Antitumor Potential of Immunomodulatory Natural Products
Abstract
1. Introduction
2. Terrestrial Environment
2.1. Plant Compounds
2.1.1. Terpenes
2.1.2. Phenolic Compounds
2.2. Therapeutic Antitumor Activity and Natural Compounds from Spices
2.3. Macromycetes
2.4. Other Sources
| Molecule | Type of Compound | Source | Tumor | Immuno System’s Role | References |
|---|---|---|---|---|---|
| Andrographolide (1) | Terpene | Andrographis paniculata | Human epidermal carcinoma (KB, ED50 1.5 µg/mL); lymphocytic leukemia (P388, ED50 1.0 µg/mL) | Stimulate antigen specific and non-specific immune responses in mice | [29] |
| Triptolide (5) | Terpene | Chinese Tripterygium Hook F (TWHF) | Solid tumor cells | Apoptosis induced by TNFα, inhibition of NF-kB | [44] |
| Zerumbone (4) | Terpene | Zingiberaceae | Human cancer cell lines of the ovary (Coav-3) breast (MCF-7) promyelocytic leukemia (HL-60) and colon adenocarcinoma HCT116 | Immunosuppressive effects via inhibition of AP-1 and NF-kB | [36,37,38,39] |
| β-Carotene (6); Lutein (7) | Carotenoids | Plant | Lung human cancer, mammary tumor bearing mice model | Stimulate NK cell activities, increase the number of leukocyte immune cells, CD4/CD8 ratio, and surface expression of MHC I molecules Stimulation effect on IFN-γ mRNA expression; suppression of IL-10 in splenocytes | [47,48,49] |
| β-Sitosterol (8) | Terpene | Plant | Human cancer cell line of the colon (HT-29) and prostate (LNCaP) | stimulated blood lymphocyte proliferation in vitro; enhanced lytic and cytotoxic activities of NK cells | [53,161] |
| Wogonin (9), Baicalein (10), Baicalin (11) | Flavones | Scutellaria baicalenis | Breast, prostate, and lung human cancer | Activation of NF-kB factor; cell cycle regulation | [60] |
| Geraniin (12), | Phenolic compounds | Phyllanthus emblica Linnaeus | Human cancer cell line of the breast (MCF-7) and embryonic fibroblast (HELF) | Promoted the level of serum IL-18 and NK cell cytotoxicity, suggesting stimulation of macrophages, thereby upregulating the NK cell-mediated antitumor immune response | [79] |
| Kaempferol 3-β−d-glucopyranoside (13) | |||||
| Kaempferol (14) | |||||
| Quercetin 3-β-d-glucopyranoside (15) | |||||
| Quercetin (16) | |||||
| Isocorilagin (17) | |||||
| Ruitin (18) | Phenolic compounds | Diospyros kaki L. | Leukemia, colorectal, neuroblastoma, melanotic melanoma and prostate human cancer. | Increase of IL-18; Upregulation of NK cells | [83,84,85] |
| Myricetin (19) | |||||
| Epigallocatechin-3-gallate (20) | Phenolic compound | Camelia sinesis | Human lung cancer cell lines | Induction of apoptosis and suppression of NF-kB pathway Inhibition of PD-L1 | [89,90,91,97] |
| Resveratrol (21) | Phennolic compound | Grapes and red wine | Brest, oral, liver, prostate and colon human cancer | Inhibition of citokyne production, (IFN-γ, IL-2, TNF-α and IL-12); block the activation of transcription factor NF-kB | [104,105,106,110] |
| Piperine (22) | Alkaloid | Piper nigrum | Human colon cancer cell lines | Cytotoxic activity of NK cells; suppression of the relase of Th2 cytokines IL-4 and IL-10; enhance murine splenocyte proliferation | [112,115,116,117,121] |
| Eugenol (23) | Phenolic compound | Cardamom | Inhibition of tumor formation in vivo | Cytotoxic activity of NK cells; suppression of the relase of Th2 cytokines IL-4 and IL-10 | [112,123,124,125,126] |
| Curcumin (24) | Phenolic compound | Curcuma longa | Human breast cancer | Modulation of NF-kB; reduction of IL-6; inhibit inflammation-mediated PD-L1 expression | [129,130,131,133,134] |
