Abstract
Three new polyketides, eutyketides A and B (1 and 2) and cytosporin X (3), along with four known compounds (4–7), were obtained from the marine-derived fungus Eutypella scoparia. The planar structures of 1 and 2 were elucidated by extensive HRMS and 1D and 2D NMR analyses. Their relative configurations of C-13 and C-14 were determined with chemical conversions by introducing an acetonylidene group. The absolute configurations of 1–3 were determined by comparing their experimental electronic circular dichroism (ECD) data with their computed ECD results. All of the isolated compounds were tested for their anti-inflammatory activities on lipopolysaccharide-induced nitric oxide production in RAW 264.7 macrophages. Compounds 5 and 6 showed stronger anti-inflammatory activities than the other compounds, with the inhibition of 49.0% and 54.9% at a concentration of 50.0 µg/mL, respectively.
1. Introduction
Eutypella species, which are one genus of the ubiquitous fungi, are widely distributed in many extreme environments, including Antarctica, tropical forests, and marine organisms [1,2,3]. Chemical investigations of Eutypella species have resulted in diverse metabolites, including γ-lactones, benzopyrans, cysporins, terpenoids, and nitrogen-containing compounds [4,5]. Among them, many bioactive secondary metabolites were obtained, such as antibacterial scoparasin B [5], cytotoxic phenochalasin B [6], and antitumor diaporthein B [7]. The Eutypella genus has become an attractive target for discovering leading compounds due to its remarkable biological activity and novel complex structures. In recent years, a lot of work has been carried out on the isolation, total synthesis, pharmacological research, and drug development for the genus Eutypella [8,9,10].
As part of our ongoing investigation of bioactive natural products from marine-derived fungi [11,12,13,14,15,16], the strain Eutypella scoparia HBU-91 attracted our attention because the EtOAc extract of the culture showed anti-inflammatory activity. As a result, the new eutyketides A and B (1 and 2) and cytosporin X (3), together with four known compounds (4–7) (Figure 1), were obtained by using silica gel and LH-20 column chromatography and semipreparative HPLC. Structurally, compounds 1 and 2 were a pair of epimers with vic-diol unit on their side chain, while 3 exhibited a skeleton characterized by a polyketide moiety and a terpenoid part. All of the isolated compounds were tested for their anti-inflammatory activities. Herein, we report their isolation, structure elucidation, and biological activities.
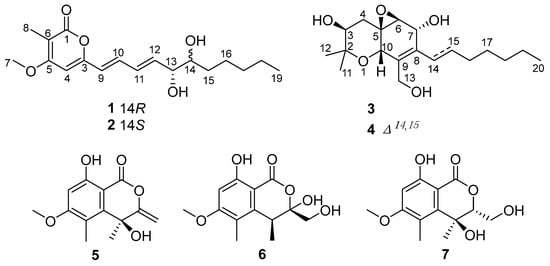
Figure 1.
Chemical structures of compounds 1−7.
2. Results and Discussion
2.1. Structural Elucidation
Eutyketide A (1) was obtained as a pale yellow oil. The molecular formula of 1 was determined to be C18H26O5 based on the HRESIMS of the pseudomolecular ion (m/z 345.1669 [M + Na]+, calcd for C18H26O5Na, 345.1672), indicating six degrees of unsaturation. The IR spectrum suggested the presence of hydroxy (3385 cm−1), double bond (1558 cm−1), and ester carbonyl (1683 cm−1) functionalities. The 1H NMR spectrum (Table 1) showed resonances for five olefinic protons [δH 7.13 (dd, J = 15.0, 11.5 Hz), 6.42 (dd, J = 15.0, 11.5 Hz), 6.08 (d, J = 15.0 Hz), 6.07 (s), and 6.05 (dd, J = 15.0, 6.0 Hz)], two oxymethines [δH 4.23 (dd, J = 6.0, 4.2 Hz) and 3.72 (m)], a methoxy [δH 3.87 (s)], and two methyls [δH 1.93 (s) and 0.88 (t, J = 6.6 Hz)]. 13C NMR combined with HSQC spectra (Table 1) of 1 displayed 18 carbon resonances that could be assignable to 9 sp2 deshielded carbons, including a α,β-unsaturated carbonyl [δC 165.0, (C-1)] and 8 olefinic carbons [δC 157.3 (C-3), 96.1 (C-4), 165.8 (C-5), 103.2 (C-6), 122.8 (C-9), 134.8 (C-10), 130.8 (C-11), and 137.5 (C-12)], and 9 sp3 deshielded carbons, including a methoxy [δC 56.4, (C-7)], 2 oxymethines [δC 75.2 (C-13) and 74.6 (C-14)], 4 methylenes [δC 32.2 (C-15), 31.9 (C-17), 25.7 (C-16), and 22.7 (C-18)], and 2 methyls [δC 8.9 (C-8) and 14.1 (C-19)]. The 1H and 13C NMR data revealed that 1 shares the same carbon framework as graphostrin I, a polyketide obtained from the Atlantic hydrothermal fungus Graphostroma sp. MCCC 3A00421 [17]. The main differences between them were the presence of a methyl at C-6 and a vic-diol [−OHCH−CHOH−] substructure at C-13/14 in 1 instead of the group [−CH2−CH2−] and the absence of the hydroxy group at C-18 in graphostrin I. The above differences were confirmed by the COSY cross-peaks of H-12/H-13/H-14/H2-15 and H2-17/H2-18/H3-19 and the HMBC correlations from H-8 to C-1, C-5, and C-6, from H-13 to C-11 and C-15, and from H-14 to C-12 and C-16, respectively (Figure 2). In addition, trans geometries at C-9−C-10 and C-11−C-12 double bonds were assigned by the large coupling constants (J9,10 = 15.0 Hz and J11,12 = 15.0 Hz) [17]. By detailed analysis of its 2D NMR spectra, the planar structure of 1 was assigned.

