction of pigment structures is less than that of classical methods.
In conclusion, combinations of both intrinsic and extrinsic influences are responsible for the stability of pigment during the extraction process. From the perspective of extraction technologies, many new advances in methods have been proposed. However, most establishments and the optimization of technologies are chasing a higher yield and recovery to meet the demand of industrial production, which usually comes at the cost of the destruction of pigment structure. So far, liquid–liquid extraction and solvent extraction remain those most common approaches used in carotenoids and chlorophylls extraction. On the other hand, the operating parameters also show an impact on the stability of pigments, in which temperature plays a decisive role in the majority of extraction technologies. UAE leads to great results in PBP extraction yield, but unsuitable high temperatures seriously impact on its stability. Moreover, for food pigments, the potential safety concerns, especially in the methods with organic solvents, cannot be ignored.
Inherently, pigment stability also relies on the microalgal cell characteristics, pigment properties, and its location in cell. The location of lutein in microalgal cell limits the mass transfer rate, similarly, the robust cell wall of H. pluvialis is hard to be broken, requiring more extreme strategies that are responsible for more serious damage of pigment structures. In addition, some pigments have special poor thermostability and pH-stability, or poor tolerance to organic solvents. As protein pigments, proteolysis and denaturation of PBPs happen under unsuitable conditions, which directly damage the structure and physiological activity of pigment molecules. Therefore, more and deeper targeted research is demanded to optimize the obtention strategy for different microalgal pigments, so as to simultaneously achieve higher yield and better product quality, for which the stability of pigments is the most basic premise.
6.2. Pigment Stability in Food Processing and Improving Strategies
In food manufacturing, preservation, storage and preparation, faster degradation of natural pigments happens frequently. Compared with extraction, the regulation mechanism of operation conditions on pigment stability in this period is extremely complex, with synergistic or antagonistic effects. Several technologies have been established to overcome the instability issue and guarantee their coloring capability.
Chlorophylls display high susceptibility to oxygen, light, temperature, and pH variations. Based on unfavorable situations, chlorophyll discoloration and decomposition take place immediately to limit their commercial-scale application as food colorants. Substituting unstable magnesium ions by copper or zinc ions can generate blue/green metallo-chlorophylls with high stability, but it is required that metal salt contents should exceed the FDA-established limit contents [154]. Physical encapsulation represents an approach, where a coating material protects and accurately releases sensitive bioactive compounds. Wall materials can protect chlorophylls against light degradation and achieve high storage stability, antioxidant activity and water solubility. Nevertheless, high encapsulation efficiency of food pigments requires the coating material to be biocompatible, biodegradable and low toxicity. While appropriate cooking conditions and food matrices can minimize thermal degradation, using chlorophylls as food colorants remains restricted by their vulnerability to acidic environments (pH 3.5–5.0) and illumination [52], thus more investigations of stable chlorophylls are demanded.
Carotenoids are relatively resistant pigments due to their lipid-soluble properties, but their degradation in processing is still noteworthy. Thermal processing contributes in their structural destruction, depriving the nutritional and organoleptic characteristics of foods. High temperatures during baking, refining, frying and other processes trigger the isomerization as well as later carotenoid decomposition [155]. Carotenoids also have resistance to extreme pH levels; nevertheless, bases and acids can induce trans- and cis-isomerization of certain de-esterifications, rearrangements and double bonds. Therefore, particular attention should be paid to pH control in the processing of food and beverages. For compound juices, carotenoid content experienced a sharp reduction when the pH was adjusted to 7–8 while increased at pH ranges of 3–6 [156]. When exposed to oxygen for long periods, oxidation reactions affect the majority of carotenoids in food products, causing the color fading of products. In the end, exposure to light also triggers the breakdown of carotenoids and generates the loss of their stability and biological function of provitamin A. Foods and beverages containing carotenoids are suggested to be packaged in opaque materials. The existing approach to improve carotenoids stabilization consist of encapsulation. β-carotene needs appropriate wall material and spray-drying conditions. When combined with freeze-drying, spray-drying within microcapsules can prevent the decomposition and enhance its stability in the presence of light, ultraviolet light, high temperatures as well as humidity.
Those major factors that affect PBP stability include pH, temperature, and light. pH has been identified as a major factor affecting PBPs dissociation and aggregation in solution and they should be handled at the best pH level to avoid decomposing. With a pH of about 7.0, the PC stable hexameric form predominates the aggregation, avoiding denaturation and stability decreasing [157]. PE is stable at pH = 4.0–10.0, for its secondary structure adopts the stable conformation (hexameric form) at such pH levels [158]. Maintaining stability at a wide pH promotes its applications into the food industry. PBPs, the protein pigments, are degraded manly because of denaturation. An increasing temperature drives the decrease in a-helix quantity, causing stability loss. Thus, food products containing PBPs are recommended to be handled and preserved at a low temperature, and preferable to be preserved under the lower than ambient temperature because they are susceptible to microbiological decomposition [157]. PBPs are also sensitive to light, among which PC shows degradation after light exposure at the 100 μmol photons/m2/s intensity [159]. When exposed to long-term light, it tends to lose chromophores, leading to the loss of color and stability.
The usage of additives is the simplest way of improving PBP stability, especially thermal stability. This method requires no costly or complicated device. Glucose, sodium chloride and sucrose are the protein-stabilizing agents by covering PC surface to protect its chemical structure and increasing water surface tension [157,160]. Benzoic acid displays antimicrobial and antioxidant activity, with great capability of preserving and increasing PBP stability [161]. However, toxicity and flavor of the heavily used additives must be considered in the food industry. Beet pectin is a suitable material for phycobilins complex formation, and it can protect color while enhancing alcalase, bromelain and papain degradation [162]. Microencapsulation was also proven to be capable for improving thermal stability of PBP and resisting gastric acids.
Overall, microalgal pigments display susceptibility to oxygen, light, temperature, and pH variations in different degrees, ordinary cooking methods were proven to adversely affect their stability. Unfavorable cooking methods and storage conditions will speed up their degradation and discoloration, leading to the loss of their coloring capability and nutritional value. Further research focusing on the stabilization of natural pigments and their chemical interaction with food matrix is needed. To date, the bottleneck has been addressed via several approaches, which show capability to overcome the instability issue, guarantee their coloring capability and strengthen their incorporation in foods. However, only scarce methods are currently available in the real food industry.
7. Economic Analysis of Microalgal Pigments in Foods
Consumers’ proclivity towards nutritive, attractive, and clean-label food products has driven a distinct move of market interest towards natural ingredients, especially within the food industry. Microalgae are one of the major photosynthesizers of naturally derived commercial pigments with abundant availability and maintenance, the growing interest of market in the exploitation of microalgae field has been confirmed in many reports [2]. Countries such as the Germany, USA and China annually generate over 19,000 tons of dry microalgae biomass, which is worthy of around USD 5.7 billion [4]. In terms of major microalgae-extracted pigments, their commercial generation was estimated to be about 10,000, 4000, 1000 and 200 tons/year for Spirulina, Chlorella, Dunaliella and Haematococcus, respectively [163].
The market value of PBP was predicted to continue increasing rapidly in upcoming years, owing to the increasing demand of natural green and blue shades from food (especially confectionery and beverages), pharmaceutical, nutraceutical and the cosmetics industry [2]. Manufacturers are chasing 100% pure and natural food-grade pigments with antioxidation effects, great nutrient levels as well as vibrant pigments from Spirulina, the largest source of PBP. Spirulina refers to one of the most worldwide cultured microalgae, which may reach above USD 779 million of marker value by 2026. Meanwhile, remarkable CAGR may grow in the Asia-Pacific region, which provides more opportunities for manufacturers. Among Spirulina-pigments, PC reached a marker value of USD 112.3 million in 2018, which is expected to exceed USD 232.9 million by the end of 2025 [2]. PC from Spirulina is already used in the food, beverages, and cosmetic industries (such as Bloo Tonic®, M&Ms® chocolates and B-blue Spirulina drink) with an ever-increasing in quantity demand. When used as food supplements, PC has been produced in liquid and powder form by several companies (e.g., Spirulysat®, Electric Sky®).