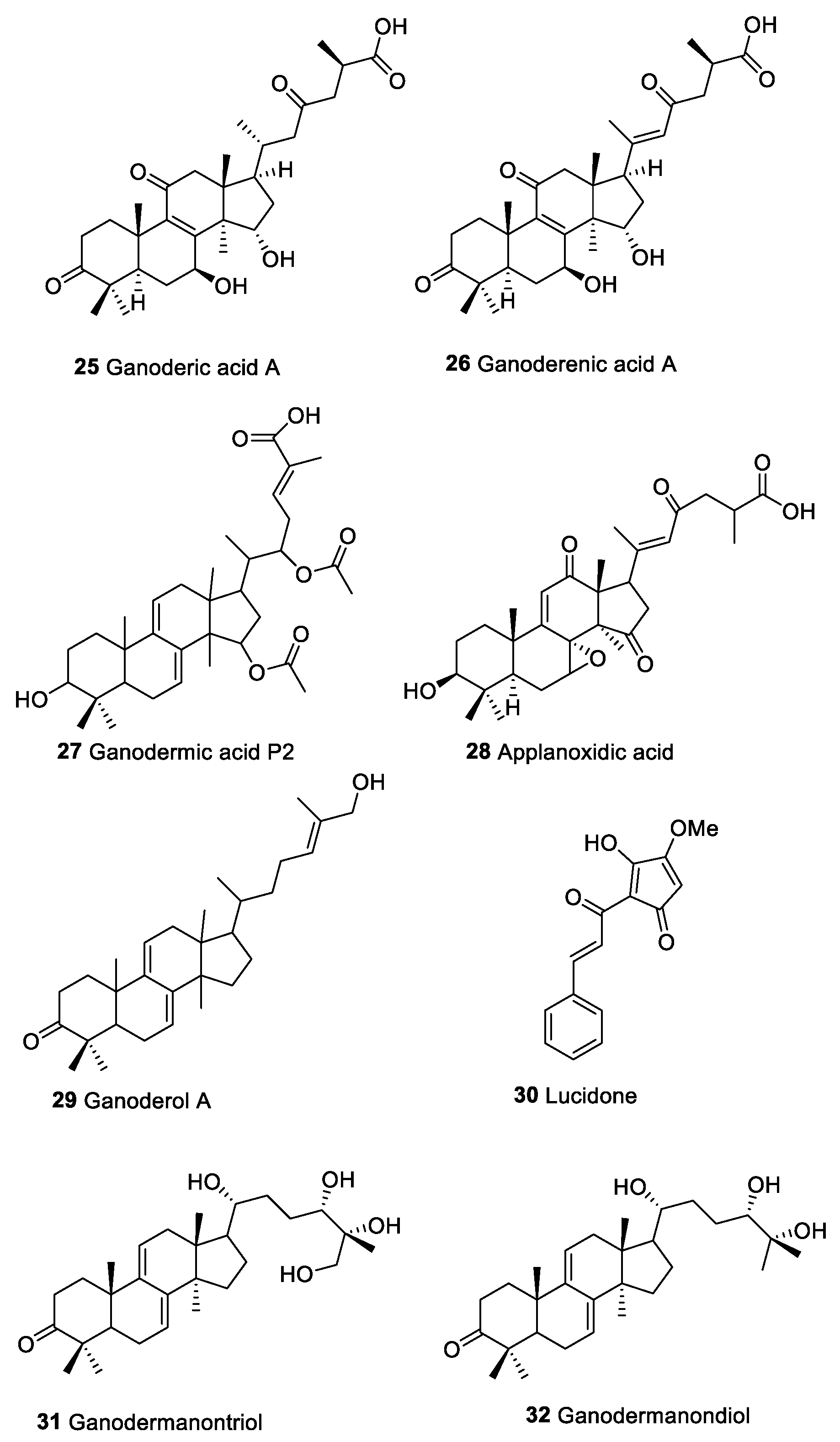
| Ganoderic acids (25) | Triterpenoid compounds | Macromycetes | Act on immune effecter cells such as hematpoietic stem cells, lymphocytes, macrophages, T cells, DCs, and NK cells Activation NF-kB pathway and modulate Ras/Erk, c-myc, CREB protein and MAPK | [139] | |
| Ganoderenic acids (26) | |||||
| Ganodermic acids P2 (27) | |||||
| Applanoxidic acid (28) | |||||
| Ganoderol A (29) | |||||
| Lucidone (30) | |||||
| Ganodermanontriol (31) | |||||
| Ganodermanondiol (32) | |||||
| Caffeic acid phenethyl ester (CAPE, 33) | Phenolic compounds | Propolis | Oral human cancer and human cancer cell lines of the promyelocytic leukemia (HL-60) | Inhibition of T cell receptor-mediated T cell proliferation | [150,151,154,158,159] |
| Artepilin C (34) |
3. Compounds from Marine Environment
| Molecule | Source | Tumor | Immuno System’s Role | References |
|---|---|---|---|---|
| Glycopeptide | Alexandrium minutum | A549 Lung adenocarcinoma cell line | Mitophagy and ICD inducer | [202] |
| Polyunsaturated aldehydes | diatoms | Programmed cell death in lung and colon adenocarcinoma | Induce the release of ATP and others immune signals which are known as ICD inducers | [21,163,202,203,204,205] |
| Coibamida A (35) | Leptolyngbya sp. | Breast camcer | Caspase-independent cell death and ICD inducer | [168] |
| Dioxinodehydroeckol (36) | Ecklonia cava | Human cancer cell line of the breast (MCF-7) | Induction of apoptosis through NF-kB family and NF-kB-dependent pathway | [169] |
| Astaxanthin (37) | Seaweeds | Antitumoral activity in the post-initiation phase of carcinogen-induced colon and oral cancer models | Improves antitumor immune responses by inhibiting lipid peroxidation induced by stress | [170,171,172,173] |
| α Galactosylceramide (38) | Sponge | Antitumor effects in mice | Stimulation of NKT cells to produce both Th1 and Th2 cytokines | [174] |
| α-Sulfoquinovosides (39) | Marine microalgae | Synthetic β-sulfoquinovosides derivative as adjuvant in vaccine against a murine B16F10 melanoma cell line | Maturation of human DCs. | [176,177] |
| Didemin B (41) | Trididemnum solidum | Inhibition of lymphocyte activation | [181,182,183] | |
| Lissoclibadin 2 (42) | Lissoclinum cf. badium | Human colon cancer lines (DLD-1) and (HCT116), breast cancer lines MDA-MB-231, renal cancer line ACHN; non-small-cell lung cancer line NCI-H460 | Increase of IL-8 production | [187] |
| 2,3-Dimethoxy-5-(3′,7′-dimethyl-octa-20(E),6′-dienyl)-[1,4] benzoquinone (43) | Aplidium glabrum | JB6 CI41 cancer cell | Inhibition of p53; Increase transcription of AP-1 and NF-kB | [186,188] |
| Lepadin A (45) | Clavelina lepadiformis sp. B | Human lung carcinoma, melanoma, and multiple myeloma | Mouse DCs | [189] |
| Bryostatin 1 (46) | Bugula neritina | Antitumor activity against leukemia, lymphoma ovarian cancer, and melanoma | Activation of PKC family; Stimulation of cytokine production | [193,194] |
4. Conclusions: Future Prospects of Natural Compounds as Potential Anti-Cancer Agents
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Thomford, N.E.; Senthebane, D.A.; Rowe, A.; Munro, D.; Seele, P.; Maroyi, A.; Dzobo, K. Natural Products for Drug Discovery in the 21st Century: Innovations for Novel Drug Discovery. Int. J. Mol. Sci. 2018, 19, 1578. [Google Scholar] [CrossRef]
- Reid, R.G.; Sarker, S.D. Isolation of Natural Products by Low-Pressure Column Chromatography. Methods Mol. Biol. 2012, 864, 155–187. [Google Scholar] [CrossRef]
