Metabolic Profiling, Antiviral Activity and the Microbiome of Some Mauritian Soft Corals
Abstract
:1. Introduction
2. Results
2.1. Metabolic Profiling
| Soft Corals | Metabolites Name | Average Rt (min) | Biological Properties | References |
|---|---|---|---|---|
| L. patulum | 2-amino-4-hydroxypyrimidine-5-carboxylic acid | 4.586 | Antibacterial | [24] |
| Cyclopamine | 10.841 | Antiviral (RSV, BRSV); Antitumor | [28,39,40] | |
| Bruceine D | 11.117 | Antitumor | [25] | |
| 5-(hydroxymethyl)pyrimidine-2,4-diol | 9.145 | - | - | |
| 2-(3,5-Dimethyl-7-oxo-7H-furo[3,2-g]chromen-6-yl)-N-[3-(2-oxo-1-pyrrolidinyl)propyl]acetamide | 11.771 | - | - | |
| Eurycomalactone | 11.27 | Antiviral (HCoV-OC43 and SARS-CoV-2 strains); Anticancer | [29,41] | |
| Neosolaniol | 11.223 | - | - | |
| Quinaldic acid | 11.117 | Antiviral (influenza A/H5N1); Antibacterial; Anticancer; | [30,42,43] | |
| Terbutaline | 9.151 | Bronchodilator; Anti-inflammatory; Antiarthritic | [26,27] | |
| Tetrahydrozoline HCl | 11.323 | Topical nasal and conjunctival decongestant | [44] | |
| S. polydactyla | 2-((6-((6,7-dimethoxy-3,4-dihydroisoquinolin-2(1H)-yl)methyl)-4-oxo-4H-pyran-3-yl)oxy)-N-(3,4-dimethoxyphenethyl)acetamide | 13.187 | - | - |
| 2-(3,4-dimethoxyphenyl)-7-methoxy-4H-chromen-4-one | 12.817 | - | - | |
| 24-epimakisterone A | 11.834 | - | - | |
| 7-amino-flunitrazepam | 13.568 | - | - | |
| Erianin | 13.521 | Antitumor; Antiviral (Human enterovirus 68); Anti-inflammation | [31,32,33,34] | |
| Linderane | 12.927 | - | - | |
| Pregn-4-ene-3,20-dione | 12.254 | - | - | |
| Methotrexate | 0.674 | Anticancer; Immunosuppressant | [45,46] | |
| C. simplex | Ketoconazole | 0.39 | Antifungal; Antiviral (HSV-1/HSV-2); Anticancer; Anti-inflammatory | [35,36,37,38] |
| L. crassum | Methyl 3-(6-((4-formylpiperazin-1-yl)methyl)-3-hydroxy-4-oxo-4H-pyran-2-yl)-3-(4-((1-methyl-1H-imidazol-2-yl)methoxy)phenyl)propanoate | 6.329 | - | - |
2.2. Associated Bacterial Communities
| Soft Corals | Associated Bacteria | Biological Compounds | Biological Properties | References |
|---|---|---|---|---|
| L.p | Salipiger mucosus | Exopolysaccharides | Antiviral; Antiangiogenic | [62] |
| Psychrobacter marincola | Capsular polysaccharides | Antitumor | [64] | |
| Psychrobacter sp. | Bile acid derivative | Antibacterial, Cytotoxic to tumor cell lines | [65] | |
| Propionibacterium sp. | Propionic acid | Antiviral | [63] | |
| Paracoccus marcussi | - | Antibacterial | [59] | |
| Kocuria palustris | Alkaloids | Antifungal | [67] | |
| Exiguobacterium sp. | - | Antibacterial | [60] | |
| Brachybacterium sp. | Exopolysaccharides | Antibacterial | [61] | |
| L.p/C.s | Mycobacterium vaccae | - | Cancer treatment | [66] |
| C.s | Gordonia sp. | - | Antimicrobial | [68] |
| C.s | Dermobacter sp. | Imidazolium compound | Antibacterial | [69] |
| C.s | Actinomyces sp. | Tetradecanoic acid, pentadecanoic acid, n-hexadecanoic acid | Antifungal; Antimicrobial | [70,71] |
| L.p/S.p | Streptomyces sp. | Polyketides, alkaloids and terpenoids Strepchloritides A and B, Polyketone, 9(10H)-acridanone | Antiviral (H1N1, SARS-CoV-2, white spot syndrome virus (WSSV)) | [72,73,74,75] |
| L.p/S.p/L.c/C.s | Lactobacillus sp. | Intercellular polysaccharides/exocellular polysaccharides | Prebiotics with bifidogenic effect | [76] |
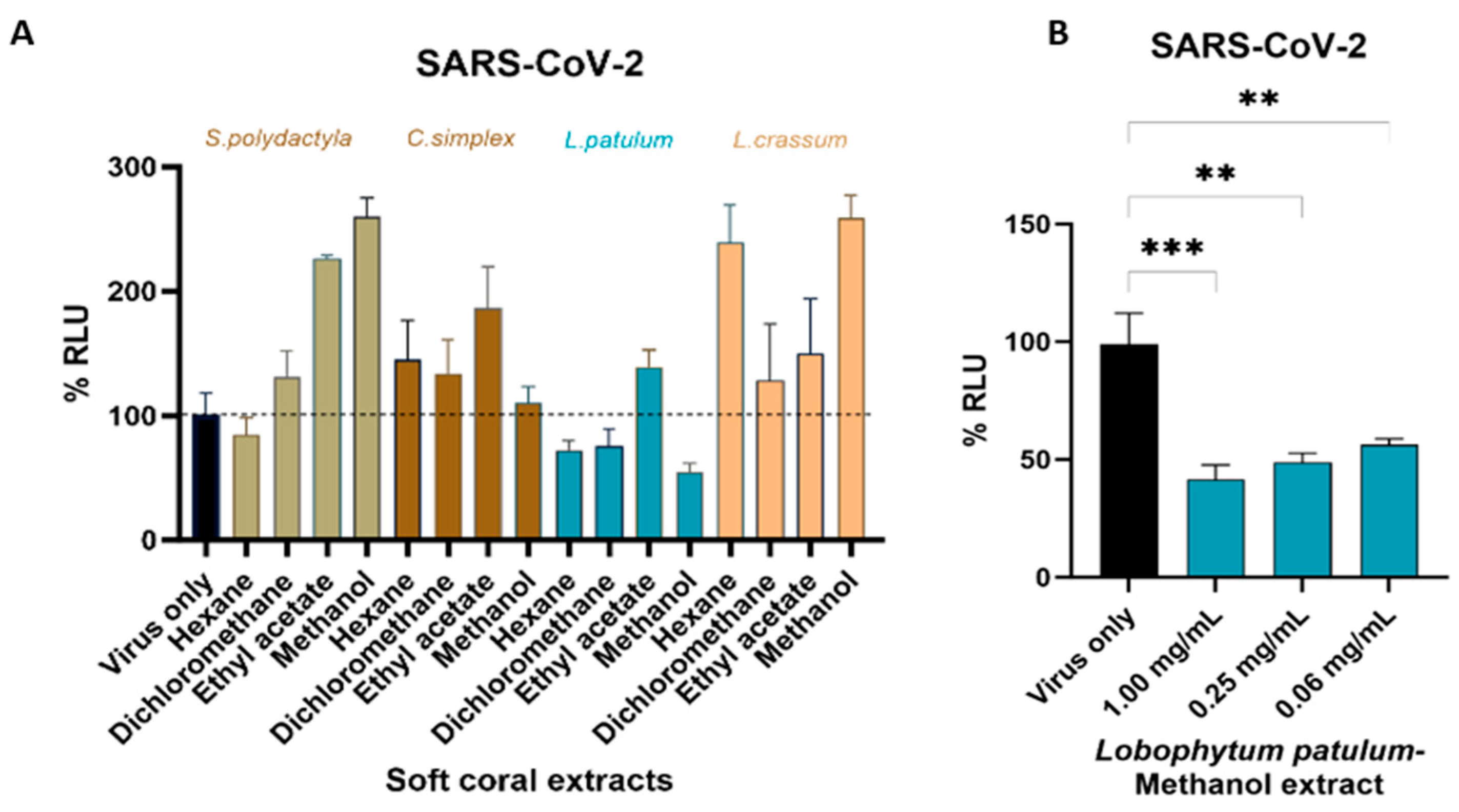
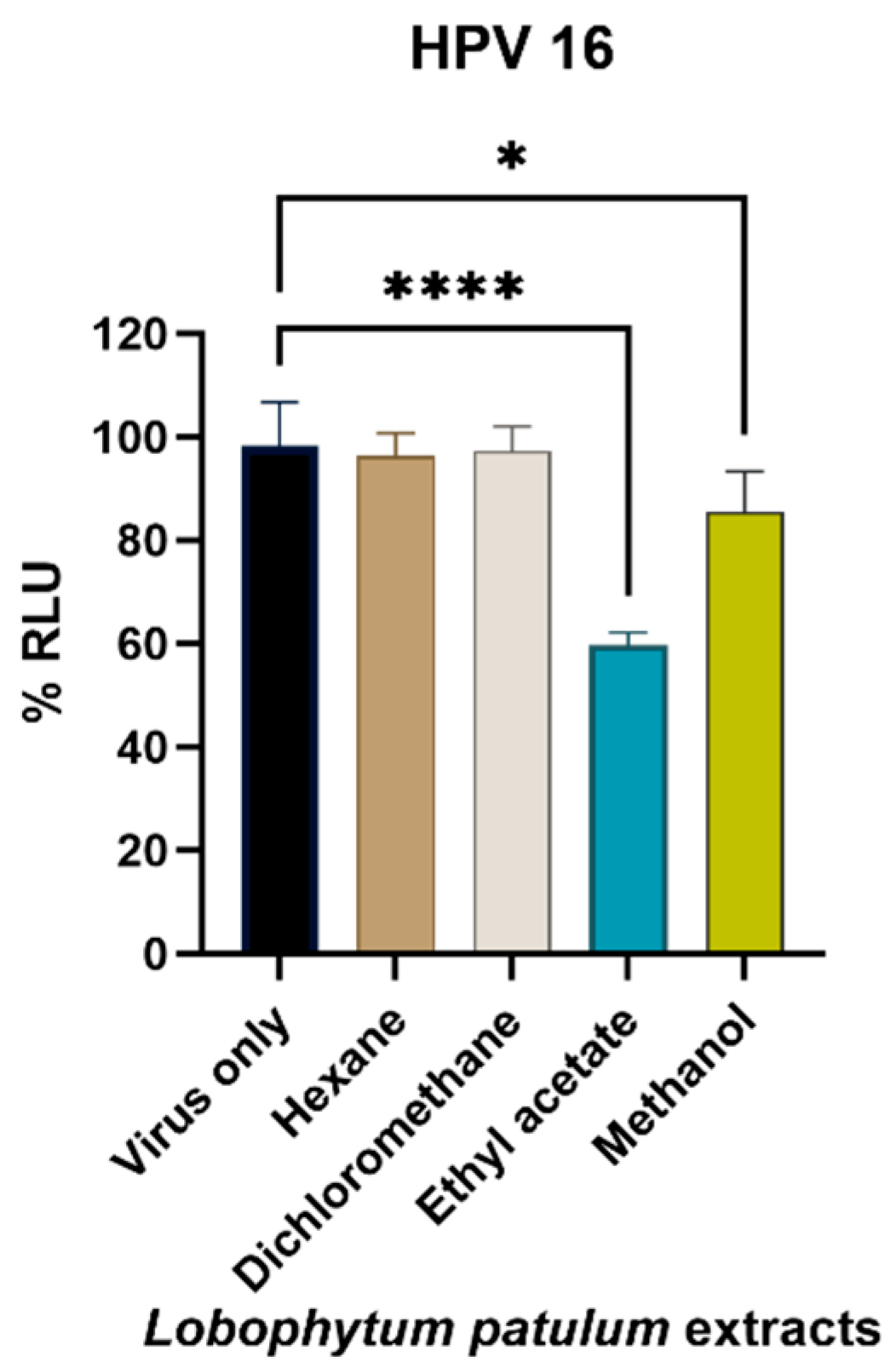
2.3. Cell-Viability Analysis
2.4. Antiviral Activity
3. Discussion
4. Materials and Methods
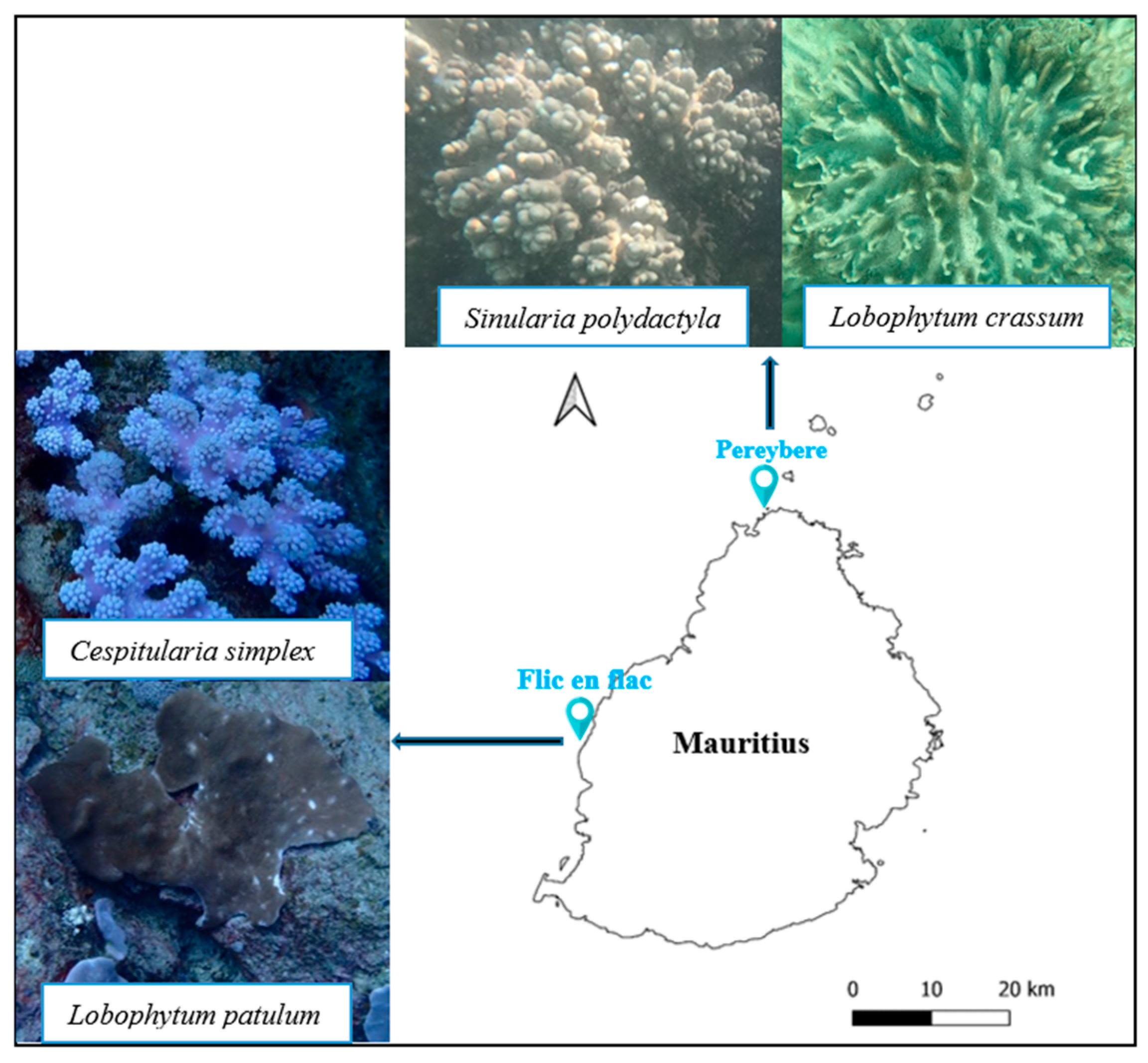
4.1. Soft Coral Materials
4.1.1. Preparation of Extracts
4.1.2. Metabolic Profiling
4.2. Metagenomics Analysis
4.3. Bioassays
4.3.1. Cell Culture
4.3.2. Preparation of SARS-CoV-2 and HPV16 Pseudovirions
4.3.3. Cytotoxicity Assay
4.3.4. Inhibiting SARS-CoV-2 Pseudovirus Entry into HEK293T–ACE2 Cells
4.3.5. Inhibiting HPV Entry in HaCaT Cells