Table 1.
1H (600 MHz) and 13C (150 MHz) NMR Data of 1 and 2 in CDCl3.
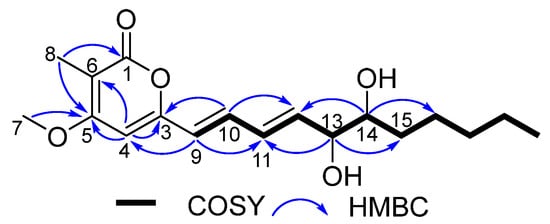
Figure 2.
COSY and key HMBC correlations of compounds 1 and 2.
Eutyketide B (2) was also obtained as a pale yellow oil. It exhibited the same molecular formula as 1, C18H26O5, according to the pseudomolecular ion at m/z 345.1669 [M + Na]+ in the HRESIMS spectrum. Detailed analysis of the 1H and 13C NMR spectra of 2 (Table 1) revealed that its 1D NMR data were similar to those of 1. The differences were attributable to the signals [δH 4.05 (m, H-13) and 3.51 (m, H-14); δC 138.7 (C-12), 74.4 (C-13), and 33.2 (C-15) in 2 vs. δH 4.23 (dd, J = 6.0, 4.2 Hz, H-13) and 3.72 (m, H-14); δC 137.5 (C-12), 75.2 (C-13), and 32.2 (C-15) in 1], indicating that the structural differences between them should be located in this part of the structure (C-13 and C-14). Thus, it was deduced that 1 and 2 were either C-13 or C-14 epimers.
The structural differences between 1 and 2 and their relative configurations were elucidated on the basis of chemical conversions and 1D NOE experiments. Treatment of 1 and 2 with 2,2-dimethoxypropane in the presence of TsOH afforded 1a and 2a as the acetonide products. In the selective NOE of 1a (Figure 3), irradiation of H-13 at δH 4.59 and H-14 at δH 4.17 resulted in the enhancement of H3-21, indicating that H-13 and H-14 should be placed on the same face of 1. In the selective NOE of 2a (Figure 3), irradiation of H-13 at δH 4.09 led to the enhancement of H3-21, while irradiation of H-14 at δH 3.70 caused the enhancement of H3-22, suggesting that H-13 and H-14 should be placed on the opposite side of 2.
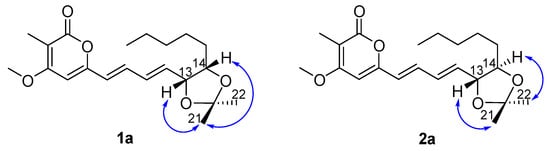
Figure 3.
Structures and 1D NOE correlations of the acetonide products of 1a and 2a.
The calculation of the solution conformers is the most time-demanding part of the ECD calculation in conformationally flexible molecules and may be aided by simplifying the input geometry to reduce the number of conformers and save computational time [18]. For example, alkyl side chains and unsaturated side chains with isolated chromophores in an achiral environment could be simplified by truncation [18]. The absolute configuration of the hydroxyl group at C-13 was affected by the conjugate system, which can be determined by ECD calculation [19]. For 1 and 2, the absolute configurations of C-13 and C-14 were determined by comparing their experimental electronic circular dichroism (ECD) results with the computed results of their simplified model compounds. The group of C-15 to C-19 was a saturated alkyl side chain with no chromophore and had a negligible effect on the ECD spectrum. Thus, the C-15 to C-19 alkyl substituent was truncated to a methyl group as model compound 1b (Figure 4). Molecules of (13S,14S)-1b, (13R,14R)-1b, (13R,14S)-1b, and (13S,14R)-1b were chosen for ECD calculations, which were carried out at the B3LYP/6-311+G(d,p) level in MeOH using the PCM model. The predicted ECD spectrum of (13R,14R)-1b matched well with the experimental ECD curve of 1, and the predicted ECD spectrum of (13R,14S)-1b was in good agreement with the experimental ECD data of 2 (Figure 4). Therefore, the absolute configurations of 1 and 2 could be defined as 13R,14R and 13R,14S, respectively.
