Applications of Chitosan in Surgical and Post-Surgical Materials
Abstract
1. Introduction
2. Properties of Chitosan
3. Chitosan-Based Materials and Devices
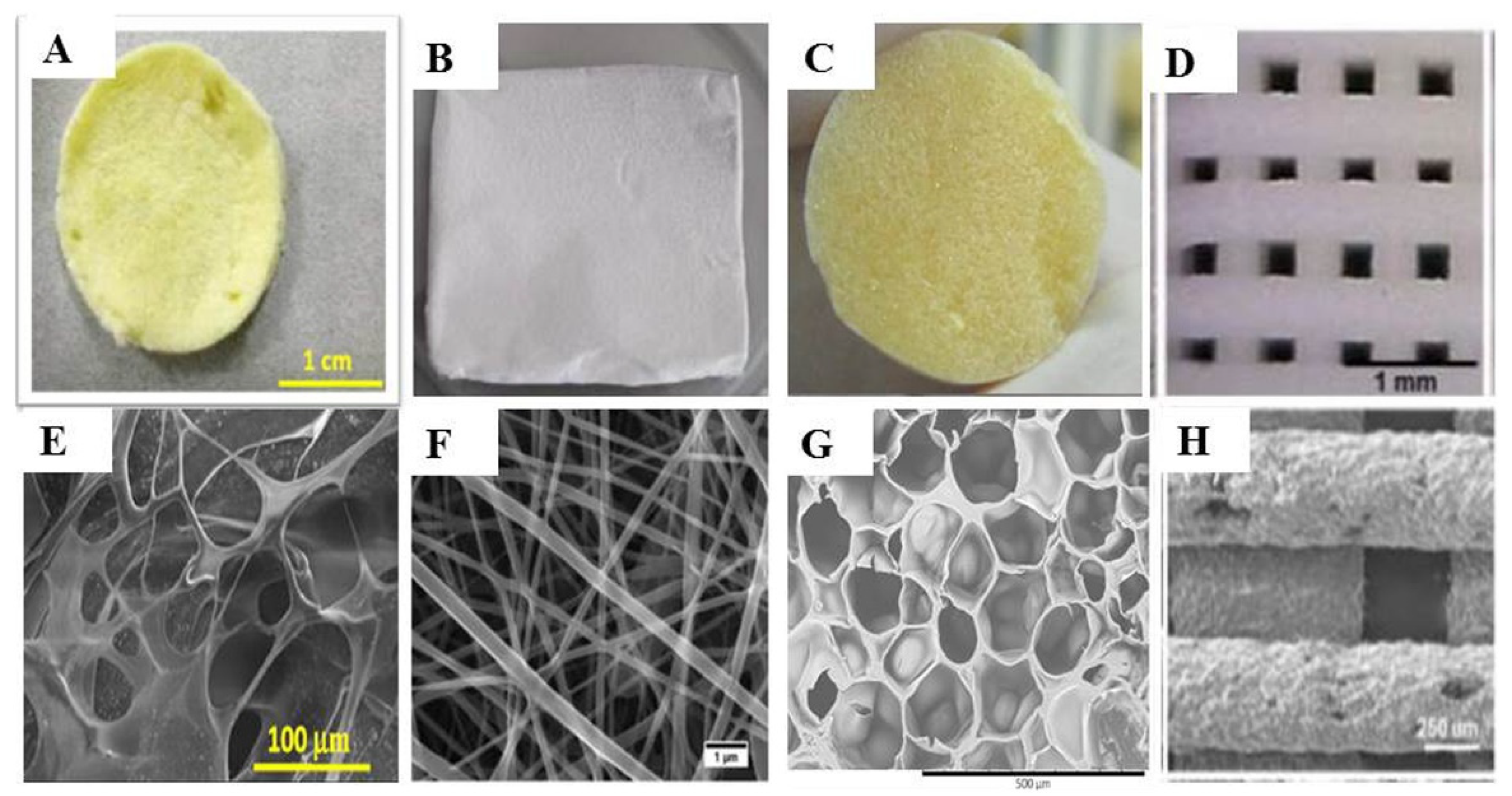
3.1. Scaffolds
3.2. Sponges
3.3. Meshes
3.4. Membranes
3.5. Hydrogels
3.6. Nanofibers and Nanoparticles
4. Surgical and Post-Surgical Applications of Chitosan-Based Devices
4.1. Nerve Regeneration
4.2. Bone Regeneration
- Inclusion of nanohydroxyapatite/chitosan microspheres in chitosan membranes, with better results than pure chitosan membranes in terms of mechanical properties [105].
- Incorporation of halloysite chitosan-modified nanotubes in thermosensitive hydrogels of chitosan/glycerophosphate, thus improving the mechanical properties and proliferation of the encapsulated stem cells with respect to the hydrogel [106].
4.3. Cartilage Regeneration and Viscosupplementation
4.4. Soft Tissue Regeneration
4.4.1. Skin and Mucosa Wound Healing
4.4.2. Cornea Damage
4.4.3. Gastric Ulcer
4.4.4. Chronic Tympanic Membrane Perforation
4.5. Sutures
4.6. Hemostasis
4.7. Other Surgical Applications of Chitosan
5. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hoogeboom, T.J.; Dronkers, J.J.; Hulzebos, E.H.; Van Meeteren, N.L. Merits of exercise therapy before and after major surgery. Curr. Opin. Anaesthesiol. 2014, 27, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Global Surgery 2030. The Lancet Comission on Global Surgery. Available online: https://b6cf2cfd-eb09-4859-92a9-a8f002c3bcef.filesusr.com/ugd/346076_713dd3f8bb594739810d84c1928ef61a.pdf (accessed on 13 May 2022).
- Scalise, A.; Calamita, R.; Tartaglione, C.; Pierangeli, M.; Bolletta, E.; Gioacchini, M.; Gesuita, R.; Di Benedetto, G. Improving wound healing and preventing surgical site complications of closed surgical incisions: A possible role of Incisional Negative Pressure Wound Therapy. A systematic review of the literature. Int. Wound J. 2015, 13, 1260–1281. [Google Scholar] [CrossRef] [PubMed]
- Kolasiński, W. Surgical site infections—Review of current knowledge, methods of prevention. Pol. J. Surg. 2018, 91, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Ghadimi, K.; Levy, J.; Welsby, I. Perioperative management of the bleeding patient. Br. J. Anaesth. 2016, 117 (Suppl. S3), iii18–iii30. [Google Scholar] [CrossRef]
- Sahana, T.G.; Rekha, P.D. Biopolymers: Applications in wound healing and skin tissue engineering. Mol. Biol. Rep. 2018, 45, 2857–2867. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.Y.; Jiang, L.J.; Cao, P.P.; Li, J.B.; Chen, X.G. Glycerophosphate-based chitosan thermosensitive hydrogels and their biomedical applications. Carbohydr. Polym. 2015, 117, 524–536. [Google Scholar] [CrossRef]
- Kozusko, S.D.; Riccio, C.; Goulart, M.; Bumgardner, J.; Jing, X.L.; Konofaos, P. Chitosan as a Bone Scaffold Biomaterial. J. Craniofacial Surg. 2018, 29, 1788–1793. [Google Scholar] [CrossRef]
- Shariatinia, Z.; Jalali, A.M. Chitosan-based hydrogels: Preparation, properties and applications. Int. J. Biol. Macromol. 2018, 115, 194–220. [Google Scholar] [CrossRef]
- Jain, R.; Wairkar, S. Recent developments and clinical applications of surgical glues: An overview. Int. J. Biol. Macromol. 2019, 137, 95–106. [Google Scholar] [CrossRef]
- Deprés-Tremblay, G.; Chevrier, A.; Snow, M.; Hurtig, M.B.; Rodeo, S.; Buschmann, M.D. Rotator cuff repair: A review of surgical techniques, animal models, and new technologies under development. J. Shoulder Elb. Surg. 2016, 25, 2078–2085. [Google Scholar] [CrossRef]
- Foster, L.J.R.; Thomson, K.; Marçal, H.; Butt, J.; Watson, S.L.; Wakefield, D. Chitosan−Vancomysin Composite Biomaterial as a Laser Activated Surgical Adhesive with Regional Antimicrobial Activity. Biomacromolecules 2010, 11, 3563–3570. [Google Scholar] [CrossRef] [PubMed]
- Lestari, W.; Yusry, W.N.A.W.; Haris, M.S.; Jaswir, I.; Idrus, E. A glimpse on the function of chitosan as a dental hemostatic agent. Jpn. Dent. Sci. Rev. 2020, 56, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Bhavsar, C.; Momin, M.; Gharat, S.; Omri, A. Functionalized and graft copolymers of chitosan and its pharmaceutical applications. Expert Opin. Drug Deliv. 2016, 14, 1189–1204. [Google Scholar] [CrossRef] [PubMed]
- Bellich, B.; D’Agostino, I.; Semeraro, S.; Gamini, A.; Cesàro, A. “The Good, the Bad and the Ugly” of Chitosans. Mar. Drugs 2016, 14, 99. [Google Scholar] [CrossRef]
- Kumar, A.; Vimal, A.; Kumar, A. Why Chitosan? From properties to perspective of mucosal drug delivery. Int. J. Biol. Macromol. 2016, 91, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.-C.; Yoo, S.-Y.; Kim, H.; Lee, J. Chitosan-Based Multifunctional Platforms for Local Delivery of Therapeutics. Mar. Drugs 2017, 15, 60. [Google Scholar] [CrossRef]
- Mohammed, M.A.; Syeda, J.T.M.; Wasan, K.M.; Wasan, E.K. An Overview of Chitosan Nanoparticles and Its Application in Non-Parenteral Drug Delivery. Pharmaceutics 2017, 9, 53. [Google Scholar] [CrossRef]
- Wang, W.; Meng, Q.; Li, Q.; Liu, J.; Zhou, M.; Jin, Z.; Zhao, K. Chitosan Derivatives and Their Application in Biomedicine. Int. J. Mol. Sci. 2020, 21, 487. [Google Scholar] [CrossRef]
- Ma, J.; Wang, Y.; Lu, R. Mechanism and Application of Chitosan and Its Derivatives in Promoting Permeation in Transdermal Drug Delivery Systems: A Review. Pharmaceuticals 2022, 15, 459. [Google Scholar] [CrossRef]
- Cazorla-Luna, R.; Martín-Illana, A.; Notario-Pérez, F.; Ruiz-Caro, R.; Veiga, M.-D. Naturally Occurring Polyelectrolytes and Their Use for the Development of Complex-Based Mucoadhesive Drug Delivery Systems: An Overview. Polymers 2021, 13, 2241. [Google Scholar] [CrossRef]
- Casadidio, C.; Peregrina, D.V.; Gigliobianco, M.R.; Deng, S.; Censi, R.; Di Martino, P. Chitin and Chitosans: Characteristics, Eco-Friendly Processes, and Applications in Cosmetic Science. Mar. Drugs 2019, 17, 369. [Google Scholar] [CrossRef] [PubMed]
- Cazorla-Luna, R.; Martín-Illana, A.; Notario-Pérez, F.; Bedoya, L.M.; Tamayo, A.; Ruiz-Caro, R.; Rubio, J.; Veiga, M.-D. Vaginal Polyelectrolyte Layer-by-Layer Films Based on Chitosan Derivatives and Eudragit® S100 for pH Responsive Release of Tenofovir. Mar. Drugs 2020, 18, 44. [Google Scholar] [CrossRef] [PubMed]
- Ways, T.M.M.; Lau, W.M.; Khutoryanskiy, V.V. Chitosan and Its Derivatives for Application in Mucoadhesive Drug Delivery Systems. Polymers 2018, 10, 267. [Google Scholar] [CrossRef] [PubMed]
- Notario-Pérez, F.; Martín-Illana, A.; Cazorla-Luna, R.; Ruiz-Caro, R.; Bedoya, L.-M.; Tamayo, A.; Rubio, J.; Veiga, M.-D. Influence of Chitosan Swelling Behaviour on Controlled Release of Tenofovir from Mucoadhesive Vaginal Systems for Prevention of Sexual Transmission of HIV. Mar. Drugs 2017, 15, 50. [Google Scholar] [CrossRef]