4.3.6. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jahajeeah, D.; Bhoyroo, V.; Ranghoo-Sanmukhiya, M. An Assessment of Soft Coral Community (Octocorallia; Alcyonacea) around Mauritius and Rodrigues Islands—New Records of Soft Corals. Reg. Stud. Mar. Sci. 2021, 47, 101976. [Google Scholar] [CrossRef]
- Lim, F.S. Bioactive Natural Products from the Soft Coral, Sinularia Sp. Master’s Thesis, Universiti Tunku Abdul Rahman, Kuala Lumpur, Malaysia, 2014. [Google Scholar]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine Natural Products. Nat. Prod. Rep. 2020, 37, 175–223. [Google Scholar] [CrossRef] [PubMed]
- Tammam, M.A.; Rárová, L.; Kvasnicová, M.; Gonzalez, G.; Emam, A.M.; Mahdy, A.; Strnad, M.; Ioannou, E.; Roussis, V. Bioactive Steroids from the Red Sea Soft Coral Sinularia Polydactyla. Mar. Drugs 2020, 18, 632. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Ibrahim, A.; Arafa, A.S. Anti-H5N1 Virus Metabolites from the Red Sea Soft Coral, Sinularia Candidula. Tetrahedron Lett. 2013, 54, 2377–2381. [Google Scholar] [CrossRef]
- Jahajeeah, D.; Bhoyroo, V. Antimicrobial properties and metabolite profiling of the ethyl acetate fractions of Sinularia Polydactyla and Cespitularia Simplex surrounding Mauritius Island. Indo Pac. J. Ocean Life 2023, 7, 133–142. [Google Scholar] [CrossRef]
- Aratake, S.; Tomura, T.; Saitoh, S.; Yokokura, R.; Kawanishi, Y.; Shinjo, R.; Reimer, J.D.; Tanaka, J.; Maekawa, H. Soft Coral Sarcophyton (Cnidaria: Anthozoa: Octocorallia) Species Diversity and Chemotypes. PLoS ONE 2012, 7, e30410. [Google Scholar] [CrossRef]
- Said, G.; Hou, X.-M.; Liu, X.; Chao, R.; Jiang, Y.-Y.; Zheng, J.-Y.; Shao, C.-L. Antimicrobial and Cytotoxic Activities of Secondary Metabolites from the Soft Coral Derived Fungus Aspergillus Sp. Chem. Nat. Compd. 2019, 55, 531–533. [Google Scholar] [CrossRef]
- Singh, R.; Chauhan, N.; Kuddus, M. Exploring the Therapeutic Potential of Marine-Derived Bioactive Compounds against COVID-19. Environ. Sci. Pollut. Res. Int. 2021, 28, 52798–52809. [Google Scholar] [CrossRef]
- Lu, R.; Zhao, X.; Li, J.; Niu, P.; Yang, B.; Wu, H.; Wang, W.; Song, H.; Huang, B.; Zhu, N.; et al. Genomic Characterisation and Epidemiology of 2019 Novel Coronavirus: Implications for Virus Origins and Receptor Binding. Lancet 2020, 395, 565–574. [Google Scholar] [CrossRef]
- Abdelhafez, O.H.; Fahim, J.R.; Mustafa, M.; AboulMagd, A.M.; Desoukey, S.Y.; Hayallah, A.M.; Kamel, M.S.; Abdelmohsen, U.R. Natural Metabolites from the Soft Coral Nephthea Sp. as Potential SARS-CoV-2 Main Protease Inhibitors. Nat. Prod. Res. 2022, 36, 2893–2896. [Google Scholar] [CrossRef]
- Ibrahim, M.A.A.; Abdelrahman, A.H.M.; Atia, M.A.M.; Mohamed, T.A.; Moustafa, M.F.; Hakami, A.R.; Khalifa, S.A.M.; Alhumaydhi, F.A.; Alrumaihi, F.; Abidi, S.H.; et al. Blue Biotechnology: Computational Screening of Sarcophyton Cembranoid Diterpenes for SARS-CoV-2 Main Protease Inhibition. Mar. Drugs 2021, 19, 391. [Google Scholar] [CrossRef]
- Avalon, N.E.; Nafie, J.; De Marco Verissimo, C.; Warrensford, L.C.; Dietrick, S.G.; Pittman, A.R.; Young, R.M.; Kearns, F.L.; Smalley, T.; Binning, J.M.; et al. Tuaimenal A, a Meroterpene from the Irish Deep-Sea Soft Coral Duva Florida, Displays Inhibition of the SARS-CoV-2 3CLpro Enzyme. J. Nat. Prod. 2022, 85, 1315–1323. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.E.; Mahmud, A.S.; Miller, I.F.; Rajeev, M.; Rasambainarivo, F.; Rice, B.L.; Takahashi, S.; Tatem, A.J.; Wagner, C.E.; Wang, L.-F.; et al. Infectious Disease in an Era of Global Change. Nat. Rev. Microbiol. 2022, 20, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Fradet-Turcotte, A.; Archambault, J. Recent Advances in the Search for Antiviral Agents against Human Papillomaviruses. Antivir. Ther. 2007, 12, 431–451. [Google Scholar] [CrossRef] [PubMed]
