Monocaprin Enhances Bioavailability of Fucoxanthin in Diabetic/Obese KK-Ay Mice
Abstract
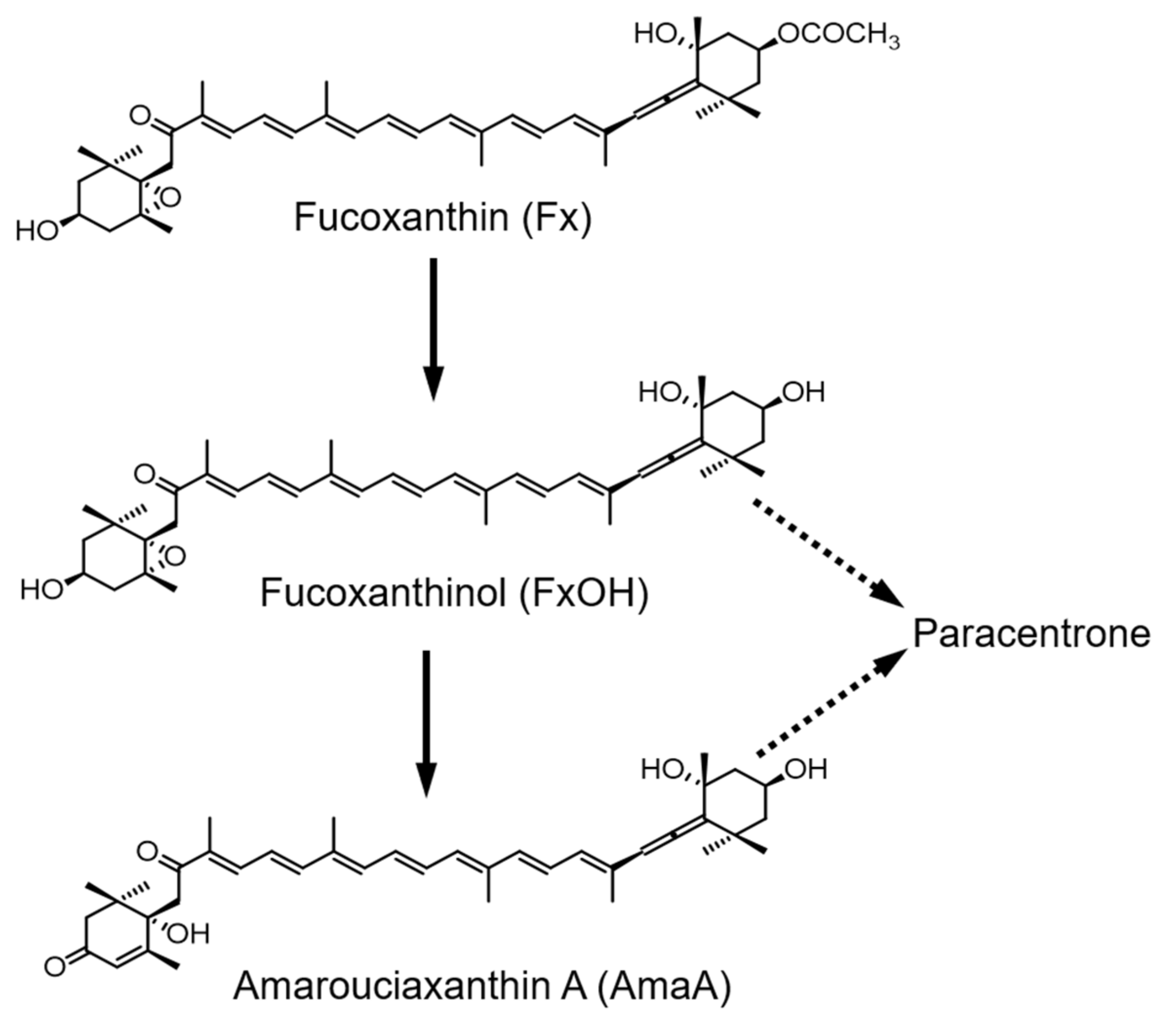
:1. Introduction
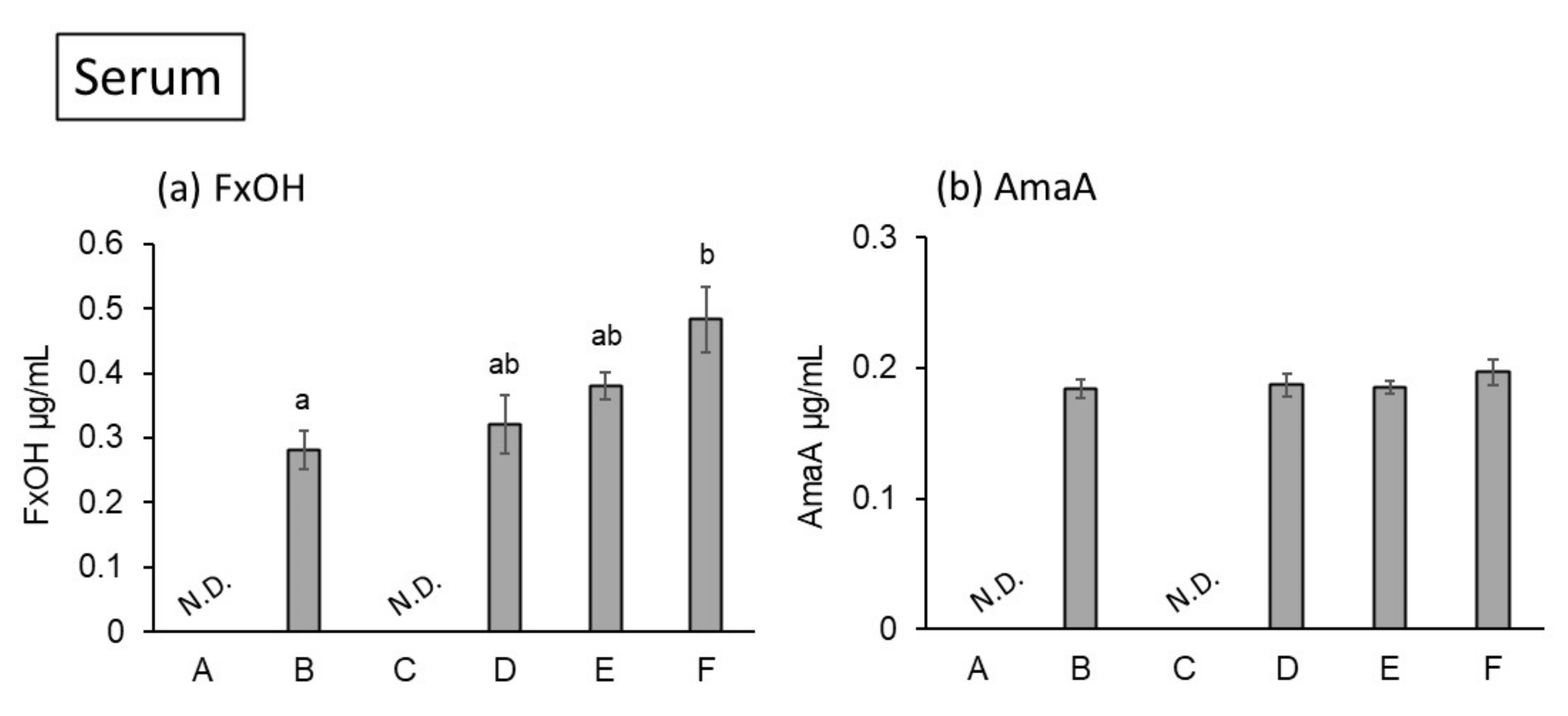
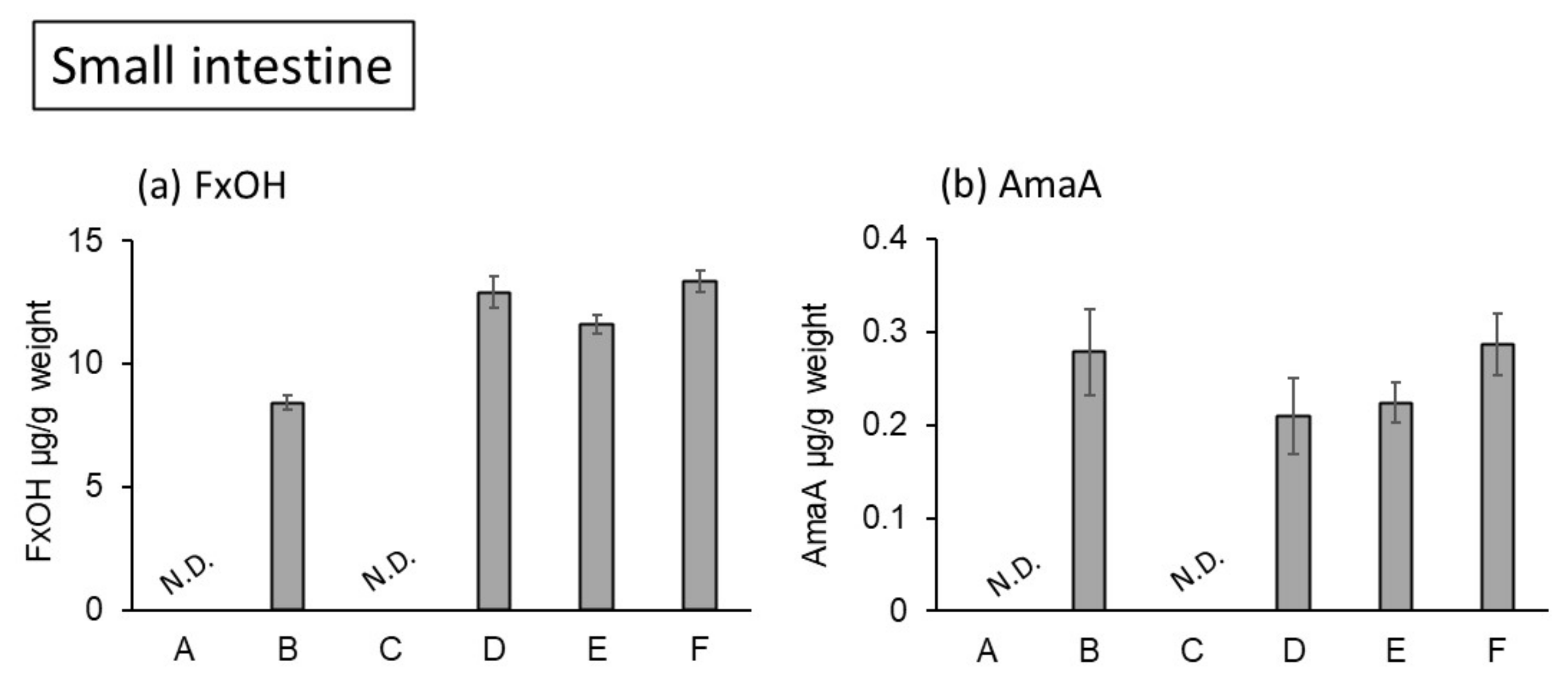
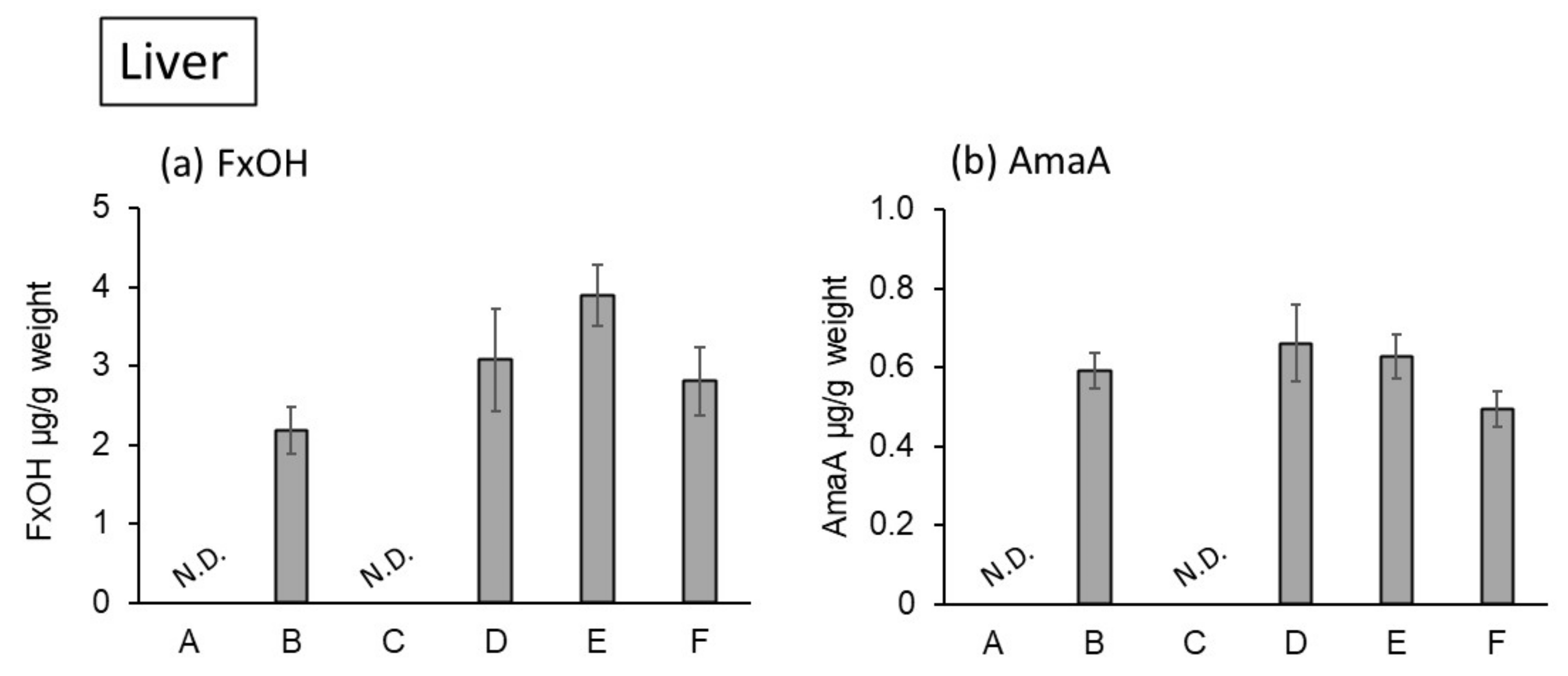
2. Results and Discussion
3. Materials and Methods
3.1. Chemicals
3.2. Animal Experiments
3.3. HPLC Analysis of Fucoxanthin Metabolites
3.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maeda, H.; Hosokawa, M.; Sashima, T.; Funayama, K.; Miyashita, K. Fucoxanthin from edible seaweed, Undaria pinnatifida, shows antiobesity effect through UCP1 expression in white adipose tissues. Biochem. Biophys. Res. Commun. 2005, 332, 329–397. [Google Scholar] [CrossRef] [PubMed]
- Hosokawa, M.; Miyashita, T.; Nishikawa, S.; Emi, S.; Tsukui, T.; Beppu, F.; Okada, T.; Miyashita, K. Fucoxanthin regulates adipocytokine mRNA expression in white adipose tissue of diabetic/obese KK-Ay mice. Arch. Biochem. Biophys. 2010, 1, 17–25. [Google Scholar] [CrossRef]
- Abidov, M.; Ramazanov, Z.; Seifulla, R.; Grachev, S. The effects of Xanthigen in the weight management of obese premenopausal women with non-alcoholic fatty liver disease and normal liver fat. Diabetes Obes. Metab. 