In the case of chlorophylls, based on the report from Value Market Research, it reached a marker value of USD 300 million globally, and may surpass USD 463.7 million by 2025 [2]. As one of its major sources, Chlorella may reach the market value of USD 210.15 million by 2024, of which Europe still controls the major market sharing.
Their simple use, good appearance, better stability and increased consumer appeal have contributed to the important position of carotenoids in the global pigment market, especially in America and Europe. The global pigment market produced by >600 organisms’ sources may reach 1.84 billion USD by 2026, which is comprised by 2500–10,000 and 300–3000 USD/kg for astaxanthin and β-carotene, respectively. For β-carotene, its market value outpaced 520 million USD in 2020, which may reach 780 million USD in 2027, of which the β-carotene for dietary supplements accounts for over 21%. In the 1980s, D. salina began to be commercially produced by Western Biotechnology and Betatene (Australia) for β-carotene production. The company is currently owned by BASF, the biggest manufacturer of natural β-carotene from Dunaliella. To this day, D. salina is generated with the annual world-wide yield being about 1200 tons [164,165]. For β-carotene, its global market value may reach USD 618.94 million in 2026, with 3.8% increase in CAGR during 2018–2026 [2]. H. pluvialis is produced at an annual amount of above 300 tons in US, India and Israel, and the vast majority of its large-scale culture is for astaxanthin production. In 1994, the AstaReal Group first achieved prosperous astaxanthin manufacturing commercially. A Brazilian company named Ocean Drop sells food and cosmetic products incorporated with astaxanthin. In the end of 2026, the market value of astaxanthin may reach USD 800 million globally. Lutein market surpasses USD 308 million per year and is currently growing [166]. Xanthophylls are also candidate biomolecules, and their application in industrial production can be enhanced by further investigations on their novel biological sources as well as productivity efficiency.
Collectively, consumers’ antipathy against the toxic issues of synthetic food colorants has raised the increase of natural pigments in the food market. As the proportion of natural pigments in the consumer market gradually expanded and partially replaced that of synthetic manufacturers, the demand for microalgal pigments is moving on an upward swing, leading to a sharp raise of market value. Microalgae are now the most economical and eco-friendly source of natural pigments, which will continue gaining more usage in various industrial fields in the next years. Microalgal astaxanthin and β-carotene will elevate in their global sales volume, and will maintain their unassailable position in the global market until at least 2026 [167]. The market value of PBP has also risen at an impressive CAGR in recent years, mostly from the microalgae Spirulina. Chlorophylls from microalgae also gain increasing attention in the food industry, and will achieve substantial market value in the next five years. To further expand the market size and economic value of these microalgae pigments, market participants are searching for lower-cost and faster technologies, and more comprehensive regulations are demanded.
8. Future Perspective and Conclusive Remarks
It is promising to use microalgae as the candidate sources to obtain value-added biochemicals such as pigments. After decades of research and exploration, microalgal pigments have been proven to have excellent coloring ability and beneficial properties, when used in the food industry, they perform well, have an attractive appearance, various nutritional functions and guaranteed edible safety.
From the perspective of economic benefits, the market consumption of microalgal pigments has been growing at an impressive CAGR and can be expected to continuosly expand in the next five years, meanwhile consumers’ acceptance and belief in them will also achieve a higher degree. These advantages confirm its wide application range and potential value in diverse markets, and some of them have indeed been successfully produced on an industrial scale. However, inapposite culture and extraction strategy may easily lead to pigment structure alterations or irreversible destruction, impacting the product quality and value. Moreover, the large-scale culture strategies for different microalgae and efficient extraction technologies that ensure product stability have not been fully developed yet. On account of the backwardness of these technologies, the production of most microalgal pigments is still confined at experimental stage, and its commercial industrialization is far from reality. Thus, further explorations are still in need to optimize the existing methods and dig untapped potential technologies, referring to microalgae characteristics, pigment properties and biosynthetic metabolism. In general, microalgae are widely acknowledged to become a prominent and popular source of commercial food pigments in the coming future, with in-depth researches conquering those technological bottlenecks.
Author Contributions
H.S.: Conceptualization, Data curation, Software, Writing—original draft. Y.W.: Data curation, Validation, Writing—original draft. Y.H.: Validation, Visualization. B.L.: Writing—review & editing. H.M.: Writing—review & editing. S.Y.: Conceptualization, Writing—review & editing. F.C.: Conceptualization, Funding acquisition, Writing—review & editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported by Shenzhen Science and Technology R & D Fund (20220809171532001), Key Realm R&D Program of Guangdong Province (No. 2018B020206001), Guangdong Province Zhujiang Talent Program (No. 2019ZT08H476), Science and Technology Innovation Commission of Shenzhen (No. KQTD20180412181334790) and Open-ended Project of Carbon Neutral Research Institute, Fujian Normal University (TZH2022-03).
Conflicts of Interest
The authors declare that they have no known competing financial interest or personal relationships that could influence the work reported in this paper.
References
- Aschemann-Witzel, J.; Gantriis, R.F.; Fraga, P.; Perez-Cueto, F.J.A. Plant-based food and protein trend from a business perspective: Markets, consumers, and the challenges and opportunities in the future. Crit. Rev. Food Sci. Nutr. 2021, 61, 3119–3128. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.K.; Albarico, F.P.J.B.; Perumal, P.K.; Vadrale, A.P.; Ntan, C.T.; Chau, H.T.B.; Anwar, C.; Wani, H.M.U.D.; Pal, A.; Saini, R. Algae as an emerging source of bioactive pigments. Bioresour. Technol. 2022, 351, 126910. [Google Scholar] [CrossRef] [PubMed]
- Baehr, L. The Next Big Superfood Could Be Green and Slimy. Available online: https://www.businessinsider.com/algae-is-the-superfood-of-the-future-2014-6 (accessed on 22 January 2023).
