Microalgae Photo-Protectants and Related Bio-Carriers Loaded with Bioactive Entities for Skin Applications—An Insight of Microalgae Biotechnology
Abstract
1. Introduction
2. UV-Resistant Microalgae
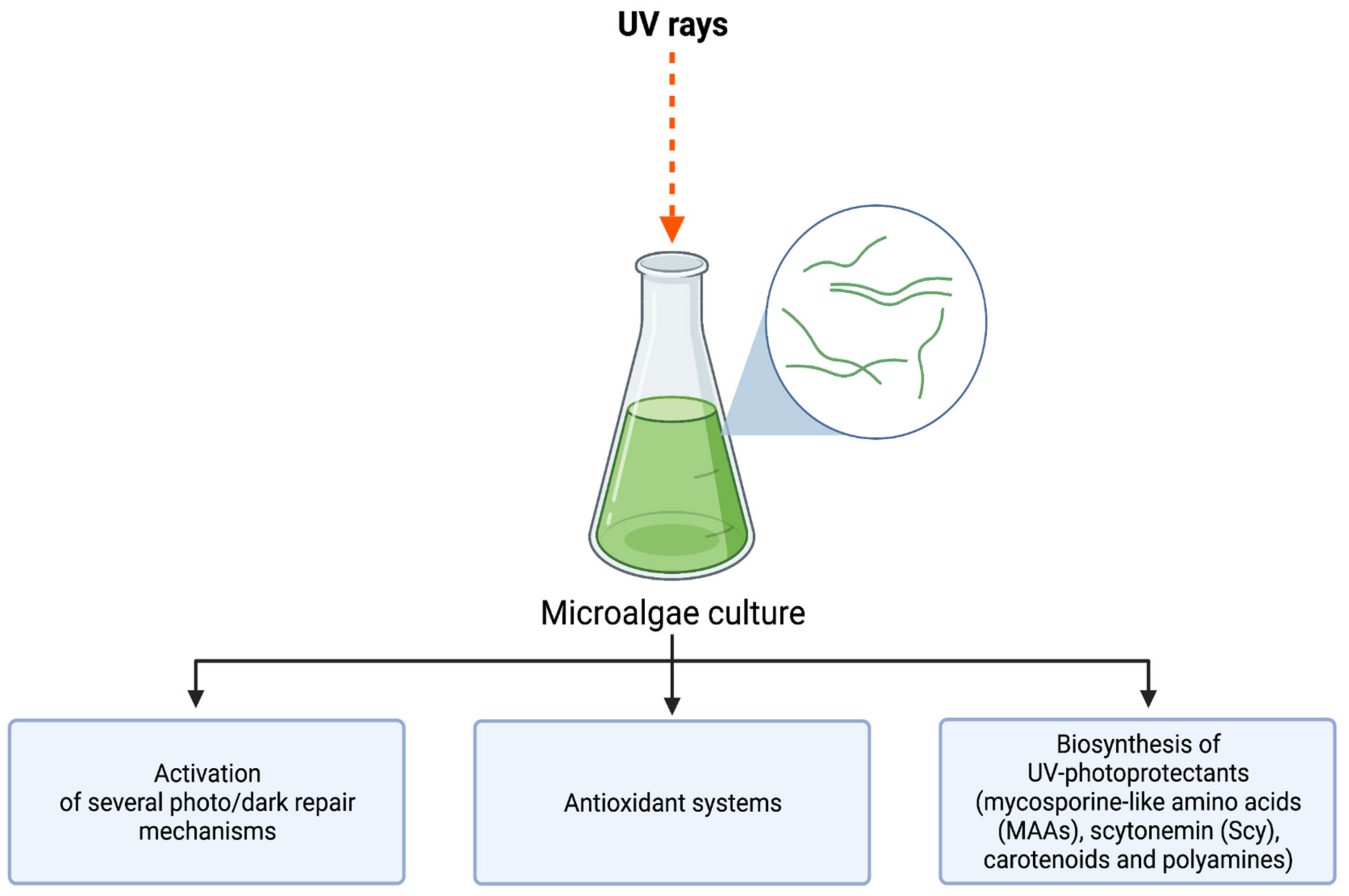
3. UV-Induced Microalgae Biosynthesis
3.1. Mycosporine-Like Amino Acids (MAAs)
3.2. Carotenoids
3.3. Sporopollenin
3.4. Scytonemin
3.5. Phenolic Compounds
4. Bio-Carriers for Skin Applications
5. Conclusions, Challenges, and Future Considerations
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roy, S. Impact of UV Radiation on Genome Stability and Human Health. In Ultraviolet Light in Human Health, Diseases and Environment; Ahmad, S., Ed.; Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2017; Volume 996. [Google Scholar]
- Suh, S.S.; Hwang, J.; Park, M.; Seo, H.H.; Kim, H.S.; Lee, J.H.; Lee, T.K. Anti-inflammation activities of mycosporine-like amino acids (MAAs) in response to UV radiation suggest potential anti-skin aging activity. Mar. Drugs 2014, 12, 5174–5187. [Google Scholar] [CrossRef] [PubMed]
- Totonchy, M.B.; Chiu, M.W. UV-based therapy. Dermatol. Clin. 2014, 32, 399–413. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, D.L.; Saladi, R.N.; Fox, J.L. Review: Ultraviolet radiation and skin cancer. Int. J. Dermatol. 2010, 49, 978–986. [Google Scholar] [CrossRef] [PubMed]
- De Gruijl, F.R.; van Kranen, H.J.; Mullenders, L.H. UV-induced DNA damage, repair, mutations and oncogenic pathways in skin cancer. J. Photochem. Photobiol. B Biol. 2001, 63, 19–27. [Google Scholar] [CrossRef]
- Poon, T.S.C.; Barnetson, R.S.; Halliday, G.M. Prevention of Immunosuppression by Sunscreens in Humans Is Unrelated to Protection from Erythema and Dependent on Protection from Ultraviolet A in the Face of Constant Ultraviolet B Protection. J. Investig. Dermatol. 2003, 121, 184–190. [Google Scholar] [CrossRef][Green Version]
- Halliday, G.M.; Agar, N.S.; Barnetson, R.S.C.; Ananthaswamy, H.N.; Jones, A.M. UV-A Fingerprint Mutations in Human Skin Cancer. Photochem. Photobiol. 2005, 81, 3–8. [Google Scholar] [CrossRef]