2010, 12, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Mikami, N.; Hosokawa, M.; Miyashita, K.; Sohma, H.; Ito, Y.M.; Kokai, Y. Reduction of HbA1c levels by fucoxanthin-enriched akamoku oil possibly involves the thrifty allele of uncoupling protein 1 (UCP1): A randomised controlled trial in normal-weight and obese Japanese adults. J. Nutr. Sci. 2017, 6, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugawara, T.; Kushiro, M.; Zhang, H.; Nara, E.; Ono, H.; Nagao, A. Lysophosphatidylcholine enhances carotenoid uptake from mixed micelles by Caco-2 human intestinal cells. J. Nutr. 2001, 131, 2921–2927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kotake-Nara, E.; Yonekura, L.; Nagao, A. Lysoglyceroglycolipids improve the intestinal absorption of micellar fucoxanthin by Caco-2 cells. J. Oleo Sci. 2015, 64, 1207–1211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Z.X.; Khalid, N.; Shu, G.F.; Zhao, Y.G.; Kobayashi, I.; Neves, M.A.; Tuwo, A.; Nakajima, M. Fucoxanthin-loaded oil-in-water emulsion-based delivery systems: Effects of natural emulsifiers on the formulation, stability, and bioaccessibility. ACS Omega 2019, 4, 10502–10509. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.X.; Qiao, Z.C.; Liu, W.Q.; Hou, Z.Q.; Zhang, D.; Huang, L.; Zhang, Y.P. Oleic acid as a protein ligand improving intestinal absorption and ocular benefit of fucoxanthin in water through protein-based encapsulation. Food Funct. 2019, 10, 4381–4395. [Google Scholar] [CrossRef] [PubMed]