- Crosbie, E.J.; Einstein, M.H.; Franceschi, S.; Kitchener, H.C. Human Papillomavirus and Cervical Cancer. Lancet 2013, 382, 889–899. [Google Scholar] [CrossRef]
- Buck, C.B.; Thompson, C.D.; Roberts, J.N.; Müller, M.; Lowy, D.R.; Schiller, J.T. Carrageenan Is a Potent Inhibitor of Papillomavirus Infection. PLoS Pathog. 2006, 2, e69. [Google Scholar] [CrossRef]
- Wang, S.-X.; Zhang, X.-S.; Guan, H.-S.; Wang, W. Potential Anti-HPV and Related Cancer Agents from Marine Resources: An Overview. Mar. Drugs 2014, 12, 2019–2035. [Google Scholar] [CrossRef]
- Campo, V.L.; Kawano, D.F.; da Silva, D.B., Jr.; Carvalho, I. Carrageenans: Biological Properties, Chemical Modifications and Structural Analysis—A Review. Carbohydr. Polym. 2009, 77, 167–180. [Google Scholar] [CrossRef]
- Wang, P.; Zhao, X.; Lv, Y.; Li, M.; Liu, X.; Li, G.; Yu, G. Structural and Compositional Characteristics of Hybrid Carrageenans from Red Algae Chondracanthus Chamissoi. Carbohydr. Polym. 2012, 89, 914–919. [Google Scholar] [CrossRef]
- Talarico, L.B.; Damonte, E.B. Interference in Dengue Virus Adsorption and Uncoating by Carrageenans. Virology 2007, 363, 473–485. [Google Scholar] [CrossRef]
- Yamada, T.; Ogamo, A.; Saito, T.; Uchiyama, H.; Nakagawa, Y. Preparation of O-Acylated Low-Molecular-Weight Carrageenans with Potent Anti-HIV Activity and Low Anticoagulant Effect. Carbohydr. Polym. 2000, 41, 115–120. [Google Scholar] [CrossRef]
- Leibbrandt, A.; Meier, C.; König-Schuster, M.; Weinmüllner, R.; Kalthoff, D.; Pflugfelder, B.; Graf, P.; Frank-Gehrke, B.; Beer, M.; Fazekas, T.; et al. Iota-Carrageenan Is a Potent Inhibitor of Influenza A Virus Infection. PLoS ONE 2010, 5, e14320. [Google Scholar] [CrossRef] [PubMed]
- Watkins, S.M.; Ghose, D.; Blain, J.M.; Grote, D.L.; Luan, C.-H.; Clare, M.; Meganathan, R.; Horn, J.R.; Hagen, T.J. Antibacterial Activity of 2-Amino-4-Hydroxypyrimidine-5-Carboxylates and Binding to Burkholderia Pseudomallei 2-C-Methyl-D-Erythritol-2,4-Cyclodiphosphate Synthase. Bioorg. Med. Chem. Lett. 2019, 29, 126660. [Google Scholar] [CrossRef] [PubMed]
- Sin, Z.W.; Bhardwaj, V.; Pandey, A.K.; Garg, M. A Brief Overview of Antitumoral Actions of Bruceine D. Explor. Target. Antitumor Ther. 2020, 1, 200–217. [Google Scholar] [CrossRef] [PubMed]
- Alotaibi, N.H.; Sarwar, M.; Alamgeer; Jabbar, Z.; Munir, M.U.; Irfan, H.M.; Akram, M.; Abbas Bukhari, S.N.; Rasul Niazi, Z.; Khan, A.Q. Pharmacological Exploration of Anti-Arthritic Potential of Terbutaline through in-Vitro and in-Vivo Experimental Models. Pak. J. Pharm. Sci. 2022, 35, 253–257. [Google Scholar]
- Spiller, H.A. Encyclopedia of Toxicology, 3rd ed.; Academic Press: Oxford, UK, 2014; pp. 484–485. [Google Scholar] [CrossRef]
- Bailly, B.; Richard, C.-A.; Sharma, G.; Wang, L.; Johansen, L.; Cao, J.; Pendharkar, V.; Sharma, D.-C.; Galloux, M.; Wang, Y.; et al. Targeting Human Respiratory Syncytial Virus Transcription Anti-Termination Factor M2-1 to Inhibit in Vivo Viral Replication. Sci. Rep. 2016, 6, 25806. [Google Scholar] [CrossRef]
- Chaingam, J.; Choonong, R.; Juengwatanatrakul, T.; Kanchanapoom, T.; Putalun, W.; Yusakul, G. Evaluation of Anti-Inflammatory Properties of Eurycoma Longifolia Jack and Eurycoma Harmandiana Pierre in Vitro Cultures and Their Constituents. Food Agric. Immunol. 2022, 33, 530–545. [Google Scholar] [CrossRef]
- Shibnev, V.A.; Garaev, T.M.; Deryabin, P.G.; Finogenova, M.P.; Botikov, A.G.; Mishin, D.V. New Carbocyclic Amino Acid Derivatives Inhibit Infection Caused by Highly Pathogenic Influenza A Virus Strain (H5N1). Bull. Exp. Biol. Med. 2016, 161, 284–287. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, T.; Sun, W.; Wang, Z.; Zuo, D.; Zhou, Z.; Li, S.; Xu, J.; Yin, F.; Hua, Y.; et al. Erianin Induces G2/M-Phase Arrest, Apoptosis, and Autophagy via the ROS/JNK Signaling Pathway in Human Osteosarcoma Cells in Vitro and in Vivo. Cell Death Dis. 2016, 7, e2247. [Google Scholar] [CrossRef]