- Fourtanier, A.; Gueniche, A.; Compan, D.; Walker, S.L.; Young, A.R. Improved Protection Against Solar-Simulated Radiation-Induced Immunosuppression by a Sunscreen with Enhanced Ultraviolet A Protection. J. Investig. Dermatol. 2000, 114, 620–627. [Google Scholar] [CrossRef][Green Version]
- Radrezza, S.; Carini, M.; Baron, G.; Aldini, G.; Negre-Salvayre, A.; D’Amato, A. Study of Carnosine’s effect on nude mice skin to prevent UV-A damage. Free Radic. Biol. Med. 2021, 173, 97–103. [Google Scholar] [CrossRef]
- Dutra, E.A.; Kedor-Hackmann, E.R.M.; Santoro, M.I.R.M. Determination of sun protection factor (SPF) of sunscreens by ultraviolet spectrophotometry. Rev. Bras. De Cienc. Farm. 2004, 40, 381–385. [Google Scholar] [CrossRef]
- Alonso, C.; Barba, C.; Rubio, L.; Scott, S.; Kilimnik, A.; Coderch, L.; Notario, J.; Parra, J.L. An ex vivo methodology to assess the lipid peroxidation in stratum corneum. J. Photochem. Photobiol. B Biol. 2009, 972, 71–76. [Google Scholar] [CrossRef]
- Sauce, R.; Pinto, C.A.S.D.O.; Ayala-Jara, C.; Prieto, Z.A.; Velasco, M.V.R.; Baby, A.R. Preliminary protocol development of a hplc-tbars-evsc (Ex vivo stratum corneum) assay for skin research: Application in a sunscreen system. Sci. Pharm. 2021, 89, 17. [Google Scholar] [CrossRef]
- World Health Organization. Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 21 June 2022).
- World Health Organization. Radiation: Ultraviolet (UV) Radiation and Skin Cancer. 2017. Available online: https://www.who.int/news-room/questions-and-answers/item/radiation-ultraviolet-(uv)-radiation-and-skin-cancer (accessed on 21 June 2022).
- European Comission. The EU in 2020. 2020. Available online: https://op.europa.eu/webpub/com/general-report-2020/en/ (accessed on 21 June 2022).
- Secretaria de Salud. Cáncer de Piel Duplica su Incidencia Cada 10 Años. 2015. Available online: https://www.gob.mx/salud/prensa/cancer-de-piel-duplica-su-incidencia-cada-10-anos (accessed on 21 June 2022).
- Celis, F. El Negocio Que Crecerá Aceleradamente Por el Cambio Climático. Forbes. 2016. Available online: https://www.forbes.com.mx/el-negocio-que-crecera-aceleradamente-por-el-cambio-climatico/ (accessed on 21 June 2022).