- Silva, S.C.; Ferreira, I.C.; Dias, M.M.; Barreiro, M.F. Microalgae-derived pigments: A 10-year bibliometric review and industry and market trend analysis. Molecules 2020, 25, 3406. [Google Scholar] [CrossRef] [PubMed]
- Schoefs, B. Chlorophyll and carotenoid analysis in food products. Properties of the pigments and methods of analysis. Trends Food Sci. Technol. 2002, 13, 361–371. [Google Scholar] [CrossRef]
- Gong, M.; Bassi, A. Carotenoids from microalgae: A review of recent developments. Biotechnol. Adv. 2016, 34, 1396–1412. [Google Scholar] [CrossRef]
- Lopez, M.J.; Hall, C.A. Physiology, Osmosis; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Patel, A.K.; Singhania, R.R.; Chen, C.-W.; Tseng, Y.-S.; Kuo, C.-H.; Wu, C.-H.; Di Dong, C. Advances in micro-and nano bubbles technology for application in biochemical processes. Environ. Technol. Innov. 2021, 23, 101729. [Google Scholar] [CrossRef]
- Ferreira, V.D.; Sant’Anna, C. Impact of culture conditions on the chlorophyll content of microalgae for biotechnological applications. World J. Microbiol. Biotechnol. 2017, 33, 8. [Google Scholar]
- Juric, S.; Juric, M.; Krol-Kilinska, Z.; Vlahovicek-Kahlina, K.; Vincekovic, M.; Dragovic-Uzelac, V.; Donsi, F. Sources, stability, encapsulation and application of natural pigments in foods. Food Rev. Int. 2022, 38, 1735–1790. [Google Scholar] [CrossRef]
- Jacob-Lopes, E.; Maroneze, M.M.; Deprá, M.C.; Sartori, R.B.; Dias, R.R.; Zepka, L.Q. Bioactive food compounds from microalgae: An innovative framework on industrial biorefineries. Curr. Opin. Food Sci. 2019, 25, 1–7. [Google Scholar] [CrossRef]
- Sigurdson, G.T.; Tang, P.; Giusti, M.M. Natural colorants: Food colorants from natural sources. Annu. Rev. Food Sci. Technol. 2017, 8, 261–280. [Google Scholar] [CrossRef]
- Beydoun, M.A.; Chen, X.; Jha, K.; Beydoun, H.A.; Zonderman, A.B.; Canas, J.A. Carotenoids, vitamin A, and their association with the metabolic syndrome: A systematic review and meta-analysis. Nutr. Rev. 2019, 77, 32–45. [Google Scholar] [CrossRef]
- Sluijs, I.; Cadier, E.; Beulens, J.; Spijkerman, A.; Van der Schouw, Y. Dietary intake of carotenoids and risk of type 2 diabetes. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 376–381. [Google Scholar] [CrossRef]
- Pogorzelska, E.; Godziszewska, J.; Brodowska, M.; Wierzbicka, A. Antioxidant potential of Haematococcus pluvialis extract rich in astaxanthin on colour and oxidative stability of raw ground pork meat during refrigerated storage. Meat Sci. 2018, 135, 54–61. [Google Scholar] [CrossRef]
- Cuellar-Bermudez, S.P.; Aguilar-Hernandez, I.; Cardenas-Chavez, D.L.; Ornelas-Soto, N.; Romero-Ogawa, M.A.; Parra-Saldivar, R. Extraction and purification of high-value metabolites from microalgae: Essential lipids, astaxanthin and phycobiliproteins. Microb. Biotechnol. 2015, 8, 190–209. [Google Scholar] [CrossRef]
- Park, J.S.; Chyun, J.H.; Kim, Y.K.; Line, L.L.; Chew, B.P. Astaxanthin decreased oxidative stress and inflammation and enhanced immune response in humans. Nutr. Metab. 2010, 7, 1–10. [Google Scholar] [CrossRef]
- Chuyen, H.V.; Eun, J.-B. Marine carotenoids: Bioactivities and potential benefits to human health. Crit. Rev. Food Sci. Nutr. 2017, 57, 2600–2610. [Google Scholar] [CrossRef]
- Yoshida, H.; Yanai, H.; Ito, K.; Tomono, Y.; Koikeda, T.; Tsukahara, H.; Tada, N. Administration of natural astaxanthin increases serum HDL-cholesterol and adiponectin in subjects with mild hyperlipidemia. Atherosclerosis 2010, 209, 520–523. [Google Scholar] [CrossRef]
- Shakeri, A.; Soheili, V.; Karimi, M.; Hosseininia, S.A.; Fazly Bazzaz, B.S. Biological activities of three natural plant pigments and their health benefits. J. Food Meas. Charact. 2018, 12, 356–361. [Google Scholar] [CrossRef]
- Du, S.-Y.; Zhang, Y.-L.; Bai, R.-X.; Ai, Z.-L.; Xie, B.-S.; Yang, H.-Y. Lutein prevents alcohol-induced liver disease in rats by modulating oxidative stress and inflammation. Int. J. Clin. Exp. Med. 2015, 8, 8785. [Google Scholar]
- Huang, Y.-M.; Dou, H.-L.; Huang, F.-F.; Xu, X.-R.; Zou, Z.-Y.; Lin, X.-M. Effect of supplemental lutein and zeaxanthin on serum, macular pigmentation, and visual performance in patients with early age-related macular degeneration. BioMed Res. Int. 2015, 2015, 564738. [Google Scholar] [CrossRef]
- Sun, Y.-X.; Liu, T.; Dai, X.-L.; Zheng, Q.-S.; Hui, B.-D.; Jiang, Z.-F. Treatment with lutein provides neuroprotection in mice subjected to transient cerebral ischemia. J. Asian Nat. Prod. Res. 2014, 16, 1084–1093. [Google Scholar] [CrossRef]
- Hosokawa, M.; Wanezaki, S.; Miyauchi, K.; Kurihara, H.; Kohno, H.; Kawabata, J.; Odashima, S.; Takahashi, K. Apoptosis-inducing effect of fucoxanthin on human leukemia cell line HL-60. Food Sci. Technol. Res. 1999, 5, 243–246. [Google Scholar] [CrossRef]
- Duppeti, H.; Chakraborty, S.; Das, B.S.; Mallick, N.; Kotamreddy, J. Rapid assessment of algal biomass and pigment contents using diffuse reflectance spectroscopy and chemometrics. Algal Res. 2017, 27, 274–285. [Google Scholar] [CrossRef]
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial applications of microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Begum, H.; Yusoff, F.M.; Banerjee, S.; Khatoon, H.; Shariff, M. Availability and Utilization of Pigments from Microalgae. Crit. Rev. Food Sci. Nutr. 2016, 56, 2209–2222. [Google Scholar] [CrossRef]
- Varela, J.C.; Pereira, H.; Vila, M.; León, R. Production of carotenoids by microalgae: Achievements and challenges. Photosynth. Res. 2015, 125, 423–436. [Google Scholar] [CrossRef]
- Rajput, A.; Singh, D.P.; Khattar, J.S.; Swatch, G.K.; Singh, Y. Evaluation of growth and carotenoid production by a green microalga Scenedesmus quadricauda PUMCC 4.1. 40. under optimized culture conditions. J. Basic Microbiol. 2022, 62, 1156–1166. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Li, X.; Ren, Y.; Zhang, H.; Mao, X.; Lao, Y.; Wang, X.; Chen, F. Boost carbon availability and value in algal cell for economic deployment of biomass. Bioresour. Technol. 2020, 300, 122640. [Google Scholar] [CrossRef] [PubMed]
- Andrade, D.; Colozzi-Filho, A.; Guedes, C.; Lima, F.; Machineski, G.; Matos, M. Principais produtos da biomassa algal e suas aplicações biotecnológicas. In Microalgas De Águas Cont. Potencialidades E Desafios Do Cultiv; Londrina: Iapar, Brazil, 2014; pp. 265–343. [Google Scholar]
- Blanco, A.M.; Moreno, J.; Del Campo, J.A.; Rivas, J.; Guerrero, M.G. Outdoor cultivation of lutein-rich cells of Muriellopsis sp. in open ponds. Appl. Microbiol. Biotechnol. 2007, 73, 1259–1266. [Google Scholar] [CrossRef]
