Supplementation with Spirulina platensis Prevents Damage to Rat Erections in a Model of Erectile Dysfunction Promoted by Hypercaloric Diet-Induced Obesity
Abstract
1. Introduction
2. Results
2.1. Food Intake Evaluation
2.2. Caloric Intake Evaluation
2.3. Animal’s Weight Gain Evaluation
2.4. Dietary Efficacy, Feed Conversion and Weight Gain for Caloric Intake Coefficients
2.5. Experimental Assessment of the Obesity Induction
2.5.1. Murinometrics Parameters
2.5.2. Mass of White Adipose Tissue (WAT)
2.5.3. Body Adiposity Index
2.5.4. Biochemical Analysis
2.6. Penile Erection Induction
Correlation between the Parameters of Experimental Obesity and the Erectile Function of Rats
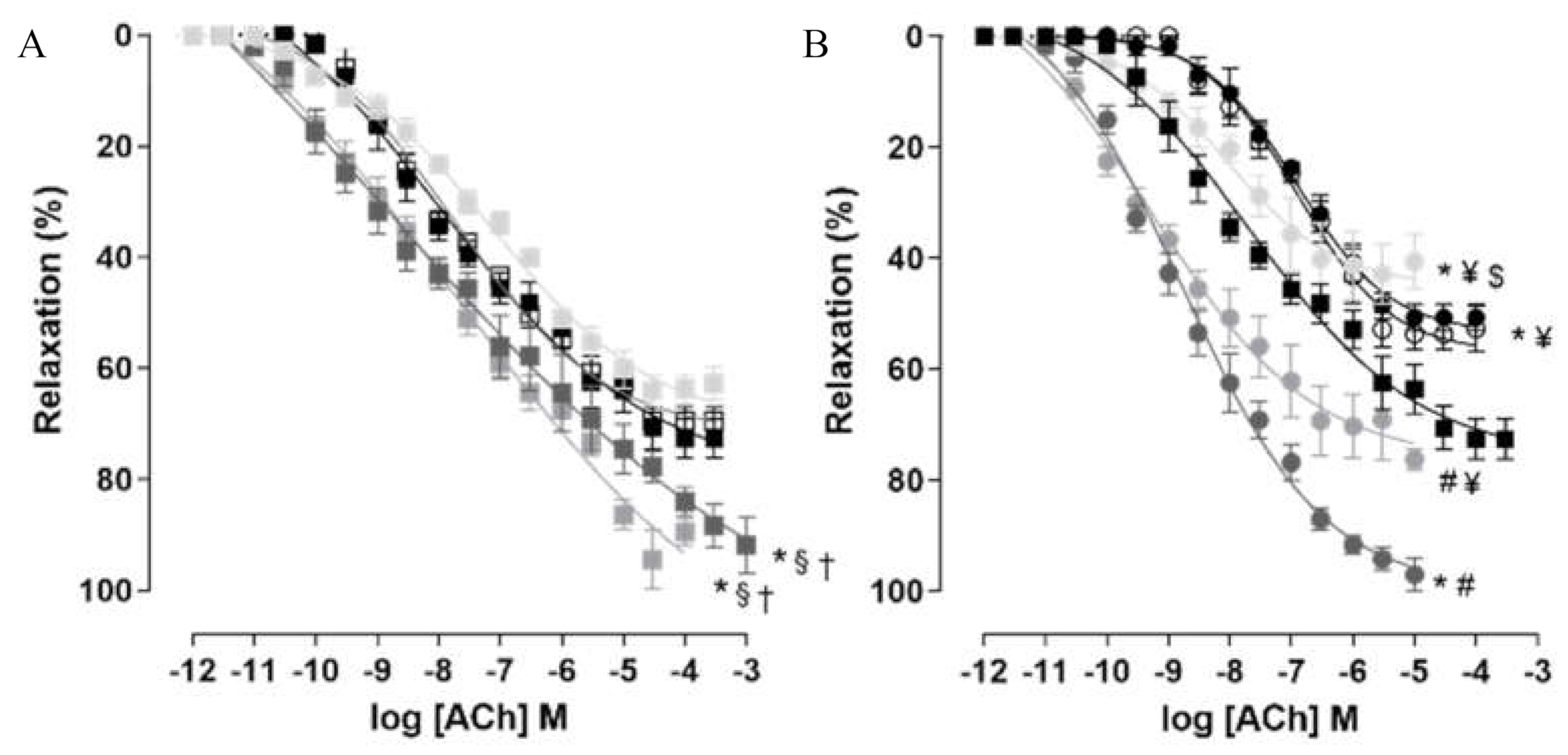
2.7. Corpus Cavernosum Reactivity
2.7.1. Contractile Reactivity Measurement
2.7.2. Relaxing Reactivity Measurement
3. Discussion
4. Materials and Methods
4.1. Test Product
4.2. Animals
4.3. Diets
4.4. Experimental Groups
4.5. Chemicals
4.6. Food Intake and Animal’s Weight Gain Evaluation
4.7. Dietary Efficacy, Feed Conversion and Weight Gain for Caloric Intake Coefficients
4.8. Experimental Assessment of the Obesity Induction
4.8.1. Murinometrics Parameters
4.8.2. Mass of White Adipose Tissue
4.8.3. Body Adiposity Index
4.8.4. Adipose Tissue Morphometry
4.9. Biochemical Analysis
4.10. Penile Erection Induction
4.11. Corpus Cavernosum Reactivity
4.11.1. Contractile Reactivity Measurement
4.11.2. Relaxing Reactivity Measurement
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- National Institutes of Health. Consensus development conference statement on impotence. Int. J. Impot. Res. 1993, 5, 181–199. [Google Scholar]
- Alves, M.A.S.G.; Queiroz, T.M.D.; Medeiros, I.A.D. Fisiologia peniana e disfunção erétil: Uma revisão de literatura. Rev. Bras. Ciên. Saúde 2012, 6, 439–444. [Google Scholar] [CrossRef][Green Version]
- Yafi, F.A.; Jenkins, L.; Albersen, M.; Corona, G.; Isidori, A.M.; Goldfarb, S.; Maggi, M.; Nelson, C.J.; Parish, S.; Salonia, A.; et al. Erectile dysfunction. Nat. Rev. Dis. Primers 2016, 2, 16003–16050. [Google Scholar] [CrossRef] [PubMed]
- Shamloul, R.; Ghanem, H. Erectile dysfunction. Lancet 2013, 381, 153–165. [Google Scholar] [CrossRef]
- Sperling, H.; Lorenz, A.; Krege, S.; Arndt, R.; Michel, M.C. An extract from the bark of Aspidosperma quebracho blanco binds to human penile α-adrenoceptors. J. Urol. 2002, 168, 160–163. [Google Scholar] [CrossRef]
- Alves, L.D.S.; Velloso, A.P.D.S. Tratamento da disfunçao erétil. Rev. Med. Minas Gerais 2005, 15, 110–113. [Google Scholar]
- Codevilla, C.F.; Castilhos, T.D.S.; Bergold, A.M. A review of analytical methods for the determination of four new phosphodiesterase type 5 inhibitors in biological samples and pharmaceutical preparations. Braz. J. Pharm. Sci. 2013, 49, 1–11. [Google Scholar] [CrossRef]
- Souza, I.L.L.; Barros, B.C.; Oliveira, G.A.; Queiroga, F.R.; Toscano, L.T.; Silva, A.S.; Silva, P.M.; Interaminense, L.F.L.; Cavalcante, F.A.; Silva, B.A. Hypercaloric diet establishes erectile dysfunction in rat: Mechanisms underlying the endotelial damage. Front. Physiol. 2017, 8, 760–775. [Google Scholar] [CrossRef]
- Tang, G.; Suter, P.M. Vitamin A, nutrition, and health values of algae: Spirulina, Chlorella and Dunaliella. J. Pharm. Nutr. Sci. 2011, 1, 111–118. [Google Scholar] [CrossRef]
- Bishop, W.M.; Zubeck, H.M. Evaluation of microalgae for use as nutraceuticals and nutritional supplements. J. Nutr. Food Sci. 2012, 2, 5. [Google Scholar] [CrossRef]