- Sticher, O. Natural Product Isolation. Nat. Prod. Rep. 2008, 25, 517–554. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Rayan, A.; Raiyn, J.; Falah, M. Nature Is the Best Source of Anticancer Drugs: Indexing Natural Products for Their Anticancer Bioactivity. PLoS ONE 2017, 12, e0187925. [Google Scholar] [CrossRef]
- Heinig, U.; Jennewein, S. Taxol: A complex diterpenoid natural product with an evolutionarily obscure origin. Afr. J. Biotechnol. 2009, 8, 1370–1385. [Google Scholar]
- Cuevas, C.; Francesch, A. Development of Yondelis® (Trabectedin, ET-743). A Semisynthetic Process Solves the Supply Problem. Nat. Prod. Rep. 2009, 26, 322–337. [Google Scholar] [CrossRef]
- Pommier, Y.; Kohlhagen, G.; Bailly, C.; Waring, M.; Mazumder, A.; Kohn, K.W. DNA Sequence-and Structure-Selective Alkylation of Guanine N2 in the DNA Minor Groove by Ecteinascidin 743, a Potent Antitumor Compound from the Caribbean Tunicate Ecteinascidia Turbinata. Biochemistry 1996, 35, 13303–13309. [Google Scholar] [CrossRef]
- Bracci, L.; Schiavoni, G.; Sistigu, A.; Belardelli, F. Immune-Based Mechanisms of Cytotoxic Chemotherapy: Implications for the Design of Novel and Rationale-Based Combined Treatments against Cancer. Cell Death Differ. 2014, 21, 15–25. [Google Scholar] [CrossRef]
- Norling, L.V.; Serhan, C.N. Profiling in Resolving Inflammatory Exudates Identifies Novel Anti-Inflammatory and pro-Resolving Mediators and Signals for Termination. J. Intern. Med. 2010, 268, 15–24. [Google Scholar] [CrossRef]
- Zhu, H.F.; Li, Y. Small-Molecule Targets in Tumor Immunotherapy. Nat. Prod. Bioprospec. 2018, 8, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Ngamkham, S.; Holden, J.E.; Smith, E.L. A Systematic Review: Mindfulness Intervention for Cancer-Related Pain. Asia-Pac. J. Oncol. Nurs. 2019, 6, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Yoest, J. Clinical Features, Predictive Correlates, and Pathophysiology of Immune-Related Adverse Events in Immune Checkpoint Inhibitor Treatments in Cancer: A Short Review. Immuno. Targets Ther. 2017, 6, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Vong, C.T.; Chen, F.; Tan, H.; Zhang, C.; Wang, N.; Cui, L.; Wang, Y.; Feng, Y. Immunomodulatory Potential of Natural Products from Herbal Medicines as Immune Checkpoints Inhibitors: Helping to Fight against Cancer via Multiple Targets. Med. Res. Rev. 2022, 42, 1246–1279. [Google Scholar] [CrossRef] [PubMed]
- Zitvogel, L.; Apetoh, L.; Ghiringhelli, F.; Kroemer, G. Immunological Aspects of Cancer Chemotherapy. Nat. Rev. Immunol. 2008, 8, 59–73. [Google Scholar] [CrossRef]
- Galluzzi, L.; Senovilla, L.; Zitvogel, L.; Kroemer, G. The Secret Ally: Immunostimulation by Anticancer Drugs. Nat. Rev. Drug Discov. 2012, 11, 215–233. [Google Scholar] [CrossRef]
- Kawano, M.; Tanaka, K.; Itonaga, I.; Iwasaki, T.; Miyazaki, M.; Ikeda, S.; Tsumura, H. Dendritic Cells Combined with Doxorubicin Induces Immunogenic Cell Death and Exhibits Antitumor Effects for Osteosarcoma. Oncol. Lett. 2016, 11, 2169–2175. [Google Scholar] [CrossRef]
- Pan, P.; Huang, Y.W.; Oshima, K.; Yearsley, M.; Zhang, J.; Arnold, M.; Yu, J.; Wang, L.S. The Immunomodulatory Potential of Natural Compounds in Tumor-Bearing Mice and Humans. Crit. Rev. Food Sci. Nutr. 2019, 59, 992–1007. [Google Scholar] [CrossRef]
- Mohamed, S.I.A.; Jantan, I.; Haque, M.A. Naturally Occurring Immunomodulators with Antitumor Activity: An Insight on Their Mechanisms of Action. Int. Immunopharmacol. 2017, 50, 291–304. [Google Scholar] [CrossRef]