- Geraldes, V.; Pinto, E. Mycosporine-Like Amino Acids (MAAs): Biology, Chemistry and Identification Features. Pharmaceuticals 2021, 14, 63. [Google Scholar] [CrossRef]
- Rosic, N.N. Mycosporine-like amino acids: Making the foundation for organic personalised sunscreens. Mar. Drugs 2019, 17, 638. [Google Scholar] [CrossRef]
- Vega, J.; Schneider, G.; Moreira, B.R.; Herrera, C.; Bonomi-Barufi, J.; Figueroa, F.L. Mycosporine-like amino acids from red macroalgae: UV-photoprotectors with potential cosmeceutical applications. Appl. Sci. 2021, 11, 5112. [Google Scholar] [CrossRef]
- Dionisio-Sese, M.L. Aquatic microalgae as potential sources of UV-screening compounds. Philipp. J. Sci. 2010, 139, 5–16. [Google Scholar]
- Sen, S.; Mallick, N. Mycosporine-like amino acids: Algal metabolites shaping the safety and sustainability profiles of commercial sunscreens. Algal Res. 2021, 58, 102425. [Google Scholar] [CrossRef]
- Yousuf, A. Fundamentals of Microalgae Cultivation. In Microalgae Cultivation for Biofuels Production; Academic Press: New York, NY, USA, 2020; pp. 1–9. [Google Scholar]
- Milito, A.; Castellano, I.; Damiani, E. From Sea to Skin: Is There a Future for Natural Photoprotectants? Mar. Drugs 2021, 19, 379. [Google Scholar] [CrossRef]
- Huang, J.J.; Cheung, P.C. Enhancement of polyunsaturated fatty acids and total carotenoid production in microalgae by ultraviolet band A (UVA, 365 nm) radiation. J. Agric. Food Chem. 2011, 59, 4629–4636. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Madamwar, D.; Nakamoto, H.; Incharoensakdi, A. Resilience and self-regulation processes of microalgae under UV radiation stress. J. Photochem. Photobiol. C Photochem. Rev. 2019, 43, 100322. [Google Scholar] [CrossRef]
- Núñez-Pons, L.; Avila, C.; Romano, G.; Verde, C.; Giordano, D. UV-protective compounds in marine organisms from the southern ocean. Mar. Drugs 2018, 16, 336. [Google Scholar] [CrossRef]
- Buma, A.G.; Wright, S.W.; van den Enden, R.; van de Poll, W.H.; Davidson, A.T. PAR acclimation and UVBR-induced DNA damage in Antarctic marine microalgae. Mar. Ecol. Prog. Ser. 2006, 315, 33–42. [Google Scholar] [CrossRef]
- Xiong, F.; Kopecky, J.; Nedbal, L. The occurrence of UV-B absorbing mycosporine-like amino acids in freshwater and terrestrial microalgae (Chlorophyta). Aquat. Bot. 1999, 63, 37–49. [Google Scholar] [CrossRef]
- Kováčik, J.; Klejdus, B.; Bačkor, M. Physiological responses of Scenedesmus quadricauda (Chlorophyceae) to UV-A and UV-C light. Photochem. Photobiol. 2010, 86, 612–616. [Google Scholar] [CrossRef]
- Guihéneuf, F.; Fouqueray, M.; Mimouni, V.; Ulmann, L.; Jacquette, B.; Tremblin, G. Effect of UV stress on the fatty acid and lipid class composition in two marine microalgae Pavlova lutheri (Pavlovophyceae) and Odontella aurita (Bacillariophyceae). J. Appl. Phycol. 2010, 22, 629–638. [Google Scholar] [CrossRef]
- Fouqueray, M.; Mouget, J.L.; Morant-Manceau, A.; Tremblin, G. Dynamics of short-term acclimation to UV radiation in marine diatoms. J. Photochem. Photobiol. B Biol. 2007, 89, 1–8. [Google Scholar] [CrossRef]
- Chen, L.; Deng, S.; De Philippis, R.; Tian, W.; Wu, H.; Wang, J. UV-B resistance as a criterion for the selection of desert microalgae to be utilized for inoculating desert soils. J. Appl. Phycol. 2013, 25, 1009–1015. [Google Scholar] [CrossRef]
- Yarkent, Ç.; Gürlek, C.; Oncel, S.S. Potential of microalgal compounds in trending natural cosmetics: A review. Sustain. Chem. Pharm. 2020, 17, 100304. [Google Scholar] [CrossRef]
