Isolation and Characterization of a Serratia rubidaea from a Shallow Water Hydrothermal Vent
Abstract
1. Introduction
2. Results and Discussion
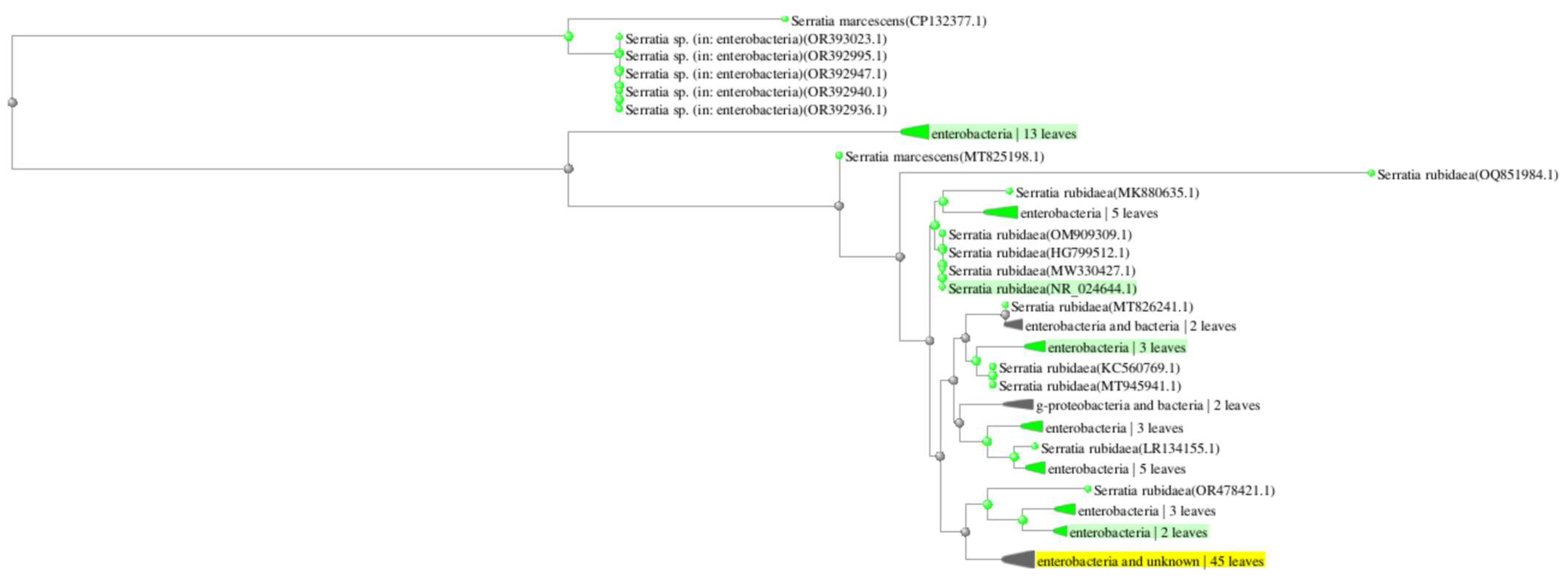
2.1. Bacterial Identification
2.2. Assessing Growth Conditions
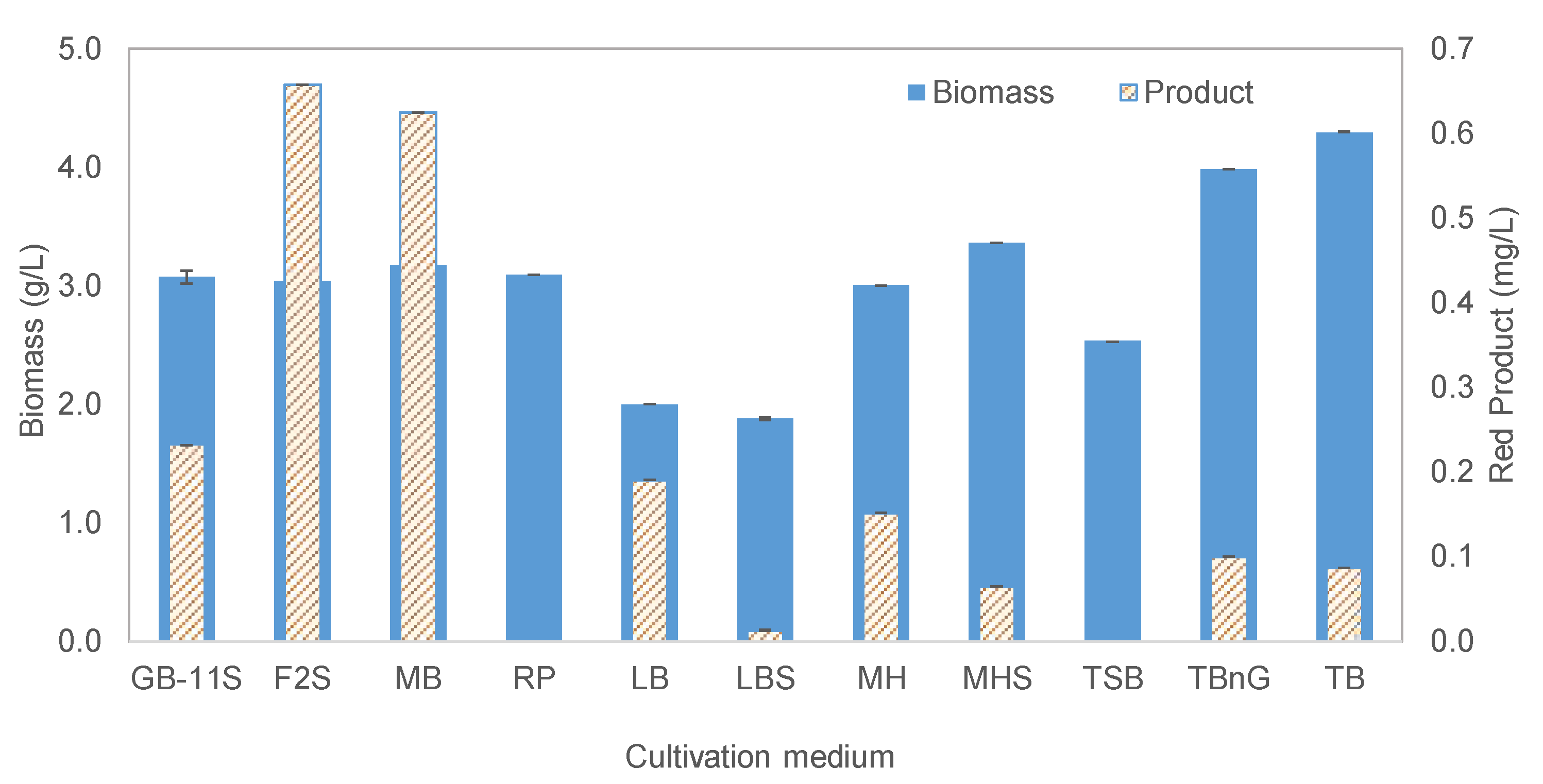
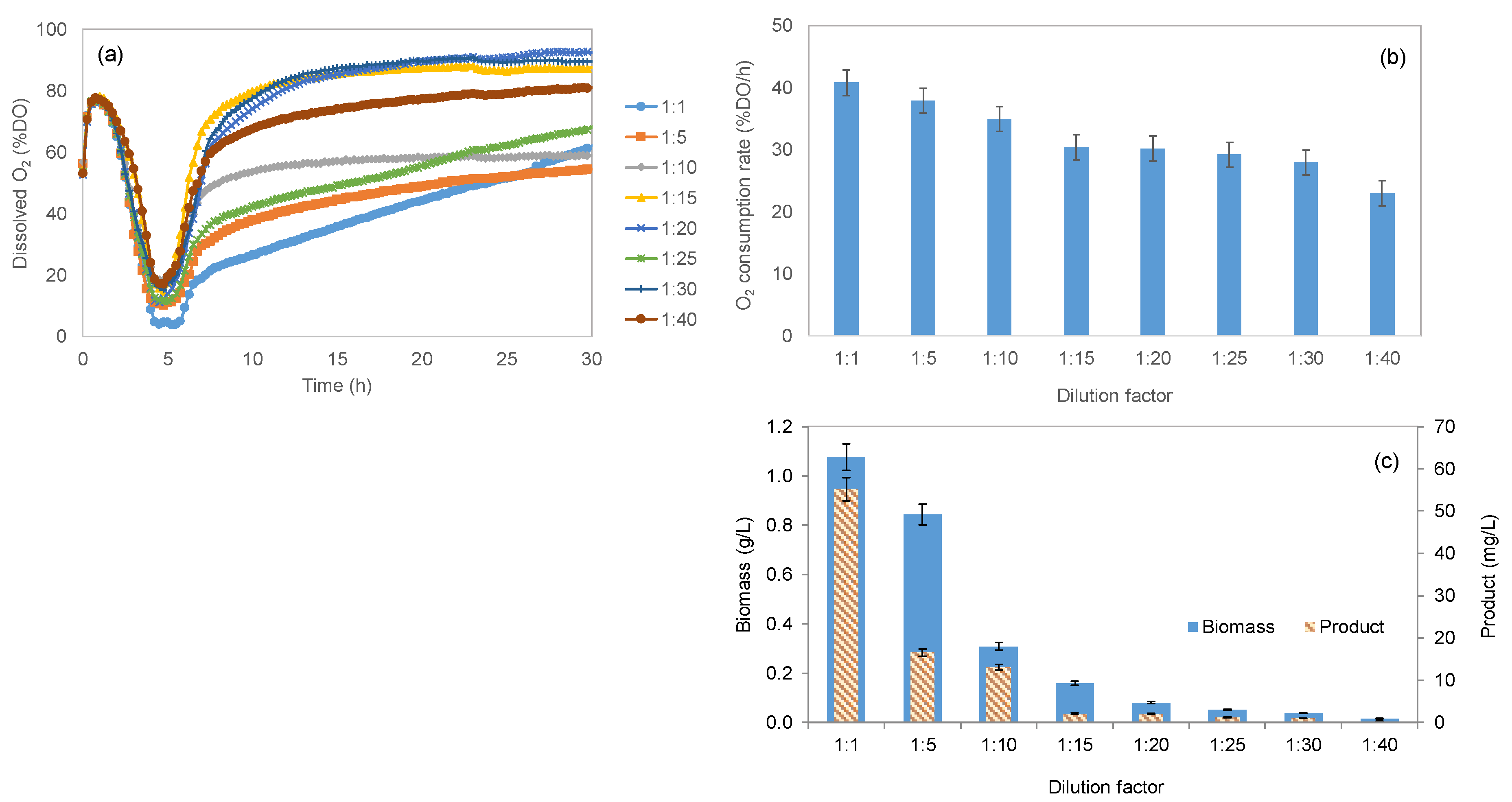
2.2.1. Effect of Nutrient Concentration
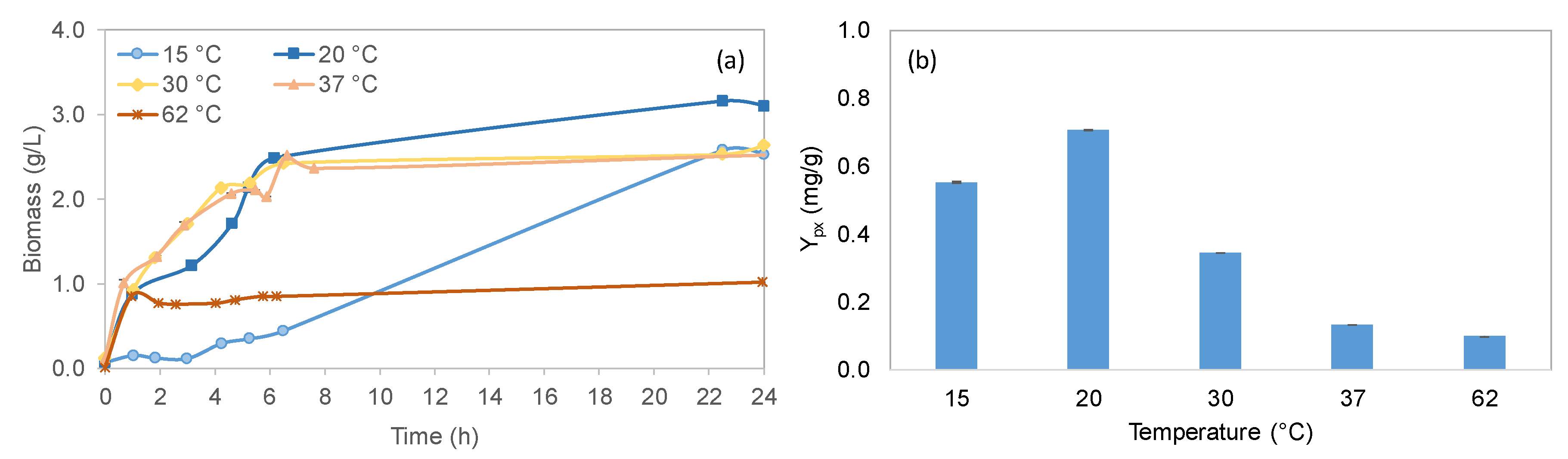
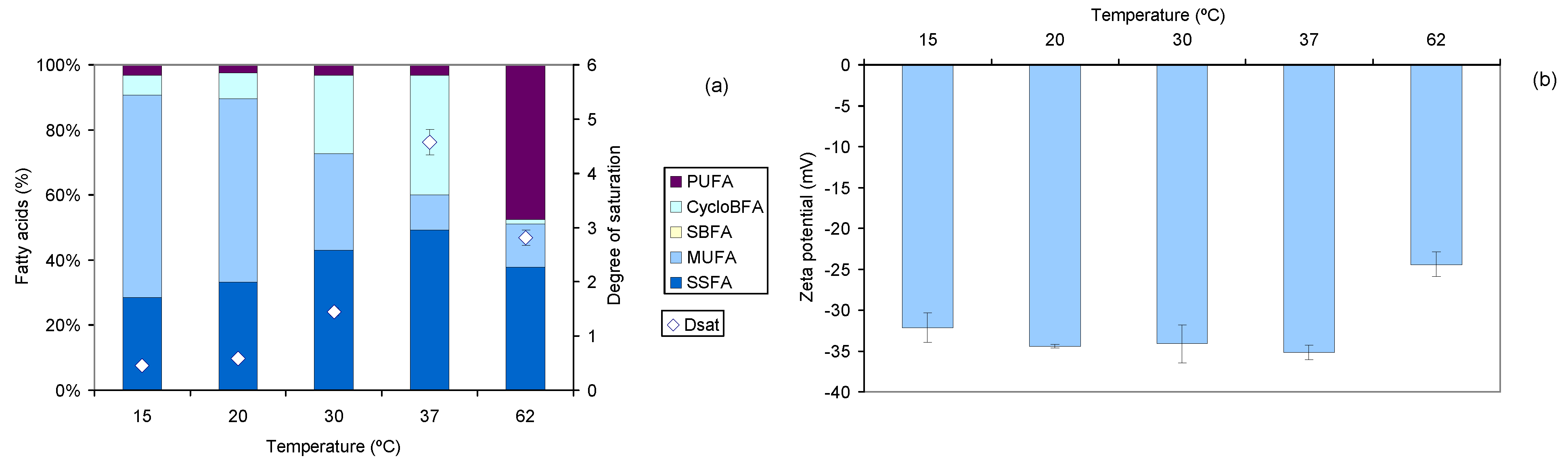
2.2.2. Effect of Temperature
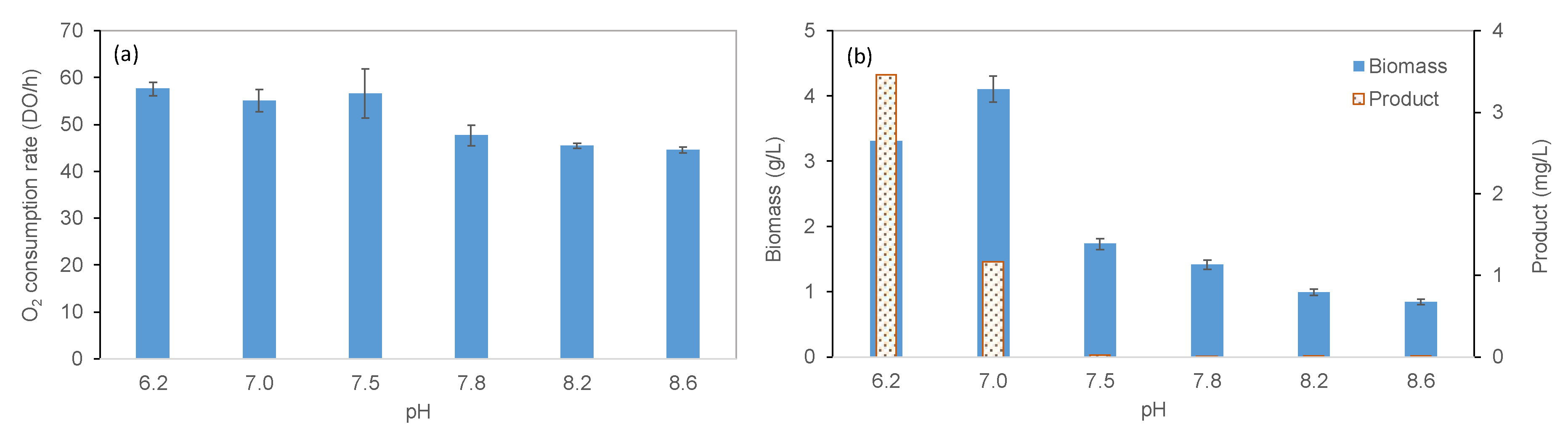
2.2.3. Effect of pH
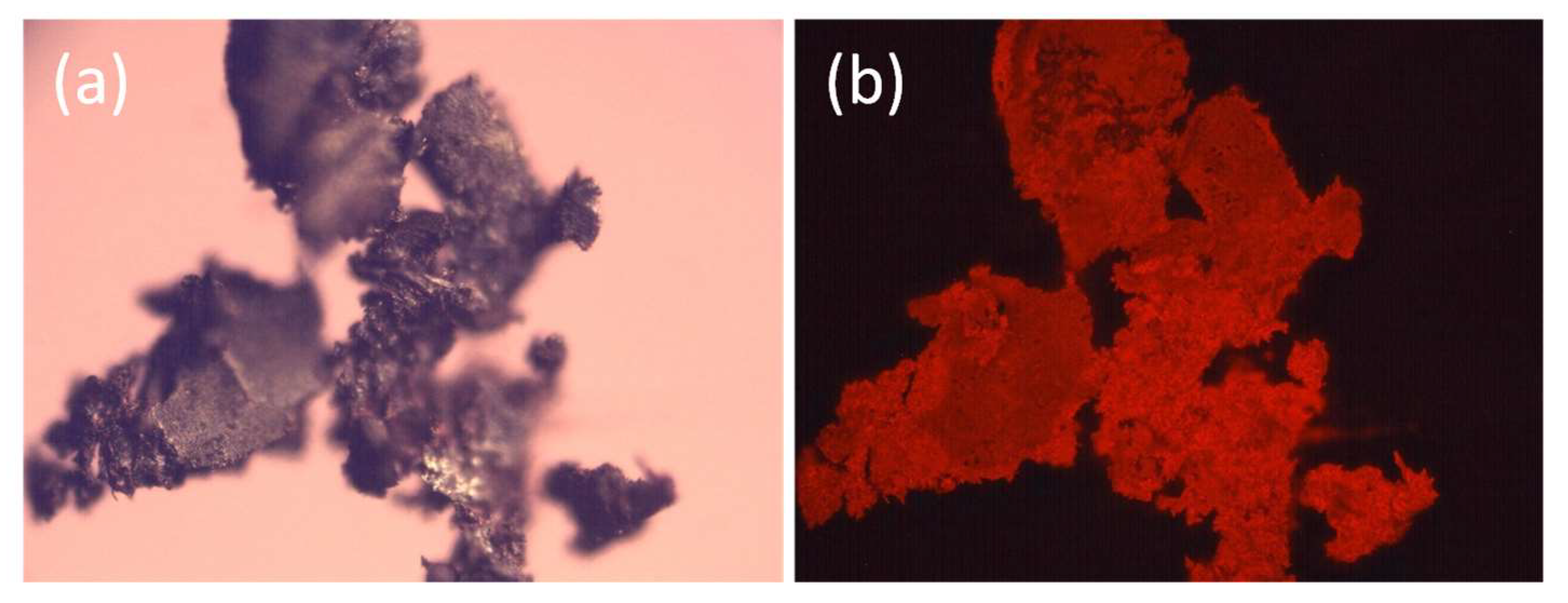
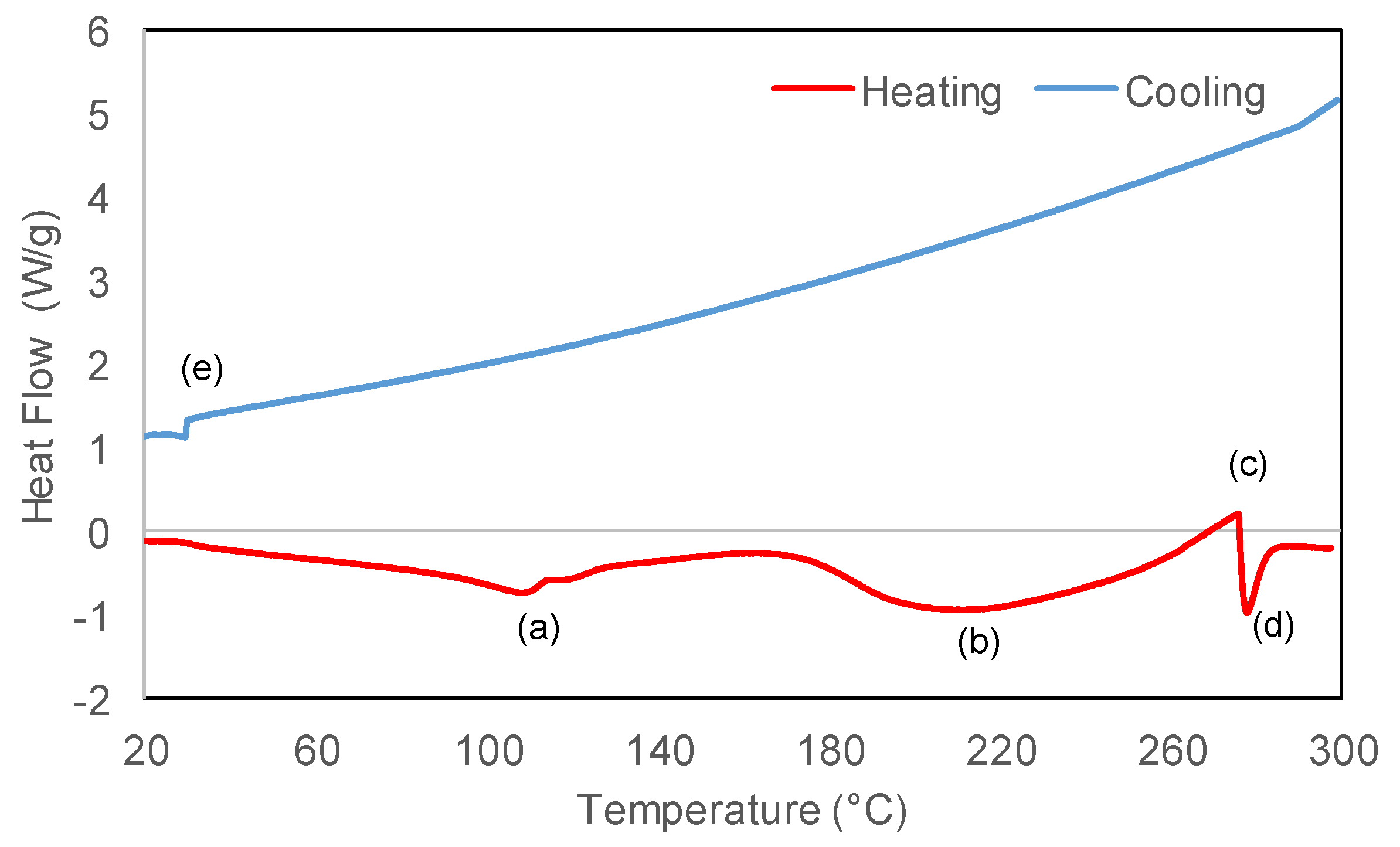
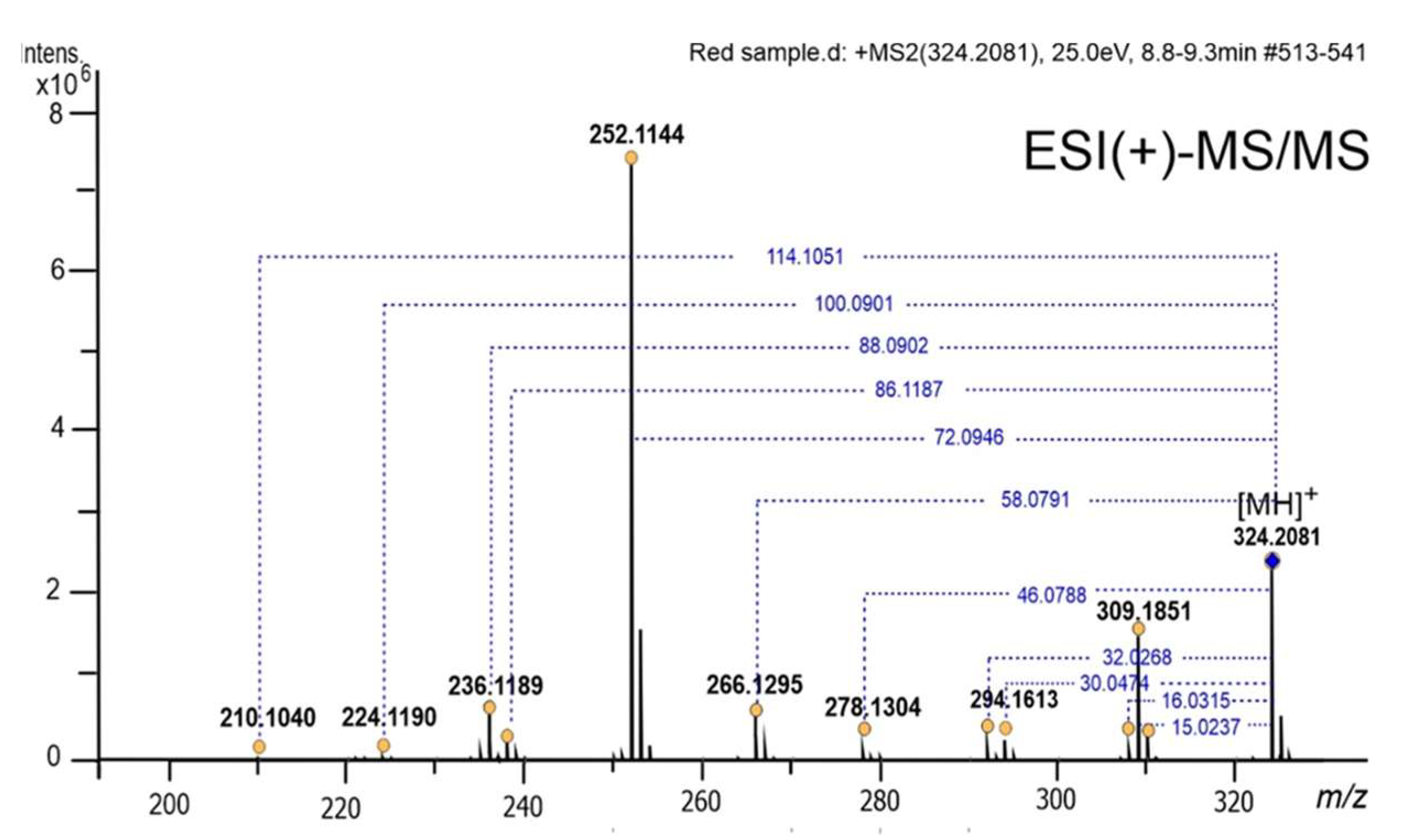
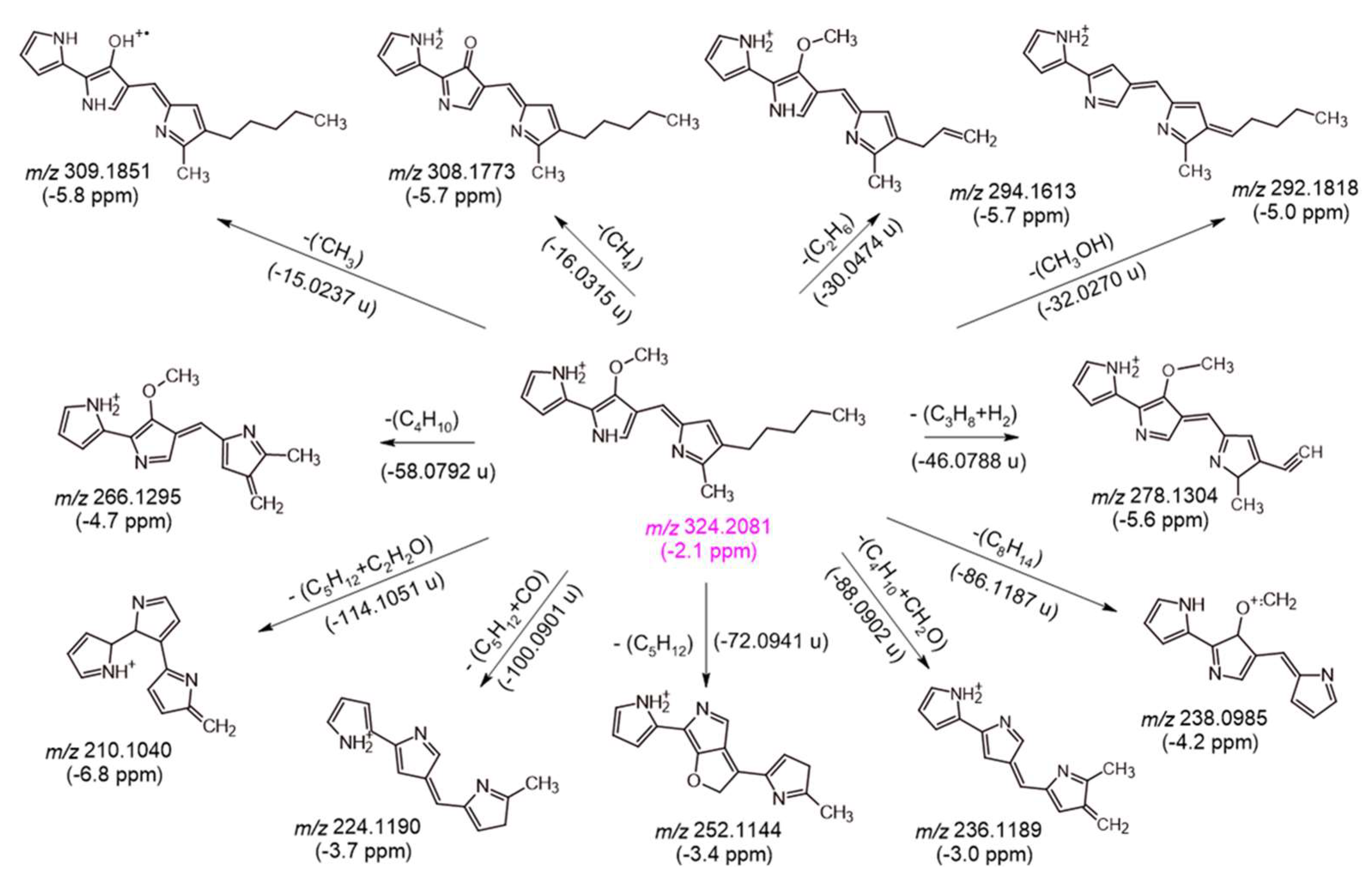
2.3. Product Identification and Characterization
3. Materials and Methods
3.1. Sample Collection and Bacterial Isolation
3.2. Bacterial Identification
3.3. Bacterial Growth
3.3.1. Shaken Flasks
3.3.2. Microtiter Plates (MTPs) with Oxygen and pH Monitoring
3.4. Bacterial Adaptation
3.4.1. Zeta Potential
3.4.2. Lipid Composition
3.5. Product Characterization
3.5.1. Extraction Procedure
3.5.2. Thin-Layer Chromatography
3.6. Analytical Methods
3.6.1. UV-VIS Spectroscopy
3.6.2. Fluorescence Microscopy and Spectroscopy
3.6.3. Nuclear Magnetic Resonance (NMR)
3.6.4. Elemental Analysis
3.6.5. Fourier-Transform Infrared Spectroscopy-Attenuated Total Reflectance (FTIR-ATR)
3.6.6. Differential Scanning Calorimetry (DSC)
3.6.7. Gas Chromatography-Mass Spectrometry (GC-MS)
3.6.8. Ultra-High Resolution Qq Time-of-Flight Mass Spectrometry
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rodrigues, C.J.C.; Pereira, R.F.S.; Fernandes, P.; Cabral, J.M.S.; de Carvalho, C.C.C.R. Cultivation-based strategies to find efficient marine biocatalysts. Biotechnol. J. 2017, 12, 1700036. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.M.; Valentão, P.; Andrade, P.B. Marine natural pigments: Chemistry, distribution and analysis. Dye. Pigment. 2014, 111, 124–134. [Google Scholar] [CrossRef]
- Higashino, T.; Imahori, H. Porphyrins as excellent dyes for dye-sensitized solar cells: Recent developments and insights. Dalt. Trans. 2015, 44, 448–463. [Google Scholar] [CrossRef] [PubMed]