- Martín-Illana, A.; Cazorla-Luna, R.; Notario-Pérez, F.; Bedoya, L.M.; Rubio, J.; Tamayo, A.; Ruiz-Caro, R.; Veiga, M.D. Smart vaginal bilayer films of Tenofovir based on Eudragit® L100/natural polymer for the prevention of the sexual transmission of HIV. Int. J. Pharm. 2021, 602, 120665. [Google Scholar] [CrossRef]
- Notario-Pérez, F.; Cazorla-Luna, R.; Martín-Illana, A.; Ruiz-Caro, R.; Tamayo, A.; Rubio, J.; Veiga, M.-D. Optimization of tenofovir release from mucoadhesive vaginal tablets by polymer combination to prevent sexual transmission of HIV. Carbohydr. Polym. 2018, 179, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Karim, S.; Akhter, H.; Burzangi, A.S.; Alkreathy, H.; Alharthy, B.; Kotta, S.; Shadab; Rashid, A.; Afzal, O.; Altamimi, A.S.A.; et al. Phytosterol-Loaded Surface-Tailored Bioactive-Polymer Nanoparticles for Cancer Treatment: Optimization, In Vitro Cell Viability, Antioxidant Activity, and Stability Studies. Gels 2022, 8, 219. [Google Scholar] [CrossRef]
- Sheu, M.-T.; Tseng, Y.-C.; Ho, H.-O.; Chiu, C.-C. Development and characterization of a gastroretentive dosage form composed of chitosan and hydroxyethyl cellulose for alendronate. Drug Des. Dev. Ther. 2013, 8, 67–78. [Google Scholar] [CrossRef][Green Version]
- Getyala, A.; Gangadharappa, H.; Prasad, M.; Reddy, M.; Kumar, T. Formulation and Evaluation of Non-Effervescent Floating Tablets of Losartan Potassium. Curr. Drug Deliv. 2013, 10, 620–629. [Google Scholar] [CrossRef]
- Stager, M.A.; Erickson, C.B.; Payne, K.A.; Krebs, M.D. Fabrication of Size-Controlled and Emulsion-Free Chitosan-Genipin Microgels for Tissue Engineering Applications. J. Vis. Exp. 2022, 182, e63857. [Google Scholar] [CrossRef]
- Kabirkoohian, A.; Bakhshi, H.; Irani, S.; Sharifi, F. Chemical Immobilization of Carboxymethyl Chitosan on Polycaprolactone Nanofibers as Osteochondral Scaffolds. Appl. Biochem. Biotechnol. 2022, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Xu, L.; Dai, J.; Yi, X.; He, J.; Li, H. N, O-carboxymethyl chitosan/oxidized cellulose composite sponge containing ε-poly-l-lysine as a potential wound dressing for the prevention and treatment of postoperative adhesion. Int. J. Biol. Macromol. 2022, 209, 2151–2164. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Rajoka, M.S.R.; Ke, Z.; Mehwish, H.M.; Deng, W.; Li, J.; Qin, W.; Zhao, L.; Wu, Y. Effect of Squid Cartilage Chitosan Molecular Structure on the Properties of Its Monofilament as an Absorbable Surgical Suture. Polymers 2022, 14, 1306. [Google Scholar] [CrossRef] [PubMed]
- Madden, J.; Vaughan, E.; Thompson, M.; Riordan, A.O.; Galvin, P.; Iacopino, D.; Teixeira, S.R. Electrochemical sensor for enzymatic lactate detection based on laser-scribed graphitic carbon modified with platinum, chitosan and lactate oxidase. Talanta 2022, 246, 123492. [Google Scholar] [CrossRef]
- Saravanan, S.; Leena, R.S.; Selvamurugan, N. Chitosan based biocomposite scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2016, 93, 1354–1365. [Google Scholar] [CrossRef]
- Thein-Han, W.; Saikhun, J.; Pholpramoo, C.; Misra, R.; Kitiyanant, Y. Chitosan–gelatin scaffolds for tissue engineering: Physico-chemical properties and biological response of buffalo embryonic stem cells and transfectant of GFP–buffalo embryonic stem cells. Acta Biomater. 2009, 5, 3453–3466. [Google Scholar] [CrossRef]
- Rodríguez-Vázquez, M.; Vega-Ruiz, B.; Ramos-Zúñiga, R.; Saldaña-Koppel, D.A.; Quiñones-Olvera, L.F. Chitosan and Its Potential Use as a Scaffold for Tissue Engineering in Regenerative Medicine. BioMed Res. Int. 2015, 2015, 821279. [Google Scholar] [CrossRef]
- Madihally, S.; Matthew, H.W. Porous chitosan scaffolds for tissue engineering. Biomaterials 1999, 20, 1133–1142. [Google Scholar] [CrossRef]
- Sahai, N.; Gogoi, M.; Tewari, R.P. 3D Printed Chitosan Composite Scaffold for Chondrocytes Differentiation. Curr. Med. Imaging 2021, 17, 832–842. [Google Scholar] [CrossRef]
- Chollakup, R.; Uttayarat, P.; Chworos, A.; Smitthipong, W. Noncovalent Sericin-Chitosan Scaffold: Physical Properties and Low Cytotoxicity Effect. Int. J. Mol. Sci. 2020, 21, 775. [Google Scholar] [CrossRef]
- Soundarya, S.P.; Menon, A.H.; Chandran, S.V.; Selvamurugan, N. Bone tissue engineering: Scaffold preparation using chitosan and other biomaterials with different design and fabrication techniques. Int. J. Biol. Macromol. 2018, 119, 1228–1239. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Li, M.; Li, N.; Wan, G.; Li, Y.; Ali, M.A.; Tang, K. One-step fabrication of chitosan sponge and its potential for rapid hemostasis in deep trauma. Biomed. Mater. 2020, 16, 015010. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Sun, Y.; Nie, J.; Lu, W.; Yang, L.; Zhang, Z.; Yin, H.; Wang, Z.; Hu, Q. Using absorbable chitosan hemostatic sponges as a promising surgical dressing. Int. J. Biol. Macromol. 2015, 75, 322–329. [Google Scholar] [CrossRef]
- Phaechamud, T.; Charoenteeraboon, J. Antibacterial Activity and Drug Release of Chitosan Sponge Containing Doxycycline Hyclate. AAPS PharmSciTech 2008, 9, 829–835. [Google Scholar] [CrossRef]
- De Castro, G.P.; Dowling, M.B.; Kilbourne, M.; Keledjian, K.; Driscoll, I.R.; Raghavan, S.R.; Hess, J.R.; Scalea, T.M.; Bochicchio, G.V. Determination of efficacy of novel modified chitosan sponge dressing in a lethal arterial injury model in swine. J. Trauma Acute Care Surg. 2012, 72, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zhou, W.; Deng, W.; Xu, C.; Cai, Y.; Wang, X. Antibacterial and Hemostatic Thiol-Modified Chitosan-Immobilized AgNPs Composite Sponges. ACS Appl. Mater. Interfaces 2020, 12, 20307–20320. [Google Scholar] [CrossRef]
- Du, X.; Wu, L.; Yan, H.; Jiang, Z.; Li, S.; Li, W.; Bai, Y.; Wang, H.; Cheng, Z.; Kong, D.; et al. Microchannelled alkylated chitosan sponge to treat noncompressible hemorrhages and facilitate wound healing. Nat. Commun. 2021, 12, 4733. [Google Scholar] [CrossRef]
- Lan, G.; Lu, B.; Wang, T.; Wang, L.; Chen, J.; Yu, K.; Liu, J.; Dai, F.; Wu, D. Chitosan/gelatin composite sponge is an absorbable surgical hemostatic agent. Colloids Surf. B Biointerfaces 2015, 136, 1026–1034. [Google Scholar] [CrossRef]
- Lu, B.; Wang, T.; Li, Z.; Dai, F.; Lv, L.; Tang, F.; Yu, K.; Liu, J.; Lan, G. Healing of skin wounds with a chitosan–gelatin sponge loaded with tannins and platelet-rich plasma. Int. J. Biol. Macromol. 2016, 82, 884–891. [Google Scholar] [CrossRef]
- Piasecka-Zelga, J.; Zelga, P.; Szulc, J.; Wietecha, J.; Ciechańska, D. An In Vivo biocompatibility study of surgical meshes made from bacterial cellulose modified with chitosan. Int. J. Biol. Macromol. 2018, 116, 1119–1127. [Google Scholar] [CrossRef]