- Thormar, H.; Hilmarsson, H.; Bergsson, G. Stable concentrated emulsions of the 1-monoglyceride of capric acid (Monocaprin) with microbicidal activities against the food-borne bacteria Campylobacter jejuni, Salmonella spp., and Escherichia coli. Appl. Environ. Microbiol. 2006, 72, 522–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skulason, S.; Holbrook, W.P.; Thormar, H.; Gunnarsson, G.B.; Kristmundsdottir, T. A study of the clinical activity of a gel combining monocaprin and doxycycline: A novel treatment for herpes labialis. K. Oral Pathol. Med. 2012, 41, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Nagao, K.; Yanagita, T. Medium-chain fatty acids: Functional lipids for the prevention and treatment of the metabolic syndrome. Pharmacol. Res. 2010, 61, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Asai, A.; Sugawara, T.; Ono, H.; Nagao, A. Biotransformation of fucoxanthinol into amarouciaxanthin A in mice and HepG2 cells: Formation and cytotoxicity of fucoxanthin metabolites. Drug Metab. Dispos. 2004, 32, 205–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Airanthi, M.K.W.A.; Sasaki, N.; Iwasaki, S.; Baba, N.; Abe, M.; Hosokawa, M.; Miyashita, K. Effect of brown seaweed lipids on fatty acid composition and lipid hydroperoxide levels of mouse liver. J. Agric. Food Chem. 2011, 59, 4156–4163. [Google Scholar] [CrossRef] [PubMed]
