Alkaline Phosphatase PhoD Mutation Induces Fatty Acid and Long-Chain Polyunsaturated Fatty Acid (LC-PUFA)-Bound Phospholipid Production in the Model Diatom Phaeodactylum tricornutum
Abstract
1. Introduction
2. Results
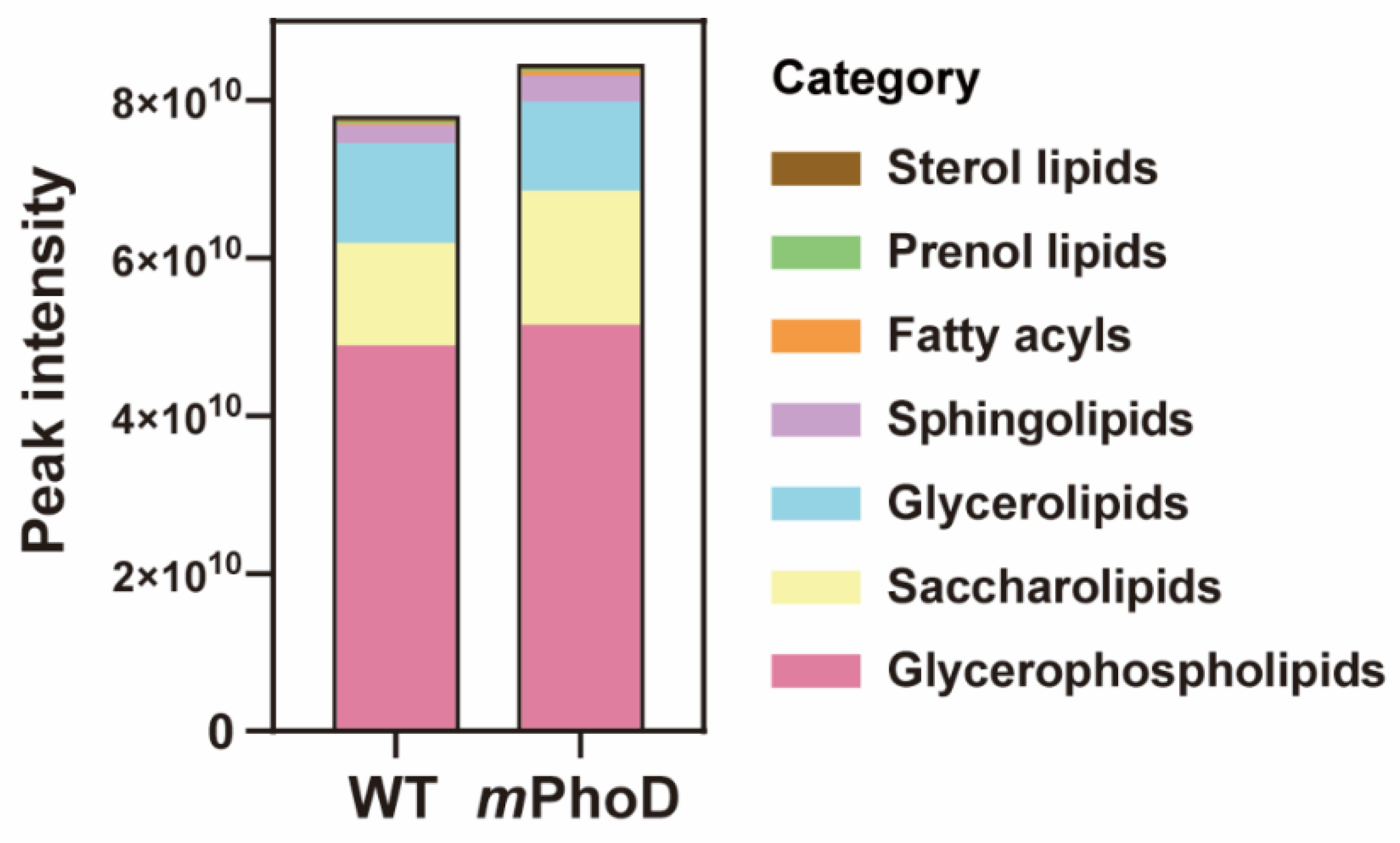
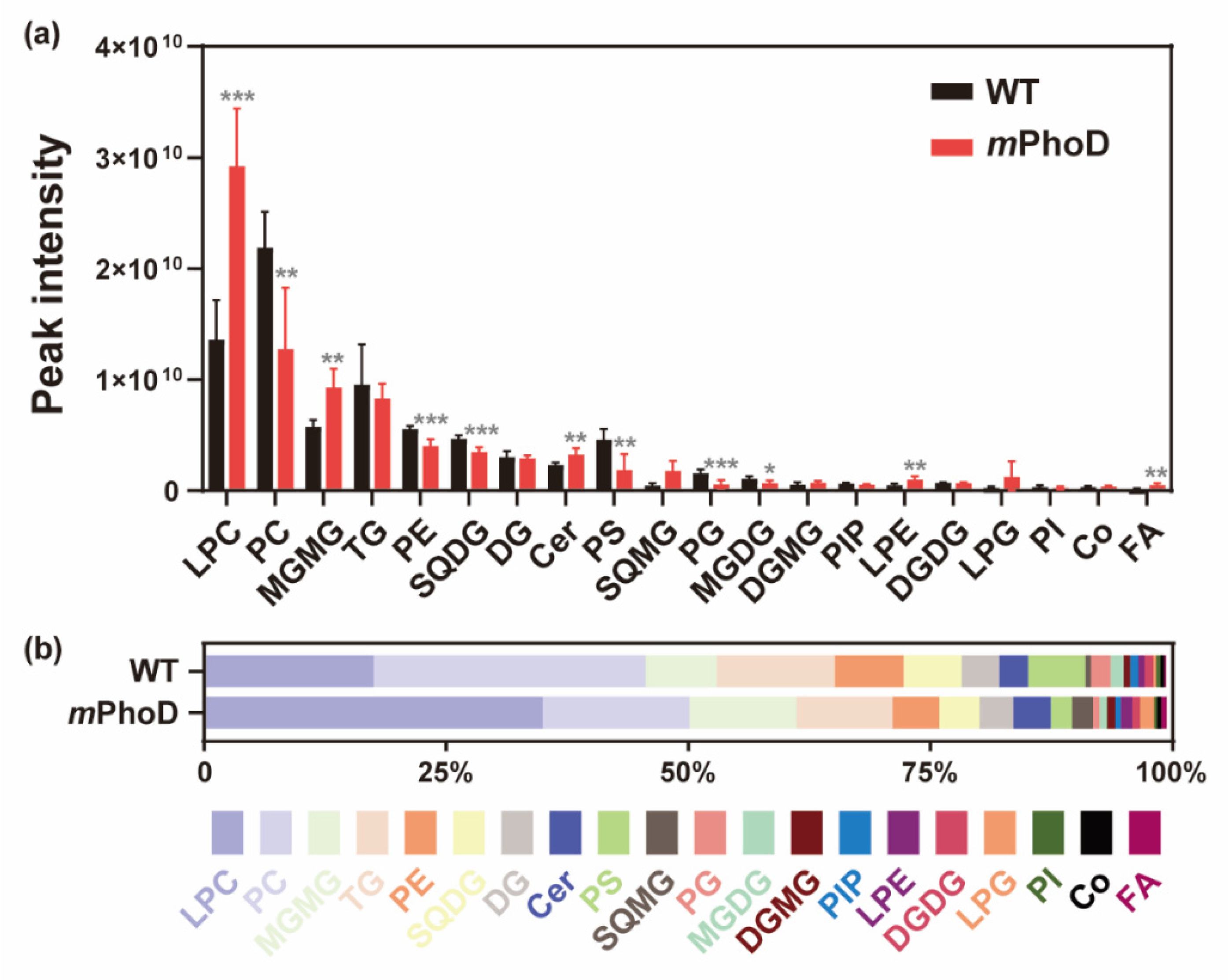
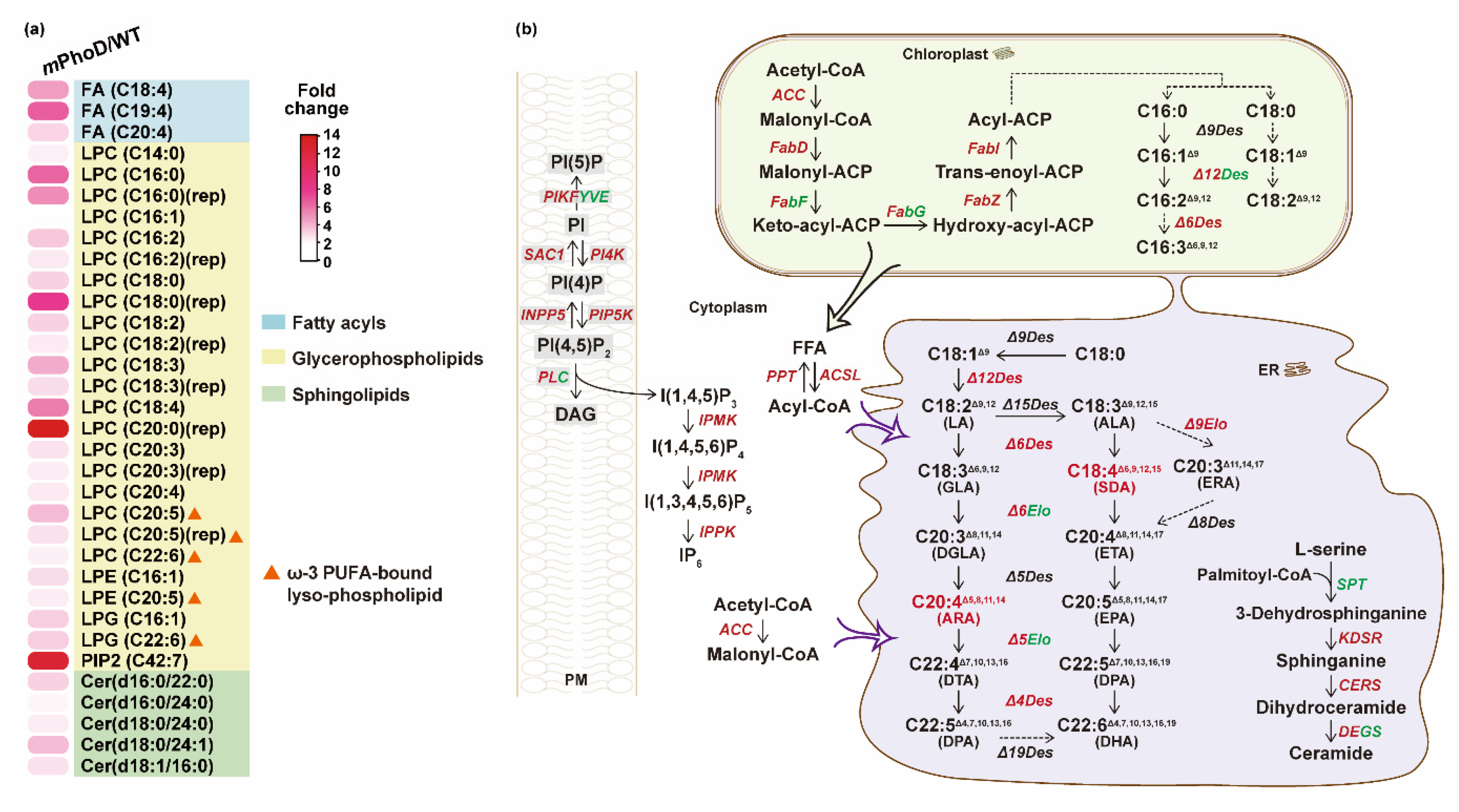
2.1. Lipidomic Profile in mPhoD Cells
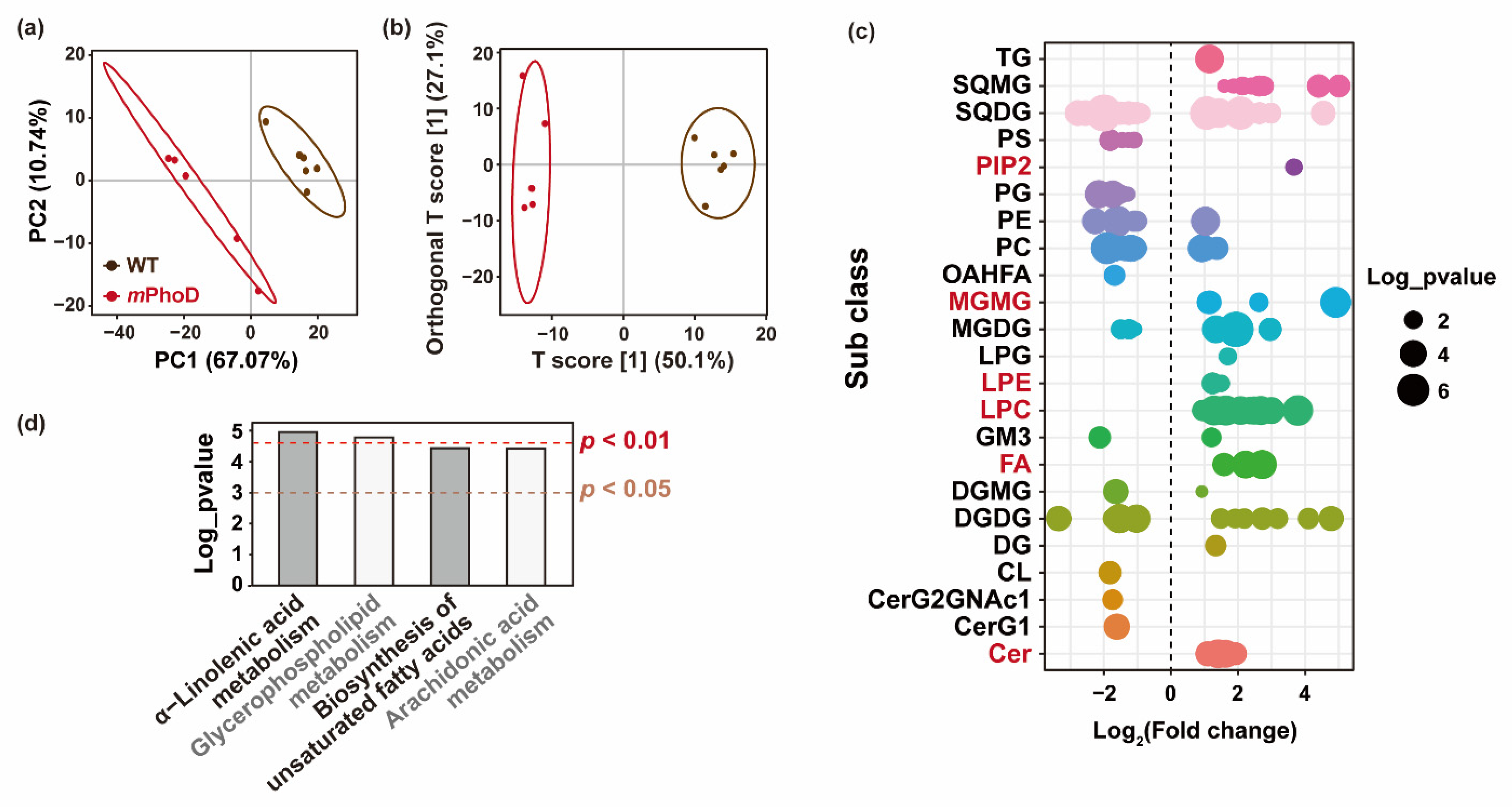
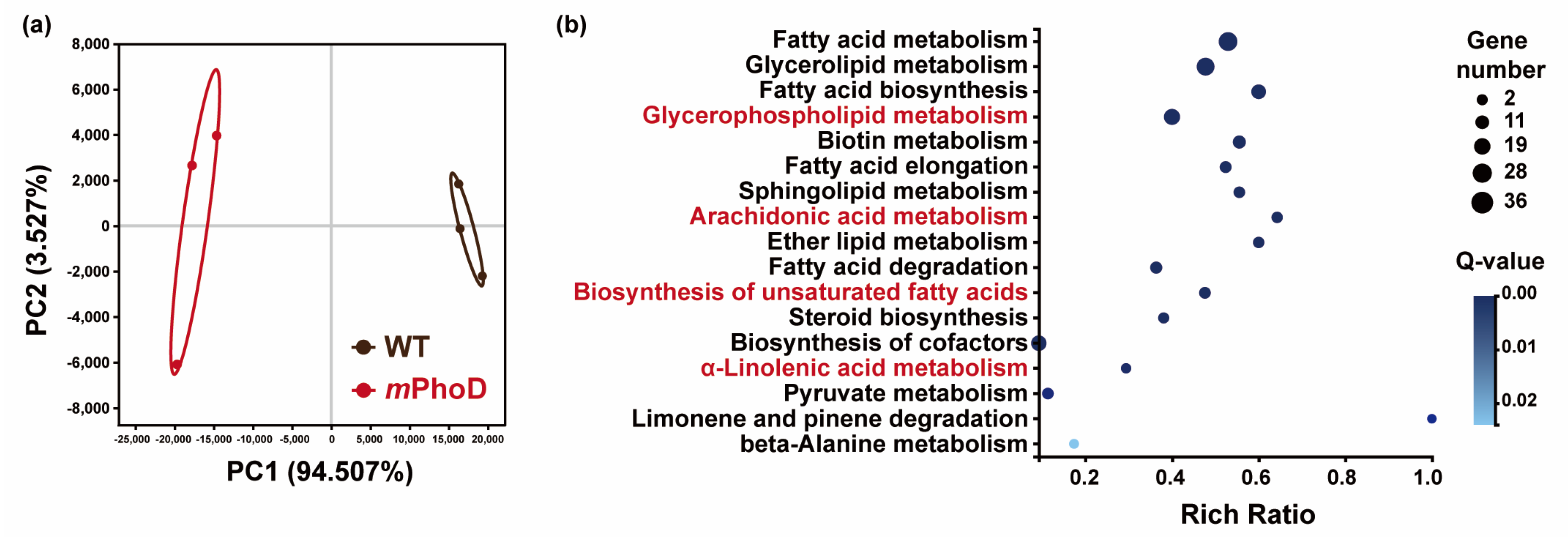
2.2. Differential Lipids and Lipid Metabolism-Related DEGs in the mPhoD/WT Comparison
2.3. Increased FA, PUFAs, LPC, PIP2, and Cer Synthesis in Both Transcriptomic and Lipidomic Levels after PhoD_45757 Mutation
3. Discussion
3.1. Improved Ceramide Synthesis after PhoD_45757 Mutation
3.2. PhoD_45757 Mutation Could Improve the Production and Potential Bioavailability of PUFAs
4. Materials and Methods
4.1. Mutant Culture and Cell Collection
4.2. Lipid Extraction and UPLC-MS Analysis
4.3. Lipid Identification and Annotation
4.4. Gene Expression Analysis and Differentially Expressed Genes Identification
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| FA | Fatty acid |
| PUFA | Polyunsaturated fatty acid |
| LC-PUFA | Long-chain polyunsaturated fatty acid |
| LPC | Lysophosphatidylcholine |
| LPE | Lysophosphatidylethanolamine |
| LPI | Lysophosphatidylinositol |
| LPG | Lysophosphatidylglycerol |
| Cer | Ceramide |
| MGMG | Monogalactosylmonoacylglycerol |
| PIP2 | Phosphatidylinositol bisphosphate |
| DHA | Docosahexaenoic acid |
| EPA | Eicosapentaenoic acid |
| ARA | Arachidonic acid |
References
- Guschina, I.A.; Harwood, J.L. Lipids and lipid metabolism in eukaryotic algae. Prog. Lipid Res. 2006, 45, 160–186. [Google Scholar] [CrossRef] [PubMed]