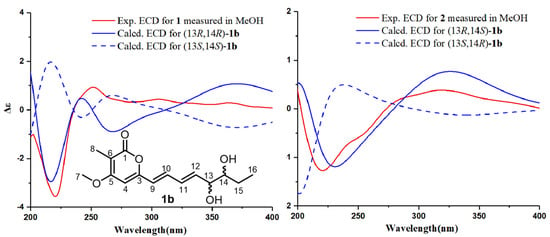
Figure 4.
Calculated ECD spectra of (13R,14R)-1b, (13S,14S)-1b, (13R,14S)-1b, and (13S,14R)-1b and the experimental ECD spectra of 1 and 2.
To the best of our knowledge, compounds 1 and 2 are very similar to prosolanapyrones and their congeners [20]. They share the same pyranone framework with long alkyl side chains. In addition to the conjugate double bonds, compounds 1 and 2 also contain a vic-diol unit on their side chains, while prosolanapyrones just possess double bonds on their side chains.
Cytosporin X (3) was obtained as a colorless oil. The molecular formula C19H32O5 was determined for 3 from the pseudomolecular ion peak at m/z 363.2132 [M + Na]+ (calcd 363.2142 for C19H32O5Na), which is consistent with four degrees of unsaturation. The IR spectrum of 3 at 3402 and 1652 cm-1 suggested the presence of hydroxyl and double bond groups. The 1H NMR spectrum of 3 displayed resonances for four oxygenated methine protons [δH 4.40 (s), 4.26 (d, J = 3.6 Hz), 3.67 (d, J = 12.0 Hz), and 3.24 (d, J = 3.6 Hz)], an oxygenated methylene proton [δH 4.24 (d, J = 12.0 Hz) and 4.04 (d, J = 12.0 Hz)], two singlet methyl protons [δH 1.32 (s) and 1.30 (s)], a terminal methyl proton [δH 0.87 (t, J = 6.6 Hz)], and a series of multiplet signals (Table 2). The 13C NMR spectrum of 3 revealed 19 resonances, including 2 olefinic carbons [δC 128.3 (C-8) and 138.1 (C-9)], 2 oxygenated quaternary carbons [δC 56.1 (C-5) and 77.2 (C-2)], 4 oxygenated methine carbons [δC 60.1 (C-6), 67.2 (C-7), 68.6 (C-10), and 73.5 (C-3)], an oxygenated methylene carbon [δC 62.2 (C-13)], 7 methylene carbons [δC 22.7 (C-19), 29.0 (C-15), 29.2 (C-17), 29.8 (C-16), 30.6 (C-14), 31.9 (C-18), and 35.6 (C-4)], and 3 methy carbons [δC 28.0 (C-12), 16.3 (C-11), and 14.2 (C-20)] (Table 2). The NMR data revealed that 3 belongs to the family of hexahydrobenzopyrane skeletons and is characterized by a polyketide moiety and a terpenoid part (the red part in Figure 5) with a tricyclic structure containing a hexahydrobenzopyrane moiety fused with an oxirane ring [1]. Careful comparison of the NMR data of 3 with those of the known hexahydrobenzopyrane cytosporin D (4) indicated that the structure of 3 is closely related to 4. The notable difference between them lay in the presence of two methylene signals [δH 2.27 and 2.16 (H-14), 1.33 and 1.42 (H-15); δC 30.6 (C-14) and 29.0 (C-15) in 3] and the absence of two olefinic methine signals [δH 6.48 (H-14), 6.15 (H-15); δC 124.8 (C-14) and 135.9 (C-15) in 4]. The COSY cross-peaks of H-14/15/16 and the key HMBC correlations from H-14 to C-7/C-9 and from H-15 to C-8/C-17 (Figure 5) confirmed the above difference. Therefore, the planar structure of 3 was established.

Table 2.
1H (600 MHz) and 13C (150 MHz) NMR Data of 3 in CDCl3.
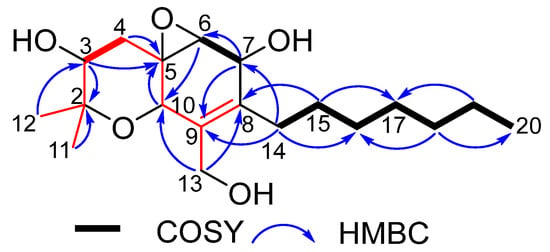
Figure 5.