- Saha, T.; Houshyar, S.; Sarker, S.R.; Pyreddy, S.; Dekiwadia, C.; Nasa, Z.; Padhye, R.; Wang, X. Nanodiamond-chitosan functionalized hernia mesh for biocompatibility and antimicrobial activity. J. Biomed. Mater. Res. Part A 2021, 109, 2449–2461. [Google Scholar] [CrossRef] [PubMed]
- Altınel, Y.; Öztürk, E.; Özkaya, G.; Akyıldız, E.Ü.; Ulcay, Y.; Özgüç, H. The effect of a chitosan coating on the adhesive potential and tensile strength of polypropylene meshes. Hernia 2012, 16, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Han, X.; Chen, J.; Pan, Y.; Yang, M.; Lu, L.; Yang, J.; Suo, Z.; Lu, T. Hydrogel–mesh composite for wound closure. Proc. Natl. Acad. Sci. USA 2021, 118, e2103457118. [Google Scholar] [CrossRef]
- Dooley, T.P.; Ellis, A.L.; Belousova, M.; Petersen, D.; Decarlo, A.A. Dense chitosan surgical membranes produced by a coincident compression-dehydration process. J. Biomater. Sci. Polym. Ed. 2012, 24, 621–643. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ma, S.; Chen, Z.; Qiao, F.; Sun, Y.; Yang, X.; Deng, X.; Cen, L.; Cai, Q.; Wu, M.; Zhang, X.; et al. Guided bone regeneration with tripolyphosphate cross-linked asymmetric chitosan membrane. J. Dent. 2014, 42, 1603–1612. [Google Scholar] [CrossRef]
- Cárdenas, G.; Anaya, P.; von Plessing, C.; Rojas, C.; Sepúlveda, J. Chitosan composite films. Biomedical applications. J. Mater. Sci. Mater. Electron. 2007, 19, 2397–2405. [Google Scholar] [CrossRef] [PubMed]
- Ku, Y.; Shim, I.K.; Lee, J.Y.; Park, Y.J.; Rhee, S.-H.; Nam, S.H.; Park, J.B.; Chung, C.P.; Lee, S.J. Chitosan/poly(L-lactic acid) multilayered membrane for guided tissue regeneration. J. Biomed. Mater. Res. Part A 2008, 90, 766–772. [Google Scholar] [CrossRef]
- Wedmore, I.; McManus, J.G.; Pusateri, A.E.; Holcomb, J.B. A Special Report on the Chitosan-based Hemostatic Dressing: Experience in Current Combat Operations. J. Trauma Inj. Infect. Crit. Care 2006, 60, 655–658. [Google Scholar] [CrossRef]
- Devlin, J.J.; Kircher, S.; Kozen, B.G.; Littlejohn, L.F.; Johnson, A.S. Comparison of ChitoFlex®, CELOX™, and QuikClot® in Control of Hemorrhage. J. Emerg. Med. 2009, 41, 237–245. [Google Scholar] [CrossRef]
- Paulo, N.M.; de Brito e Silva, M.S.; Moraes, A.M.; Rodrigues, A.P.; De Menezes, L.B.; Miguel, M.P.; De Lima, F.G.; Faria, A.D.M.; Lima, L.M.L. Use of chitosan membrane associated with polypropylene mesh to prevent peritoneal adhesion in rats. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 91, 221–227. [Google Scholar] [CrossRef]
- Xu, C.; Lei, C.; Meng, L.; Wang, C.; Song, Y. Chitosan as a barrier membrane material in periodontal tissue regeneration. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100, 1435–1443. [Google Scholar] [CrossRef] [PubMed]
- Harikumar, K.; Nandakumar, K. Management of periodontal furcation defects by guided tissue regeneration using collagen—Chitosan as a barrier membrane. Int. J. Oral Health Dent. 2017, 3, 210–213. [Google Scholar] [CrossRef]
- Ma, S.; Adayi, A.; Liu, Z.; Li, M.; Wu, M.; Xiao, L.; Sun, Y.; Cai, Q.; Yang, X.; Zhang, X.; et al. Asymmetric Collagen/chitosan Membrane Containing Minocycline-loaded Chitosan Nanoparticles for Guided Bone Regeneration. Sci. Rep. 2016, 6, 31822. [Google Scholar] [CrossRef] [PubMed]
- Croisier, F.; Jérôme, C. Chitosan-based biomaterials for tissue engineering. Eur. Polym. J. 2013, 49, 780–792. [Google Scholar] [CrossRef]
- Oryan, A.; Sahvieh, S. Effectiveness of chitosan scaffold in skin, bone and cartilage healing. Int. J. Biol. Macromol. 2017, 104 Pt A, 1003–1011. [Google Scholar] [CrossRef]
- Berger, J.; Reist, M.; Mayer, J.; Felt, O.; Gurny, R. Structure and interactions in chitosan hydrogels formed by complexation or aggregation for biomedical applications. Eur. J. Pharm. Biopharm. 2003, 57, 35–52. [Google Scholar] [CrossRef]
- Dambies, L.; Vincent, T.; Domard, A.A.; Guibal, E. Preparation of Chitosan Gel Beads by Ionotropic Molybdate Gelation. Biomacromolecules 2001, 2, 1198–1205. [Google Scholar] [CrossRef]
- Zhao, Y.; Xiao, A.; Wu, P.; Chen, F.; Zhang, Q.; Liang, M.X.; Han, X.; Shi, X.; Li, Y.; Chen, Y. Fabrication of Hydroxypropyl Chitosan/Soy Protein Isolate Hydrogel for Effective Hemorrhage Control. Tissue Eng. Part A 2021, 27, 788–795. [Google Scholar] [CrossRef]
- Muşat, V.; Anghel, E.M.; Zaharia, A.; Atkinson, I.; Mocioiu, O.C.; Buşilă, M.; Alexandru, P. A Chitosan–Agarose Polysaccharide-Based Hydrogel for Biomimetic Remineralization of Dental Enamel. Biomolecules 2021, 11, 1137. [Google Scholar] [CrossRef]
- Cui, Z.-K.; Kim, S.; Baljon, J.J.; Wu, B.M.; Aghaloo, T.; Lee, M. Microporous methacrylated glycol chitosan-montmorillonite nanocomposite hydrogel for bone tissue engineering. Nat. Commun. 2019, 10, 3523. [Google Scholar] [CrossRef]
- Pawar, V.; Dhanka, M.; Srivastava, R. Cefuroxime conjugated chitosan hydrogel for treatment of wound infections. Colloids Surf. B Biointerfaces 2019, 173, 776–787. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.-C.; Lin, W.-J.; Ling, T.-Y.; Young, T.-H. Sustained release of adipose-derived stem cells by thermosensitive chitosan/gelatin hydrogel for therapeutic angiogenesis. Acta Biomater. 2017, 51, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.-H.; Yao, C.-J.; Liao, M.-H.; Lin, P.-I.; Liu, S.-H.; Chen, R.-M. Chitosan nanofiber scaffold improves bone healing via stimulating trabecular bone production due to upregulation of the Runx2/osteocalcin/alkaline phosphatase signaling pathway. Int. J. Nanomed. 2015, 10, 5941–5954. [Google Scholar] [CrossRef]
- Guo, S.; He, L.; Yang, R.; Chen, B.; Xie, X.; Jiang, B.; Weidong, T.; Ding, Y. Enhanced effects of electrospun collagen-chitosan nanofiber membranes on guided bone regeneration. J. Biomater. Sci. Polym. Ed. 2019, 31, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Correa, V.L.R.; Martins, J.A.; de Souza, T.R.; Rincon, G.D.C.N.; Miguel, M.P.; de Menezes, L.B.; Amaral, A.C. Melatonin loaded lecithin-chitosan nanoparticles improved the wound healing in diabetic rats. Int. J. Biol. Macromol. 2020, 162, 1465–1475. [Google Scholar] [CrossRef] [PubMed]
- Gregory, H.; Phillips, J.B. Materials for peripheral nerve repair constructs: Natural proteins or synthetic polymers? Neurochem. Int. 2020, 143, 104953. [Google Scholar] [CrossRef]
- Boecker, A.; Daeschler, S.; Kneser, U.; Harhaus, L. Relevance and Recent Developments of Chitosan in Peripheral Nerve Surgery. Front. Cell. Neurosci. 2019, 13, 104. [Google Scholar] [CrossRef]
- Medovent Reaxon® Nerve Guide. Available online: https://medovent.de/wp-content/uploads/2015/12/REAXON_GB_mail.pdf (accessed on 4 May 2022).