- Kou, J.; Dou, D.; Yang, L. Porphyrin photosensitizers in photodynamic therapy and its applications. Oncotarget 2017, 8, 81591–81603. [Google Scholar] [CrossRef] [PubMed]
- Kijjoa, A.; Sawangwong, P. Drugs and cosmetics from the Sea. Mar. Drugs 2004, 2, 73–82. [Google Scholar] [CrossRef]
- Kiuru, P.; Muller, C.D.; Vuorela, H.; Yli-Kauhaluoma, J. Exploring marine resources for bioactive compounds. Planta Med. 2014, 80, 1234–1246. [Google Scholar] [CrossRef]
- de Carvalho, C.C.C.R.; Fernandes, P. Production of metabolites as bacterial responses to the marine environment. Mar. Drugs 2010, 8, 705–727. [Google Scholar] [CrossRef]
- Romano, G.; Costantini, M.; Sansone, C.; Lauritano, C.; Ruocco, N.; Ianora, A. Marine microorganisms as a promising and sustainable source of bioactive molecules. Mar. Environ. Res. 2017, 128, 58–69. [Google Scholar] [CrossRef]
- Heipieper, H.J.; Weber, F.J.; Sikkema, J.; Keweloh, H.; de Bont, J.A.M. Mechanisms of resistance of whole cells to toxic organic solvents. Trends Biotechnol. 1994, 12, 409–415. [Google Scholar] [CrossRef]
- Weber, F.J.; de Bont, J.A.M. Adaptation mechanism of microorganisms to toxic effects of organic solvents on membranes. Biochim. Biophys. Acta 1996, 1286, 225–245. [Google Scholar] [CrossRef]
- Kawauchi, K.; Shibutani, K.; Yagisawa, H.; Kamata, H.; Nakatsuji, S.; Anzai, H.; Yokoyama, Y.; Ikegami, Y.; Moriyama, Y.; Hirata, H. A possible immunosuppressant, cycloprodigiosin hydrochloride, obtained from Pseudoalteromonas denitrificans. Biochem. Biophys. Res. Commun. 1997, 237, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.X.; Withall, D.M.; Challis, G.L.; Thomson, R.J. Structure, chemical synthesis, and biosynthesis of prodiginine natural products. Chem. Rev. 2016, 116, 7818–7853. [Google Scholar] [CrossRef] [PubMed]
- Williamson, N.R.; Fineran, P.C.; Leeper, F.J.; Salmond, G.P.C. The biosynthesis and regulation of bacterila prodiginines. Nat. Rev. Microbiol. 2006, 4, 887–899. [Google Scholar] [CrossRef] [PubMed]
- Ponjavic, M.; Malagurski, I.; Lazic, J.; Jeremic, S.; Pavlovic, V.; Prlainovic, N.; Maksimovic, V.; Cosovic, V.; Atanase, L.I.; Freitas, F.; et al. Advancing PHBV biomedical potential with the incorporation of bacterial biopigment prodigiosin. Int. J. Mol. Sci. 2023, 24, 1906. [Google Scholar] [CrossRef]
