Abstract
Rheumatoid arthritis (RA) is an invalidating chronic autoimmune disorder characterized by joint inflammation and progressive bone damage. Dietary intervention is an important component in the treatment of RA to mitigate oxidative stress, a major pathogenic driver of the disease. Alongside traditional sources of antioxidants, microalgae—a diverse group of photosynthetic prokaryotes and eukaryotes—are emerging as anti-inflammatory and immunomodulatory food supplements. Several species accumulate therapeutic metabolites—mainly lipids and pigments—which interfere in the pro-inflammatory pathways involved in RA and other chronic inflammatory conditions. The advancement of the clinical uses of microalgae requires the continuous exploration of phytoplankton biodiversity and chemodiversity, followed by the domestication of wild strains into reliable producers of said metabolites. In addition, the tractability of microalgal genomes offers unprecedented possibilities to establish photosynthetic microbes as light-driven biofactories of heterologous immunotherapeutics. Here, we review the evidence-based anti-inflammatory mechanisms of microalgal metabolites and provide a detailed coverage of the genetic engineering strategies to enhance the yields of endogenous compounds and to develop innovative bioproducts.
Keywords:
photosynthesis; polyunsaturated fatty acids; carotenoids; oxylipins; xanthophylls; antioxidants; functional foods; synthetic biology; genetic engineering; inflammation; rheumatoid arthritis; autoimmunity; docosahexaenoic acid (DHA); eicosapenteanoic acid (EPA); astaxanthin; rheumatology; interleukins; chloroplast; molecular pharming; novel foods; bioeconomy 1. Introduction
Chronic inflammation is a defining feature of autoimmune diseases, a group of conditions in which immunological self-tolerance is disturbed due to the recognition of autoantigens by immune cells. Rheumatoid arthritis (RA), the most common chronic inflammatory arthropathy [1,2], is a systemic autoimmune disorder affecting the synovial joints, with a higher incidence in women [3]. RA displays a complex pathophysiology involving the upregulation of pro-inflammatory mediators (interleukins, ILs) and enhanced production of reactive oxygen species (ROS) [4,5]. Both genetic and modifiable lifestyle factors contribute to the risk of RA predisposition [6,7], with diet highly influencing disease activity [8,9]. In particular, a high antioxidant intake is known to reduce onset risk and to ameliorate the clinical course of the disease [10], therefore, the identification of new sources of antioxidant and anti-inflammatory molecules is of high clinical relevance.
Microalgae are photosynthetic prokaryotes and eukaryotes adapted to diverse environments, including extreme habitats [11,12], which are consumed in human nutrition as sources of proteins and other bioactive compounds [13,14,15,16,17]. Several species are non-toxic producers of essential vitamins, lipids, and pigments of therapeutic value [18,19,20,21,22], which could be employed as complementary agents in the management of chronic inflammatory diseases. Moreover, the fast life cycle and light-powered autotrophic metabolism of microalgae allows for large-scale cultivation with lower inputs compared with heterotrophic microorganisms [23].
In this review, we summarize the evidence-based putative interference of microalgal compounds in pro-inflammatory pathways involved in the pathogenesis of RA and discuss how bioprospecting for novel pharmacologically relevant strains, and their domestication, can advance the clinical use of photosynthetic microorganisms. Lastly, we provide an update on the available genetic engineering strategies to enhance the production of endogenous microalgal metabolites and introduce emerging approaches to achieve light-driven conversion of CO2 into high-value recombinant biopharmaceuticals.
Pathogenesis and Mediators of Rheumatoid Arthritis
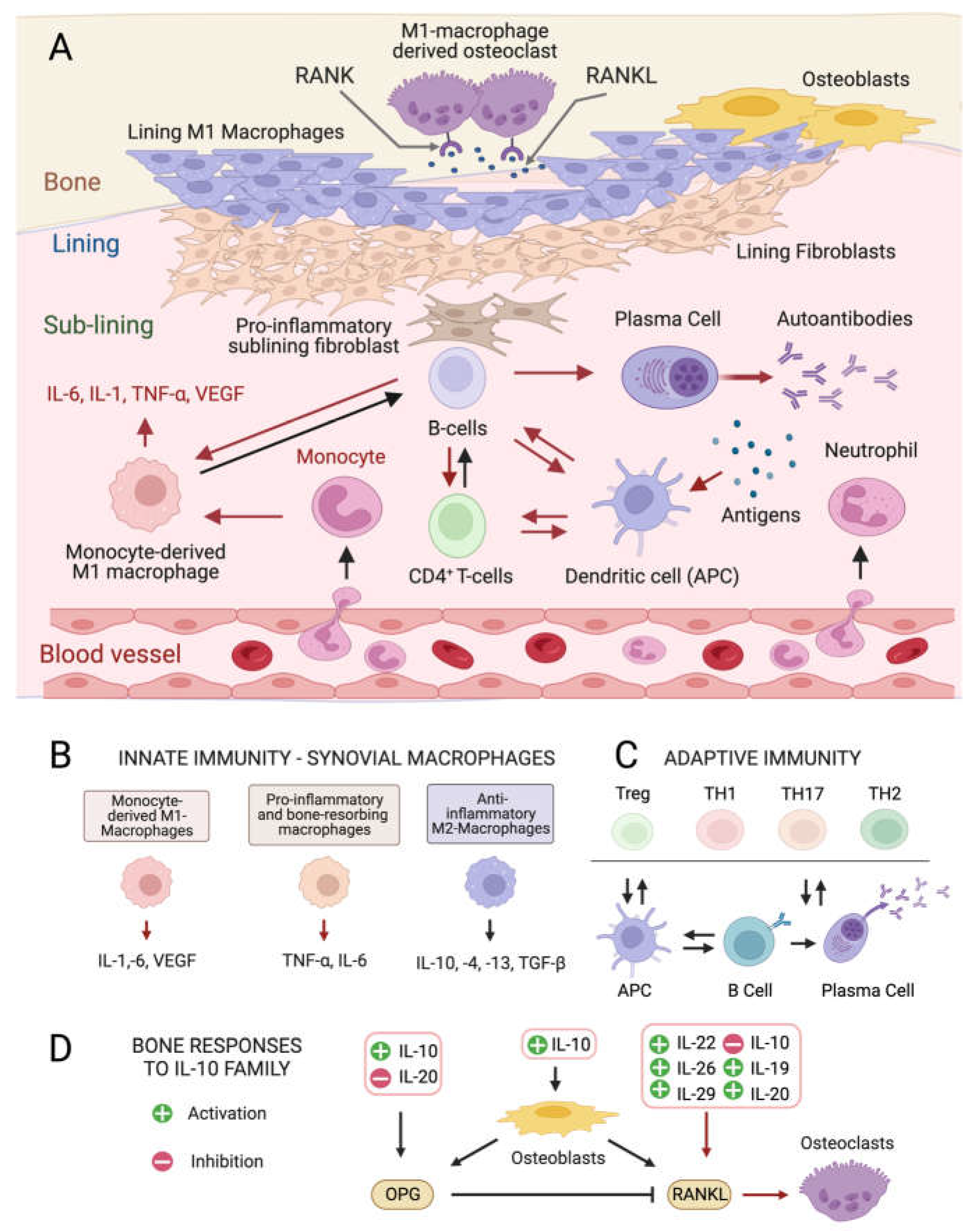
Although the exact etiology of RA remains unknown, the balance between immune cells and the production of inflammatory ILs in the connective tissue that lines the joint capsule (synovium) is altered in the disease onset and progression [4,5]. The healthy synovium consists of a thin lining layer of fibroblasts covering a connective tissue surrounded by blood vessels and enriched in fibroblasts, and innate and adaptive immune cells: the sub-lining layer [24] (Figure 1A). In RA, the lining layer is hyperplastic while the sub-lining layer is infiltrated with B-cells, monocyte-derived macrophages, autoantibody-secreting plasma cells, and differentiated cytotoxic CD4+ T-cells involved in the breakdown of tissue tolerance [25,26,27]. The release of pro-inflammatory ILs by monocyte-derived M1 macrophages, and osteoclast activation cause progressive bone resorption [28], and autoantibodies produced by differentiated plasma cells further contribute to joint damage [29].
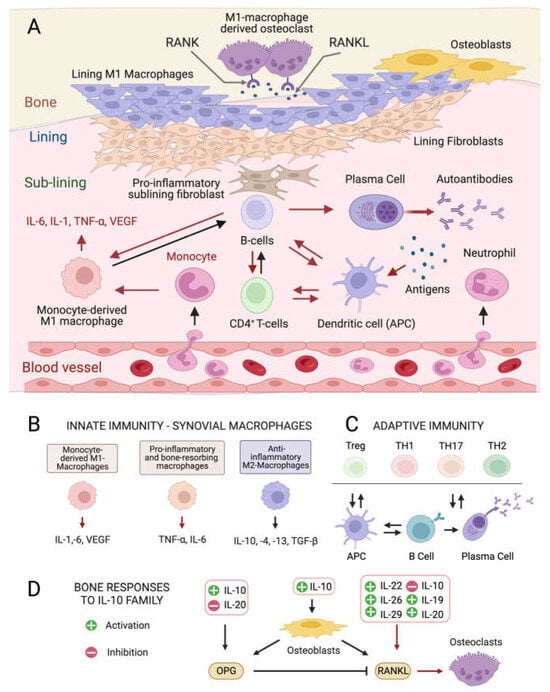
Figure 1.
Cellular composition of the synovial membrane, its interaction with bone tissue and immune cell types, and related mediators involved in inflamed RA joints. (A) In healthy conditions, the synovium consist of a thin lining layer of lining fibroblasts in association with lining M1 directly exposed to the bone tissue. The underlying sub-lining layer is a connective tissue enriched in blood vessels, adipocytes, fibroblasts, and both innate and adaptive immune cells. The inflamed RA synovium is characterized by a hyperplastic lining layer surrounded by proinflammatory sub-lining fibroblasts and a massive infiltration of B-cells, monocyte-derived macrophages, autoantibody-secreting plasma cells, and differentiated cytotoxic effector memory CD4+ T-cells in the sub-lining layer. The secretion of pro-inflammatory interleukins (IL-1: interleukin 1; IL-6: interleukin 6; TNF- α: tumor necrosis factor-alpha) by activated immune cells stimulates the production of the soluble cytokine Mediator Receptor Activator of Nuclear Factor Kappa-Β Ligand (RANKL), which binds to its receptor RANK on monocytes and macrophages causing their differentiation into bone-resorbing osteoclasts. Red arrows indicate pro-inflammatory processes. (B) Interleukin (IL) isoforms produced by different types of synovial innate immune cells and macrophages (IL-6: interleukin 6; IL-1: interleukin 1; TNF-α: tumor necrosis factor-alpha; TNF-β: tumor necrosis factor-beta; VEGF: vascular endothelial growth factor). (C) Differentiation and interconversion of adaptive immune cells (Treg: regulatory T cells; TH1: T helper 1 cells; TH 17: T helper 17 cells; TH2: T helper 2 cells; B cells; APCs: antigen-presenting cells). (D) Effects of secreted ILs on bone-remodeling processes depending on the osteoprotegerin (OPG)–RANKL axis, which regulates the differentiation of osteoclasts in bone-resorbing osteoclasts. Figures created with BioRender.com, accessed on 15 November 2023.
At the intracellular level, three main pro-inflammatory signaling kinase cascades responding to soluble mediators are involved in RA, all being influenced by microalgal metabolites: the Nuclear Factor Kappa-Β (NF-kB)-mediated pathway [30], the Janus kinase 2/Signal Transducers and Activators of Transcription 3 (JAK2/STAT3) pathway [31], and the Jun N-terminal kinase (JNK)/p38 Mitogen-Activated Protein Kinase (p38 MAPK) pathway [32], the latter being predominant in the lining layer and endothelial cells.
Pro-inflammatory ILs (Figure 1D) [33] stimulate the production of the soluble cytokine Mediator Receptor Activator of Nuclear Factor Kappa-Β Ligand (RANKL) by immune cells. RANKL binds its receptor RANK on monocytes and macrophages causing their differentiation into bone-resorbing osteoclasts [34]. Other ILs produced by anti-inflammatory M2 macrophages positively influence the osteoprotegerin (OPG)–RANKL ratio, promoting bone homeostasis [35] (Figure 1D), while the IL-10 family (Figure 1D) appears to play dual roles. Some members stimulate osteogenesis and suppress the synthesis of pro-inflammatory mediators like IL-6, Tumor Necrosis Factor-α (TNF-α), and Vascular endothelial growth factor (VEGF) [36], while others activate the NF-kB and MAPK pathways, stimulating TNF-α, IL-1β, and RANKL production by synovial fibroblasts, promoting osteoclastogenesis.
In conclusion, given the diversified nature of factors regulating the balance between pro- and anti-inflammatory mediators in RA, there is a strong interest in discovering novel molecules to be used as complementary tools alongside conventional therapies.
2. Anti-Inflammatory and Immunomodulatory Metabolites from Microalgae
2.1. Carotenes and Xanthophylls
Like bacteria, fungi, and plants, microalgae synthetize C40 lipophilic pigments consisting of a polyene chain of conjugated double bonds (Figure 2) with terminally linked ionone rings known as carotenoids [37,38]. β-carotene (Figure 2B)—a structural element of photosystems [39]—and the antioxidant lycopene (Figure 2A) are anti-inflammatory carotenes [40,41] usually introduced in the diet with carrots (Daucus carota) and tomatoes (Solanum lycopersicum), respectively, although present also in microalgae [42].
Xanthophylls are oxygenated carotenoids containing hydroxyl and ketone groups in the ionone rings, which serve different functions in phototrophs. The non-ketolated xanthophyll lutein (Figure 2C) participates in light-harvesting and photoprotection, while the ketocarotenoid astaxanthin (ASTX, Figure 2D) scavenges harmful ROS generated by photosynthetic electron transport under excess light [43]. Lutein is an anti-inflammatory carotenoid [44] abundantly found in green leafy vegetables and egg yolk, while ASTX is a potent antioxidant uniquely synthetized by a few microalgal species.
Abiotic stresses induce a hypercarotenogenic response in several chlorophytes, including the halophile Dunaliella salina (Chlorophyceae), which overaccumulates β-carotene in lipid bodies (plastoglobules) inside the chloroplast [45], and in the freshwater species Haematococcus pluvialis (Haematococcaceae), which forms haematocysts filled with ASTX-rich cytoplasmic lipid droplets [46]. Other microalgal xanthophylls with anti-inflammatory and immunomodulatory properties are fucoxanthin and diatoxanthin produced by several diatoms (stramenopiles) and by the haptophyte Tisochrysis lutea (Coccolithophyceae) (Figure 2E,F) [47,48]. As discussed in the following paragraphs, carotenoids appear to interfere in all major pro-inflammatory pathways implicated in the onset and progression of RA.
Astaxanthin: The Red Gold of Algae
With recognized safety for human consumption [49], approved Novel Food status [50], and an established role in promoting bone homeostasis in degenerative skeletal diseases [51], ASTX is the microalgal pigment of highest biopharmaceutical value.
Clinical studies have shown that ASTX intake reduces the levels of systemic inflammatory biomarkers [52,53] and potentiates the pain-relieving effect of anti-inflammatory therapies [54]. The pharmacological effects of ASTX derive from its strong antioxidant-activity mediated via ROS quenching [55] (Figure 2G, top panel) and direct free radical scavenging [56,57] (Figure 2G, bottom panel). This amphipathic molecule is symmetrically arranged within the lipid bilayer [58], thus exerting antioxidant activity on both intra- and extracellular environments. Notably, the higher number of hydroxyl groups compared with other carotenoids confers to ASTX superior ROS-detoxifying capacity [59]. Early studies showed that ASTX suppressed ROS production [60,61,62,63] and secretion of pro-inflammatory ILs by cultured human-activated monocytes [64]. Moreover ASTX stimulated the expression of ROS-scavenging enzymes in chondrocytes challenged with IL-1β [65], and inhibited pro-inflammatory and osteoclastogenic gene expression in macrophages challenged with RANKL [66]. Lastly, the administration of ASTX promoted cartilage health in animal models of arthritis and osteoasthritis [67,68,69].
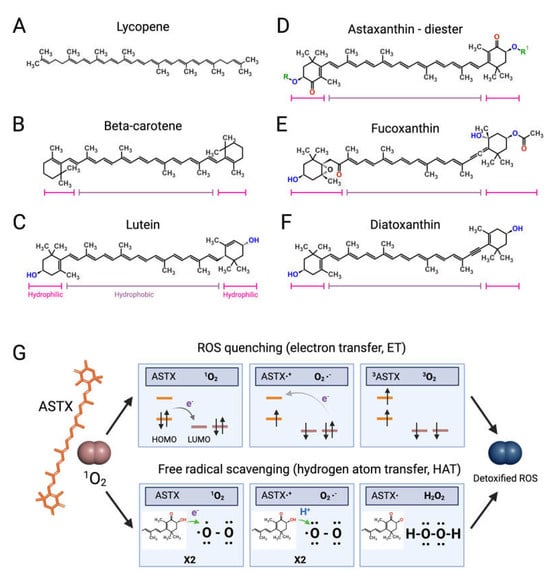
Figure 2.
Structures of microalgal carotenoids and ROS detoxification mechanisms of astaxanthin. Lycopene (A), beta-carotene (B), lutein (C), astaxanthin (ASTX, (D)), fucoxanthin (E), and diatoxanthin (F). Pink and purple bars indicate the hydrophobic and hydrophilic regions of the molecules, respectively; in red, the oxygen of the keto groups, and in blue, the oxygen of the carboxylic groups; in green, the R and R’ functional groups of astaxanthin. Panel (G) outlines the two main routes of ASTX-mediated singlet molecule oxygen (1O2) detoxification. The top pathway is based on an electron transfer process involving: (i) the formation of a weakly bound ASTX-1O2 complex followed by direct electron transfer from the highest occupied molecular orbital (HOMO) of ASTX to the lowest unoccupied molecular orbital (LUMO) of singlet oxygen (1O2), and the formation of radicals; (ii) a reverse reaction restoring the electron distribution between the two molecules. The overall process converts 1O2 to its triplet unreactive form (3O2) upon spin inversion, while ASTX is restored from 3ASTX via internal conversion [55]. The bottom pathway shows the free radical scavenging activity based on a two-step transfer involving both an electron and proton (H+) from ASTX to 1O2. The formed hydrogen peroxide is readily removed by peroxidase enzymes while ASTX is spontaneously restored by ascorbate. These mechanisms of action are iterative, meaning that a single ASTX molecule can perform multiple ROS detoxification cycles. Figures created with BioRender.com, accessed on 15 November 2023.
Figure 2.
Structures of microalgal carotenoids and ROS detoxification mechanisms of astaxanthin. Lycopene (A), beta-carotene (B), lutein (C), astaxanthin (ASTX, (D)), fucoxanthin (E), and diatoxanthin (F). Pink and purple bars indicate the hydrophobic and hydrophilic regions of the molecules, respectively; in red, the oxygen of the keto groups, and in blue, the oxygen of the carboxylic groups; in green, the R and R’ functional groups of astaxanthin. Panel (G) outlines the two main routes of ASTX-mediated singlet molecule oxygen (1O2) detoxification. The top pathway is based on an electron transfer process involving: (i) the formation of a weakly bound ASTX-1O2 complex followed by direct electron transfer from the highest occupied molecular orbital (HOMO) of ASTX to the lowest unoccupied molecular orbital (LUMO) of singlet oxygen (1O2), and the formation of radicals; (ii) a reverse reaction restoring the electron distribution between the two molecules. The overall process converts 1O2 to its triplet unreactive form (3O2) upon spin inversion, while ASTX is restored from 3ASTX via internal conversion [55]. The bottom pathway shows the free radical scavenging activity based on a two-step transfer involving both an electron and proton (H+) from ASTX to 1O2. The formed hydrogen peroxide is readily removed by peroxidase enzymes while ASTX is spontaneously restored by ascorbate. These mechanisms of action are iterative, meaning that a single ASTX molecule can perform multiple ROS detoxification cycles. Figures created with BioRender.com, accessed on 15 November 2023.
Notably, the esterified biological form of ASTX displays higher bioavailability compared with synthetic ester-free derivatives [70,71,72,73], suggesting the need to improve its production from natural sources. However, a major limitation to the clinical use of ASTX lies in its low solubility in gastrointestinal fluids [74], requiring encapsulation in polysaccharide, lipid, and protein nanoparticles to enhance its delivery and release [75,76,77,78,79,80].
2.2. Anti-Inflammatory Mechanisms of Action of Astaxanthin and Other Carotenoids
2.2.1. NF-κB Pathway
ASTX and β-carotene interfere with the NF-κB pathway blocking the translocation of the NF-κB transcription factor to the nucleus, thereby suppressing ROS and pro-inflammatory gene expression. This effect is likely mediated through targeting the Inhibitor of the NF-κB γ subunit (IKK-γ) of the IkB kinase complex [81,82,83,84]. This prevents the phosphorylation and subsequent proteasome-mediated degradation of the IkBα binding factor, which abolishes the release of NF-κB [30]. A similar inhibitory effect has been proposed for fucoxanthin and diatoxanthin [85,86].