COSY and key HMBC correlations of 3.
The relative configuration of 3 was determined by analysis of the NOESY data (Figure 6). The NOESY correlations of H-3/H3-12, H3-11/H-10, H-10/H-7, H-10/H-4β, and H-4α/H-6 indicated that H-3 and H-6 were situated on the same side of the molecule with an α-orientation, while C-5, C-6, H-7, and H-10 were accordingly assigned to be β-configured. In addition, the observed NOEs are consistent with the structure and relative configuration of 4.
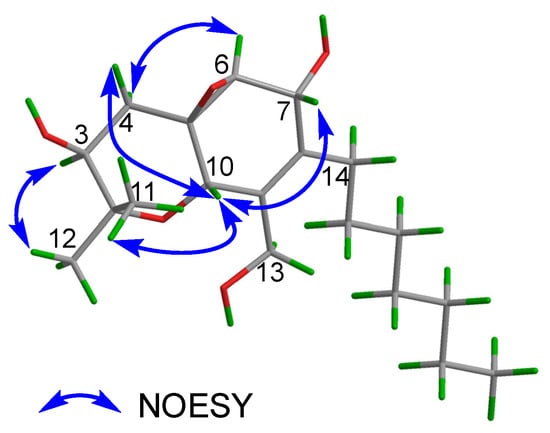
Figure 6.
Key NOESY correlations of 3.
The absolute configuration of 3 was determined on the basis of ECD calculations. Compound 3 had a long flexible side chain with no chromophore. Thus, the side chain was truncated to two methyl groups attached at C-8, and model compound 3a was used for ECD calculations. The calculations were carried out for (3S,5R,6S,7R,10S)-3a and (3R,5S,6R,7S,10R)-3a at the B3LYP/6-311+G(d,p) level using the PCM model (MeOH). The calculated ECD curve of (3S,5R,6S,7R,10S)-3a matched well with the experimental ECD data of 3 (Figure 7). Therefore, the absolute configuration of 3 was defined as 3S,5R,6S,7R,10S.
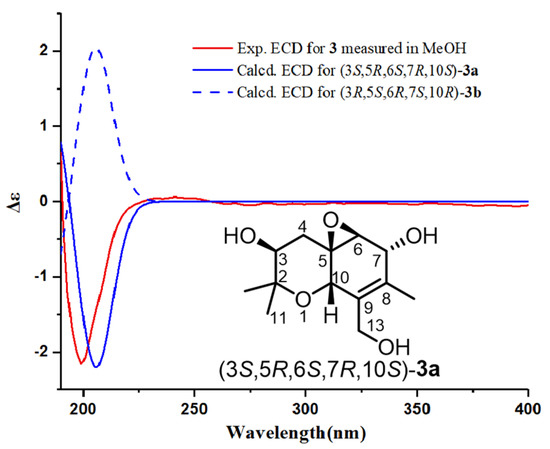
Figure 7.
Calculated ECD spectra of (3S,5R,6S,7R,10S)-3a and (3R,5S,6R,7S,10R)-3a and the experimental ECD spectrum of 3.
The known compounds 4−7 were identified as cytosporin D (4) [1], 4,8-dihydroxy-6-methoxy-4,5-dimethyl-3-methyleneisochroman-1-one (5) [21], banksialactone A (6) [22], and 4,8-dihydroxy-3-(hydr-oxymethyl)-6-methoxy-4,5-dimethylisochroman-1-one (7) [23], respectively, by comparing their NMR and MS data with reported values.
2.2. Anti-Inflammatory Activity
The anti-inflammatory activities of 1−7 were tested by evaluating their influence on nitric oxide (NO) production in RAW264.7 cells induced by lipopolysaccharide (LPS). Compounds 5 and 6 showed stronger anti-inflammatory activities than other compounds, with inhibition rates of 49.0%, 32.1%, and 27.4% for 5 and 54.9%, 35.9%, and 21.1% for 6 at concentrations of 50.0, 25.0, and 12.5 µg/mL, respectively. Moreover, 5 was also active at 6.25 µg/mL with 24.1% inhibition. In addition, 1 exhibited 20.3% inhibition when tested at 6.25 µg/mL (Table S2).