- Perez-Tomas, R.; Vinas, M. New insights on the antitumoral properties of prodiginines. Curr. Med. Chem. 2010, 17, 2222–2231. [Google Scholar] [CrossRef]
- Branco, P.C.; Pontes, C.A.; Rezende-Teixeira, P.; Amengual-Rigo, P.; Alves-Fernandes, D.K.; Maria-Engler, S.S.; da Silva, A.B.; Pessoa, O.D.L.; Jimenez, P.C.; Mollasalehi, N.; et al. Survivin modulation in the antimelanoma activity of prodiginines. Eur. J. Pharmacol. 2020, 888, 173465. [Google Scholar] [CrossRef]
- Jeong, H.; Yim, J.H.; Lee, C.; Choi, S.; Park, Y.K.; Yoon, S.H.; Hur, C.; Kang, H.; Kim, D.; Lee, H.H.; et al. Genomic blueprint of Hahella chejuensis, a marine microbe producing an algicidal agent. Nucleic Acids Res. 2005, 33, 7066–7073. [Google Scholar] [CrossRef]
- Han, R.; Xiang, R.; Li, J.; Wang, F.; Wang, C. High-level production of microbial prodigiosin: A review. J. Basic Microbiol. 2021, 61, 506–523. [Google Scholar] [CrossRef]
- Karnaker, V.K.; Ashraf, A.A.; Veetil, S.S.; Chand, B.; Nair, S.; Varma, S.R. A rare occurrence of Serratia rubidaea in a patient with ear discharge. J. Infect. Public Health 2023, 16, 1–3. [Google Scholar] [CrossRef]
- Kunitsky, C.; Osterhout, G.; Sasser, M. Identification of microorganisms using fatty acid methyl ester (FAME) analysis and the MIDI Sherlock Microbial Identification System. In Encyclopedia of Rapid Microbiological Methods; Bethesda, MD, USA, 2006; pp. 1–17. [Google Scholar]
- de Carvalho, C.C.C.R.; Caramujo, M.J. The various roles of fatty acids. Molecules 2018, 23, 2583. [Google Scholar] [CrossRef]
- Elkenawy, N.M.; Yassin, A.S.; Elhifnawy, H.N.; Amin, M.A. Optimization of prodigiosin production by Serratia marcescens using crude glycerol and enhancing production using gamma radiation. Biotechnol. Rep. 2017, 14, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Kram, K.E.; Finkel, S.E. Rich medium composition affects Escherichia coli survival, glycation, and mutation frequency during long-term batch culture. Appl. Environ. Microbiol. 2015, 81, 4442–4450. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.P.; Green, J.A.; Rappo-Port, D.A. Studies on pigmentation of Serratia marcescens. I. Spectral and paper chromatographic properties of prodigiosin. J. Bacteriol. 1956, 71, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Jannasch, H.W. Growth of marine bacteria at limiting concentrations of organic carbon in seawater. Limnol. Oceanogr. 1967, 12, 264–271. [Google Scholar] [CrossRef]