- Holman, B.W.B.; Malau-Aduli, A.E.O. Spirulina as a livestock supplement and animal feed. J. Anim. Physiol. Anim. Nutr. 2013, 97, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Samuels, R.; Mani, U.V.; Iyer, U.M.; Nayak, U.S. Hypocholesterolemic effect of Spirulina in patients with hyperlipidemic nephrotic syndrome. J. Med. Food 2002, 5, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Mazokopakis, E.E.; Starakis, I.K.; Papadomanolaki, M.G.; Mavroeidi, N.G.; Ganotakis, E.S. The hypolipidaemic effects of Spirulina (Arthrospira platensis) supplementation in a Cretan population: A prospective study. J. Sci. Food Agric. 2014, 94, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Nagaoka, S.; Shimizu, K.; Kaneko, H.; Shibayama, F.; Morikawa, K.; Kanamaru, Y.; Otsuka, A.; Hirashi, T.; Kato, T. A novel protein C-phycocyanin plays a crucial role in the hypocholesterolemic action of Spirulina platensis concentrate in rats. J. Nutr. 2005, 135, 2425–2430. [Google Scholar] [CrossRef] [PubMed]
- Ku, C.S.; Yang, Y.; Park, Y.; Lee, J. Health benefits of blue-green algae: Prevention of cardiovascular disease and nonalcoholic fatty liver disease. J. Med. Food 2013, 16, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.X.; Park, Y.K.; Lee, J.Y. Anti-inflammatory effects of Spirulina platensis extract via the modulation of histone deacetylases. Nutrients 2016, 8, 381. [Google Scholar] [CrossRef]
- Becker, E.W.; Jakober, B.; Luft, D.; Schmulling, R.M. Clinical and biochemical evaluations of the alga Spirulina with regard to its application in the treatment of obesity. A double-blind cross-over study. Nutr. Rep. Int. 1986, 33, 565–574. [Google Scholar]
- Zeinalian, R.; Farhangi, M.A.; Shariat, A.; Saghafi-Asl, M. The effects of Spirulina platensis on anthropometric indices, appetite, lipid profile and serum vascular endothelial growth factor (VEGF) in obese individuals: A randomized double blinded placebo controlled trial. BMC Complement. Altern. Med. 2017, 17, 225. [Google Scholar] [CrossRef]
- Estrada, J.P.; Bescós, P.B.; Del Fresno, A.V. Antioxidant activity of different fractions of Spirulina platensis protean extract. Il Fármaco 2011, 56, 497–500. [Google Scholar] [CrossRef]
- Hwang, J.H.; Chen, J.C.; Chan, Y.C. Effects of C-phycocyanin and Spirulina on salicylate-induced tinnitus, expression of NMDA receptor and inflammatory genes. PLoS ONE 2013, 8, 23–32. [Google Scholar] [CrossRef]
- Bashandy, S.A.; El Awdan, S.A.; Ebaid, H.; Alhazza, I.M. Antioxidant potential of Spirulina platensis mitigates oxidative stress and reprotoxicity induced by sodium arsenite in male rats. Oxid. Med. Cell. Longev. 2016, 2016, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Brito, A.F.; Silva, A.S.; Souza, A.A.; Ferreira, P.B.; Souza, I.L.L.; Araujo, L.C.C.; Félix, G.S.; Sampaio, R.S.; Silva, M.C.C.; Tavares, R.L.; et al. Supplementation with Spirulina platensis Modulates Aortic Vascular Reactivity through Nitric Oxide and Antioxidant Activity. Oxid. Med. Cell. Longev. 2019, 2019, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Marcel, A.K.; Ekali, L.G.; Eugene, S.; Arnold, O.E.; Sandrine, E.D.; Von der Weid, D.; GbaguidI, E.; Ngogang, J.; Mbanya, J.C. The effect of Spirulina platensis versus soybean on insulin resistance in HIV-infected patients: A randomized pilot study. Nutrients 2011, 3, 712–724. [Google Scholar] [CrossRef] [PubMed]
- Ali, E.A.; Barakat, B.M.; Hassan, R. Antioxidant and angiostatic effect of Spirulina platensis suspension in complete Freund’s adjuvant-induced arthritis in rats. PLoS ONE 2015, 10, e0121523. [Google Scholar] [CrossRef]
- Ibrahim, A.E.; Abdel-Daim, M.M. Modulating effects of Spirulina platensis against tilmicosin-induced cardiotoxicity in mice. Cell J. 2015, 17, 137. [Google Scholar] [CrossRef]
- Araujo, L.C.C.; Brito, A.F.; Souza, I.L.L.; Ferreira, P.B.; Vasconcelos, L.H.C.; Silva, A.S.; Silva, B.A. Spirulina platensis Supplementation Coupled to Strength Exercise Improves Redox Balance and Reduces Intestinal Contractile Reactivity in Rat Ileum. Mar. Drugs 2020, 18, 89. [Google Scholar] [CrossRef]