3. Materials and Methods
3.1. General Experimental Procedures
The OR data were recorded on a JASCO P-2000 spectrometer (Jasco Corp., Tokyo, Japan) in MeOH. ECD and UV spectra were measured by MOS450-SFM300 (Biologic, Grenoble, France) and a Perkin-Elmer model 241 spectrophotometers (Perkin-Elmer Corp., Waltham, MA, USA), respectively, with samples dissolved in MeOH. IR spectra were acquired on an FTIR-8400 spectrometer (Shimadzu, Kyoto, Japan) using KBr pellets. NMR data were recorded on a Bruker AV-600 spectrometer (Bruker Corp., Rheinstetten, Germany) with TMS as the internal standard. HRESIMS spectra were obtained from a Bruker apex-ultra 7.0T spectrometer (Bruker Corp., Rheinstetten, Germany). HPLC separation was performed on the Shimadzu LC-20AT system (Shimadzu, Kyoto, Japan) using an RP-18 HPLC column (Waters, Worcester, MA, USA, 10 × 250 mm, 5 μm).
3.2. Isolation of Fungal Material
3.2.1. Fungal Material
The fungal strain Eutypella scoparia HBU-91 (GenBank, OM892669) was collected from the Bohai Sea (Huanghua, China, Apr. 2017). The strain was deposited in the College of Pharmaceutical Sciences, Hebei University, Baoding, China.
3.2.2. Fermentation and Purification
Fermentation was carried out for the fungus E. scoparia using rice medium (170 mL water and 200 g rice in 1 L Erlenmeyer flasks, 200 flasks) at 28 °C for 40 days. After cultivation, the fermented rice substrate was extracted with a mixture of CH2Cl2/MeOH (1:1, 500 mL for each flask) five times and EtOAc five times successively to produce a residue (40.0 g), which was further subjected to silica gel column chromatography (CC), eluting with EtOAc−petroleum ether (PE) stepped gradient elution (0%–100%), to afford six fractions, Fr.1−Fr.6. Fr.2 was separated by Sephadex LH-20 CC (PE−CH2Cl2−MeOH (v/v, 2:1:1)) to afford four subfractions, Fr.2-1−Fr.2-4. Then, Fr.2-2 was fractionated by silica gel CC (PE−EtOAc, 5:1) and further purified by semipreparative HPLC (MeOH−H2O, 70:30, 2.0 mL/min) to afford 6 (2.3 mg) and 5 (3.9 mg). Fr.4 was separated by Sephadex LH-20 CC (MeOH−CH2Cl2 (v/v, 1:1)) to give subfractions Fr.4-1−Fr.4-3. Fr.4-1 was fractionated by silica gel CC (PE−Acetone, 3:1) and further purified by semipreparative HPLC (MeOH−H2O, 70:30, 2.0 mL/min) to give 4 (15.9 mg). Compound 3 (15.5 mg) was obtained from Fr.4-2 under the same conditions as 4. Fr.4-3 was fractionated by semipreparative HPLC (MeOH−H2O, 60:40, 2 mL/min) to provide 1 (4.0 mg) and 2 (3.2 mg). Fr.5 was separated by Sephadex LH-20 CC (MeOH−CH2Cl2 (v/v, 1:1)) to give subfractions Fr.5-1 and Fr.5-2. Fr.5-2 was fractionated by silica gel CC (CH2Cl2/MeOH, 80:1) and further purified by semipreparative HPLC (MeOH−H2O, 50:50, 2.0 mL/min) to afford 7 (4.8 mg).
Eutylactone A (1): pale yellow oil; +105.8 (c 0.1, MeOH); UV (MeOH) λmax (log ε) 225 (2.85), 396 (2.36) nm; ECD (1.04 mM, MeOH) λmax (Δε) 221 (−3.5), 251 (+0.9) nm; IR (KBr) vmax 3385, 2932, 2341, 1683, 1558, 1027 cm−1; 1H and 13C NMR data see Table 1; HRESIMS m/z 345.1669 [M + Na]+ (calcd for C18H26O5Na, 345.1672 [M + Na]+).
Eutylactone B (2); pale yellow oil; +9.5 (c 0.1, MeOH); UV (MeOH) λmax (log ε) 223 (2.85), 392 (2.39) nm; ECD (1.04 mM, MeOH) λmax (Δε) 221 (−1.3), 319 (+0.4) nm; IR (KBr) vmax 3300, 2929, 2359,1679, 1556, 1027 cm−1; 1H and 13C NMR data see Table 1; HRESIMS m/z 345.1669 [M + Na]+ (calcd for C18H26O5Na, 345.1672 [M + Na]+).
Cytosporin X (3); pale yellow oil; −314.5 (c 0.1, MeOH); UV (MeOH) λmax (log ε) 254 (2.64), 335 (2.04) nm; ECD (0.98 mM, MeOH) λmax (Δε) 199 (−2.15) nm; IR (KBr) vmax 3402, 2918, 2351, 1652, 1018 cm−1; 1H and 13C NMR data see Table 2; HRESIMS m/z 363.2132 [M + Na]+ (calcd for C19H32O5Na, 363.2142 [M + Na]+); 379.1871 [M + K]+ (calcd for C19H32O5K, 379.1881 [M + K]+).