- Meng, X.; Yu, X.; Liu, C.; Wang, Y.; Song, F.; Huan, C.; Huo, W.; Zhang, S.; Li, Z.; Zhang, J.; et al. Effect of Ingredients from Chinese Herbs on Enterovirus D68 Production. Phytother. Res. 2019, 33, 174–186. [Google Scholar] [CrossRef]
- Zhang, T.; Ouyang, H.; Mei, X.; Lu, B.; Yu, Z.; Chen, K.; Wang, Z.; Ji, L. Erianin Alleviates Diabetic Retinopathy by Reducing Retinal Inflammation Initiated by Microglial Cells via Inhibiting Hyperglycemia-Mediated ERK1/2-NF-κB Signaling Pathway. FASEB J. 2019, 33, 11776–11790. [Google Scholar] [CrossRef] [PubMed]
- Dou, B.; Hu, W.; Song, M.; Lee, R.J.; Zhang, X.; Wang, D. Anti-Inflammation of Erianin in Dextran Sulphate Sodium-Induced Ulcerative Colitis Mice Model via Collaborative Regulation of TLR4 and STAT3. Chem. Biol. Interact. 2020, 324, 109089. [Google Scholar] [CrossRef] [PubMed]
- Khoza, S.; Moyo, I.; Ncube, D. Comparative Hepatotoxicity of Fluconazole, Ketoconazole, Itraconazole, Terbinafine, and Griseofulvin in Rats. J. Toxicol. 2017, 2017, 6746989. [Google Scholar] [CrossRef] [PubMed]
- Pottage, J.C., Jr.; Kessler, H.A.; Goodrich, J.M.; Chase, R.; Benson, C.A.; Kapell, K.; Levin, S. In Vitro Activity of Ketoconazole against Herpes Simplex Virus. Antimicrob. Agents Chemother. 1986, 30, 215–219. [Google Scholar] [CrossRef]
- Lou, D.; Cui, X.; Bao, S.-S.; Sun, W.; Pan, W.-H.; Chen, M.-C.; Dong, Y.-Y.; Hu, G.-X.; Chen, R.-J.; Wang, Z. Effects of Ketoconazole, Voriconazole, and Itraconazole on the Pharmacokinetics of Apatinib in Rats. Drug Dev. Ind. Pharm. 2019, 45, 689–693. [Google Scholar] [CrossRef]
- Van Cutsem, J.; Van Gerven, F.; Cauwenbergh, G.; Odds, F.; Janssen, P.A.J. The Antiinflammatory Effects of Ketoconazole: A Comparative Study with Hydrocortisone Acetate in a Model Using Living and Killed Staphylococcus Aureus on the Skin of Guinea Pigs. J. Am. Acad. Dermatol. 1991, 25, 257–261. [Google Scholar] [CrossRef]
- Fix, J.; Descamps, D.; Galloux, M.; Ferret, C.; Bouguyon, E.; Zohari, S.; Näslund, K.; Hägglund, S.; Altmeyer, R.; Valarcher, J.-F.; et al. Screening Antivirals with a mCherry-Expressing Recombinant Bovine Respiratory Syncytial Virus: A Proof of Concept Using Cyclopamine. Vet. Res. 2023, 54, 36. [Google Scholar] [CrossRef]
- Zhang, B.; Jiang, T.; Shen, S.; She, X.; Tuo, Y.; Hu, Y.; Pang, Z.; Jiang, X. Cyclopamine Disrupts Tumor Extracellular Matrix and Improves the Distribution and Efficacy of Nanotherapeutics in Pancreatic Cancer. Biomaterials 2016, 103, 12–21. [Google Scholar] [CrossRef]
- Dukaew, N.; Konishi, T.; Chairatvit, K.; Autsavapromporn, N.; Soonthornchareonnon, N.; Wongnoppavich, A. Enhancement of Radiosensitivity by Eurycomalactone in Human NSCLC Cells Through G2/M Cell Cycle Arrest and Delayed DNA Double-Strand Break Repair. Oncol. Res. 2020, 28, 161–175. [Google Scholar] [CrossRef]
- Lee, C.H.; Lee, H.S. Growth inhibiting activity of quinaldic acid isolated from Ephedra pachyclada against intestinal bacteria. J. Korean Soc. Appl. Biol. Chem. 2009, 52, 331–335. [Google Scholar] [CrossRef]
- Langner, E.; Walczak, K.; Jeleniewicz, W.; Turski, W.A.; Rajtar, G. Quinaldic Acid Inhibits Proliferation of Colon Cancer Ht-29 Cells in Vitro: Effects on Signaling Pathways. Eur. J. Pharmacol. 2015, 757, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Shadoul, W.; Kariem, E.; Ibrahim, K.; Adam, M. Simultaneous determination of etrahydrozoline hydrochloride and antazoline hydrochloride in ophthalmic solutions using hplc. AJPS 2011, 1, 29–38. [Google Scholar]
- Koźmiński, P.; Halik, P.K.; Chesori, R.; Gniazdowska, E. Overview of Dual-Acting Drug Methotrexate in Different Neurological Diseases, Autoimmune Pathologies and Cancers. Int. J. Mol. Sci. 2020, 21, 3483. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.S.L.; Cronstein, B.N. Mechanisms of Action of Methotrexate. Bull. Hosp. Jt. Dis. 2013, 71 (Suppl. S1), S5–S8. [Google Scholar]