- Estadella, D.; Oyama, L.M.; Dâmaso, A.R.; Ribeiro, E.B.; Nascimento, C.M.O. Effect of palatable hyperlipidic diet on lipid metabolism of sedentary and exercised rats. Nutrition 2004, 20, 218–224. [Google Scholar] [CrossRef]
- Bernardes, D.; Manzoni, M.S.J.; SOUZA, C.P.; Tenório, N.; Dâmaso, A.R. Efeitos da dieta hiperlipídica e do treinamento de natação sobre o metabolismo de recuperação ao exercício em ratos. Rev. Bras. Educ. Fís Esporte 2004, 18, 191–200. [Google Scholar] [CrossRef]
- Hariri, N.; Thibault, L. High-fat diet-induced obesity in animal models. Nutr. Res. Rev. 2010, 23, 270–299. [Google Scholar] [CrossRef]
- Masago, F. Avaliação Preliminar do Efeito da Physalis angulata L. sobre a Obesidade Induzida por Dieta Hipercalórica; Universidade Estadual Paulista Júlio de Mesquita Filho: Sao Paulo, Brazil, 2011. [Google Scholar]
- Himaya, A.; Fantino, M.; Antoine, J.M.; Brondel, L.; Louis-Sylvestre, J. Satiety power of dietary fat: A new appraisal. Am. J. Clin. Nutr. 1997, 65, 1410–1418. [Google Scholar] [CrossRef]
- Konturek, S.J.; Konturek, P.C.; Pawlik, T.; Brzozowski, T. Brain-gut axis and its role in the control of food intake. J. Physiol. Pharmacol. 2004, 55, 137–154. [Google Scholar] [PubMed]
- FDA. Viagra and Increased Appetite—FDA Reports. Available online: http://www.ehealthme.com/ds/viagra/increased%20appetite/#print (accessed on 10 December 2019).
- Nascimento, A.F.; Sugizaki, M.M.; Leopoldo, A.S.; Lima-Leopoldo, A.P.; Luvizotto, R.A.; Nogueira, C.R.; Cicogna, A.C. A hypercaloric pellet-diet cycle induces obesity and co-morbidities in Wistar rats. Arq. Bras. Endocrinol. Metabol. 2008, 52, 968–974. [Google Scholar] [CrossRef] [PubMed]
- Ravagnani, F.C.D.P.; Ravagnani, C.D.F.C.; Braga Neto, J.A.; Voltarelli, F.A.; Zavala, A.A.Z.; Habitante, C.A.; Inouye, C.M. Efeito de dietas hiperlipídicas com extrato de baru e chocolate sobre a área de adipócitos de ratos submetidos ao exercício físico. Rev. Bras. Med. Esporte 2012, 18, 190–194. [Google Scholar] [CrossRef]
- Porrini, M.; Santangelo, A.; Crovetti, R.; Riso, P.; Testolin, G.; Blundell, J.E. Weight, protein, fat, and timing of preloads affect food intake. Physiol. Behav. 1997, 62, 563–570. [Google Scholar] [CrossRef]
- Mikkelsen, P.B.; Toubro, S.; Astrup, A. Effect of fat-reduced diets on 24-h energy expenditure: Comparisons between animal protein, vegetable protein, and carbohydrate. Am. J. Clin. Nutr. 2000, 72, 1135–1141. [Google Scholar] [CrossRef]
- Mitschke, M.M.; Hoffmann, L.S.; Gnad, T.; Scholz, D.; Kruithoff, K.; Mayer, P.; HAAS, B.; Sassmann, A.; Pfeifer, A.; Kilić, A. Increased cGMP promotes healthy expansion and browning of white adipose tissue. FASEB J. 2013, 27, 1621–1630. [Google Scholar] [CrossRef]
- Fernandes, H.J.; Paulino, M.F.; Martins, R.G.R.; Valadares Filho, S.D.C.; Torres, R.D.A.; Paiva, L.M.; Moraes, G.F.B.K.D. Ganho de peso, conversão alimentar, ingestão diária de nutrientes e digestibilidade de garrotes não-castrados de três grupos genéticos em recria e terminação. R. Br. Zootec. 2004, 33, 2403–2411. [Google Scholar] [CrossRef][Green Version]
- Lupatini, F. Avaliação do Efeito de Variáveis Produtivas na Conversão Alimentar de Frangos de Corte. Master’s Thesis, Universidade Federal de Goiás, Goiânia, Brazil, 2015. [Google Scholar]
- Malafaia, A.B.; Nassif, P.A.N.; Ribas, C.A.P.M.; Ariede, B.L.; Sue, K.N.; Cruz, M.A. Indução de obesidade com sacarose em ratos. ABCD Arq. Bras. Cir. Dig. 2013, 26, 17–21. [Google Scholar] [CrossRef]
- Thibault, L.; Woods, S.C.; Westerterp-Plantenga, M.S. The utility of animal models of human energy homeostasis. Br. J. Nutr. 2004, 92, S41–S45. [Google Scholar] [CrossRef]
