Seaweed Phenolics as Natural Antioxidants, Aquafeed Additives, Veterinary Treatments and Cross-Linkers for Microencapsulation
Abstract
:1. Introduction
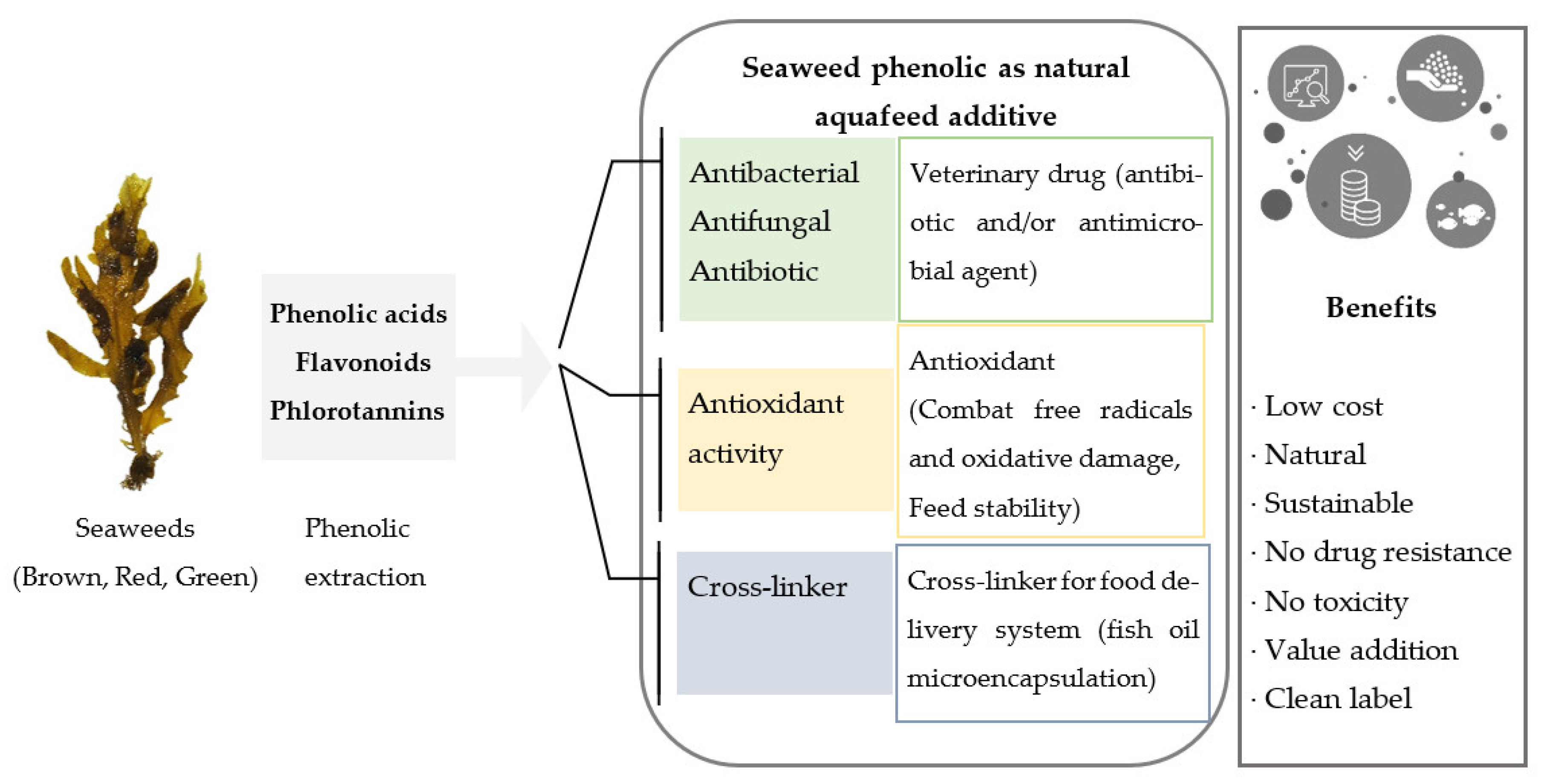
2. Seaweed Phenolics as Sustainable Aquafeed Additives
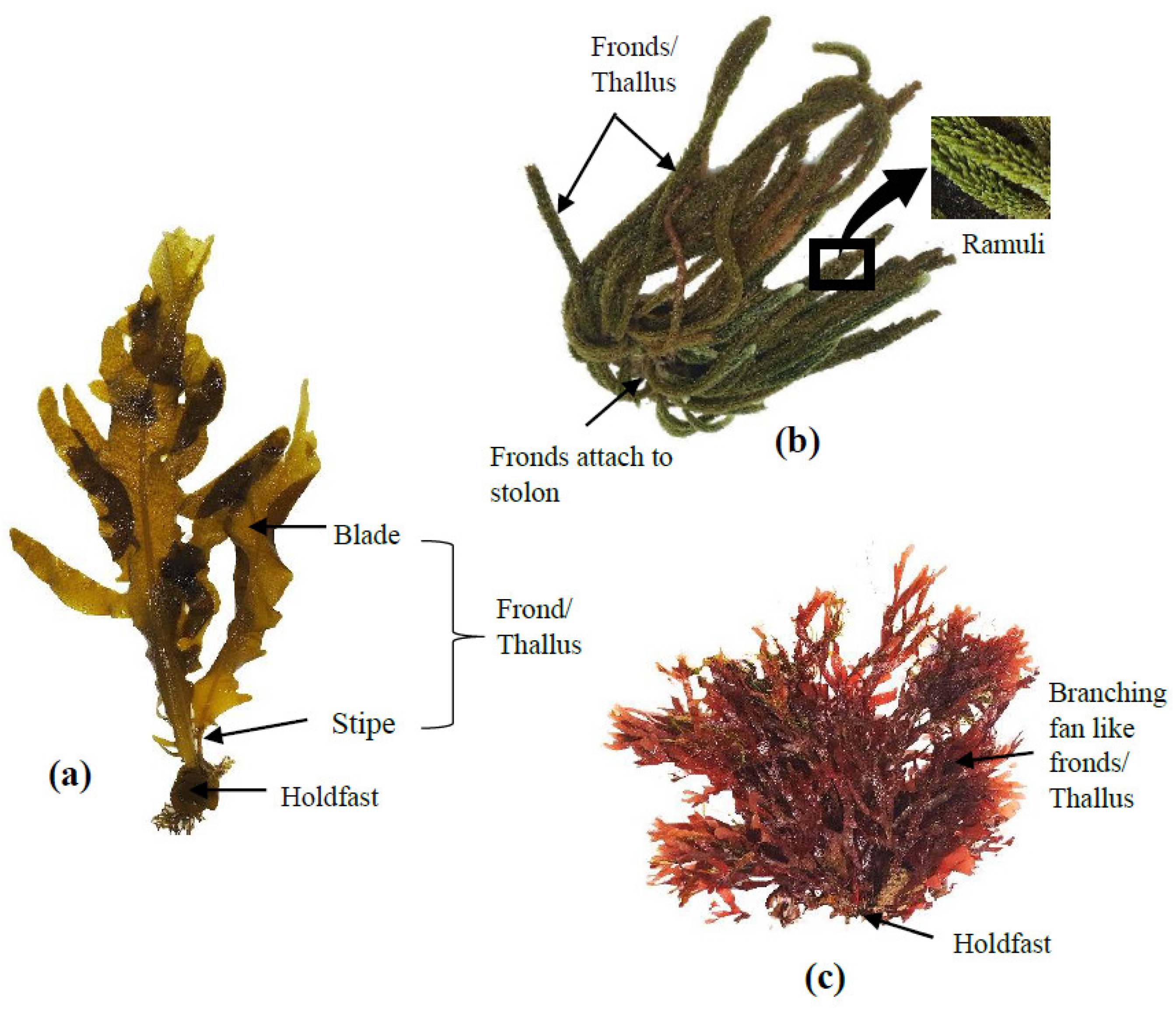
3. Overview of Seaweeds
4. Seaweed Phenolics Compounds
4.1. Classification of Phenolic Compounds
4.2. Phenolic Compounds Found in Seaweeds
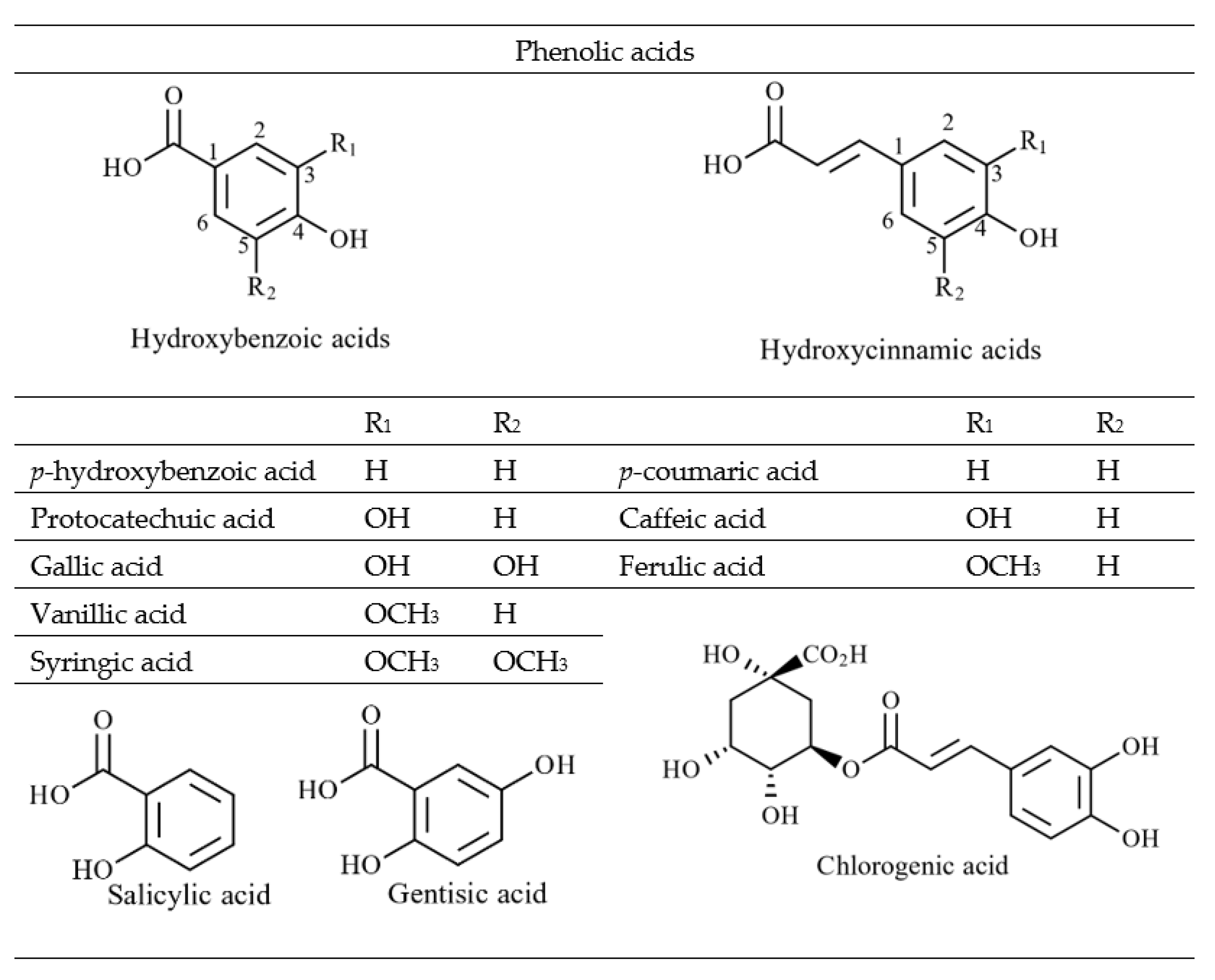
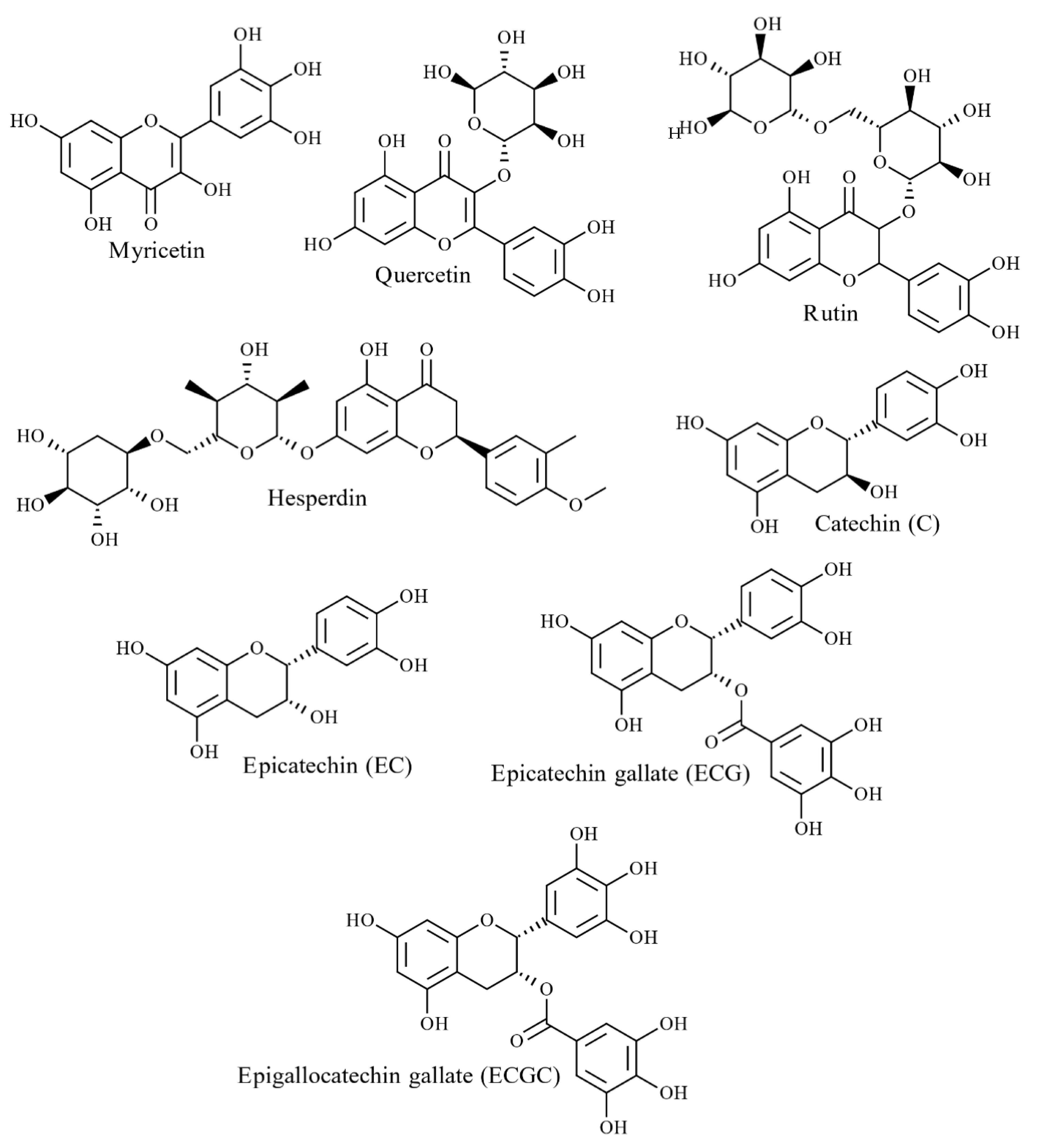
4.2.1. Phenolic Acids and Flavonoids
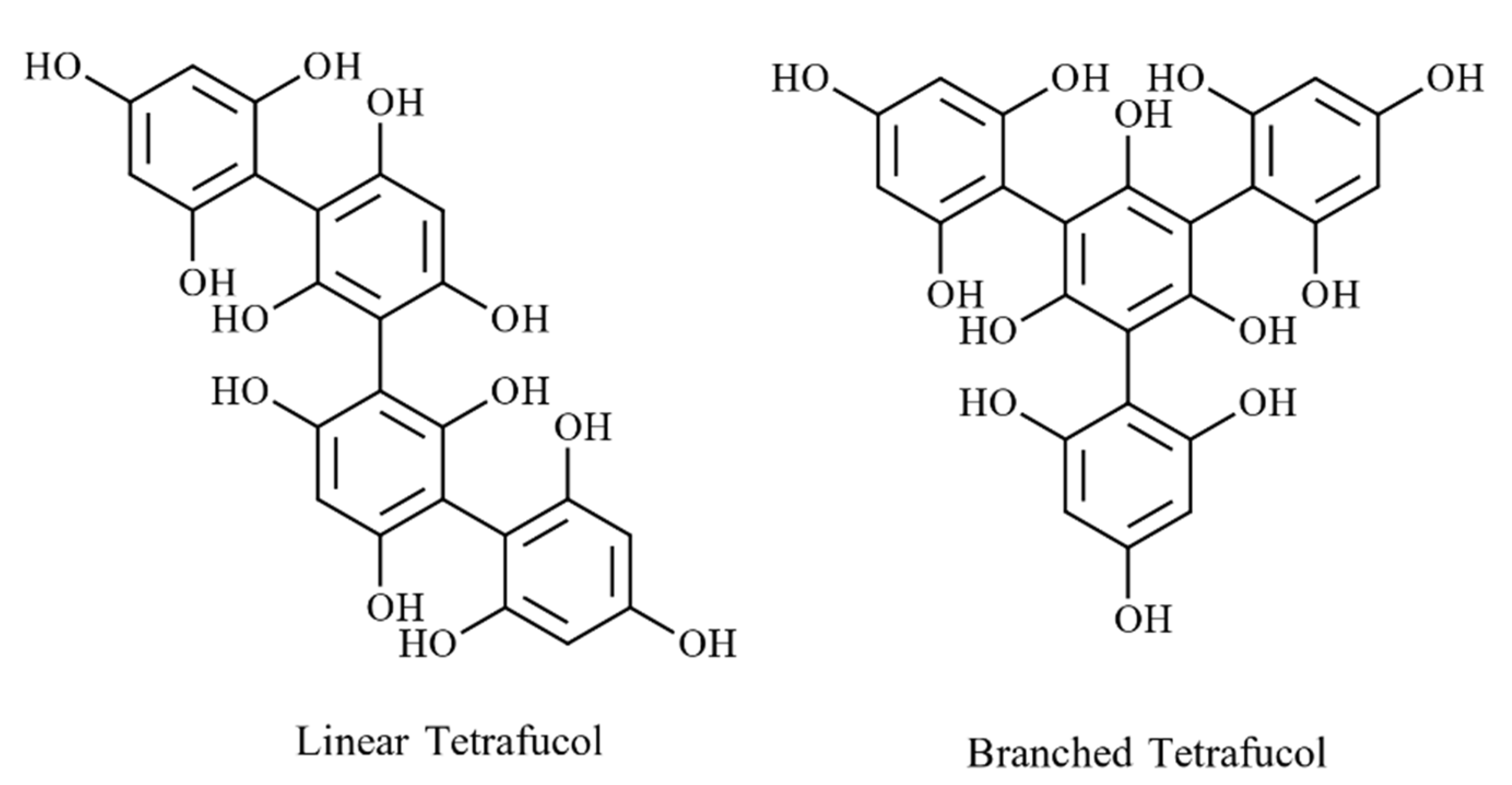
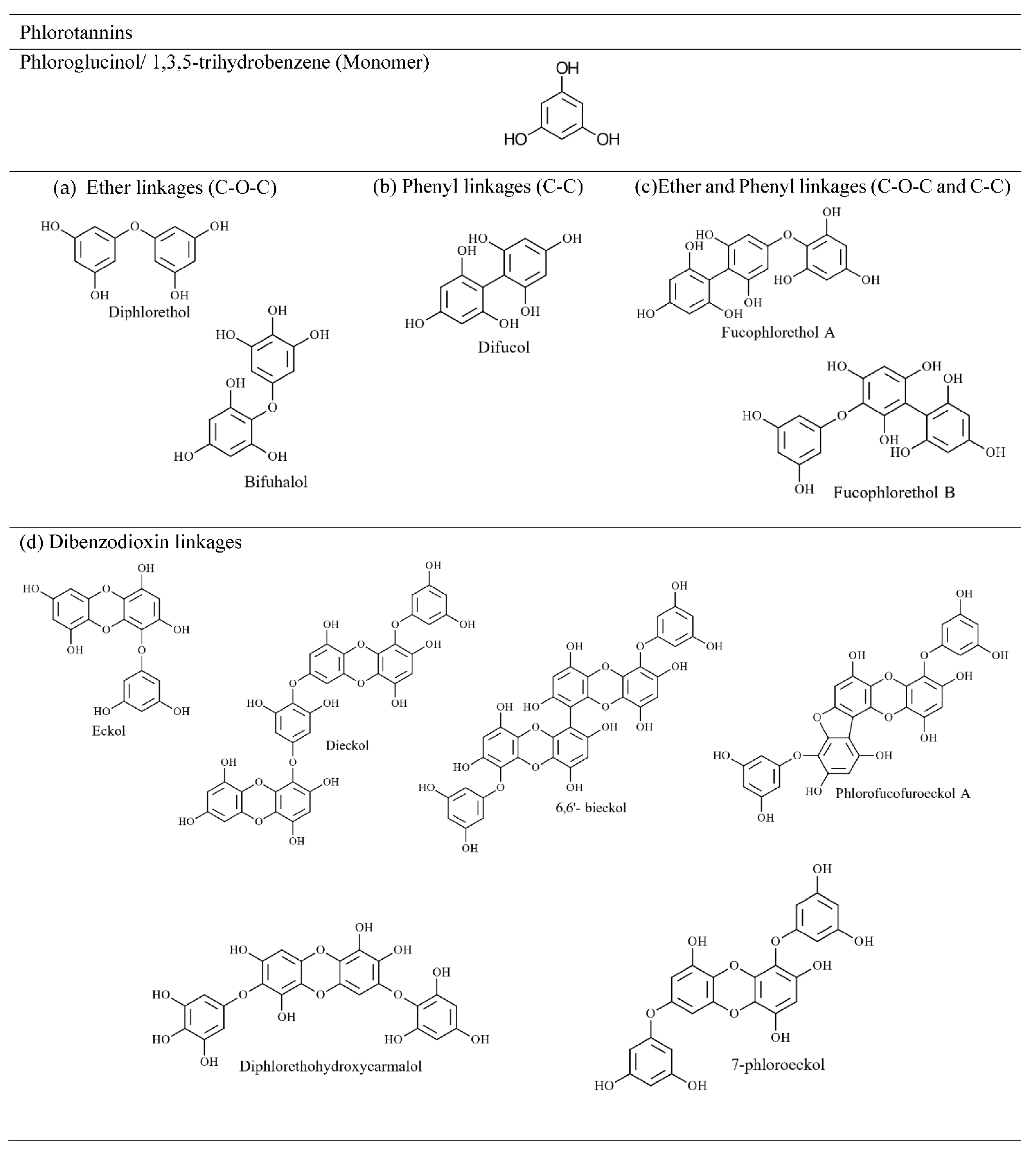
4.2.2. Phlorotannin
4.3. Occurrence and Biosynthesis
4.4. Variation of Phenolic Content
5. Extraction and Identification of Seaweed Phenolic Compounds
5.1. Sample Handling and Pre-Treatments
5.2. Extraction of Seaweed Phenolics
5.3. Separation and Purification of Phenolics from Crude Seaweed Extracts
5.4. Identification and Structural Elucidation
6. Seaweed Phenolics as Veterinary Treatments in Aquaculture
7. Seaweed Phenolics as Natural Antioxidants in Aquafeed
7.1. Importance of Antioxidants in Aquaculture Management
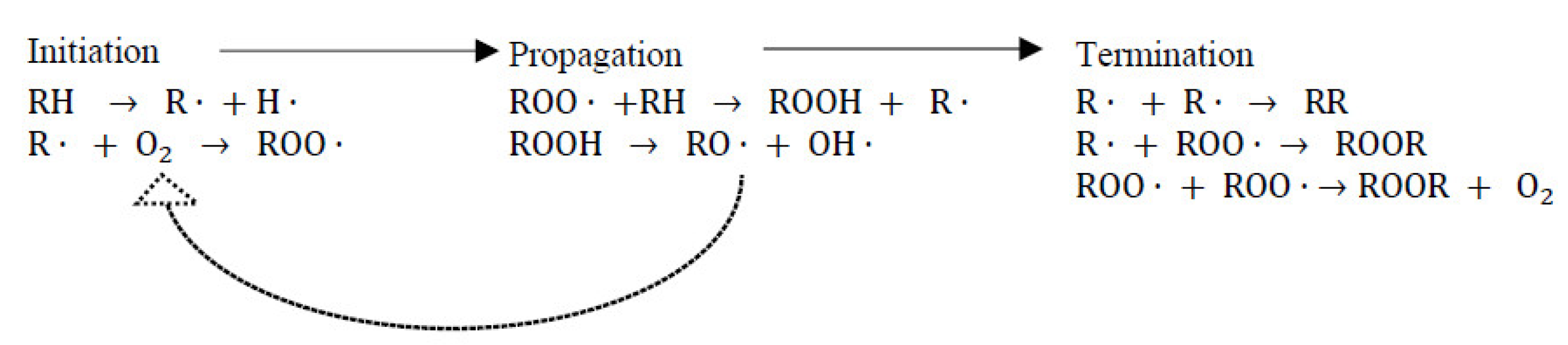