- Takatani, N.; Taya, D.; Katsuki, A.; Beppu, F.; Yamano, Y.; Wada, A.; Miyashita, K.; Hosokawa, M. Identification of paracentrone in fucoxanthin-fed mice and anti-inflammatory effect against lipopolysaccharide-stimulated macrophages and adipocytes. Mol. Food Nutr. Res. 2021, 65, 2000405. [Google Scholar] [CrossRef] [PubMed]
- Koo, S.Y.; Mok, I.K.; Pan, C.H.; Kim, S.M. Preparation of fucoxanthin-loaded nanoparticles composed of casein and chitosan with improved fucoxanthin bioavailability. J. Agric. Food Chem. 2016, 64, 9428–9435. [Google Scholar] [CrossRef] [PubMed]
- Li, D.H.; Zhang, Q.; Huang, L.; Chen, Z.H.; Zou, C.; Ma, Y.; Cao, M.J.; Liu, G.M.; Liu, Y.X.; Wang, Y.B. Fabricating hydrophilic particles with oleic acid and bovine serum albumin to improve the dispersibility and bioaccessibility of fucoxanthin in water. Food Hydrocoll. 2021, 118, 106752. [Google Scholar] [CrossRef]
- Kumagai, K.; Nebashi, N.; Muromachi, A.; Nakano, Y.; Ito, Y.; Nagasawa, T. Emulsified fucoxanthin increases stability and absorption in rats. Nippon. Shokuhin Kagaku Kogaku Kaishi 2018, 65, 349–356. [Google Scholar] [CrossRef] [Green Version]