- Rodolfi, L.; Chini Zittelli, G.; Bassi, N.; Padovani, G.; Biondi, N.; Bonini, G.; Tredici, M.R. Microalgae for oil: Strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnol. Bioeng. 2009, 102, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Schenk, P.M.; Thomas-Hall, S.R.; Stephens, E.; Marx, U.C.; Mussgnug, J.H.; Posten, C.; Kruse, O.; Hankamer, B. Second generation biofuels: High-efficiency microalgae for Biodiesel production. BioEnergy Res. 2008, 1, 20–43. [Google Scholar] [CrossRef]
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Halim, R.; Danquah, M.K.; Webley, P.A. Extraction of oil from microalgae for biodiesel production: A review. Biotechnol. Adv. 2012, 30, 709–732. [Google Scholar] [CrossRef]
- Khozin-Goldberg, I. Lipid metabolism in microalgae. In The Physiology of Microalgae; Michael, A., Borowitzka, J.B., Raven, J.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 413–484. [Google Scholar]
- Innis, S.M. Dietary omega 3 fatty acids and the developing brain. Brain Res. 2008, 1237, 35–43. [Google Scholar] [CrossRef]
- Dalsgaard, J.; John, M.S.; Kattner, G.; Müller-Navarra, D.; Hagen, W. Fatty acid trophic markers in the pelagic marine environment. Adv. Mar. Biol. 2003, 46, 225–340. [Google Scholar]
- Harris, W.S.; Miller, M.; Tighe, A.P.; Davidson, M.H.; Schaefer, E.J. Omega-3 fatty acids and coronary heart disease risk: Clinical and mechanistic perspectives. Atherosclerosis 2008, 197, 12–24. [Google Scholar] [CrossRef]
- Lagarde, M.; Bernoud, N.; Brossard, N.; Lemaitre-Delaunay, D.; Thiès, F.; Croset, M.; Lecerf, J. Lysophosphatidylcholine as a Preferred Carrier Form of Docosahexaenoic Acid to the Brain. J. Mol. Neurosci. 2001, 16, 201–204, discussion 215–221. [Google Scholar] [CrossRef]
- Calder, P.C. Functional roles of fatty acids and their effects on human health. J. Parenter. Enter. Nutr. 2015, 39 (Suppl. S1), 18S–32S. [Google Scholar] [CrossRef]
- Tallima, H.; El Ridi, R. Arachidonic acid: Physiological roles and potential health benefits—A review. J. Adv. Res. 2018, 11, 33–41. [Google Scholar] [CrossRef] [PubMed]