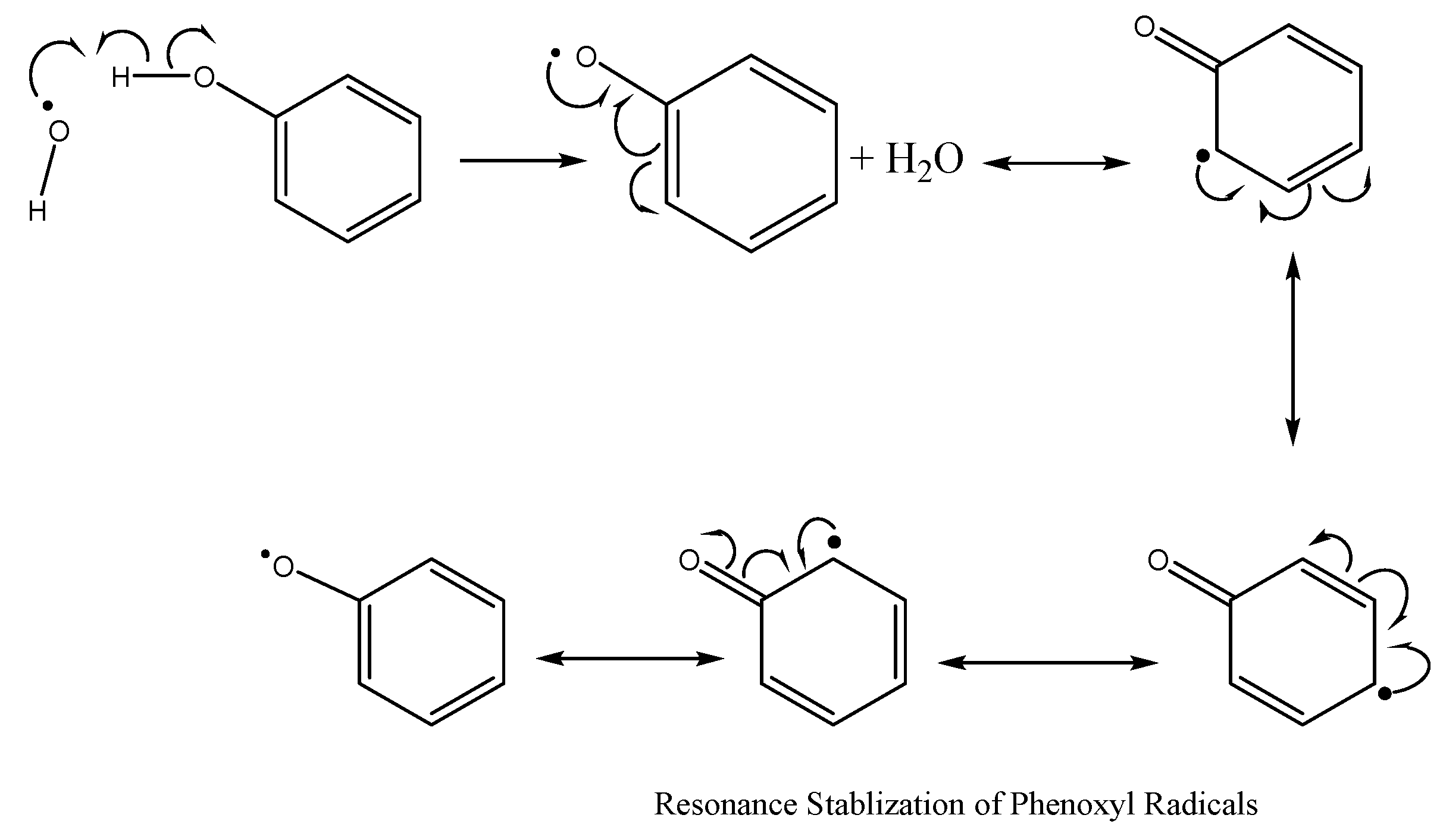
7.1.1. Combatting Free Radicals and Oxidative Damage
7.1.2. Feed Stability and Prolonged Shelf Life
7.2. Synthetic Antioxidants in Aquafeed and Their Future Viability
7.3. Antioxidant Capacity of Seaweed Phenolic Compounds
7.4. Antioxidant Activity of Seaweed Phenolics in Feed/Ingredient/Animal Model Systems
8. Seaweed Phenolics as Crosslinkers in Food Delivery Systems (Microencapsulation)
8.1. Seaweed as a Source of Biomaterials for Microencapsulation
8.2. Microencapsulated Oil in Aquafeed
8.3. Phenolics as Cross-Linkers in Complex Coacervation
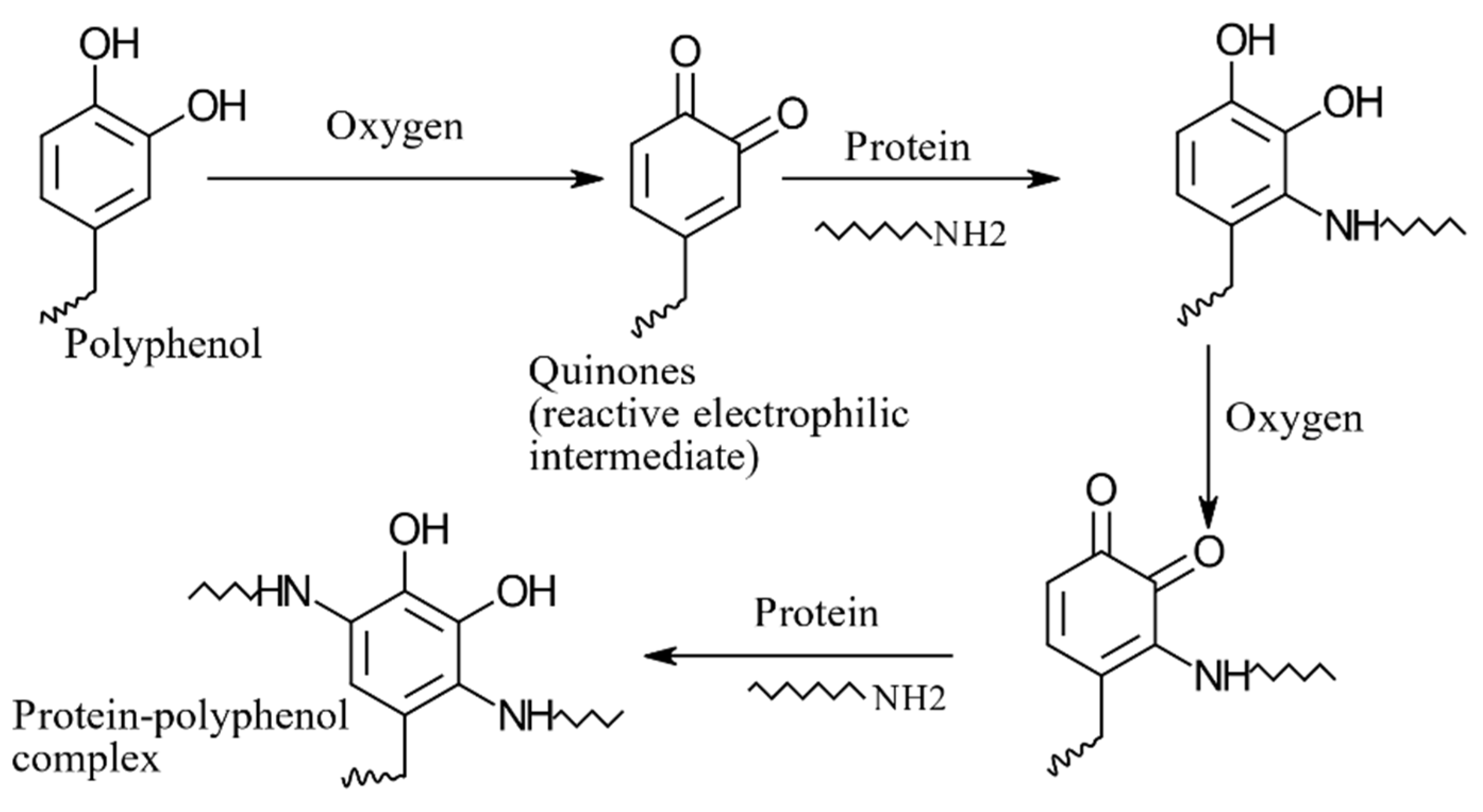
8.4. Protein–Polyphenol Interactions
8.5. Factors Effecting Protein–Polyphenol Interactions
9. Safety, Legal and Ethical Aspects of Seaweed in Aqua Feed Products
10. Conclusions & Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- UN. UN DESA Policy Brief No. 130: Why Population Growth Matters for Sustainable Development. Available online: https://www.un.org/development/desa/dpad/publication/un-desa-policy-brief-no-130-why-population-growth-matters-for-sustainable-development/#:~:text=Since%20the%20middle%20of%20the,11%20billion%20by%20around%202100 (accessed on 16 May 2022).