- Bąk, M.; Gutlowska, O.; Wagner, E.; Gosk, J. The role of chitin and chitosan in peripheral nerve reconstruction. Polym. Med. 2017, 47, 43–47. [Google Scholar] [CrossRef]
- Wei, Z.; Hong, F.F.; Cao, Z.; Zhao, S.-Y.; Chen, L. In Situ Fabrication of Nerve Growth Factor Encapsulated Chitosan Nanoparticles in Oxidized Bacterial Nanocellulose for Rat Sciatic Nerve Regeneration. Biomacromolecules 2021, 22, 4988–4999. [Google Scholar] [CrossRef]
- Fornasari, B.E.; Carta, G.; Gambarotta, G.; Raimondo, S. Natural-Based Biomaterials for Peripheral Nerve Injury Repair. Front. Bioeng. Biotechnol. 2020, 8, 554257. [Google Scholar] [CrossRef]
- Dolkhani, S.; Najafpour, A.; Mohammadi, R. Fabrication and transplantation of chitosan-selenium biodegradable nanocomposite conduit on transected sciatic nerve: A novel study in rat model. Neurol. Res. 2020, 42, 439–450. [Google Scholar] [CrossRef] [PubMed]
- Haastert-Talini, K.; Geuna, S.; Dahlin, L.B.; Meyer, C.; Stenberg, L.; Freier, T.; Heimann, C.; Barwig, C.; Pinto, L.F.; Raimondo, S.; et al. Chitosan tubes of varying degrees of acetylation for bridging peripheral nerve defects. Biomaterials 2013, 34, 9886–9904. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, N.K.; Khan, T.R.; Mejias, C.; Paniello, R.C. Nerve transection repair using laser-activated chitosan in a rat model. Laryngoscope 2017, 127, E253–E257. [Google Scholar] [CrossRef] [PubMed]
- Deng, P.; Chen, F.; Zhang, H.; Chen, Y.; Zhou, J. Multifunctional Double-Layer Composite Hydrogel Conduit Based on Chitosan for Peripheral Nerve Repairing. Adv. Healthc. Mater. 2022, e2200115. [Google Scholar] [CrossRef] [PubMed]
- Şahin, M.M.; Cayonu, M.; Dinc, S.K.; Ozkocer, E.; Ilhan, M.; Uzunoğlu, E.; Elmas, C.; Yılmaz, M.; Akkol, E. Effects of chitosan and platelet-rich plasma on facial nerve regeneration in an animal model. Eur. Arch. Oto-Rhino-Laryngol. 2021, 279, 987–994. [Google Scholar] [CrossRef]
- Dietzmeyer, N.; Förthmann, M.; Leonhard, J.; Helmecke, O.; Brandenberger, C.; Freier, T.; Haastert-Talini, K. Two-Chambered Chitosan Nerve Guides with Increased Bendability Support Recovery of Skilled Forelimb Reaching Similar to Autologous Nerve Grafts in the Rat 10 mm Median Nerve Injury and Repair Model. Front. Cell. Neurosci. 2019, 13, 149. [Google Scholar] [CrossRef]
- Rao, F.; Wang, Y.; Zhang, D.; Lu, C.; Cao, Z.; Sui, J.; Wu, M.; Zhang, Y.; Pi, W.; Wang, B.; et al. Aligned chitosan nanofiber hydrogel grafted with peptides mimicking bioactive brain-derived neurotrophic factor and vascular endothelial growth factor repair long-distance sciatic nerve defects in rats. Theranostics 2020, 10, 1590–1603. [Google Scholar] [CrossRef]
- Pop, N.; Nan, A.; Urda-Cimpean, A.; Florea, A.; Toma, V.; Moldovan, R.; Decea, N.; Mitrea, D.; Orasan, R. Chitosan Functionalized Magnetic Nanoparticles to Provide Neural Regeneration and Recovery after Experimental Model Induced Peripheral Nerve Injury. Biomolecules 2021, 11, 676. [Google Scholar] [CrossRef]
- Nawrotek, K.; Mąkiewicz, M.; Zawadzki, D. Fabrication and Characterization of Polycaprolactone/Chitosan—Hydroxyapatite Hybrid Implants for Peripheral Nerve Regeneration. Polymers 2021, 13, 775. [Google Scholar] [CrossRef]
- Mohajer, M.A.; Raisi, A.; Farjanikish, G.; Mohammadi, R. Effect of Local Administration of Verapamil Combined with Chitosan Based Hybrid Nanofiber Conduit on Transected Sciatic Nerve in Rat. Bull. Emerg. Trauma 2019, 7, 28–34. [Google Scholar] [CrossRef]
- Zheng, F.; Li, R.; He, Q.; Koral, K.; Tao, J.; Fan, L.; Xiang, R.; Ma, J.; Wang, N.; Yin, Y.; et al. The electrostimulation and scar inhibition effect of chitosan/oxidized hydroxyethyl cellulose/reduced graphene oxide/asiaticoside liposome based hydrogel on peripheral nerve regeneration In Vitro. Mater. Sci. Eng. C 2019, 109, 110560. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wen, W.; Hu, M.; Bi, W.; Chen, L.; Liu, S.; Chen, P.; Tan, X.; Wallace, M.; Hindle, A.; et al. Chitosan conduits combined with nerve growth factor microspheres repair facial nerve defects. Neural Regen. Res. 2013, 8, 3139–3147. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Tian, C.; Wu, P.; Chen, F.; Xiao, A.; Ye, Q.; Shi, X.; Wang, Z.; Han, X.; Chen, Y. Hydroxypropyl Chitosan/Soy Protein Isolate Conduits Promote Peripheral Nerve Regeneration. Tissue Eng. Part A 2022, 28, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, K.; Liu, Z.; Wang, Z.; Wu, H. Valproic acid-labeled chitosan nanoparticles promote recovery of neuronal injury after spinal cord injury. Aging 2020, 12, 8953–8967. [Google Scholar] [CrossRef]
- Huang, Z.-B.; Chen, T.-N.; Yang, B.; Wang, P.-B.; Mu, N.; Ma, K.; Wang, S.; Yang, C.-Y.; Lai, Y.; Feng, H.; et al. Graphene oxide-composited chitosan scaffold contributes to functional recovery of injured spinal cord in rats. Neural Regen. Res. 2021, 16, 1829–1835. [Google Scholar] [CrossRef]
- Guo, L.; Liang, Z.; Yang, L.; Du, W.; Yu, T.; Tang, H.; Li, C.; Qiu, H. The role of natural polymers in bone tissue engineering. J. Control. Release 2021, 338, 571–582. [Google Scholar] [CrossRef]
- Abinaya, B.; Prasith, T.P.; Ashwin, B.; Chandran, S.V.; Selvamurugan, N. Chitosan in Surface Modification for Bone Tissue Engineering Applications. Biotechnol. J. 2019, 14, e1900171. [Google Scholar] [CrossRef]
- Venkatesan, J.; Kim, S.-K. Chitosan Composites for Bone Tissue Engineering—An Overview. Mar. Drugs 2010, 8, 2252–2266. [Google Scholar] [CrossRef]
- LogithKumar, R.; KeshavNarayan, A.; Dhivya, S.; Chawla, A.; Saravanan, S.; Selvamurugan, N. A review of chitosan and its derivatives in bone tissue engineering. Carbohydr. Polym. 2016, 151, 172–188. [Google Scholar] [CrossRef]
- Sukpaita, T.; Chirachanchai, S.; Pimkhaokham, A.; Ampornaramveth, R.S. Chitosan-Based Scaffold for Mineralized Tissues Regeneration. Mar. Drugs 2021, 19, 551. [Google Scholar] [CrossRef]
- Brun, P.; Zamuner, A.; Battocchio, C.; Cassari, L.; Todesco, M.; Graziani, V.; Iucci, G.; Marsotto, M.; Tortora, L.; Secchi, V.; et al. Bio-Functionalized Chitosan for Bone Tissue Engineering. Int. J. Mol. Sci. 2021, 22, 5916. [Google Scholar] [CrossRef] [PubMed]