- Joshi, D.; Mohandass, C.; Dhale, M. Effect of UV-B Radiation and Desiccation Stress on Photoprotective Compounds Accumulation in Marine Leptolyngbya sp. Appl. Biochem. Biotechnol. 2018, 184, 35–47. [Google Scholar] [CrossRef]
- Singh, D.K.; Pathak, J.; Pandey, A.; Singh, V.; Ahmed, H.; Kumar, D.; Rajneesh Sinha, R.P. Response of a rice-field cyanobacterium Anabaena sp. HKAR-7 upon exposure to ultraviolet-B radiation and ammonium chloride. Environ. Sustain. 2021, 4, 95–105. [Google Scholar] [CrossRef]
- Ariede, M.B.; Morocho-Jácome, A.L.; Candido, T.M.; Lourenço, F.R.; Kato, E.T.M.; Lima, F.V.; Rosado, C.; Velasco, M.V.R.; Carvalho, J.C.M.; Baby, A.R. Is the Botryococcus braunii dry biomass an adjuvant for anti-UVB topical formulations? Sci. Pharm. 2020, 88, 22. [Google Scholar] [CrossRef]
- Chekanov, K.; Shibzukhova, K.; Lobakova, E.; Solovchenko, A. Differential Responses to UV-A Stress Recorded in Carotenogenic Microalgae Haematococcus rubicundus, Bracteacoccus aggregatus, and Deasonia sp. Plants 2022, 11, 1431. [Google Scholar] [CrossRef] [PubMed]
- Stoyneva-Gärtner, M.; Uzunov, B.; Gärtner, G. Enigmatic microalgae from aeroterrestrial and extreme habitats in cosmetics: The potential of the untapped natural sources. Cosmetics 2020, 7, 27. [Google Scholar] [CrossRef]
- Gao, Y.; Cui, Y.; Xiong, W.; Li, X.; Wu, Q. Effect of UV-C on Algal Evolution and Differences in Growth Rate, Pigmentation and Photosynthesis Between Prokaryotic and Eukaryotic Algae. Photochem. Photobiol. 2009, 85, 774–782. [Google Scholar] [CrossRef] [PubMed]
- Ganapathy, K.; Chidambaram, K.; Janarthanan, R.; Ramasamy, R. Effect of UV-B radiation on growth, photosynthetic activity and metabolic activities of Chlorella vulgaris. J. Microbiol. Biotechnol. 2017, 6, 53–60. [Google Scholar]
- Zaytseva, A.; Chekanov, K.; Zaytsev, P.; Bakhareva, D.; Gorelova, O.; Kochkin, D.; Lobakova, E. Sunscreen Effect Exerted by Secondary Carotenoids and Mycosporine-like Amino Acids in the Aeroterrestrial Chlorophyte Coelastrella rubescens under High Light and UV-A Irradiation. Plants 2021, 10, 2601. [Google Scholar] [CrossRef]
- White, A.L.; Jahnke, L.S. Contrasting Effects of UV-A and UV-B on Photosynthesis and Photoprotection of β-carotene in two Dunaliella spp. Plant Cell Physiol. 2002, 43, 877–884. [Google Scholar] [CrossRef]
- Muhaemin, M. Dynamic response of ultra violet absorbing in Dunaliella sp. Maspari J. Mar. Sci. Res. 2011, 3, 20–23. [Google Scholar]
- Hernando, M.; Schloss, I.; Roy, S.; Ferreyra, G. Photoacclimation to long-term ultraviolet radiation exposure of natural sub-antarctic phytoplankton communities: Fixed surface incubations versus mixed mesocosms. Photochem. Photobiol. 2006, 82, 923–935. [Google Scholar] [CrossRef]
- Bhandari, R.R.; Sharma, P.K. Photosynthetic and biochemical characterization of pigments and UV-absorbing compounds in Phormidium tenue due to UV-B radiation. J. Appl. Phycol. 2011, 23, 283–292. [Google Scholar] [CrossRef]
- Ryu, B.; Himaya, S.W.A.; Kim, S.K. Applications of microalgae-derived active ingredients as cosmeceuticals. In Handbook of Marine Microalgae; Academic Press: New York, NY, USA, 2015; pp. 309–316. [Google Scholar]
- Rastogi, R.P.; Incharoensakdi, A. Characterization of UV-screening compounds, mycosporine-like amino acids, and scytonemin in the cyanobacterium Lyngbya sp. CU2555. FEMS Microbiol. 2014, 87, 244–256. [Google Scholar] [CrossRef]
- Srinivas, R.; Ochs, C. Effect of UV-A irradiance on lipid accumulation in Nannochloropsis oculata. Photochem. Photobiol. 2012, 88, 684–689. [Google Scholar] [CrossRef]