- Fernández-Sevilla, J.M.; Acién Fernández, F.; Molina Grima, E. Biotechnological production of lutein and its applications. Appl. Microbiol. Biotechnol. 2010, 86, 27–40. [Google Scholar] [CrossRef]
- Li, Y.L.; Sun, H.; Wu, T.; Fu, Y.L.; He, Y.J.; Mao, X.M.; Chen, F. Storage carbon metabolism of Isochrysis zhangjiangensis under different light intensities and its application for co-production of fucoxanthin and stearidonic acid. Bioresour. Technol. 2019, 282, 94–102. [Google Scholar] [CrossRef]
- Kim, S.M.; Kang, S.-W.; Kwon, O.-N.; Chung, D.; Pan, C.-H. Fucoxanthin as a major carotenoid in Isochrysis aff. galbana: Characterization of extraction for commercial application. J. Korean Soc. Appl. Biol. Chem. 2012, 55, 477–483. [Google Scholar] [CrossRef]
- Mohamadnia, S.; Tavakoli, O.; Faramarzi, M.A. Enhancing production of fucoxanthin by the optimization of culture media of the microalga Tisochrysis lutea. Aquaculture 2021, 533, 736074. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Y.; Chen, L.; Cheng, W.; Liu, T. Combined production of fucoxanthin and EPA from two diatom strains Phaeodactylum tricornutum and Cylindrotheca fusiformis cultures. Bioprocess Biosyst. Eng. 2018, 41, 1061–1071. [Google Scholar] [CrossRef]
- Ishika, T.; Moheimani, N.R.; Bahri, P.A.; Laird, D.W.; Blair, S.; Parlevliet, D. Halo-adapted microalgae for fucoxanthin production: Effect of incremental increase in salinity. Algal Res. 2017, 28, 66–73. [Google Scholar] [CrossRef]
- Bermejo, R.; Acién, F.G.; Ibáñez, M.J.; Fernández, J.M.; Molina, E.; Alvarez-Pez, J.M. Preparative purification of B-phycoerythrin from the microalga Porphyridium cruentum by expanded-bed adsorption chromatography. J. Chromatogr. 2003, 790, 317–325. [Google Scholar] [CrossRef]
- Ruiz-Domínguez, M.; Fuentes, J.L.; Mendiola, J.; Cerezal-Mezquita, P.; Morales, J.; Vílchez, C.; Ibáñez, E. Bioprospecting of cyanobacterium in Chilean coastal desert, Geitlerinema sp. molecular identification and pressurized liquid extraction of bioactive compounds. Food Bioprod. Process. 2021, 128, 227–239. [Google Scholar] [CrossRef]
- Dufossé, L.; Galaup, P.; Yaron, A.; Arad, S.M.; Blanc, P.; Murthy, K.N.C.; Ravishankar, G.A. Microorganisms and microalgae as sources of pigments for food use: A scientific oddity or an industrial reality? Trends Food Sci. Technol. 2005, 16, 389–406. [Google Scholar] [CrossRef]
- Alonso-Gonzalez, A.; Orive, E.; David, H.; Garcia-Etxebarria, K.; Garrido, J.L.; Laza-Martinez, A.; Seoane, S. Scaly green flagellates from Spanish Atlantic coastal waters: Molecular, ultrastructural and pigment analyses. Bot. Mar. 2014, 57, 379–402. [Google Scholar] [CrossRef]
- Fithriani, D.; Ambarwaty, D.; Nurhayati. Identification of Bioactive Compounds from Nannochloropsis sp. 4th ed. In Proceedings of the EMBRIO International Symposium (EIS)/7th International Symposium of East-Asia-Fisheries-and-Technologists-Association (EAFTA), Bogor, Indonesia, 5–6 August 2019. [Google Scholar]
- Heydarizadeh, P.; Poirier, I.; Loizeau, D.; Ulmann, L.; Mimouni, V.; Schoefs, B.; Bertrand, M. Plastids of Marine Phytoplankton Produce Bioactive Pigments and Lipids. Mar. Drugs 2013, 11, 3425–3471. [Google Scholar] [CrossRef]
- Mimouni, V.; Ulmann, L.; Pasquet, V.; Mathieu, M.; Picot, L.; Bougaran, G.; Cadoret, J.-P.; Morant-Manceau, A.; Schoefs, B. The Potential of Microalgae for the Production of Bioactive Molecules of Pharmaceutical Interest. Curr. Pharm. Biotechnol. 2012, 13, 2733–2750. [Google Scholar] [CrossRef] [PubMed]
- Ngamwonglumlert, L.; Devahastin, S.; Chiewchan, N. Molecular structure, stability and cytotoxicity of natural green colorants produced from Centella asiatica L. leaves treated by steaming and metal complexations. Food Chem. 2017, 232, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Christaki, E.; Bonos, E.; Florou-Paneri, P. Innovative microalgae pigments as functional ingredients in nutrition. In Handbook of Marine Microalgae; Elsevier: Amsterdam, The Netherlands, 2015; pp. 233–243. [Google Scholar]
- Carvalho, A.P.; Silva, S.O.; Baptista, J.M.; Malcata, F.X. Light requirements in microalgal photobioreactors: An overview of biophotonic aspects. Appl. Microbiol. Biot 2011, 89, 1275–1288. [Google Scholar] [CrossRef]
- Marawaha, R.K.; Bansal, D.; Kaur, S.; Trehan, A. Wheat grass juice reduces transfusion requirement in patients with thalassemia major: A pilot study. Indian Pediatr. 2004, 41, 716–720. [Google Scholar] [PubMed]
- Ferruzzi, M.G.; Blakeslee, J. Digestion, absorption, and cancer preventative activity of dietary chlorophyll derivatives. Nutr. Res. 2007, 27, 1–12. [Google Scholar] [CrossRef]
- Zepka, L.Q.; Jacob-Lopes, E.; Roca, M. Catabolism and bioactive properties of chlorophylls. Curr. Opin. Food Sci. 2019, 26, 94–100. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B. Natural food pigments and colorants. In Bioactive Molecules in Food; Springer: Berlin/Heidelberg, Germany, 2019; pp. 867–901. [Google Scholar]
- Gateau, H.; Solymosi, K.; Marchand, J.; Schoefs, B. Carotenoids of Microalgae Used in Food Industry and Medicine. Mini-Rev. Med. Chem. 2017, 17, 1140–1172. [Google Scholar] [CrossRef]
- Borowitzka, M.A. High-value products from microalgae—Their development and commercialisation. J. Appl. Phycol. 2013, 25, 743–756. [Google Scholar] [CrossRef]
- Chiu, H.F.; Liao, J.Y.; Lu, Y.Y.; Han, Y.C.; Shen, Y.C.; Venkatakrishnan, K.; Golovinskaia, O.; Wang, C.K. Anti-proliferative, anti-inflammatory and pro-apoptotic effects of Dunaliella salina on human KB oral carcinoma cells. J. Food Biochem. 2017, 41, e12349. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.-S. Carotenoid extraction methods: A review of recent developments. Food Chem. 2018, 240, 90–103. [Google Scholar] [CrossRef]
- Cezare-Gomes, E.A.; Mejia-da-Silva, L.D.; Perez-Mora, L.S.; Matsudo, M.C.; Ferreira-Camargo, L.S.; Singh, A.K.; de Carvalho, J.C.M. Potential of Microalgae Carotenoids for Industrial Application. Appl. Biochem. Biotechnol. 2019, 188, 602–634. [Google Scholar] [CrossRef]
- Ohi, Y.; Namiki, T.; Katatae, M.; Tsukahara, H.; Kitamura, A. Effects of the addition of the natural carotenoid astaxanthin from microalgae Haematococcus pluvialis on the physical properties of bread. Nippon Shokuhin Kagaku Kogaku Kaishi = J. Jpn. Soc. Food Sci. Technol. 2009, 56, 579–584. [Google Scholar] [CrossRef]
- Berman, J.; Zorrilla-López, U.; Farré, G.; Zhu, C.; Sandmann, G.; Twyman, R.M.; Capell, T.; Christou, P. Nutritionally important carotenoids as consumer products. Phytochem. Rev. 2015, 14, 727–743. [Google Scholar] [CrossRef]