The Mitogen- and Stress-activated protein Kinase-1 (MSK1) is a nucleus-localized factor, which activates the NF-κB pathway [87] and the transcriptional regulator cAMP-responsive Element-Binding Protein (CREB) [88]. Phosphorylated CREB binds CREB-Responsive Elements (CRE) promoting pro-inflammatory gene expression [89]. These events are suppressed by ASTX, which inhibits MSK1 autophosphorylation [90]. Lastly, in silico simulations suggested that ASTX and β-carotene extracellularly interact with IL-6 and TNF-α, preventing their binding to membrane receptors [91]. ASTX may also interact with the NF-κB-Inducing Kinase (NIK) and block the phosphorylation of the IKK-α subunit of the IkB α kinase complex, suppressing the NF-κB pathway [92].
2.2.2. JAK2/STAT3 and JNK/p38 MAPK Pathways
β-carotene and ASTX further modulate the pro-inflammatory pathways mediated by the JNK/p38 MAPK [93] and JAK2/STAT3 kinases [84,94], the latter responding to IL-6 in the pathogenesis of RA and osteoarthritis [31,95]. Phosphorylation of STAT3 dimers by JAK2 induces nuclear translocation and the differentiation of CD4+ T cells into the highly reactive T helper 17 (Th17) cell type [96]. TNFs and IL-1 activate the JNK/p38 MAPK pathway starting a phosphorylation cascade ending with JNK/p38 MAPK nuclear translocation [97], and phosphorylation of pro-inflammatory transcription factors (ELK1, MEF2, ATF2, and STAT1) [98] and of the MAPK-activated kinase 2 (MK2), which, in turn, targets the tristetrapolin (TTP) factor, promoting stabilization of IL mRNAs [99]. These oxidant-sensitive inflammatory pathways are also modulated by lutein [100], as reported using extracts enriched in this xanthophyll from different species of the chlorophyte genus Tetraselmis (Chlorodendrophyceae) [101].
2.2.3. Other Pro-Inflammatory Pathways Targeted by Microalgal Carotenoids
Mitochondrial disfunction is a key pathogenic driver in RA [102], and ASTX was reported to attenuate organellar ROS production in human chondrocytes treated with IL-1β [69]. In addition to suppressing pro-inflammatory pathways, ASTX is also suggested to promote cartilage homeostasis via the transcriptional regulator nuclear factor-erythroid 2-related factor 2 (Nrf2) [68,93]. ASTX is suggested to stabilize and promote the nuclear translocation of Nfr2, which binds so-called antioxidant response elements (AREs), enhancing the expression of anti-inflammatory and ROS-detoxifying genes [103,104].
2.3. Lipids and Their Derivatives
A hallmark of RA is the altered fatty acid profile of the synovium [105,106], while the intake of polyunsaturated fatty acids (PUFAs) correlates with joint health and mitigates the risk of RA onset [107]. Microalgal mass cultivation is a more sustainable way to derive functional lipids compared with cold water fish [108,109,110,111,112]. Phytoplankton occupies the lowest trophic level in oceans and freshwater basins, representing the primary PUFAs producer in aquatic food webs [113]. Global warning and ocean acidification are predicted to affect phytoplankton ecology [114,115], thus reducing PUFAs availability to higher trophic levels and, eventually, putting at risk the supply for human nutrition [116]. Moreover, upon stress acclimation, microalgae synthetise a wider range of anti-inflammatory and immunomodulatory lipids compared to animals [117,118,119,120,121].
2.3.1. Long-Chain Polyunsaturated Fatty Acids
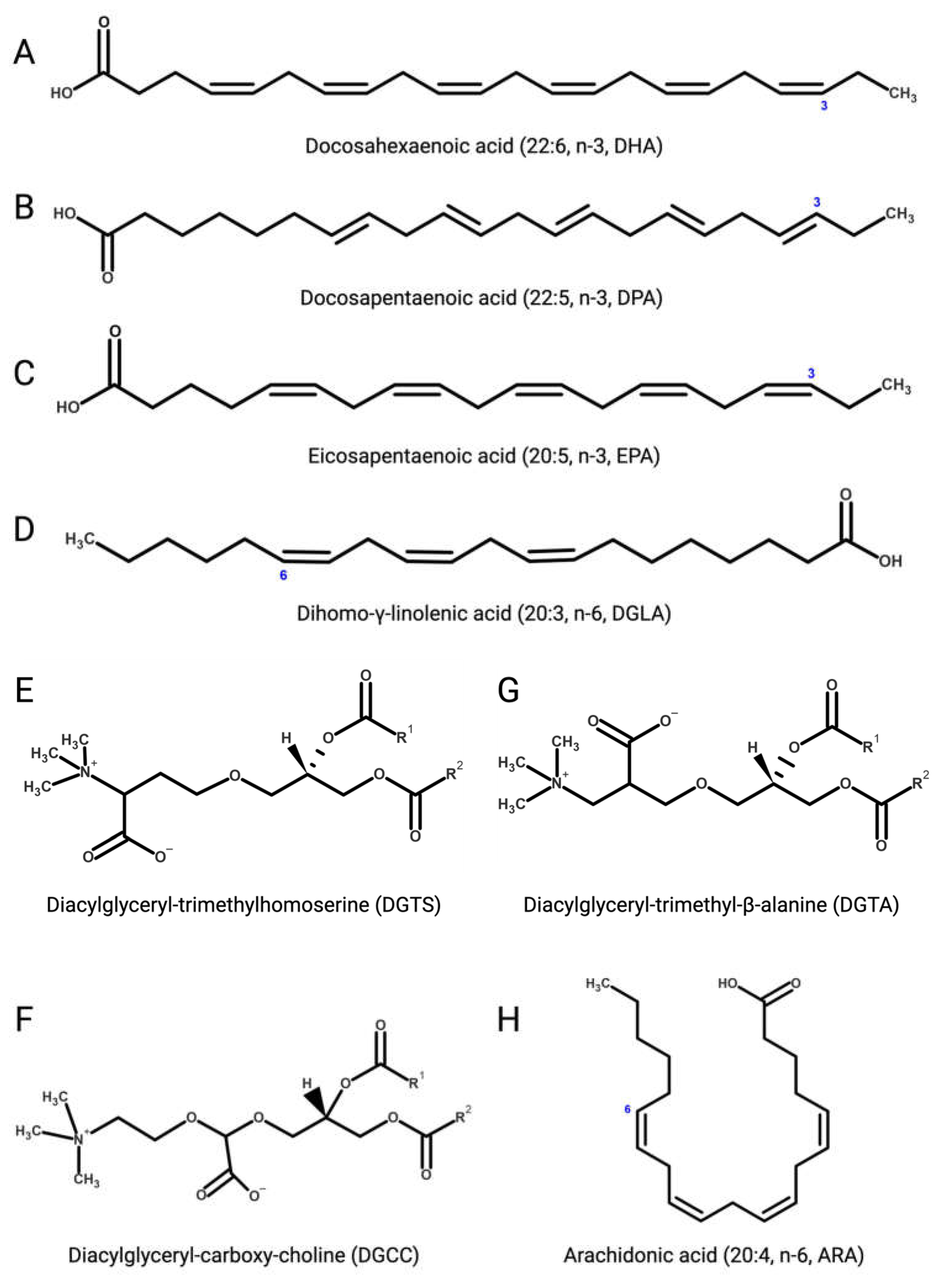
Several microalgae accumulate very long-chain PUFAs [122,123], including the omega-3 (ω-3, n-3) PUFAs [124] α-linolenic (18:3), docosahexaenoic (DHA, 22:6, n-3, Figure 3A), docosapentaenoic (DPA, n-3, 22:5, Figure 3B), and eicosapentaenoic (EPA, 20:5, n-3, Figure 3C) acids but also ω-6 PUFAs like arachidonic (ARA, 20:4, n-6, Figure 3H), γ-linolenic (18:3), linoleic (18:2), and dihomo-γ-linolenic (DGLA, 20:3, n-6, Figure 3D) acids. These molecules are biosynthetic precursors of anti-inflammatory signaling molecules and interfere with pro-inflammatory pathways [125,126].
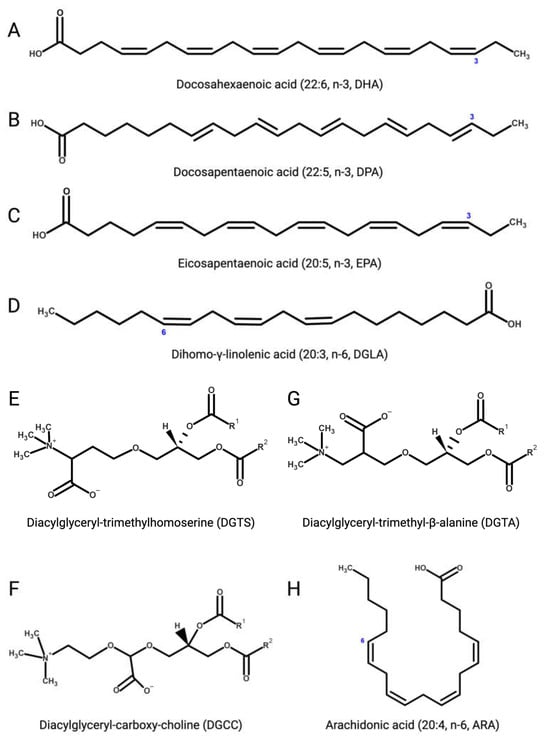
Figure 3.
(A) Docosahexaenoic (DHA, 22:6, n-3); (B) docosapentaenoic (DPA, 22:5, n-3); (C) eicosapentaenoic (EPA, 20:5, n-3); (D) dihomo-γ-linolenic (DGLA, 20:3, n-6); (E) 1,2-diacylglyceryl-3-O-4’-(N,N,N-trimethyl)-homoserine (DGTS); (F) 1,2-diacylglyceryl-3-O-carboxy-(hydroxymethyl)-choline (DGCC); (G) 1,2-diacylglyceryl-3-O-2’-(hydroxymethyl)-(N,N,N-trimethyl)-β-alanine (DGTA); (H) arachidonic (ARA, 20:4, n-6). Figures created with BioRender.com, accessed on 15 November 2023.
The administration of lipids from the DGLA-hyperproducing freshwater chlorophyte Lobosphaera incisa (Trebouxiophyceae) suppressed the expression of the NF-κB pathway-related genes in an animal model of chronic inflammation [127]. Similarly, lipid extracts from the haptophyte Pavlova lutheri (Prymnesiophyceae), an EPA- and DHA-hyperaccumulating strain, inhibited IL-6 and TNF-α production in activated human macrophages, possibly through suppressing the NF-κB pathway [128].
Several heterotrophic marine microorganisms are strong DHA producers, such as the dinoflagellate Crypthecodinium cohnii (Dinophyceae) and, above all, the thraustochytrids protists Thraustochytrium spp., Aurantiochytrium (formerly Schizochytrium) limacinum (Labyrinthulomycetes) [129,130,131,132,133], and related genera [134]. Although these microorganisms cannot exploit light energy to drive their metabolism, they are capable of fermenting plant biomass hydrolysates, affording cost-effective heterotrophic cultivation using renewable feedstocks [135,136,137,138,139,140,141]. Arguably, the most valuable heterotrophic sources of DHA are Schizochytrium spp., which recently obtained Novel Food status [142,143]. Notably, a recent human clinical trial investigated the supplementation of an EPA- and DHA-enriched oil from a Schizochytrium sp. in RA patients, reporting beneficial effects on joint health and the blood levels of inflammatory markers [144].
2.3.2. Betaine Lipids
Betaine lipids are anti-inflammatory and immunomodulatory glycerolipids in which the phosphate and carbohydrate moieties attached to the glycerol backbone are replaced with positively charged ether-bond betaine groups. Betaine lipids are widely distributed in all clades of eukaryotic microalgae, where they act as acyl group donors upon membrane lipid remodelling and during the accumulation of storage neutral lipids [145,146].
Betaine lipids derive from the turnover of membrane phospholipids under abiotic stresses, mainly temperature and nutrient starvation [147,148,149]. The betaine lipid 1,2-diacylglyceryl-3-O-4’-(N,N,N-trimethyl)-homoserine (DGTS, Figure 3E) is the most abundant betaine lipid in microalgae, followed by 1,2-diacylglyceryl-3-O-carboxy-(hydroxymethyl)-choline (DGCC, Figure 3F), and 1,2-diacylglyceryl-3-O-2’-(hydroxymethyl)-(N,N,N-trimethyl)-β-alanine (DGTA, Figure 3G), with evidence of DGTS-mediated inhibition of the IKK-β kinase causing suppression of the NF-κB pathway and secretion of pro-inflammatory ILs by Th1 and Th2 cells, as recently reported with extracts from the oleaginous chlorophyte Chromochloris zofingiensis (Chlorophyceae) [150].
2.3.3. Oxylipins
The eicosanoids, prostaglandins and leukotrienes, and the thromboxanes, are hormone-like oxygenated metabolites of C20 fatty acids involved in the modulation and resolution of inflammation in the RA synovium [106]. In mammals, oxylipins are produced by the enzyme phospholipase A2 via release of sn-2 PUFAs from membrane phospholipids. Typical oxylipin precursors are linoleic, α-linolenic, and ARA, which are substrates of cyclooxygenases, lipoxygenases, and cytochrome P450 enzymes, respectively [151].
Several microalgal species are known to accumulate prostaglandin-like oxylipins [152,153,154]. Their synthesis can occur either enzymatically via animal-like biosynthetic pathways [155], as in diatoms Skeletonema marinoi and Thalassiosira rotula (Bacillariophyceae) [156,157,158], or via spontaneous oxidation of ARA, EPA, and DHA in T. lutea [159]. The latter, known as isoprostanoids, are functional lipids which regulate bone health through preventing osteoclast differentiation [160].
The oxylipins isolated from the chlorophytes Chlamydomonas debaryana (Chlorophyceae) and Nannochloropsis gaditana inhibited TNF-α production in cultured macrophages [161], while the oral administration of biomass of the former suppressed the production of pro-inflammatory ILs in an animal model of chronic inflammation, possibly through inhibiting the NF-κB pathway [162,163]
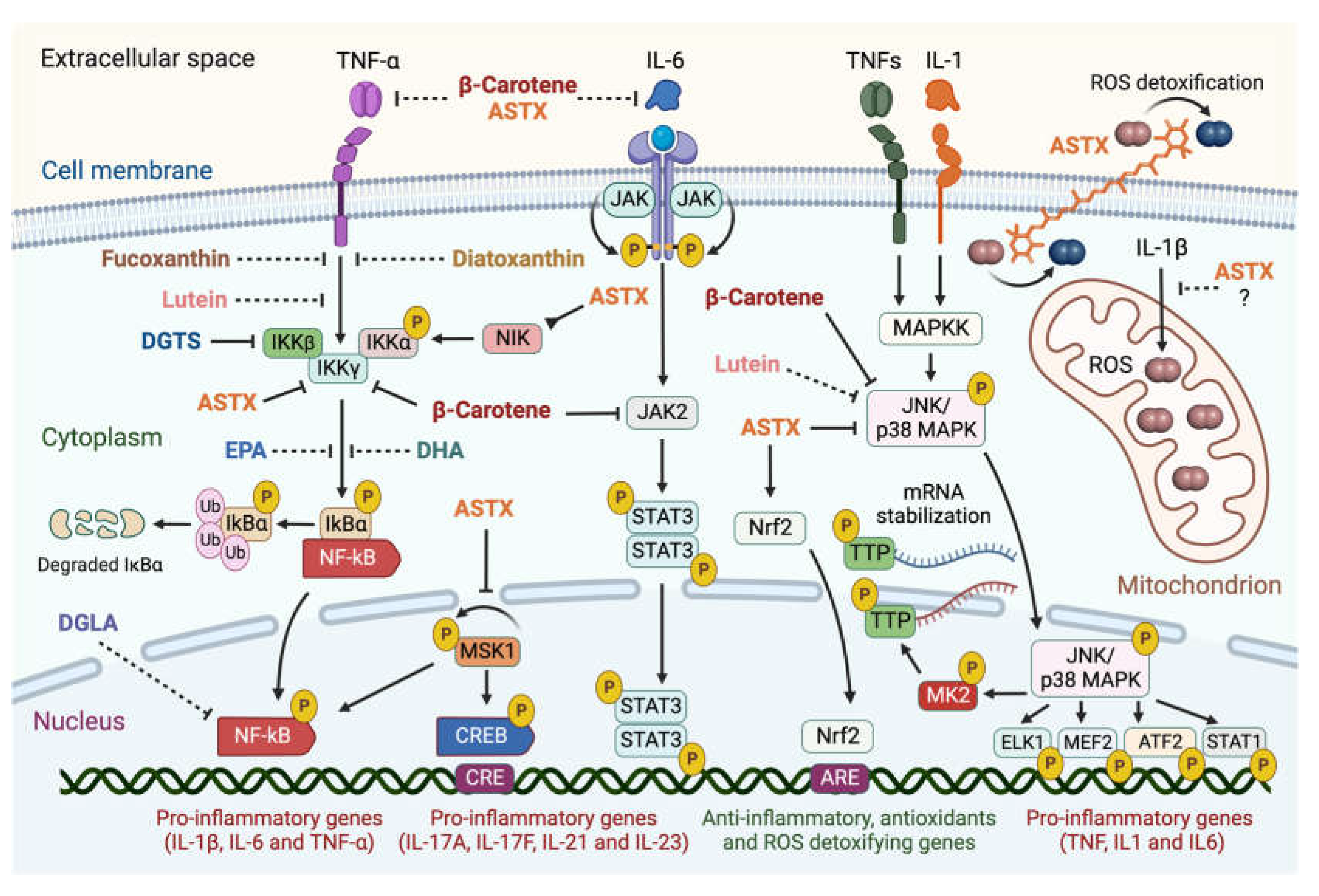
Figure 4 summarizes the evidence-based interference of the above-mentioned microalgal carotenoids and lipids with the main intracellular pro-inflammatory pathways involved in the pathogenesis of RA.
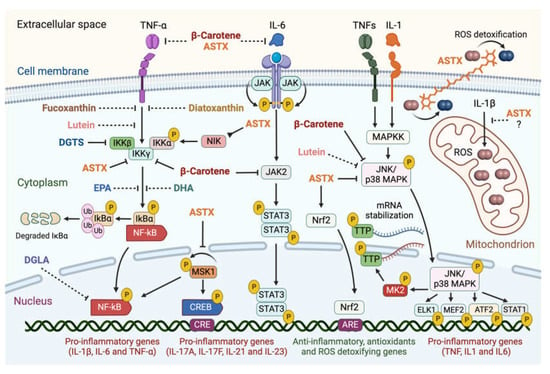
Figure 4.
Summary of evidence-based interference of selected microalgal metabolites with the intracellular pro-inflammatory signaling pathways involved in the pathogenesis of RA. Microalgal carotenoids and lipids exert inhibitory effects on major intracellular pro-inflammatory signaling pathways involved in the onset and progression of rheumatoid arthritis. Blunt-ended solid lines indicate an inhibitory pharmacological effect, while dashed lines suggest proposed interference. Astaxanthin (ASTX), fucoxanthin, diatoxanthin, and β-carotene target different subunits of the Inhibitor of κB (IkB) kinase complex, preventing the phosphorylation-dependent release of the pro-inflammatory transcriptional activator NF-kB and its nuclear translocation. ASTX further acts upstream of this pathway through inhibiting the NF-κB-Inducing Kinase (NIK), preventing the autophosphorylation of the Mitogen- and Stress-activated protein Kinase-1 (MSK1), a nucleus-localized effector which activates NF-κB and the cAMP-responsive Element-Binding Protein (CREB) pro-inflammatory transcription factor. ASTX and β-carotene also inhibit the JNK/p38 MAPK signaling cascade, blocking the nuclear translocation of the JNK/p38 MAPK complex, and thus phosphorylation of downstream targets: the pro-inflammatory transcription factors ELK1, MEF2, ATF2, and STAT1, and the MAPK-activated kinase 2 (MK2) responsible for stabilizing IL mRNAs. β-carotene inhibits the JAK2/STAT3 pathway through blocking phosphorylation of the pro-inflammatory transcriptional activator STAT3 by JAK2 and its nuclear translocation. ASTX positively regulates the nuclear factor-erythroid 2-Related factor 2 (Nrf2)-mediated pathway involved in antioxidant and anti-inflammatory gene expression. ASTX and β-carotene are proposed to directly bind interleukin 6 (IL-6) and tumor necrosis factor-alpha (TNF-α), blocking their receptor interaction and activation of the downstream pathways. Lutein is suggested to inhibit both NF-kB and JNK/p38 MAPK pathways. ASTX detoxifies free radicals on both sides of the lipid bilayer and appears to suppress mitochondrial ROS production. The betaine lipid DGTS interferes with the activity of the IKKβ subunit of the IkB kinase complex, while the PUFAs docosahexaenoic (DHA), eicosapentaenoic (EPA), and dihomo-γ-linolenic (DGLA) acids modulate the NF-kB signaling cascade through targeting unknown pathway components. Figures created with BioRender.com, accessed on 15 November 2023.
3. Bioprospecting and Domestication of Pharmacologically Relevant Microalgae
Of the over 70,000 estimated existing algal strains [164], only a few species are used in human nutrition and health. Bioprospecting for novel pharmacologically relevant species [165,166] requires scrupulous large-scale screening of phytoplankton biodiversity and chemodiversity [167], and the establishment of optimal cultivation strategies [168]. Inhospitable environments are excellent ecosystems to discover species with industrial applications since extremophiles are physiologically adapted to harsh conditions and hyperaccumulate pigments and lipids [11,12,169,170,171].