- Kirchman, D.L. Growth rates of microbes in the oceans. Ann. Rev. Mar. Sci. 2016, 8, 285–309. [Google Scholar] [CrossRef]
- Marques, M.P.C.; Walshe, K.; Doyle, S.; Fernandes, P.; de Carvalho, C.C.C.R. Anchoring high-throughput screening methods to scale-up bioproduction of siderophores. Process Biochem. 2012, 47, 416–421. [Google Scholar] [CrossRef][Green Version]
- Carvalho, M.D.R.; Mateus, A.; Nunes, J.C.; Carvalho, J.M. Chemistry of the Ferraria thermal water, S. Miguel Island, Azores: Mixing and precipitation processes. Environ. Earth Sci. 2011, 64, 539–547. [Google Scholar] [CrossRef]
- Romanowski, E.G.; Lehner, K.M.; Martin, N.C.; Patel, K.R.; Callaghan, J.D.; Stella, N.A.; Shanks, R.M.Q. Thermoregulation of prodigiosin biosynthesis by Serratia marcescens is controlled at the transcriptional level and requires HexS. Pol. J. Microbiol. 2019, 68, 43–50. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, L.; Pan, X.; Osire, T.; Fang, H.; Zhang, H.; Yang, S.-T.; Yang, T.; Rao, Z. Improved prodigiosin production by relieving CpxR temperature-sensitive inhibition. Front. Bioeng. Biotechnol. 2020, 8, 344. [Google Scholar] [CrossRef]
- Santos, R.; de Carvalho, C.C.C.R.; Stevenson, A.; Grant, I.R.; Hallsworth, J.E. Extraordinary solute-stress tolerance contributes to the environmental tenacity of mycobacteria. Environ. Microbiol. Rep. 2015, 7, 746–764. [Google Scholar] [CrossRef]
- Ernst, R.; Ejsing, C.S.; Antonny, B. Homeoviscous adaptation and the regulation of membrane lipids. J. Mol. Biol. 2016, 428, 4776–4791. [Google Scholar] [CrossRef] [PubMed]
- Eze, M.O. Phase transitions in phospholipid bilayers: Lateral phase separations play vital roles in biomembranes. Biochem. Educ. 1991, 19, 204–208. [Google Scholar] [CrossRef]