- Moody, R.; Wilson, K.; Jaworowski, A.; Plebanski, M. Natural Compounds with Potential to Modulate Cancer Therapies and Self-Reactive Immune Cells. Cancers 2020, 12, 673. [Google Scholar] [CrossRef]
- Sansone, C.; Bruno, A.; Piscitelli, C.; Baci, D.; Fontana, A.; Brunet, C.; Noonan, D.M.; Albini, A. Natural Compounds of Marine Origin as Inducers of Immunogenic Cell Death (ICD): Potential Role for Cancer Interception and Therapy. Cells 2021, 10, 1–20. [Google Scholar] [CrossRef]
- Katanaev, V.L.; di Falco, S.; Khotimchenko, Y. The Anticancer Drug Discovery Potential of Marine Invertebrates from Russian Pacific. Marine Drugs 2019, 17, 474. [Google Scholar] [CrossRef]
- Riccio, G.; Lauritano, C. Microalgae with Immunomodulatory Activities. Marine Drugs 2020, 18, 2. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Kunnumakkara, A.B.; Harlkumar, K.B.; Tharakan, S.T.; Sung, B.; Anand, P. Potential of Spice-Derived Phytochemicals for Cancer Prevention. Planta Med. 2008, 74, 1560–1569. [Google Scholar] [CrossRef]
- Shen, Y.-C.; Chen, C.-F.; Chiou, W.-F. Andrographolide Prevents Oxygen Radical Production by Human Neutrophils: Possible Mechanism(s) Involved in Its Anti-Intinflammatory Effect. Br. J. Pharmacol. 2002, 135, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Shihman Chang, R.; Ding, L.; Gai-qing, C.; Ze-lin, Z.; SMITHt, K.M. Dehydroandrographolide Succinic Acid Monoester as an Inhibitor against the Human Immunodeficiency Virus (43225). Proc. Soc. Exp. Biol. Med. 1991, 197, 59–66. [Google Scholar] [CrossRef]
- Hossain, S.; Urbi, Z.; Karuniawati, H.; Mohiuddin, R.B.; Moh Qrimida, A.; Allzrag, A.M.M.; Ming, L.C.; Pagano, E.; Capasso, R. Andrographis paniculata (Burm. f.) Wall. ex Nees: An Updated Review of Phytochemistry, Antimicrobial Pharmacology, and Clinical Safety and Efficacy. Life 2021, 11, 348. [Google Scholar] [CrossRef]
- Puri, A.; Saxena, R.; Saxena, R.P.; Saxena, K.C.; Srivastava, V.; Tandón, J.S. Immunostimulant Agents from Andrographis Paniculata1. J. Nat. Prod. 1993, 56, 995–999. [Google Scholar] [CrossRef]
- Ajaya Kumar, R.; Sridevi, K.; Vijaya Kumar, N.; Nanduri, S.; Rajagopal, S. Anticancer and Immunostimulatory Compounds from Andrographis Paniculata. J. Ethnopharmacol. 2004, 92, 291–295. [Google Scholar] [CrossRef]
- Ghazalee, N.S.; Jantan, I.; Arshad, L.; Haque, M.A. Immunosuppressive Effects of the Standardized Extract of Zingiber Zerumbet on Innate Immune Responses in Wistar Rats. Phytother. Res. 2019, 33, 929–938. [Google Scholar] [CrossRef]
- Girisa, S.; Shabnam, B.; Monisha, J.; Fan, L.; Halim, C.E.; Arfuso, F.; Ahn, K.S.; Sethi, G.; Kunnumakkara, A.B. Potential of Zerumbone as an Anti-Cancer Agent. Molecules 2019, 24, 734. [Google Scholar] [CrossRef] [PubMed]
- Arshad, L.; Jantan, I.; Bukhari, S.N.A.; Haque, M.A. Immunosuppressive Effects of Natural α,β-Unsaturated Carbonyl-Based Compounds, and Their Analogs and Derivatives, on Immune Cells: A Review. Front. Pharmacol. 2017, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Prasannan, R.; Kalesh, K.A.; Shanmugam, M.K.; Nachiyappan, A.; Ramachandran, L.; Nguyen, A.H.; Kumar, A.P.; Lakshmanan, M.; Ahn, K.S.; Sethi, G. Key Cell Signaling Pathways Modulated by Zerumbone: Role in the Prevention and Treatment of Cancer. Biochem. Pharmacol. 2012, 84, 1268–1276. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.A.; Jantan, I.; Arshad, L.; Bukhari, S.N.A. Exploring the Immunomodulatory and Anticancer Properties of Zerumbone. Food Funct. 2017, 8, 3410–3431. [Google Scholar] [CrossRef] [PubMed]