Among cryophilic species, the extracts of two Antarctic chlorophytes, Chloromonas reticulata (Chlorophyceae) and Micractinium simplicissimus (Trebouxiophyceae), were recently reported to exert an anti-inflammatory effect on activated macrophages [172,173]. At the other extreme, the rhodophyte Cyanidioschyzon merolae (Cyanidiophyceae) thrives at 40 °C and low pH, producing heat-stable carotenoids [174], while a stress-resilient strain of the marine chlorophyte species Tetraselmis striata [175] accumulates anti-inflammatory carotenoids and lipids [176]. A PUFAs-hyperproducing rhodophyte strain of the Galdieria genus (Cyanidiophyceae) was identified in acid thermal springs, and its lipid content could be enhanced via cultivation at temperatures below its optimal range [177]. Finally, the extracts of a chlorophyte Mucidosphaerium sp. (Trebouxiophyceae) isolated from a similar environment suppressed pro-inflammatory gene expression in human fibroblasts, as well as mitochondrial ROS production and inflammation in cultured synoviocytes [178,179].
Turning Wild Species into “Unicellular Medicinal Crops”
Although the accumulation of therapeutic compounds in microalgae can be accrued through abiotic stress challenges [180,181,182,183,184,185], wild organisms are usually not suited for industrial applications. For instance, ASTX production via mass cultivation H. pluvialis is restrained by its slow growth and elevated risk of pest contamination [186,187].
Adaptive laboratory evolution and random mutagenesis are powerful strategies for strain improvement based, respectively, on spontaneous and enhanced mutation rates [188,189] (Figure 5A). This approach generated lipid- and carotenoid-hyperproducing strains of chlorophyte Chlorella vulgaris (Trebouxiophyceae) [190,191], enabling the discovery of new genetic targets to enhance lutein content [192]. Similarly, a simultaneous enhancement of ASTX and EPA was reported in the Nannochloropsis species gaditiana [193] and in the Tetraselmis striata [194], while DHA accrual was achieved in Schizochytrium sp. [195,196,197], C. cohnii [198], and P. lutheri [199]. It should be noted, however, that evolved strains are susceptible to the risk of retromutation and trait drift [200].
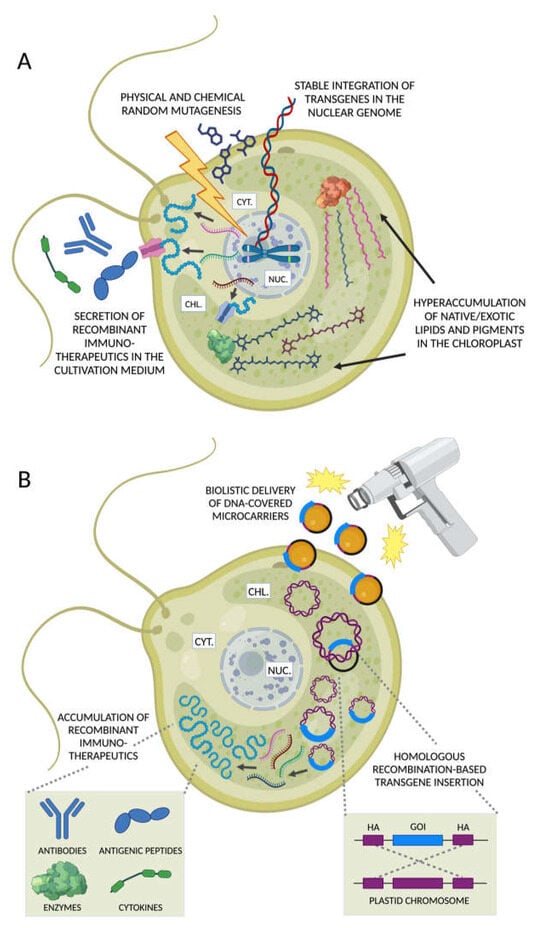
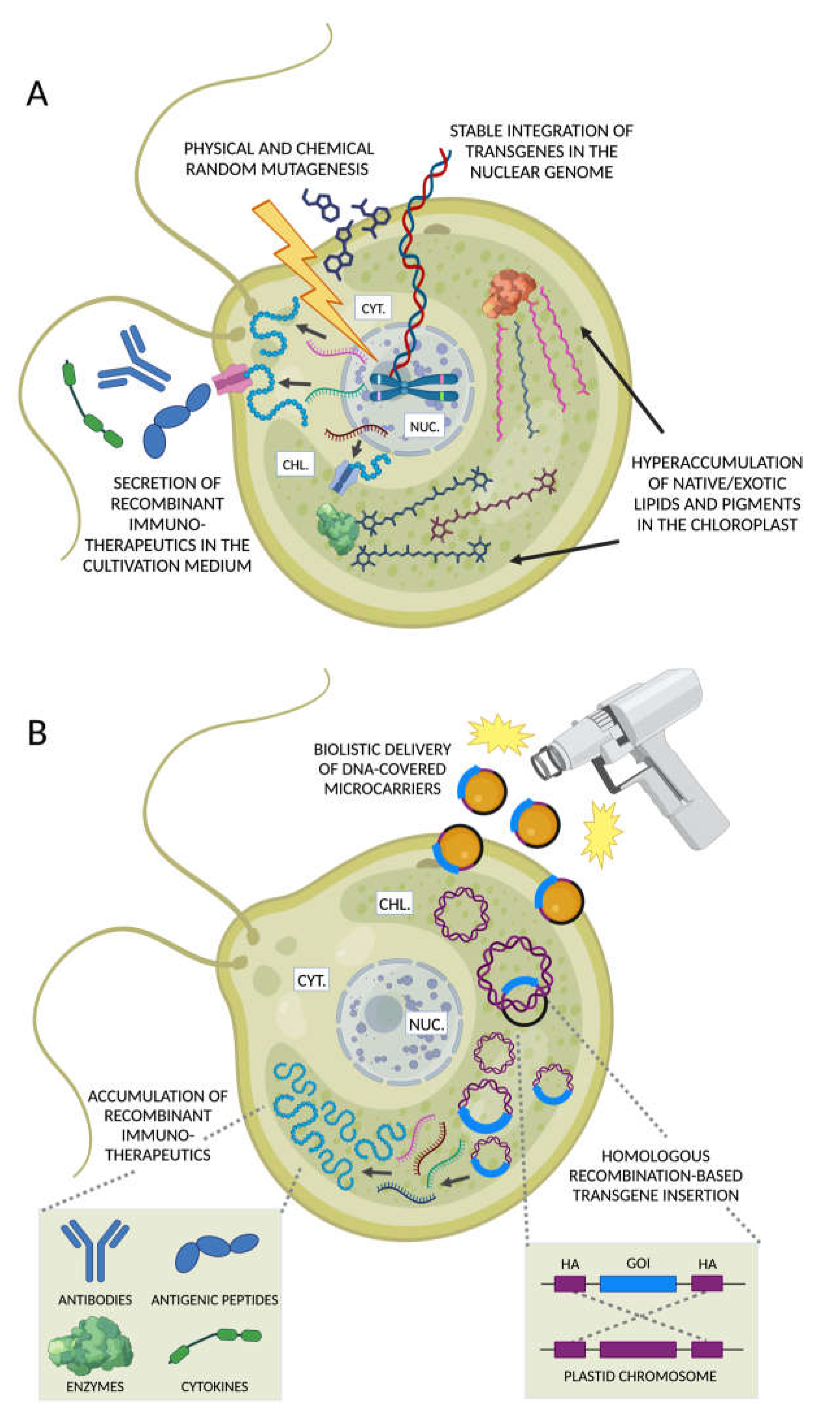
Figure 5.
Nuclear and chloroplast engineering to produce immunotherapeutics in microalgae. (A) Foreign DNA sequences can be introduced in the nuclear genome of microalgae resulting in the stable integration of transgene(s). Trait evolution can be achieved through random mutagenesis using physical (e.g., UV radiation, violet flash) or chemical agents followed by the identification of phenotypes of interest. The recombinant protein products synthetized on cytoplasmic ribosomes can be either accumulated in the cell or secreted in the cultivation medium to facilitate their recovery. Alternatively, recombinant metabolic enzymes can be targeted to the chloroplast to enhance the biosynthesis of native long-chain polyunsaturated fatty acids (PUFAs), or even exotic metabolites with immunomodulatory/anti-inflammatory properties. (B) Transgenes are introduced in the chloroplast via biolistic delivery and targeted to defined chromosomal loci exploiting homologous recombination-enabled homology arm sequences (HA, purple) flanking the gene of interest (GOI, blue segment). The high copy number of plastid chromosomes ensures a significantly greater synthesis of different classes of recombinant immunotherapeutics compared with the engineering of the nuclear haploid genome. Figures created with BioRender.com, accessed on 15 November 2023.
4. Biomanufacturing of Immunomodulatory Metabolites
4.1. Engineering Carotenoid Metabolism
Although microalgae can potentially substitute plants in the production of carotenoids like lutein [201,202], their yields are still lagging behind heterotrophic microorganisms [203,204,205,206]. Genetic engineering can greatly enhance pigment productivity in microalgae [207] but requires a detailed knowledge of algal genomes and of the transcriptional networks regulating carotenoid biosynthesis to manipulate key metabolic genes [208,209,210,211] (Figure 5A). In this respect, a model organism for ketocarotenoid biosynthesis is C. zofingiensis [212,213], from which the β-carotene ketolase (BKT) enzyme was identified as a rate-limiting factor for ASTX production [214], while the chlorophytes D. salina and Desmodesmus spp. (Chlorophyceae) are useful resources for β-carotene and lutein metabolism, respectively [215,216].
Carotenoid enhancement and the production of non-native ASTX isomers can be achieved through different engineering strategies [217], such as: (i) heterologous expression of chaperones stabilizing biosynthetic enzymes [218,219] and cyanobacterial proteins to improve pigment storage capacity [220], (ii) revival of endogenous silent BKT genes [221,222], and (iii) multigene overexpression to circumvent several biosynthetic bottlenecks [223]. Simultaneous enhancement of fucoxanthin and β-carotene was achieved with a similar approach in the diatom Phaeodactylum tricornutum (Bacillariophyceae) through overexpressing endogenous biosynthetic genes [224,225] and a plastoglobule protein to augment pigment sequestration [226]. Ketocarotenoids engineering was recently reported in the industrially relevant species C. merolae via heterologous expression of two biosynthetic genes [227], and in the thraustochytrid A. limacinum via overexpression of an endogenous β-carotene hydroxylase gene [228].
4.2. Synthetic Long-Chain Carotenoids
Algal chloroplasts provide excellent metabolic chassis to engineer the synthesis of non-native pigments with superior therapeutic properties [229], such as carotenoids with extended polyene chains and extra hydroxyl groups [230,231]. In this respect, extremophilic microorganisms are invaluable genetic resources to implement novel metabolic pathways in microalgae [232,233,234]. Archaea produce long-chain (C50) carotenoids through adding isoprene (C5) units to lycopene (C40) [235]. This biosynthetic pathway was successfully introduced in bacteria to produce C50 ASTX [236,237] and non-natural C60 ketocarotenoids [238], and it would be of extreme interest to verify its feasibility in microalgae to accumulate extremely valuable synthetic metabolites.
4.3. Enhancing PUFAs Accumulation
Microalgae can be engineered to maximize the yields of the endogenous anti-inflammatory lipids [239]. Overexpression of endogenous or heterologous fatty acid biosynthesis genes is a standard approach to enhance PUFAs productivity [240], and the availability of transcriptomes investigating stress adaptation is crucial to identify new factors to modulate lipid profiles [241]. For instance, P. lutheri, different Prasinophyte species of the genus Ostreococcus (Mamiellophyceae), and the diatom Fragilariopsis cylindrus (Bacillariophyceae) are model species for studying DHA and EPA biosynthesis [242,243,244]. Transcriptomics analysis of Aurantiochytrium revealed fatty acid synthase isoforms involved in DHA production under nitrogen starvation [245], while an acyl-CoA binding protein related to lipid droplet remodeling and EPA synthesis in P. tricornutum was recently suggested as a target to enhance the yields of therapeutic PUFAs [246].
Lipid enhancement was reported in the oleaginous chlorophyte Neochloris oleoabundans (Chlorophyceae) via heterologous expression of Kennedy pathway genes from the chlorophyte Acutodesmus obliquus (Chlorophyceae) [247], while DHA was increased in P. tricornutum through overexpressing the Δ-6 desaturase [248], and the heterologous Δ-5 elongase and acyl-CoA-dependent Δ6-desaturase from Ostreococcus tauri [249].
DHA enhancement was achieved in Aurantiochytrium sp. via overexpression of glucose-6-phosphate dehydrogenase to increase NADPH regeneration for fatty acid biosynthesis [250], and in a Schizochytrium sp. via overexpression of ATP-citrate lyase and acetyl-CoA carboxylase [251]. Lastly, EPA was increased in D. salina [252] with a heterologous Δ-6 desaturase gene from T. pseudonana [253].
Prostaglandin biosynthesis was engineered in the oleaginous diatom Fistulifera solaris (Bacillariophyceae), a natural producer of C20 PUFAs, via expression of a cyclooxygenase gene from the red macroalga Agarophyton vermiculophyllum (Gracilariaceae), resulting in the highest reported heterologous prostaglandin production in a photosynthetic host.
5. Production of Heterologous Immunotherapeutics in Microalgae
Microalgae can be genetically engineered [254] to produce heterologous biopharmaceuticals [255,256], including human immune-related proteins [257,258,259,260].
Nuclear transgenesis affords eukaryotic-like post-translational modifications of recombinant proteins (mainly N-terminal glycosylation) [261,262] and extracellular secretion to facilitate recovery [258,263] (Figure 5A). Although nuclear transgenesis results in low expression titers and requires intensive screening efforts to identify high-yielding strains [264], combinatorial transgene assembly and synthetic regulatory elements can greatly improve translation rates [265,266].
As in plants [267], the algal chloroplast genome affords high-level accumulation of recombinant therapeutics [268,269,270,271] (Figure 5B). The prokaryotic features of the polyploid chloroplast genome (plastome) enable efficient homologous recombination-based transgene insertion and multigene expression with synthetic operons [272]. A rich genetic toolkit allows plastome manipulation in non-model species [273], including marker-free and metabolism-dependent selection strategies [274,275,276]. Among these, the type D phosphite dehydrogenase (PtxD) gene, which enables the oxidization of non-assimilable phosphite ions in phosphate, is a biosafe solution to maintain axenic cultures [277,278], especially in mixotrophic conditions where pest contamination risk is high [279].
Fast Tracking Microalgal Immunotherapeutics: The Time Is Now
Among newly proposed therapeutic agents for autoimmune diseases, fragment crystallisable (Fc) multimers against autoantibodies and peptides targeting Fc/Fcγ receptors are highly promising candidates [280]. Since microalgae can assemble full-length human monoclonal antibodies binding Fcγ receptors [281,282,283], it should be possible to produce antibodies targeting RA mediators [284]. Notably, polycistronic nuclear gene expression was reported in microalgae [285], potentially affording simultaneous production of multiple immunotherapeutic variants.
Viral nanoparticles are emerging tools with diagnostic and therapeutic applications in autoimmune diseases [286,287]. This strategy was pioneered in the plant Nicotiana benthamiana via self-assembled nanoparticles exposing RA autoantigens, which induced immunotolerance in animal RA models [288]. Although few studies have explored the use of viral vectors to produce recombinant therapeutics in microalgae so far [289,290], the ongoing characterization of microalgae-infecting viruses [291,292,293,294] should facilitate this approach in photosynthetic microbes [295,296].
6. Final Observations, Comments, and Outlook
Microalgae are key players in the transition towards a bioeconomy [297], and are the focus of intense academic and private research [298,299]. In Europe, the number of companies engaging in microalgae production is growing [300], and so is the list of patents describing their therapeutic uses in inflammatory diseases [301]. Microalgal biotechnology, however, is still restrained by low product yields and recovery [302,303,304,305,306], high operating costs, and barriers to market entry [307,308], especially for engineered strains [309].
6.1. Building a Good Reputation
Arguably, the biggest obstacle to the nutritional uses of photosynthetic microbes is their recognition as safe [310]. Currently, only five eukaryotic microalgae and three cyanoprokaryotes hold Novel Food status [311]. The occasional finding of hazardous contaminants such as heavy metals, toxins, pathogens, and pesticides in the harvested algal biomass [312,313] calls for rigorous adherence to good manufacturing practice in the microalgal food industry [314,315], especially in the case of emerging strains [316]. An even greater barrier is the fear of horizontal transfer of antibiotic resistance genes from engineered strains to the gut microbiome and human pathogens [317], thus the adoption of marker-free selection [274] and metabolic markers [275] are expected to promote a more favourable attitude towards engineered microalgae and their derived products.
6.2. A Road Map for Clinical Uses of Microalgae in Chronic Inflammation
To advance the applications of microalgae in autoimmune diseases, the therapeutic potential of novel classes of bioactive compounds should be explored. For example, some microalgal strains are sustainable sources of the lipid-based prohormone vitamin D3, the biosynthetic precursor of the biologically active 1,25-dihydroxyvitamin D (calcitriol) [318,319] whose deficiency is a major risk factor for RA [320,321]. Several microalgae accumulate bioactive ergosterol and β-sitosterol [322,323], precursors of vitamin D2 (ergocalciferol) and D3, respectively [324,325]. Moreover, the chlorophyte Botryococcus braunii (Trebouxiophyceae) and Schizochytrium mangrovei can be engineered to hyperaccumulate squalene, an anti-inflammatory triterpene and precursor of ergosterol [326,327,328,329,330,331].
The photosynthetic protist Euglena gracilis (Euglenoidea), a recently authorised Novel Food species [332], also deserves attention, being a natural producer of anti-inflammatory carotenoids, DHA [333,334,335], and paramylon, an immunomodulatory (1,3)-β-glucan [336] with reported inhibitory activity towards Th17 cells [337].
Finally, photosynthetic prokaryotes are still largely unexplored biomanufacturing platforms, despite producing a vast repertoire of unique compounds [338], including molecules with antioxidant and anti-inflammatory properties [339,340] like the pigment–protein complex phycocyanin, which is a selective inhibitor of pro-inflammatory oxylipin synthesis [341,342], and various polysaccharides [343,344,345] and peptides [346,347,348]. Last but not least, an increasing number of genetic engineering and synthetic biology tools [349] can be employed to produce recombinant therapeutics in cyanoprokaryotes [350], as already reported for immunomodulatory proteins [351].
7. Conclusions
The exploration of microalgal biodiversity and chemodiversity is a promising approach to discover new complementary therapeutic approaches for the management of RA and related chronic inflammatory conditions. Indeed, several preclinical studies have highlighted multiple mechanisms of action of microalgal compounds and their interference with pro-inflammatory pathways. Moreover, the availability of advanced genetic engineering tools holds tremendous potential to develop innovative biopharmaceuticals in photosynthetic microbes and expand their clinical applications.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study.