3.2.3. Acetonide Formation of 1 and 2
A mixture of 1 (1.0 mg), 2,2-dimethoxypropane (2.0 mL), and p-TsOH (0.2 mg) was stirred at room temperature for 0.5 h. Saturated aqueous NaHCO3 (6.0 mL) was then added, and the reaction mixture was extracted with EtOAc (24 mL × 3). The organic solvents were removed with a high-vacuum pump, and the crude mixture was subjected to preparative HPLC to obtain acetonide product 1a (0.96 mg). Acetonide product 2a (0.91 mg) was obtained from 2 under the same conditions as 1a.
Compound 1a: 1H NMR (600 MHz, CDCl3) δ 7.17 (1H, dd, J = 15.1, 10.9 Hz, H-10), 6.74 (1H, dd, J = 15.0, 5.6 Hz, H-11), 6.37 (1H, dd, J = 15.0, 10.9 Hz, H-9), 6.06 (1H, s, H-4), 5.96 (1H, dd, J = 15.0, 7.2 Hz, H-12), 4.59 (1H, m, H-13), 4.35 (1H, s, H-14), 3.88 (3H, m, H-7), 1.94 (3H, s, H-8), 1.38 (3H, s, H-21), 1.34 (3H, s, H-22), 1.30-1.50 (8H, m, H-15/16/17/18), 0.88 (3H, t, J = 6.6 Hz, H-19).
Compound 2a: 1H NMR (600 MHz, CDCl3) δ 7.15 (1H, dd, J = 15.0, 11.1 Hz, H-10), 6.43 (1H, dd, J = 15.0, 11.1 Hz, H-11), 6.09 (1H, d, J = 15.0 Hz, H-9), 6.06 (1H, s, H-4), 5.94 (1H, dd, J = 15.0, 7.2 Hz, H-12), 4.09 (1H, m, H-13), 3.88 (3H, s, H-8), 3.70 (1H, m, H-14), 1.95 (3H, s, H-8), 1.26 (3H, s, H-21), 1.25 (3H, s, H-22), 1.30-1.50 (8H, m, H-15/16/17/18), 0.89 (3H, t, J = 6.6 Hz, H-19).
3.3. Computational Section
A conformational search for the molecules was carried out using the MMFF94S force field and Compute VOA software, with relative energies ranging from 0−10.0 kcal/mol energy, respectively. The conformers were optimized at the B3LYP/6-31G(d)//B3LYP/6-311+G(d) levels with Gaussian 09 software [24]. Then, stable conformers with relative energy within a 2.5 kcal/mol energy window were chosen for ECD calculations at the B3LYP/6-311+G(d,p) level in methanol using the PCM model, with a total of 60 excited states. A standard deviation of 0.3 eV was used for ECD simulations. Boltzmann statistics were applied for the final simulations of the ECD spectra by the software SpecDis 1.64 [25].
3.4. Cell Culture and Viability Assay
Compounds 1−7 were first tested for their cytotoxic effects on RAW264.7 cells at 3.13, 6.25, 12.5, 25.0, and 50.0 µg/mL. Murine monocytic RAW264.7 macrophages were cultivated at 37 °C with 5% CO2 in Dulbecco’s modified Eagle’s medium (DMEM), which was added with 10% (v/v) fetal bovine serum (FBS) as well as 1% (v/v) penicillin/streptomycin. RAW264.7 cells were grown in 96-well plates and then incubated with the tested compounds for 24 h. A solution of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) at a concentration of 5.0 mg/mL was substituted for the culture medium. After incubation at 37 °C for 4 h, the MTT solution was removed, and DMSO was chosen for the dissolution of the formazan crystals. The absorbance was measured at 540 nm with a microplate reader [26]. Compounds 3−7 exhibited no toxicity at a concentration of 50.0 µg/mL, 1 showed no toxicity at a concentration of 25.0 µg/mL, and 2 displayed no toxicity at a concentration of 6.25 µg/mL.
3.5. Inhibition of NO Production Assay
The Griess assay was applied to evaluate the production of NO through the level of nitrite (NO2) in the medium [27]. RAW264.7 cells were inoculated into 96-well plates, and then LPS at a concentration of 1.0 μg/mL was added to induce inflammation. The tested compounds at different concentrations were added to the above mixture. The Griess reaction was used for the quantification of NO production in the supernatant. The absorbance was measured at 540 nm with a microplate reader. All of the experiments were carried out in triplicate.