- Nakayama, M.; Suzuki, K.; Toda, M.; Okubo, S.; Hara, Y.; Shimamura, T. Inhibition of the Infectivity of Influenza Virus by Tea Polyphenols. Antivir. Res. 1993, 21, 289–299. [Google Scholar] [CrossRef]
- Song, J.-M.; Lee, K.-H.; Seong, B.-L. Antiviral Effect of Catechins in Green Tea on Influenza Virus. Antivir. Res. 2005, 68, 66–74. [Google Scholar] [CrossRef]
- Williamson, M.P.; McCormick, T.G.; Nance, C.L.; Shearer, W.T. Epigallocatechin Gallate, the Main Polyphenol in Green Tea, Binds to the T-Cell Receptor, CD4: Potential for HIV-1 Therapy. J. Allergy Clin. Immunol. 2006, 118, 1369–1374. [Google Scholar] [CrossRef]
- Nishimura, H.; Okamoto, M.; Dapat, I.; Katsumi, M.; Oshitani, H. Inactivation of SARS-CoV-2 by Catechins from Green Tea. Jpn. J. Infect. Dis. 2021, 74, 421–423. [Google Scholar] [CrossRef]
- Lyu, S.-Y.; Rhim, J.-Y.; Park, W.-B. Antiherpetic Activities of Flavonoids against Herpes Simplex Virus Type 1 (HSV-1) and Type 2 (HSV-2) in Vitro. Arch. Pharm. Res. 2005, 28, 1293–1301. [Google Scholar] [CrossRef]
- Tyring, S.K. Effect of Sinecatechins on HPV-Activated Cell Growth and Induction of Apoptosis. J. Clin. Aesthet. Dermatol. 2012, 5, 34–41. [Google Scholar]
- Montalvão, S.; Leino, T.O.; Kiuru, P.S.; Lillsunde, K.-E.; Yli-Kauhaluoma, J.; Tammela, P. Synthesis and Biological Evaluation of 2-Aminobenzothiazole and Benzimidazole Analogs Based on the Clathrodin Structure. Arch. Pharm. 2016, 349, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Melano, I.; Kuo, L.-L.; Lo, Y.-C.; Sung, P.-W.; Tien, N.; Su, W.-C. Effects of Basic Amino Acids and Their Derivatives on SARS-CoV-2 and Influenza-A Virus Infection. Viruses 2021, 13, 1301. [Google Scholar] [CrossRef]
- Jang, Y.; Shin, J.S.; Lee, J.-Y.; Shin, H.; Kim, S.J.; Kim, M. In Vitro and In Vivo Antiviral Activity of Nylidrin by Targeting the Hemagglutinin 2-Mediated Membrane Fusion of Influenza A Virus. Viruses 2020, 12, 581. [Google Scholar] [CrossRef]
- Harmenberg, J.; Åkesson-Johansson, A.; Gräslund, A.; Malmfors, T.; Bergman, J.; Wahren, B.; Åkerfeldt, S.; Lundblad, L.; Cox, S. The Mechanism of Action of the Anti-Herpes Virus Compound 2,3-Dimethyl-6(2-Dimethylaminoethyl)-6H-Indolo-(2,3-B)quinoxaline. Antiviral Res. 1991, 15, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-J.; Yu, K.-M.; Kim, Y.-I.; Kim, S.-M.; Kim, E.-H.; Kim, S.-G.; Kim, E.J.; Casel, M.A.B.; Rollon, R.; Jang, S.-G.; et al. Antiviral Efficacies of FDA-Approved Drugs against SARS-CoV-2 Infection in Ferrets. MBio 2020, 11, e01114-20. [Google Scholar] [CrossRef] [PubMed]
- Marrazzo, J.M.; Rabe, L.; Kelly, C.; Richardson, B.; Deal, C.; Schwartz, J.L.; Chirenje, Z.M.; Piper, J.; Morrow, R.A.; Hendrix, C.W.; et al. Tenofovir Gel for Prevention of Herpes Simplex Virus Type 2 Acquisition: Findings From the VOICE Trial. J. Infect. Dis. 2019, 219, 1940–1947. [Google Scholar] [CrossRef]
- Leinberger, J.; Holste, J.; Bunk, B.; Freese, H.M.; Spröer, C.; Dlugosch, L.; Kück, A.-C.; Schulz, S.; Brinkhoff, T. High Potential for Secondary Metabolite Production of Paracoccus Marcusii CP157, Isolated from the Crustacean Cancer Pagurus. Front. Microbiol. 2021, 12, 688754. [Google Scholar] [CrossRef]
- Cavanaugh, N.T.; Parthasarathy, A.; Wong, N.H.; Steiner, K.K.; Chu, J.; Adjei, J.; Hudson, A.O. Exiguobacterium Sp. Is Endowed with Antibiotic Properties against Gram Positive and Negative Bacteria. BMC Res. Notes 2021, 14, 230. [Google Scholar] [CrossRef]
- Orsod, M.; Joseph, M.; Huyop, F. Characterization of Exopolysaccharides Produced by Bacillus cereus and Brachybacterium sp. Isolated from Asian Sea Bass (Lates calcarifer). MJM 2012, 8, 170–174. [Google Scholar] [CrossRef]
- Llamas, I.; Mata, J.A.; Tallon, R.; Bressollier, P.; Urdaci, M.C.; Quesada, E.; Béjar, V. Characterization of the Exopolysaccharide Produced by Salipiger Mucosus A3, a Halophilic Species Belonging to the Alphaproteobacteria, Isolated on the Spanish Mediterranean Seaboard. Mar. Drugs 2010, 8, 2240–2251. [Google Scholar] [CrossRef]