- Helbling, E.W.; Gao, K.; Ai, H.; Ma, Z.; Villafañe, V.E. Differential responses of Nostoc sphaeroides and Arthrospira platensis to solar ultraviolet radiation exposure. J. Appl. Phycol. 2006, 18, 57–66. [Google Scholar] [CrossRef]
- Singh, S.P.; Häder, D.P.; Sinha, R.P. Cyanobacteria and ultraviolet radiation (UVR) stress: Mitigation strategies. Ageing Res. Rev. 2010, 9, 79–90. [Google Scholar] [CrossRef]
- Singh, S.P.; Kumari, S.; Rastogi, R.P.; Singh, K.L.; Sinha, R.P. Mycosporine-like amino acids (MAAs): Chemical structure, biosynthesis and significance as UV-absorbing/screening compounds. Indian J. Exp. Biol. 2008, 46, 7–17. [Google Scholar]
- Pope, M.A.; Spence, E.; Seralvo, V.; Gacesa, R.; Heidelberger, S.; Weston, A.J.; Long, P.F. O-Methyltransferase is shared between the pentose phosphate and shikimate pathways and is essential for mycosporine-like amino acid biosynthesis in Anabaena variabilis ATCC 29413. ChemBioChem 2015, 16, 320–327. [Google Scholar] [CrossRef]
- Fuentes-Tristan, S.; Parra-Saldivar, R.; Iqbal, H.M.; Carrillo-Nieves, D. Bioinspired biomolecules: Mycosporine-like amino acids and scytonemin from Lyngbya sp. with UV-protection potentialities. J. Photochem. Photobiol. B Biol. 2019, 201, 111684. [Google Scholar] [CrossRef]
- Pathak, J.; Ahmed, H.; Singh, S.P.; Häder, D.P.; Sinha, R.P. Genetic regulation of scytonemin and mycosporine-like amino acids (MAAs) biosynthesis in cyanobacteria. Plant Gene 2019, 17, 100172. [Google Scholar] [CrossRef]
- Conde, F.; Churio, M.; Previtali, C. The photoprotector mechanism of mycosporine-like amino acids. Excited-state properties and photostability of porphyra-334 in aqueous solution. J. Photochem. Photobiol. B Biol. 2000, 56, 139–144. [Google Scholar] [CrossRef]
- Singh, A.; Čížková, M.; Bišová, K.; Vítová, M. Exploring mycosporine-like amino acids (MAAs) as safe and natural protective agents against UV-induced skin damage. Antioxidants 2021, 10, 683. [Google Scholar] [CrossRef]
- Zaki, N.A.A.; Mahmud, S.; Omar, A.F. Ultraviolet protection properties of commercial sunscreens and sunscreens containing zno nanorods. J. Phys. Conf. Ser. 2018, 1083, 012012. [Google Scholar] [CrossRef]
- Holzinger, A.; Karsten, U. Desiccation stress and tolerance in green algae: Consequences for ultrastructure, physiological and molecular mechanisms. Front. Plant Sci. 2013, 4, 327. [Google Scholar] [CrossRef]
- Del Mondo, A.; Sansone, C.; Brunet, C. Insights into the biosynthesis pathway of phenolic compounds in microalgae. Comput. Struct. Biotechnol. J. 2022, 20, 1901–1913. [Google Scholar] [CrossRef]
- Horvat, G.; Pantić, M.; Knez, Ž.; Novak, Z. Encapsulation and drug release of poorly water soluble nifedipine from bio-carriers. J. Non-Cryst. Solids 2018, 481, 486–493. [Google Scholar] [CrossRef]
- Jagur-Grodzinski, J. Polymeric gels and hydrogels for biomedical and pharmaceutical applications. Polym. Adv. Technol. 2010, 21, 27–47. [Google Scholar] [CrossRef]
- Huang, H.J.; Yuan, W.K.; Chen, X.D. Microencapsulation based on emulsification for producing pharmaceutical products: A literature review. Dev. Chem. Eng. Miner. Process. 2006, 14, 515–544. [Google Scholar] [CrossRef]
- Khanmohammadi, M.; Elmizadeh, H.; Ghasemi, K. Investigation of size and morphology of chitosan nanoparticles used in drug delivery system employing chemometric technique. Iran. J. Pharm. Res. IJPR 2015, 14, 665. [Google Scholar]
- Aydınoğlu, D.; Ünal, M. Evaluation of the influence of spirulina microalgae on the drug delivery characteristics of genipin cross-linked chitosan hydrogels. Int. J. Polym. Mater. Polym. Biomater. 2019, 68, 1020–1033. [Google Scholar] [CrossRef]