- de Carvalho, C.C.C.R. Adaptation of Rhodococcus erythropolis cells for growth and bioremediation under extreme conditions. Res. Microbiol. 2012, 163, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Grogan, D.W.; Cronan, J.E. Cyclopropane ring formation in membrane lipids of bacteria. Microbiol. Mol. Biol. Rev. 1997, 61, 429–441. [Google Scholar] [CrossRef]
- Okuyama, H.; Orikasa, Y.; Nishida, T.; Watanabe, K.; Morita, N. Bacterial genes responsible for the biosynthesis of eicosapentaenoic and docosahexaenoic acids and their heterologous expression. Appl. Environ. Microbiol. 2007, 73, 665–670. [Google Scholar] [CrossRef]
- Russell, N.J.; Nichols, D.S. Polyunsaturated fatty acids in marine bacteria—A dogma rewritten. Microbiology 1999, 145, 767–779. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, C.C.C.R.; Marques, M.P.C.; Hachicho, N.; Heipieper, H.J. Rapid adaptation of Rhodococcus erythropolis cells to salt stress by synthesizing polyunsaturated fatty acids. Appl. Microbiol. Biotechnol. 2014, 98, 5599–5606. [Google Scholar] [CrossRef]
- De Santis, A.; Varela, Y.; Sot, J.; D’Errico, G.; Goñi, F.M.; Alonso, A. Omega-3 polyunsaturated fatty acids do not fluidify bilayers in the liquid-crystalline state. Sci. Rep. 2018, 8, 16240. [Google Scholar] [CrossRef]
- Halder, S.; Yadav, K.K.; Sarkar, R.; Mukherjee, S.; Saha, P.; Haldar, S.; Karmakar, S.; Sen, T. Alteration of zeta potential and membrane permeability in bacteria: A study with cationic agents. Springerplus 2015, 4, 672. [Google Scholar] [CrossRef]
- Siliakus, M.F.; Van Der Oost, J.; Kengen, S.W.M. Adaptations of archaeal and bacterial membranes to variations in temperature, pH and pressure. Extremophiles 2017, 21, 651–670. [Google Scholar] [CrossRef]
- Krulwich, T.A.; Sachs, G.; Padan, E. Molecular aspects of bacterial pH sensing and homeostasis. Nat. Rev. Microbiol. 2011, 9, 330–343. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Mangwani, N. Ocean acidification and marine microorganisms: Responses and consequences. Oceanologia 2015, 57, 349–361. [Google Scholar] [CrossRef]
- Giovannetti, R. The Use of Spectrophotometry UV-Vis for the Study of Porphyrins; Uddin, J., Ed.; Intech: London, UK, 2012; ISBN 978-953-51-0664-7. [Google Scholar]