- Rao, A.R.; Sindhuja, H.; Dharmesh, S.M.; Sankar, K.U.; Sarada, R.; Ravishankar, G.A. Effective inhibition of skin cancer, tyrosinase, and antioxidative properties by astaxanthin and astaxanthin esters from the green alga Haematococcus pluvialis. J. Agric. Food Chem. 2013, 61, 3842–3851. [Google Scholar] [CrossRef]
- D’Alessandro, E.B.; Antoniosi Filho, N.R. Concepts and studies on lipid and pigments of microalgae: A review. Renew. Sustain. Energy Rev. 2016, 58, 832–841. [Google Scholar] [CrossRef]
- Sun, H.; Yang, S.; Zhao, W.; Kong, Q.; Zhu, C.; Fu, X.; Zhang, F.; Liu, Z.; Zhan, Y.; Mou, H.; et al. Fucoxanthin from marine microalgae: A promising bioactive compound for industrial production and food application. Crit. Rev. Food Sci. Nutr. 2022, 1–17. [Google Scholar] [CrossRef]
- Li, Y.; Liu, L.; Sun, P.; Zhang, Y.; Wu, T.; Sun, H.; Cheng, K.-W.; Chen, F. Fucoxanthinol from the diatom Nitzschia laevis ameliorates neuroinflammatory responses in lipopolysaccharide-stimulated BV-2 microglia. Mar. Drugs 2020, 18, 116. [Google Scholar] [CrossRef]
- Mishra, S.K.; Shrivastav, A.; Mishra, S. Effect of preservatives for food grade C-PC from Spirulina platensis. Process Biochem. 2008, 43, 339–345. [Google Scholar] [CrossRef]
- Mohammadi-Gouraji, E.; Soleimanian-Zad, S.; Ghiaci, M. Phycocyanin-enriched yogurt and its antibacterial and physicochemical properties during 21 days of storage. LWT 2019, 102, 230–236. [Google Scholar] [CrossRef]
- Sekar, S.; Chandramohan, M. Phycobiliproteins as a commodity: Trends in applied research, patents and commercialization. J. Appl. Phycol. 2008, 20, 113–136. [Google Scholar] [CrossRef]
- Patel, A.K.; Joun, J.M.; Hong, M.E.; Sim, S.J. Effect of light conditions on mixotrophic cultivation of green microalgae. Bioresour. Technol. 2019, 282, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Madhyastha, H.; Vatsala, T. Pigment production in Spirulina fussiformis in different photophysical conditions. Biomol. Eng. 2007, 24, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, U.; Pathak, N. Effect of different conditions on the production of chlorophyll by Spirulina platensis. J. Algal Biomass Utln 2010, 1, 89–99. [Google Scholar]
- Pisal, D.S.; Lele, S.S. Carotenoid production from microalga, Dunaliella salina. Indian J. Biotechnol. 2005, 4, 476–483. [Google Scholar]
- Novoveská, L.; Ross, M.E.; Stanley, M.S.; Pradelles, R.; Wasiolek, V.; Sassi, J.-F. Microalgal carotenoids: A review of production, current markets, regulations, and future direction. Mar. Drugs 2019, 17, 640. [Google Scholar] [CrossRef]
- Imamoglu, E.; Dalay, M.C.; Sukan, F.V. Influences of different stress media and high light intensities on accumulation of astaxanthin in the green alga Haematococcus pluvialis. New Biotech. 2009, 26, 199–204. [Google Scholar] [CrossRef]
- Ho, S.-H.; Chan, M.-C.; Liu, C.-C.; Chen, C.-Y.; Lee, W.-L.; Lee, D.-J.; Chang, J.-S. Enhancing lutein productivity of an indigenous microalga Scenedesmus obliquus FSP-3 using light-related strategies. Bioresour. Technol. 2014, 152, 275–282. [Google Scholar] [CrossRef]
- Zhao, X.; Ma, R.; Liu, X.; Ho, S.-H.; Xie, Y.; Chen, J. Strategies related to light quality and temperature to improve lutein production of marine microalga Chlamydomonas sp. Bioprocess Biosyst. Eng. 2019, 42, 435–443. [Google Scholar] [CrossRef]
- Ma, R.; Zhao, X.; Xie, Y.; Ho, S.-H.; Chen, J. Enhancing lutein productivity of Chlamydomonas sp. via high-intensity light exposure with corresponding carotenogenic genes expression profiles. Bioresour. Technol. 2019, 275, 416–420. [Google Scholar] [CrossRef]
- George, B.; Pancha, I.; Desai, C.; Chokshi, K.; Paliwal, C.; Ghosh, T.; Mishra, S. Effects of different media composition, light intensity and photoperiod on morphology and physiology of freshwater microalgae Ankistrodesmus falcatus—A potential strain for bio-fuel production. Bioresour. Technol. 2014, 171, 367–374. [Google Scholar] [CrossRef]
- Kim, Z.-H.; Kim, S.-H.; Lee, H.-S.; Lee, C.-G. Enhanced production of astaxanthin by flashing light using Haematococcus pluvialis. Enzym. Microb. Technol. 2006, 39, 414–419. [Google Scholar] [CrossRef]
- Fatma, T. Screening of cyanobacteria for phycobiliproteins and effect of different environmental stress on its yield. Bull. Environ. Contam. Toxicol. 2009, 83, 509–515. [Google Scholar]
- Chen, W.-C.; Hsu, Y.-C.; Chang, J.-S.; Ho, S.-H.; Wang, L.-F.; Wei, Y.-H. Enhancing production of lutein by a mixotrophic cultivation system using microalga Scenedesmus obliquus CWL-1. Bioresour. Technol. 2019, 291, 121891. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, P.; Wu, Y.; Zhang, L. Effect of light quality on growth, ultrastructure, pigments, and membrane lipids of Pyropia haitanensis. J. Appl. Phycol. 2020, 32, 4189–4197. [Google Scholar] [CrossRef]
- Wu, H. Effect of different light qualities on growth, pigment content, chlorophyll fluorescence, and antioxidant enzyme activity in the red alga Pyropia haitanensis (Bangiales, Rhodophyta). BioMed Res. Int. 2016, 2016, 7383918. [Google Scholar] [CrossRef]
- Mohsenpour, S.F.; Richards, B.; Willoughby, N. Spectral conversion of light for enhanced microalgae growth rates and photosynthetic pigment production. Bioresour. Technol. 2012, 125, 75–81. [Google Scholar] [CrossRef]
- Domınguez-Bocanegra, A.; Legarreta, I.G.; Jeronimo, F.M.; Campocosio, A.T. Influence of environmental and nutritional factors in the production of astaxanthin from Haematococcus pluvialis. Bioresour. Technol. 2004, 92, 209–214. [Google Scholar] [CrossRef]
- Ip, P.-F.; Chen, F. Production of astaxanthin by the green microalga Chlorella zofingiensis in the dark. Process Biochem. 2005, 40, 733–738. [Google Scholar] [CrossRef]
- Kang, C.D.; An, J.Y.; Park, T.H.; Sim, S.J. Astaxanthin biosynthesis from simultaneous N and P uptake by the green alga Haematococcus pluvialis in primary-treated wastewater. Biochem. Eng. J. 2006, 31, 234–238. [Google Scholar] [CrossRef]
- Rodrigues, D.B.; Flores, É.M.; Barin, J.S.; Mercadante, A.Z.; Jacob-Lopes, E.; Zepka, L.Q. Production of carotenoids from microalgae cultivated using agroindustrial wastes. Food Res. Int. 2014, 65, 144–148. [Google Scholar] [CrossRef]
- Del Campo, J.A.; Rodrıguez, H.; Moreno, J.; Vargas, M.A.; Rivas, J.N.; Guerrero, M.G. Lutein production by Muriellopsis sp. in an outdoor tubular photobioreactor. J. Biotechnol. 2001, 85, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Chen, F.; Chen, G.; Zhang, X.; Liu, L.; Zhang, H. Enhanced production of lutein in heterotrophic Chlorella protothecoides by oxidative stress. Sci. China Ser. Life Sci. 2008, 51, 1088–1093. [Google Scholar] [CrossRef] [PubMed]