- Gao, Y.; Zhao, D.; Chang, M.W.; Ahmad, Z.; Li, J.S. Optimising the shell thicknessto-radius ratio for the fabrication of oil-encapsulated polymeric microspheres. Chem. Eng. J. 2016, 284, 963–971. [Google Scholar] [CrossRef]
- Karakaş, C.Y.; Özçimen, D. A novel approach to production of Chlorella protothecoides oil loaded nanoparticles via electrospraying method: Modelling of critical parameters for particle sizing. Biotechnol. Appl. Biochem. 2020, 68, 659–668. [Google Scholar] [CrossRef]
- Agrawal, P.; Pramanik, K. Chitosan-poly(vinyl alcohol) nanofibers by free surface electrospinning for tissue engineering applications. Tissue Eng. Regen. Med. 2016, 13, 485–497. [Google Scholar] [CrossRef]
- İnan, B.; Özçimen, D. Preparation and characterization of microalgal oil loaded alginate/poly (vinyl alcohol) electrosprayed nanoparticles. Food Bioprod. Process. 2021, 129, 105–114. [Google Scholar] [CrossRef]
- Morais, F.P.; Simões, R.M.S.; Curto, J.M.R. Biopolymeric Delivery Systems for Cosmetic Applications Using Chlorella vulgaris Algae and Tea Tree Essential Oil. Polymers 2020, 12, 2689. [Google Scholar] [CrossRef] [PubMed]
- Bi, L.; Chen, Y.P.; Wang, C.; Su, J.; Pan, G. Microalgae-derived cellulose/inorganic nanocomposite rattle-type microspheres as an advanced sensor for pollutant detection. Chem. Eng. J. 2020, 395, 125073. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Venugopal, J.; Huang, Z.M.; Lim, C.T.; Ramakrishna, S. Crosslinking of the electrospun gelatin nanofibers. Polymer 2006, 47, 2911–2917. [Google Scholar] [CrossRef]
- Kwak, H.W.; Kang, M.J.; Bae, J.H.; Hur, S.B.; Kim, I.S.; Park, Y.H.; Lee, K.H. Fabrication of Phaeodactylum tricornutum extract-loaded gelatin nanofibrous mats exhibiting antimicrobial activity. Int. J. Biol. Macromol. 2014, 63, 198–204. [Google Scholar] [CrossRef]
- Bao Ha, T.L.; Minh, T.; Nguyen, D.; Minh, D. Naturally Derived Biomaterials: Preparation and Application. In Regenerative Medicine and Tissue Engineering; IntechOpen: Rijeka, Croatia, 2013. [Google Scholar]
- Ching, S.H.; Bansal, N.; Bhandari, B. Alginate gel particles–A review of production techniques and physical properties. Crit. Rev. Food Sci. Nutr. 2017, 57, 1133–1152. [Google Scholar] [CrossRef]
- Jain, D.; Bar-Shalom, D. Alginate drug delivery systems: Application in context of pharmaceutical and biomedical research. Drug Dev. Ind. Pharm. 2014, 40, 1576–1584. [Google Scholar] [CrossRef]
- Tønnesen, H.H.; Karlsen, J. Alginate in drug delivery systems. Drug Dev. Ind. Pharm. 2002, 28, 621–630. [Google Scholar] [CrossRef]
- Choi, Y.S.; Lee, S.B.; Hong, S.R.; Lee, Y.M.; Song, K.W.; Park, M.H. Studies on gelatin-based sponges. Part III: A comparative study of cross-linked gelatin/alginate, gelatin/hyaluronate and chitosan/hyaluronate sponges and their application as a wound dressing in full-thickness skin defect of rat. J. Mater. Sci. Mater. Med. 2001, 12, 67–73. [Google Scholar] [CrossRef]
- Boucard, N.; Viton, C.; Agay, D.; Mari, E.; Roger, T.; Chancerelle, Y.; Domard, A. The use of physical hydrogels of chitosan for skin regeneration following third-degree burns. Biomaterials 2007, 28, 3478–3488. [Google Scholar] [CrossRef]
- Nayyef, S.H.; Thalij, K.M. The Antibacterial activity of Spirulina platensis aqueous extract and Chitosan nanoparticles on bacterial isolates from different human Sources. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2020; Volume 928, p. 062027. [Google Scholar]
- Nascimento, A.V.; Singh, A.; Bousbaa, H.; Ferreira, D.; Sarmento, B.; Amiji, M.M. Mad2 checkpoint gene silencing using epidermal growth factor receptor-targeted chitosan nanoparticles in non-small cell lung cancer model. Mol. Pharm. 2014, 11, 3515–3527. [Google Scholar] [CrossRef]