- Hubbard, R.; Rimington, C. The biosynthesis of prodigiosin, the tripyrrylmethene pigment from Bacillus prodigiosus (Serratia marcescens). Biochem. J. 1950, 46, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.H. The Identification of Prodigiosin and Similar Compounds. Ph.D. Thesis, Iowa State University of Sciences and Technology, Ames, IA, USA, 1965. [Google Scholar]
- Darshan, N.; Manonmani, H.K. Prodigiosin and its potential applications. J. Food Sci. Technol. 2013, 52, 5393–5407. [Google Scholar] [CrossRef] [PubMed]
- So, P.T.; Dong, C.Y. Fluorescence Spectrophotometry. In Encyclopedia of Life Sciences; Macmillan Publishers: New York, NY, USA, 2001; pp. 1–4. [Google Scholar]
- Darshan, N.; Manonmani, H.K. Prodigiosin inhibits motility and activates bacterial cell death revealing molecular biomarkers of programmed cell death. AMB Express 2016, 6, 50. [Google Scholar] [CrossRef]
- Dunn, W.B.; Ellis, D.I. Metabolomics: Current analytical platforms and methodologies. Trends Anal. Chem. 2005, 24, 285–294. [Google Scholar] [CrossRef]
- Trenerry, V.C.; Rochfort, S.J. Natural products research and metabolomics. In Comprehensive Natural Products II: Chemistry and Biology; Liu, H.-W., Mander, L., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2010; Volume 9, pp. 595–628. ISBN 9780080453828. [Google Scholar]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J. Spectrometric Identification of Organic Compounds, 7th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005; ISBN 0471393622. [Google Scholar]
- Swann, G.E.A.; Patwardhan, S.V. Application of Fourier Transform Infrared Spectroscopy (FTIR) for assessing biogenic silica sample purity in geochemical analyses and palaeoenvironmental research. Clim. Past 2011, 7, 65–74. [Google Scholar] [CrossRef]
- Roy, D.R.; Shah, E.V.; Mondal Roy, S. Optical activity of Co-porphyrin in the light of IR and Raman spectroscopy: A critical DFT investigation. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 190, 121–128. [Google Scholar] [CrossRef]
- Jehlička, J.; Němec, I.; Varnali, T.; Culka, A.; Svatoš, A.; Frank, O.; Oren, A.; Edwards, H.G.M. The pink pigment prodigiosin: Vibrational spectroscopy and DFT calculations. Dye. Pigment. 2016, 134, 234–243. [Google Scholar] [CrossRef]