Acknowledgments
E.A.C. acknowledges the support of the post-doctoral research fellowship “Borsa Valeria e Vincenzo Landi per Ricerche nel Campo della Genetica Agraria” from the Accademia Nazionale dei Lincei. R.C. acknowledges the financial support of the European Research Council (ERC) Advanced Grant 101053983-GrInSun (Harvesting Light for Life: Green Proteins at the Interface between Sun Energy and Biosphere, Scientific Coordinator: Roberto Bassi).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Finckh, A.; Gilbert, B.; Hodkinson, B.; Bae, S.-C.; Thomas, R.; Deane, K.D.; Alpizar-Rodriguez, D.; Lauper, K. Global epidemiology of rheumatoid arthritis. Nat. Rev. Rheumatol. 2022, 18, 591–602. [Google Scholar] [CrossRef]
- Meyer, A.; Cirpus, P.; Ott, C.; Schlecker, R.; Zähringer, U.; Heinz, E. Biosynthesis of Docosahexaenoic Acid in Euglena gracilis: Biochemical and Molecular Evidence for the Involvement of a Δ4-Fatty Acyl Group Desaturase. Biochemistry 2003, 42, 9779–9788. [Google Scholar] [CrossRef]
- Kvien, T.K.; Uhlig, T.; Ødegård, S.; Heiberg, M.S. Epidemiological aspects of rheumatoid arthritis: The sex ratio. Ann. N. Y Acad. Sci. 2006, 1069, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.; Kwon, E.J.; Lee, J.J. Rheumatoid Arthritis: Pathogenic Roles of Diverse Immune Cells. Int. J. Mol. Sci. 2022, 23, 905. [Google Scholar] [CrossRef] [PubMed]
- Tardito, S.; Martinelli, G.; Soldano, S.; Paolino, S.; Pacini, G.; Patane, M.; Alessandri, E.; Smith, V.; Cutolo, M. Macrophage M1/M2 polarization and rheumatoid arthritis: A systematic review. Autoimmun. Rev. 2019, 18, 102397. [Google Scholar] [CrossRef] [PubMed]
- Dedmon, L.E. The genetics of rheumatoid arthritis. Rheumatology 2020, 59, 2661–2670. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, C.; Keyßer, G. Lifestyle Factors and Their Influence on Rheumatoid Arthritis: A Narrative Review. J. Clin. Med. 2022, 11, 7179. [Google Scholar] [CrossRef] [PubMed]
- Philippou, E.; Petersson, S.D.; Rodomar, C.; Nikiphorou, E. Rheumatoid arthritis and dietary interventions: Systematic review of clinical trials. Nutr. Rev. 2021, 79, 410–428. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J.; Sjöblom, H.; Gjertsson, I.; Ulven, S.M.; Lindqvist, H.M.; Bärebring, L. Do Interventions with Diet or Dietary Supplements Reduce the Disease Activity Score in Rheumatoid Arthritis? A Systematic Review of Randomized Controlled Trials. Nutrients 2020, 12, 2991. [Google Scholar] [CrossRef] [PubMed]
- Cutolo, M.; Nikiphorou, E. Nutrition and Diet in Rheumatoid Arthritis. Nutrients 2022, 14, 888. [Google Scholar] [CrossRef]
- Malavasi, V.; Soru, S.; Cao, G. Extremophile Microalgae: The potential for biotechnological application. J. Phycol. 2020, 56, 559–573. [Google Scholar] [CrossRef] [PubMed]
- Varshney, P.; Mikulic, P.; Vonshak, A.; Beardall, J.; Wangikar, P.P. Extremophilic micro-algae and their potential contribution in biotechnology. Bioresour. Technol. 2015, 184, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Williamson, E.; Ross, I.L.; Wall, B.T.; Hankamer, B. Microalgae: Potential novel protein for sustainable human nutrition. Trends Plant Sci. 2021, 49, D1004–D1011. [Google Scholar] [CrossRef] [PubMed]
- Niccolai, A.; Chini Zittelli, G.; Rodolfi, L.; Biondi, N.; Tredici, M.R. Microalgae of interest as food source: Biochemical composition and digestibility. Algal Res. 2019, 42, 101617. [Google Scholar] [CrossRef]
- Ampofo, J.; Abbey, L. Microalgae: Bioactive Composition, Health Benefits, Safety and Prospects as Potential High-Value Ingredients for the Functional Food Industry. Foods 2022, 11, 1744. [Google Scholar] [CrossRef]
- Barone, G.D.; Cernava, T.; Ullmann, J.; Liu, J.; Lio, E.; Germann, A.T.; Nakielski, A.; Russo, D.A.; Chavkin, T.; Knufmann, K.; et al. Recent developments in the production and utilization of photosynthetic microorganisms for food applications. Heliyon 2023, 9, e14708. [Google Scholar] [CrossRef]
- Levasseur, W.; Perré, P.; Pozzobon, V. A review of high value-added molecules production by microalgae in light of the classification. Biotechnol. Adv. 2020, 41, 107545. [Google Scholar] [CrossRef]
- Borowitzka, M.A. Chapter 9—Microalgae in Medicine and Human Health: A Historical Perspective. In Microalgae in Health and Disease Prevention; Levine, I.A., Fleurence, J., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 195–210. [Google Scholar]
- Riccio, G.; Lauritano, C. Microalgae with Immunomodulatory Activities. Mar. Drugs 2019, 18, 2. [Google Scholar] [CrossRef]
- Abu-Ghosh, S.; Dubinsky, Z.; Verdelho, V.; Iluz, D. Unconventional high-value products from microalgae: A review. Bioresour. Technol. 2021, 329, 124895. [Google Scholar] [CrossRef]
- Saide, A.; Martínez, K.A.; Ianora, A.; Lauritano, C. Unlocking the Health Potential of Microalgae as Sustainable Sources of Bioactive Compounds. Int. J. Mol. Sci. 2021, 22, 4383. [Google Scholar] [CrossRef]
- Del Mondo, A.; Smerilli, A.; Sané, E.; Sansone, C.; Brunet, C. Challenging microalgal vitamins for human health. Microb. Cell Factories 2020, 19, 201. [Google Scholar] [CrossRef] [PubMed]
- Novoveská, L.; Nielsen, S.L.; Eroldoğan, O.T.; Haznedaroglu, B.Z.; Rinkevich, B.; Fazi, S.; Robbens, J.; Vasquez, M.; Einarsson, H. Overview and Challenges of Large-Scale Cultivation of Photosynthetic Microalgae and Cyanobacteria. Mar. Drugs 2023, 21, 445. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.A.; Arayssi, T.; Duray, P.; Schumacher, H.R. Immunohistochemistry of normal human knee synovium: A quantitative study. Ann. Rheum. Dis. 2004, 63, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wei, K.; Slowikowski, K.; Fonseka, C.Y.; Rao, D.A.; Kelly, S.; Goodman, S.M.; Tabechian, D.; Hughes, L.B.; Salomon-Escoto, K.; et al. Defining inflammatory cell states in rheumatoid arthritis joint synovial tissues by integrating single-cell transcriptomics and mass cytometry. Nat. Immunol. 2019, 20, 928–942. [Google Scholar] [CrossRef] [PubMed]
- Cutolo, M.; Campitiello, R.; Gotelli, E.; Soldano, S. The Role of M1/M2 Macrophage Polarization in Rheumatoid Arthritis Synovitis. Front. Immunol. 2022, 13, 867260. [Google Scholar] [CrossRef] [PubMed]
- Makowski, L.; Chaib, M.; Rathmell, J.C. Immunometabolism: From basic mechanisms to translation. Immunol. Rev. 2020, 295, 5–14. [Google Scholar] [CrossRef]
- McInnes, I.B.; Schett, G. The pathogenesis of rheumatoid arthritis. N. Engl. J. Med. 2011, 365, 2205–2219. [Google Scholar] [CrossRef]
- Fukui, S.; Iwamoto, N.; Takatani, A.; Igawa, T.; Shimizu, T.; Umeda, M.; Nishino, A.; Horai, Y.; Hirai, Y.; Koga, T.; et al. M1 and M2 Monocytes in Rheumatoid Arthritis: A Contribution of Imbalance of M1/M2 Monocytes to Osteoclastogenesis. Front. Immunol. 2017, 8, 1958. [Google Scholar] [CrossRef]
- Brown, K.D.; Claudio, E.; Siebenlist, U. The roles of the classical and alternative nuclear factor-κB pathways: Potential implications for autoimmunity and rheumatoid arthritis. Arthritis Res. Ther. 2008, 10, 212. [Google Scholar] [CrossRef]
- Malemud, C.J. The role of the JAK/STAT signal pathway in rheumatoid arthritis. Ther. Adv. Musculoskelet. Dis. 2018, 10, 117–127. [Google Scholar] [CrossRef]
- Schett, G.; Zwerina, J.; Firestein, G. The p38 mitogen-activated protein kinase (MAPK) pathway in rheumatoid arthritis. Ann. Rheum. Dis. 2008, 67, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, N.; Takayanagi, H. Mechanisms of joint destruction in rheumatoid arthritis—Immune cell-fibroblast-bone interactions. Nat. Rev. Rheumatol. 2022, 18, 415–429. [Google Scholar] [CrossRef] [PubMed]
- Ono, T.; Hayashi, M.; Sasaki, F.; Nakashima, T. RANKL biology: Bone metabolism, the immune system, and beyond. Inflamm. Regen. 2020, 40, 2. [Google Scholar] [CrossRef] [PubMed]
- Ni, S.; Shan, F.; Geng, J. Interleukin-10 family members: Biology and role in the bone and joint diseases. Int. Immunopharmacol. 2022, 108, 108881. [Google Scholar] [CrossRef] [PubMed]
- Müller, R.D.; John, T.; Kohl, B.; Oberholzer, A.; Gust, T.; Hostmann, A.; Hellmuth, M.; Laface, D.; Hutchins, B.; Laube, G.; et al. IL-10 overexpression differentially affects cartilage matrix gene expression in response to TNF-alpha in human articular chondrocytes in vitro. Cytokine 2008, 44, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Sandmann, G. Diversity and origin of carotenoid biosynthesis: Its history of coevolution towards plant photosynthesis. New Phytol. 2021, 232, 479–493. [Google Scholar] [CrossRef]
- Takaichi, S. Carotenoids in algae: Distributions, biosyntheses and functions. Mar. Drugs 2011, 9, 1101–1118. [Google Scholar] [CrossRef]
- Gao, J.; Wang, H.; Yuan, Q.; Feng, Y. Structure and Function of the Photosystem Supercomplexes. Front. Plant Sci. 2018, 9, 357. [Google Scholar] [CrossRef]
- Anjani, G.; Ayustaningwarno, F.; Eviana, R. Critical review on the immunomodulatory activities of carrot’s β-carotene and other bioactive compounds. J. Funct. Foods 2022, 99, 105303. [Google Scholar] [CrossRef]
- Moia, V.M.; Leal Portilho, F.; Almeida Pádua, T.; Barbosa Corrêa, L.; Ricci-Junior, E.; Cruz Rosas, E.; Magalhaes Rebelo Alencar, L.; Savio Mendes Sinfronio, F.; Sampson, A.; Hussain Iram, S.; et al. Lycopene used as Anti-inflammatory Nanodrug for the Treatment of Rheumathoid Arthritis: Animal assay, Pharmacokinetics, ABC Transporter and Tissue Deposition. Colloids Surf. B Biointerfaces 2020, 188, 110814. [Google Scholar] [CrossRef]
- Renju, G.L.; Muraleedhara Kurup, G.; Saritha Kumari, C.H. Anti-inflammatory activity of lycopene isolated from Chlorella marina on Type II Collagen induced arthritis in Sprague Dawley rats. Immunopharmacol. Immunotoxicol. 2013, 35, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Caferri, R.; Guardini, Z.; Bassi, R.; Dall’Osto, L. Chapter Two—Assessing photoprotective functions of carotenoids in photosynthetic systems of plants and green algae. In Methods in Enzymology; Wurtzel, E.T., Ed.; Academic Press: Cambridge, MA, USA, 2022; Volume 674, pp. 53–84. [Google Scholar]
- Zhao, K.; Zhou, T.; Yang, J.; Li, Y.; Qin, J.; Wang, S.; Li, D.; Chen, J.; Zheng, W.V. Lutein shows a protective effect against the aging of mesenchymal stem cells by downregulating inflammation. Int. Immunopharmacol. 2023, 116, 109749. [Google Scholar] [CrossRef]
- Pick, U.; Zarka, A.; Boussiba, S.; Davidi, L. A hypothesis about the origin of carotenoid lipid droplets in the green algae Dunaliella and Haematococcus. Planta 2019, 249, 31–47. [Google Scholar] [CrossRef] [PubMed]
- Chekanov, K.; Lukyanov, A.; Boussiba, S.; Aflalo, C.; Solovchenko, A. Modulation of photosynthetic activity and photoprotection in Haematococcus pluvialis cells during their conversion into haematocysts and back. Photosynth. Res. 2016, 128, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Bigagli, E.; D’Ambrosio, M.; Cinci, L.; Niccolai, A.; Biondi, N.; Rodolfi, L.; Dos Santos Nascimiento, L.B.; Tredici, M.R.; Luceri, C. A Comparative In Vitro Evaluation of the Anti-Inflammatory Effects of a Tisochrysis lutea Extract and Fucoxanthin. Mar. Drugs 2021, 19, 334. [Google Scholar] [CrossRef] [PubMed]
- Sansone, C.; Pistelli, L.; Del Mondo, A.; Calabrone, L.; Fontana, A.; Noonan, D.M.; Albini, A.; Brunet, C. The Microalgal Diatoxanthin Inflects the Cytokine Storm in SARS-CoV-2 Stimulated ACE2 Overexpressing Lung Cells. Antioxidants 2022, 11, 1515. [Google Scholar] [CrossRef] [PubMed]
- Satoh, A.; Tsuji, S.; Okada, Y.; Murakami, N.; Urami, M.; Nakagawa, K.; Ishikura, M.; Katagiri, M.; Koga, Y.; Shirasawa, T. Preliminary Clinical Evaluation of Toxicity and Efficacy of A New Astaxanthin-rich Haematococcus pluvialis Extract. J. Clin. Biochem. Nutr. 2009, 44, 280–284. [Google Scholar] [CrossRef]
- Turck, D.; Castenmiller, J.; de Henauw, S.; Hirsch-Ernst, K.I.; Kearney, J.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; Pelaez, C.; et al. Safety of astaxanthin for its use as a novel food in food supplements. EFSA J. 2020, 18, e05993. [Google Scholar]
- Valenti, M.T.; Perduca, M.; Romanelli, M.G.; Mottes, M.; Dalle Carbonare, L. A potential role for astaxanthin in the treatment of bone diseases (Review). Mol. Med. Rep. 2020, 22, 1695–1701. [Google Scholar] [CrossRef]
- Donoso, A.; González-Durán, J.; Muñoz, A.A.; González, P.A.; Agurto-Muñoz, C. Therapeutic uses of natural astaxanthin: An evidence-based review focused on human clinical trials. Pharmacol. Res. 2021, 166, 105479. [Google Scholar] [CrossRef]
- Hajizadeh-Sharafabad, F.; Zahabi, E.S.; Malekahmadi, M.; Zarrin, R.; Alizadeh, M. Carotenoids supplementation and inflammation: A systematic review and meta-analysis of randomized clinical trials. Crit. Rev. Food Sci. Nutr. 2022, 62, 8161–8177. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, H.; Ding, K.; He, S.; Li, G.; Qu, J.; Qiao, Y.; Zhang, L.; Sui, X.; Fan, C.; et al. Astaxanthin intake alleviates gouty arthritis in patients and rats by modulating the levels of various inflammatory markers. J. Funct. Foods 2021, 87, 104823. [Google Scholar] [CrossRef]
- Tamura, H.; Ishikita, H. Quenching of Singlet Oxygen by Carotenoids via Ultrafast Superexchange Dynamics. J. Phys. Chem. A 2020, 124, 5081–5088. [Google Scholar] [CrossRef]
- Dose, J.; Matsugo, S.; Yokokawa, H.; Koshida, Y.; Okazaki, S.; Seidel, U.; Eggersdorfer, M.; Rimbach, G.; Esatbeyoglu, T. Free Radical Scavenging and Cellular Antioxidant Properties of Astaxanthin. Int. J. Mol. Sci. 2016, 17, 103. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Sun, Z.; Sun, P.; Chen, T.; Chen, F. Microalgal carotenoids: Beneficial effects and potential in human health. Food Funct. 2014, 5, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Kim, B.; Lee, J.-Y. Astaxanthin Structure, Metabolism, and Health Benefits. 2014. Available online: https://www.jscimedcentral.com/public/assets/articles/nutrition-1-1003.pdf (accessed on 15 November 2023).
- Fukuzawa, K.; Inokami, Y.; Tokumura, A.; Terao, J.; Suzuki, A. Rate constants for quenching singlet oxygen and activities for inhibiting lipid peroxidation of carotenoids and α-tocopherol in liposomes. Lipids 1998, 33, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Bolin, A.P.; Macedo, R.C.; Marin, D.P.; Barros, M.P.; Otton, R. Astaxanthin prevents in vitro auto-oxidative injury in human lymphocytes. Cell Biol. Toxicol. 2010, 26, 457–467. [Google Scholar] [CrossRef]
- Macedo, R.C.; Bolin, A.P.; Marin, D.P.; Otton, R. Astaxanthin addition improves human neutrophils function: In vitro study. Eur. J. Nutr. 2010, 49, 447–457. [Google Scholar] [CrossRef]
- Guerra, B.A.; Otton, R. Impact of the carotenoid astaxanthin on phagocytic capacity and ROS/RNS production of human neutrophils treated with free fatty acids and high glucose. Int. Immunopharmacol. 2011, 11, 2220–2226. [Google Scholar] [CrossRef]
- Guerra, B.A.; Bolin, A.P.; Otton, R. Carbonyl stress and a combination of astaxanthin/vitamin C induce biochemical changes in human neutrophils. Toxicol Vitr. 2012, 26, 1181–1190. [Google Scholar] [CrossRef]
- Speranza, L.; Pesce, M.; Patruno, A.; Franceschelli, S.; Lutiis, M.A.d.; Grilli, A.; Felaco, M. Astaxanthin Treatment Reduced Oxidative Induced Pro-Inflammatory Cytokines Secretion in U937: SHP-1 as a Novel Biological Target. Mar. Drugs 2012, 10, 890–899. [Google Scholar] [CrossRef] [PubMed]
- Kimble, L.; Mathison, B.; Chew, B.P. Astaxanthin mediates inflammatory biomarkers associated with arthritis in human chondrosarcoma cells induced with interleukin-1β. FASEB J. 2013, 27, 638.6. [Google Scholar] [CrossRef]
- Mamun-Or-Rashid, A.N.M.; Lucy, T.T.; Yagi, M.; Yonei, Y. Inhibitory Effects of Astaxanthin on CML-HSA-Induced Inflammatory and RANKL-Induced Osteoclastogenic Gene Expression in RAW 264.7 Cells. Biomedicines 2022, 10, 54. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Dhaliwal, N.; Dhaliwal, J.; Dharavath, R.N.; Chopra, K. Astaxanthin attenuates oxidative stress and inflammatory responses in complete Freund-adjuvant-induced arthritis in rats. Pharmacol. Rep. 2020, 72, 104–114. [Google Scholar] [CrossRef]
- Sun, K.; Luo, J.; Jing, X.; Guo, J.; Yao, X.; Hao, X.; Ye, Y.; Liang, S.; Lin, J.; Wang, G.; et al. Astaxanthin protects against osteoarthritis via Nrf2: A guardian of cartilage homeostasis. Aging 2019, 11, 10513–10531. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, Z.; Peng, P.; Gong, Z.; Huang, J.; Peng, H. Astaxanthin attenuates osteoarthritis progression via inhibiting ferroptosis and regulating mitochondrial function in chondrocytes. Chem.-Biol. Interact. 2022, 366, 110148. [Google Scholar] [CrossRef]
- Budriesi, R.; Micucci, M.; Daglia, M.; Corazza, I.; Biotti, G.; Mattioli, L.B. Chemical Features and Biological Effects of Astaxanthin Extracted from Haematococcus pluvialis Flotow: Focus on Gastrointestinal System. Biol. Life Sci. Forum 2022, 12, 31. [Google Scholar]
- Régnier, P.; Bastias, J.; Rodriguez-Ruiz, V.; Caballero-Casero, N.; Caballo, C.; Sicilia, D.; Fuentes, A.; Maire, M.; Crepin, M.; Letourneur, D.; et al. Astaxanthin from Haematococcus pluvialis Prevents Oxidative Stress on Human Endothelial Cells without Toxicity. Mar. Drugs 2015, 13, 2857–2874. [Google Scholar] [CrossRef]
- Capelli, B.; Bagchi, D.; Cysewski, G.R. Synthetic astaxanthin is significantly inferior to algal-based astaxanthin as an antioxidant and may not be suitable as a human nutraceutical supplement. Nutrafoods 2013, 12, 145–152. [Google Scholar] [CrossRef]
- Yang, L.; Qiao, X.; Gu, J.; Li, X.; Cao, Y.; Xu, J.; Xue, C. Influence of molecular structure of astaxanthin esters on their stability and bioavailability. Food Chem. 2021, 343, 128497. [Google Scholar] [CrossRef]
- Madhavi, D.; Kagan, D.; Seshadri, S. A Study on the Bioavailability of a Proprietary, Sustained-release Formulation of Astaxanthin. Integr. Med. 2018, 17, 38–42. [Google Scholar]