- UN. How certain are the United Nations global population projections? Popul. Facts 2019/6, 1. Available online: https://www.un.org/en/development/desa/population/publications/pdf/popfacts/PopFacts_2019-6.pdf (accessed on 5 April 2022).
- FAO. The State of World Fisheries and Aquaculture 2018—Meeting the Sustainable Development Goals; Food and Agriculture Organization of the United Nations: Rome, Italy, 2018; ISBN 978-92-5-130562-1. [Google Scholar]
- FAO. Fishery and Aquaculture Statistics. Available online: http://www.fao.org/fishery/statistics/en (accessed on 5 April 2022).
- You, W.; Hedgecock, D. Boom-and-bust production cycles in animal seafood aquaculture. Rev. Aquac. 2019, 11, 1045–1060. [Google Scholar] [CrossRef]
- Rizzo, C.; Genovese, G.; Morabito, M.; Faggio, C.; Pagano, M.; Spanò, A.; Zammuto, V.; Minicante, S.A.; Manghisi, A.; Cigala, R.M. Potential antibacterial activity of marine macroalgae against pathogens relevant for aquaculture and human health. J. Pure Appl. Microbiol. 2017, 11, 1695–1706. [Google Scholar] [CrossRef]
- Reverter, M.; Bontemps, N.; Lecchini, D.; Banaigs, B.; Sasal, P. Use of plant extracts in fish aquaculture as an alternative to chemotherapy: Current status and future perspectives. Aquaculture 2014, 433, 50–61. [Google Scholar] [CrossRef]
- Aklakur, M. Natural antioxidants from sea: A potential industrial perspective in aquafeed formulation. Rev. Aquac. 2018, 10, 385–399. [Google Scholar] [CrossRef]
- Cian, R.E.; Bacchetta, C.; Rossi, A.; Cazenave, J.; Drago, S.R. Red seaweed Pyropia columbina as antioxidant supplement in feed for cultured juvenile Pacú (Piaractus mesopotamicus). J. Appl. Phycol. 2019, 31, 1455–1465. [Google Scholar] [CrossRef]
- Fontagné-Dicharry, S.; Lataillade, E.; Surget, A.; Larroquet, L.; Cluzeaud, M.; Kaushik, S. Antioxidant defense system is altered by dietary oxidized lipid in first-feeding rainbow trout (Oncorhynchus mykiss). Aquaculture 2014, 424, 220–227. [Google Scholar] [CrossRef]
- Biller-Takahashi, J.D.; Takahashi, L.S.; Mingatto, F.E.; Urbinati, E.C. The immune system is limited by oxidative stress: Dietary selenium promotes optimal antioxidative status and greatest immune defense in pacu Piaractus mesopotamicus. Fish Shellfish Immunol. 2015, 47, 360–367. [Google Scholar] [CrossRef]
- Rumsey, G.L. Antioxidants in Compounded Feeds. Available online: http://www.fao.org/3/x5738e/x5738e0b.htm (accessed on 29 July 2020).
- ECOLEX. Commission Implementing Regulation (EU) 2017/962 Suspending the Authorisation of Ethoxyquin as a Feed Additive for All Animal Species and Categories. In 2017/962; Food and Agriculture Organization of the United Nations, Ed.; The Official Journal of the European Union: 2017; Volume 60. Available online: https://eur-lex.europa.eu/eli/reg_impl/2017/962/oj (accessed on 13 July 2020).
- Lundebye, A.-K.; Hove, H.; Måge, A.; Bohne, V.; Hamre, K. Levels of synthetic antioxidants (ethoxyquin, butylated hydroxytoluene and butylated hydroxyanisole) in fish feed and commercially farmed fish. Food Addit. Contam. Part A 2010, 27, 1652–1657. [Google Scholar] [CrossRef]
- Błaszczyk, A.; Skolimowski, J. Cytotoxicity and genotoxicity of ethoxyquin used as an antioxidant. Food Rev. Int. 2015, 31, 222–235. [Google Scholar] [CrossRef]
- FDA. Food Additives Permitted in Feed and Drinking Water of Animals. Available online: https://www.ecfr.gov/cgi-bin/text-idx?SID=37d1b1c88427be27d41991d6d47c8e44&mc=true&node=pt21.6.573&rgn=div5#se21.6.573_1380 (accessed on 13 July 2020).
- Manage, P.M. Heavy Use of Antibiotics in Aquaculture; Emerging Human and Animal Health Problems—A review. Sri Lanka J. Aquat. Sci. 2018, 23, 13–27. [Google Scholar] [CrossRef]
- Kraan, S.; Martin, P.; Mair, C. Natural and Sustainable Seaweed Formula that Replaces Synthetic Additives in Fish Feed. U.S. Patent Application 15/665,303, 18 January 2018. [Google Scholar]
- Plentex Aquafeed. Available online: https://www.plentex.com.au/site/markets/aquafeed (accessed on 16 May 2022).
- Teves, J.F.C.; Ragaza, J.A. The quest for indigenous aquafeed ingredients: A review. Rev. Aquac. 2016, 8, 154–171. [Google Scholar] [CrossRef]
- Wan, A.H.; Davies, S.J.; Soler-Vila, A.; Fitzgerald, R.; Johnson, M.P. Macroalgae as a sustainable aquafeed ingredient. Rev. Aquac. 2019, 11, 458–492. [Google Scholar] [CrossRef]
- Stanley, J.G.; Jones, J.B. Feeding algae to fish. Aquaculture 1976, 7, 219–223. [Google Scholar] [CrossRef]
- Black, W. Seaweed as a poultry food. World’s Poult. Sci. J. 1954, 10, 33–35. [Google Scholar] [CrossRef]
- Lorbeer, A.; Tham, R.; Zhang, W. Potential products from the highly diverse and endemic macroalgae of Southern Australia and pathways for their sustainable production. J. Appl. Phycol. 2013, 25, 717–732. [Google Scholar] [CrossRef]
- Ragaza, J.A.; Koshio, S.; Mamauag, R.E.; Ishikawa, M.; Yokoyama, S.; Villamor, S.S. Dietary supplemental effects of red seaweed Eucheuma denticulatum on growth performance, carcass composition and blood chemistry of juvenile Japanese flounder, Paralichthys olivaceus. Aquac. Res. 2015, 46, 647–657. [Google Scholar] [CrossRef]
- Thorp, J.H.; Rogers, D.C. Chapter 4—A Primer on Ecological Relationships among Freshwater Invertebrates. In Field Guide to Freshwater Invertebrates of North America; Thorp, J.H., Rogers, D.C., Eds.; Academic Press: Boston, MA, USA, 2011; pp. 37–46. [Google Scholar]
- Omont, A.; Quiroz-Guzman, E.; Tovar-Ramirez, D.; Peña-Rodríguez, A. Effect of diets supplemented with different seaweed extracts on growth performance and digestive enzyme activities of juvenile white shrimp Litopenaeus vannamei. J. Appl. Phycol. 2019, 31, 1433–1442. [Google Scholar] [CrossRef]
- Kamunde, C.; Sappal, R.; Melegy, T.M. Brown seaweed (AquaArom) supplementation increases food intake and improves growth, antioxidant status and resistance to temperature stress in Atlantic salmon, Salmo salar. PLoS ONE 2019, 14, e0219792. [Google Scholar] [CrossRef] [Green Version]
- Wilke, T.; Faulkner, S.; Murphy, L.; Kealy, L.; Kraan, S.; Brouns, F. Seaweed enrichment of feed supplied to farm-raised Atlantic salmon (Salmo salar) is associated with higher total fatty acid and LC n-3 PUFA concentrations in fish flesh. Eur. J. Lipid Sci. Technol. 2015, 117, 767–772. [Google Scholar] [CrossRef]
- Moutinho, S.; Linares, F.; Rodríguez, J.; Sousa, V.; Valente, L. Inclusion of 10% seaweed meal in diets for juvenile and on-growing life stages of Senegalese sole (Solea senegalensis). J. Appl. Phycol. 2018, 30, 3589–3601. [Google Scholar] [CrossRef]
- Fernandes, H.; Salgado, J.M.; Martins, N.; Peres, H.; Oliva-Teles, A.; Belo, I. Sequential bioprocessing of Ulva rigida to produce lignocellulolytic enzymes and to improve its nutritional value as aquaculture feed. Bioresour. Technol. 2019, 281, 277–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Santos, S.A.; Félix, R.; Pais, A.; Rocha, S.M.; Silvestre, A.J. The Quest for Phenolic Compounds from Macroalgae: A Review of Extraction and Identification Methodologies. Biomolecules 2019, 9, 847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freitas, A.C.; Pereira, L.; Rodrigues, D.; Carvalho, A.P.; Panteleitchouk, T.; Gomes, A.M.; Duarte, A.C. Marine Functional Foods. In Springer Handbook of Marine Biotechnology; Springer: Berlin/Heidelberg, Germany, 2015; pp. 969–994. [Google Scholar] [CrossRef]
- Ma, W.C.J.; Chung, H.Y.; Ang, P.O.; Kim, J.-S. Enhancement of bromophenol levels in aquacultured silver seabream (Sparus sarba). J. Agric. Food Chem. 2005, 53, 2133–2139. [Google Scholar] [CrossRef]
- ABARES. Australian Seaweed Production. Available online: https://www.agriculture.gov.au/abares/research-topics/fisheries/fisheries-and-aquaculture-statistics/australian-seaweed-production (accessed on 12 August 2020).
- McHugh, D. A Guide to the Seaweed Industry FAO Fisheries Technical Paper 441; Food and Agriculture Organization of the United Nations: Rome, Italy, 2003; Available online: http://www.fao.org/3/y4765e/y4765e.pdf (accessed on 12 August 2020).
- Guiry, M.D.; Guiry, G.M. AlgaeBase. World-Wide Electronic Publication, National University of Ireland, Galway. 2022. Available online: http://www.algaebase.org (accessed on 16 May 2022).
- Santos, S.A.; Vilela, C.; Freire, C.S.; Abreu, M.H.; Rocha, S.M.; Silvestre, A.J. Chlorophyta and Rhodophyta macroalgae: A source of health promoting phytochemicals. Food Chem. 2015, 183, 122–128. [Google Scholar] [CrossRef]
- Ferdouse, F.; Holdt, S.L.; Smith, R.; Murúa, P.; Yang, Z. The global status of seaweed production, trade and utilization. Globefish Res. Programme 2018, 124, I. Available online: https://www.proquest.com/openview/63a9872d1ea30c63f92d5d8acfcd6e35/1?pq-origsite=gscholar&cbl=237312 (accessed on 13 August 2020).
- Buschmann, A.H.; Camus, C.; Infante, J.; Neori, A.; Israel, Á.; Hernández-González, M.C.; Pereda, S.V.; Gomez-Pinchetti, J.L.; Golberg, A.; Tadmor-Shalev, N. Seaweed production: Overview of the global state of exploitation, farming and emerging research activity. Eur. J. Phycol. 2017, 52, 391–406. [Google Scholar] [CrossRef]
- RIRDC. Cultivated Seaweed. Available online: https://www.agrifutures.com.au/farm-diversity/cultivated-seaweed/#:~:text=Growing%20regions,Victoria%20and%20New%20South%20Wales. (accessed on 13 August 2020).