4. Conclusions
In summary, three new polyketides (1−3), together with four known compounds (4−7), were isolated from the marine-derived fungus Eutypella scoparia. Chemical conversions and TDDFT ECD calculations were used to determine the absolute configurations of 1−3. Compounds 1, 5, and 6 exhibited certain anti-inflammatory activities on nitric oxide (NO) production in RAW264.7 cells induced by lipopolysaccharide (LPS). Our findings will contribute to the diversity of these fungal metabolites.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/md20080486/s1. Figures S1−S29: 1D and 2D NMR, HRESIMS, and IR and UV spectra of compounds 1−3 and 1D NOE of 1a and 2a; Table S1: Cytotoxic activity data of compounds 1−7; Table S2: Anti-inflammatory activity data of compounds 1−7; Tables S3−S8: The coordinates for the lowest-energy conformers of 1b and 3a in ECD calculations.
Author Contributions
Conceptualization, Y.-H.Z. and F.C.; methodology, Y.-H.Z.; software, H.-F.D.; validation, Y.-H.Z. and H.-F.D.; formal analysis, Y.-H.Z. and W.-B.G.; investigation, Y.-H.Z.; resources, F.C.; data curation, Y.-H.Z. and W.L.; writing—original draft preparation, Y.-H.Z.; writing—review and editing, F.C. and C.-Y.W., visualization, F.C.; supervision, F.C. and C.-Y.W.; project administration, F.C. and C.-Y.W.; funding acquisition, F.C. and C.-Y.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (No. 41830535), Shandong Provincial Natural Science Foundation (Major Basic Research Projects) (ZR2019ZD18), the Program of Open Studio for Druggability Research of Marine Natural Products, Pilot National Laboratory for Marine Science and Technology (Qingdao, China) directed by Kai-Xian Chen and Yue-Wei Guo, and the Taishan Scholars Program, China, Natural Science Foundation of Hebei Province of China (No. H2021201059).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data are contained within the article or Supplementary Material.
Acknowledgments
We would like to thank the High Performance Computer Center of Hebei University for providing computational services.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ciavatta, M.L.; Lopez-Gresa, M.P.; Gavagnin, M.; Nicoletti, R.; Manzo, E.; Mollo, E.; Guo, Y.W.; Cimino, G. Cytosporin-related compounds from the marine-derived fungus Eutypella scoparia. Tetrahedron 2008, 64, 5365–5369. [Google Scholar] [CrossRef]
- Bergero, R.; Girlanda, M.; Varese, G.C.; Intili, D.; Luppi, A.M. Psychrooligotrophic fungi from arctic soils of Franz Joseph Land. Polar Biol. 1999, 21, 361–368. [Google Scholar] [CrossRef]
- Yuan, Z.Q. Fungi and associated tree diseases in Melville Island, Northern Territory, Australia. Aust. Syst. Bot. 1996, 9, 337–360. [Google Scholar] [CrossRef]
- Isaka, M.; Palasarn, S.; Lapanun, S.; Chanthaket, R.; Boonyuen, N.; Lumyong, S. γ-Lactones and ent-eudesmane sesquiterpenes from the endophytic fungus Eutypella sp. BCC 13199. J. Nat. Prod. 2009, 72, 1720–1722. [Google Scholar] [CrossRef]
- Pongcharoen, W.; Rukachaisirikul, V.; Phongpaichit, S.; Rungjindamai, N.; Sakayaroj, J. Pimarane diterpene and cytochalasin derivatives from the endophytic fungus Eutypella scoparia PSU-D44. J. Nat. Prod. 2006, 69, 856–858. [Google Scholar] [CrossRef] [PubMed]
- Kongprapan, T.; Rukachaisirikul, V.; Saithong, S.; Phongpaichit, S.; Poonsuwan, W.; Sakayaroj, J. Cytotoxic cytochalasins from the endophytic fungus Eutypella scoparia PSU-H267. Phytochem. Lett. 2015, 13, 171–176. [Google Scholar] [CrossRef]
- Li, D.; Sun, L.; Chen, Y.; Zhang, W.; Zhang, Q.; Wang., L. Manufacture Method and Application of Diaporthein B in Antitumor agents. CN Patent 102168119A, 31 August 2011. [Google Scholar]
- Liu, H.; Zhang, L.; Chen, Y.; Li, S.; Tan, G.; Sun, Z.; Pan, Q.; Ye, W.; Li, H.; Zhang, W. Cytotoxic pimarane-type diterpenes from the marine sediment-derived fungus Eutypella sp. FS46. Nat. Prod. Res. 2017, 31, 404–410. [Google Scholar] [CrossRef]