- Liu, X.; Xie, J.; Zhou, L.; Zhang, J.; Chen, Z.; Xiao, J.; Cao, Y.; Xiao, H. Recent advances in health benefits and bioavailability of dietary astaxanthin and its isomers. Food Chem. 2023, 404, 134605. [Google Scholar] [CrossRef] [PubMed]
- Jafari, Z.; Bigham, A.; Sadeghi, S.; Dehdashti, S.M.; Rabiee, N.; Abedivash, A.; Bagherzadeh, M.; Nasseri, B.; Karimi-Maleh, H.; Sharifi, E.; et al. Nanotechnology-Abetted Astaxanthin Formulations in Multimodel Therapeutic and Biomedical Applications. J. Med. Chem. 2022, 65, 2–36. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Lee, J.-Y.; Luo, Y. Health benefits of astaxanthin and its encapsulation for improving bioavailability: A review. J. Agric. Food Res. 2023, 14, 100685. [Google Scholar] [CrossRef]
- Abdol Wahab, N.R.; Meor Mohd Affandi, M.M.R.; Fakurazi, S.; Alias, E.; Hassan, H. Nanocarrier System: State-of-the-Art in Oral Delivery of Astaxanthin. Antioxidants 2022, 11, 1676. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wang, J.; Feng, J.; Xuan, R. Research progress of Astaxanthin nano-based drug delivery system: Applications, prospects and challenges? Front. Pharmacol. 2023, 14, 1102888. [Google Scholar] [CrossRef] [PubMed]
- Hien, H.T.M.; Oanh, H.T.; Quynh, Q.T.; Thu, N.T.H.; Van Hanh, N.; Hong, D.D.; Hoang, M.H. Astaxanthin-loaded nanoparticles enhance its cell uptake, antioxidant and hypolipidemic activities in multiple cell lines. J. Drug Deliv. Sci. Technol. 2023, 80, 104133. [Google Scholar] [CrossRef]
- Bai, S.-K.; Lee, S.-J.; Na, H.-J.; Ha, K.-S.; Han, J.-A.; Lee, H.; Kwon, Y.-G.; Chung, C.-K.; Kim, Y.-M. β-Carotene inhibits inflammatory gene expression in lipopolysaccharide-stimulated macrophages by suppressing redox-based NF-κB activation. Exp. Mol. Med. 2005, 37, 323–334. [Google Scholar] [CrossRef]
- Lee, S.J.; Bai, S.K.; Lee, K.S.; Namkoong, S.; Na, H.J.; Ha, K.S.; Han, J.A.; Yim, S.V.; Chang, K.; Kwon, Y.G.; et al. Astaxanthin inhibits nitric oxide production and inflammatory gene expression by suppressing I(kappa)B kinase-dependent NF-κB activation. Mol. Cells 2003, 16, 97–105. [Google Scholar]
- Priyadarshini, L.; Aggarwal, A. Astaxanthin inhibits cytokines production and inflammatory gene expression by suppressing IκB kinase-dependent nuclear factor κB activation in pre and postpartum Murrah buffaloes during different seasons. Vet. World 2018, 11, 782–788. [Google Scholar] [CrossRef]
- Li, R.; Hong, P.; Zheng, X. β-carotene attenuates lipopolysaccharide-induced inflammation via inhibition of the NF-κB, JAK2/STAT3 and JNK/p38 MAPK signaling pathways in macrophages. Anim. Sci. J. 2019, 90, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.H.; Shin, H.-Y.; Park, J.-H.; Koo, S.Y.; Kim, S.M.; Yang, S.-H. Fucoxanthin from microalgae Phaeodactylum tricornutum inhibits pro-inflammatory cytokines by regulating both NF-κB and NLRP3 inflammasome activation. Sci. Rep. 2021, 11, 543. [Google Scholar] [CrossRef] [PubMed]
- Pistelli, L.; Sansone, C.; Smerilli, A.; Festa, M.; Noonan, D.M.; Albini, A.; Brunet, C. MMP-9 and IL-1β as Targets for Diatoxanthin and Related Microalgal Pigments: Potential Chemopreventive and Photoprotective Agents. Mar. Drugs 2021, 19, 354. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, L.; De Wilde, G.; Van Damme, P.; Vanden Berghe, W.; Haegeman, G. Transcriptional activation of the NF-κB p65 subunit by mitogen- and stress-activated protein kinase-1 (MSK1). EMBO J. 2003, 22, 1313–1324. [Google Scholar] [CrossRef] [PubMed]
- Takeba, Y.; Suzuki, N.; Wakisaka, S.; Takeno, M.; Kaneko, A.; Asai, T.; Sakane, T. Involvement of cAMP responsive element binding protein (CREB) in the synovial cell hyperfunction in patients with rheumatoid arthritis. Clin. Exp. Rheumatol. 2000, 18, 47–55. [Google Scholar] [PubMed]
- Wen, A.Y.; Sakamoto, K.M.; Miller, L.S. The role of the transcription factor CREB in immune function. J. Immunol. 2010, 185, 6413–6419. [Google Scholar] [CrossRef] [PubMed]
- Terazawa, S.; Nakajima, H.; Shingo, M.; Niwano, T.; Imokawa, G. Astaxanthin attenuates the UVB-induced secretion of prostaglandin E2 and interleukin-8 in human keratinocytes by interrupting MSK1 phosphorylation in a ROS depletion–independent manner. Exp. Dermatol. 2012, 21, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Widyaningrum, D.; Oktafika, R.A.; Cecilia, D. Microalgae pigments as a promising immunomodulating food ingredient: In silico study. IOP Conf. Ser. Earth Environ. Sci. 2022, 998, 012056. [Google Scholar] [CrossRef]
- Pflug, K.M.; Sitcheran, R. Targeting NF-κB-Inducing Kinase (NIK) in Immunity, Inflammation, and Cancer. Int. J. Mol. Sci. 2020, 21, 8470. [Google Scholar] [CrossRef]
- Zhao, L.; Tao, X.; Wan, C.; Dong, D.; Wang, C.; Xi, Q.; Liu, Y.; Song, T. Astaxanthin alleviates inflammatory pain by regulating the p38 mitogen-activated protein kinase and nuclear factor-erythroid factor 2-related factor/heme oxygenase-1 pathways in mice. Food Funct. 2021, 12, 12381–12394. [Google Scholar] [CrossRef]
- Yang, Y.; Li, R.; Hui, J.; Li, L.; Zheng, X. β-Carotene attenuates LPS-induced rat intestinal inflammation via modulating autophagy and regulating the JAK2/STAT3 and JNK/p38 MAPK signaling pathways. J. Food Biochem. 2021, 45, e13544. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Ning, K.; Sun, M.-l.; Zhang, X.-A. Regulation and therapy, the role of JAK2/STAT3 signaling pathway in OA: A systematic review. Cell Commun. Signal. 2023, 21, 67. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.K.; Chen, Z.; Larjo, A.; Kanduri, K.; Nousiainen, K.; Äijo, T.; Ricaño-Ponce, I.; Hrdlickova, B.; Tuomela, S.; Laajala, E.; et al. Genome-wide Analysis of STAT3-Mediated Transcription during Early Human Th17 Cell Differentiation. Cell Rep. 2017, 19, 1888–1901. [Google Scholar] [CrossRef] [PubMed]
- Maik-Rachline, G.; Zehorai, E.; Hanoch, T.; Blenis, J.; Seger, R. The nuclear translocation of the kinases p38 and JNK promotes inflammation-induced cancer. Sci. Signal 2018, 11, eaao3428. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Kim, S.C.; Yu, T.; Yi, Y.-S.; Rhee, M.H.; Sung, G.-H.; Yoo, B.C.; Cho, J.Y. Functional Roles of p38 Mitogen-Activated Protein Kinase in Macrophage-Mediated Inflammatory Responses. Mediat. Inflamm. 2014, 2014, 352371. [Google Scholar] [CrossRef] [PubMed]
- O’Neil, J.D.; Ammit, A.J.; Clark, A.R. MAPK p38 regulates inflammatory gene expression via tristetraprolin: Doing good by stealth. Int. J. Biochem. Cell Biol. 2018, 94, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.J.; Kim, H. Lutein as a Modulator of Oxidative Stress-Mediated Inflammatory Diseases. Antioxidants 2021, 10, 1448. [Google Scholar] [CrossRef]
- Kim, E.A.; Kang, N.; Heo, S.Y.; Oh, J.Y.; Lee, S.H.; Cha, S.H.; Kim, W.K.; Heo, S.J. Antioxidant, Antiviral, and Anti-Inflammatory Activities of Lutein-Enriched Extract of Tetraselmis Species. Mar. Drugs 2023, 21, 369. [Google Scholar] [CrossRef]
- Clayton, S.A.; MacDonald, L.; Kurowska-Stolarska, M.; Clark, A.R. Mitochondria as Key Players in the Pathogenesis and Treatment of Rheumatoid Arthritis. Front. Immunol. 2021, 12, 673916. [Google Scholar] [CrossRef]
- Kim, J.; Cha, Y.N.; Surh, Y.J. A protective role of nuclear factor-erythroid 2-related factor-2 (Nrf2) in inflammatory disorders. Mutat. Res. 2010, 690, 12–23. [Google Scholar] [CrossRef]
- Ferrándiz, M.L.; Nacher-Juan, J.; Alcaraz, M.J. Nrf2 as a therapeutic target for rheumatic diseases. Biochem. Pharmacol. 2018, 152, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Sigaux, J.; Bellicha, A.; Buscail, C.; Julia, C.; Flipo, R.M.; Cantagrel, A.; Laporte, F.; Beal, C.; Boissier, M.C.; Semerano, L. Serum Fatty Acid Profiles Are Associated with Disease Activity in Early Rheumatoid Arthritis: Results from the ESPOIR Cohort. Nutrients 2022, 14, 2947. [Google Scholar] [CrossRef] [PubMed]
- Mustonen, A.-M.; Nieminen, P. Fatty Acids and Oxylipins in Osteoarthritis and Rheumatoid Arthritis—A Complex Field with Significant Potential for Future Treatments. Curr. Rheumatol. Rep. 2021, 23, 41. [Google Scholar] [CrossRef] [PubMed]
- Hahn, J.; Cook, N.R.; Alexander, E.K.; Friedman, S.; Walter, J.; Bubes, V.; Kotler, G.; Lee, I.-M.; Manson, J.E.; Costenbader, K.H. Vitamin D and marine omega 3 fatty acid supplementation and incident autoimmune disease: VITAL randomized controlled trial. BMJ 2022, 376, e066452. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Li, T.; Du, S.; Chen, H.; Wang, Q. Microalgal polyunsaturated fatty acids: Hotspots and production techniques. Front. Bioeng. Biotechnol. 2023, 11, 1146881. [Google Scholar] [CrossRef] [PubMed]
- Demets, R.; Foubert, I. Chapter 1—Traditional and novel sources of long-chain omega-3 fatty acids. In Omega-3 Delivery Systems; García-Moreno, P.J., Jacobsen, C., Moltke Sørensen, A.-D., Yesiltas, B., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 3–23. [Google Scholar]
- Fernandes, T.; Cordeiro, N. Microalgae as Sustainable Biofactories to Produce High-Value Lipids: Biodiversity, Exploitation, and Biotechnological Applications. Mar. Drugs 2021, 19, 573. [Google Scholar] [CrossRef]
- Remize, M.; Brunel, Y.; Silva, J.L.; Berthon, J.Y.; Filaire, E. Microalgae n-3 PUFAs Production and Use in Food and Feed Industries. Mar. Drugs 2021, 19, 113. [Google Scholar] [CrossRef]
- Razali, W.A.W.; Pandhal, J. Outdoor pilot-scale cultivation and techno economic assessment of a novel omega-3 eicosapentaenoic acid over-producing Nannochloropsis oculata strain. Bioresour. Technol. Rep. 2023, 24, 10168. [Google Scholar] [CrossRef]
- Brett, M.; Müller-Navarra, D. The role of highly unsaturated fatty acids in aquatic foodweb processes. Freshw. Biol. 1997, 38, 483–499. [Google Scholar] [CrossRef]
- Hixson, S.M.; Arts, M.T. Climate warming is predicted to reduce omega-3, long-chain, polyunsaturated fatty acid production in phytoplankton. Glob. Chang. Biol. 2016, 22, 2744–2755. [Google Scholar] [CrossRef]
- Wang, T.; Tong, S.; Liu, N.; Li, F.; Wells, M.L.; Gao, K. The fatty acid content of plankton is changing in subtropical coastal waters as a result of OA: Results from a mesocosm study. Mar. Environ. Res. 2017, 132, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Puccinelli, E.; Sardenne, F.; Pecquerie, L.; Fawcett, S.E.; Machu, E.; Soudant, P. Omega-3 Pathways in Upwelling Systems: The Link to Nitrogen Supply. Front. Mar. Sci. 2021, 8, 664601. [Google Scholar] [CrossRef]
- Li-Beisson, Y.; Thelen, J.J.; Fedosejevs, E.; Harwood, J.L. The lipid biochemistry of eukaryotic algae. Progress. Lipid Res. 2019, 74, 31–68. [Google Scholar] [CrossRef] [PubMed]
- Lopes, D.; Aveiro, S.S.; Conde, T.; Rey, F.; Couto, D.; Melo, T.; Moreira, A.S.P.; Domingues, M.R. Chapter 6—Algal lipids: Structural diversity, analysis and applications. In Functional Ingredients from Algae for Foods and Nutraceuticals, 2nd ed.; Dominguez, H., Pereira, L., Kraan, S., Eds.; Woodhead Publishing: Sawston, UK, 2023; pp. 335–396. [Google Scholar]
- Kugler, A.; Zorin, B.; Didi-Cohen, S.; Sibiryak, M.; Gorelova, O.; Ismagulova, T.; Kokabi, K.; Kumari, P.; Lukyanov, A.; Boussiba, S.; et al. Long-Chain Polyunsaturated Fatty Acids in the Green Microalga Lobosphaera incisa Contribute to Tolerance to Abiotic Stresses. Plant Cell Physiol. 2019, 60, 1205–1223. [Google Scholar] [CrossRef] [PubMed]
- Rousch, J.M.; Bingham, S.E.; Sommerfeld, M.R. Changes in fatty acid profiles of thermo-intolerant and thermo-tolerant marine diatoms during temperature stress. J. Exp. Mar. Biol. Ecol. 2003, 295, 145–156. [Google Scholar] [CrossRef]
- Conde, T.A.; Zabetakis, I.; Tsoupras, A.; Medina, I.; Costa, M.; Silva, J.; Neves, B.; Domingues, P.; Domingues, M.R. Microalgal Lipid Extracts Have Potential to Modulate the Inflammatory Response: A Critical Review. Int. J. Mol. Sci. 2021, 22, 9825. [Google Scholar] [CrossRef]
- Khozin-Goldberg, I.; Leu, S.; Boussiba, S. Microalgae as a Source for VLC-PUFA Production. Subcell. Biochem. 2016, 86, 471–510. [Google Scholar]
- Taipale, S.; Peltomaa, E.; Salmi, P. Variation in ω-3 and ω-6 Polyunsaturated Fatty Acids Produced by Different Phytoplankton Taxa at Early and Late Growth Phase. Biomolecules 2020, 10, 559. [Google Scholar] [CrossRef]
- Barta, D.G.; Coman, V.; Vodnar, D.C. Microalgae as sources of omega-3 polyunsaturated fatty acids: Biotechnological aspects. Algal Res. 2021, 58, 102410. [Google Scholar] [CrossRef]
- Lupette, J.; Benning, C. Human health benefits of very-long-chain polyunsaturated fatty acids from microalgae. Biochimie 2020, 178, 15–25. [Google Scholar] [CrossRef]
- Nauroth, J.M.; Liu, Y.C.; Van Elswyk, M.; Bell, R.; Hall, E.B.; Chung, G.; Arterburn, L.M. Docosahexaenoic acid (DHA) and docosapentaenoic acid (DPAn-6) algal oils reduce inflammatory mediators in human peripheral mononuclear cells in vitro and paw edema in vivo. Lipids 2010, 45, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Novichkova, E.; Nayak, S.; Boussiba, S.; Gopas, J.; Zilberg, D.; Khozin-Goldberg, I. Dietary Application of the Microalga Lobosphaera incisa P127 Reduces Severity of Intestinal Inflammation, Modulates Gut-Associated Gene Expression, and Microbiome in the Zebrafish Model of IBD. Mol. Nutr. Food Res. 2023, 67, 2200253. [Google Scholar] [CrossRef] [PubMed]
- Robertson, R.C.; Guihéneuf, F.; Bahar, B.; Schmid, M.; Stengel, D.B.; Fitzgerald, G.F.; Ross, R.P.; Stanton, C. The Anti-Inflammatory Effect of Algae-Derived Lipid Extracts on Lipopolysaccharide (LPS)-Stimulated Human THP-1 Macrophages. Mar. Drugs 2015, 13, 5402–5424. [Google Scholar] [CrossRef] [PubMed]
- Morabito, C.; Bournaud, C.; Maës, C.; Schuler, M.; Aiese Cigliano, R.; Dellero, Y.; Maréchal, E.; Amato, A.; Rébeillé, F. The lipid metabolism in thraustochytrids. Progress Lipid Res. 2019, 76, 101007. [Google Scholar] [CrossRef] [PubMed]
- Ngoc Mai, D.T.; Ha, N.C.; Thom, L.T.; Hong, D.D. Initial studies on squalene from some marine microalgae isolated in Vietnam. Acad. J. Biol. 2013, 35, 333–341. [Google Scholar] [CrossRef]
- Chen, X.; He, Y.; Ye, H.; Xie, Y.; Sen, B.; Jiao, N.; Wang, G. Different carbon and nitrogen sources regulated docosahexaenoic acid (DHA) production of Thraustochytriidae sp. PKU#SW8 through a fully functional polyunsaturated fatty acid (PUFA) synthase gene (pfaB). Bioresour. Technol. 2020, 318, 124273. [Google Scholar]
- Leyton, A.; Shene, C.; Chisti, Y.; Asenjo, J.A. Production of Carotenoids and Phospholipids by Thraustochytrium sp. in Batch and Repeated-Batch Culture. Mar. Drugs 2022, 20, 416. [Google Scholar] [CrossRef] [PubMed]
- Jaritkhuan, S.; Suanjit, S. Species diversity and polyunsaturated fatty acid content of thraustochytrids from fallen mangrove leaves in Chon Buri province, Thailand. Agric. Nat. Resour. 2018, 52, 24–32. [Google Scholar] [CrossRef]
- Dellero, Y.; Cagnac, O.; Rose, S.; Seddiki, K.; Cussac, M.; Morabito, C.; Lupette, J.; Aiese Cigliano, R.; Sanseverino, W.; Kuntz, M.; et al. Proposal of a new thraustochytrid genus Hondaea gen. nov. and comparison of its lipid dynamics with the closely related pseudo-cryptic genus Aurantiochytrium. Algal Res. 2018, 35, 125–141. [Google Scholar] [CrossRef]
- Olsen, P.M.; Kósa, G.; Klüver, M.; Kohler, A.; Shapaval, V.; Horn, S.J. Production of docosahexaenoic acid from spruce sugars using Aurantiochytrium limacinum. Bioresour. Technol. 2023, 376, 128827. [Google Scholar] [CrossRef]
- Aini, U.N.; Lunprom, S.; Reungsang, A.; Salakkam, A. Docosahexaenoic acid (DHA) production by Aurantiochytrium limacinum using cassava pulp hydrolysate as an alternative low-cost carbon source. Front. Mar. Sci. 2022, 9, 985119. [Google Scholar] [CrossRef]
- Didrihsone, E.; Dubencovs, K.; Grube, M.; Shvirksts, K.; Suleiko, A.; Suleiko, A.; Vanags, J. Crypthecodinium cohnii Growth and Omega Fatty Acid Production in Mediums Supplemented with Extract from Recycled Biomass. Mar. Drugs 2022, 20, 68. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Fu, Z.; Zhu, Y.; He, J.; Ma, L.; Bu, D. Enhancing docosahexaenoic acid production of Schizochytrium sp. by optimizing fermentation using central composite design. BMC Biotechnol. 2022, 22, 39. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Liefeldt, S.; Rova, U.; Christakopoulos, P.; Matsakas, L. Co-production of DHA and squalene by thraustochytrid from forest biomass. Sci. Rep. 2020, 10, 1992. [Google Scholar] [CrossRef]
- Leong, H.Y.; Su, C.-A.; Lee, B.-S.; Lan, J.C.-W.; Law, C.L.; Chang, J.-S.; Show, P.L. Development of Aurantiochytrium limacinum SR21 cultivation using salt-rich waste feedstock for docosahexaenoic acid production and application of natural colourant in food product. Bioresour. Technol. 2019, 271, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Rova, U.; Christakopoulos, P.; Matsakas, L. Simultaneous production of DHA and squalene from Aurantiochytrium sp. grown on forest biomass hydrolysates. Biotechnol. Biofuels 2019, 12, 255. [Google Scholar] [CrossRef]
- Allen, K.M.; Habte-Tsion, H.-M.; Thompson, K.R.; Filer, K.; Tidwell, J.H.; Kumar, V. Freshwater microalgae (Schizochytrium sp.) as a substitute to fish oil for shrimp feed. Sci. Rep. 2019, 9, 6178. [Google Scholar] [CrossRef]
- EFSA Panel on Nutrition; Novel Foods and Food Allergens (NDA); Turck, D.; Bohn, T.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; et al. Safety of oil from Schizochytrium sp. (strain ATCC 20889) for use in infant and follow-on formula as a novel food pursuant to Regulation (EU) 2015/2283. EFSA J. 2022, 20, e07083. [Google Scholar]
- Dawczynski, C.; Dittrich, M.; Neumann, T.; Goetze, K.; Welzel, A.; Oelzner, P.; Völker, S.; Schaible, A.M.; Troisi, F.; Thomas, L.; et al. Docosahexaenoic acid in the treatment of rheumatoid arthritis: A double-blind, placebo-controlled, randomized cross-over study with microalgae vs. sunflower oil. Clin. Nutr. 2018, 37, 494–504. [Google Scholar] [CrossRef]