- Murray, M.; Dordevic, A.L.; Ryan, L.; Bonham, M.P. An emerging trend in functional foods for the prevention of cardiovascular disease and diabetes: Marine algal polyphenols. Crit. Rev. Food Sci. Nutr. 2018, 58, 1342–1358. [Google Scholar] [CrossRef]
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Dietary phenolics: Chemistry, bioavailability and effects on health. Nat. Prod. Rep. 2009, 26, 1001–1043. [Google Scholar] [CrossRef]
- Laura, A.; Moreno-Escamilla, J.O.; Rodrigo-García, J.; Alvarez-Parrilla, E. Phenolic Compounds. In Postharvest Physiology and Biochemistry of Fruits and Vegetables; Elsevier: Cambridge, UK, 2019; pp. 253–271. [Google Scholar] [CrossRef]
- Gómez-Guzmán, M.; Rodríguez-Nogales, A.; Algieri, F.; Gálvez, J. Potential role of seaweed polyphenols in cardiovascular-associated disorders. Mar. Drugs 2018, 16, 250. [Google Scholar] [CrossRef] [Green Version]
- Neveu, V.; Perez-Jiménez, J.; Vos, F.; Crespy, V.; du Chaffaut, L.; Mennen, L.; Knox, C.; Eisner, R.; Cruz, J.; Wishart, D. Phenol-Explorer: An online comprehensive database on polyphenol contents in foods. Database 2010, 2010, bap024. [Google Scholar] [CrossRef] [PubMed]
- Sanjeewa, K.K.A.; Kim, E.-A.; Son, K.-T.; Jeon, Y.-J. Bioactive properties and potentials cosmeceutical applications of phlorotannins isolated from brown seaweeds: A review. J. Photochem. Photobiol. B Biol. 2016, 162, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Agregan, R.; Munekata, P.E.; Franco, D.; Dominguez, R.; Carballo, J.; Lorenzo, J.M. Phenolic compounds from three brown seaweed species using LC-DAD–ESI-MS/MS. Food Res. Int. 2017, 99, 979–985. [Google Scholar] [CrossRef] [PubMed]
- López, A.; Rico, M.; Rivero, A.; de Tangil, M.S. The effects of solvents on the phenolic contents and antioxidant activity of Stypocaulon scoparium algae extracts. Food Chem. 2011, 125, 1104–1109. [Google Scholar] [CrossRef]
- Ragan, M.A. Phlorotannins, brown algal polyphenols. Prog. Phycol. Res. 1986, 4, 177–241. [Google Scholar]
- Yoshie-Stark, Y.; Hsieh, Y.-P.; Suzuki, T. Distribution of flavonoids and related compounds from seaweeds in Japan. J.-Tokyo Univ. Fish. 2003, 89, 1–6. [Google Scholar]
- Onofrejová, L.; Vašíčková, J.; Klejdus, B.; Stratil, P.; Mišurcová, L.; Kráčmar, S.; Kopecký, J.; Vacek, J. Bioactive phenols in algae: The application of pressurized-liquid and solid-phase extraction techniques. J. Pharm. Biomed. Anal. 2010, 51, 464–470. [Google Scholar] [CrossRef]
- Klejdus, B.; Lojková, L.; Plaza, M.; Šnóblová, M.; Štěrbová, D. Hyphenated technique for the extraction and determination of isoflavones in algae: Ultrasound-assisted supercritical fluid extraction followed by fast chromatography with tandem mass spectrometry. J. Chromatogr. A 2010, 1217, 7956–7965. [Google Scholar] [CrossRef]
- Jones, B.; Smullen, R.; Carton, A. Flavour enhancement of freshwater farmed barramundi (Lates calcarifer), through dietary enrichment with cultivated sea lettuce, Ulva ohnoi. Aquaculture 2016, 454, 192–198. [Google Scholar] [CrossRef]
- Hartmann, A.; Ganzera, M.; Karsten, U.; Skhirtladze, A.; Stuppner, H. Phytochemical and analytical characterization of novel sulfated coumarins in the marine green macroalga Dasycladus vermicularis (Scopoli) Krasser. Molecules 2018, 23, 2735. [Google Scholar] [CrossRef] [Green Version]
- Imbs, T.; Zvyagintseva, T. Phlorotannins are polyphenolic metabolites of brown algae. Russ. J. Mar. Biol. 2018, 44, 263–273. [Google Scholar] [CrossRef]
- Heffernan, N.; Brunton, N.P.; FitzGerald, R.J.; Smyth, T.J. Profiling of the molecular weight and structural isomer abundance of macroalgae-derived phlorotannins. Mar. Drugs 2015, 13, 509–528. [Google Scholar] [CrossRef] [PubMed]
- Vo, T.-S.; Ngo, D.-H.; Kim, S.-K. Marine algae as a potential pharmaceutical source for anti-allergic therapeutics. Process Biochem. 2012, 47, 386–394. [Google Scholar] [CrossRef]
- Kim, S.-K. Handbook of Marine Macroalgae: Biotechnology and Applied Phycology; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Singh, I.P.; Bharate, S.B. Phloroglucinol compounds of natural origin. Nat. Prod. Rep. 2006, 23, 558–591. [Google Scholar] [CrossRef]
- Glombitza, K.-W.; Rauwald, H.-W.; Eckhardt, G. Fucole, polyhydroxyoligophenyle aus Fucus vesiculosus. Phytochemistry 1975, 14, 1403–1405. [Google Scholar] [CrossRef]
- Catarino, M.D.; Silva, A.; Cardoso, S.M. Fucaceae: A source of bioactive phlorotannins. Int. J. Mol. Sci. 2017, 18, 1327. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Fu, X.; Duan, D.; Liu, X.; Xu, J.; Gao, X. Extraction and identification of phlorotannins from the brown alga, Sargassum fusiforme (Harvey) Setchell. Mar. Drugs 2017, 15, 49. [Google Scholar] [CrossRef] [Green Version]
- Olate-Gallegos, C.; Barriga, A.; Vergara, C.; Fredes, C.; García, P.; Giménez, B.; Robert, P. Identification of Polyphenols from Chilean Brown Seaweeds Extracts by LC-DAD-ESI-MS/MS. J. Aquat. Food Prod. Technol. 2019, 28, 375–391. [Google Scholar] [CrossRef]
- Shibata, T.; Fujimoto, K.; Nagayama, K.; Yamaguchi, K.; Nakamura, T. Inhibitory activity of brown algal phlorotannins against hyaluronidase. Int. J. Food Sci. Technol. 2002, 37, 703–709. [Google Scholar] [CrossRef]
- Montero, L.; Herrero, M.; Ibáñez, E.; Cifuentes, A. Separation and characterization of phlorotannins from brown algae Cystoseira abies-marina by comprehensive two-dimensional liquid chromatography. Electrophoresis 2014, 35, 1644–1651. [Google Scholar] [CrossRef] [Green Version]
- Steevensz, A.J.; MacKinnon, S.L.; Hankinson, R.; Craft, C.; Connan, S.; Stengel, D.B.; Melanson, J.E. Profiling phlorotannins in brown macroalgae by liquid chromatography–high resolution mass spectrometry. Phytochem. Anal. 2012, 23, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Leyton, A.; Pezoa-Conte, R.; Barriga, A.; Buschmann, A.; Mäki-Arvela, P.; Mikkola, J.-P.; Lienqueo, M. Identification and efficient extraction method of phlorotannins from the brown seaweed Macrocystis pyrifera using an orthogonal experimental design. Algal Res. 2016, 16, 201–208. [Google Scholar] [CrossRef]
- Schoenwaelder, M.E. The occurrence and cellular significance of physodes in brown algae. Phycologia 2002, 41, 125–139. [Google Scholar] [CrossRef]
- Singh, I.P.; Sidana, J. 5-Phlorotannins. In Functional Ingredients from Algae for Foods and Nutraceuticals; Domínguez, H., Ed.; Woodhead Publishing: Sawston, UK, 2013; pp. 181–204. [Google Scholar]
- Schoenwaelder, M.E.; Clayton, M.N. The presence of phenolic compounds in isolated cell walls of brown algae. Phycologia 1999, 38, 161–166. [Google Scholar] [CrossRef]
- Koivikko, R.; Loponen, J.; Honkanen, T.; Jormalainen, V. Contents of soluble, cell-wall-bound and exuded phlorotannins in the brown alga Fucus vesiculosus, with implications on their ecological functions. J. Chem. Ecol. 2005, 31, 195–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baardseth, E. A method of estimating the physode content in brown algae. Rep. Norw. Inst. Seaweed Res. 1958, 20, 1–16. [Google Scholar]
- Jacobsen, C.; Sørensen, A.-D.M.; Holdt, S.L.; Akoh, C.C.; Hermund, D.B. Source, extraction, characterization, and applications of novel antioxidants from seaweed. Annu. Rev. Food Sci. Technol. 2019, 10, 541–568. [Google Scholar] [CrossRef]
- Shibata, T.; Kawaguchi, S.; Hama, Y.; Inagaki, M.; Yamaguchi, K.; Nakamura, T. Local and chemical distribution of phlorotannins in brown algae. J. Appl. Phycol. 2004, 16, 291–296. [Google Scholar] [CrossRef]
- Rugiu, L.; Panova, M.; Pereyra, R.T.; Jormalainen, V. Gene regulatory response to hyposalinity in the brown seaweed Fucus vesiculosus. BMC Genom. 2020, 21, 42. [Google Scholar] [CrossRef] [Green Version]
- Stengel, D.B.; Connan, S.; Popper, Z.A. Algal chemodiversity and bioactivity: Sources of natural variability and implications for commercial application. Biotechnol. Adv. 2011, 29, 483–501. [Google Scholar] [CrossRef]
- Mabeau, S.; Fleurence, J. Seaweed in food products: Biochemical and nutritional aspects. Trends Food Sci. Technol. 1993, 4, 103–107. [Google Scholar] [CrossRef]
- Connan, S.; Goulard, F.; Stiger, V.; Deslandes, E.; Gall, E.A. Interspecific and temporal variation in phlorotannin levels in an assemblage of brown algae. Bot. Mar. 2004, 47, 410–416. [Google Scholar] [CrossRef]
- Tabassum, M.R.; Xia, A.; Murphy, J.D. Seasonal variation of chemical composition and biomethane production from the brown seaweed Ascophyllum nodosum. Bioresour. Technol. 2016, 216, 219–226. [Google Scholar] [CrossRef]
- Abdala-Díaz, R.T.; Cabello-Pasini, A.; Márquez-Garrido, E.; López-Figueroa, F. Intra-thallus variation of phenolic compounds, antioxidant activity, and phenolsulphatase activity in Cystoseira tamariscifolia (Phaeophyceae) from southern Spain. Cienc. Mar. 2014, 40, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Van Hees, D.H.; Olsen, Y.S.; Wernberg, T.; Van Alstyne, K.L.; Kendrick, G.A. Phenolic concentrations of brown seaweeds and relationships to nearshore environmental gradients in Western Australia. Mar. Biol. 2017, 164, 74. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, A. Studies on Phenol Content and Heavy Metal Uptake in Fucoids; Bird, C.J., Ragan, M.A., Eds.; Springer: Dordrecht, The Netherlands, 1984; pp. 498–504. [Google Scholar] [CrossRef]
- Dang, T.T.; Van Vuong, Q.; Schreider, M.J.; Bowyer, M.C.; Van Altena, I.A.; Scarlett, C.J. Optimisation of ultrasound-assisted extraction conditions for phenolic content and antioxidant activities of the alga Hormosira banksii using response surface methodology. J. Appl. Phycol. 2017, 29, 3161–3173. [Google Scholar] [CrossRef]
- Tanniou, A.; Vandanjon, L.; Incera, M.; Leon, E.S.; Husa, V.; Le Grand, J.; Nicolas, J.-L.; Poupart, N.; Kervarec, N.; Engelen, A. Assessment of the spatial variability of phenolic contents and associated bioactivities in the invasive alga Sargassum muticum sampled along its European range from Norway to Portugal. J. Appl. Phycol. 2014, 26, 1215–1230. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, P.; Gorain, P.C.; Paul, I.; Bose, R.; Bhadoria, P.; Pal, R. Investigation on the effects of nitrate and salinity stress on the antioxidant properties of green algae with special reference to the use of processed biomass as potent fish feed ingredient. Aquac. Int. 2020, 28, 211–234. [Google Scholar] [CrossRef]
- Deniaud-Bouët, E.; Kervarec, N.; Michel, G.; Tonon, T.; Kloareg, B.; Hervé, C. Chemical and enzymatic fractionation of cell walls from Fucales: Insights into the structure of the extracellular matrix of brown algae. Ann. Bot. 2014, 114, 1203–1216. [Google Scholar] [CrossRef] [Green Version]
- Quan, T.H.; Benjakul, S.; Sae-leaw, T.; Balange, A.K.; Maqsood, S. Protein–polyphenol conjugates: Antioxidant property, functionalities and their applications. Trends Food Sci. Technol. 2019, 91, 507–517. [Google Scholar] [CrossRef]
- Habeebullah, S.F.K.; Alagarsamy, S.; Sattari, Z.; Al-Haddad, S.; Fakhraldeen, S.; Al-Ghunaim, A.; Al-Yamani, F. Enzyme-assisted extraction of bioactive compounds from brown seaweeds and characterization. J. Appl. Phycol. 2019, 32, 615–629. [Google Scholar] [CrossRef]
- Naczk, M.; Shahidi, F. Extraction and analysis of phenolics in food. J. Chromatogr. A 2004, 1054, 95–111. [Google Scholar] [CrossRef]
- Gereniu, C.R.N.; Saravana, P.S.; Getachew, A.T.; Chun, B.-S. Characteristics of functional materials recovered from Solomon Islands red seaweed (Kappaphycus alvarezii) using pressurized hot water extraction. J. Appl. Phycol. 2017, 29, 1609–1621. [Google Scholar] [CrossRef]
- Charoensiddhi, S.; Franco, C.; Su, P.; Zhang, W. Improved antioxidant activities of brown seaweed Ecklonia radiata extracts prepared by microwave-assisted enzymatic extraction. J. Appl. Phycol. 2015, 27, 2049–2058. [Google Scholar] [CrossRef]
- Topuz, O.K.; Gokoglu, N.; Yerlikaya, P.; Ucak, I.; Gumus, B. Optimization of antioxidant activity and phenolic compound extraction conditions from red seaweed (Laurencia obtuse). J. Aquat. Food Prod. Technol. 2016, 25, 414–422. [Google Scholar] [CrossRef]
- Gião, M.S.; Pereira, C.I.; Fonseca, S.C.; Pintado, M.E.; Malcata, F.X. Effect of particle size upon the extent of extraction of antioxidant power from the plants Agrimonia eupatoria, Salvia sp. and Satureja montana. Food Chem. 2009, 117, 412–416. [Google Scholar] [CrossRef]
- Norra, I.; Aminah, A.; Suri, R. Effects of drying methods, solvent extraction and particle size of Malaysian brown seaweed, Sargassum sp. on the total phenolic and free radical scavenging activity. Int. Food Res. J. 2016, 23, 1558. [Google Scholar]
- Cruces, E.; Rojas-Lillo, Y.; Ramirez-Kushel, E.; Atala, E.; López-Alarcón, C.; Lissi, E.; Gómez, I. Comparison of different techniques for the preservation and extraction of phlorotannins in the kelp Lessonia spicata (Phaeophyceae): Assays of DPPH, ORAC-PGR, and ORAC-FL as testing methods. J. Appl. Phycol. 2016, 28, 573–580. [Google Scholar] [CrossRef]
- Farvin, K.S.; Jacobsen, C. Phenolic compounds and antioxidant activities of selected species of seaweeds from Danish coast. Food Chem. 2013, 138, 1670–1681. [Google Scholar] [CrossRef]
- Jiménez-Escrig, A.; Gómez-Ordóñez, E.; Rupérez, P. Brown and red seaweeds as potential sources of antioxidant nutraceuticals. J. Appl. Phycol. 2012, 24, 1123–1132. [Google Scholar] [CrossRef]
- Jiménez-Escrig, A.; Jiménez-Jiménez, I.; Pulido, R.; Saura-Calixto, F. Antioxidant activity of fresh and processed edible seaweeds. J. Sci. Food Agric. 2001, 81, 530–534. [Google Scholar] [CrossRef]
- Keyrouz, R.; Abasq, M.-L.; Le Bourvellec, C.; Blanc, N.; Audibert, L.; ArGall, E.; Hauchard, D. Total phenolic contents, radical scavenging and cyclic voltammetry of seaweeds from Brittany. Food Chem. 2011, 126, 831–836. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Jonsdottir, R.; Ólafsdóttir, G. Total phenolic compounds, radical scavenging and metal chelation of extracts from Icelandic seaweeds. Food Chem. 2009, 116, 240–248. [Google Scholar] [CrossRef]
- Kallithraka, S.; Garcia-Viguera, C.; Bridle, P.; Bakker, J. Survey of solvents for the extraction of grape seed phenolics. Phytochem. Anal. 1995, 6, 265–267. [Google Scholar] [CrossRef]
- Liu, W.; Wang, H.; Yao, W.; Gao, X.; Yu, L. Effects of sulfation on the physicochemical and functional properties of a water-insoluble polysaccharide preparation from Ganoderma lucidum. J. Agric. Food Chem. 2010, 58, 3336–3341. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, N.A.; Ibrahim, D.; Sulaiman, S.F.; Supardy, A. Assessment of antioxidant activity, total phenolic content and in-vitro toxicity of Malaysian red seaweed, Acanthophora spicifera. J. Chem. Pharm. Res. 2011, 3, 182–191. [Google Scholar]