- Murakami, A.; Shigemori, T.; Ohigashi, H. International Conference on Diet, Nutrition, and Cancer Zingiberaceous and Citrus Constituents, 1-Acetoxychavicol Acetate, Zerumbone, Auraptene, and Nobiletin, Suppress Lipopolysaccharide-Induced Cyclooxygenase-2 Expression in RAW264.7 Murine Macrophages through Different Modes of Action. J. Nutr. 2005, 135, 2987S–2992S. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, A.; Kaneko, Y.; Murakami, A.; Ohigashi, H. Zerumbone Suppresses Phorbol Ester-Induced Expression of Multiple Scavenger Receptor Genes in THP-1 Human Monocytic Cells. Biosci. Biotechnol. Biochem. 2007, 71, 935–945. [Google Scholar] [CrossRef][Green Version]
- Murakami, A.; Takahashi, D.; Kinoshita, T.; Koshimizu, K.; Kim, H.W.; Yoshihiro, A.; Nakamura, Y.; Jiwajinda, S.; Terao, J.; Ohigashi, H. Zerumbone, a Southeast Asian Ginger Sesquiterpene, Markedly Suppresses Free Radical Generation, Proinflammatory Protein Production, and Cancer Cell Proliferation Accompanied by Apoptosis: The α,β-Unsaturated Carbonyl Group Is a Prerequisite. Carcinogenesis 2002, 23, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Abdel Wahab, S.I.; Abdul, A.B.; Alzubairi, A.S.; Mohamed Elhassan, M.; Mohan, S. In Vitro Ultramorphological Assessment of Apoptosis Induced by Zerumbone on (HeLa). J. Biomed. Biotechnol. 2009, 2009. [Google Scholar] [CrossRef]
- Yodkeeree, S.; Sung, B.; Limtrakul, P.; Aggarwal, B.B. Zerumbone Enhances TRAIL-Induced Apoptosis through the Induction of Death Receptors in Human Colon Cancer Cells: Evidence for an Essential Role of Reactive Oxygen Species. Cancer Res. 2009, 69, 6581–6589. [Google Scholar] [CrossRef]
- Kupchan, S.M.; Court, W.A.; Dailey, R.G., Jr.; Gilmore, C.J.; Bryan, R.F. Triptolide and Tripdiolide, Novel Antileukemic Diterpenoid Triepoxides from Tripterygium Wilfordii. J. Am. Chem. Soc. 1972, 94, 7194–7195. [Google Scholar] [CrossRef]
- Tao, X.; Cai, J.J.; Lipsky, P.E. The Identity of Immunosuppressive Components of the Ethyl Acetate Extract and Chloroform Methanol Extract. J. Pharmacol. Exp. Ther. 1995, 272, 1305–1312. [Google Scholar] [PubMed]
- Tao, X.; Davis, L.S.; Hashhoto, K.; Lipsky, R.E. The Chinese Herbal Remedy, T2. Inhibits Mitogen-Induced Cytokine Gene Transcription by T Cells, but Not Initial Signal Transduction. J. Pharmacol. Exp.Ther. 1996, 276, 316–325. [Google Scholar]
- Qiu, D.; Zhao, G.; Aoki, Y.; Shi, L.; Uyei, A.; Nazarian, S.; Ng, J.C.H.; Kao, P.N. Immunosuppressant PG490 (Triptolide) Inhibits T-Cell Interleukin-2 Expression at the Level of Purine-Box/Nuclear Factor of Activated T-Cells and NF-ΚB Transcriptional Activation. J. Biol. Chem. 1999, 274, 13443–13450. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Chang, W.T.; Qiu, D.; Kao, P.N.; Rosen, G.D. PG490 (Triptolide) Cooperates with Tumor Necrosis Factor-α to Induce Apoptosis in Tumor Cells. J. Biol. Chem. 1999, 274, 13451–13455. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.T.; Kang, J.J.; Lee, K.Y.; Wei, K.; Anderson, E.; Gotmare, S.; Ross, J.A.; Rosen, G.D. Triptolide and Chemotherapy Cooperate in Tumor Cell Apoptosis. A Role for the P53 Pathway. J. Biol. Chem. 2001, 276, 2221–2227. [Google Scholar] [CrossRef] [PubMed]
- Rongzhen, X.; Hongjian, J. C14-Hydroxyl Esterified Amino Acid Derivatives of Triptolide, and Preparation Method and Use Thereof. CN Patent. EP3248981A1. Available online: https://patents.google.com/patent/EP3248981A1/en (accessed on 13 April 2022).