- Cañavate, J.P.; Armada, I.; Ríos, J.L.; Hachero-Cruzado, I. Exploring occurrence and molecular diversity of betaine lipids across taxonomy of marine microalgae. Phytochemistry 2016, 124, 68–78. [Google Scholar] [CrossRef]
- Hoffmann, D.Y.; Shachar-Hill, Y. Do betaine lipids replace phosphatidylcholine as fatty acid editing hubs in microalgae? Front. Plant Sci. 2023, 14, 1077347. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.; Van Mooy, B.A.S.; Heithoff, A.; Dyhrman, S.T. Phosphorus supply drives rapid turnover of membrane phospholipids in the diatom Thalassiosira pseudonana. ISME J. 2011, 5, 1057–1060. [Google Scholar] [CrossRef] [PubMed]
- Murakami, H.; Nobusawa, T.; Hori, K.; Shimojima, M.; Ohta, H. Betaine Lipid Is Crucial for Adapting to Low Temperature and Phosphate Deficiency in Nannochloropsis. Plant Physiol. 2018, 177, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Oishi, Y.; Otaki, R.; Iijima, Y.; Kumagai, E.; Aoki, M.; Tsuzuki, M.; Fujiwara, S.; Sato, N. Diacylglyceryl-N,N,N-trimethylhomoserine-dependent lipid remodeling in a green alga, Chlorella kessleri. Commun. Biol. 2022, 5, 19. [Google Scholar] [CrossRef]
- Leitner, P.D.; Jakschitz, T.; Gstir, R.; Stuppner, S.; Perkams, S.; Kruus, M.; Trockenbacher, A.; Griesbeck, C.; Bonn, G.K.; Huber, L.A.; et al. Anti-Inflammatory Extract from Soil Algae Chromochloris zofingiensis Targeting TNFR/NF-κB Signaling at Different Levels. Cells 2022, 11, 1407. [Google Scholar] [CrossRef]
- Biringer, R.G. The enzymology of the human prostanoid pathway. Mol. Biol. Rep. 2020, 47, 4569–4586. [Google Scholar] [CrossRef]
- Di Costanzo, F.; Di Dato, V.; Ianora, A.; Romano, G. Prostaglandins in Marine Organisms: A Review. Mar. Drugs 2019, 17, 428. [Google Scholar] [CrossRef]
- Blasio, M.; Balzano, S. Fatty Acids Derivatives From Eukaryotic Microalgae, Pathways and Potential Applications. Front. Microbiol. 2021, 12, 718933. [Google Scholar] [CrossRef]
- Linares-Maurizi, A.; Reversat, G.; Awad, R.; Bultel-Poncé, V.; Oger, C.; Galano, J.-M.; Balas, L.; Durbec, A.; Bertrand-Michel, J.; Durand, T.; et al. Bioactive Oxylipins Profile in Marine Microalgae. Mar. Drugs 2023, 21, 136. [Google Scholar] [CrossRef]
- Orefice, I.; Romano, G.; Di Dato, V. Chapter Ten—The biosynthesis and metabolism of prostaglandins in microalgae. In Advances in Botanical Research; Rébeillé, F., Maréchal, E., Eds.; Academic Press: Cambridge, MA, USA, 2022; Volume 101, pp. 375–436. [Google Scholar]
- Di Dato, V.; Orefice, I.; Amato, A.; Fontanarosa, C.; Amoresano, A.; Cutignano, A.; Ianora, A.; Romano, G. Animal-like prostaglandins in marine microalgae. ISME J. 2017, 11, 1722–1726. [Google Scholar] [CrossRef]
- Di Dato, V.; Barbarinaldi, R.; Amato, A.; Di Costanzo, F.; Fontanarosa, C.; Perna, A.; Amoresano, A.; Esposito, F.; Cutignano, A.; Ianora, A.; et al. Variation in prostaglandin metabolism during growth of the diatom Thalassiosira rotula. Sci. Rep. 2020, 10, 5374. [Google Scholar] [CrossRef] [PubMed]
- Di Dato, V.; Di Costanzo, F.; Barbarinaldi, R.; Perna, A.; Ianora, A.; Romano, G. Unveiling the presence of biosynthetic pathways for bioactive compounds in the Thalassiosira rotula transcriptome. Sci. Rep. 2019, 9, 9893. [Google Scholar] [CrossRef] [PubMed]
- Vigor, C.; Oger, C.; Reversat, G.; Rocher, A.; Zhou, B.; Linares-Maurizi, A.; Guy, A.; Bultel-Poncé, V.; Galano, J.M.; Vercauteren, J.; et al. Isoprostanoid Profiling of Marine Microalgae. Biomolecules 2020, 10, 1073. [Google Scholar] [CrossRef] [PubMed]
- Lwin, S.; Brooks, J.; Jacobson, R.; Danks, L.; Lundberg, K.; Milne, G.; Morrow, J.; Edwards, J.R. Isoprostane levels are altered in rheumatoid arthritis and suppress NFκB activity to inhibit osteoclast formation. Bone 2011, 48, S136. [Google Scholar] [CrossRef]
- de Los Reyes, C.; Ávila-Román, J.; Ortega, M.J.; de la Jara, A.; García-Mauriño, S.; Motilva, V.; Zubía, E. Oxylipins from the microalgae Chlamydomonas debaryana and Nannochloropsis gaditana and their activity as TNF-α inhibitors. Phytochemistry 2014, 102, 152–161. [Google Scholar] [CrossRef]
- Ávila-Román, J.; Talero, E.; Rodríguez-Luna, A.; García-Mauriño, S.; Motilva, V. Anti-inflammatory effects of an oxylipin-containing lyophilised biomass from a microalga in a murine recurrent colitis model. Br. J. Nutr. 2016, 116, 2044–2052. [Google Scholar] [CrossRef]
- Ávila-Román, J.; Talero, E.; de Los Reyes, C.; García-Mauriño, S.; Motilva, V. Microalgae-derived oxylipins decrease inflammatory mediators by regulating the subcellular location of NFκB and PPAR-γ. Pharmacol. Res. 2018, 128, 220–230. [Google Scholar] [CrossRef]
- Guiry, M.D. How Many Species of Algae are There? J. Phycol. 2012, 48, 1057–1063. [Google Scholar] [CrossRef]
- Serive, B.; Nicolau, E.; Bérard, J.-B.; Kaas, R.; Pasquet, V.; Picot, L.; Cadoret, J.-P. Community analysis of pigment patterns from 37 microalgae strains reveals new carotenoids and porphyrins characteristic of distinct strains and taxonomic groups. PLoS ONE 2017, 12, e0171872. [Google Scholar] [CrossRef]
- Saeed, M.U.; Hussain, N.; Shahbaz, A.; Hameed, T.; Iqbal, H.M.N.; Bilal, M. Bioprospecting microalgae and cyanobacteria for biopharmaceutical applications. J. Basic Microbiol. 2021, 62, 1110–1124. [Google Scholar] [CrossRef]
- Vincent, F.; Ibarbalz, F.M.; Bowler, C. Chapter 15—Global marine phytoplankton revealed by the Tara Oceans expedition. In Advances in Phytoplankton Ecology; Clementson, L.A., Eriksen, R.S., Willis, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 531–561. [Google Scholar]
- Wolf, J.; Ross, I.L.; Radzun, K.A.; Jakob, G.; Stephens, E.; Hankamer, B. High-throughput screen for high performance microalgae strain selection and integrated media design. Algal Res. 2015, 11, 313–325. [Google Scholar] [CrossRef]
- Lafarga, T.; Sánchez-Zurano, A.; Morillas-España, A.; Acién-Fernández, F.G. Extremophile microalgae as feedstock for high-value carotenoids: A review. Int. J. Food Sci. Technol. 2021, 56, 4934–4941. [Google Scholar] [CrossRef]
- Montuori, E.; Saggiomo, M.; Lauritano, C. Microalgae from Cold Environments and Their Possible Biotechnological Applications. Mar. Drugs 2023, 21, 292. [Google Scholar] [CrossRef] [PubMed]
- Steinrücken, P.; Erga, S.R.; Mjøs, S.A.; Kleivdal, H.; Prestegard, S.K. Bioprospecting North Atlantic microalgae with fast growth and high polyunsaturated fatty acid (PUFA) content for microalgae-based technologies. Algal Res. 2017, 26, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Suh, S.-S.; Hong, J.-M.; Kim, E.J.; Jung, S.W.; Chae, H.; Kim, J.E.; Kim, J.H.; Kim, I.-C.; Kim, S. Antarctic freshwater microalga, Chloromonas reticulata, suppresses inflammation and carcinogenesis. Int. J. Med. Sci. 2019, 16, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Chae, H.J.; Seo, J.B.; Kim, S.H.; Youn, E.J.; Kim, S.; Suh, S.S. Antarctic Freshwater Microalga, Micractinium simplicissimum, Suppresses Inflammation. J. Nanosci. Nanotechnol. 2021, 21, 4098–4103. [Google Scholar] [CrossRef]
- Villegas-Valencia, M.; González-Portela, R.E.; de Freitas, B.B.; Al Jahdali, A.; Romero-Villegas, G.I.; Malibari, R.; Kapoore, R.V.; Fuentes-Grünewald, C.; Lauersen, K.J. Cultivation of the polyextremophile Cyanidioschyzon merolae 10D during summer conditions on the coast of the Red Sea and its adaptation to hypersaline sea water. Front. Microbiol. 2023, 14, 1157151. [Google Scholar] [CrossRef]
- Schüler, L.M.; Gangadhar, K.N.; Duarte, P.; Placines, C.; Molina-Márquez, A.M.; Léon-Bañares, R.; Sousa, V.S.; Varela, J.; Barreira, L. Improvement of carotenoid extraction from a recently isolated, robust microalga, Tetraselmis sp. CTP4 (chlorophyta). Bioprocess Biosyst. Eng. 2020, 43, 785–796. [Google Scholar] [CrossRef]
- Silva, M.; Kamberovic, F.; Uota, S.T.; Kovan, I.-M.; Viegas, C.S.B.; Simes, D.C.; Gangadhar, K.N.; Varela, J.; Barreira, L. Microalgae as Potential Sources of Bioactive Compounds for Functional Foods and Pharmaceuticals. Appl. Sci. 2022, 12, 5877. [Google Scholar] [CrossRef]
- López, G.; Yate, C.; Ramos, F.A.; Cala, M.P.; Restrepo, S.; Baena, S. Production of Polyunsaturated Fatty Acids and Lipids from Autotrophic, Mixotrophic and Heterotrophic cultivation of Galdieria sp. strain USBA-GBX-832. Sci. Rep. 2019, 9, 10791. [Google Scholar] [CrossRef]
- Miyata, M.; Iwata, S.; Mifude, C.K.; Tajima, M.; Kameyama, M.; Ihara, M.; Matsui, T.; Yamagishi, S.I.; Ishitobi, H.; Miyaki, S.; et al. A Novel Mucidosphaerium sp. Downregulates Inflammatory Gene Expression in Skin and Articular Cells. Altern. Ther. Health Med. 2021, 27, 28–34. [Google Scholar]
- Kaseda, K.; Mifude, C.K.; Ishitobi, H.; Miyaki, S. Mitochondrial Regulation in the Pathogenic Process of Inflammatory Arthritis by Microalgal Mucidosphaerium Species. Of 2017, 6, 17–19. [Google Scholar]
- Ren, X.; Liu, Y.; Fan, C.; Hong, H.; Wu, W.; Zhang, W.; Wang, Y. Production, Processing, and Protection of Microalgal n-3 PUFA-Rich Oil. Foods 2022, 11, 1215. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Sun, H.; Deng, J.; Huang, J.; Chen, F. Carotenoid Production from Microalgae: Biosynthesis, Salinity Responses and Novel Biotechnologies. Mar. Drugs 2021, 19, 713. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.-Q.; Wang, L.-R.; Zhang, Z.-X.; Sun, X.-M.; Huang, H. Stresses as First-Line Tools for Enhancing Lipid and Carotenoid Production in Microalgae. Front. Bioeng. Biotechnol. 2020, 8, 610. [Google Scholar] [CrossRef]
- Sui, Y.; Muys, M.; Van de Waal, D.B.; D’Adamo, S.; Vermeir, P.; Fernandes, T.V.; Vlaeminck, S.E. Enhancement of co-production of nutritional protein and carotenoids in Dunaliella salina using a two-phase cultivation assisted by nitrogen level and light intensity. Bioresour. Technol. 2019, 287, 121398. [Google Scholar] [CrossRef]
- Yin, F.-W.; Zhang, Y.-T.; Jiang, J.-Y.; Guo, D.-S.; Gao, S.; Gao, Z. Efficient docosahexaenoic acid production by Schizochytrium sp. via a two-phase pH control strategy using ammonia and citric acid as pH regulators. Process Biochem. 2019, 77, 1–7. [Google Scholar] [CrossRef]
- Montero-Lobato, Z.; Vázquez, M.; Navarro, F.; Fuentes, J.L.; Bermejo, E.; Garbayo, I.; Vílchez, C.; Cuaresma, M. Chemically-Induced Production of Anti-Inflammatory Molecules in Microalgae. Mar. Drugs 2018, 16, 478. [Google Scholar] [CrossRef]
- Dawidziuk, A.; Popiel, D.; Luboinska, M.; Grzebyk, M.; Wisniewski, M.; Koczyk, G. Assessing contamination of microalgal astaxanthin producer Haematococcus cultures with high-resolution melting curve analysis. J. Appl. Genet. 2017, 58, 277–285. [Google Scholar] [CrossRef]
- Jannel, S.; Caro, Y.; Bermudes, M.; Petit, T. Novel Insights into the Biotechnological Production of Haematococcus pluvialis-Derived Astaxanthin: Advances and Key Challenges to Allow Its Industrial Use as Novel Food Ingredient. J. Mar. Sci. Eng. 2020, 8, 789. [Google Scholar] [CrossRef]
- Arora, N.; Philippidis, G.P. Microalgae strain improvement strategies: Random mutagenesis and adaptive laboratory evolution. Trends Plant Sci. 2021, 26, 1199–1200. [Google Scholar] [CrossRef]
- Trovão, M.; Schüler, L.M.; Machado, A.; Bombo, G.; Navalho, S.; Barros, A.; Pereira, H.; Silva, J.; Freitas, F.; Varela, J. Random Mutagenesis as a Promising Tool for Microalgal Strain Improvement towards Industrial Production. Mar. Drugs 2022, 20, 440. [Google Scholar] [CrossRef] [PubMed]
- Varunraj, R.; Priyadharshini, U.; Vijay, K.; Balamurugan, S. Adaptive laboratory evolution empowers lipids and biomass overproduction in Chlorella vulgaris for environmental applications. Environ. Res. 2023, 238 Pt 1, 117125. [Google Scholar] [CrossRef] [PubMed]
- Guardini, Z.; Dall’Osto, L.; Barera, S.; Jaberi, M.; Cazzaniga, S.; Vitulo, N.; Bassi, R. High Carotenoid Mutants of Chlorella vulgaris Show Enhanced Biomass Yield under High Irradiance. Plants 2021, 10, 911. [Google Scholar] [CrossRef] [PubMed]
- McQuillan, J.L.; Cutolo, E.A.; Evans, C.; Pandhal, J. Proteomic characterization of a lutein-hyperaccumulating Chlamydomonas reinhardtii mutant reveals photoprotection-related factors as targets for increasing cellular carotenoid content. Biotechnol. Biofuels Bioprod. 2023, 16, 166. [Google Scholar] [CrossRef]
- Cecchin, M.; Cazzaniga, S.; Martini, F.; Paltrinieri, S.; Bossi, S.; Maffei, M.E.; Ballottari, M. Astaxanthin and eicosapentaenoic acid production by S4, a new mutant strain of Nannochloropsis gaditana. Microb. Cell Factories 2022, 21, 117. [Google Scholar] [CrossRef]
- Schüler, L.M.; Bombo, G.; Duarte, P.; Santos, T.F.; Maia, I.B.; Pinheiro, F.; Marques, J.; Jacinto, R.; Schulze, P.S.C.; Pereira, H.; et al. Carotenoid biosynthetic gene expression, pigment and n-3 fatty acid contents in carotenoid-rich Tetraselmis striata CTP4 strains under heat stress combined with high light. Bioresour. Technol. 2021, 337, 125385. [Google Scholar] [CrossRef]
- Sun, X.-M.; Ren, L.-J.; Bi, Z.-Q.; Ji, X.-J.; Zhao, Q.-Y.; Jiang, L.; Huang, H. Development of a cooperative two-factor adaptive-evolution method to enhance lipid production and prevent lipid peroxidation in Schizochytrium sp. Biotechnol. Biofuels 2018, 11, 65. [Google Scholar] [CrossRef]
- Ren, L.; Sun, X.; Zhang, L.; Huang, H.; Zhao, Q. Exergy analysis for docosahexaenoic acid production by fermentation and strain improvement by adaptive laboratory evolution for Schizochytrium sp. Bioresour. Technol. 2020, 298, 122562. [Google Scholar] [CrossRef]
- Sun, X.M.; Ren, L.J.; Ji, X.J.; Chen, S.L.; Guo, D.S.; Huang, H. Adaptive evolution of Schizochytrium sp. by continuous high oxygen stimulations to enhance docosahexaenoic acid synthesis. Bioresour. Technol. 2016, 211, 374–381. [Google Scholar] [CrossRef]
- Diao, J.; Song, X.; Cui, J.; Liu, L.; Shi, M.; Wang, F.; Zhang, W. Rewiring metabolic network by chemical modulator based laboratory evolution doubles lipid production in Crypthecodinium cohnii. Metab. Eng. 2019, 51, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Meireles, L.A.; Guedes, A.C.; Malcata, F.X. Increase of the yields of eicosapentaenoic and docosahexaenoic acids by the microalga Pavlova lutheri following random mutagenesis. Biotechnol. Bioeng. 2003, 81, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Jebali, A.; Sanchez, M.R.; Hanschen, E.R.; Starkenburg, S.R.; Corcoran, A.A. Trait drift in microalgae and applications for strain improvement. Biotechnol. Adv. 2022, 60, 108034. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.H.; Lee, D.J.; Chang, J.S. Lutein production from biomass: Marigold flowers versus microalgae. Bioresour. Technol. 2015, 184, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Rova, U.; Christakopoulos, P.; Matsakas, L. Microalgal lutein biosynthesis: Recent trends and challenges to enhance the lutein content in microalgal cell factories. Front. Mar. Sci. 2022, 9, 1015419. [Google Scholar] [CrossRef]
- Dufossé, L. Back to nature, microbial production of pigments and colorants for food use. Adv. Food Nutr. Res. 2022, 102, 93–122. [Google Scholar] [PubMed]
- Li, C.; Swofford, C.A.; Sinskey, A.J. Modular engineering for microbial production of carotenoids. Metab. Eng. Commun. 2020, 10, e00118. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Peng, H.; Yang, C.; Guo, W.; Wang, M.; Li, G.; Liu, D. Metabolic Engineering of Model Microorganisms for the Production of Xanthophyll. Microorganisms 2023, 11, 1252. [Google Scholar] [CrossRef]
- Seeger, J.; Wendisch, V.F.; Henke, N.A. Extraction and Purification of Highly Active Astaxanthin from Corynebacterium glutamicum Fermentation Broth. Mar. Drugs 2023, 21, 530. [Google Scholar] [CrossRef]
- Kato, Y.; Hasunuma, T. Metabolic Engineering for Carotenoid Production Using Eukaryotic Microalgae and Prokaryotic Cyanobacteria. In Carotenoids: Biosynthetic and Biofunctional Approaches; Misawa, N., Ed.; Springer Singapore: Singapore, 2021; pp. 121–135. [Google Scholar]
- Fachet, M.; Witte, C.; Flassig, R.J.; Rihko-Struckmann, L.K.; McKie-Krisberg, Z.; Polle, J.E.W.; Sundmacher, K. Reconstruction and analysis of a carbon-core metabolic network for Dunaliella salina. BMC Bioinform. 2020, 21, 1–9. [Google Scholar] [CrossRef]
- Narang, P.K.; Dey, J.; Mahapatra, S.R.; Roy, R.; Kushwaha, G.S.; Misra, N.; Suar, M.; Raina, V. Genome-based identification and comparative analysis of enzymes for carotenoid biosynthesis in microalgae. World J. Microbiol. Biotechnol. 2021, 38, 8. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Liu, J.; Ma, R.; Zou, Y.; Ho, S.-H.; Chen, J.; Xie, Y. Functional Characterization of Lycopene β- and ε-Cyclases from a Lutein-Enriched Green Microalga Chlorella sorokiniana FZU60. Mar. Drugs 2023, 21, 418. [Google Scholar] [CrossRef] [PubMed]
- Grigoriev, I.V.; Hayes, R.D.; Calhoun, S.; Kamel, B.; Wang, A.; Ahrendt, S.; Dusheyko, S.; Nikitin, R.; Mondo, S.J.; Salamov, A.; et al. PhycoCosm, a comparative algal genomics resource. Nucleic Acids Res. 2020, 49, D1004–D1011. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ye, Y.; Bai, F.; Liu, J. The oleaginous astaxanthin-producing alga Chromochloris zofingiensis: Potential from production to an emerging model for studying lipid metabolism and carotenogenesis. Biotechnol. Biofuels 2021, 14, 119. [Google Scholar] [CrossRef] [PubMed]