- Takatani, N.; Kono, Y.; Beppu, F.; Okamatsu-Ogura, Y.; Yamano, Y.; Miyashita, K.; Hosokawa, M. Fucoxanthin inhibits hepatic oxidative stress, inflammation, and fibrosis in diet-induced nonalcoholic steatohepatitis model mice. Biochem. Biophys. Res. Commun. 2020, 528, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Xu, Y.; Zhao, L.; Yan, H.; Wang, S.; Wang, D. The stability and bioaccessibility of fucoxanthin in spray-dried microcapsules based on various biopolymers. RCS Adv. 2018, 8, 35138–35149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
| Ingredient (g/kg Diet) | A: Control | B: SO 0.2% | C: Monocaprin 0.5% | D: SO 0.2% +Monocaprin 0.125% | E: SO 0.2% +Monocaprin 0.25% | F: SO 0.2% +Monocaprin 0.5% |
|---|---|---|---|---|---|---|
| Soybean oil | 98.000 | 98.000 | 93.000 | 96.750 | 95.500 | 93.000 |
| Seaweed oil (SO) | - | 2.000 | - | 2.000 | 2.000 | 2.000 |
| (Fx in diet) | - | (0.100) | - | (0.100) | (0.100) | (0.100) |
| MCT | 2.000 | - | 2.000 | - | - | - |
| Monocaprin | - | - | 5.000 | 1.250 | 2.500 | 5.000 |
| Corn starch | 374.120 | 374.120 | 374.120 | 374.120 | 374.120 | 374.120 |
| Dextrinized cornstarch | 124.240 | 124.240 | 124.240 | 124.240 | 124.240 | 124.240 |
| Casein | 207.000 | 207.000 | 207.000 | 207.000 | 207.000 | 207.000 |
| Sucrose | 94.120 | 94.120 | 94.120 | 94.120 | 94.120 | 94.120 |
| Cellulose | 50.000 | 50.000 | 50.000 | 50.000 | 50.000 | 50.000 |
| AIN-93 mineral mixture | 35.000 | 35.000 | 35.000 | 35.000 | 35.000 | 35.000 |