- Soriente, A.; Fasolino, I.; Gomez-Sánchez, A.; Prokhorov, E.; Buonocore, G.G.; Luna-Barcenas, G.; Ambrosio, L.; Raucci, M.G. Chitosan/hydroxyapatite nanocomposite scaffolds to modulate osteogenic and inflammatory response. J. Biomed. Mater. Res. Part A 2021, 110, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Niu, L.; Li, J.; Du, J.; Wei, Y.; Hu, Y.; Lian, X.; Chen, W.; Wang, K. Reinforced chitosan membranes by microspheres for guided bone regeneration. J. Mech. Behav. Biomed. Mater. 2018, 81, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Kazemi-Aghdam, F.; Jahed, V.; Dehghan-Niri, M.; Ganji, F.; Vasheghani-Farahani, E. Injectable chitosan hydrogel embedding modified Halloysite nanotubes for bone tissue engineering. Carbohydr. Polym. 2021, 269, 118311. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Pan, Y.; Liu, Y.; Dai, K.; Zhang, Q.; Wang, J. Effect of sulfated chitosan hydrogel on vascularization and osteogenesis. Carbohydr. Polym. 2022, 281, 119059. [Google Scholar] [CrossRef]
- Sukul, M.; Sahariah, P.; Lauzon, H.L.; Borges, J.; Másson, M.; Mano, J.F.; Haugen, H.J.; Reseland, J.E. In Vitro biological response of human osteoblasts in 3D chitosan sponges with controlled degree of deacetylation and molecular weight. Carbohydr. Polym. 2020, 254, 117434. [Google Scholar] [CrossRef]
- Murali, V.P.; Guerra, F.D.; Ghadri, N.; Christian, J.M.; Stein, S.H.; Jennings, J.A.; Smith, R.A.; Bumgardner, J.D. Simvastatin loaded chitosan guided bone regeneration membranes stimulate bone healing. J. Periodontal Res. 2021, 56, 877–884. [Google Scholar] [CrossRef]
- Liu, G.; Sun, J.; Gong, M.; Xing, F.; Wu, S.; Xiang, Z. Urine-derived stem cells loaded onto a chitosan-optimized biphasic calcium-phosphate scaffold for repairing large segmental bone defects in rabbits. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 2014–2029. [Google Scholar] [CrossRef]
- Nandi, S.K.; Kundu, B.; Basu, D. Protein growth factors loaded highly porous chitosan scaffold: A comparison of bone healing properties. Mater. Sci. Eng. C 2013, 33, 1267–1275. [Google Scholar] [CrossRef]
- Xu, W.; Tan, W.; Li, C.; Wu, K.; Zeng, X.; Xiao, L. Metformin-loaded β-TCP/CTS/SBA-15 composite scaffolds promote alveolar bone regeneration in a rat model of periodontitis. J. Mater. Sci. Mater. Med. 2021, 32, 1037–1042. [Google Scholar] [CrossRef]
- Niu, X.; Wang, L.; Xu, M.; Qin, M.; Zhao, L.; Wei, Y.; Hu, Y.; Lian, X.; Liang, Z.; Chen, S.; et al. Electrospun polyamide-6/chitosan nanofibers reinforced nano-hydroxyapatite/polyamide-6 composite bilayered membranes for guided bone regeneration. Carbohydr. Polym. 2021, 260, 117769. [Google Scholar] [CrossRef] [PubMed]
- Tamburaci, S.; Tihminlioglu, F. Development of Si doped nano hydroxyapatite reinforced bilayer chitosan nanocomposite barrier membranes for guided bone regeneration. Mater. Sci. Eng. C 2021, 128, 112298. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Ge, K.; Xu, Y.; Wang, Y.; Lu, M.; Wei, Y.; Zhu, Q.; Han, X.; Huang, Q.; Cao, Z. Controlled release of minocycline in hydroxyapatite/chitosan composite for periodontal bone defect repair. Dent. Mater. J. 2022, 41, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Varoni, E.; Vijayakumar, S.; Canciani, E.; Cochis, A.; De Nardo, L.; Lodi, G.; Rimondini, L.; Cerruti, M. Chitosan-Based Trilayer Scaffold for Multitissue Periodontal Regeneration. J. Dent. Res. 2017, 97, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.-Z.; Beucken, J.J.V.D.; Cai, X.; Yu, N.; Jansen, J.A.; Yang, F. Periodontal Tissue Regeneration Using Enzymatically Solidified Chitosan Hydrogels With or Without Cell Loading. Tissue Eng. Part A 2015, 21, 1066–1076. [Google Scholar] [CrossRef]
- Barbu, H.M.; Andreescu, C.F.; Comaneanu, M.R.; Referendaru, D.; Mijiritsky, E. Maxillary Sinus Floor Augmentation to Enable One-Stage Implant Placement by Using Bovine Bone Substitute and Platelet-Rich Fibrin. BioMed Res. Int. 2018, 2018, 6562958. [Google Scholar] [CrossRef]
- Li, D.; Zhao, L.; Cong, M.; Liu, L.; Yan, G.; Li, Z.; Li, B.; Yu, W.; Sun, H.; Yang, B. Injectable thermosensitive chitosan/gelatin-based hydrogel carried erythropoietin to effectively enhance maxillary sinus floor augmentation In Vivo. Dent. Mater. 2020, 36, e229–e240. [Google Scholar] [CrossRef]
- Schwarz, F.; Derks, J.; Monje, A.; Wang, H.-L. Peri-implantitis. J. Clin. Periodontol. 2018, 45, S246–S266. [Google Scholar] [CrossRef]
- Hussain, B.; Karaca, E.O.; Kuru, B.E.; Gursoy, H.; Haugen, H.J.; Wohlfahrt, J.C. Treatment of residual pockets using an oscillating chitosan device versus regular curettes alone—A randomized, feasibility parallel-arm clinical trial. J. Periodontol. 2021, 93, 780–789. [Google Scholar] [CrossRef]
- Kotsakis, G.A.; Olmedo, D.G. Peri-implantitis is not periodontitis: Scientific discoveries shed light on microbiome-biomaterial interactions that may determine disease phenotype. Periodontology 2000 2021, 86, 231–240. [Google Scholar] [CrossRef]
- Koldsland, O.C.; Aass, A.M. Supportive treatment following peri-implantitis surgery; an RCT using titanium curettes or chitosan brushes. J. Clin. Periodontol. 2020, 47, 1259–1267. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zhang, Q.; Wang, Y. Preparation and characterization of antibacterial and anti-inflammatory hyaluronic acid-chitosan-dexamethasone hydrogels for peri-implantitis repair. J. Biomater. Appl. 2021, 36, 1141–1150. [Google Scholar] [CrossRef] [PubMed]
- Min, Q.; Tian, D.; Zhang, Y.; Wang, C.; Wan, Y.; Wu, J. Strong and Elastic Chitosan/Silk Fibroin Hydrogels Incorporated with Growth-Factor-Loaded Microspheres for Cartilage Tissue Engineering. Biomimetics 2022, 7, 41. [Google Scholar] [CrossRef] [PubMed]
- Abarrategi, A.; Lópiz-Morales, Y.; Ramos, V.; Civantos, A.; López-Durán, L.; Marco, F.; López-Lacomba, J.L. Chitosan scaffolds for osteochondral tissue regeneration. J. Biomed. Mater. Res. Part A 2010, 95, 1132–1141. [Google Scholar] [CrossRef] [PubMed]
- Comblain, F.; Rocasalbas, G.; Gauthier, S.; Henrotin, Y. Chitosan: A promising polymer for cartilage repair and viscosupplementation. Bio.-Med. Mater. Eng. 2017, 28, S209–S215. [Google Scholar] [CrossRef]
- Santin, M. 14—Bone tissue engineering. In Bone Repair Biomaterials, 1st ed.; Planell, J.A., Best, S.M., Lacroix, D., Merolli, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2009; pp. 378–422. [Google Scholar] [CrossRef]
- Akmeşe, R.; Ertan, M.B.; Kocaoğlu, H. Comparison of Chitosan-Based Liquid Scaffold and Hyaluronic Acid–Based Soft Scaffold for Treatment of Talus Osteochondral Lesions. Foot Ankle Int. 2020, 41, 1240–1248. [Google Scholar] [CrossRef]
- Hoemann, C.D.; Hurtig, M.; Rossomacha, E.; Sun, J.; Chevrier, A.; Shive, M.S.; Buschmann, M.D. Chitosan-Glycerol Phosphate/Blood Implants Improve Hyaline Cartilage Repair in Ovine Microfracture Defects. J. Bone Jt. Surg. 2005, 87, 2671–2686. [Google Scholar] [CrossRef]
- Zheng, D.; Chen, T.; Han, L.; Lv, S.; Yin, J.; Yang, K.; Wang, Y.; Xu, N. Synergetic integrations of bone marrow stem cells and transforming growth factor-β1 loaded chitosan nanoparticles blended silk fibroin injectable hydrogel to enhance repair and regeneration potential in articular cartilage tissue. Int. Wound J. 2022, 1–16. [Google Scholar] [CrossRef]