- Roth, M.S.; Cokus, S.J.; Gallaher, S.D.; Walter, A.; Lopez, D.; Erickson, E.; Endelman, B.; Westcott, D.; Larabell, C.A.; Merchant, S.S.; et al. Chromosome-level genome assembly and transcriptome of the green alga Chromochloris zofingiensis illuminates astaxanthin production. Proc. Natl. Acad. Sci. USA 2017, 114, E4296–E4305. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Huang, J.C. Defining the biosynthesis of ketocarotenoids in Chromochloris zofingiensis. Plant Divers. 2020, 42, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kim, J.; Lee, S.; Khanh, N.; Li, Z.; Polle, J.E.W.; Jin, E. Deciphering the β-carotene hyperaccumulation in Dunaliella by the comprehensive analysis of Dunaliella salina and Dunaliella tertiolecta under high light conditions. Plant Cell Environ. 2023, 47, 213–229. [Google Scholar] [CrossRef] [PubMed]
- Yeh, T.-J.; Tseng, Y.-F.; Chen, Y.-C.; Hsiao, Y.; Lee, P.-C.; Chen, T.-J.; Chen, C.-Y.; Kao, C.-Y.; Chang, J.-S.; Chen, J.-C.; et al. Transcriptome and physiological analysis of a lutein-producing alga Desmodesmus sp. reveals the molecular mechanisms for high lutein productivity. Algal Res. 2017, 21, 103–119. [Google Scholar] [CrossRef]
- Velmurugan, A.; Kodiveri Muthukaliannan, G. Genetic manipulation for carotenoid production in microalgae an overview. Curr. Res. Biotechnol. 2022, 4, 221–228. [Google Scholar] [CrossRef]
- Yazdani, M.; Croen, M.G.; Fish, T.L.; Thannhauser, T.W.; Ahner, B.A. Overexpression of native ORANGE (OR) and OR mutant protein in Chlamydomonas reinhardtii enhances carotenoid and ABA accumulation and increases resistance to abiotic stress. Metab. Eng. 2021, 68, 94–105. [Google Scholar] [CrossRef]
- Kumari, S.; Vira, C.; Lali, A.M.; Prakash, G. Heterologous expression of a mutant Orange gene from Brassica oleracea increases carotenoids and induces phenotypic changes in the microalga Chlamydomonas reinhardtii. Algal Res. 2020, 47, 101871. [Google Scholar] [CrossRef]
- Pivato, M.; Perozeni, F.; Licausi, F.; Cazzaniga, S.; Ballottari, M. Heterologous expression of cyanobacterial Orange Carotenoid Protein (OCP2) as a soluble carrier of ketocarotenoids in Chlamydomonas reinhardtii. Algal Res. 2021, 55, 102255. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.T.; Kaldenhoff, R. Metabolic engineering of ketocarotenoids biosynthetic pathway in Chlamydomonas reinhardtii strain CC-4102. Sci. Rep. 2020, 10, 10688. [Google Scholar] [CrossRef] [PubMed]
- Perozeni, F.; Cazzaniga, S.; Baier, T.; Zanoni, F.; Zoccatelli, G.; Lauersen, K.J.; Wobbe, L.; Ballottari, M. Turning a green alga red: Engineering astaxanthin biosynthesis by intragenic pseudogene revival in Chlamydomonas reinhardtii. Plant Biotechnol. J. 2020, 18, 2053–2067. [Google Scholar] [CrossRef] [PubMed]
- Amendola, S.; Kneip, J.S.; Meyer, F.; Perozeni, F.; Cazzaniga, S.; Lauersen, K.J.; Ballottari, M.; Baier, T. Metabolic Engineering for Efficient Ketocarotenoid Accumulation in the Green Microalga Chlamydomonas reinhardtii. ACS Synth. Biol. 2023, 12, 820–831. [Google Scholar] [CrossRef] [PubMed]
- Hao, T.B.; Lu, Y.; Zhang, Z.H.; Liu, S.F.; Wang, X.; Yang, W.D.; Balamurugan, S.; Li, H.Y. Hyperaccumulation of fucoxanthin by enhancing methylerythritol phosphate pathway in Phaeodactylum tricornutum. Appl. Microbiol. Biotechnol. 2021, 105, 8783–8793. [Google Scholar]
- Cen, S.-Y.; Li, D.-W.; Huang, X.-L.; Huang, D.; Balamurugan, S.; Liu, W.-J.; Zheng, J.-W.; Yang, W.-D.; Li, H.-Y. Crucial carotenogenic genes elevate hyperaccumulation of both fucoxanthin and β-carotene in Phaeodactylum tricornutum. Algal Res. 2022, 64, 102691. [Google Scholar] [CrossRef]
- Jiang, E.-Y.; Fan, Y.; Phung, N.-V.; Xia, W.-Y.; Hu, G.-R.; Li, F.-L. Overexpression of plastid lipid-associated protein in marine diatom enhances the xanthophyll synthesis and storage. Front. Microbiol. 2023, 14, 1143017. [Google Scholar] [CrossRef]
- Seger, M.; Mammadova, F.; Villegas-Valencia, M.; Bastos de Freitas, B.; Chang, C.; Isachsen, I.; Hemstreet, H.; Abualsaud, F.; Boring, M.; Lammers, P.J.; et al. Engineered ketocarotenoid biosynthesis in the polyextremophilic red microalga Cyanidioschyzon merolae 10D. Metab. Eng. Commun. 2023, 17, e00226. [Google Scholar] [CrossRef]
- Sueishi, Y.; Ishikawa, M.; Yoshioka, D.; Endoh, N.; Oowada, S.; Shimmei, M.; Fujii, H.; Kotake, Y. Oxygen radical absorbance capacity (ORAC) of cyclodextrin-solubilized flavonoids, resveratrol and astaxanthin as measured with the ORAC-EPR method. J. Clin. Biochem. Nutr. 2012, 50, 127–132. [Google Scholar] [CrossRef]
- Larrea-Alvarez, M.; Purton, S. Multigenic engineering of the chloroplast genome in the green alga Chlamydomonas reinhardtii. Microbiology 2020, 166, 510. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, M.; Takaichi, S.; Steiger, S.; Wang, Z.-Y.; Sandmann, G. Novel hydroxycarotenoids with improved antioxidative properties produced by gene combination in Escherichia coli. Nat. Biotechnol. 2000, 18, 843–846. [Google Scholar] [CrossRef] [PubMed]
- Umeno, D.; Arnold, F.H. Evolution of a pathway to novel long-chain carotenoids. J. Bacteriol. 2004, 186, 1531–1536. [Google Scholar] [CrossRef] [PubMed]
- Jehlička, J.; Edwards, H.G.; Oren, A. Bacterioruberin and salinixanthin carotenoids of extremely halophilic Archaea and Bacteria: A Raman spectroscopic study. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 106, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Mandelli, F.; Miranda, V.S.; Rodrigues, E.; Mercadante, A.Z. Identification of carotenoids with high antioxidant capacity produced by extremophile microorganisms. World J. Microbiol. Biotechnol. 2012, 28, 1781–1790. [Google Scholar] [CrossRef] [PubMed]
- Grivard, A.; Goubet, I.; Duarte Filho, L.M.S.; Thiéry, V.; Chevalier, S.; de Oliveira-Junior, R.G.; El Aouad, N.; Guedes da Silva Almeida, J.R.; Sitarek, P.; Quintans-Junior, L.J.; et al. Archaea Carotenoids: Natural Pigments with Unexplored Innovative Potential. Mar. Drugs 2022, 20, 524. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yatsunami, R.; Ando, A.; Miyoko, N.; Fukui, T.; Takaichi, S.; Nakamura, S. Complete biosynthetic pathway of the C50 carotenoid bacterioruberin from lycopene in the extremely halophilic archaeon Haloarcula japonica. J. Bacteriol. 2015, 197, 1614–1623. [Google Scholar] [CrossRef]
- Heider, S.A.E.; Peters-Wendisch, P.; Wendisch, V.F.; Beekwilder, J.; Brautaset, T. Metabolic engineering for the microbial production of carotenoids and related products with a focus on the rare C50 carotenoids. Appl. Microbiol. Biotechnol. 2014, 98, 4355–4368. [Google Scholar] [CrossRef]
- Furubayashi, M.; Ikezumi, M.; Takaichi, S.; Maoka, T.; Hemmi, H.; Ogawa, T.; Saito, K.; Tobias, A.V.; Umeno, D. A highly selective biosynthetic pathway to non-natural C50 carotenoids assembled from moderately selective enzymes. Nat. Commun. 2015, 6, 7534. [Google Scholar] [CrossRef]
- Li, L.; Furubayashi, M.; Wang, S.; Maoka, T.; Kawai-Noma, S.; Saito, K.; Umeno, D. Genetically engineered biosynthetic pathways for nonnatural C60 carotenoids using C5-elongases and C50-cyclases in Escherichia coli. Sci. Rep. 2019, 9, 2982. [Google Scholar] [CrossRef]
- Jakhwal, P.; Kumar Biswas, J.; Tiwari, A.; Kwon, E.E.; Bhatnagar, A. Genetic and non-genetic tailoring of microalgae for the enhanced production of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA)—A review. Bioresour. Technol. 2022, 344, 126250. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, C.F.; Südfeld, C.; Naduthodi, M.I.S.; Weusthuis, R.A.; Barbosa, M.J.; Wijffels, R.H.; D’Adamo, S. Genetic engineering of microalgae for enhanced lipid production. Biotechnol. Adv. 2021, 52, 107836. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Xie, X.; Meesapyodsuk, D. Molecular mechanisms for biosynthesis and assembly of nutritionally important very long chain polyunsaturated fatty acids in microorganisms. Progress Lipid Res. 2020, 79, 101047. [Google Scholar] [CrossRef] [PubMed]
- Hulatt, C.J.; Wijffels, R.H.; Posewitz, M.C. The Genome of the Haptophyte Diacronema lutheri (Pavlova lutheri, Pavlovales): A Model for Lipid Biosynthesis in Eukaryotic Algae. Genome Biol. Evol. 2021, 13, evab178. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.-R.; Robert, S.S.; Petrie, J.R.; Frampton, D.M.F.; Mansour, M.P.; Blackburn, S.I.; Nichols, P.D.; Green, A.G.; Singh, S.P. Isolation and characterization of genes from the marine microalga Pavlova salina encoding three front-end desaturases involved in docosahexaenoic acid biosynthesis. Phytochemistry 2007, 68, 785–796. [Google Scholar] [CrossRef] [PubMed]
- Vaezi, R.; Napier, J.A.; Sayanova, O. Identification and Functional Characterization of Genes Encoding Omega-3 Polyunsaturated Fatty Acid Biosynthetic Activities from Unicellular Microalgae. Mar. Drugs 2013, 11, 5116–5129. [Google Scholar] [CrossRef]
- Heggeset, T.M.B.; Ertesvåg, H.; Liu, B.; Ellingsen, T.E.; Vadstein, O.; Aasen, I.M. Lipid and DHA-production in Aurantiochytrium sp.—Responses to nitrogen starvation and oxygen limitation revealed by analyses of production kinetics and global transcriptomes. Sci. Rep. 2019, 9, 19470. [Google Scholar] [CrossRef]
- Leyland, B.; Novichkova, E.; Dolui, A.K.; Jallet, D.; Daboussi, F.; Legeret, B.; Li, Z.; Li-Beisson, Y.; Boussiba, S.; Khozin-Goldberg, I. Acyl-CoA binding protein is required for lipid droplet degradation in the diatom Phaeodactylum tricornutum. Plant Physiol. 2023. [Google Scholar] [CrossRef]
- Muñoz, C.F.; Weusthuis, R.A.; D’Adamo, S.; Wijffels, R.H. Effect of Single and Combined Expression of Lysophosphatidic Acid Acyltransferase, Glycerol-3-Phosphate Acyltransferase, and Diacylglycerol Acyltransferase on Lipid Accumulation and Composition in Neochloris oleoabundans. Front. Plant Sci. 2019, 10, 1573. [Google Scholar] [CrossRef]
- Zhu, B.-H.; Tu, C.-C.; Shi, H.-P.; Yang, G.-P.; Pan, K.-H. Overexpression of endogenous delta-6 fatty acid desaturase gene enhances eicosapentaenoic acid accumulation in Phaeodactylum tricornutum. Process Biochem. 2017, 57, 43–49. [Google Scholar] [CrossRef]
- Hamilton, M.L.; Haslam, R.P.; Napier, J.A.; Sayanova, O. Metabolic engineering of Phaeodactylum tricornutum for the enhanced accumulation of omega-3 long chain polyunsaturated fatty acids. Metab. Eng. 2014, 22, 3–9. [Google Scholar] [CrossRef]
- Cui, G.-Z.; Ma, Z.; Liu, Y.-J.; Feng, Y.; Sun, Z.; Cheng, Y.; Song, X.; Cui, Q. Overexpression of glucose-6-phosphate dehydrogenase enhanced the polyunsaturated fatty acid composition of Aurantiochytrium sp. SD116. Algal Res. 2016, 19, 138–145. [Google Scholar] [CrossRef]
- Han, X.; Zhao, Z.; Wen, Y.; Chen, Z. Enhancement of docosahexaenoic acid production by overexpression of ATP-citrate lyase and acetyl-CoA carboxylase in Schizochytrium sp. Biotechnol. Biofuels 2020, 13, 131. [Google Scholar] [CrossRef] [PubMed]
- Celente, G.d.S.; Rizzetti, T.M.; Sui, Y.; Schneider, R.d.C.d.S. Potential use of microalga Dunaliella salina for bioproducts with industrial relevance. Biomass Bioenergy 2022, 167, 106647. [Google Scholar]
- Shi, H.; Luo, X.; Wu, R.; Yue, X. Production of eicosapentaenoic acid by application of a delta-6 desaturase with the highest ALA catalytic activity in algae. Microb. Cell Factories 2018, 17, 7. [Google Scholar] [CrossRef]
- Sproles, A.E.; Fields, F.J.; Smalley, T.N.; Le, C.H.; Badary, A.; Mayfield, S.P. Recent advancements in the genetic engineering of microalgae. Algal Res. 2021, 53, 102158. [Google Scholar] [CrossRef]
- Banerjee, A.; Ward, V. Production of recombinant and therapeutic proteins in microalgae. Curr. Opin. Biotechnol. 2022, 78, 102784. [Google Scholar] [CrossRef] [PubMed]
- Arias, C.A.D.; Oliveira, C.F.M.d.; Molino, J.V.D.; Ferreira-Camargo, L.S.; Matsudo, M.C.; Carvalho, J.C.M.d. Production of Recombinant Biopharmaceuticals in Chlamydomonas reinhardtii. Int. J. Plant Biol. 2023, 14, 39–52. [Google Scholar] [CrossRef]
- El-Ayouty, Y.; El-Manawy, I.; Nasih, S.; Hamdy, E.; Kebeish, R. Engineering Chlamydomonas reinhardtii for Expression of Functionally Active Human Interferon-α. Mol. Biotechnol. 2019, 61, 134–144. [Google Scholar] [CrossRef]
- Torres-Tiji, Y.; Fields, F.J.; Yang, Y.; Heredia, V.; Horn, S.J.; Keremane, S.R.; Jin, M.M.; Mayfield, S.P. Optimized production of a bioactive human recombinant protein from the microalgae Chlamydomonas reinhardtii grown at high density in a fed-batch bioreactor. Algal Res. 2022, 66, 102786. [Google Scholar] [CrossRef]
- Dehghani, J.; Adibkia, K.; Movafeghi, A.; Pourseif, M.M.; Omidi, Y. Designing a new generation of expression toolkits for engineering of green microalgae; robust production of human interleukin-2. Bioimpacts 2020, 10, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Smyth, D.J.; Ren, B.; White, M.P.J.; McManus, C.; Webster, H.; Shek, V.; Evans, C.; Pandhal, J.; Fields, F.; Maizels, R.M.; et al. Oral delivery of a functional algal-expressed TGF-β mimic halts colitis in a murine DSS model. J. Biotechnol. 2021, 340, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mathieu-Rivet, E.; Lerouge, P.; Bardor, M. Chlamydomonas reinhardtii: Protein Glycosylation and Production of Biopharmaceuticals. In Chlamydomonas: Biotechnology and Biomedicine; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Van Landuyt, L.; Lonigro, C.; Meuris, L.; Callewaert, N. Customized protein glycosylation to improve biopharmaceutical function and targeting. Curr. Opin. Biotechnol. 2019, 60, 17–28. [Google Scholar] [CrossRef]
- Ramos-Martinez, E.M.; Fimognari, L.; Sakuragi, Y. High-yield secretion of recombinant proteins from the microalga Chlamydomonas reinhardtii. Plant Biotechnol. J. 2017, 15, 1214–1224. [Google Scholar] [CrossRef] [PubMed]
- Sproles, A.E.; Berndt, A.; Fields, F.J.; Mayfield, S.P. Improved high-throughput screening technique to rapidly isolate Chlamydomonas transformants expressing recombinant proteins. Appl. Microbiol. Biotechnol. 2022, 106, 1677–1689. [Google Scholar] [CrossRef] [PubMed]
- Perozeni, F.; Baier, T. Current Nuclear Engineering Strategies in the Green Microalga Chlamydomonas reinhardtii. Life 2023, 13, 1566. [Google Scholar] [CrossRef] [PubMed]
- McQuillan, J.L.; Berndt, A.J.; Sproles, A.E.; Mayfield, S.P.; Pandhal, J. Novel cis-regulatory elements as synthetic promoters to drive recombinant protein expression from the Chlamydomonas reinhardtii nuclear genome. New Biotechnol. 2022, 68, 9–18. [Google Scholar] [CrossRef]
- LaManna, L.; Chou, C.-H.; Lei, H.; Barton, E.R.; Maliga, P. Chloroplast transformation for bioencapsulation and oral delivery using the immunoglobulin G fragment crystallizable (Fc) domain. Sci. Rep. 2023, 13, 18916. [Google Scholar] [CrossRef]
- Carrera-Pacheco, S.E.; Hankamer, B.; Oey, M. Environmental and nuclear influences on microalgal chloroplast gene expression. Trends Plant Sci. 2023, 28, 955–967. [Google Scholar] [CrossRef]
- Dyo, Y.M.; Purton, S. The algal chloroplast as a synthetic biology platform for production of therapeutic proteins. Microbiology 2018, 164, 113–121. [Google Scholar] [CrossRef]
- Akram, M.; Khan, M.A.; Ahmed, N.; Bhatti, R.; Pervaiz, R.; Malik, K.; Tahir, S.; Abbas, R.; Ashraf, F.; Ali, Q. Cloning and expression of an anti-cancerous cytokine: Human IL-29 gene in Chlamydomonas reinhardtii. AMB Express 2023, 13, 23. [Google Scholar] [CrossRef] [PubMed]
- Taunt, H.N.; Stoffels, L.; Purton, S. Green biologics: The algal chloroplast as a platform for making biopharmaceuticals. Bioengineered 2018, 9, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Yeon, J.; Miller, S.M.; Dejtisakdi, W. New Synthetic Operon Vectors for Expressing Multiple Proteins in the Chlamydomonas reinhardtii Chloroplast. Genes 2023, 14, 368. [Google Scholar] [CrossRef] [PubMed]
- Cutolo, E.A.; Mandalà, G.; Dall’Osto, L.; Bassi, R. Harnessing the Algal Chloroplast for Heterologous Protein Production. Microorganisms 2022, 10, 743. [Google Scholar] [CrossRef] [PubMed]
- Taunt, H.N.; Jackson, H.O.; Gunnarsson, Í.N.; Pervaiz, R.; Purton, S. Accelerating Chloroplast Engineering: A New System for Rapid Generation of Marker-Free Transplastomic Lines of Chlamydomonas reinhardtii. Microorganisms 2023, 11, 1967. [Google Scholar] [CrossRef]
- Cutolo, E.; Tosoni, M.; Barera, S.; Herrera-Estrella, L.; Dall’Osto, L.; Bassi, R. A Phosphite Dehydrogenase Variant with Promiscuous Access to Nicotinamide Cofactor Pools Sustains Fast Phosphite-Dependent Growth of Transplastomic Chlamydomonas reinhardtii. Plants 2020, 9, 473. [Google Scholar] [CrossRef]
- Dahlin, L.R.; Guarnieri, M.T. Heterologous expression of phosphite dehydrogenase in the chloroplast or nucleus enables phosphite utilization and genetic selection in Picochlorum spp. Algal Res. 2022, 62, 102604. [Google Scholar] [CrossRef]
- Cutolo, E.; Tosoni, M.; Barera, S.; Herrera-Estrella, L.; Dall’Osto, L.; Bassi, R. A chimeric hydrolase-PTXD transgene enables chloroplast-based heterologous protein expression and non-sterile cultivation of Chlamydomonas reinhardtii. Algal Res. 2021, 59, 102429. [Google Scholar] [CrossRef]
- Changko, S.; Rajakumar, P.D.; Young, R.E.B.; Purton, S. The phosphite oxidoreductase gene, ptxD as a bio-contained chloroplast marker and crop-protection tool for algal biotechnology using Chlamydomonas. Appl. Microbiol. Biotechnol. 2020, 104, 675–686. [Google Scholar] [CrossRef]
- Liaqat, F.; Khazi, M.I.; Bahadar, A.; He, L.; Aslam, A.; Liaquat, R.; Agathos, S.N.; Li, J. Mixotrophic cultivation of microalgae for carotenoid production. Rev. Aquac. 2023, 15, 35–61. [Google Scholar] [CrossRef]
- Zuercher, A.W.; Spirig, R.; Baz Morelli, A.; Rowe, T.; Käsermann, F. Next-generation Fc receptor–targeting biologics for autoimmune diseases. Autoimmun. Rev. 2019, 18, 102366. [Google Scholar] [CrossRef] [PubMed]
- Vanier, G.; Stelter, S.; Vanier, J.; Hempel, F.; Maier, U.G.; Lerouge, P.; Ma, J.; Bardor, M. Alga-Made Anti-Hepatitis B Antibody Binds to Human Fcγ Receptors. Biotechnol. J. 2018, 13, 1700496. [Google Scholar] [CrossRef] [PubMed]