- Sun, L.; Li, D.; Tao, M.; Chen, Y.; Zhang, Q.; Dan, F.; Zhang, W. Two new polyketides from a marine sediment-derived fungus Eutypella scoparia FS26. Nat. Prod. Res. 2013, 27, 1298–1304. [Google Scholar] [CrossRef]
- Sun, L.; Li, D.; Tao, M.; Chen, Y.; Dan, F.; Zhang, W. Scopararanes C–G: New oxygenated pimarane diterpenes from the marine sediment-derived fungus Eutypella scoparia FS26. Mar. Drugs 2012, 10, 539–550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, F.; Meng, Z.H.; Mu, X.; Yue, Y.F.; Zhu, H.J. Absolute configuration of bioactive azaphilones from the marine-derived fungus Pleosporales sp. CF09-1. J. Nat. Prod. 2019, 82, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.; Meng, Z.H.; Wang, P.; Luo, D.Q.; Zhu, H.J. Dipleosporalones A and B, dimeric azaphilones from a marine-derived Pleosporales sp. fungus. J. Nat. Prod. 2020, 83, 1283–1287. [Google Scholar] [CrossRef]
- Liu, Y.F.; Yue, Y.F.; Feng, L.X.; Zhu, H.J.; Cao, F. Asperienes A–D, bioactive sesquiterpenes from the marine-derived fungus Aspergillus flavus. Mar. Drugs 2019, 17, 550–559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, X.C.; Xu, L.L.; Yang, R.Y.; Yang, M.Y.; Hu, L.D.; Zhu, H.J.; Cao, F. Anti-vibrio indole-diterpenoids and C-25 epimeric steroids from the marine-derived fungus Penicillium janthinellum. Front. Chem. 2019, 7, 80. [Google Scholar] [CrossRef] [Green Version]
- Meng, Z.H.; Sun, T.T.; Zhao, G.Z.; Yue, Y.F.; Chang, Q.H.; Zhu, H.J.; Cao, F. Marine-derived fungi as a source of bioactive indole alkaloids with diversified structures. Mar. Life Sci. Technol. 2021, 3, 44–61. [Google Scholar] [CrossRef]
- Hai, Y.; Wei, M.Y.; Wang, C.Y.; Gu, Y.C.; Shao, C.L. The intriguing chemistry and biology of sulfur-containing natural products from marine microorganisms (1987–2020). Mar. Life Sci. Technol. 2021, 3, 488–518. [Google Scholar] [CrossRef]
- Niu, S.; Liu, Q.; Xia, J.M.; Xie, C.L.; Luo, Z.H.; Shao, Z.; Liu, G.; Yang, X.W. Polyketides from the deep-sea-derived fungus Graphostroma sp. MCCC 3A00421 showed potent antifood allergic activities. J. Agric. Food Chem. 2018, 66, 1369–1376. [Google Scholar] [CrossRef]
- Mándi, A.; Kurtán, T. Applications of OR/ECD/VCD to the structure elucidation of natural products. Nat. Prod. Rep. 2019, 36, 889–918. [Google Scholar] [CrossRef]
- Ren, J.; Ding, S.S.; Zhu, A.; Cao, F.; Zhu, H.J. Bioactive azaphilone derivatives from the fungus Talaromyces aculeatus. J. Nat. Prod. 2017, 80, 2199–2203. [Google Scholar] [CrossRef]
- Oikawa, H.; Kobayashi, T.; Katayama, K.; Suzuki, Y.; Ichihara, A. Total synthesis of (−)-solanapyrone a via enzymatic Diels−Alder reaction of prosolanapyrone. J. Org. Chem. 1998, 63, 8748–8756. [Google Scholar] [CrossRef]
- Tayone, W.C.; Honma, M.; Kanamaru, S.; Noguchi, S.; Tanaka, K.; Nehira, T.; Hashimoto, M. Stereochemical investigations of isochromenones and isobenzofuranones isolated from Leptosphaeria sp. KTC 727. J. Nat. Prod. 2011, 74, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Tayone, W.C.; Kanamaru, S.; Honma, M.; Tanaka, K.; Nehira, T.; Hashimoto, M. Absolute stereochemistry of novel isochromanone derivatives from Leptosphaeria sp. KTC 727. Biosci. Biotechnol. Biochem. 2011, 75, 2390–2393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Elimat, T.; Raja, H.A.; Figueroa, M.; Falkinham, J.O., III.; Oberlies, N.H. Isochromenones, isobenzofuranone, and tetrahydronaphthalenes produced by Paraphoma radicina, a fungus isolated from a freshwater habitat. Phytochemistry 2014, 104, 114–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Bruhn, T.; Schaumlöffel, A.; Hemberger, Y.; Bringmann, G. SpecDis: Quantifying the comparison of calculated and experimental electronic circular dichroism spectra. Chirality 2013, 25, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Griess, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite and [15N] in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).