- Cani, P.D.; Bibiloni, R.; Knauf, C.; Waget, A.; Neyrinck, A.M.; Delzenne, N.M.; Burcelin, R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet–induced obesity and diabetes in mice. Diabetes 2008, 57, 1470–1481. [Google Scholar] [CrossRef]
- Mauer, M.M.; Harris, R.B.; Bartness, T.J. The regulation of total body fat: Lessons learned from lipectomy studies. Neurosci. Biobehav. Rev. 2001, 25, 15–28. [Google Scholar] [CrossRef]
- Jang, I.; Hwang, D.; Lee, J.; Chae, K.; Kim, Y.; Kang, T.; Kang, T.; Cho, J. Physiological difference between dietary obesity-susceptible and obesity-resistant Sprague Dawley rats in response to moderate high fat diet. Exp. Anim. 2003, 52, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Woods, S.C.; Seeley, R.J.; Rushing, P.A.; D’Alessio, D.; Tso, P. A controlled high-fat diet induces an obese syndrome in rats. J. Nutr. 2003, 133, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Dourmashkin, J.T.; Chang, G.Q.; Gayles, E.C.; Hill, J.O.; Fried, S.K.; Julien, C.; Leibowitz, S.F. Different forms of obesity as a function of diet composition. Int. J. Obes. 2005, 29, 1368–1378. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Duarte, A.C.G.O.; Fonseca, D.F.; Manzoni, M.S.J.; Soave, C.F.; Sene-Fiorese, M.; Dâmaso, A.R. High-fat diet and secretory capacity of insulin in rats. Rev. Nutr. 2006, 19, 341–348. [Google Scholar] [CrossRef][Green Version]
- Souza, I.L.L.; Ferreira, E.D.S.; Diniz, A.F.; Carvalho, M.T.D.L.; Queiroga, F.R.; Toscano, L.T.; Silva, A.S.; Silva, P.M.; Cavalcante, F.A.; Silva, B.A. Effects of Redox Disturbances on Intestinal Contractile Reactivity in Rats Fed with a Hypercaloric Diet. Oxid. Med. Cell. Longev. 2018, 2018, 1–8. [Google Scholar] [CrossRef]
- Andersson, K.E. Mechanisms of penile erection and basis for pharmacological treatment of erectile dysfunction. Pharmacol. Rev. 2011, 63, 811–859. [Google Scholar] [CrossRef]
- Corbin, J.D. Mechanisms of action of PDE5 inhibition in erectile dysfunction. Int. J. Impot. Res. 2004, 16, S4–S7. [Google Scholar] [CrossRef]
- Spiegel, M.R. Correlação de Pearson. Estatística, 3rd ed.; Pearson Makron Books: São Paulo, Brazil, 1993; pp. 98–108. [Google Scholar]
- Toque, H.A.; Silva, F.H.; Calixto, M.C.; Lintomen, L.; Schenka, A.A.; Saad, M.J.; Zanesco, A.; Antunes, E. High-fat diet associated with obesity induces impairment of mouse corpus cavernosum responses. BJU Int. 2010, 107, 1628–1634. [Google Scholar] [CrossRef]
- Toque, H.A.; Nunes, K.P.; Yao, L.; Liao, J.K.; Webb, C.; Caldwell, R.B.; Caldwell, W. Activated Rho kinase mediates diabetes-induced elevation of vascular arginase activation and contributes to impaired corpora cavernosa relaxation: Possible involvement of p38 MAPK activation. J. Sex. Med. 2013, 10, 1502–1515. [Google Scholar] [CrossRef]
- Silva, F.H.; Mônica, F.Z.; Báu, F.R.; Brufnerotto, A.F.; Priviero, F.; Toque, H.A.; Antunes, E. Superoxide anion production by NADPH oxidase plays a major role in erectile dysfunction in middle-aged rats: Prevention by antioxidant therapy. J. Sex. Med. 2013, 10, 960–971. [Google Scholar] [CrossRef] [PubMed]
- Prieto, D.; Riviera, L.; Recio, P.; Rubio, J.L.R.; Hernández, M.; García-Sacristán, A. Role of nitric oxide in the relaxation elicited by sildenafil in penile resistance arteries. J. Urol. 2006, 175, 1164–1170. [Google Scholar] [CrossRef]
- Sherwin, C.M.; Christiansen, S.B.; Duncan, I.J.H.; Erhard, H.W.; Lay, D.C.; Mench, J.A.; O’Connor, C.E.; Petherick, C.J. Guidelines for the ethical use of animals in applied animal behaviour research. Appl. Anim. Behav. Sci. 2003, 81, 291–305. [Google Scholar] [CrossRef]
- Brasil Ministério da Ciência, Tecnologia e Inovação. Conselho Nacional de Experimentação Animal. Guia Brasileiro de Produção, Manutenção ou Utilização de Animais em Atividades de Ensino ou Pesquisa Científica: Fascículo 1; Introdução geral: Brasília, Brasil, 2016.