- Hempel, F.; Maurer, M.; Brockmann, B.; Mayer, C.; Biedenkopf, N.; Kelterbaum, A.; Becker, S.; Maier, U.G. From hybridomas to a robust microalgal-based production platform: Molecular design of a diatom secreting monoclonal antibodies directed against the Marburg virus nucleoprotein. Microb. Cell Factories 2017, 16, 131. [Google Scholar] [CrossRef] [PubMed]
- Vanier, G.; Hempel, F.; Chan, P.; Rodamer, M.; Vaudry, D.; Maier, U.G.; Lerouge, P.; Bardor, M. Biochemical Characterization of Human Anti-Hepatitis B Monoclonal Antibody Produced in the Microalgae Phaeodactylum tricornutum. PLoS ONE 2015, 10, e0139282. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.H.; Kim, K.; Choi, C.-I. Pharmacogenomics of Monoclonal Antibodies for the Treatment of Rheumatoid Arthritis. J. Pers. Med. 2022, 12, 1265. [Google Scholar] [CrossRef]
- Gallaher, S.D.; Craig, R.J.; Ganesan, I.; Purvine, S.O.; McCorkle, S.R.; Grimwood, J.; Strenkert, D.; Davidi, L.; Roth, M.S.; Jeffers, T.L.; et al. Widespread polycistronic gene expression in green algae. Proc. Natl. Acad. Sci. USA 2021, 118, e2017714118. [Google Scholar] [CrossRef]
- Tinazzi, E.; Merlin, M.; Bason, C.; Beri, R.; Zampieri, R.; Lico, C.; Bartoloni, E.; Puccetti, A.; Lunardi, C.; Pezzotti, M.; et al. Plant-Derived Chimeric Virus Particles for the Diagnosis of Primary Sjögren Syndrome. Front. Plant Sci. 2015, 6, 1080. [Google Scholar] [CrossRef]
- Clarke, J. Harnessing plant viruses to treat autoimmune diseases. Nat. Rev. Rheumatol. 2020, 16, 352. [Google Scholar] [CrossRef]
- Zampieri, R.; Brozzetti, A.; Pericolini, E.; Bartoloni, E.; Gabrielli, E.; Roselletti, E.; Lomonosoff, G.; Meshcheriakova, Y.; Santi, L.; Imperatori, F.; et al. Prevention and treatment of autoimmune diseases with plant virus nanoparticles. Sci. Adv. 2020, 6, eaaz0295. [Google Scholar] [CrossRef]
- Malla, A.; Rosales-Mendoza, S.; Phoolcharoen, W.; Vimolmangkang, S. Efficient Transient Expression of Recombinant Proteins Using DNA Viral Vectors in Freshwater Microalgal Species. Front. Plant Sci. 2021, 12, 650820. [Google Scholar] [CrossRef]
- Bañuelos-Hernández, B.; Monreal-Escalante, E.; González-Ortega, O.; Angulo, C.; Rosales-Mendoza, S. Algevir: An Expression System for Microalgae Based on Viral Vectors. Front. Microbiol. 2017, 8, 1100. [Google Scholar] [CrossRef] [PubMed]
- Quispe, C.F.; Esmael, A.; Sonderman, O.; McQuinn, M.; Agarkova, I.; Battah, M.; Duncan, G.A.; Dunigan, D.D.; Smith, T.P.L.; De Castro, C.; et al. Characterization of a new chlorovirus type with permissive and non-permissive features on phylogenetically related algal strains. Virology 2017, 500, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Esmael, A.; Agarkova, I.V.; Dunigan, D.D.; Zhou, Y.; Van Etten, J.L. Viral DNA Accumulation Regulates Replication Efficiency of Chlorovirus OSy-NE5 in Two Closely Related Chlorella variabilis Strains. Viruses 2023, 15, 1341. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.R.; Hazzouri, K.M.; Lauersen, K.J.; Jaiswal, A.; Chaiboonchoe, A.; Mystikou, A.; Fu, W.; Daakour, S.; Dohai, B.; Alzahmi, A.; et al. Large-scale genome sequencing reveals the driving forces of viruses in microalgal evolution. Cell Host Microbe 2021, 29, 250–266. [Google Scholar] [CrossRef] [PubMed]
- Wilson, W.H.; Van Etten, J.L.; Allen, M.J. The Phycodnaviridae: The story of how tiny giants rule the world. Curr. Top. Microbiol. Immunol. 2009, 328, 1–42. [Google Scholar] [PubMed]
- D’Adamo, S.; Kormelink, R.; Martens, D.; Barbosa, M.J.; Wijffels, R.H. Prospects for viruses infecting eukaryotic microalgae in biotechnology. Biotechnol. Adv. 2021, 54, 107790. [Google Scholar] [CrossRef] [PubMed]
- Bolaños-Martínez, O.C.; Mahendran, G.; Rosales-Mendoza, S.; Vimolmangkang, S. Current Status and Perspective on the Use of Viral-Based Vectors in Eukaryotic Microalgae. Mar. Drugs 2022, 20, 434. [Google Scholar] [CrossRef]
- Fernández, F.G.A.; Reis, A.; Wijffels, R.H.; Barbosa, M.; Verdelho, V.; Llamas, B. The role of microalgae in the bioeconomy. New Biotechnol. 2021, 61, 99–107. [Google Scholar] [CrossRef]
- Fabris, M.; Abbriano, R.M.; Pernice, M.; Sutherland, D.L.; Commault, A.S.; Hall, C.C.; Labeeuw, L.; McCauley, J.I.; Kuzhiuparambil, U.; Ray, P.; et al. Emerging Technologies in Algal Biotechnology: Toward the Establishment of a Sustainable, Algae-Based Bioeconomy. Front. Plant Sci. 2020, 11, 279. [Google Scholar] [CrossRef]
- Rumin, J.; Nicolau, E.; Junior, R.G.O.; Fuentes-Grünewald, C.; Picot, L. Analysis of Scientific Research Driving Microalgae Market Opportunities in Europe. Mar. Drugs 2020, 18, 264. [Google Scholar] [CrossRef]
- Araújo, R.; Vázquez Calderón, F.; Sánchez López, J.; Azevedo, I.C.; Bruhn, A.; Fluch, S.; Garcia Tasende, M.; Ghaderiardakani, F.; Ilmjärv, T.; Laurans, M.; et al. Current Status of the Algae Production Industry in Europe: An Emerging Sector of the Blue Bioeconomy. Front. Mar. Sci. 2021, 7, 626389. [Google Scholar] [CrossRef]
- Kapoor, S.; Nailwal, N.; Kumar, M.; Barve, K. Recent Patents and Discovery of Anti-inflammatory Agents from Marine Source. Recent. Pat. Inflamm. Allergy Drug Discov. 2019, 13, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Tzima, S.; Georgiopoulou, I.; Louli, V.; Magoulas, K. Recent Advances in Supercritical CO2 Extraction of Pigments, Lipids and Bioactive Compounds from Microalgae. Molecules 2023, 28, 1410. [Google Scholar] [CrossRef] [PubMed]
- Karan, H.; Roles, J.; Hankamer, B.; Ross, I.L. Targeting greens and yellows: A solar biorefinery analysis for the microalgae-based co-production of pigments, proteins, and fuel. Algal Res. 2023, 74, 103187. [Google Scholar] [CrossRef]
- Kholany, M.; Coutinho, J.A.P.; Ventura, S.P.M. Carotenoid Production from Microalgae: The Portuguese Scenario. Molecules 2022, 27, 2540. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, M.; Saraiva, J.A.; Martins, A.P.; Pinto, C.A.; Prieto, M.A.; Simal-Gandara, J.; Cao, H.; Xiao, J.; Barba, F.J. Extraction of lipids from microalgae using classical and innovative approaches. Food Chem. 2022, 384, 132236. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Duan, C.; Yi, S.; Gao, Z.; Xiao, C.; Agathos, S.N.; Wang, G.; Li, J. Biotechnological production of astaxanthin from the microalga Haematococcus pluvialis. Biotechnol. Adv. 2020, 43, 107602. [Google Scholar] [CrossRef]
- Rumin, J.; Junior, R.G.d.O.; Bérard, J.-B.; Picot, L. Improving Microalgae Research and Marketing in the European Atlantic Area: Analysis of Major Gaps and Barriers Limiting Sector Development. Mar. Drugs 2021, 19, 319. [Google Scholar] [CrossRef]
- Mendes, M.C.; Navalho, S.; Ferreira, A.; Paulino, C.; Figueiredo, D.; Silva, D.; Gao, F.; Gama, F.; Bombo, G.; Jacinto, R.; et al. Algae as Food in Europe: An Overview of Species Diversity and Their Application. Foods 2022, 11, 1871. [Google Scholar] [CrossRef]
- Spicer, A.; Molnar, A. Gene Editing of Microalgae: Scientific Progress and Regulatory Challenges in Europe. Biology 2018, 7, 21. [Google Scholar] [CrossRef]
- Vilatte, A.; Spencer-Milnes, X.; Jackson, H.O.; Purton, S.; Parker, B. Spray Drying Is a Viable Technology for the Preservation of Recombinant Proteins in Microalgae. Microorganisms 2023, 11, 512. [Google Scholar] [CrossRef] [PubMed]
- Commission, E.; Centre, J.R.; Araújo, R.; Peteiro, C. Algae as Food and Food Supplements in Europe; Publications Office: Luxembourg, 2021.
- Rzymski, P.; Budzulak, J.; Niedzielski, P.; Klimaszyk, P.; Proch, J.; Kozak, L.; Poniedziałek, B. Essential and toxic elements in commercial microalgal food supplements. J. Appl. Phycol. 2019, 31, 3567–3579. [Google Scholar] [CrossRef]
- Muys, M.; Sui, Y.; Schwaiger, B.; Lesueur, C.; Vandenheuvel, D.; Vermeir, P.; Vlaeminck, S.E. High variability in nutritional value and safety of commercially available Chlorella and Spirulina biomass indicates the need for smart production strategies. Bioresour. Technol. 2019, 275, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Rzymski, P.; Niedzielski, P.; Kaczmarek, N.; Jurczak, T.; Klimaszyk, P. The multidisciplinary approach to safety and toxicity assessment of microalgae-based food supplements following clinical cases of poisoning. Harmful Algae 2015, 46, 34–42. [Google Scholar] [CrossRef]
- Ferreira de Oliveira, A.P.; Bragotto, A.P.A. Microalgae-based products: Food and public health. Future Foods 2022, 6, 100157. [Google Scholar] [CrossRef]
- Molino, A.; Iovine, A.; Casella, P.; Mehariya, S.; Chianese, S.; Cerbone, A.; Rimauro, J.; Musmarra, D. Microalgae Characterization for Consolidated and New Application in Human Food, Animal Feed and Nutraceuticals. Int. J. Environ. Res. Public. Health 2018, 15, 2436. [Google Scholar] [CrossRef]
- McInnes, R.S.; McCallum, G.E.; Lamberte, L.E.; van Schaik, W. Horizontal transfer of antibiotic resistance genes in the human gut microbiome. Curr. Opin. Microbiol. 2020, 53, 35–43. [Google Scholar] [CrossRef]
- Ljubic, A.; Jacobsen, C.; Holdt, S.L.; Jakobsen, J. Microalgae Nannochloropsis oceanica as a future new natural source of vitamin D(3). Food Chem. 2020, 320, 126627. [Google Scholar] [CrossRef]
- Ljubic, A.; Thulesen, E.T.; Jacobsen, C.; Jakobsen, J. UVB exposure stimulates production of vitamin D3 in selected microalgae. Algal Res. 2021, 59, 102472. [Google Scholar] [CrossRef]
- Mateen, S.; Moin, S.; Shahzad, S.; Khan, A.Q. Level of inflammatory cytokines in rheumatoid arthritis patients: Correlation with 25-hydroxy vitamin D and reactive oxygen species. PLoS ONE 2017, 12, e0178879. [Google Scholar] [CrossRef]
- Athanassiou, L.; Kostoglou-Athanassiou, I.; Koutsilieris, M.; Shoenfeld, Y. Vitamin D and Autoimmune Rheumatic Diseases. Biomolecules 2023, 13, 709. [Google Scholar] [CrossRef] [PubMed]
- Kühn, J.; Brandsch, C.; Kiourtzidis, M.; Nier, A.; Bieler, S.; Matthäus, B.; Griehl, C.; Stangl, G.I. Microalgae-derived sterols do not reduce the bioavailability of oral vitamin D(3) in mice. Int. J. Vitam. Nutr. Res. 2022. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jeon, Y.-J.; Kim, J.-I. In vitro and in vivo anti-inflammatory activities of a sterol-enriched fraction from freshwater green alga, Spirogyra sp. Fish. Aquat. Sci. 2020, 23, 27. [Google Scholar] [CrossRef]
- Fagundes, M.B.; Vendruscolo, R.G.; Wagner, R. Chapter 21—Sterols from microalgae. In Handbook of Microalgae-Based Processes and Products; Jacob-Lopes, E., Maroneze, M.M., Queiroz, M.I., Zepka, L.Q., Eds.; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar]
- Randhir, A.; Laird, D.W.; Maker, G.; Trengove, R.; Moheimani, N.R. Microalgae: A potential sustainable commercial source of sterols. Algal Res. 2020, 46, 101772. [Google Scholar] [CrossRef]
- Cárdeno, A.; Aparicio-Soto, M.; Montserrat-de la Paz, S.; Bermudez, B.; Muriana, F.J.G.; Alarcón-de-la-Lastra, C. Squalene targets pro- and anti-inflammatory mediators and pathways to modulate over-activation of neutrophils, monocytes and macrophages. J. Funct. Foods 2015, 14, 779–790. [Google Scholar] [CrossRef]
- Fan, K.W.; Aki, T.; Chen, F.; Jiang, Y. Enhanced production of squalene in the thraustochytrid Aurantiochytrium mangrovei by medium optimization and treatment with terbinafine. World J. Microbiol. Biotechnol. 2010, 26, 1303–1309. [Google Scholar] [CrossRef]
- Hong, W.K.; Heo, S.Y.; Park, H.M.; Kim, C.H.; Sohn, J.H.; Kondo, A.; Seo, J.W. Characterization of a squalene synthase from the thraustochytrid microalga Aurantiochytrium sp. KRS101. J. Microbiol. Biotechnol. 2013, 23, 759–765. [Google Scholar] [CrossRef]
- Nakazawa, A.; Matsuura, H.; Kose, R.; Kato, S.; Honda, D.; Inouye, I.; Kaya, K.; Watanabe, M.M. Optimization of culture conditions of the thraustochytrid Aurantiochytrium sp. strain 18W-13a for squalene production. Bioresour. Technol. 2012, 109, 287–291. [Google Scholar] [CrossRef]
- Okada, S.; Devarenne, T.P.; Chappell, J. Molecular Characterization of Squalene Synthase from the Green Microalga Botryococcus braunii, Race B. Arch. Biochem. Biophys. 2000, 373, 307–317. [Google Scholar] [CrossRef]
- Duan, R.; Pan, X.; Li, K.; Yang, Q.; Cui, X.; Zheng, Y.; Lu, Y.; Yao, C.; Ling, X. Metabolism balance regulation for squalene production by disturbing triglyceride (TAG) synthesis in Schizochytrium sp. Algal Res. 2023, 69, 102946. [Google Scholar] [CrossRef]
- EFSA Panel on Nutrition, Novel Foods; Food Allergens (NDA); Turck, D.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Kearney, J.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; et al. Safety of dried whole cell Euglena gracilis as a novel food pursuant to Regulation (EU) 2015/2283. EFSA J. 2020, 18, e06100. [Google Scholar] [PubMed]
- Yao, R.; Fu, W.; Du, M.; Chen, Z.-X.; Lei, A.-P.; Wang, J.-X. Carotenoids Biosynthesis, Accumulation, and Applications of a Model Microalga Euglenagracilis. Mar. Drugs 2022, 20, 496. [Google Scholar] [CrossRef] [PubMed]
- Piovan, A.; Filippini, R.; Corbioli, G.; Costa, V.D.; Giunco, E.M.V.; Burbello, G.; Pagetta, A.; Giusti, P.; Zusso, M. Carotenoid Extract Derived from Euglena gracilis Overcomes Lipopolysaccharide-Induced Neuroinflammation in Microglia: Role of NF-κB and Nrf2 Signaling Pathways. Mol. Neurobiol. 2021, 58, 3515–3528. [Google Scholar] [CrossRef] [PubMed]
- Brun, P.; Piovan, A.; Caniato, R.; Dalla Costa, V.; Pauletto, A.; Filippini, R. Anti-Inflammatory Activities of Euglena gracilis Extracts. Microorganisms 2021, 9, 2058. [Google Scholar] [CrossRef] [PubMed]
- Feuzing, F.; Mbakidi, J.P.; Marchal, L.; Bouquillon, S.; Leroy, E. A review of paramylon processing routes from microalga biomass to non-derivatized and chemically modified products. Carbohydr. Polym. 2022, 288, 119181. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Nakashima, A.; Igarashi, M.; Saito, K.; Konno, M.; Yamazaki, N.; Takimoto, H. Euglena gracilis Z and its carbohydrate storage substance relieve arthritis symptoms by modulating Th17 immunity. PLoS ONE 2018, 13, e0191462. [Google Scholar] [CrossRef] [PubMed]
- Dittmann, E.; Gugger, M.; Sivonen, K.; Fewer, D.P. Natural Product Biosynthetic Diversity and Comparative Genomics of the Cyanobacteria. Trends Microbiol. 2015, 23, 642–652. [Google Scholar] [CrossRef]
- Pagels, F.; Guedes, A.C.; Vasconcelos, V.; Lopes, G. Chapter 4—Anti-inflammatory compounds from cyanobacteria. In The Pharmacological Potential of Cyanobacteria; Lopes, G., Silva, M., Vasconcelos, V., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 81–105. [Google Scholar]
- Wu, Q.; Liu, L.; Miron, A.; Klímová, B.; Wan, D.; Kuča, K. The antioxidant, immunomodulatory, and anti-inflammatory activities of Spirulina: An overview. Arch. Toxicol. 2016, 90, 1817–1840. [Google Scholar] [CrossRef]
- Reddy, M.C.; Subhashini, J.; Mahipal, S.V.; Bhat, V.B.; Srinivas Reddy, P.; Kiranmai, G.; Madyastha, K.M.; Reddanna, P. C-Phycocyanin, a selective cyclooxygenase-2 inhibitor, induces apoptosis in lipopolysaccharide-stimulated RAW 264.7 macrophages. Biochem. Biophys. Res. Commun. 2003, 304, 385–392. [Google Scholar] [CrossRef]
- Jensen, G.S.; Attridge, V.L.; Beaman, J.L.; Guthrie, J.; Ehmann, A.; Benson, K.F. Antioxidant and anti-inflammatory properties of an aqueous cyanophyta extract derived from Arthrospira platensis: Contribution to bioactivities by the non-phycocyanin aqueous fraction. J. Med. Food 2015, 18, 535–541. [Google Scholar] [CrossRef]
- Ngatu, N.R.; Okajima, M.K.; Yokogawa, M.; Hirota, R.; Eitoku, M.; Muzembo, B.A.; Dumavibhat, N.; Takaishi, M.; Sano, S.; Kaneko, T.; et al. Anti-inflammatory effects of sacran, a novel polysaccharide from Aphanothece sacrum, on 2,4,6-trinitrochlorobenzene–induced allergic dermatitis in vivo. Ann. Allergy Asthma Immunol. 2012, 108, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Motoyama, K.; Tanida, Y.; Hata, K.; Hayashi, T.; Hashim, I.I.A.; Higashi, T.; Ishitsuka, Y.; Kondo, Y.; Irie, T.; Kaneko, S.; et al. Anti-inflammatory Effects of Novel Polysaccharide Sacran Extracted from Cyanobacterium Aphanothece sacrum in Various Inflammatory Animal Models. Biol. Pharm. Bull. 2016, 39, 1172–1178. [Google Scholar] [CrossRef] [PubMed]
- Zampieri, R.M.; Adessi, A.; Caldara, F.; Codato, A.; Furlan, M.; Rampazzo, C.; De Philippis, R.; La Rocca, N.; Dalla Valle, L. Anti-Inflammatory Activity of Exopolysaccharides from Phormidium sp. ETS05, the Most Abundant Cyanobacterium of the Therapeutic Euganean Thermal Muds, Using the Zebrafish Model. Biomolecules 2020, 10, 582. [Google Scholar] [CrossRef] [PubMed]
- Vo, T.-S.; Ryu, B.; Kim, S.-K. Purification of novel anti-inflammatory peptides from enzymatic hydrolysate of the edible microalgal Spirulina maxima. J. Funct. Foods 2013, 5, 1336–1346. [Google Scholar] [CrossRef]
- Kirk, R.D.; He, H.; Wahome, P.G.; Wu, S.; Carter, G.T.; Bertin, M.J. New Micropeptins with Anti-Neuroinflammatory Activity Isolated from a Cyanobacterial Bloom. ACS Omega 2021, 6, 15472–15478. [Google Scholar] [CrossRef]
- Kirk, R.D.; Picard, K.; Christian, J.A.; Johnson, S.L.; DeBoef, B.; Bertin, M.J. Unnarmicin D, an Anti-inflammatory Cyanobacterial Metabolite with δ and μ Opioid Binding Activity Discovered via a Pipeline Approach Designed to Target Neurotherapeutics. ACS Chem. Neurosci. 2020, 11, 4478–4488. [Google Scholar] [CrossRef] [PubMed]
- Santos-Merino, M.; Singh, A.K.; Ducat, D.C. New Applications of Synthetic Biology Tools for Cyanobacterial Metabolic Engineering. Front. Bioeng. Biotechnol. 2019, 7, 33. [Google Scholar] [CrossRef]
- Jester, B.W.; Zhao, H.; Gewe, M.; Adame, T.; Perruzza, L.; Bolick, D.T.; Agosti, J.; Khuong, N.; Kuestner, R.; Gamble, C.; et al. Development of spirulina for the manufacture and oral delivery of protein therapeutics. Nat. Biotechnol. 2022, 40, 956–964. [Google Scholar] [CrossRef]
- Betterle, N.; Hidalgo Martinez, D.; Melis, A. Cyanobacterial Production of Biopharmaceutical and Biotherapeutic Proteins. Front. Plant Sci. 2020, 11, 237. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).