- Palma, M.; Piñeiro, Z.; Barroso, C.G. Stability of phenolic compounds during extraction with superheated solvents. J. Chromatogr. A 2001, 921, 169–174. [Google Scholar] [CrossRef]
- Obluchinskaya, E.D.; Pozharitskaya, O.N.; Zakharova, L.V.; Daurtseva, A.V.; Flisyuk, E.V.; Shikov, A.N. Efficacy of natural deep eutectic solvents for extraction of hydrophilic and lipophilic compounds from Fucus vesiculosus. Molecules 2021, 26, 4198. [Google Scholar] [CrossRef]
- Richter, B.E.; Jones, B.A.; Ezzell, J.L.; Porter, N.L.; Avdalovic, N.; Pohl, C. Accelerated solvent extraction: A technique for sample preparation. Anal. Chem. 1996, 68, 1033–1039. [Google Scholar] [CrossRef]
- Plaza, M.; Abrahamsson, V.; Turner, C. Extraction and neoformation of antioxidant compounds by pressurized hot water extraction from apple byproducts. J. Agric. Food Chem. 2013, 61, 5500–5510. [Google Scholar] [CrossRef]
- Putnik, P.; Lorenzo, J.M.; Barba, F.J.; Roohinejad, S.; Režek Jambrak, A.; Granato, D.; Montesano, D.; Bursać Kovačević, D. Novel food processing and extraction technologies of high-added value compounds from plant materials. Foods 2018, 7, 106. [Google Scholar] [CrossRef] [Green Version]
- Pereira, C.G.; Meireles, M.A.A. Supercritical fluid extraction of bioactive compounds: Fundamentals, applications and economic perspectives. Food Bioprocess Technol. 2010, 3, 340–372. [Google Scholar] [CrossRef]
- Díaz-Reinoso, B.; Moure, A.; Domínguez, H.; Parajó, J.C. Supercritical CO2 extraction and purification of compounds with antioxidant activity. J. Agric. Food Chem. 2006, 54, 2441–2469. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Jónsdóttir, R.; Liu, H.; Gu, L.; Kristinsson, H.G.; Raghavan, S.; Ólafsdóttir, G. Antioxidant capacities of phlorotannins extracted from the brown algae Fucus vesiculosus. J. Agric. Food Chem. 2012, 60, 5874–5883. [Google Scholar] [CrossRef] [PubMed]
- Catarino, M.D.; Silva, A.; Mateus, N.; Cardoso, S.M. Optimization of phlorotannins extraction from Fucus vesiculosus and evaluation of their potential to prevent metabolic disorders. Mar. Drugs 2019, 17, 162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, B.; Robinson, N.A.; Warner, R.D.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A. LC-ESI-QTOF-MS/MS Characterization of Seaweed Phenolics and Their Antioxidant Potential. Mar. Drugs 2020, 18, 331. [Google Scholar] [CrossRef] [PubMed]
- Hermund, D.B. 10-Antioxidant Properties of Seaweed-Derived Substances. In Bioactive Seaweeds for Food Applications; Qin, Y., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 201–221. [Google Scholar] [CrossRef]
- Montero, L.; Sánchez-Camargo, A.P.; García-Cañas, V.; Tanniou, A.; Stiger-Pouvreau, V.; Russo, M.; Rastrelli, L.; Cifuentes, A.; Herrero, M.; Ibáñez, E. Anti-proliferative activity and chemical characterization by comprehensive two-dimensional liquid chromatography coupled to mass spectrometry of phlorotannins from the brown macroalga Sargassum muticum collected on North-Atlantic coasts. J. Chromatogr. A 2016, 1428, 115–125. [Google Scholar] [CrossRef]
- Stiger-Pouvreau, V.; Jegou, C.; Cerantola, S.; Guérard, F.; Le Lann, K. Phlorotannins in Sargassaceae Species from Brittany (France): Interesting Molecules for Ecophysiological and Valorisation Purposes. In Advances in Botanical Research; Elsevier: Amsterdam, The Netherlands, 2014; Volume 71, pp. 379–411. [Google Scholar] [CrossRef]
- Thanigaivel, S.; Hindu, S.V.; Vijayakumar, S.; Mukherjee, A.; Chandrasekaran, N.; Thomas, J. Differential solvent extraction of two seaweeds and their efficacy in controlling Aeromonas salmonicida infection in Oreochromis mossambicus: A novel therapeutic approach. Aquaculture 2015, 443, 56–64. [Google Scholar] [CrossRef]
- Thirunavukkarasu, R.; Pandiyan, P.; Balaraman, D.; Subaramaniyan, K.; Edward Gnana Jothi, G.; Manikkam, S.; Sadaiyappan, B. Isolation of bioactive compound from marine seaweeds against fish pathogenic bacteria Vibrio alginolyticus (VA09) and characterisation by FTIR. J. Coast. Life Med. 2013, 1, 26–33. [Google Scholar] [CrossRef]
- Thanigaivel, S.; Vijayakumar, S.; Mukherjee, A.; Chandrasekaran, N.; Thomas, J. Antioxidant and antibacterial activity of Chaetomorpha antennina against shrimp pathogen Vibrio parahaemolyticus. Aquaculture 2014, 433, 467–475. [Google Scholar] [CrossRef]
- Wei, Y.; Liu, Q.; Xu, C.; Yu, J.; Zhao, L.; Guo, Q. Damage to the membrane permeability and cell death of Vibrio parahaemolyticus caused by phlorotannins with low molecular weight from Sargassum thunbergii. J. Aquat. Food Prod. Technol. 2016, 25, 323–333. [Google Scholar] [CrossRef]
- González-Colunga, D.; Antunes-Ricardo, M.; Gutiérrez-Uribe, J.A.; Cruz-Suárez, L.E. Bioactivity-guided identification of anti-AHPND (acute hepatopancreatic necrosis disease) metabolites of Ecklonia arborea. J. Appl. Phycol. 2019, 31, 3189–3199. [Google Scholar] [CrossRef]
- Latifah, L.; Soekamto, N.; Tahir, A. Preliminary study: Padina australis Hauck’s antibacterial activity and phytochemical test against pathogenic shrimp bacteria. J. Phys. Conf. Ser. 2019, 1341, 022005. [Google Scholar] [CrossRef] [Green Version]
- Thanigaivel, S.; Chandrasekaran, N.; Mukherjee, A.; Thomas, J. Protective efficacy of microencapsulated seaweed extracts for preventing Aeromonas infections in Oreochromis mossambicus. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2019, 218, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Ford, L.; Stratakos, A.C.; Theodoridou, K.; Dick, J.T.; Sheldrake, G.N.; Linton, M.; Corcionivoschi, N.; Walsh, P.J. Polyphenols from Brown Seaweeds as a Potential Antimicrobial Agent in Animal Feeds. ACS Omega 2020, 5, 9093–9103. [Google Scholar] [CrossRef] [Green Version]
- Cox, S.; Abu-Ghannam, N.; Gupta, S. An assessment of the antioxidant and antimicrobial activity of six species of edible Irish seaweeds. Int. Food Res. J. 2010, 17, 205–220. [Google Scholar]
- Kumaran, S.; Deivasigamani, B.; Alagappan, K.; Sakthivel, M.; Karthikeyan, R. Antibiotic resistant E. coli strains from seafood and its susceptibility to seaweed extracts. Asian Pac. J. Trop. Med. 2010, 3, 412–420. [Google Scholar] [CrossRef] [Green Version]
- Vijayabaskar, P.; Shiyamala, V. Antibacterial activities of brown marine algae (Sargassum wightii and Turbinaria ornata) from the Gulf of Mannar Biosphere Reserve. Adv. Biol. Res. 2011, 5, 99–102. [Google Scholar]
- Kumar, I.N.; Barot, M.; Kumar, R. Phytochemical analysis and antifungal activity of selected seaweeds from. J. Coast. Life Med. 2014, 2, 535–540. [Google Scholar]
- Khaled, N.; Hiba, M.; Asma, C. Antioxidant and antifungal activities of Padina pavonica and Sargassum vulgare from the Lebanese Mediterranean Coast. Adv. Environ. Biol. 2012, 6, 42–48. [Google Scholar]
- Martelli, F.; Cirlini, M.; Lazzi, C.; Neviani, E.; Bernini, V. Edible seaweeds and spirulina extracts for food application: In vitro and in situ evaluation of antimicrobial activity towards foodborne pathogenic bacteria. Foods 2020, 9, 1442. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.A.; Jin, S.E.; Ahn, B.R.; Lee, C.M.; Choi, J.S. Anti-inflammatory activity of edible brown alga Eisenia bicyclis and its constituents fucosterol and phlorotannins in LPS-stimulated RAW264. 7 macrophages. Food Chem. Toxicol. 2013, 59, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.; Zhang, W.; Begbie, A.; Pukala, T.L.; Smid, S.D. Ecklonia radiata extract containing eckol protects neuronal cells against Aβ1–42 evoked toxicity and reduces aggregate density. Food Funct. 2020, 11, 6509–6516. [Google Scholar] [CrossRef] [PubMed]
- Nazarudin, M.; Yasin, I.; Mazli, N.; Saadi, A.; Azizee, M.; Nooraini, M.; Saad, N.; Ferdous, U.; Fakhrulddin, I. Preliminary screening of antioxidant and cytotoxic potential of green seaweed, Halimeda opuntia (Linnaeus) Lamouroux. Saudi J. Biol. Sci. 2022, 29, 2698–2705. [Google Scholar] [CrossRef] [PubMed]
- Corona, G.; Coman, M.M.; Guo, Y.; Hotchkiss, S.; Gill, C.; Yaqoob, P.; Spencer, J.P.; Rowland, I. Effect of simulated gastrointestinal digestion and fermentation on polyphenolic content and bioactivity of brown seaweed phlorotannin-rich extracts. Mol. Nutr. Food Res. 2017, 61, 1700223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Namvar, F.; Mohamad, R.; Baharara, J.; Zafar-Balanejad, S.; Fargahi, F.; Rahman, H.S. Antioxidant, antiproliferative, and antiangiogenesis effects of polyphenol-rich seaweed (Sargassum muticum). BioMed Res. Int. 2013, 2013, 604787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanjana, K.; Radtanatip, T.; Asuvapongpatana, S.; Withyachumnarnkul, B.; Wongprasert, K. Solvent extracts of the red seaweed Gracilaria fisheri prevent Vibrio harveyi infections in the black tiger shrimp Penaeus monodon. Fish Shellfish Immunol. 2011, 30, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.E.; Jung, Y.C.; Jung, I.; Lee, H.-W.; Youn, H.-Y.; Lee, J.S. Anti-inflammatory effects of ethanolic extract from Sargassum horneri (Turner) C. Agardh on lipopolysaccharide-stimulated macrophage activation via NF-κB pathway regulation. Immunol. Investig. 2015, 44, 137–146. [Google Scholar] [CrossRef]
- Sugiura, Y.; Tanaka, R.; Katsuzaki, H.; Imai, K.; Matsushita, T. The anti-inflammatory effects of phlorotannins from Eisenia arborea on mouse ear edema by inflammatory inducers. J. Funct. Foods 2013, 5, 2019–2023. [Google Scholar] [CrossRef]
- Yang, B.; Li, Y.; Yang, Z.; Xue, L.; Zhang, M.; Chen, G.; Chinnathambi, A.; Alahmadi, T.A.; Liu, X. Anti-inflammatory and anti-cell proliferative effects of dieckol in the prevention and treatment of colon cancer induced by 1,2-dimethyl hydrazine in experimental animals. Pharmacogn. Mag. 2020, 16, 851. [Google Scholar]
- Phasanasophon, K.; Kim, S.M. Anti-inflammatory activity of the phlorotannin trifuhalol A using LPS-stimulated RAW264. 7 cells through NF-κB and MAPK main signaling pathways. Nat. Prod. Commun. 2019, 14, 1934578X19849798. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.-I.; Woo, J.-H.; Seo, Y.-J.; Lee, K.-T.; Lim, Y.; Choi, J.-H. Protective effect of brown alga phlorotannins against hyper-inflammatory responses in lipopolysaccharide-induced sepsis models. J. Agric. Food Chem. 2016, 64, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Rempe, C.S.; Burris, K.P.; Lenaghan, S.C.; Stewart, C.N., Jr. The potential of systems biology to discover antibacterial mechanisms of plant phenolics. Front. Microbiol. 2017, 8, 422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scalbert, A. Antimicrobial properties of tannins. Phytochemistry 1991, 30, 3875–3883. [Google Scholar] [CrossRef]
- Gupta, S.; Abu-Ghannam, N. Recent developments in the application of seaweeds or seaweed extracts as a means for enhancing the safety and quality attributes of foods. Innov. Food Sci. Emerg. Technol. 2011, 12, 600–609. [Google Scholar] [CrossRef]
- Abbas, M.; Saeed, F.; Suleria, H.A.R. Marine Bioactive Compounds: Innovative Trends in Food and Medicine. In Plant-and Marine-Based Phytochemicals for Human Health: Attributes, Potential, and Use; Apple Academic Press: New York, NY, USA, 2018; pp. 61–82. [Google Scholar]
- Wu, Y.; Wen, J.; Ma, Y.; Ma, X.; Chen, Y. Epidemiology of foodborne disease outbreaks caused by Vibrio parahaemolyticus, China, 2003–2008. Food Control 2014, 46, 197–202. [Google Scholar] [CrossRef]
- Miller, S.I. Antibiotic resistance and regulation of the gram-negative bacterial outer membrane barrier by host innate immune molecules. mBio 2016, 7, e01541-16. [Google Scholar] [CrossRef] [Green Version]
- Pérez, M.J.; Falqué, E.; Domínguez, H. Antimicrobial action of compounds from marine seaweed. Mar. Drugs 2016, 14, 52. [Google Scholar] [CrossRef] [Green Version]
- El Shafay, S.M.; Ali, S.S.; El-Sheekh, M.M. Antimicrobial activity of some seaweeds species from Red sea, against multidrug resistant bacteria. Egypt. J. Aquat. Res. 2016, 42, 65–74. [Google Scholar] [CrossRef] [Green Version]
- Del Río, L.A. ROS and RNS in plant physiology: An overview. J. Exp. Bot. 2015, 66, 2827–2837. [Google Scholar] [CrossRef] [Green Version]
- Machu, L.; Misurcova, L.; Vavra Ambrozova, J.; Orsavova, J.; Mlcek, J.; Sochor, J.; Jurikova, T. Phenolic content and antioxidant capacity in algal food products. Molecules 2015, 20, 1118–1133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agregán, R.; Munekata, P.E.; Domínguez, R.; Carballo, J.; Franco, D.; Lorenzo, J.M. Proximate composition, phenolic content and in vitro antioxidant activity of aqueous extracts of the seaweeds Ascophyllum nodosum, Bifurcaria bifurcata and Fucus vesiculosus. Effect of addition of the extracts on the oxidative stability of canola oil under accelerated storage conditions. Food Res. Int. 2017, 99, 986–994. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects–A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Frankel, E.N. Chapter 3—Photooxidation of Unsaturated Fats. In Lipid Oxidation, 2nd ed.; Frankel, E.N., Ed.; Woodhead Publishing: Sawston, UK, 2012; pp. 51–66. [Google Scholar]
- Bhuvaneswari, G.H. 3-Degradability of Polymers. In Recycling of Polyurethane Foams; Thomas, S., Rane, A.V., Kanny, K., Abitha, V.K., Thomas, M.G., Eds.; William Andrew Publishing: Norwich, NY, USA, 2018; pp. 29–44. [Google Scholar]
- EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP). Safety and efficacy of ethoxyquin (6-ethoxy-1,2-dihydro-2,2,4-trimethylquinoline) for all animal species. EFSA J. 2015, 13, 4272. [Google Scholar]
- Kranawetvogl, A.; Elsinghorst, P.W. Determination of the Synthetic Antioxidant Ethoxyquin and Its Metabolites in Fish and Fishery Products Using Liquid Chromatography–Fluorescence Detection and Stable-Isotope Dilution Analysis–Liquid Chromatography–Tandem Mass Spectrometry. J. Agric. Food Chem. 2019, 67, 6650–6657. [Google Scholar] [CrossRef]
- Merel, S.; Regueiro, J.; Berntssen, M.H.; Hannisdal, R.; Ørnsrud, R.; Negreira, N. Identification of ethoxyquin and its transformation products in salmon after controlled dietary exposure via fish feed. Food Chem. 2019, 289, 259–268. [Google Scholar] [CrossRef]
- EUR-Lex on Maximum Residue Levels of Pesticides in or on Food and Feed of Plant and Animal Origin and Amending Council Directive 91/414/EEC. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32005R0396 (accessed on 17 May 2022).