- Ramanathan, S.; Wolynec, C.; Cutting, W. Antiviral Principles of Propionibacteria-Isolation and Activity of Propionins B and C. Proc. Soc. Exp. Biol. Med. 1968, 129, 73–77. [Google Scholar] [CrossRef]
- Kokoulin, M.S.; Kuzmich, A.S.; Romanenko, L.A.; Chikalovets, I.V.; Chernikov, O.V. Structure and in Vitro Bioactivity against Cancer Cells of the Capsular Polysaccharide from the Marine Bacterium Psychrobacter Marincola. Mar. Drugs 2020, 18, 268. [Google Scholar] [CrossRef]
- Li, H.; Shinde, P.B.; Lee, H.J.; Yoo, E.S.; Lee, C.-O.; Hong, J.; Choi, S.H.; Jung, J.H. Bile Acid Derivatives from a Sponge-Associated Bacterium Psychrobacter Sp. Arch. Pharm. Res. 2009, 32, 857–862. [Google Scholar] [CrossRef]
- Noguera-Ortega, E.; Guallar-Garrido, S.; Julián, E. Mycobacteria-Based Vaccines as Immunotherapy for Non-Urological Cancers. Cancers 2020, 12, 1802. [Google Scholar] [CrossRef] [PubMed]
- Setiawan, A.; Setiawan, F.; Juliasih, N.L.G.R.; Widyastuti, W.; Laila, A.; Setiawan, W.A.; Djailani, F.M.; Mulyono, M.; Hendri, J.; Arai, M. Fungicide Activity of Culture Extract from Kocuria Palustris 19C38A1 against Fusarium Oxysporum. J. Fungi 2022, 8, 280. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Xu, M.; Liu, H.; Yu, T.; Guo, P.; Liu, W.; Jin, X. Antimicrobial Compounds Were Isolated from the Secondary Metabolites of Gordonia, a Resident of Intestinal Tract of Periplaneta Americana. AMB Express 2021, 11, 111. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.R.; Kim, J.; Yu, J.S.; Lee, B.S.; Kim, K.H.; Kim, C.S. Isolation, Structure Elucidation, Total Synthesis, and Biosynthesis of Dermazolium A, an Antibacterial Imidazolium Metabolite of a Vaginal Bacterium Dermabacter Vaginalis. Arch. Pharm. Res. 2023, 46, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Sangkanu, S.; Rukachaisirikul, V.; Suriyachadkun, C.; Phongpaichit, S. Evaluation of Antibacterial Potential of Mangrove Sediment-Derived Actinomycetes. Microb. Pathog. 2017, 112, 303–312. [Google Scholar] [CrossRef]
- Sangkanu, S.; Rukachaisirikul, V.; Suriyachadkun, C.; Phongpaichit, S. Antifungal Activity of Marine-derived Actinomycetes against Talaromyces Marneffei. J. Appl. Microbiol. 2021, 130, 1508–1522. [Google Scholar] [CrossRef]
- Liu, H.; Chen, Z.; Zhu, G.; Wang, L.; Du, Y.; Wang, Y.; Zhu, W. Phenolic Polyketides from the Marine Alga-Derived Streptomyces Sp. OUCMDZ-3434. Tetrahedron 2017, 73, 5451–5455. [Google Scholar] [CrossRef]
- Wang, P.; Xi, L.; Liu, P.; Wang, Y.; Wang, W.; Huang, Y.; Zhu, W. Diketopiperazine Derivatives from the Marine-Derived Actinomycete Streptomyces Sp. FXJ7.328. Mar. Drugs 2013, 11, 1035–1049. [Google Scholar] [CrossRef] [PubMed]
- Carter-Timofte, M.E.; Arulanandam, R.; Kurmasheva, N.; Fu, K.; Laroche, G.; Taha, Z.; van der Horst, D.; Cassin, L.; van der Sluis, R.M.; Palermo, E.; et al. Antiviral Potential of the Antimicrobial Drug Atovaquone against SARS-CoV-2 and Emerging Variants of Concern. ACS Infect. Dis. 2021, 7, 3034–3051. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, Y.; Kobayashi, T.; Izawa, M. Indanostatin, a New Neuroprotective Compound from Streptomyces Sp. J. Antibiot. 2013, 66, 731–733. [Google Scholar] [CrossRef] [PubMed]
- Hongpattarakere, T.; Cherntong, N.; Wichienchot, S.; Kolida, S.; Rastall, R.A. In Vitro Prebiotic Evaluation of Exopolysaccharides Produced by Marine Isolated Lactic Acid Bacteria. Carbohydr. Polym. 2012, 87, 846–852. [Google Scholar] [CrossRef]
- Changyun, W.; Haiyan, L.; Changlun, S.; Yanan, W.; Liang, L.; Huashi, G. Chemical defensive substances of soft corals and gorgonians. Acta Ecol. Sin. 2008, 28, 2320–2328. [Google Scholar] [CrossRef]
- Jimenez, C. Marine natural products in medicinal chemistry. ACS Med. Chem. Lett. 2018, 9, 959–961. [Google Scholar] [CrossRef]