- De Luca, M.; Pappalardo, I.; Limongi, A.R.; Viviano, E.; Radice, R.P.; Todisco, S.; Martelli, G.; Infantino, V.; Vassallo, A. Lipids from microalgae for cosmetic applications. Cosmetics 2021, 8, 52. [Google Scholar] [CrossRef]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for biodiesel production and other applications: A review. Renew. Sustain. Energy Rev. 2010, 14, 217–232. [Google Scholar] [CrossRef]
- Sayanova, O.; Mimouni, V.; Ulmann, L.; Morant-Manceau, A.; Pasquet, V.; Schoefs, B.; Napier, J.A.; Olga, S.; Virginie, M.; Lionel, U.; et al. Modulation of lipid biosynthesis by stress in diatoms. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160407. [Google Scholar] [CrossRef]
- Moll, K.; Gardner, R.; Eustance, E.; Gerlach, R.; Peyton, B. Combining multiple nutrient stresses and bicarbonate addition to promote lipid accumulation in the diatom RGd-1. Algal Res. 2014, 5, 7–15. [Google Scholar] [CrossRef][Green Version]
- Levitan, O.; Dinamarca, J.; Zelzion, E.; Lun, D.S.; Guerra, L.T.; Kim, M.K.; Kim, J.; Van Mooy, B.A.S.; Bhattacharya, D.; Falkowski, P.G. Remodeling of intermediate metabolism in the diatom Phaeodactylum tricornutum under nitrogen stress. Proc. Natl. Acad. Sci. USA 2015, 112, 412–417. [Google Scholar] [CrossRef]
- Converti, A.; Casazza, A.A.; Ortiz, E.Y.; Perego, P.; Del Borghi, M. Effect of temperature and nitrogen concentration on the growth and lipid content of Nannochloropsis oculata and Chlorella vulgaris for biodiesel production. Chem. Eng. Process. Process. Intensif. 2009, 48, 1146–1151. [Google Scholar] [CrossRef]
- Xin, L.; Hong-Ying, H.; Ke, G.; Ying-Xue, S. Effects of different nitrogen and phosphorus concentrations on the growth, nutrient uptake, and lipid accumulation of a freshwater microalga Scenedesmus sp. Bioresour. Technol. 2010, 101, 5494–5500. [Google Scholar] [CrossRef]
- Liu, Z.-Y.; Wang, G.-C.; Zhou, B.-C. Effect of iron on growth and lipid accumulation in Chlorella vulgaris. Bioresour. Technol. 2008, 99, 4717–4722. [Google Scholar] [CrossRef]