- Zhang, Z.; Lin, S.; Yan, Y.; You, X.; Ye, H. Enhanced efficacy of transforming growth factor-β1 loaded an injectable cross-linked thiolated chitosan and carboxymethyl cellulose-based hydrogels for cartilage tissue engineering. J. Biomater. Sci. Polym. Ed. 2021, 32, 2402–2422. [Google Scholar] [CrossRef]
- Li, P.; Fu, L.; Liao, Z.; Peng, Y.; Ning, C.; Gao, C.; Zhang, D.; Sui, X.; Lin, Y.; Liu, S.; et al. Chitosan hydrogel/3D-printed poly(ε-caprolactone) hybrid scaffold containing synovial mesenchymal stem cells for cartilage regeneration based on tetrahedral framework nucleic acid recruitment. Biomaterials 2021, 278, 121131. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, Y.; Yan, S.; Gong, L.; Wang, J.; Chen, X.; Cui, L.; Yin, J. Repair of an articular cartilage defect using adipose-derived stem cells loaded on a polyelectrolyte complex scaffold based on poly(l-glutamic acid) and chitosan. Acta Biomater. 2013, 9, 7276–7288. [Google Scholar] [CrossRef] [PubMed]
- Scognamiglio, F.; Travan, A.; Donati, I.; Borgogna, M.; Marsich, E. A hydrogel system based on a lactose-modified chitosan for viscosupplementation in osteoarthritis. Carbohydr. Polym. 2020, 248, 116787. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Jiang, J.; Zhao, J.; Chen, S.; Yan, X. Regulating Preparation of Functional Alginate-Chitosan Three-Dimensional Scaffold for Skin Tissue Engineering. Int. J. Nanomed. 2019, 14, 8891–8903. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Chhabra, P.; Tyagi, P.; Bhatnagar, A.; Mittal, G. Optimization, characterization, and efficacy evaluation of 2% chitosan scaffold for tissue engineering and wound healing. J. Pharm. Bioallied Sci. 2016, 8, 300–308. [Google Scholar] [CrossRef]
- Raftery, R.; O’Brien, F.J.; Cryan, S.-A. Chitosan for Gene Delivery and Orthopedic Tissue Engineering Applications. Molecules 2013, 18, 5611–5647. [Google Scholar] [CrossRef]
- Ryu, J.H.; Choi, J.S.; Park, E.; Eom, M.R.; Jo, S.; Lee, M.S.; Kwon, S.K.; Lee, H. Chitosan oral patches inspired by mussel adhesion. J. Control. Release 2019, 317, 57–66. [Google Scholar] [CrossRef]
- Yu, N.; Li, Y.; Wang, Y.; Xu, H.; Ye, F.; Fu, Q. Healing effect of carboxymethyl chitosan-plantamajoside hydrogel on burn wound skin. Burns 2022, in press. [Google Scholar] [CrossRef]
- Shaheen, T.I.; Abdelhameed, M.F.; Zaghloul, S.; Montaser, A. In Vivo assessment of the durable, green and in situ bio-functional cotton fabrics based carboxymethyl chitosan nanohybrid for wound healing application. Int. J. Biol. Macromol. 2022, 209, 485–497. [Google Scholar] [CrossRef]
- Fathalla, Z.; Mustafa, W.W.; Abdelkader, H.; Moharram, H.; Sabry, A.M.; Alany, R.G. Hybrid thermosensitive-mucoadhesive in situ forming gels for enhanced corneal wound healing effect of L-carnosine. Drug Deliv. 2022, 29, 374–385. [Google Scholar] [CrossRef]
- Kim, J.H.; Bae, J.-H.; Lim, K.T.; Choung, P.-H.; Park, J.-S.; Choi, S.J.; Im, A.L.; Lee, E.T.; Choung, Y.-H.; Chung, J.H. Development of water-insoluble chitosan patch scaffold to repair traumatic tympanic membrane perforations. J. Biomed. Mater. Res. Part A 2008, 90, 446–455. [Google Scholar] [CrossRef]
- Kim, J.H.; Choi, S.J.; Park, J.-S.; Lim, K.T.; Choung, P.-H.; Kim, S.W.; Bin Lee, J.; Chung, J.H.; Choung, Y.-H. Tympanic Membrane Regeneration Using a Water-Soluble Chitosan Patch. Tissue Eng. Part A 2010, 16, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Prabha, S.; Sowndarya, J.; Ram, P.J.V.S.; Rubini, D.; Hari, B.N.V.; Aruni, W.; Nithyanand, P. Chitosan-Coated Surgical Sutures Prevent Adherence and Biofilms of Mixed Microbial Communities. Curr. Microbiol. 2021, 78, 502–512. [Google Scholar] [CrossRef] [PubMed]
- Madrazo-Jimenez, M.; Rodriguez-Caballero, A.; Figallo, M.A.S.; Garrido-Serrano, R.; Corrales, A.G.; Pérez, J.L.G.; Torres-Lagares, D. The effects of a topical gel containing chitosan, 0.2% chlorhexidine, allantoin and despanthenol on the wound healing process subsequent to impacted lower third molar extraction. Med. Oral Patol. Oral Y Cir. Buccal 2016, 21, e696–e702. [Google Scholar] [CrossRef][Green Version]
- Hasibuan, P.A.Z.; Tanjung, M.; Gea, S.; Pasaribu, K.M.; Harahap, M.; Perangin-Angin, Y.A.; Prayoga, A.; Ginting, J.G. Antimicrobial and antihemolytic properties of a CNF/AgNP-chitosan film: A potential wound dressing material. Heliyon 2021, 7, e08197. [Google Scholar] [CrossRef]
- Ruprai, H.; Shanu, A.; Mawad, D.; Hook, J.M.; Kilian, K.; George, L.; Wuhrer, R.; Houang, J.; Myers, S.; Lauto, A. Porous chitosan adhesives with L-DOPA for enhanced photochemical tissue bonding. Acta Biomater. 2019, 101, 314–326. [Google Scholar] [CrossRef]
- Dong, Q.; Wu, D.; Li, M.; Dong, W. Polysaccharides, as biological macromolecule-based scaffolding biomaterials in cornea tissue engineering: A review. Tissue Cell 2022, 76, 101782. [Google Scholar] [CrossRef]
- Tang, Q.; Lu, B.; He, J.; Chen, X.; Fu, Q.; Han, H.; Luo, C.; Yin, H.; Qin, Z.; Lyu, D.; et al. Exosomes-loaded thermosensitive hydrogels for corneal epithelium and stroma regeneration. Biomaterials 2021, 280, 121320. [Google Scholar] [CrossRef]
- Tayebi, T.; Baradaran-Rafii, A.; Hajifathali, A.; Rahimpour, A.; Zali, H.; Shaabani, A.; Niknejad, H. Biofabrication of chitosan/chitosan nanoparticles/polycaprolactone transparent membrane for corneal endothelial tissue engineering. Sci. Rep. 2021, 11, 7060. [Google Scholar] [CrossRef]
- Feng, L.; Liu, R.; Zhang, X.; Li, J.; Zhu, L.; Li, Z.; Li, W.; Zhang, A. Thermo-Gelling Dendronized Chitosans as Biomimetic Scaffolds for Corneal Tissue Engineering. ACS Appl. Mater. Interfaces 2021, 13, 49369–49379. [Google Scholar] [CrossRef]
- Huang, Z.; Shi, Y.; Wang, H.; Chun, C.; Chen, L.; Wang, K.; Lu, Z.; Zhao, Y.; Li, X. Protective Effects of Chitosan-Bilirubin Nanoparticles Against Ethanol-Induced Gastric Ulcers. Int. J. Nanomed. 2021, 16, 8235–8250. [Google Scholar] [CrossRef]
- Niaz, T.; Ihsan, A.; Abbasi, R.; Shabbir, S.; Noor, T.; Imran, M. Chitosan-albumin based core shell-corona nano-antimicrobials to eradicate resistant gastric pathogen. Int. J. Biol. Macromol. 2019, 138, 1006–1018. [Google Scholar] [CrossRef] [PubMed]
- Maeng, J.H.; Bang, B.W.; Lee, E.; Kim, J.; Gil Kim, H.; Lee, D.H.; Yang, S.-G. Endoscopic application of EGF-chitosan hydrogel for precipitated healing of GI peptic ulcers and mucosectomy-induced ulcers. J. Mater. Sci. Mater. Med. 2013, 25, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.Y.; Shen, Y.; Wang, J.T.; Eikelboom, R.H.; Dilley, R.J. Animal models of chronic tympanic membrane perforation: In response to plasminogen initiates and potentiates the healing of acute and chronic tympanic membrane perforations in mice. Clin. Transl. Med. 2014, 3, 5. [Google Scholar] [CrossRef] [PubMed]