- Anwar, M.M.; Shalaby, M.; Embaby, A.M.; Saeed, H.; Agwa, M.M.; Hussein, A. Prodigiosin/PU-H71 as a novel potential combined therapy for triple negative breast cancer (TNBC): Preclinical insights. Sci. Rep. 2020, 10, 14706. [Google Scholar] [CrossRef]
- Cox, R.; Charles, H.P. Porphyrin-accumulating mutants of Escherichia coli. J. Bacteriol. 1973, 113, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Domröse, A.; Klein, A.S.; Hage-Hülsmann, J.; Thies, S.; Svensson, V.; Classen, T.; Pietruszka, J.; Jaeger, K.E.; Drepper, T.; Loeschcke, A. Efficient recombinant production of prodigiosin in Pseudomonas putida. Front. Microbiol. 2015, 6, 972. [Google Scholar] [CrossRef] [PubMed]
- Williamson, N.R.; Simonsen, H.T.; Ahmed, R.A.A.; Goldet, G.; Slater, H.; Woodley, L.; Leeper, F.J.; Salmond, G.P.C. Biosynthesis of the red antibiotic, prodigiosin, in Serratia: Identification of a novel 2-methyl-3-n-amyl- pyrrole (MAP) assembly pathway, definition of the terminal condensing enzyme, and implications for undecylprodigiosin biosynthesis in Strepto. Mol. Microbiol. 2005, 56, 971–989. [Google Scholar] [CrossRef]
- Nakamura, K.; Kitamura, K. Process for Preparation of Prodigiosin. U.S. Patent 4266028A, 5 May 1981. [Google Scholar]
- Nunes, C.; Mahendrasingam, A.; Suryanarayanan, R. Quantification of crystallinity in substantially amorphous materials by synchrotron X-ray powder diffractometry. Pharm. Res. 2005, 22, 1942–1953. [Google Scholar] [CrossRef]
- Rochfort, S. Metabolomics reviewed: A new “omics” platform technology for systems biology and implications for natural products research. J. Nat. Prod. 2005, 68, 1813–1820. [Google Scholar] [CrossRef]
- Vijay, D.; Alshamsi, N.S.; Moussa, Z.; Akhtar, M.K. Extraction of the Anticancer and Antimicrobial Agent, Prodigiosin, from Vibrio gazogenes PB1 and Its Identification by 1D and 2D NMR. Molecules 2022, 27, 6030. [Google Scholar] [CrossRef] [PubMed]
- Haddix, P.L.; Shanks, R.M.Q. Prodigiosin pigment of Serratia marcescens is associated with increased biomass production. Arch. Microbiol. 2018, 200, 989–999. [Google Scholar] [CrossRef]
- Pereira, R.F.S.; de Carvalho, C.C.C.R. Optimization of multiparameters for increased yields of cytochrome B5 in bioreactors. Molecules 2021, 26, 4148. [Google Scholar] [CrossRef]