- Van Mooy, B.A.S.; Fredricks, H.F.; Pedler, B.E.; Dyhrman, S.T.; Karl, D.M.; Koblížek, M.; Lomas, M.W.; Mincer, T.J.; Moore, L.R.; Moutin, T.; et al. Phytoplankton in the ocean use non-phosphorus lipids in response to phosphorus scarcity. Nature 2009, 458, 69–72. [Google Scholar] [CrossRef]
- Martin, P.; Van Mooy, B.A.; Heithoff, A.; Dyhrman, S.T. Phosphorus supply drives rapid turnover of membrane phospholipids in the diatom Thalassiosira pseudonana. ISME J. 2011, 5, 1057–1060. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Liu, M.; Ding, W.; Liu, J. Novel insights into phosphorus deprivation boosted lipid synthesis in the marine alga Nannochloropsis oceanica without compromising biomass production. J. Agric. Food Chem. 2020, 68, 11488–11502. [Google Scholar] [CrossRef] [PubMed]
- Bai, F.; Yu, L.; Shi, J.; Li-Beisson, Y.; Liu, J. Long-chain acyl-CoA synthetases activate fatty acids for lipid synthesis, remodeling and energy production in Chlamydomonas. New Phytol. 2022, 233, 823–837. [Google Scholar] [CrossRef]
- Liu, J.; Sun, Z.; Mao, X.; Gerken, H.; Wang, X.; Yang, W. Multiomics analysis reveals a distinct mechanism of oleaginousness in the emerging model alga Chromochloris zofingiensis. Plant J. 2019, 98, 1060–1077. [Google Scholar] [CrossRef]
- Balamurugan, S.; Wang, X.; Wang, H.-L.; An, C.-J.; Li, H.; Li, D.-W.; Yang, W.-D.; Liu, J.-S.; Li, H.-Y. Occurrence of plastidial triacylglycerol synthesis and the potential regulatory role of AGPAT in the model diatom Phaeodactylum tricornutum. Biotechnol. Biofuels 2017, 10, 97. [Google Scholar] [CrossRef]
- Haslam, R.P.; Hamilton, M.L.; Economou, C.K.; Smith, R.; Hassall, K.L.; Napier, J.A.; Sayanova, O. Overexpression of an endogenous type 2 diacylglycerol acyltransferase in the marine diatom Phaeodactylum tricornutum enhances lipid production and omega-3 long-chain polyunsaturated fatty acid content. Biotechnol. Biofuels 2020, 13, 87. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.-H.; Shi, H.-P.; Yang, G.-P.; Lv, N.-N.; Yang, M.; Pan, K.-H. Silencing UDP-glucose pyrophosphorylase gene in Phaeodactylum tricornutum affects carbon allocation. New Biotechnol. 2016, 33, 237–244. [Google Scholar] [CrossRef]