- Seonwoo, H.; Kim, S.W.; Kim, J.; Chunjie, T.; Lim, K.T.; Kim, Y.J.; Pandey, S.; Choung, P.-H.; Choung, Y.-H.; Chung, J.H. Regeneration of Chronic Tympanic Membrane Perforation Using an EGF-Releasing Chitosan Patch. Tissue Eng. Part A 2013, 19, 2097–2107. [Google Scholar] [CrossRef] [PubMed]
- Shehata, A.; Mohamed, S. Chitosan patch scaffold for repair of chronic safe tympanic membrane perforation. Egypt. J. Otolaryngol. 2014, 30, 311–316. [Google Scholar] [CrossRef]
- Kim, J.; Kim, S.W.; Choi, S.J.; Lim, K.T.; Bin Lee, J.; Seonwoo, H.; Choung, P.-H.; Park, K.; Cho, C.-S.; Choung, Y.-H.; et al. A Healing Method of Tympanic Membrane Perforations Using Three-Dimensional Porous Chitosan Scaffolds. Tissue Eng. Part A 2011, 17, 2763–2772. [Google Scholar] [CrossRef]
- da Silva, M.C.; da Silva, H.N.; Cruz, R.D.C.A.L.; Amoah, S.K.S.; Silva, S.M.D.L.; Fook, M.V.L. N-Acetyl-D-Glucosamine-Loaded Chitosan Filaments Biodegradable and Biocompatible for Use as Absorbable Surgical Suture Materials. Materials 2019, 12, 1807. [Google Scholar] [CrossRef]
- Debbabi, F.; Gargoubi, S.; Ayed, M.A.H.; Ben Abdessalem, S. Development and characterization of antibacterial braided polyamide suture coated with chitosan-citric acid biopolymer. J. Biomater. Appl. 2017, 32, 384–398. [Google Scholar] [CrossRef]
- Mohammadi, H.; Alihosseini, F.; Hosseini, S.A. Improving physical and biological properties of nylon monofilament as suture by Chitosan/Hyaluronic acid. Int. J. Biol. Macromol. 2020, 164, 3394–3402. [Google Scholar] [CrossRef]
- Hrachovinová, I. Diagnostic strategies in disorders of hemostasis. Vnitr. Lek. 2018, 64, 537–544. [Google Scholar] [CrossRef]
- Müller, J.; Pekrul, I.; Pötzsch, B.; Berning, B.; Oldenburg, J.; Spannagl, M. Laboratory Monitoring in Emicizumab-Treated Persons with Hemophilia A. Thromb. Haemost. 2019, 119, 1384–1393. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Lu, S.; Cheng, Y.; Kong, S.; Li, S.; Li, C.; Yang, L. Investigation of the Effects of Molecular Parameters on the Hemostatic Properties of Chitosan. Molecules 2018, 23, 3147. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Lu, S.; Hu, Z.; Zhang, B.; Li, S.; Hong, P. Marine collagen peptide grafted carboxymethyl chitosan: Optimization preparation and coagulation evaluation. Int. J. Biol. Macromol. 2020, 164, 3953–3964. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-W.; Liu, C.-C.; Cherng, J.-H.; Lin, C.-S.; Chang, S.-J.; Hong, Z.-J.; Chiu, Y.-K.; Hsu, S.-D. Biological Effects of Chitosan-Based Dressing on Hemostasis Mechanism. Polymers 2019, 11, 1906. [Google Scholar] [CrossRef]
- Wang, C.-H.; Cherng, J.-H.; Liu, C.-C.; Fang, T.-J.; Hong, Z.-J.; Chang, S.-J.; Fan, G.-Y.; Hsu, S.-D. Procoagulant and Antimicrobial Effects of Chitosan in Wound Healing. Int. J. Mol. Sci. 2021, 22, 7067. [Google Scholar] [CrossRef] [PubMed]
- Seethamsetty, S.; Sarepally, G.; Sanober, A.; Qureshi, Y.; Fatima, U.; Arif, S.M. A comparative evaluation of the effectiveness of chitosan-based dressing and conventional method of hemostasis in patients on oral antithrombotic therapy without therapy interruption. J. Pharm. Bioallied Sci. 2019, 11 (Suppl. S1), S18–S23. [Google Scholar] [CrossRef]
- Rodriguez-Merchan, E.C. Local fibrin glue and chitosan-based dressings in haemophilia surgery. Blood Coagul. Fibrinolysis 2012, 23, 473–476. [Google Scholar] [CrossRef]
- Logun, M.T.; Dowling, M.B.; Raghavan, S.R.; Wallace, M.L.; Schmiedt, C.; Stice, S.; Karumbaiah, L. Expanding Hydrophobically Modified Chitosan Foam for Internal Surgical Hemostasis: Safety Evaluation in a Murine Model. J. Surg. Res. 2019, 239, 269–277. [Google Scholar] [CrossRef]
- Chong, G.O.; Lee, Y.H.; Jeon, S.Y.; Yang, H.-Y.; An, S.-H. Efficacy of a chitosan tampon in the loop electrosurgical excision procedure: A prospective randomized controlled study. Sci. Rep. 2020, 10, 6017. [Google Scholar] [CrossRef]
- Valentine, R.; Athanasiadis, T.; Moratti, S.; Robinson, S.; Wormald, P.-J. The Efficacy of a Novel Chitosan Gel on Hemostasis after Endoscopic Sinus Surgery in a Sheep Model of Chronic Rhinosinusitis. Am. J. Rhinol. Allergy 2009, 23, 71–75. [Google Scholar] [CrossRef]
- Valentine, R.; Athanasiadis, T.; Moratti, S.; Hanton, L.; Robinson, S.; Wormald, P.-J. The Efficacy of a Novel Chitosan Gel on Hemostasis and Wound Healing after Endoscopic Sinus Surgery. Am. J. Rhinol. Allergy 2010, 24, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.-J.; An, S.-Y.; Yeon, J.-Y.; Shim, W.S.; Mo, J.-H. Effect of a Chitosan Gel on Hemostasis and Prevention of Adhesion After Endoscopic Sinus Surgery. Clin. Exp. Otorhinolaryngol. 2016, 9, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Yang, L.; Hong, L.; Hu, Q. A chitosan hydrogel sealant with self-contractile characteristic: From rapid and long-term hemorrhage control to wound closure and repair. Carbohydr. Polym. 2021, 271, 118428. [Google Scholar] [CrossRef] [PubMed]
- Bartley, J. Should chitosan and tranexamic acid be combined for improved hemostasis after sinus surgery? Med. Hypotheses 2013, 81, 1036–1038. [Google Scholar] [CrossRef] [PubMed]
- Misgav, M.; Lubetszki, A.; Brutman-Barazani, T.; Martinowitz, U.; Kenet, G. The hemostatic efficacy of chitosan-pads in hemodialysis patients with significant bleeding tendency. J. Vasc. Access 2017, 18, 220–224. [Google Scholar] [CrossRef]
- Pathan, A.Z.; Aijaz, S.; Sheikh, S.; Sattar, S. Randomized trial comparing radial hemostasis techniques; catechol conjugated chitosan pad (InnoSEAL) versus pneumatic compression band. Catheter. Cardiovasc. Interv. 2021, 98, E181–E187. [Google Scholar] [CrossRef]
- Roberts, J.S.; Niu, J.; Pastor-Cervantes, J.A. Comparison of Hemostasis Times with a Chitosan-Based Hemostatic Pad (Clo-SurPlus Radial™) vs Mechanical Compression (TR Band®) Following Transradial Access: A pilot Study. Cardiovasc. Revascularization Med. 2018, 20, 871–874. [Google Scholar] [CrossRef]
- D’Almeida, M.; Attik, N.; Amalric, J.; Brunon, C.; Renaud, F.; Abouelleil, H.; Toury, B.; Grosgogeat, B. Chitosan coating as an antibacterial surface for biomedical applications. PLoS ONE 2017, 12, e0189537. [Google Scholar] [CrossRef]
- Keirouz, A.; Radacsi, N.; Ren, Q.; Dommann, A.; Beldi, G.; Maniura-Weber, K.; Rossi, R.M.; Fortunato, G. Nylon-6/chitosan core/shell antimicrobial nanofibers for the prevention of mesh-associated surgical site infection. J. Nanobiotechnol. 2020, 18, 51. [Google Scholar] [CrossRef]