- Choi, Y.K. Emerging and re-emerging fatal viral diseases. Exp. Mol. Med. 2021, 53, 711–712. [Google Scholar] [CrossRef]
- Rashid, M.A.; Gustafson, K.R.; Boyd, M.R. HIV-inhibitory cembrane derivatives from a Philippines collection of the soft coral Lobophytum species. J. Nat. Prod. 2000, 63, 531–533. [Google Scholar] [CrossRef]
- Cheng, S.-Y.; Wen, Z.-H.; Chiou, S.-F.; Hsu, C.-H.; Wang, S.-K.; Dai, C.-F.; Chiang, M.Y.; Duh, C.-Y. Durumolides A–E, Anti-Inflammatory and Antibacterial Cembranolides from the Soft Coral Lobophytum Durum. Tetrahedron 2008, 64, 9698–9704. [Google Scholar] [CrossRef]
- Cheng, S.-Y.; Chen, P.-W.; Chen, H.-P.; Wang, S.-K.; Duh, C.-Y. New Cembranolides from the Dongsha Atoll Soft Coral Lobophytum Durum. Mar. Drugs 2011, 9, 1307–1318. [Google Scholar] [CrossRef]
- Xia, Z.-Y.; Sun, M.-M.; Jin, Y.; Yao, L.-G.; Su, M.-Z.; Liang, L.-F.; Wang, H.; Guo, Y.-W. Lobosteroids A-F: Six New Highly Oxidized Steroids from the Chinese Soft Coral Lobophytum Sp. Mar. Drugs 2023, 21, 457. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-H.; Ahmed, A.F.; Yang, T.-S.; Lin, Y.-C.; Huang, C.-Y.; Hwang, T.-L.; Sheu, J.-H. Isolation of Lobane and Prenyleudesmane Diterpenoids from the Soft Coral Lobophytum Varium. Mar. Drugs 2020, 18, 223. [Google Scholar] [CrossRef] [PubMed]
- Mhatre, S.; Gurav, N.; Shah, M.; Patravale, V. Entry-Inhibitory Role of Catechins against SARS-CoV-2 and Its UK Variant. Comput. Biol. Med. 2021, 135, 104560. [Google Scholar] [CrossRef] [PubMed]
- Ocky, K.; Radjasa; Wiese, J.; Sabdono, A.; Imhoff, J. Coral as Source of Bacteria with Antimicrobial Activity. J. Coast. Dev. ISSN 2008, 11, 1410–5217. [Google Scholar]
- Van de Water, J.A.J.M.; Allemand, D.; Ferrier-Pagès, C. Host-Microbe Interactions in Octocoral Holobionts—Recent Advances and Perspectives. Microbiome 2018, 6, 64. [Google Scholar] [CrossRef]
- Romano, S.; Jackson, S.A.; Patry, S.; Dobson, A.D.W. Extending the “One Strain Many Compounds” (OSMAC) Principle to Marine Microorganisms. Mar. Drugs 2018, 16, 244. [Google Scholar] [CrossRef]
- Kausar, S.; Said Khan, F.; Ishaq Mujeeb Ur Rehman, M.; Akram, M.; Riaz, M.; Rasool, G.; Hamid Khan, A.; Saleem, I.; Shamim, S.; Malik, A. A Review: Mechanism of Action of Antiviral Drugs. Int. J. Immunopathol. Pharmacol. 2021, 35, 20587384211002621. [Google Scholar] [CrossRef]
- McFadden, C.S.; Alderslade, P.; van Ofwegen, L.P.; Johnsen, H.; Rusmevichientong, A. Phylogenetic Relationships within the Tropical Soft Coral Genera Sarcophyton and Lobophytum (Anthozoa, Octocorallia). Invertebr. Biol. 2006, 125, 288–305. [Google Scholar] [CrossRef]
- Arokiyaraj, S.; Perinbam, K.; Agastian, P.; Mohan Kumar, R. Phytochemical Analysis and Antibacterial Activity of Vitex Agnus-Castus. Int. J. Green Pharm. IJGP 2009, 3, 162–164. [Google Scholar] [CrossRef]
- Gong, X.; Wang, J.; Zhang, M.; Wang, P.; Wang, C.; Shi, R.; Zang, E.; Zhang, M.; Zhang, C.; Li, M. Bioactivity, Compounds Isolated, Chemical Qualitative, and Quantitative Analysis of Cymbaria Daurica Extracts. Front. Pharmacol. 2020, 11, 48. [Google Scholar] [CrossRef]
- Wang, Z.; Chu, Y.; Zhang, Y.; Chen, Y.; Zhang, J.; Chen, X. Investigation of Potential Toxic Components Based on the Identification of Genkwa Flos Chemical Constituents and Their Metabolites by High-Performance Liquid Chromatography Coupled with a Q Exactive High-Resolution Benchtop Quadrupole Orbitrap Mass Spectrometer. J. Sep. Sci. 2018, 41, 3328–3338. [Google Scholar] [CrossRef]
- Lu, T.; Liu, Y.; Zhou, L.; Liao, Q.; Nie, Y.; Wang, X.; Lei, X.; Hong, P.; Feng, Y.; Hu, X.; et al. The Screening for Marine Fungal Strains with High Potential in Alkaloids Production by in Situ Colony Assay and LC-MS/MS Based Secondary Metabolic Profiling. Front. Microbiol. 2023, 14, 1144328. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Maruyama, F.; Kato, H.; Toyoda, A.; Dozono, A.; Ohtsubo, Y.; Nagata, Y.; Fujiyama, A.; Tsuda, M.; Kurokawa, K. Design and Experimental Application of a Novel Non-Degenerate Universal Primer Set That Amplifies Prokaryotic 16S rRNA Genes with a Low Possibility to Amplify Eukaryotic rRNA Genes. DNA Res. 2014, 21, 217–227. [Google Scholar] [CrossRef]