- Yang, J.; Pan, Y.; Bowler, C.; Zhang, L.; Hu, H. Knockdown of phosphoenolpyruvate carboxykinase increases carbon flux to lipid synthesis in Phaeodactylum tricornutum. Algal Res. 2016, 15, 50–58. [Google Scholar] [CrossRef]
- Li, J.; Zhang, K.; Li, L.; Wang, Y.; Lin, S. Unsuspected functions of alkaline phosphatase PhoD in the diatom Phaeodactylum tricornutum. Algal Res. 2022, 68, 102873. [Google Scholar] [CrossRef]
- Zhang, K.; Li, J.; Zhou, Z.; Huang, R.; Lin, S. Roles of Alkaline phosphatase phoa in algal metabolic regulation under phosphorus-replete conditions. J. Phycol. 2021, 57, 703–707. [Google Scholar] [CrossRef]
- Edwards, B.R. Lipid biogeochemistry and modern lipidomic techniques. Annu. Rev. Mar. Sci. 2023, 15, 485–508. [Google Scholar] [CrossRef]
- Wenk, M.R. The emerging field of lipidomics. Nat. Rev. Drug Discov. 2005, 4, 594–610. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Banerjee, C.; Negi, S.; Chang, J.-S.; Shukla, P. Improvements in algal lipid production: A systems biology and gene editing approach. Crit. Rev. Biotechnol. 2018, 38, 369–385. [Google Scholar] [CrossRef] [PubMed]
- Radakovits, R.; Jinkerson, R.E.; Darzins, A.; Posewitz, M.C. Genetic engineering of algae for enhanced biofuel production. Eukaryot. Cell 2010, 9, 486–501. [Google Scholar] [CrossRef] [PubMed]
- Artamonova, E.; Svenning, J.; Vasskog, T.; Hansen, E.; Eilertsen, H. Analysis of phospholipids and neutral lipids in three common northern cold water diatoms: Coscinodiscus concinnus, Porosira glacialis, and Chaetoceros socialis, by ultra-high performance liquid chromatography-mass spectrometry. J. Appl. Phycol. 2017, 29, 1241–1249. [Google Scholar] [CrossRef]
- van Blitterswijk, W.J.; van der Luit, A.H.; Veldman, R.J.; Verheij, M.; Borst, J. Ceramide: Second messenger or modulator of membrane structure and dynamics? Biochem. J. 2003, 369, 199–211. [Google Scholar] [CrossRef]