- Chaneva, G.; Furnadzhieva, S.; Minkova, K.; Lukavsky, J. Effect of light and temperature on the cyanobacterium Arthronema africanum-a prospective phycobiliprotein-producing strain. J. Appl. Phycol. 2007, 19, 537–544. [Google Scholar] [CrossRef]
- Lv, J.M.; Cheng, L.H.; Xu, X.H.; Zhang, L.; Chen, H.L. Enhanced lipid production of Chlorella vulgaris by adjustment of cultivation conditions. Bioresour. Technol. 2010, 101, 6797–6804. [Google Scholar] [CrossRef] [PubMed]
- Mojaat, M.; Pruvost, J.; Foucault, A.; Legrand, J. Effect of organic carbon sources and Fe2+ ions on growth and β-carotene accumulation by Dunaliella salina. Biochem. Eng. J. 2008, 39, 177–184. [Google Scholar] [CrossRef]
- Esakkimuthu, S.; Krishnamurthy, V.; Govindarajan, R.; Swaminathan, K. Augmentation and starvation of calcium, magnesium, phosphate on lipid production of Scenedesmus obliquus. Biomass Bioenergy 2016, 88, 126–134. [Google Scholar] [CrossRef]
- Cakmak, T.; Angun, P.; Demiray, Y.E.; Ozkan, A.D.; Elibol, Z.; Tekinay, T. Differential effects of nitrogen and sulfur deprivation on growth and biodiesel feedstock production of Chlamydomonas reinhardtii. Biotechnol. Bioeng. 2012, 109, 1947–1957. [Google Scholar] [CrossRef]
- Jerez, C.G.; Malapascua, J.R.; Sergejevová, M.; Figueroa, F.L.; Masojídek, J. Effect of nutrient starvation under high irradiance on lipid and starch accumulation in Chlorella fusca (Chlorophyta). Mar. Biotechnol. 2016, 18, 24–36. [Google Scholar] [CrossRef]
- Yamazaki, T.; Konosu, E.; Takeshita, T.; Hirata, A.; Ota, S.; Kazama, Y.; Abe, T.; Kawano, S. Independent regulation of the lipid and starch synthesis pathways by sulfate metabolites in the green microalga Parachlorella kessleri under sulfur starvation conditions. Algal Res. 2018, 36, 37–47. [Google Scholar] [CrossRef]
- Scarsini, M.; Marchand, J.; Schoefs, B. Carotenoid Overproduction in Microalgae: Biochemical and Genetic Engineering. In Pigments from Microalgae Handbook; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Boynton, J.E.; Gillham, N.W.; Harris, E.H.; Hosler, J.P.; Johnson, A.M.; Jones, A.R.; Randolphanderson, B.L.; Robertson, D.; Klein, T.M.; Shark, K.B.; et al. Chloroplast Transformation in Chlamydomonas with High-Velocity Microprojectiles. Science 1988, 240, 1534–1538. [Google Scholar] [CrossRef]
- Purton, S.; Szaub, J.B.; Wannathong, T.; Young, R.; Economou, C.K. Genetic engineering of algal chloroplasts: Progress and prospects. Russ. J. Plant Physiol. 2013, 60, 491–499. [Google Scholar] [CrossRef]
- Kilian, O.; Benemann, C.S.E.; Niyogi, K.K.; Vick, B. High-efficiency homologous recombination in the oil-producing alga Nannochloropsis sp. Proc. Natl. Acad. Sci. USA 2011, 108, 21265–21269. [Google Scholar] [CrossRef]
- Radakovits, R.; Eduafo, P.M.; Posewitz, M.C. Genetic engineering of fatty acid chain length in Phaeodactylum tricornutum. Metab. Eng. 2011, 13, 89–95. [Google Scholar] [CrossRef]
- Liu, J.; Chen, F. Biology and Industrial Applications of Chlorella: Advances and Prospects. Adv. Biochem. Eng. Biot. 2016, 153, 1–35. [Google Scholar]
- Yang, B.; Liu, J.; Liu, B.; Sun, P.P.; Ma, X.N.; Jiang, Y.; Wei, D.; Chen, F. Development of a stable genetic system for Chlorella vulgaris-A promising green alga for CO2 biomitigation. Algal Res. 2015, 12, 134–141. [Google Scholar] [CrossRef]
- Bock, R. Engineering Plastid Genomes: Methods, Tools, and Applications in Basic Research and Biotechnology. Annu. Rev. Plant Biol. 2015, 66, 211–241. [Google Scholar] [CrossRef]
- Couso, I.; Vila, M.; Rodriguez, H.; Vargas, M.A.; Leon, R. Overexpression of an Exogenous Phytoene Synthase Gene in the Unicellular Alga Chlamydomonas reinhardtii Leads to an Increase in the Content of Carotenoids. Biotechnol. Prog. 2011, 27, 54–60. [Google Scholar] [CrossRef]
- Kadono, T.; Kira, N.; Suzuki, K.; Iwata, O.; Ohama, T.; Okada, S.; Nishimura, T.; Akakabe, M.; Tsuda, M.; Adachi, M. Effect of an Introduced Phytoene Synthase Gene Expression on Carotenoid Biosynthesis in the Marine Diatom Phaeodactylum tricornutum. Mar. Drugs 2015, 13, 5334–5357. [Google Scholar] [CrossRef]
- Galarza, J.I.; Gimpel, J.A.; Rojas, V.; Arredondo-Vega, B.O.; Henriquez, V. Over-accumulation of astaxanthin in Haematococcus pluvialis through chloroplast genetic engineering. Algal Res.-Biomass Biofuels Bioprod. 2018, 31, 291–297. [Google Scholar] [CrossRef]
- Lin, B.; Cui, Y.L.; Yan, M.Y.; Wang, Y.C.; Gao, Z.Q.; Meng, C.X.; Qin, S. Construction of astaxanthin metabolic pathway in the green microalga Dunaliella viridis. Algal Res.-Biomass Biofuels Bioprod. 2019, 44, 101697. [Google Scholar] [CrossRef]
- Tran, Q.G.; Cho, K.; Kim, U.; Yun, J.H.; Cho, D.H.; Heo, J.; Park, S.B.; Kim, J.W.; Lee, Y.J.; Ramanan, R.; et al. Enhancement of beta-carotene production by regulating the autophagy-carotenoid biosynthesis seesaw in Chlamydomonas reinhardtii. Bioresour. Technol. 2019, 292, 121937. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Wu, T.; Chen, S.H.Y.; Ren, Y.; Yang, S.; Huang, J.; Mou, H.; Chen, F. Powerful tools for productivity improvements in microalgal production. Renew. Sustain. Energy Rev. 2021, 152, 111609. [Google Scholar] [CrossRef]
- Sun, H.; Ren, Y.; Fan, Y.; Lu, X.; Zhao, W.; Chen, F. Systematic metabolic tools reveal underlying mechanism of product biosynthesis in Chromochloris zofingiensis. Bioresour. Technol. 2021, 337, 125406. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Sun, H.; Zhao, W.; Cheng, K.-W.; Chen, F.; Liu, B. A Hetero-Photoautotrophic Two-Stage Cultivation Process for Production of Fucoxanthin by the Marine Diatom Nitzschia laevis. Mar. Drugs 2018, 16, 219. [Google Scholar] [CrossRef]
- Li, Y.; Sun, H.; Wang, Y.; Yang, S.; Wang, J.; Wu, T.; Lu, X.; Chu, Y.; Chen, F. Integrated metabolic tools reveal carbon alternative in Isochrysis zhangjiangensis for fucoxanthin improvement. Bioresour. Technol. 2021, 347, 126401. [Google Scholar] [CrossRef]
- Sun, H.; Kong, Q.; Geng, Z.; Duan, L.; Yang, M.; Guan, B. Enhancement of cell biomass and cell activity of astaxanthin-rich Haematococcus pluvialis. Bioresour. Technol. 2015, 186, 67–73. [Google Scholar] [CrossRef]
- Sun, H.; Ren, Y.; Lao, Y.; Li, X.; Chen, F. A novel fed-batch strategy enhances lipid and astaxanthin productivity without compromising biomass of Chromochloris zofingiensis. Bioresour. Technol. 2020, 308, 123306. [Google Scholar] [CrossRef]