- Pawar, V.; Bulbake, U.; Khan, W.; Srivastava, R. Chitosan sponges as a sustained release carrier system for the prophylaxis of orthopedic implant-associated infections. Int. J. Biol. Macromol. 2019, 134, 100–112. [Google Scholar] [CrossRef]
- Lauto, A.; Hook, J.; Doran, M.; Camacho, F.; Poole-Warren, L.; Avolio, A.; Foster, L. Chitosan adhesive for laser tissue repair: In Vitro characterization. Lasers Surg. Med. 2005, 36, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Foster, L.J.R.; Karsten, E. A Chitosan Based, Laser Activated Thin Film Surgical Adhesive, “SurgiLux”: Preparation and Demonstration. J. Vis. Exp. 2012, 68, e3527. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, J.G.; Seetharam, S.; Ding, C.; Feliz, J.; Doherty, E.; Ingber, D.E. Direct Bonding of Chitosan Biomaterials to Tissues Using Transglutaminase for Surgical Repair or Device Implantation. Tissue Eng. Part A 2017, 23, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, B.; Soman, D.; Payanam, U.; Laurent, A.; Labarre, D.; Jayakrishnan, A. A novel injectable tissue adhesive based on oxidized dextran and chitosan. Acta Biomater. 2017, 53, 343–354. [Google Scholar] [CrossRef]
- Zhu, L.; Peng, L.; Zhang, Y.-Q. The Processing of Chitosan and Its Derivatives and Their Application for Postoperative Anti-Adhesion. Mini-Rev. Med. Chem. 2015, 15, 330–337. [Google Scholar] [CrossRef]
- Aussel, A.; Montembault, A.; Malaise, S.; Foulc, M.P.; Faure, W.; Cornet, S.; Aid, R.; Chaouat, M.; Delair, T.; Letourneur, D.; et al. In Vitro Mechanical Property Evaluation of Chitosan-Based Hydrogels Intended for Vascular Graft Development. J. Cardiovasc. Transl. Res. 2017, 10, 480–488. [Google Scholar] [CrossRef]
- Aussel, A.; Thébaud, N.B.; Bérard, X.; Brizzi, V.; Delmond, S.; Bareille, R.; Siadous, R.; James, C.; Ripoche, J.; Durand, M.; et al. Chitosan-based hydrogels for developing a small-diameter vascular graft: In Vitro and in vivo evaluation. Biomed. Mater. 2017, 12, 065003. [Google Scholar] [CrossRef]
- Kang, J.; Oh, S.-J.; Lee, H.M.; Jeong, K.-J.; Shin, N.; Lee, I.-W.; Lee, J. In Vivo Study of Mastoid Obliteration Using Hydroxyapatite-Chitosan Patch. J. Biomed. Nanotechnol. 2017, 13, 1715–1724. [Google Scholar] [CrossRef]
- Zong, W.; Chen, J.; Han, W.; Chen, J.; Wang, Y.; Wang, W.; Cheng, G.; Ou, L.; Yu, Y. Preparation of chitosan/amino multiwalled carbon nanotubes nanocomposite beads for bilirubin adsorption in hemoperfusion. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 106, 96–103. [Google Scholar] [CrossRef]
- Ghosh, P.; Das, M.; Rameshbabu, A.P.; Das, D.; Datta, S.; Pal, S.; Panda, A.B.; Dhara, S. Chitosan Derivatives Cross-Linked with Iodinated 2,5-Dimethoxy-2,5-dihydrofuran for Non-Invasive Imaging. ACS Appl. Mater. Interfaces 2014, 6, 17926–17936. [Google Scholar] [CrossRef]






| Chitosan Form/System | Treated Nerve | Main Results | Reference |
|---|---|---|---|
| Chitosan–selenium biodegradable nanocomposite conduit | Sciatic | Number and diameter of myelinated fibers significantly higher against chitosan | [83] |
| Chitosan tubes with different degrees of acetylation | Sciatic | Intermediate degree of acetylation as the best choice in terms of degradation and regeneration efficacy | [84] |
| Laser-activated chitosan | Posterior tibial | Good functional recovery and tensile strength with laser-activated chitosan | [85] |
| Double-layer composite hydrogel conduit based on chitosan | Sciatic | Significant regeneration against chitosan hollow conduit and repair ability comparable to autologous transplantation when loaded with 7,8-dihydroxyflavone | [86] |
| Chitosan gel absorbed into Spongostan® | Facial | Positive effect of chitosan gel in nerve healing and better results when combined with platelet-rich plasma | [87] |
| Corrugated chitosan-film-enhanced chitosan nerve guides | Median | Accelerated functional recovery and thicker myelin sheats against other nerve guides | [88] |
| Aligned chitosan nanofiber hydrogel grafted with peptides as conduit filler | Sciatic | Enhanced nerve regeneration, secretion of neurotrophic factors, vascular penetration, and functional recovery than other conduits | [89] |
| Chitosan functionalized magnetic nanoparticles | Sciatic | Nerve outreach without surgical intervention and better functional outcome versus without treatment | [90] |
| Polycaprolactone/chitosan–hydroxyapatite hybrid implants | Peripheral | Possibility of controlling the diffusion of oxygen and nutrients and invariable mechanical properties for up to 28 days | [91] |
| Chitosan Form/System | Purpose | Main Results | Reference |
|---|---|---|---|
| Chatechol-conjugated chitosan patch | Oral mucositis | Mucoadhesive patches with enhanced healing properties through sustained release of triamcinolone acetonide. | [139] |
| Carboxymethyl chitosan/alginate-plantamajoside hydrogel | Burn wound skin | Reduces inflammation, increases collagen deposition, promotes cell migration and proliferation, and accelerates skin scald repair. | [140] |
| Cotton fabrics coated with carboxymethyl chitosan | Damaged skin | Antibacterial properties against S. aureus and E. coli and accelerated reepithelization. | [141] |
| Poloxamer 407/methylcellulose chitosan thermosensitive gel | Cornea damage | Good spreading ability, mucoadhesion, and ocular biocompatibility; accelerated corneal healing. | [142] |
| Chitosan patches | Tympanic perforation | More effective than spontaneous healing in tympanic regeneration. | [143,144] |
| Chitosan-coated filaments | Surgical suture | Significant reduction of biofilm formation. | [145] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Notario-Pérez, F.; Martín-Illana, A.; Cazorla-Luna, R.; Ruiz-Caro, R.; Veiga, M.D. Applications of Chitosan in Surgical and Post-Surgical Materials. Mar. Drugs 2022, 20, 396. https://doi.org/10.3390/md20060396
Notario-Pérez F, Martín-Illana A, Cazorla-Luna R, Ruiz-Caro R, Veiga MD. Applications of Chitosan in Surgical and Post-Surgical Materials. Marine Drugs. 2022; 20(6):396. https://doi.org/10.3390/md20060396
Chicago/Turabian StyleNotario-Pérez, Fernando, Araceli Martín-Illana, Raúl Cazorla-Luna, Roberto Ruiz-Caro, and María Dolores Veiga. 2022. "Applications of Chitosan in Surgical and Post-Surgical Materials" Marine Drugs 20, no. 6: 396. https://doi.org/10.3390/md20060396
APA StyleNotario-Pérez, F., Martín-Illana, A., Cazorla-Luna, R., Ruiz-Caro, R., & Veiga, M. D. (2022). Applications of Chitosan in Surgical and Post-Surgical Materials. Marine Drugs, 20(6), 396. https://doi.org/10.3390/md20060396