- Montes, L.R.; Ruiz-Argüello, M.B.; Goñi, F.M.; Alonso, A. Membrane restructuring via ceramide results in enhanced solute efflux. J. Biol. Chem. 2002, 277, 11788–11794. [Google Scholar] [CrossRef] [PubMed]
- Hannun, Y.A.; Luberto, C. Ceramide in the Eukaryotic Stress Response. Trends Cell Biol. 2000, 10, 73–80. [Google Scholar] [CrossRef]
- Elhady, S.S.; Habib, E.S.; Abdelhameed, R.F.A.; Goda, M.S.; Hazem, R.M.; Mehanna, E.T.; Helal, M.A.; Hosny, K.M.; Diri, R.M.; Hassanean, H.A.; et al. Anticancer Effects of New Ceramides Isolated from the Red Sea Red Algae Hypnea musciformis in a Model of Ehrlich Ascites Carcinoma: LC-HRMS Analysis Profile and Molecular Modeling. Mar. Drugs 2022, 20, 63. [Google Scholar] [CrossRef]
- Alasalvar, C.; Shahidi, F.; Quantick, P. Food and health applications of marine nutraceuticals: A review. In Seafoods—Quality, Technology and Nutraceutical Applications; Alasalvar, C., Taylor, T., Eds.; Springer: Berlin/Heidelberg, Germany, 2002; pp. 175–204. [Google Scholar]
- Gladyshev, M.I.; Sushchik, N.N.; Makhutova, O.N. Production of EPA and DHA in aquatic ecosystems and their transfer to the land. Prostaglandins Other Lipid Mediat. 2013, 107, 117–126. [Google Scholar] [CrossRef]
- Salem, N., Jr.; Eggersdorfer, M. Is the world supply of omega-3 fatty acids adequate for optimal human nutrition? Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Lordan, R.; Redfern, S.; Tsoupras, A.; Zabetakis, I. Inflammation and cardiovascular disease: Are marine phospholipids the answer? Food Funct. 2020, 11, 2861–2885. [Google Scholar] [CrossRef] [PubMed]
- Ahmmed, M.K.; Ahmmed, F.; Tian, H.; Carne, A.; Bekhit, A.E.D. Marine omega-3 (n-3) phospholipids: A comprehensive review of their properties, sources, bioavailability, and relation to brain health. Compr. Rev. Food Sci. Food Saf. 2020, 19, 64–123. [Google Scholar] [CrossRef] [PubMed]