- Ragelle, H.; Riva, R.; Vandermeulen, G.; Naeye, B.; Pourcelle, V.; Le Duff, C.S.; D’Haese, C.; Nysten, B.; Braeckmans, K.; De Smedt, S.C. Chitosan nanoparticles for siRNA delivery: Optimizing formulation to increase stability and efficiency. J. Control. Release 2014, 176, 54–63. [Google Scholar] [CrossRef]
- Al-Nimry, S.; Dayah, A.A.; Hasan, I.; Daghmash, R. Cosmetic, biomedical and pharmaceutical applications of fish gelatin/hydrolysates. Mar. Drugs 2021, 19, 145. [Google Scholar] [CrossRef]
- Fu, L.; Zhang, J.; Yang, G. Present status and applications of bacterial cellulose-based materials for skin tissue repair. Carbohydr. Polym. 2013, 92, 1432–1442. [Google Scholar] [CrossRef]

| Microalgae | UV Type | Units/Range | Resistance Factor | Type of Study | Culture Media | Growth Temperature | Reference |
|---|---|---|---|---|---|---|---|
| Anabaena spp. Leptolyngbya sp. | UV-B | 1 W m−2 for 4 h/day | Scytonemin, MAAs | Exposure to UV-B radiation | BG-11 (without nitrogen sources) with NH4Cl | 28 ± 2 °C | [35,36] |
| Arthrospira platensis | UV-A | 320 nm | Sporopollenin, Scytonemin, MAAs | Exposure to UV-A irradiance | BG-11 medium | 23 °C | [36] |
| Botryococcus braunii | UV-B | 250 to 450 nm | Self-emulsifying base cetearyl alcohol (and) dicetyl hosphate (and) ceteth-10 phosphate (Crodafos® CES) | Analysis of photoprotection activity | CHU medium | 25 ± 1 °C | [37] |
| Haematococcus rubicundus, Bracteacoccus aggregatus, and Deasonia sp. | UV-A | 250 to 400 nm | MAAs, Carotenoids | Exposure to UV-A radiation | BG-11 | 25 °C | [38] |
| Chaetoceros dichaeta | UV-B | 400 to 700 nm | Antioxidant enzyme superoxide dismutase | Acclimation to PAR | GP5 medium | 1.0 ± 0.5 °C | [27,28] |
| Synechococcus sp. PCC7942 (Cyanophyta), Synechocystis sp. PCC6803 (Cyanophyta), Chlorella protothecoides (Chlorophyta), Chlamydomonas reinhardtii (Chlorophyta), Phaeodactylum tricornutum (Bacillariophyta), Alexandrium tamarense (Pyrrhophyta) and Dicrateria zhanjiangensis (Chrysophyta) | UV-C | 0.01 to 0.20 W m−2 | Chlorophyll a (Chl a), Carotenoids | Exposure to different UV-C irradiances | BG-11 medium | 25 ± 1 °C | [40] |
| Chlorella vulgaris, Chlorococcum citriforme | UV-B | 1 to 5 W m−2 | Sporopollenin, Scytonemin, MAAs | Exposure to UV-B radiation | Bold Basal medium | 25 ± 1 °C | [41] |
| Coelastrella rubescens | UV-A | 380 to 415 nm, power of 2.9 W m−2 | Carotenoids, MAAs, Sporopollenin | Exposure to high fluxes of visible light and UV-A | BG-11 medium | 24 °C | [42] |
| Dunaliella spp. (Dunaliella bardawil, and Dunaliella salina) | UV-B, UV-A | 110 mmol m−2s−1 for UV-A | β-carotene | Exposure to UV-A and UV-B radiation | Medium with NaCl | 26 °C | [43] |
| Dunaliella spp. | NR | 2 W m−2 | MAAs | Long-term exposure to artificial UV radiation | NR | NR | [44] |
| Scenedesmus sp. | UV-B | 2 to 15 W m−2 | MAAs (mycosporine-Gly, palythine, asterina, shinorine and porphyra) | Exposure to extreme UV-B irradiance | NR | 26 °C | [29] |
| Eutreptiella sp. | UV-B | 250 to 750 nm | Xanthophylls, MAAs | Tested under fixed light | f/2 medium | 10 °C | [45] |
| Halamphora coffeaeformis | UV-B, UV-A | NR | Recovery of the photosynthetic parameters | Exposure to UV-A and UV-B radiation | Artificial seawater | NR | [32] |
| Haematococcus lacustris | NR | 500 lux | Astaxanthin | Exposure to artificial UV radiation | Bold Basal medium | 24 °C | [34] |
| Leptolyngbya tenuis | UV-B | 0.8 ± 0.1 mW cm−2 | Scytonemin, MAAs | Exposure to UVB-R | ASN III medium | 30 ± 2 °C | [34,46] |