- US-FAD. Part 573—Food additives permitted in feed and drinking water of animals. In Code of Federal Regulations, FAD. Available online: https://www.accessdata.fda.gov/ (accessed on 1 November 2019).
- Byrne, J. Ethoxyquin Authorization Suspended in the EU—Are there Viable Alternatives? In Feed Navigator; William Reed Business Media Ltd.: Crawley RH, UK, 2020; Volume 2020, Available online: Feednavigator.com (accessed on 31 July 2020).
- Karakaya, S. Bioavailability of phenolic compounds. Crit. Rev. Food Sci. Nutr. 2004, 44, 453–464. [Google Scholar] [CrossRef]
- Folin, O.; Denis, W. On phosphotungstic-phosphomolybdic compounds as color reagents. J. Biol. Chem. 1912, 12, 239–243. [Google Scholar] [CrossRef]
- Devi, K.P.; Suganthy, N.; Kesika, P.; Pandian, S.K. Bioprotective properties of seaweeds: In vitro evaluation of antioxidant activity and antimicrobial activity against food borne bacteria in relation to polyphenolic content. BMC Complement. Altern. Med. 2008, 8, 38. [Google Scholar] [CrossRef] [Green Version]
- Corsetto, P.A.; Montorfano, G.; Zava, S.; Colombo, I.; Ingadottir, B.; Jonsdottir, R.; Sveinsdottir, K.; Rizzo, A.M. Characterization of antioxidant potential of seaweed extracts for enrichment of convenience food. Antioxidants 2020, 9, 249. [Google Scholar] [CrossRef] [Green Version]
- Dinh, T.V.; Saravana, P.S.; Woo, H.C.; Chun, B.S. Ionic liquid-assisted subcritical water enhances the extraction of phenolics from brown seaweed and its antioxidant activity. Sep. Purif. Technol. 2018, 196, 287–299. [Google Scholar] [CrossRef]
- Chakraborty, K.; Joseph, D.; Praveen, N.K. Antioxidant activities and phenolic contents of three red seaweeds (Division: Rhodophyta) harvested from the Gulf of Mannar of Peninsular India. J. Food Sci. Technol. 2015, 52, 1924–1935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matanjun, P.; Mohamed, S.; Mustapha, N.M.; Muhammad, K.; Ming, C.H. Antioxidant activities and phenolics content of eight species of seaweeds from north Borneo. J. Appl. Phycol. 2008, 20, 367. [Google Scholar] [CrossRef]
- Rattaya, S.; Benjakul, S.; Prodpran, T. Extraction, antioxidative, and antimicrobial activities of brown seaweed extracts, Turbinaria ornata and Sargassum polycystum, grown in Thailand. Int. Aquat. Res. 2015, 7, 1–16. [Google Scholar] [CrossRef]
- Sanz-Pintos, N.; Pérez-Jiménez, J.; Buschmann, A.H.; Vergara-Salinas, J.R.; Pérez-Correa, J.R.; Saura-Calixto, F. Macromolecular antioxidants and dietary fiber in edible seaweeds. J. Food Sci. 2017, 82, 289–295. [Google Scholar] [CrossRef]
- Sathya, R.; Kanaga, N.; Sankar, P.; Jeeva, S. Antioxidant properties of phlorotannins from brown seaweed Cystoseira trinodis (Forsskål) C. Agardh. Arab. J. Chem. 2017, 10, S2608–S2614. [Google Scholar] [CrossRef] [Green Version]
- Shipeng, Y.; Woo, H.-C.; Choi, J.-H.; Park, Y.-B.; Chun, B.-S. Measurement of antioxidant activities and phenolic and flavonoid contents of the brown seaweed Sargassum horneri: Comparison of supercritical CO2 and various solvent extractions. Fish. Aquat. Sci. 2015, 18, 123–130. [Google Scholar] [CrossRef] [Green Version]
- Vega, J.; Álvarez-Gómez, F.; Güenaga, L.; Figueroa, F.L.; Gómez-Pinchetti, J.L. Antioxidant activity of extracts from marine macroalgae, wild-collected and cultivated, in an integrated multi-trophic aquaculture system. Aquaculture 2020, 522, 735088. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, J.; Shen, J.; Silva, A.; Dennis, D.A.; Barrow, C.J. A simple 96-well microplate method for estimation of total polyphenol content in seaweeds. J. Appl. Phycol. 2006, 18, 445–450. [Google Scholar] [CrossRef] [Green Version]
- Fu, C.; Ho, C.; Yong, W.; Abas, F.; Tan, T.; Tan, C. Extraction of phenolic antioxidants from four selected seaweeds obtained from Sabah. Int. Food Res. J. 2016, 23, 2363. [Google Scholar]
- Srikong, W.; Bovornreungroj, N.; Mittraparparthorn, P.; Bovornreungroj, P. Antibacterial and antioxidant activities of differential solvent extractions from the green seaweed Ulva intestinalis. ScienceAsia 2017, 43, 88–95. [Google Scholar] [CrossRef] [Green Version]
- Rajauria, G.; Jaiswal, A.K.; Abu-Gannam, N.; Gupta, S. Antimicrobial, antioxidant and free radical-scavenging capacity of brown seaweed Himanthalia elongata from western coast of Ireland. J. Food Biochem. 2013, 37, 322–335. [Google Scholar] [CrossRef]
- Belda, M.; Sanchez, D.; Bover, E.; Prieto, B.; Padrón, C.; Cejalvo, D.; Lloris, J.M. Extraction of polyphenols in Himanthalia elongata and determination by high performance liquid chromatography with diode array detector prior to its potential use against oxidative stress. J. Chromatogr. B 2016, 1033, 334–341. [Google Scholar] [CrossRef]
- Poole, J.; Diop, A.; Rainville, L.-C.; Barnabé, S. Bioextracting Polyphenols from the Brown Seaweed Ascophyllum nodosum from Québec’s North Shore Coastline. Ind. Biotechnol. 2019, 15, 212–218. [Google Scholar] [CrossRef] [Green Version]
- Shahidi, F.; Alasalvar, C.; Liyana-Pathirana, C.M. Antioxidant phytochemicals in hazelnut kernel (Corylus avellana L.) and hazelnut byproducts. J. Agric. Food Chem. 2007, 55, 1212–1220. [Google Scholar] [CrossRef]
- Parys, S.; Rosenbaum, A.; Kehraus, S.; Reher, G.; Glombitza, K.-W.; König, G.M. Evaluation of quantitative methods for the determination of polyphenols in algal extracts. J. Nat. Prod. 2007, 70, 1865–1870. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.-J.; Zhang, W.-W.; Li, X.-M.; Wang, B.-G. Evaluation of antioxidant property of extract and fractions obtained from a red alga, Polysiphonia urceolata. Food Chem. 2006, 95, 37–43. [Google Scholar] [CrossRef]
- Hemat, R. Fat and muscle dysfunction. In Andropathy; Urotext: Dublin, Ireland, 2007; pp. 83–85. [Google Scholar]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Fernando, I.S.; Kim, M.; Son, K.-T.; Jeong, Y.; Jeon, Y.-J. Antioxidant activity of marine algal polyphenolic compounds: A mechanistic approach. J. Med. Food 2016, 19, 615–628. [Google Scholar] [CrossRef]
- Amić, D.; Davidović-Amić, D.; Bešlo, D.; Trinajstić, N. Structure-radical scavenging activity relationships of flavonoids. Croat. Chem. Acta 2003, 76, 55–61. [Google Scholar]
- Xiaojun, Y.; Xiancui, L.; Chengxu, Z.; Xiao, F. Prevention of fish oil rancidity by phlorotannins from Sargassum kjellmanianum. J. Appl. Phycol. 1996, 8, 201–203. [Google Scholar] [CrossRef]
- Farvin, K.S.; Jacobsen, C. Antioxidant activity of seaweed extracts: In vitro assays, evaluation in 5% fish oil-in-water emulsions and characterization. J. Am. Oil Chem. Soc. 2015, 92, 571–587. [Google Scholar] [CrossRef]
- Kang, M.-C.; Cha, S.H.; Wijesinghe, W.; Kang, S.-M.; Lee, S.-H.; Kim, E.-A.; Song, C.B.; Jeon, Y.-J. Protective effect of marine algae phlorotannins against AAPH-induced oxidative stress in zebrafish embryo. Food Chem. 2013, 138, 950–955. [Google Scholar] [CrossRef]
- Kim, E.-A.; Kang, M.-C.; Lee, J.-H.; Kang, N.; Lee, W.; Oh, J.-Y.; Yang, H.-W.; Lee, J.-S.; Jeon, Y.-J. Protective effect of marine brown algal polyphenols against oxidative stressed zebrafish with high glucose. RSC Adv. 2015, 5, 25738–25746. [Google Scholar] [CrossRef]
- Cunha, L.; Grenha, A. Sulfated seaweed polysaccharides as multifunctional materials in drug delivery applications. Mar. Drugs 2016, 14, 42. [Google Scholar] [CrossRef]
- Nkurunziza, D.; Sivagnanam, S.P.; Park, J.-S.; Cho, Y.-J.; Chun, B.S. Effect of wall materials on the spray drying encapsulation of brown seaweed bioactive compounds obtained by subcritical water extraction. Algal Res. 2021, 58, 102381. [Google Scholar] [CrossRef]
- Hindu, S.V.; Thanigaivel, S.; Vijayakumar, S.; Chandrasekaran, N.; Mukherjee, A.; Thomas, J. Effect of microencapsulated probiotic Bacillus vireti 01-polysaccharide extract of Gracilaria folifera with alginate-chitosan on immunity, antioxidant activity and disease resistance of Macrobrachium rosenbergii against Aeromonas hydrophila infection. Fish Shellfish Immunol. 2018, 73, 112–120. [Google Scholar] [CrossRef]
- Sachindra, N.M.; Sato, E.; Maeda, H.; Hosokawa, M.; Niwano, Y.; Kohno, M.; Miyashita, K. Radical scavenging and singlet oxygen quenching activity of marine carotenoid fucoxanthin and its metabolites. J. Agric. Food Chem. 2007, 55, 8516–8522. [Google Scholar] [CrossRef]
- Indrawati, R.; Sukowijoyo, H.; Wijayanti, R.D.E.; Limantara, L. Encapsulation of brown seaweed pigment by freeze drying: Characterization and its stability during storage. Procedia Chem. 2015, 14, 353–360. [Google Scholar] [CrossRef] [Green Version]
- Barbosa, M.; Borsarelli, C.; Mercadante, A. Light stability of spray-dried bixin encapsulated with different edible polysaccharide preparations. Food Res. Int. 2005, 38, 989–994. [Google Scholar] [CrossRef]
- Raveendran, S.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Pharmaceutically versatile sulfated polysaccharide based bionano platforms. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 605–626. [Google Scholar] [CrossRef] [PubMed]
- Sezer, A.D.; Akbuğa, J. The design of biodegradable ofloxacin-based core-shell microspheres: Influence of the formulation parameters on in vitro characterization. Pharm. Dev. Technol. 2012, 17, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Sezer, A.; Akbuğa, J. Fucosphere—New microsphere carriers for peptide and protein delivery: Preparation and in vitro characterization. J. Microencapsul. 2006, 23, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T. Lipid nutrition in fish. Comp. Biochem. Physiol. Part B Comp. Biochem. 1982, 73, 3–15. [Google Scholar] [CrossRef]
- Matsuo, N. Studies on the toxicity of fish oil. J. Biochem. 1954, 41, 481–487. [Google Scholar] [CrossRef]
- Ma, Q.; Li, L.-Y.; Le, J.-Y.; Lu, D.-L.; Qiao, F.; Zhang, M.-L.; Du, Z.-Y.; Li, D.-L. Dietary microencapsulated oil improves immune function and intestinal health in Nile tilapia fed with high-fat diet. Aquaculture 2018, 496, 19–29. [Google Scholar] [CrossRef]
- Wang, B.; Akanbi, T.O.; Agyei, D.; Holland, B.J.; Barrow, C.J. Coacervation Technique as an Encapsulation and Delivery Tool for Hydrophobic Biofunctional Compounds. In Role of Materials Science in Food Bioengineering; Academic Press: Cambridge, MA, USA, 2018; pp. 235–261. [Google Scholar] [CrossRef]
- Xia, Q.; Wang, B.; Akanbi, T.O.; Li, R.; Yang, W.; Adhikari, B.; Barrow, C.J. Microencapsulation of lipase produced omega-3 concentrates resulted in complex coacervates with unexpectedly high oxidative stability. J. Funct. Foods 2017, 35, 499–506. [Google Scholar] [CrossRef]
- Sanchez, C.; Renard, D. Stability and structure of protein–polysaccharide coacervates in the presence of protein aggregates. Int. J. Pharm. 2002, 242, 319–324. [Google Scholar] [CrossRef]
- Cao, N.; Fu, Y.; He, J. Mechanical properties of gelatin films cross-linked, respectively, by ferulic acid and tannin acid. Food Hydrocoll. 2007, 21, 575–584. [Google Scholar] [CrossRef]
- Muhoza, B.; Xia, S.; Zhang, X. Gelatin and high methyl pectin coacervates crosslinked with tannic acid: The characterization, rheological properties, and application for peppermint oil microencapsulation. Food Hydrocoll. 2019, 97, 105174. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, Z. Effects of gelatin-polyphenol and gelatin–genipin cross-linking on the structure of gelatin hydrogels. Int. J. Food Prop. 2017, 20 (Suppl. 3), S2822–S2832. [Google Scholar] [CrossRef]
- Fraga-Corral, M.; García-Oliveira, P.; Pereira, A.G.; Lourenço-Lopes, C.; Jimenez-Lopez, C.; Prieto, M.A.; Simal-Gandara, J. Technological application of tannin-based extracts. Molecules 2020, 25, 614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anvari, M.; Chung, D. Dynamic rheological and structural characterization of fish gelatin–gum Arabic coacervate gels cross-linked by tannic acid. Food Hydrocoll. 2016, 60, 516–524. [Google Scholar] [CrossRef]
- Mohseni, F.; Goli, S.A.H. Encapsulation of flaxseed oil in the tertiary conjugate of oxidized tannic acid-gelatin and flaxseed (Linum usitatissimum) mucilage. Int. J. Biol. Macromol. 2019, 140, 959–964. [Google Scholar] [CrossRef]
- Strauss, G.; Gibson, S.M. Plant phenolics as cross-linkers of gelatin gels and gelatin-based coacervates for use as food ingredients. Food Hydrocoll. 2004, 18, 81–89. [Google Scholar] [CrossRef]