- Zou, T.-B.; Jia, Q.; Li, H.-W.; Wang, C.-X.; Wu, H.-F. Response surface methodology for ultrasound-assisted extraction of astaxanthin from Haematococcus pluvialis. Mar. Drugs 2013, 11, 1644–1655. [Google Scholar] [CrossRef]
- Silveira, S.T.; Burkert, J.d.M.; Costa, J.A.V.; Burkert, C.A.V.; Kalil, S.J. Optimization of phycocyanin extraction from Spirulina platensis using factorial design. Bioresour. Technol. 2007, 98, 1629–1634. [Google Scholar] [CrossRef]
- Tavanandi, H.A.; Vanjari, P.; Raghavarao, K. Synergistic method for extraction of high purity Allophycocyanin from dry biomass of Arthrospira platensis and utilization of spent biomass for recovery of carotenoids. Sep. Purif. Technol. 2019, 225, 97–111. [Google Scholar] [CrossRef]
- Spiden, E.M.; Yap, B.H.; Hill, D.R.; Kentish, S.E.; Scales, P.J.; Martin, G.J. Quantitative evaluation of the ease of rupture of industrially promising microalgae by high pressure homogenization. Bioresour. Technol. 2013, 140, 165–171. [Google Scholar] [CrossRef]
- Bernaerts, T.M.; Verstreken, H.; Dejonghe, C.; Gheysen, L.; Foubert, I.; Grauwet, T.; Van Loey, A.M. Cell disruption of Nannochloropsis sp. improves in vitro bioaccessibility of carotenoids and ω3-LC-PUFA. J. Funct. Foods 2020, 65, 103770. [Google Scholar] [CrossRef]
- Cha, K.H.; Lee, H.J.; Koo, S.Y.; Song, D.-G.; Lee, D.-U.; Pan, C.-H. Optimization of pressurized liquid extraction of carotenoids and chlorophylls from Chlorella vulgaris. J. Agric. Food Chem. 2010, 58, 793–797. [Google Scholar] [CrossRef]
- Herrero, M.; Jaime, L.; Martín-Álvarez, P.J.; Cifuentes, A.; Ibáñez, E. Optimization of the extraction of antioxidants from Dunaliella salina microalga by pressurized liquids. J. Agric. Food Chem. 2006, 54, 5597–5603. [Google Scholar] [CrossRef]
- Rodríguez-Meizoso, I.; Jaime, L.; Santoyo, S.; Cifuentes, A.; Garcia-Blairsy Reina, G.; Senorans, F.; Ibáñez, E. Pressurized fluid extraction of bioactive compounds from Phormidium species. J. Agric. Food Chem. 2008, 56, 3517–3523. [Google Scholar] [CrossRef]
- Amaro, H.M.; Guedes, A.C.; Preto, M.A.; Sousa-Pinto, I.; Malcata, F.X. Gloeothece sp. as a Nutraceutical Source—An improved method of extraction of carotenoids and fatty acids. Mar. Drugs 2018, 16, 327. [Google Scholar] [CrossRef]
- Juin, C.; Chérouvrier, J.-R.; Thiéry, V.; Gagez, A.-L.; Bérard, J.-B.; Joguet, N.; Kaas, R.; Cadoret, J.-P.; Picot, L. Microwave-assisted extraction of phycobiliproteins from Porphyridium purpureum. Appl. Biochem. Biotechnol. 2015, 175, 1–15. [Google Scholar] [CrossRef]
- Pasquet, V.; Chérouvrier, J.-R.; Farhat, F.; Thiéry, V.; Piot, J.-M.; Bérard, J.-B.; Kaas, R.; Serive, B.; Patrice, T.; Cadoret, J.-P. Study on the microalgal pigments extraction process: Performance of microwave assisted extraction. Process Biochem. 2011, 46, 59–67. [Google Scholar] [CrossRef]
- Dey, S.; Rathod, V.K. Ultrasound assisted extraction of β-carotene from Spirulina platensis. Ultrason. Sonochem. 2013, 20, 271–276. [Google Scholar] [CrossRef]
- Fernandes, A.S.; Nogara, G.P.; Menezes, C.R.; Cichoski, A.J.; Mercadante, A.Z.; Jacob-Lopes, E.; Zepka, L.Q. Identification of chlorophyll molecules with peroxyl radical scavenger capacity in microalgae Phormidium autumnale using ultrasound-assisted extraction. Food Res. Int. 2017, 99, 1036–1041. [Google Scholar] [CrossRef]
- Martínez, J.M.; Luengo, E.; Saldaña, G.; Álvarez, I.; Raso, J. C-phycocyanin extraction assisted by pulsed electric field from Artrosphira platensis. Food Res. Int. 2017, 99, 1042–1047. [Google Scholar] [CrossRef] [PubMed]
- Chittapun, S.; Jonjaroen, V.; Khumrangsee, K.; Charoenrat, T. C-phycocyanin extraction from two freshwater cyanobacteria by freeze thaw and pulsed electric field techniques to improve extraction efficiency and purity. Algal Res. 2020, 46, 101789. [Google Scholar] [CrossRef]
- Martínez, J.M.; Delso, C.; Álvarez, I.; Raso, J. Pulsed electric field permeabilization and extraction of phycoerythrin from Porphyridium cruentum. Algal Res. 2019, 37, 51–56. [Google Scholar] [CrossRef]
- Parniakov, O.; Barba, F.J.; Grimi, N.; Marchal, L.; Jubeau, S.; Lebovka, N.; Vorobiev, E. Pulsed electric field assisted extraction of nutritionally valuable compounds from microalgae Nannochloropsis spp. using the binary mixture of organic solvents and water. Innov. Food Sci. Emerg. Technol. 2015, 27, 79–85. [Google Scholar] [CrossRef]
- Martínez, J.M.; Gojkovic, Z.; Ferro, L.; Maza, M.; Álvarez, I.; Raso, J.; Funk, C. Use of pulsed electric field permeabilization to extract astaxanthin from the Nordic microalga Haematococcus pluvialis. Bioresour. Technol. 2019, 289, 121694. [Google Scholar] [CrossRef]
- Jaime, L.; Mendiola, J.A.; Ibáñez, E.; Martin-Álvarez, P.J.; Cifuentes, A.; Reglero, G.; Señoráns, F.J. β-Carotene isomer composition of sub-and supercritical carbon dioxide extracts. Antioxidant activity measurement. J. Agric. Food Chem. 2007, 55, 10585–10590. [Google Scholar] [CrossRef]
- Hosseini, S.R.P.; Tavakoli, O.; Sarrafzadeh, M.H. Experimental optimization of SC-CO2 extraction of carotenoids from Dunaliella salina. J. Supercrit. Fluids 2017, 121, 89–95. [Google Scholar] [CrossRef]
- Liau, B.-C.; Shen, C.-T.; Liang, F.-P.; Hong, S.-E.; Hsu, S.-L.; Jong, T.-T.; Chang, C.-M.J. Supercritical fluids extraction and anti-solvent purification of carotenoids from microalgae and associated bioactivity. J. Supercrit. Fluids 2010, 55, 169–175. [Google Scholar] [CrossRef]
- Macıas-Sánchez, M.; Mantell, C.; Rodrıguez, M.; de La Ossa, E.M.; Lubián, L.; Montero, O. Supercritical fluid extraction of carotenoids and chlorophyll a from Nannochloropsis gaditana. J. Food Eng. 2005, 66, 245–251. [Google Scholar] [CrossRef]
- Guedes, A.C.; Gião, M.S.; Matias, A.A.; Nunes, A.V.; Pintado, M.E.; Duarte, C.M.; Malcata, F.X. Supercritical fluid extraction of carotenoids and chlorophylls a, b and c, from a wild strain of Scenedesmus obliquus for use in food processing. J. Food Eng. 2013, 116, 478–482. [Google Scholar] [CrossRef]
- Sanzo, G.D.; Mehariya, S.; Martino, M.; Larocca, V.; Casella, P.; Chianese, S.; Musmarra, D.; Balducchi, R.; Molino, A. Supercritical carbon dioxide extraction of astaxanthin, lutein, and fatty acids from Haematococcus pluvialis microalgae. Mar. Drugs 2018, 16, 334. [Google Scholar] [CrossRef]
- Nobre, B.; Marcelo, F.; Passos, R.; Beirão, L.; Palavra, A.; Gouveia, L.; Mendes, R. Supercritical carbon dioxide extraction of astaxanthin and other carotenoids from the microalga Haematococcus pluvialis. Eur. Food Res. Technol. 2006, 223, 787–790. [Google Scholar] [CrossRef]