- Vadivel, V.; Pugalenthi, M. Studies on the incorporation of velvet bean (Mucuna pruriens var. utilis) as an alternative protein source in poultry feed and its effect on growth performance of broiler chickens. Trop. Anim. Health Pro. 2010, 42, 1367–1376. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.A. Method for determination of PER and NPR. In Evaluation of Protein Quality; Food and Nutrition Board, Committee on Protein Quality, Eds.; National Academy of Sciences: Washington, DC, USA, 1963; pp. 31–32. [Google Scholar]
- Lee, M.O. Determination of the surface area of the white rat with its application to the expression of metabolic results. Am. J. Physiol. 1929, 89, 24–33. [Google Scholar] [CrossRef]
- Novelli, E.L.B.; Diniz, Y.S.; Galhardi, C.M.; Ebaid, G.M.X.; Rodrigues, H.G.; Mani, F.; Fernandes, A.; Cigogna, A.C.; Novelli Filho, J.L.V.B. Anthropometrical parameters and markers of obesity in rats. Lab. Anim. 2007, 41, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Howard, D.W.; Lewis, E.J.; Keller, B.J.; Smith, C.S. Histological Techniques for Marine Bivalve Molluscs and Crustaceans, 2nd ed.; NOAA, National Centers for Coastal Ocean Science: Oxford, UK, 2004; p. 218.
- Okafor, O.Y.; Erukainure, O.L.; Ajiboye, J.A.; Adejobi, R.O.; Owolabi, F.O.; Kosoko, S.B. Modulatory effect of pineapple peel extract on lipid peroxidation, catalase activity and hepatic biomarker levels in blood plasma of alcohol–induced oxidative stressed rats. Asian Pac. J. Trop. Biomed. 