- Alsharif, S.M.; Waznah, M.S.; Ismaeil, M.; El-Sayed, W.S. 16S rDNA-Based Diversity Analysis of Bacterial Communities Associated with Soft Corals of the Red Sea, Al Rayyis, White Head, KSA. J. Taibah Univ. Sci. 2023, 17, 2156762. [Google Scholar] [CrossRef]
- Buck, C.B.; Pastrana, D.V.; Lowy, D.R.; Schiller, J.T. Efficient Intracellular Assembly of Papillomaviral Vectors. J. Virol. 2004, 78, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Buck, C.B.; Pastrana, D.V.; Lowy, D.R.; Schiller, J.T. Generation of HPV Pseudovirions Using Transfection and Their Use in Neutralization Assays. Methods Mol. Med. 2005, 119, 445–462. [Google Scholar] [CrossRef]
- Mou, H.; Quinlan, B.D.; Peng, H.; Liu, G.; Guo, Y.; Peng, S.; Zhang, L.; Davis-Gardner, M.E.; Gardner, M.R.; Crynen, G.; et al. Mutations Derived from Horseshoe Bat ACE2 Orthologs Enhance ACE2-Fc Neutralization of SARS-CoV-2. PLoS Pathog. 2021, 17, e1009501. [Google Scholar] [CrossRef]
- Rogers, T.F.; Zhao, F.; Huang, D.; Beutler, N.; Burns, A.; He, W.-T.; Limbo, O.; Smith, C.; Song, G.; Woehl, J.; et al. Isolation of Potent SARS-CoV-2 Neutralizing Antibodies and Protection from Disease in a Small Animal Model. Science 2020, 369, 956–963. [Google Scholar] [CrossRef]
- Schäfer, G.; Graham, L.M.; Lang, D.M.; Blumenthal, M.J.; Bergant Marušič, M.; Katz, A.A. Vimentin Modulates Infectious Internalization of Human Papillomavirus 16 Pseudovirions. J. Virol. 2017, 91, e00307-17. [Google Scholar] [CrossRef]
- Bergant Marušič, M.; Ozbun, M.A.; Campos, S.K.; Myers, M.P.; Banks, L. Human Papillomavirus L2 Facilitates Viral Escape from Late Endosomes via Sorting Nexin 17. Traffic 2012, 13, 455–467. [Google Scholar] [CrossRef]
- Van Meerloo, J.; Kaspers, G.J.L.; Cloos, J. Cell Sensitivity Assays: The MTT Assay. In Cancer Cell Culture: Methods and Protocols; Cree, I.A., Ed.; Humana Press: Totowa, NJ, USA, 2011; pp. 237–245. [Google Scholar] [CrossRef]
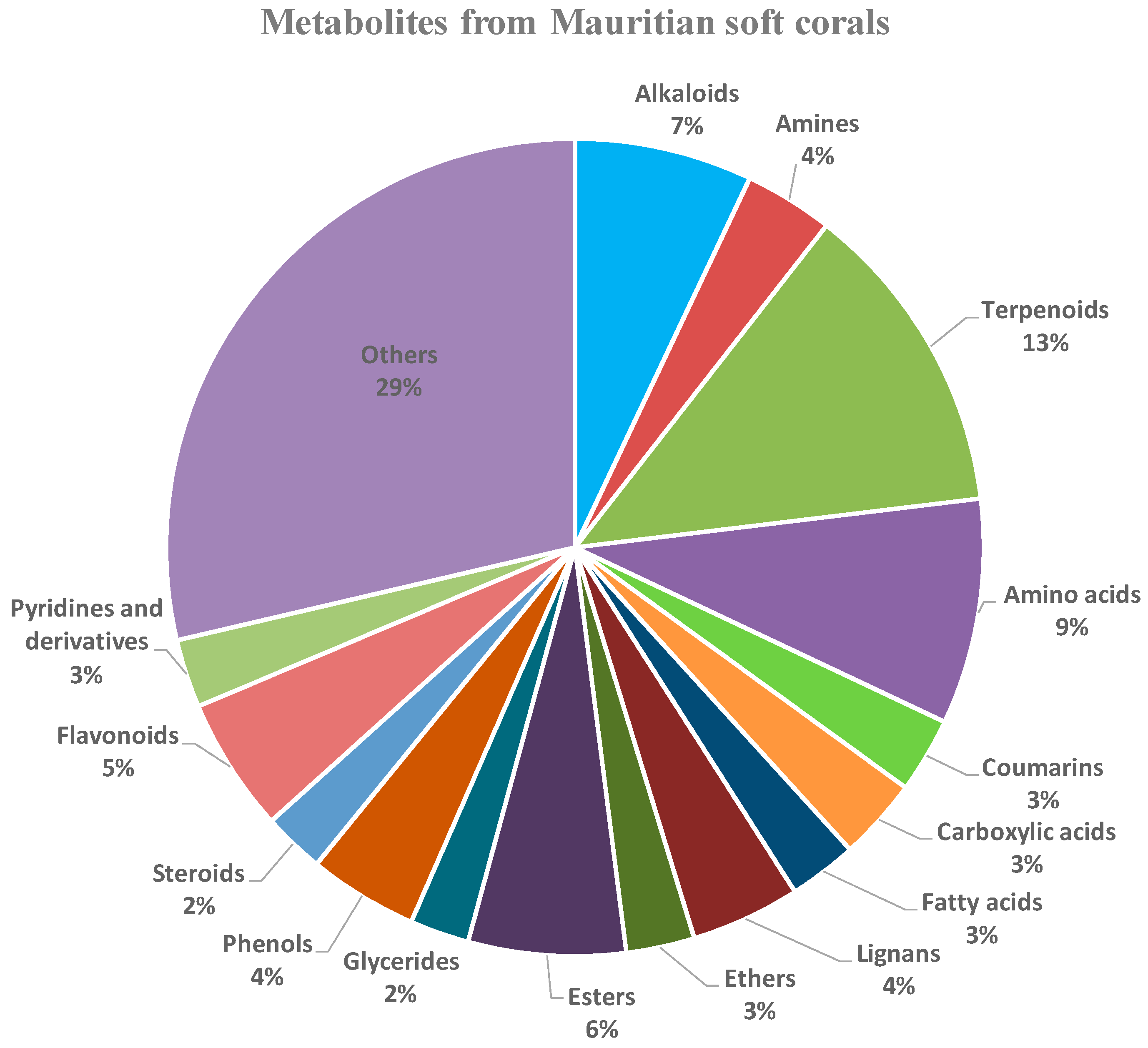
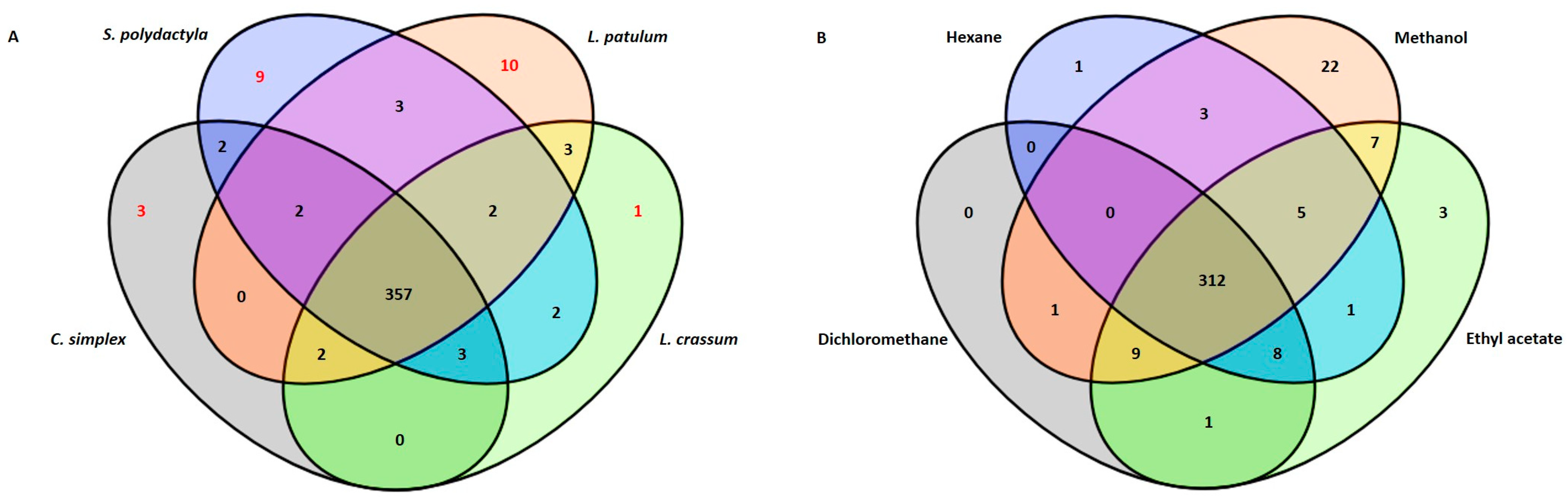


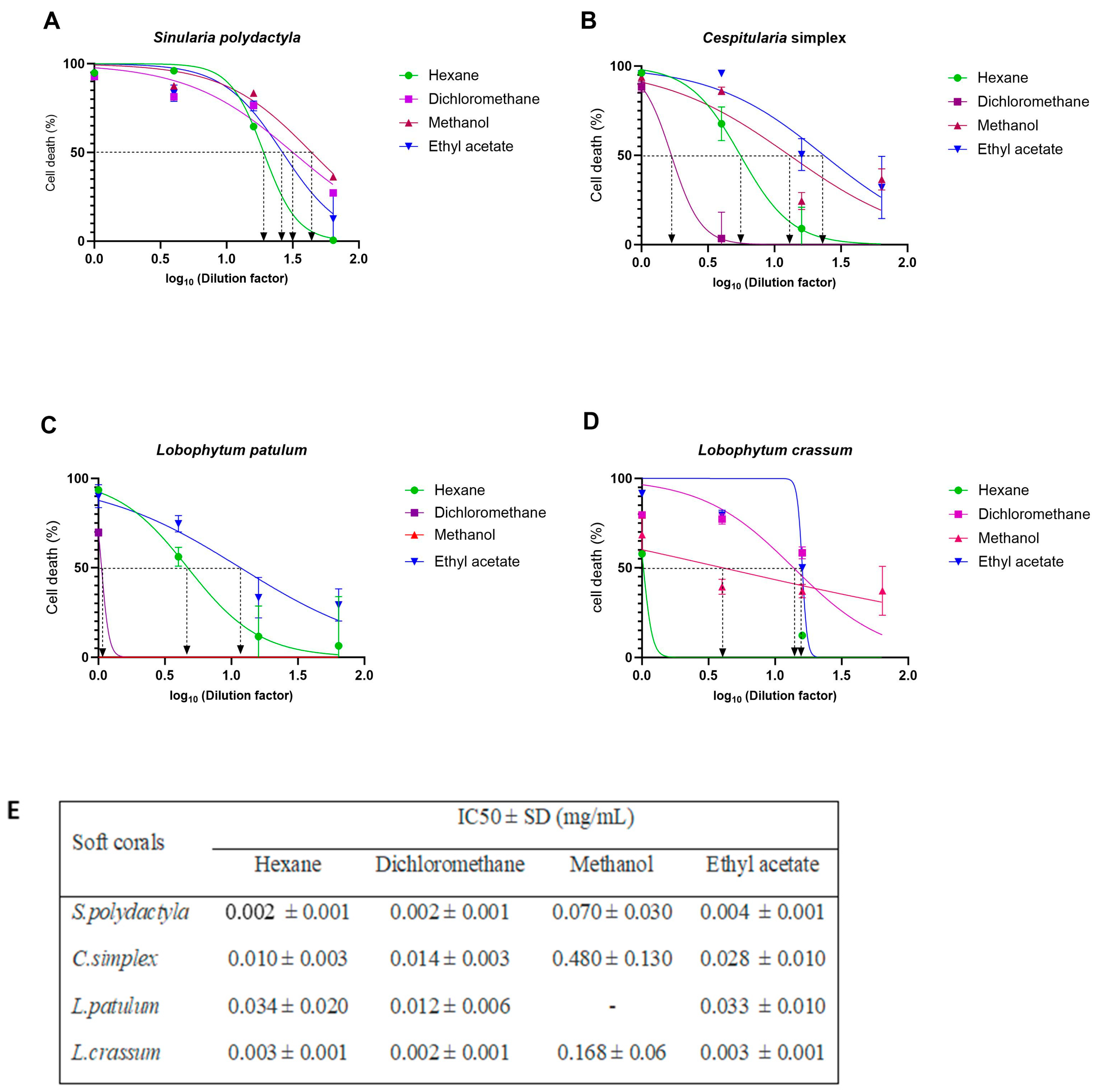
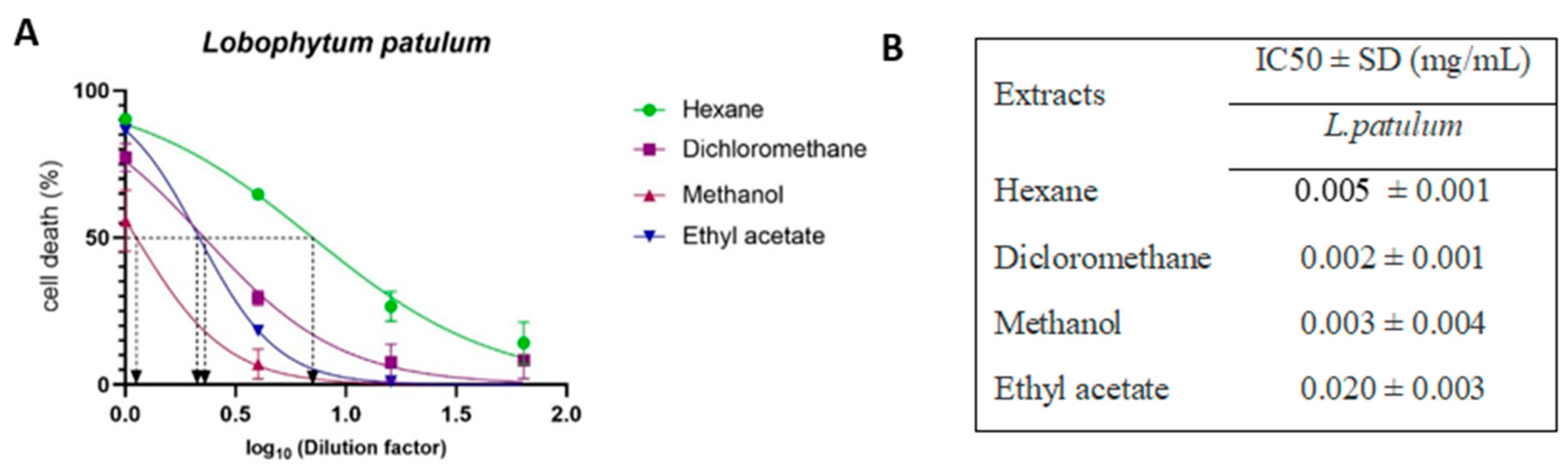
| Soft Corals | Metabolite Name | Antiviral Activities | References |
|---|---|---|---|
| S. polydactyla,
C. simplex, L. patulum, L. crassum | Catechin | Human immunodeficiency virus; Herpes simplex virus; Influenza virus; Hepatitis B and C virus; SARS-CoV-2; Human papilloma virus | [47,48,49,50,51,52] |
| 2-Aminobenzothiazole | Hepatitis C virus | [53] | |
| Lysine | SARS-CoV-2; Influenza A virus | [54] | |
| Nylidrin | Influenza A virus | [55] | |
| Quinoxaline | Herpes simplex virus | [56] | |
| Tenofovir | SARS-CoV-2; Herpes simplex virus; Human immunodeficiency virus | [57,58] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jahajeeah, D.; Ranghoo-Sanmukhiya, M.; Schäfer, G. Metabolic Profiling, Antiviral Activity and the Microbiome of Some Mauritian Soft Corals. Mar. Drugs 2023, 21, 574. https://doi.org/10.3390/md21110574
Jahajeeah D, Ranghoo-Sanmukhiya M, Schäfer G. Metabolic Profiling, Antiviral Activity and the Microbiome of Some Mauritian Soft Corals. Marine Drugs. 2023; 21(11):574. https://doi.org/10.3390/md21110574
Chicago/Turabian StyleJahajeeah, Deeya, Mala Ranghoo-Sanmukhiya, and Georgia Schäfer. 2023. "Metabolic Profiling, Antiviral Activity and the Microbiome of Some Mauritian Soft Corals" Marine Drugs 21, no. 11: 574. https://doi.org/10.3390/md21110574
APA StyleJahajeeah, D., Ranghoo-Sanmukhiya, M., & Schäfer, G. (2023). Metabolic Profiling, Antiviral Activity and the Microbiome of Some Mauritian Soft Corals. Marine Drugs, 21(11), 574. https://doi.org/10.3390/md21110574