| AIN-93 vitamin mixture | 10.000 | 10.000 | 10.000 | 10.000 | 10.000 | 10.000 |
| l-Cystine | 3.000 | 3.000 | 3.000 | 3.000 | 3.000 | 3.000 |
| Choline bitartrate | 2.500 | 2.500 | 2.500 | 2.500 | 2.500 | 2.500 |
| tert-Butyl hydroquinone | 0.014 | 0.014 | 0.014 | 0.014 | 0.014 | 0.014 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagata, K.; Takatani, N.; Beppu, F.; Abe, A.; Tominaga, E.; Fukuhara, T.; Ozeki, M.; Hosokawa, M. Monocaprin Enhances Bioavailability of Fucoxanthin in Diabetic/Obese KK-Ay Mice. Mar. Drugs 2022, 20, 446. https://doi.org/10.3390/md20070446
Nagata K, Takatani N, Beppu F, Abe A, Tominaga E, Fukuhara T, Ozeki M, Hosokawa M. Monocaprin Enhances Bioavailability of Fucoxanthin in Diabetic/Obese KK-Ay Mice. Marine Drugs. 2022; 20(7):446. https://doi.org/10.3390/md20070446
Chicago/Turabian StyleNagata, Kodai, Naoki Takatani, Fumiaki Beppu, Aya Abe, Etsuko Tominaga, Tomohisa Fukuhara, Makoto Ozeki, and Masashi Hosokawa. 2022. "Monocaprin Enhances Bioavailability of Fucoxanthin in Diabetic/Obese KK-Ay Mice" Marine Drugs 20, no. 7: 446. https://doi.org/10.3390/md20070446
APA StyleNagata, K., Takatani, N., Beppu, F., Abe, A., Tominaga, E., Fukuhara, T., Ozeki, M., & Hosokawa, M. (2022). Monocaprin Enhances Bioavailability of Fucoxanthin in Diabetic/Obese KK-Ay Mice. Marine Drugs, 20(7), 446. https://doi.org/10.3390/md20070446