- Cortes, M.A.L.R.M.; de Carvalho, C.C.C.R. Effect of carbon sources on lipid accumulation in Rhodococcus cells. Biochem. Eng. J. 2015, 94, 100–105. [Google Scholar] [CrossRef]




| Fatty Acid (%) | Temperature (°C) | ||||
|---|---|---|---|---|---|
| 15 | 20 | 30 | 37 | 62 | |
| 12:0 | 0.23 | 0.26 | 0.31 | 0.54 | 8.80 |
| 14:0 | 1.53 | 2.22 | 3.58 | 5.21 | 0.00 |
| 15:0 | 0.35 | 1.03 | 1.21 | 1.17 | 0.00 |
| 15:3 ω3c | 0.09 | 0.08 | 0.13 | 0.15 | 3.87 |
| 16:0 | 25.73 | 28.39 | 36.66 | 40.85 | 14.85 |
| 16:1 ω5c | 0.29 | 0.24 | 0.14 | 0.11 | 0.00 |
| 16:1 ω7c | 33.43 | 29.48 | 13.16 | 3.35 | 4.66 |
| 16:4 ω3c | 2.12 | 1.60 | 1.57 | 1.42 | 1.47 |
| 17:0 | 0.23 | 0.42 | 0.51 | 0.63 | 0.00 |
| 17:0 cyclo ω7c | 5.72 | 7.42 | 23.39 | 32.06 | 1.34 |
| 18:0 | 0.38 | 0.91 | 0.68 | 0.86 | 14.05 |
| 18:1 ω7c | 28.56 | 26.50 | 16.03 | 7.20 | 7.44 |
| 18:1 ω9c | 0.00 | 0.08 | 0.35 | 0.11 | 1.29 |
| 18:3 ω6c | 1.05 | 0.91 | 1.44 | 1.71 | 42.23 |
| 19:0 cyclo ω7c | 0.27 | 0.44 | 0.84 | 4.61 | 0.00 |
| Location | GPS Coordinates | TH2O (°) | Tair (°) | pH | Conductivity (mS/cm) |
|---|---|---|---|---|---|
| S. Miguel, Azores | 37°51′30″ N 25°51′7″ W | 28 | 18 | 8.91 | 66.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, R.F.S.; Ferreira, M.J.; Oliveira, M.C.; Serra, M.C.; de Carvalho, C.C.C.R. Isolation and Characterization of a Serratia rubidaea from a Shallow Water Hydrothermal Vent. Mar. Drugs 2023, 21, 599. https://doi.org/10.3390/md21120599
Pereira RFS, Ferreira MJ, Oliveira MC, Serra MC, de Carvalho CCCR. Isolation and Characterization of a Serratia rubidaea from a Shallow Water Hydrothermal Vent. Marine Drugs. 2023; 21(12):599. https://doi.org/10.3390/md21120599
Chicago/Turabian StylePereira, Ricardo F. S., Maria J. Ferreira, M. Conceição Oliveira, Maria C. Serra, and Carla C. C. R. de Carvalho. 2023. "Isolation and Characterization of a Serratia rubidaea from a Shallow Water Hydrothermal Vent" Marine Drugs 21, no. 12: 599. https://doi.org/10.3390/md21120599
APA StylePereira, R. F. S., Ferreira, M. J., Oliveira, M. C., Serra, M. C., & de Carvalho, C. C. C. R. (2023). Isolation and Characterization of a Serratia rubidaea from a Shallow Water Hydrothermal Vent. Marine Drugs, 21(12), 599. https://doi.org/10.3390/md21120599