- Zhan, F.; Yang, J.; Li, J.; Wang, Y.; Li, B. Characteristics of the interaction mechanism between tannic acid and sodium caseinate using multispectroscopic and thermodynamics methods. Food Hydrocoll. 2018, 75, 81–87. [Google Scholar] [CrossRef]
- Zhang, X.; Do, M.D.; Casey, P.; Sulistio, A.; Qiao, G.G.; Lundin, L.; Lillford, P.; Kosaraju, S. Chemical cross-linking gelatin with natural phenolic compounds as studied by high-resolution NMR spectroscopy. Biomacromolecules 2010, 11, 1125–1132. [Google Scholar] [CrossRef]
- Zhang, Z.-Q.; Pan, C.-H.; Chung, D. Tannic acid cross-linked gelatin–gum arabic coacervate microspheres for sustained release of allyl isothiocyanate: Characterization and in vitro release study. Food Res. Int. 2011, 44, 1000–1007. [Google Scholar] [CrossRef]
- Kanakis, C.; Hasni, I.; Bourassa, P.; Tarantilis, P.; Polissiou, M.; Tajmir-Riahi, H.-A. Milk β-lactoglobulin complexes with tea polyphenols. Food Chem. 2011, 127, 1046–1055. [Google Scholar] [CrossRef]
- Thongkaew, C.; Gibis, M.; Hinrichs, J.; Weiss, J. Polyphenol interactions with whey protein isolate and whey protein isolate–pectin coacervates. Food Hydrocoll. 2014, 41, 103–112. [Google Scholar] [CrossRef]
- Bartolome, B.; Estrella, I.; Hernandez, M. Interaction of low molecular weight phenolics with proteins (BSA). J. Food Sci. 2000, 65, 617–621. [Google Scholar] [CrossRef]
- Czubinski, J.; Dwiecki, K. A review of methods used for investigation of protein–phenolic compound interactions. Int. J. Food Sci. Technol. 2017, 52, 573–585. [Google Scholar] [CrossRef]
- Le Bourvellec, C.; Renard, C. Interactions between polyphenols and macromolecules: Quantification methods and mechanisms. Crit. Rev. Food Sci. Nutr. 2012, 52, 213–248. [Google Scholar] [CrossRef] [PubMed]
- Yildirim-Elikoglu, S.; Erdem, Y.K. Interactions between milk proteins and polyphenols: Binding mechanisms, related changes, and the future trends in the dairy industry. Food Rev. Int. 2018, 34, 665–697. [Google Scholar] [CrossRef]
- Buitimea-Cantúa, N.E.; Gutiérrez-Uribe, J.A.; Serna-Saldivar, S.O. Phenolic–protein interactions: Effects on food properties and health benefits. J. Med. Food 2018, 21, 188–198. [Google Scholar] [CrossRef]
- Ozdal, T.; Capanoglu, E.; Altay, F. A review on protein–phenolic interactions and associated changes. Food Res. Int. 2013, 51, 954–970. [Google Scholar] [CrossRef]
- Hurrell, R.F.; Finot, P.-A. Nutritional Consequences of the Reactions between Proteins and Oxidized POLYPHENOLIC acids. In Nutritional and Toxicological Aspects of Food Safety; Springer: Boston, MA, USA, 1984; pp. 423–435. [Google Scholar]
- Faergemand, M.; Otte, J.; Qvist, K. Enzymatic cross-linking of whey proteins by a Ca2+-independent microbial transglutaminase from Streptomyces lydicus. Food Hydrocoll. 1997, 11, 19–25. [Google Scholar] [CrossRef]
- de Freitas, V.; Mateus, N. Structural features of procyanidin interactions with salivary proteins. J. Agric. Food Chem. 2001, 49, 940–945. [Google Scholar] [CrossRef]
- Dubeau, S.; Samson, G.; Tajmir-Riahi, H.-A. Dual effect of milk on the antioxidant capacity of green, Darjeeling, and English breakfast teas. Food Chem. 2010, 122, 539–545. [Google Scholar] [CrossRef]
- Xiao, J.; Mao, F.; Yang, F.; Zhao, Y.; Zhang, C.; Yamamoto, K. Interaction of dietary polyphenols with bovine milk proteins: Molecular structure–affinity relationship and influencing bioactivity aspects. Mol. Nutr. Food Res. 2011, 55, 1637–1645. [Google Scholar] [CrossRef]
- Alseekh, S.; Perez de Souza, L.; Benina, M.; Fernie, A.R. The style and substance of plant flavonoid decoration; towards defining both structure and function. Phytochemistry 2020, 174, 112347. [Google Scholar] [CrossRef] [PubMed]
- Rawel, H.M.; Meidtner, K.; Kroll, J. Binding of selected phenolic compounds to proteins. J. Agric. Food Chem. 2005, 53, 4228–4235. [Google Scholar] [CrossRef] [PubMed]
- Sastry, M.S.; Rao, M.N. Binding of chlorogenic acid by the isolated polyphenol-free 11 S protein of sunflower (Helianthus annuus) seed. J. Agric. Food Chem. 1990, 38, 2103–2110. [Google Scholar] [CrossRef]
- Prigent, S.V.; Gruppen, H.; Visser, A.J.; van Koningsveld, G.A.; de Jong, G.A.; Voragen, A.G. Effects of non-covalent interactions with 5-O-caffeoylquinic acid (chlorogenic acid) on the heat denaturation and solubility of globular proteins. J. Agric. Food Chem. 2003, 51, 5088–5095. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L. Edible Seaweeds of the World; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Lähteenmäki-Uutela, A.; Rahikainen, M.; Camarena-Gómez, M.T.; Piiparinen, J.; Spilling, K.; Yang, B. European Union legislation on macroalgae products. Aquac. Int. 2021, 29, 487–509. [Google Scholar] [CrossRef]
- Roleda, M.Y.; Marfaing, H.; Desnica, N.; Jónsdóttir, R.; Skjermo, J.; Rebours, C.; Nitschke, U. Variations in polyphenol and heavy metal contents of wild-harvested and cultivated seaweed bulk biomass: Health risk assessment and implication for food applications. Food Control 2019, 95, 121–134. [Google Scholar] [CrossRef]
- FSANZ Arsenic. Available online: https://www.foodstandards.gov.au/consumer/chemicals/arsenic/Pages/default.aspx (accessed on 7 August 2020).
- Winberg, P.C.; Ghosh, D.; Tapsell, L. Seaweed Culture in Integrated Multi-Trophic Aquaculture-Nutritional Benefits and Systems for Australia. 2009. Available online: https://ro.uow.edu.au/cgi/viewcontent.cgi?referer=https://scholar.google.com.au/&httpsredir=1&article=1004&context=smfc (accessed on 12 August 2021).
- Mac Monagail, M.; Cornish, L.; Morrison, L.; Araújo, R.; Critchley, A.T. Sustainable harvesting of wild seaweed resources. Eur. J. Phycol. 2017, 52, 371–390. [Google Scholar] [CrossRef]

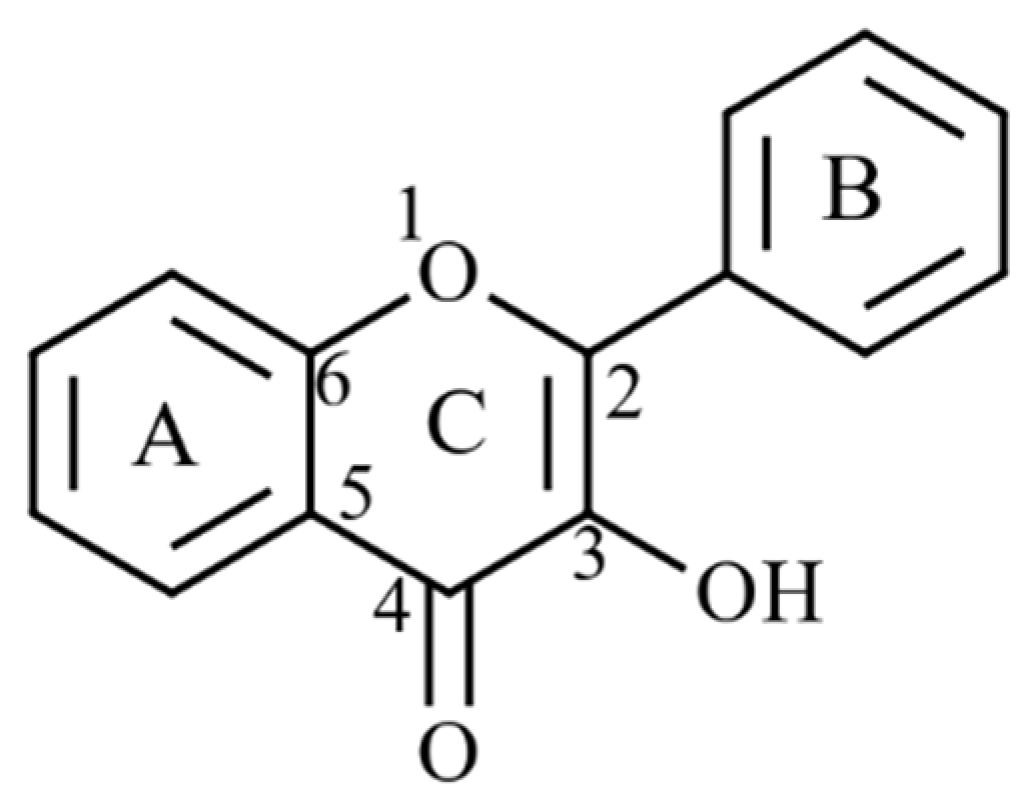

| Seaweed Species | Seaweed Extract | Polyphenol Content/ Active Compounds | Pathogenic Species | Antibiotic and Antimicrobial Properties | Reference |
|---|---|---|---|---|---|
| Gracilaria folifera (Red seaweed), Sargassum longifolium (Brown seaweed) | Ethanolic and aqueous extract (250 mg/L). | TPC; 5.2 mg GAE/g, and 2.8 mg GAE/g in ethanolic extract (Folin–Ciocalteu Assay) TFC; 9.3 mg QE/g and 8 mg QE/g in ethanolic extract | Aeromonas salmonicida | Antibacterial activity Relative percentage of survival; 90% till 120 h | [119] |
| Sargassum wightii (Brown seaweed), Ulva lacta (Green seaweed), Padina tetramatica (Brown seaweed) | Methanolic extract Diethyl ether extract Methanolic extract | Sargassum wightii exhibited high phenolic content | Vibrio alginolyticus (VA09), fish pathogenic bacteria | Antimicrobial activity; Minimum inhibitory concentration (MIC) of extracts were 25 mg/mL, 50 mg/mL and 50 mg/mL respectively | [120] |
| Sargassum muticum (Brown seaweed) | Crude acetone-water seaweed extracts and purified (SPE) methanol-water seaweed fraction | TPC; 17% DWfraction in crude extract and 1.45% DWseaweeds after SPE extract Phlorotannins; phlorethol (1H NMR and 2D NMR) | Vibrio aestuarianus Vibrio anguillarum Vibrio parahaemolyticus | Antibacterial activity; Crude extract (>50% bacterial growth inhibition) >> Purified fraction | [86] |
| Chaetomorpha antennina (Green seaweed) | Ethanolic extract | TPC; 180 mg GAE/g DW TFC; 79.6 mg QE/g DW | Vibrio parahaemolyticus (shrimp pathogen) | Antibacterial activity Extracts of 50 μL, 100 μL, 150 μL, 200 μL showed zone of inhibition 17 mm, 21 mm, 28 mm, and 36 mm respectively. | [121] |
| Sargassum thunbergii (Brown seaweed) | Ethanolic extract fraction | Low molecular weight phlorotannins (LMPs) | Vibrio parahaemolyticus (marine bacterium associated with human infection) | Antibacterial property Growth curve; LMPs (900 μg/mL) prevented cell division at logarithmic growth phase vs control group grew towards the stationary phase | [122] |
| Ecklonia Arborea (Brown seaweed) | Crude extract (CE) and phlorotannin-enriched ethyl acetate fraction (EPE). | Phlorotannins; Eckol (5.23 mg/g) and dieckol (1.67 mg/g) (HPLC/MS-TOF) | Vibrio parahaemolyticus (Acute hepatopancreatic necrosis disease (AHPND) of shrimps | EPE bactericidal activity 4.6-fold higher than CE. Minimum bactericidal Concentration; CE (3500 μg/mL) vs. EPE (750 μg/mL) | [123] |
| Cladophora glomerate, Rhizoclonium crassipellitum, Chaetomorpha aerea, Pithophora cleveana; (Green seaweed) | 50% seaweed biomass-added to fish feed Biomass includes each 25% for algal biomass | TPC; max 52.55 ± 0.01 mg GAE/g DW in algal biomass TFC; max 71.8 ± 0.21 mg QE/g DW in algal biomass | Fed to Carassius auratus (goldfish) | Skin pigmentation, Growth rate and antioxidant activities; 1.44–4-folds increase compared to the control group | [87] |
| Padina australis Hauck (Brown seaweed) | Ethyl acetate fraction (EAF) | Presence of tannin, steroid, phenolic, alkaloid and terpenoid compounds in EAF | Vibrio harveyi, Vibrio parahaemolyticus, and Aeromonas hydrophilla Shrimp pathogenic bacteria | Antimicrobial activity Average zone of inhibition for Vibrio harveyi, Vibrio parahaemolyticus and Aeromonas hydrophilla (EAF; 1.76 mm/ 2.3 mm/ 4.43 mm vs. Ciprofloxacin; 9.3 mm/ 6 mm/ 9.41) | [124] |
| Gracilaria foliifera (Red seaweed), Sargassum longifolium (Brown seaweed) | Purified ethanolic fraction of seaweed | TPC; 18.42 mg/g GAE and 14. 71 mg/g GAE TFC; 14.71 mg/g QE and 17.21 mg/g QE | Aeromonas salmonicida infection in Oreochromis mossambicus (Mozambique tilapia) | Antibiotic activity Minimal inhibitory concentration of G. folifera, S. lonfifolium, negative control, positive control (antibiotic) were 15 μg/mL, 20 μg/mL, 0 and 15 mg/mL respectively. | [125] |
| Sargassum thunbergii (Brown seaweed) | Purified ethanolic fraction | Low molecular weight phlorotannin (LMPs); 900 μg/mL | Vibrio parahaemolyticus | Antibacterial activity Growth curve; LMPs (inhibited thalli growth at logarithmic phase) vs. control (started growth in logarithmic phase); Membrane permeability- protein content of culture media; LMPs (256.79 μg/mL) vs. control (47.73 μg/mL) | [122] |
| Seaweed Species | Seaweed Extract | Polyphenol Content/Active Compounds | Antimicrobial Properties | Reference |
|---|---|---|---|---|
| Ascophyllum nodosum, Fucus serratus (Brown seaweed) | Phlorotannin extracts | TPC, A. nodosum; 37.35 mg/g (1H NMR) and 30.68 PGE/g (FC assay) TPC, F. serratus; 17.00 mg/g (1H NMR) and 36.68 PGE/g | Antimicrobial activity against Escherichia coli, Salmonella agona, and Streptococcus suis (foodborne pathogens) Minimum inhibitory Concentration; A. nodosum (1.56–0.78 mg/mL) and F. serratus (3.13mg/mL) and Minimum Bactericidal Concentration; A. nodosum (3.125–1.56 mg/mL) and F. serratus (6.25 mg/mL) | [126] |
| Himanthalia elongate (Brown seaweed) | Dried methanolic extracts | TPC; 151.3 mg GAE/g TFC; 42.5 mg QE/g Total tannin; 38.34 mg CE/g | Antimicrobial activity against L. monocytogenes, S. abony and E. faecalis, and P. aeruginosa (food borne and food spoilage bacteria); H. elongate extract, Sodium benzoate, Sodium nitrite; up to 100%, 99–89% and 98–93% inhibition respectively | [127] |
| Padina boergesenii (Brown seaweed) | Polyphenol extract | NA | Antibacterial activity against antibiotic resistant E. coli strains; most of the bacteria inhibited within 256 µg/mL concentration | [128] |
| Turbinaria ornate Sargassum wightii (Brown seaweed) | Methanolic extract | TPC; 43.72 and 35.98 mg GAE/g extract respectively | Antibacterial activities against Bacillus subtilis, E. coli, Shigella flexnerii and Staphylococcus aureus Zone of inhibition (mm); T. ornate, S. wightii and standard were max 20 mm, 18 mm and 28 mm respectively | [129] |
| Sargassum tenerrimum and Turbinaria ornate (Brown seaweed) | Chloroform extract | TPC; 3.598 mg/g and TFC; ~0.15 mg/g TPC; ~2 mg/g and TFC; ~0.1mg/g | Antifungal activity against Aspergillus niger and Penicillium janthinellum; S. tenerrimum 100 µL (20 mm,14 mm), T. ornate 100 µL (13 mm, 16 mm), Fluconazole 10 mcg (10 mm, 12 mm), Ketoconazole 10 mcg (17 mm, 20 mm), Amphotericin B 20 mcg (18 mm, 19 mm), Negative Control (8 mm, 9 mm) | [130] |
| Padina Pavonica (Brown seaweed) | Ethyl acetate fraction | TPC; 8.98 GAE/g | Antifungal activity against Candida glabrata (diameter of inhibition = 16 mm) and Candida krusei (diameter of inhibition = 14 mm) | [131] |
| Himanthalia elongate (Brown seaweed) | Ethanolic extract | TPC; 18.79 mg GAE/g | Antimicrobial activity against Salmonella spp. Listeria monocytogenes Escherichia coli Staphylococcus aureus Bacillus cereus | [132] |