2011, 1, 12–14. [Google Scholar] [CrossRef]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- Matsumoto, T.; Kakami, M.; Noguchi, E.; Kobayashi, T.; Kamata, K. Imbalance between endothelium-derived relaxing and contracting factors in mesenteric arteries from aged OLETF rats, a model of Type 2 diabetes. Am. J. Physiol. 2007, 293, H1480–H1490. [Google Scholar] [CrossRef]
- Claudino, M.A.; Priviero, F.B.; Teixeira, C.E.; Nucci, G.; Antunes, E.; Zanesco, A. Improvement in relaxation response in corpus cavernosum from trained rats. Urology 2004, 63, 1004–1008. [Google Scholar] [CrossRef]
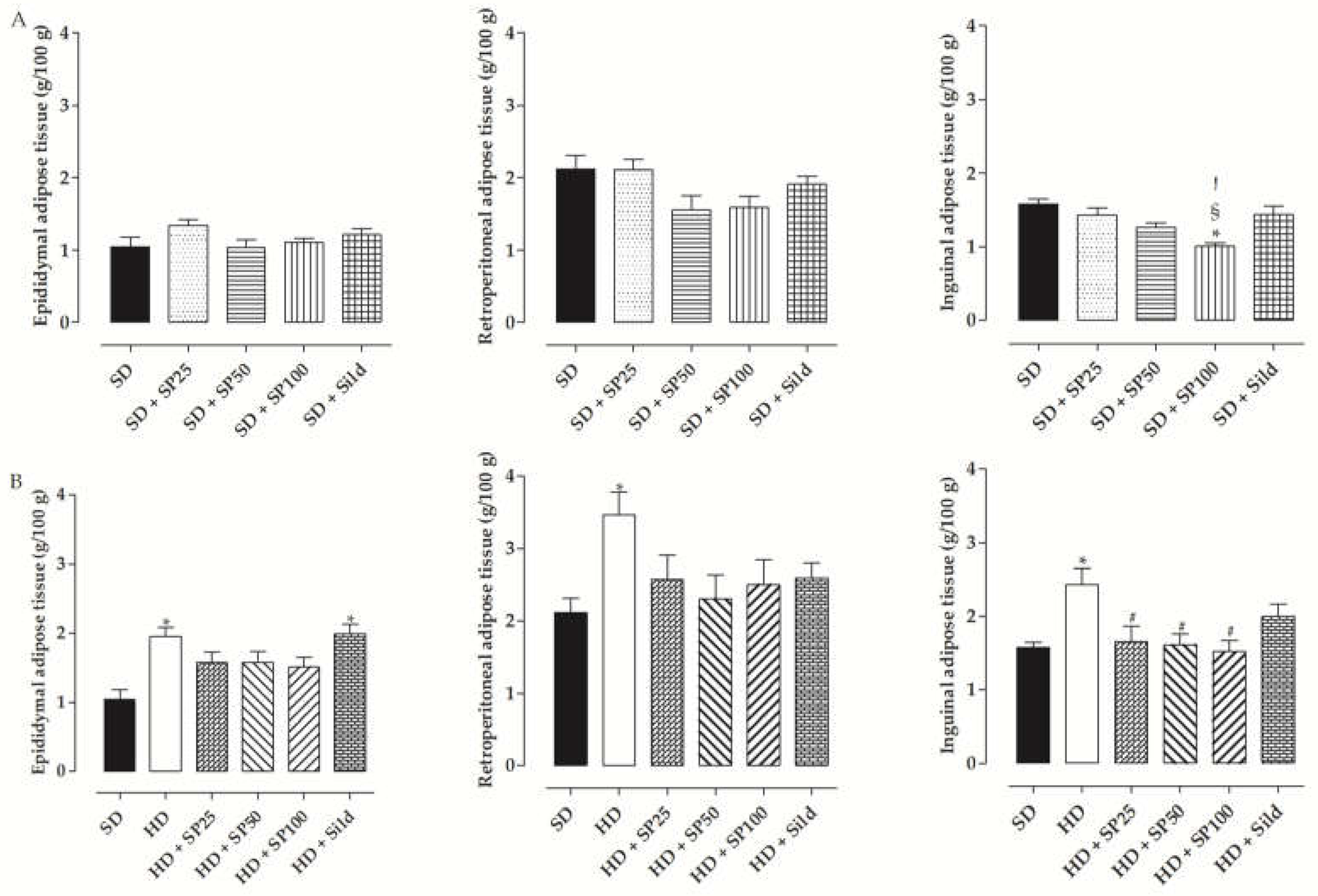
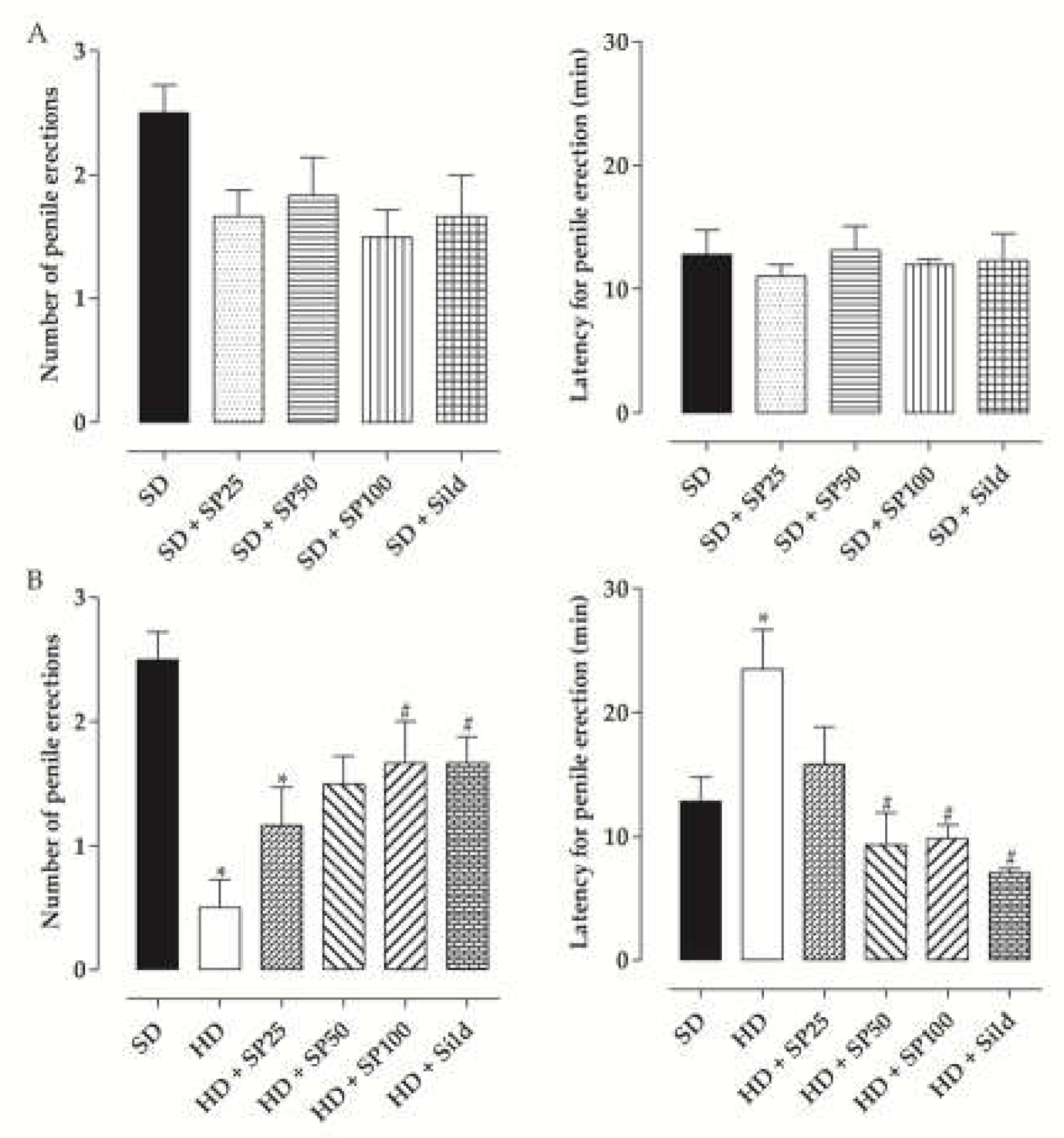
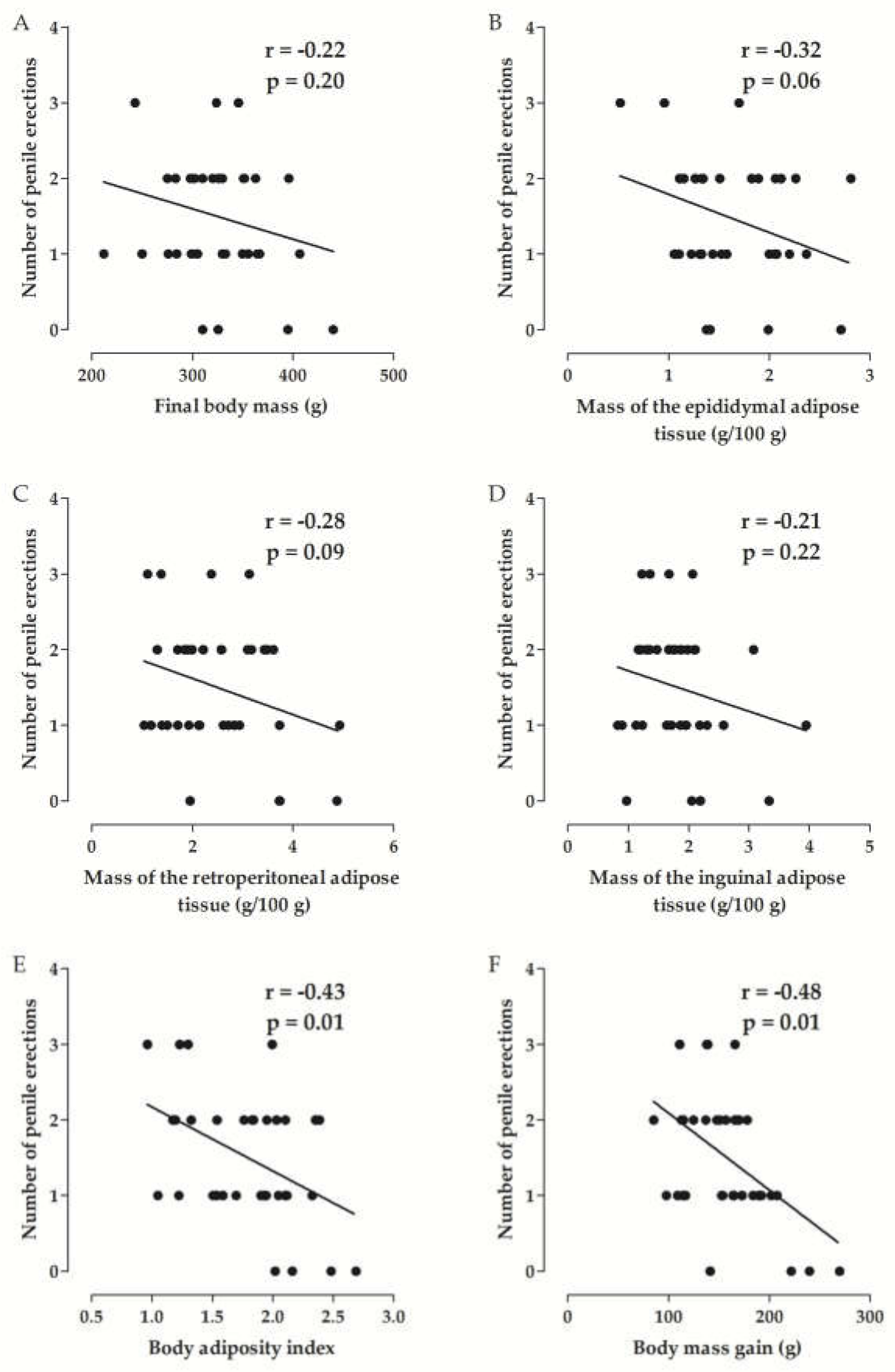

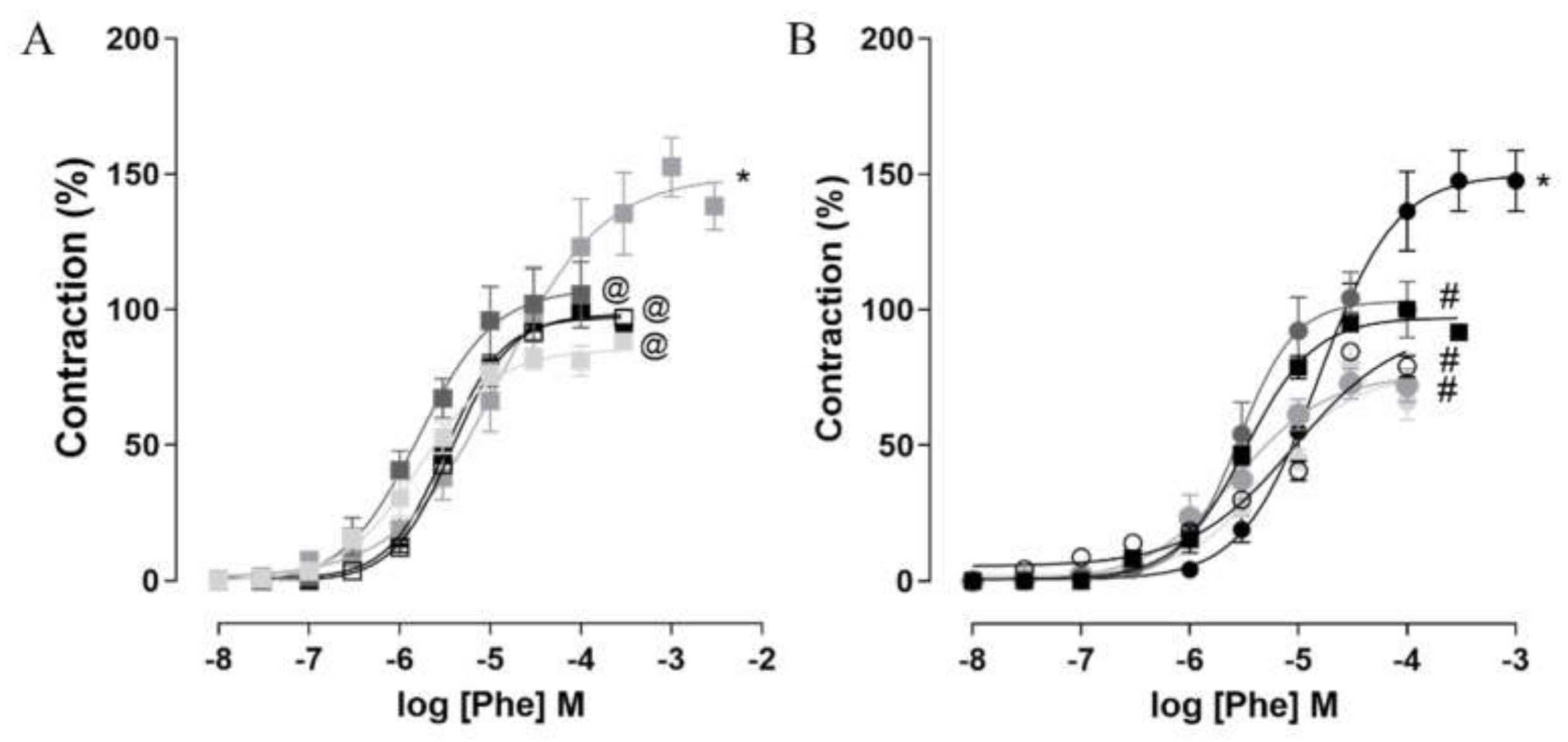
| Group | Initial Body Mass (g) | Final Body Mass (g) | Body Mass Gain (g) | Body Mass Gain (%) |
|---|---|---|---|---|
| SD | 147.6 ± 8.4 | 322.9 ± 4.0 | 166.3 ± 10.5 | 118.2 ± 2.4 |
| SD + SP25 | 168.2 ± 9.3 | 354.4 ± 14.3 @ | 186.2 ± 10.0 @ | 110.4 ± 1.5 @ |
| SD + SP50 | 157.4 ± 4.4 | 284.4 ± 10.1 | 129.1 ± 9.7 | 80.0 ± 3.4 * |
| SD + SP100 | 161.8 ± 7.8 | 338.6 ± 12.5 @ | 176.8 ± 10.3 @ | 109.0 ± 2.5 @ |
| SD + Sild | 175.7 ± 2.6 | 358.8 ± 8.7 @ | 183.1 ± 9.2 @ | 104.5 ± 1.5 @ |
| Group | Initial Body Mass (g) | Final Body Mass (g) | Body Mass Gain (g) | Body Mass Gain (%) |
|---|---|---|---|---|
| SD | 147.6 ± 8.4 | 322.9 ± 4.0 | 166.3 ± 10.5 | 118.2 ± 2.4 |
| HD | 158.1 ± 2.5 | 367.1 ± 12.8 * | 215.0 ± 11.1 * | 132.5 ± 3.0 * |