- Namin, M.H.; Ebrahimzadeh, H.; Ghareyazie, B.; Radjabian, T.; Gharavi, S.; Tafreshi, N.; Lee, H. In Vitro Expression of Apocarotenoid Genes in Crocus Sativus L. Afr. J. Biotechnol. 2009, 8, 5378–5382. [Google Scholar]
- Wyss, A. Carotene Oxygenases: A New Family of Double Bond Cleavage Enzymes. J. Nutr. 2004, 134, 246S–250S. [Google Scholar] [CrossRef]
- Bolhassani, A.; Khavari, A.; Bathaie, S.Z. Saffron and Natural Carotenoids: Biochemical Activities and Anti-Tumor Effects. Biochim. Et Biophys. Acta-Rev. Cancer 2014, 1845, 20–30. [Google Scholar] [CrossRef]
- Abar, L.; Vieira, A.R.; Aune, D.; Stevens, C.; Vingeliene, S.; Navarro Rosenblatt, D.A.; Chan, D.; Greenwood, D.C.; Norat, T. Blood Concentrations of Carotenoids and Retinol and Lung Cancer Risk: An Update of the WCRF–AICR Systematic Review of Published Prospective Studies. Cancer Med. 2016, 5, 2069–2083. [Google Scholar] [CrossRef]
- Rakic, J.M.; Liu, C.; Veeramachaneni, S.; Wu, D.; Paul, L.; Chen, C.Y.O.; Ausman, L.M.; Wang, X.D. Lycopene Inhibits Smoke-Induced Chronic Obstructive Pulmonary Disease and Lung Carcinogenesis by Modulating Reverse Cholesterol Transport in Ferrets. Cancer Prev. Res. 2019, 12, 421–432. [Google Scholar] [CrossRef]
- Eid, S.Y.; El-Readi, M.Z.; Wink, M. Carotenoids Reverse Multidrug Resistance in Cancer Cells by Interfering with ABC-Transporters. Phytomedicine 2012, 19, 977–987. [Google Scholar] [CrossRef] [PubMed]
- Awad, A.B.; Chen, Y.C.; Fink, C.S.; Hennessey, T. Beta-Sitosterol Inhibits HT-29 Human Colon Cancer Cell Growth and Alters Membrane Lipids. Anticancer Res. 1996, 16, 2797–2804. [Google Scholar] [PubMed]
- Bouic, P.J.D. The Role of Phytosterols and Phytosterolins in Immune Modulation: A Review of the Past 10 Years. Curr. Opin. Clin. Nutr. Metab. Care 2001, 4, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Moreno, J.J. Effect of Olive Oil Minor Components on Oxidative Stress and Arachidonic Acid Mobilization and Metabolism by Macrophages RAW 264.7. Free. Radic. Biol. Med. 2003, 35, 1073–1081. [Google Scholar] [CrossRef]
- Awad, A.B.; Toczek, J.; Fink, C.S. Phytosterols Decrease Prostaglandin Release in Cultured P388D 1/MAB Macrophages. Prostaglandins Leukot. Essent. Fat. Acids 2004, 70, 511–520. [Google Scholar] [CrossRef]
- Ikemoto, S.; Sugimura, K.; Yoshida, N.; Yasumoto, R.; Wada, S.; Yamamoto, K.; Kishimoto, T. Antitumor Effects of Scutellariae Radix and Its Components Baicalein, Baicalin, and Wogonin on Bladder Cancer Cell Lines. Urology 2000, 55, 951–955. [Google Scholar] [CrossRef]
- Kaplya, O.A.; Sherstoboev, E.Y.; Zueva, E.P.; Razina, T.G.; Amosova, E.N.; Krylova, S.G. Effect of Baikal Skullcap Extract Administered Alone or in Combination with Cyclophosphamide on Natural Cytotoxicity System in Mice with Lewis Lung Carcinoma. Bull. Exp. Biol. Med. 2004, 137, 471–474. [Google Scholar] [CrossRef]
- Li-Weber, M. New Therapeutic Aspects of Flavones: The Anticancer Properties of Scutellaria and Its Main Active Constituents Wogonin, Baicalein and Baicalin. Cancer Treat. Rev. 2009, 35, 57–68. [Google Scholar] [CrossRef]
- Lamer-Zarawska, E.; Wiśniewska, A.; Błach-Olszewska, Z. Anticancer Properties of Scutellaria Baicalensis Root in Aspect of Innate Immunity Regulation Przeciwnowotworowa Aktywność Tarczycy Bajkalskiej w Świetle Regulacji Wrodzonej Odporności. Adv. Clin. Exp. Med. 2010, 19, 419–428. [Google Scholar]
- Ma, Z.; Otsuyama, K.-I.; Liu, S.; Abroun, S.; Ishikawa, H.; Tsuyama, N.; Obata, M.; Li, F.-J.; Zheng, X.; Maki, Y.; et al. Baicalein, a Component of Scutellaria Radix from Huang-Lian-Jie-Du-Tang (HLJDT), Leads to Suppression of Proliferation and Induction of Apoptosis in Human Myeloma Cells. Blood 2005, 105, 3312–3318. [Google Scholar] [CrossRef]