- Montero, O.; Macías-Sánchez, M.D.; Lama, C.M.; Lubián, L.M.; Mantell, C.; Rodríguez, M.; de la Ossa, E.M. Supercritical CO2 extraction of β-carotene from a marine strain of the cyanobacterium Synechococcus species. J. Agric. Food Chem. 2005, 53, 9701–9707. [Google Scholar] [CrossRef]
- Macías-Sánchez, M.; Mantell, C.; Rodríguez, M.; de la Ossa, E.M.; Lubián, L.; Montero, O. Supercritical fluid extraction of carotenoids and chlorophyll a from Synechococcus sp. J. Supercrit. Fluids 2007, 39, 323–329. [Google Scholar] [CrossRef]
- McMillan, J.R.; Watson, I.A.; Ali, M.; Jaafar, W. Evaluation and comparison of algal cell disruption methods: Microwave, waterbath, blender, ultrasonic and laser treatment. Appl. Energy 2013, 103, 128–134. [Google Scholar] [CrossRef]
- Zhang, R.; Marchal, L.; Lebovka, N.; Vorobiev, E.; Grimi, N. Two-step procedure for selective recovery of bio-molecules from microalga Nannochloropsis oculata assisted by high voltage electrical discharges. Bioresour. Technol. 2020, 302, 122893. [Google Scholar] [CrossRef]
- Salinas-Salazar, C.; Saul Garcia-Perez, J.; Chandra, R.; Castillo-Zacarias, C.; Iqbal, H.; Parra-Saldívar, R. Methods for extraction of valuable products from microalgae biomass. In Microalgae Biotechnology for Development of Biofuel and Wastewater Treatment; Springer: Berlin/Heidelberg, Germany, 2019; pp. 245–263. [Google Scholar]
- Gong, M.; Li, X.; Bassi, A. Investigation of simultaneous lutein and lipid extraction from wet microalgae using Nile Red as solvatochromic shift probe. J. Appl. Phycol. 2018, 30, 1617–1627. [Google Scholar] [CrossRef]
- Aguilar-Machado, D.; Morales-Oyervides, L.; Contreras-Esquivel, J.C.; Aguilar, C.; Méndez-Zavala, A.; Raso, J.; Montañez, J. Effect of ohmic heating processing conditions on color stability of fungal pigments. Food Sci. Technol. Int. 2017, 23, 338–348. [Google Scholar] [CrossRef]
- Araya, B.; Gouveia, L.; Nobre, B.; Reis, A.; Chamy, R.; Poirrier, P. Evaluation of the simultaneous production of lutein and lipids using a vertical alveolar panel bioreactor for three Chlorella species. Algal Res. 2014, 6, 218–222. [Google Scholar] [CrossRef]
- Tavanandi, H.A.; Mittal, R.; Chandrasekhar, J.; Raghavarao, K. Simple and efficient method for extraction of C-Phycocyanin from dry biomass of Arthospira platensis. Algal Res. 2018, 31, 239–251. [Google Scholar] [CrossRef]
- Lee, S.Y.; Cho, J.M.; Chang, Y.K.; Oh, Y.-K. Cell disruption and lipid extraction for microalgal biorefineries: A review. Bioresour. Technol. 2017, 244, 1317–1328. [Google Scholar] [CrossRef] [PubMed]
- Low, K.L.; Idris, A.; Yusof, N.M. Novel protocol optimized for microalgae lutein used as food additives. Food Chem. 2020, 307, 125631. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.J.; Kumar, G.V.; Dash, A.; Scholz, P.; Banerjee, R. Sustainable green solvents and techniques for lipid extraction from microalgae: A review. Algal Res. 2017, 21, 138–147. [Google Scholar] [CrossRef]
- Ahirwar, A.; Khan, M.J.; Sirotiya, V.; Mourya, M.; Rai, A.; Schoefs, B.; Marchand, J.; Varjani, S.; Vinayak, V. Pulsed Electric Field-Assisted Cell Permeabilization of Microalgae (Haematococcus pluvialis) for Milking of Value-Added Compounds. Bioenergy Res. 2022, 1–14. [Google Scholar] [CrossRef]
- Khoo, K.S.; Chew, K.W.; Ooi, C.W.; Ong, H.C.; Ling, T.C.; Show, P.L. Extraction of natural astaxanthin from Haematococcus pluvialis using liquid biphasic flotation system. Bioresour. Technol. 2019, 290, 121794. [Google Scholar] [CrossRef]
- Viera, I.; Pérez-Gálvez, A.; Roca, M. Green natural colorants. Molecules 2019, 24, 154. [Google Scholar] [CrossRef]
- Ortiz, D.; Ponrajan, A.; Bonnet, J.P.; Rocheford, T.; Ferruzzi, M.G. Carotenoid stability during dry milling, storage, and extrusion processing of biofortified maize genotypes. J. Agric. Food Chem. 2018, 66, 4683–4691. [Google Scholar] [CrossRef]
- Bell, T.; Alamzad, R.; Graf, B. Effect of pH on the chemical stability of carotenoids in juice. Proc. Nutr. Soc. 2016, 75, E94. [Google Scholar] [CrossRef]
- Chaiklahan, R.; Chirasuwan, N.; Bunnag, B. Stability of phycocyanin extracted from Spirulina sp.: Influence of temperature, pH and preservatives. Process Biochem. 2012, 47, 659–664. [Google Scholar] [CrossRef]
- Gonzalez-Ramirez, E.; Andujar-Sanchez, M.; Ortiz-Salmeron, E.; Bacarizo, J.; Cuadri, C.; Mazzuca-Sobczuk, T.; Ibáñez, M.J.; Camara-Artigas, A.; Martinez-Rodriguez, S. Thermal and pH stability of the B-phycoerythrin from the red algae Porphyridium cruentum. Food Biophys. 2014, 9, 184–192. [Google Scholar] [CrossRef]
- Wu, H.-L.; Wang, G.-H.; Xiang, W.-Z.; Li, T.; He, H. Stability and antioxidant activity of food-grade phycocyanin isolated from Spirulina platensis. Int. J. Food Prop. 2016, 19, 2349–2362. [Google Scholar] [CrossRef]
- Braga, A.R.C.; Figueira, F.d.S.; Silveira, J.T.d.; Morais, M.G.d.; Costa, J.A.V.; Kalil, S.J. Improvement of thermal stability of c-phycocyanin by nanofiber and preservative agents. J. Food Process. Preserv. 2016, 40, 1264–1269. [Google Scholar] [CrossRef]
- Kannaujiya, V.K.; Sinha, R.P. Thermokinetic stability of phycocyanin and phycoerythrin in food-grade preservatives. J. Appl. Phycol. 2016, 28, 1063–1070. [Google Scholar] [CrossRef]
- Selig, M.J.; Malchione, N.M.; Gamaleldin, S.; Padilla-Zakour, O.I.; Abbaspourrad, A. Protection of blue color in a spirulina derived phycocyanin extract from proteolytic and thermal degradation via complexation with beet-pectin. Food Hydrocoll. 2018, 74, 46–52. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Goswami, S. Microalgae—A green multi-product biorefinery for future industrial prospects. Biocatal. Agric. Biotechnol. 2020, 25, 101580. [Google Scholar] [CrossRef]
- Borowitzka, M.A. Dunaliella: Biology, production, and markets. In Handbook of Microalgal Culture: Applied Phycology and Biotechnology, 2nd ed.; Wiley: Hoboken, NJ, USA, 2013; pp. 359–368. [Google Scholar]
- Paniagua-Michel, J. Microalgal nutraceuticals. In Handbook of Marine Microalgae; Elsevier: Amsterdam, The Netherlands, 2015; pp. 255–267. [Google Scholar]
- Grama, B.S.; Chader, S.; Khelifi, D.; Agathos, S.N.; Jeffryes, C. Induction of canthaxanthin production in a Dactylococcus microalga isolated from the Algerian Sahara. Bioresour. Technol. 2014, 151, 297–305. [Google Scholar] [CrossRef]
- Mulders, K.J.; Lamers, P.P.; Martens, D.E.; Wijffels, R.H. Phototrophic pigment production with microalgae: Biological constraints and opportunities. J. Phycol. 2014, 50, 229–242. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).