- Sugasini, D.; Thomas, R.; Yalagala, P.C.R.; Tai, L.M.; Subbaiah, P.V. Dietary docosahexaenoic acid (DHA) as lysophosphatidylcholine, but not as free acid, enriches brain DHA and improves memory in adult mice. Sci. Rep. 2017, 7, 11263. [Google Scholar] [CrossRef] [PubMed]
- Sugasini, D.; Yalagala, P.C.R.; Subbaiah, P.V. Efficient enrichment of retinal dha with dietary lysophosphatidylcholine-DHA: Potential application for retinopathies. Nutrients 2020, 12, 3114. [Google Scholar] [CrossRef]
- Ali, D.M.; Hogeveen, K.; Orhant, R.-M.; Kerangal, T.L.G.d.; Ergan, F.; Ulmann, L.; Pencreac’h, G. Lysophosphatidylcholine-DHA Specifically Induces Cytotoxic Effects of the MDA-MB-231 Human Breast Cancer Cell Line In Vitro—Comparative Effects with Other Lipids Containing DHA. Nutrients 2023, 15, 2137. [Google Scholar] [CrossRef]
- Zhang, K.; Li, J.; Wang, J.; Lin, X.; Li, L.; You, Y.; Wu, X.; Zhou, Z.; Lin, S. Functional differentiation and complementation of alkaline phosphatases and choreography of DOP scavenging in a marine diatom. Mol. Ecol. 2022, 31, 3389–3399. [Google Scholar] [CrossRef]
- Guillard, R.R.L. Culture of phytoplankton for feeding marine invertebrates. In Culture of Marine Invertebrate Animals; Smith, W.L., Chanley, M.H., Eds.; Springer: Boston, MA, USA, 1975; pp. 29–60. [Google Scholar]
- Kanehisa, M.; Goto, S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Bowler, C.; Allen, A.E.; Badger, J.H.; Grimwood, J.; Jabbari, K.; Kuo, A.; Grigoriev, I.V. The Phaeodactylum genome reveals the evolutionary history of diatom genomes. Nature 2008, 456, 239–244. [Google Scholar] [CrossRef]
- Rastogi, A.; Maheswari, U.; Dorrell, R.G.; Vieira, F.R.J.; Maumus, F.; Kustka, A.; McCarthy, J.; Allen, A.E.; Kersey, P.; Bowler, C.; et al. Integrative analysis of large scale transcriptome data draws a comprehensive landscape of Phaeodactylum tricornutum genome and evolutionary origin of diatoms. Sci. Rep. 2018, 8, 4834. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Yu, G.; Shi, C.; Liu, L.; Guo, Q.; Han, C.; Zhang, D.; Zhang, L.; Liu, B.; Gao, H.; et al. Majorbio Cloud: A one-stop, comprehensive bioinformatic platform for multiomics analyses. Imeta 2022, 1, 12. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Storey, J.D.; Tibshirani, R. Statistical significance for genomewide studies. Proc. Natl. Acad. Sci. USA 2003, 100, 9440–9445. [Google Scholar] [CrossRef]
- Anders, S.; Huber, W. Differential expression analysis for sequence count data. Genome Biol. 2010, 11, R106. [Google Scholar] [CrossRef]
- Wang, L.; Feng, Z.; Wang, X.; Wang, X.; Zhang, X. DEGseq: An R package for identifying differentially expressed genes from RNA-seq data. Bioinformatics 2010, 26, 136–138. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, K.; Li, J.; Cheng, J.; Lin, S. Alkaline Phosphatase PhoD Mutation Induces Fatty Acid and Long-Chain Polyunsaturated Fatty Acid (LC-PUFA)-Bound Phospholipid Production in the Model Diatom Phaeodactylum tricornutum. Mar. Drugs 2023, 21, 560. https://doi.org/10.3390/md21110560
Zhang K, Li J, Cheng J, Lin S. Alkaline Phosphatase PhoD Mutation Induces Fatty Acid and Long-Chain Polyunsaturated Fatty Acid (LC-PUFA)-Bound Phospholipid Production in the Model Diatom Phaeodactylum tricornutum. Marine Drugs. 2023; 21(11):560. https://doi.org/10.3390/md21110560
Chicago/Turabian StyleZhang, Kaidian, Jiashun Li, Jie Cheng, and Senjie Lin. 2023. "Alkaline Phosphatase PhoD Mutation Induces Fatty Acid and Long-Chain Polyunsaturated Fatty Acid (LC-PUFA)-Bound Phospholipid Production in the Model Diatom Phaeodactylum tricornutum" Marine Drugs 21, no. 11: 560. https://doi.org/10.3390/md21110560
APA StyleZhang, K., Li, J., Cheng, J., & Lin, S. (2023). Alkaline Phosphatase PhoD Mutation Induces Fatty Acid and Long-Chain Polyunsaturated Fatty Acid (LC-PUFA)-Bound Phospholipid Production in the Model Diatom Phaeodactylum tricornutum. Marine Drugs, 21(11), 560. https://doi.org/10.3390/md21110560