- Bonham, M.; Posakony, J.; Coleman, I.; Montgomery, B.; Simon, J.; Nelson, P.S. Characterization of Chemical Constituents in Scutellaria Baicalensis with Antiandrogenic and Growth-Inhibitory Activities toward Prostate Carcinoma. Clin. Cancer Res. 2005, 11, 3905–3914. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, T.; Müller, C.I.; Desmond, J.C.; Imai, Y.; Heber, D.; Koeffler, H.P. Scutellaria Baicalensis, a Herbal Medicine: Anti-Proliferative and Apoptotic Activity against Acute Lymphocytic Leukemia, Lymphoma and Myeloma Cell Lines. Leuk. Res. 2007, 31, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Chi, Y.S.; Lim, H.; Park, H.; Kim, H.P. Effects of Wogonin, a Plant Flavone from Scutellaria Radix, on Skin Inflammation: In Vivo Regulation of Inflammation-Associated Gene Expression. Biochem. Pharmacol. 2003, 66, 1271–1278. [Google Scholar] [CrossRef]
- Huang, W.H.; Lee, A.R.; Yang, C.H. Antioxidative and Anti-Inflammatory Activities of Polyhydroxyflavonoids of Scutellaria Baicalensis GEORGI. Biosci. Biotechnol. Biochem. 2006, 70, 2371–2380. [Google Scholar] [CrossRef]
- Lim, H.; Park, H.; Kim, H.P. Inhibition of Contact Dermatitis in Animal Models and Suppression of Proinflammatory Gene Expression by Topically Applied Flavonoid, Wogonin. Arch. Pharm. Res. 2004, 27, 442. [Google Scholar] [CrossRef]
- Błach-Olszewska, Z.; Jatczak, B.; Rak, A.; Lorenc, M.; Gulanowski, B.; Drobna, A.; Lamer-Zarawska, E. Production of Cytokines and Stimulation of Resistance to Viral Infection in Human Leukocytes by Scutellaria Baicalensis Flavones. J. Interferon Cytokine Res. 2008, 28, 571–581. [Google Scholar] [CrossRef]
- Chen, Y.C.; Shen, S.C.; Chen, L.G.; Lee, T.J.; Yang, L.L. Wogonin, Baicalin, and Baicalein Inhibition of Inducible Nitric Oxide Synthase and Cyclooxygenase-2 Gene Expressions Induced by Nitric Oxide Synthase Inhibitors and Lipopolysaccharide. Biochem. Pharmacol. 2001, 61, 1417–1427. [Google Scholar] [CrossRef]
- Chen, L.G.; Hung, L.Y.; Tsai, K.W.; Pan, Y.S.; da Tsai, Y.; Li, Y.Z.; Liu, Y.W. Wogonin, a Bioactive Flavonoid in Herbal Tea, Inhibits Inflammatory Cyclooxygenase-2 Gene Expression in Human Lung Epithelial Cancer Cells. Mol. Nutr. Food Res. 2008, 52, 1349–1357. [Google Scholar] [CrossRef]
- Kim, H.; Kim, Y.S.; Kim, S.Y.; Suk, K. The Plant flavonoid Wogonin Suppresses Death of Activated C6 Rat Glial Cells by Inhibiting Nitric Oxide Production. Neurosci. Lett. 2001, 309, 67–71. [Google Scholar] [CrossRef]
- Fukumura, D.; Kashiwagi, S.; Jain, R.K. The Role of Nitric Oxide in Tumour Progression. Nat. Rev. Cancer 2006, 6, 521–534. [Google Scholar] [CrossRef]
- Ke, M.; Zhang, Z.; Xu, B.; Zhao, S.; Ding, Y.; Wu, X.; Wu, R.; Lv, Y.; Dong, J. Baicalein and Baicalin Promote Antitumor Immunity by Suppressing PD-L1 Expression in Hepatocellular Carcinoma Cells. Int. Immunopharmacol. 2019, 75, 105824. [Google Scholar] [CrossRef]
- Anila, L.; Vijayalakshmi, N.R. Antioxidant Action of Flavonoids from Mangifera Indica and Emblica Officinalis in Hypercholesterolemic Rats. Food Chem. 2003, 83, 569–574. [Google Scholar] [CrossRef]
- Abesundara, K.J.M.; Matsui, T.; Matsumoto, K. α-Glucosidase Inhibitory Activity of Some Sri Lanka Plant Extracts, One of Which, Cassia Auriculata, Exerts a Strong Antihyperglycemic Effect in Rats Comparable to the Therapeutic Drug Acarbose. J. Agric. Food Chem. 2004, 52, 2541–2545. [Google Scholar] [CrossRef] [PubMed]
- Rani, P.; Khullar, N. Antimicrobial Evaluation of Some Medicinal Plants for Their Anti-Enteric Potential against Multi-Drug Resistant Salmonella Typhi. Phytother. Res. 2004, 18, 670–673. [Google Scholar] [CrossRef] [PubMed]
- Ram, M.S.; Neetu, D.; Yogesh, B.; Anju, B.; Dipti, P.; Pauline, T.; Sharma, S.K.; Sarada, S.K.S.; Ilavazhagan, G.; Kumar, D.; et al. Cyto-Protective and Immunomodulating Properties of Amla (Emblica Officinalis) on Lymphocytes: An in-Vitro Study. J. Ethnopharmacol. 2002, 81, 5–10. [Google Scholar] [CrossRef]