| HD + SP25 | 174.3 ± 17.2 | 320.7 ± 11.4 # | 148.6 ± 14.5 # | 84.0 ± 2.2 # |
| HD + SP50 | 166.5 ± 5.3 | 310.2 ± 21.7 # | 145.3 ± 13.7 # | 86.0 ± 1.9 # |
| HD + SP100 | 174.5 ± 10.5 | 308.6 ± 9.2 # | 143.1 ± 9.6 # | 77.0 ± 2.5 # |
| HD + Sild | 178.9 ± 4.0 | 344.4 ± 9.5 | 175.5 ± 6.2 | 92.0 ± 3.0 |
| Group | Glucose (mg/dL) | Triglyceride (mg/dL) | Total Cholesterol (mg/dL) | HDL-c (mg/dL) | LDL-c (mg/dL) |
|---|---|---|---|---|---|
| SD | 86.9 ± 4.5 | 67.5 ± 2.5 | 53.2 ± 3.4 | 17.4 ± 2.4 | 14.9 ± 1.6 |
| SD + SP25 | 88.1 ± 2.3 | 66.0 ± 6.0 | 44.3 ± 2.8 | 18.3 ± 2.1 | 14.4 ± 3.0 |
| SD + SP50 | 86.7 ± 3.5 | 68.0 ± 7.2 | 48.0 ± 3.4 | 19.7 ± 1.7 | 15.5 ± 2.4 |
| SD + SP100 | 78.8 ± 3.5 | 61.5 ± 4.7 | 49.8 ± 3.1 | 17.2 ± 1.3 | 18.2 ± 2.9 |
| SD + Sild | 84.3 ± 3.4 | 69.0 ± 4.1 | 49.8 ± 3.9 | 16.2 ± 1.1 | 15.9 ± 2.8 |
| HD | 91.4 ± 3.1 | 80.7 ± 7.2 | 53.0 ± 3.1 | 17.0 ± 1.8 | 14.3 ± 2.0 |
| HD + SP25 | 86.2 ± 2.6 | 65.5 ± 6.4 | 41.3 ± 5.7 | 15.8 ± 2.0 | 12.9 ± 2.3 |
| HD + SP50 | 89.7 ± 4.8 | 67.2 ± 4.8 | 48.3 ± 1.9 | 16.3 ± 1.0 | 13.5 ± 1.7 |
| HD + SP100 | 87.2 ± 2.3 | 62.0 ± 3.4 | 50.7 ± 6.2 | 17.5 ± 1.1 | 14.0 ± 1.9 |
| HD + Sild | 85.6 ± 2.4 | 64.8 ± 8.9 | 47.3 ± 4.6 | 15.2 ± 1.1 | 13.0 ± 2.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Souza, I.L.L.d.; Barros, B.C.; Ferreira, E.d.S.; Queiroga, F.R.; Vasconcelos, L.H.C.; Toscano, L.d.L.T.; Silva, A.S.; Silva, P.M.d.; Cavalcante, F.d.A.; Silva, B.A.d. Supplementation with Spirulina platensis Prevents Damage to Rat Erections in a Model of Erectile Dysfunction Promoted by Hypercaloric Diet-Induced Obesity. Mar. Drugs 2022, 20, 467. https://doi.org/10.3390/md20080467