| Lyngbya sp. | UV-B | 8 W m−2 | MAAs, Scytonemin | Exposure to UV radiation | Liquid culture medium | 23 °C | [35,47,48] |
| Nannochloropsis oculata | UV-A | 6 to 24 W m−2 | Sporopollenin, Scytonemin, mycosporine-like amino acids | Exposure to different levels of UVA radiation | Instant Ocean artificial sea water | 25 °C | [34,49] |
| Nostoc sp. | UV-B | 312 nm | Carotenoids, Scytonemin | Analysis of photosynthetic activity | BG-11 medium | 25 °C | [27,33] |
| Nostoc sphaeroides | UV-A | 320 nm | Not identified | Exposure to UVA irradiance | BG-11 medium | 23 °C | [50] |
| Odontella aurita | UV-B, UV-A | 110 kJ m−2 | D1 protein, Activation of antioxidant enzymes | Exposure to UVA-R and UVB-R | Artificial seawater | NR | [31,32] |
| Material Used | Microalgae | Component Loaded | Formulation | Purpose | Types of Tests | References |
|---|---|---|---|---|---|---|
| Chitosan | Spirulina | 5-Fluorouracil | Chitosan (1%(v/v)), Spirulina microalgae and genipin. | Drug delivery | In vitro cytotoxicity test | [65] |
| Alginate and Chitosan | Auxonochlorella protothecoides | Microalgae oil extract | PVA solution (7–8% (w/v)) with sodium alginate (2% (w/v)) and PVA solution (7–8% (w/v)) with chitosan (2–3% (w/v)) | Nanoparticle production to deliver bioactive compounds in microalgae | In vitro release testing | [66,67,68] |
| Alginate, PVA | Botryococcus braunii and Microcystis aeruginosa | Microalgae oil extract | PVA solution (8% (w/v)) and sodium alginate (2% (w/v)) | Nanoparticle production to deliver bioactive compounds in microalgae | In vitro release profile of nanoparticles | [66,69] |
| Alginate, cellulose | Chlorella vulgaris | Tea tree essential oil | Microfibrillated cellulose, nanofibrillated cellulose and carboxymethylcellulose (0.01% (m/v)) with alginate (2% (m/v)) | Cosmetic application | Differential scanning calorimetry | [70] |
| Cellulose | Chlorella pyrenoidosa | - | Microalgae powder, sodium dodecyl sulfate | Sensor for pollutant detection | - | [71] |
| Gelatin | Phaeodactylum tricornutum | Microalgae extract | P. tricornutum powder (0.5% or 1.0% (w/v)), gelatin solution | Wound dressing with antimicrobial P. tricornutum-loaded gelatin nanofiber mat | In vitro studies for antibacterial activity | [72,73] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santiesteban-Romero, B.; Martínez-Ruiz, M.; Sosa-Hernández, J.E.; Parra-Saldívar, R.; Iqbal, H.M.N. Microalgae Photo-Protectants and Related Bio-Carriers Loaded with Bioactive Entities for Skin Applications—An Insight of Microalgae Biotechnology. Mar. Drugs 2022, 20, 487. https://doi.org/10.3390/md20080487
Santiesteban-Romero B, Martínez-Ruiz M, Sosa-Hernández JE, Parra-Saldívar R, Iqbal HMN. Microalgae Photo-Protectants and Related Bio-Carriers Loaded with Bioactive Entities for Skin Applications—An Insight of Microalgae Biotechnology. Marine Drugs. 2022; 20(8):487. https://doi.org/10.3390/md20080487
Chicago/Turabian StyleSantiesteban-Romero, Berenice, Manuel Martínez-Ruiz, Juan Eduardo Sosa-Hernández, Roberto Parra-Saldívar, and Hafiz M. N. Iqbal. 2022. "Microalgae Photo-Protectants and Related Bio-Carriers Loaded with Bioactive Entities for Skin Applications—An Insight of Microalgae Biotechnology" Marine Drugs 20, no. 8: 487. https://doi.org/10.3390/md20080487
APA StyleSantiesteban-Romero, B., Martínez-Ruiz, M., Sosa-Hernández, J. E., Parra-Saldívar, R., & Iqbal, H. M. N. (2022). Microalgae Photo-Protectants and Related Bio-Carriers Loaded with Bioactive Entities for Skin Applications—An Insight of Microalgae Biotechnology. Marine Drugs, 20(8), 487. https://doi.org/10.3390/md20080487