| Seaweed Species | Seaweed Extract | Polyphenol Content/ Active Compounds | Tested Species/Cell Line | Dosage | Therapeutic Properties | Reference |
|---|---|---|---|---|---|---|
| Eisenia bicyclis (Brown seaweed) | Ethyl acetate fraction | Phlorofucofuroeckol A and dioxinodehydroeckol Dieckol and 7-phloroeckol Phloroglucinol | RAW 264.7 murine macrophages cells | >10 µg/mL >50 µg/mL >100 µg/mL | Cytotoxicity Prevent inflammatory and oxidative stress-related diseases | [133] |
| Ecklonia radiata (Brown seaweed) | Ethyl acetate fraction | TPhC; 619 PGE mg/g (eckol-type phlorotannins) | Neuronal PC-12 cell line | 100 µg/mL | Neuroprotective activity against the neurotoxic amyloid β protein (Aβ1–42) | [134] |
| Halimeda opuntia (Green seaweed) | Methanolic extract | TPC; 55.04 mg GAE/g of extract | MCF-7 & 3T3 cell lines | 25.14 µg/mL and 65 µg/mL | Cytotoxicity | [135] |
| Ascophyllum nodosum (Brown seaweed) | Ethanolic extract | High molecular weight fraction (>10 KDa) TPhC; 938.2 µg PGE/mg hydroxytrifuhalol A, C-O-C dimer of phloroglucinol, dimer diphlorethol, difucol and 7-hydroxyeckol | HT-29 cell culture | 250 μg/mL | Effect of simulated gastrointestinal digestion and fermentation | [136] |
| Sargassum muticum (Brown Seaweed) | Methanolic extract | TPC; 78.95 ± 4.33 mg GAE/ 100 g dried plant | MCF-7 and MDA-MB-231 breast cancer cell lines | 22 μg/mL and 55 μg/mL | Antioxidant, Antiproliferative, and Antiangiogenesis Effects | [137] |
| Gracilaria Fisheri (Red Seaweed) | Ethanolic extract | NA | virulent strain of Vibrio harveyi | MIC; 90 µg/mL | Immunostimulant and anti-bacterial activity | [138] |
| Sargassum horneri (Turner) C. Agardh (Brown Seaweed) | Ethanolic extract | NA | RAW 264.7 murine macrophage cell line | 200 µg/mL | Anti-inflammatory activity | [139] |
| Eisenia arborea (Brown Seaweed) | Methanol-chloroform extract | Phlorotannins (eckol, 8,8′-bieckol, phlorofucofuroeckol (PFF)- A and PFF-B | ICR mice | 0.1 mg/mouse | Anti-allergic and anti-inflammatory effects | [140] |
| NA | NA | Commercially purchased Dieckol | Rats | 20 mg/kg bwt | Anticancer, anti-inflammatory, and anti-cell proliferative effects | [141] |
| Agarum cribrosum (Brown Seaweed) | Ethyl acetate fraction | Trifuhalol A | RAW 264.7 cells | Hyaluronidase inhibitory activity (200–1000 µg/mL) Proliferation, NO production, cytokines mRNA expression (5–20 µg/mL) | Anti-inflammatory Activity | [142] |
| Ecklonia cava (Brown Seaweed) | Phlorotannin-rich extract | Dieckol 98% phloroglucinol equivalent | Mice | 50– 100 mg/kg/d | Prevent lipopolysaccharide (LPS)-induced septic shock | [143] |
| Seaweed Species | Tested Sample | Phenolic Content/Active Compounds | In Vitro Antioxidant Activities | Application | Reference |
|---|---|---|---|---|---|
| F. vesiculosus, F. serratus, F. distichus, F. spiralis (Brown seaweed) | Aqueous extracts | High levels of caffeic and gentisic acid and relatively high levels of gallic and vanillic acid | DPPH, RAP, oxidation inhibition in liposome model system. | Preserving fish oil | [98] |
| Gelidiella acerosa (Red seaweed) | Methanolic extract | TPC; 0.616 g/g GE | DPPH, Inhibition of lipid peroxidation, nitric oxide radical scavenging activity, hydrogen peroxide scavenging activity, RAP | Food preservative and therapeutic agent for oxidative stress-related disorders | [166] |
| Fucus Vesiculosus, Bifurcaria bifurcate, Ascophyllum nodosum (Brown seaweeds) | Aqueous extract | TPhC (FVE); 1.15 g PGE/100 g extract TPhC (BBE); 1.99 g PGE/100 g extract | ABTS, DPPH, ORAC and FRAP | Oil stabilizers in canola oil (500 ppm seaweed extract > 50 ppm BHT) | [154] |
| Ecklonia radiata (Brown seaweed) | Seaweed extract | TPhC; 4.4 g (PGE) 100 g−1 (DW) | FRAP, ORAC | Natural antioxidant and functional food ingredient | [93] |
| Fucus vesiculosus (Brown seaweed) | Aqueous seaweed extracts | TPC; 0.26 and 0.30 g PGE/g Phlorotannin-LMW; fucodiphlorethol A and trifucodiplorethol isomers (HPLC-DAD-ESI-MS) | ORAC, DPPH, FCA, ABTS, CAA | Antioxidant potential of enriched convenience cereals | [167] |
| Saccharina japonica (Brown seaweed) | SWE + IL extract | TPC; 39.55 mg PGE/g DW Chlorogenic, Protocatechuic, p-Hydroxybenzoic, Gentisic, Caffeic, Gallic, Syringic | DPPH, ABTS, TAC, FRAP | NA | [168] |
| Fucus vesiculosus L. (Brown seaweed) | Methanol/water extracts | TPC; 41.4 gPEGkg−1 DM | DPPH, FRAP, inhibition of copper-catalyzed LDL oxidation | NA | [100] |
| Bifurcaria bifurcate (Brown seaweed) | Organic extract (methanol and acetone) and aqueous extract | TPC; 2.0–2.5 g PGE 100 g−1 | DPPH, Reduction of power | Antioxidant nutraceuticals | [99] |
| Durvillaea antarctica, Lessonia spicata, Macrocystis integrifolia (Brown seaweed) | Ethanol/water extract | D. antarctica; TPC 5 g PGE/kg DW and L. spicata; TPC 1.21 g PGE/kg DW; phlorotannins (trimer to tetramer). M. integrifolia; TPC 3.7 g PGE/kg DW; flavonoids (glycoside forms) | FRAP, ORAC, DPPH | Food and pharmaceutical applications | [65] |
| Hypnea musciformis (Red seaweed) | Ethyl acetate fraction | TPC; 205.5 mg GAE/g | DPPH, ABTS, FCA, H2O2 scavenging activity, lipid peroxidation inhibitory activity | Food preservative | [169] |
| Ascophyllum Nodosum, Fucus vesiculosus, Fucus serratus (Brown seaweed) | Ethanol/water extract | TPC; 21.42 g PGE/100 g extract TPC; 22.71 g PGE/100 g extract TPC; 12.36 g PGE/100 g extract | DPPH A. Nodosum > F. Serratus > F. Vesiculosus | NA | [101] |
| Caulerpa lentillifera, C. racemose (Green seaweeds), Sargassum polycystum (Brown seaweed) | Methanolic extract | TPC; 42.85 mg PGE/g DW TPC; 40.36 mg PGE/g DW TPC; 45.16 mg PGE/g DW | TEAC, FRAP | Natural antioxidants | [170] |
| Sargassum sp. (Brown seaweed) | Hot water extract | TPC; 2.4 mg GAE/g DW | DPPH | Functional food ingredient | [96] |
| Turbinaria ornate (Brown seaweed) | Methanolic extract | TPC; 2.07 mg catechin/g DW | ABTS, DPPH, RAP | NA | [171] |
| Ulva sp. (Green seaweed) Gracilaria chilensis, Callophyllis concepcionensis (Red seaweeds) | Hot water extracts | TPC; 551.1 mg GAE/100 g DW TPC; 216.4 mg GAE/100 g DW TPC; 218.6 mg GAE/100 g DW (Hydrolyzable polyphenols; hydroxycinnamic acids, hydroxybenzoic acids and flavonols) | ABTS, FRAP | Natural antioxidant | [172] |
| Cystoseira trinodis (Brown seaweed) | Dichloromethane fraction from crude methanolic extract | TPC; 17.30 mg GAE/g of fraction Active compound; phlorotannins | DPPH | NA | [173] |
| Sargassum horneri (Brown seaweed) | Ethanolic extract in SC-CO2 | TPC; 0.64 ± 0.02 mg GAE/g TFC; 5.57 ± 0.05 mg catechin/g | DPPH, ABTS | Natural antioxidant | [174] |
| F. vesiculosus, F. serratus, A. nodosum (Brown seaweed) | 70% acetone extract | TPC; 24.2 g PGE/100 g extract TPC; 24.0 g PGE/100 g extract TPC; 15.9 g PGE/100 g extract | DPPH, ORAC | Natural antioxidants for functional foods and nutraceuticals | [102] |
| Halopithys incurve (Red seaweed), Fucus spiralis, Treptacantha abies-marina (Brown seaweeds) | Hydroethanolic methanolic and extracts | TPC; 4.8% of DW TPC; 3.1% of DW TPC; 3.9% of DW | DPPH, RAP | NA | [175] |
| Ascophyllum nodosum Fucus distichus Fucus evanescens (Brown seaweed) | Methanolic extract | TPC; 38.95 PGE% TPC; 30.40 PGE% TPC; 23.85 PGE% | DPPH | NA | [176] |
| Sargassum polycystum (Brown seaweed) | Ethanol/water extract | TPC; 37.41 mg GAE/g DW TFC; 4.54 mg CE/g DW | DPPH, ABTS | NA | [177] |
| Ulva intestinalis (Green seaweed) | Dichloromethane extract | TPC; 197 ± 16 mg GAE/g extract | DPPH, ABTS | Medicine, dietary supplements, cosmetics, and food industries. | [178] |
| Acanthophora spicifera (Red seaweed) | Ethyl acetate extract | TPC; 40.583 GAE; µg mg−1 DW | DPPH | Natural antioxidant | [105] |
| Himanthalia elongate (Brown seaweed) | 60% methanolic extract | TPC; 286.0 mg GAE/g TFC; 109.8 mg QE/g Condensed tannin; 35.6 mg CE/g | DPPH, FRAP, FCA, inhibition of lipid peroxidation, hydrogen peroxide scavenging activity | Natural food preservative or nutraceutical | [179] |
| Himanthalia elongate (Brown seaweed) | Ethanol/water extract | TPC; 548.33 mg AG/100 g seaweed. Phloroglucinol, Gallic Acid, Catechin, Rutin, Gentisic Acid, Chlorogenic Acid, Caffeic Acid, Coumaric, Ferulic, Myricetin and Quercetin | DPPH | NA | [180] |
| Kappaphycus alvarezii (Red seaweed) | 1% Formic acid extracts | TPC; 40 mg (100 g)−1 GAE TFC; 60 mg (100 g)−1 CE | DPPH, ABTS | NA | [92] |
| Laurencia obtuse (Red seaweed) | Ethanolic extract | TPC; 26.23 mg GAE/g seaweed | ABTS, TAA | NA | [94] |
| Macrocystis pyrifera (Brown seaweed) | Aqueous extract | TPC; 200.5 mg (GAE)/100 g DW Phlorotannin; phloroeckol and a tetrameric phloroglucinol | DPPH, TAA | Medicinal foods or therapeutics | [69] |
| Sargassum fusiforme (Brown seaweed) | Ethyl acetate fractions | TPC; 88.48 mg PGE/100 mg extract. fuhalol-type phlorotannins, phlorethols, fucophlorethols and eckol-type phlorotannins. | DRSA, FRAP | Marine antioxidants | [64] |
| Ascophyllum nodosum (Brown seaweed) | 75% (v/v aq.) 1,3-propanediol solvent extract | TPC; 100 mg/ (PEG/g) DW | DPPH | NA | [181] |
| Phenolic Compound | Biopolymers | Morphological Characteristics | Physiochemical Characteristics | Application | Reference |
|---|---|---|---|---|---|
| Tannic acid | Gelatin-high methyl pectin | Rough and irregular shape, Average particle size (47 µm) | Improved melting and gelling points, thermal stability, encapsulation efficacy (75%) | Peppermint Oil microencapsulation | [209] |
| Caffeic, Chlorogenic, Ferulic, Rutin, white grape juice, Instant coffee | Gelatin-pectin | NA | Reduced swelling, fewer free amino groups, lipophilicity, thermal stability up to 200 °C | Gelatin-pectin microparticles | [214] |
| Tannic acid–oxidized and non-oxidized form | Fish gelatin-gum arabic | NA | Improved gelling ability and mechanical properties | Complex coacervate gel | [212] |
| Tannic acid | Sodium caseinate | NA | Altered secondary structure of SC, high antioxidative properties | Complex coacervate gel | [215] |
| Caffeic, Tannic acid Oxidized form | Gelatin | NA | Decreased molecular mobility of hydrogels, thermal stability | Gelatin film (Insoluble hydrogels) | [216] |
| Tannic acid | Gelatin–gum arabic | Spherical in shape, mean cluster size 116.80 μm | High encapsulation efficiency (84%), sustained release of AITC (46% after 2h and 48% in 6h) | Allyl isothiocyanate (AITC) encapsulation | [217] |
| Fructus Chebulae extract (TPC; 360 μg polyphenols/g of gelatin) | Gelatin | Compact surface | Thermal stability, reduced swelling | Gelatin hydrogels | [210] |
| Tannic acid-oxidized form | Gelatin-flaxseed mucilage | Fine to less firm structure | High encapsulation efficacy (>90% w/w) and loading capacity, high stability, controlled release of oil | Flaxseed oil encapsulation | [213] |
| Ferulic acid, Tannin acid | Gelatin | Smooth surface, increased thickness of layers and interlayer space | High mechanical strength, decrease swelling ratios, | Gelatin film | [208] |
| Tea polyphenol (Catechin, Epicatechin, Epicatechin gallate, Epigallocatechin gallate) | Milk β-lactoglobulin | NA | Structural stabilization via an increase in β-sheet and α-helix | Milk β-lactoglobulin complexes | [218] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gunathilake, T.; Akanbi, T.O.; Suleria, H.A.R.; Nalder, T.D.; Francis, D.S.; Barrow, C.J. Seaweed Phenolics as Natural Antioxidants, Aquafeed Additives, Veterinary Treatments and Cross-Linkers for Microencapsulation. Mar. Drugs 2022, 20, 445. https://doi.org/10.3390/md20070445
Gunathilake T, Akanbi TO, Suleria HAR, Nalder TD, Francis DS, Barrow CJ. Seaweed Phenolics as Natural Antioxidants, Aquafeed Additives, Veterinary Treatments and Cross-Linkers for Microencapsulation. Marine Drugs. 2022; 20(7):445. https://doi.org/10.3390/md20070445
Chicago/Turabian StyleGunathilake, Tharuka, Taiwo O. Akanbi, Hafiz A. R. Suleria, Tim D. Nalder, David S. Francis, and Colin J. Barrow. 2022. "Seaweed Phenolics as Natural Antioxidants, Aquafeed Additives, Veterinary Treatments and Cross-Linkers for Microencapsulation" Marine Drugs 20, no. 7: 445. https://doi.org/10.3390/md20070445
APA StyleGunathilake, T., Akanbi, T. O., Suleria, H. A. R., Nalder, T. D., Francis, D. S., & Barrow, C. J. (2022). Seaweed Phenolics as Natural Antioxidants, Aquafeed Additives, Veterinary Treatments and Cross-Linkers for Microencapsulation. Marine Drugs, 20(7), 445. https://doi.org/10.3390/md20070445