Souza ILLd, Barros BC, Ferreira EdS, Queiroga FR, Vasconcelos LHC, Toscano LdLT, Silva AS, Silva PMd, Cavalcante FdA, Silva BAd. Supplementation with Spirulina platensis Prevents Damage to Rat Erections in a Model of Erectile Dysfunction Promoted by Hypercaloric Diet-Induced Obesity. Marine Drugs. 2022; 20(8):467. https://doi.org/10.3390/md20080467
Chicago/Turabian StyleSouza, Iara Leão Luna de, Bárbara Cavalcanti Barros, Elba dos Santos Ferreira, Fernando Ramos Queiroga, Luiz Henrique César Vasconcelos, Lydiane de Lima Tavares Toscano, Alexandre Sérgio Silva, Patrícia Mirella da Silva, Fabiana de Andrade Cavalcante, and Bagnólia Araújo da Silva. 2022. "Supplementation with Spirulina platensis Prevents Damage to Rat Erections in a Model of Erectile Dysfunction Promoted by Hypercaloric Diet-Induced Obesity" Marine Drugs 20, no. 8: 467. https://doi.org/10.3390/md20080467
APA StyleSouza, I. L. L. d., Barros, B. C., Ferreira, E. d. S., Queiroga, F. R., Vasconcelos, L. H. C., Toscano, L. d. L. T., Silva, A. S., Silva, P. M. d., Cavalcante, F. d. A., & Silva, B. A. d. (2022). Supplementation with Spirulina platensis Prevents Damage to Rat Erections in a Model of Erectile Dysfunction Promoted by Hypercaloric Diet-Induced Obesity. Marine Drugs, 20(8), 467. https://doi.org/10.3390/md20080467