- Jose, J.K.; Kuttan, G.; Kuttan, R. Antitumour Activity of Emblica Officinalis. J. Ethnopharmacol. 2001, 75, 65–69. [Google Scholar] [CrossRef]
- Rajeshkumar, N.V.; Joy, K.L.; Kuttan, G.; Ramsewak, R.S.; Nair, M.G.; Kuttan, R. Antitumour and Anticarcinogenic Activity of Phyllanthus Amarus Extract. J. Ethnopharmacol. 2002, 81, 17–22. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, M.; Wu, K.; Chai, X.; Yu, H.; Tao, Z.; Wang, J. Immunomodulatory and Anticancer Activities of Phenolics from Emblica Fruit (Phyllanthus Emblica L.). Food Chem. 2012, 131, 685–690. [Google Scholar] [CrossRef]
- Kawakami, K.; Nishida, H.; Tatewaki, N.; Eguchi-Kasai, K.; Anzai, K.; Eitsuka, T.; Konishi, T.; Hirayama, M. Persimmon Leaf Flavonols Enhance the Anti-Cancer Effect of Heavy Ion Radiotherapy on Murine Xenograft Tumors. J. Cancer Ther. 2013, 4, 1150–1157. [Google Scholar] [CrossRef][Green Version]
- Ling, W.D.; Du, G. Effect of Flavonoid from Diospyros Kaki Leaves on TGF- β 1 and MMP-9 in Blood Glucose Levels and Kidney Tissues of Rats with Diabetic Nephropathy. Chin. J. Exp. Tradit. Med. Form 2016, 22, 139–143. [Google Scholar]
- Sun, L.; Zhang, J.; Lu, X.; Zhang, L.; Zhang, Y. Evaluation to the Antioxidant Activity of Total Flavonoids Extract from Persimmon (Diospyros Kaki L.) Leaves. Food Chem. Toxicol. 2011, 49, 2689–2696. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wei, Y.; Zhao, S.; Zhang, M.; Yan, X.; Gao, X.; Li, J.; Gao, Y.; Zhang, A.; Gao, Y. Antitumor and Immunomodulatory Activities of Total Flavonoids Extract from Persimmon Leaves in H22 Liver Tumor-Bearing Mice. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ganeshpurkar, A.; Saluja, A.K. The Pharmacological Potential of Rutin. Saudi Pharm. J. 2017, 25, 149–164. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Zhang, Y.; Ye, X.; Xue, S.; Shi, J.; Pan, J.; Chen, Q. Inhibition Effects and Induction of Apoptosis of Flavonoids on the Prostate Cancer Cell Line PC-3 in Vitro. Food Chem. 2013, 138, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Hayat, K.; Iqbal, H.; Malik, U.; Bilal, U.; Mushtaq, S. Tea and Its Consumption: Benefits and Risks. Crit. Rev. Food Sci. Nutr. 2015, 55, 939–954. [Google Scholar] [CrossRef]
- Crespy, V.; Williamson, G. A Review of the Health Effects of Green Tea Catechins in In Vivo Animal Models. J. Nutr. 2004, 134, 3431S–3440S. [Google Scholar] [CrossRef]
- Katiyar, S.; Elmets, C.A.; Katiyar, S.K. Green Tea and Skin Cancer: Photoimmunology, Angiogenesis and DNA Repair. J. Nutr. Biochem. 2007, 18, 287–296. [Google Scholar] [CrossRef]
- Shirakami, Y.; Shimizu, M.; Moriwaki, H. Cancer Chemoprevention with Green Tea Catechins: From Bench to Bed. Current Drug Targets 2012, 13, 1842–1857. [Google Scholar] [CrossRef]
- Butt, M.S.; Sultan, M.T. Green Tea: Nature’s Defense against Malignancies. Crit. Rev. Food Sci. Nutr. 2009, 49, 463–473. [Google Scholar] [CrossRef]
- Fujiki, H.; Watanabe, T.; Sueoka, E.; Rawangkan, A.; Suganuma, M. Cancer Prevention with Green Tea and Its Principal Constituent, EGCG: From Early Investigations to Current Focus on Human Cancer Stem Cells. Mol. Cells 2018, 41, 73–82. [Google Scholar]
- Negri, A.; Naponelli, V.; Rizzi, F.; Bettuzzi, S. Molecular Targets of Epigallocatechin—Gallate (EGCG): A Special Focus on Signal Transduction and Cancer. Nutrients 2018, 10, 1936. [Google Scholar] [CrossRef] [PubMed]
- Santilli, G.; Piotrowska, I.; Cantilena, S.; Chayka, O.; D’Alicarnasso, M.; Morgenstern, D.A.; Himoudi, N.; Pearson, K.; Anderson, J.; Thrasher, A.J.; et al. Polyphenol e Enhances the Antitumor Immune Response in Neuroblastoma by Inactivating Myeloid Suppressor Cells. Clin. Cancer Res. 2013, 19, 1116–1125. [Google Scholar] [CrossRef] [PubMed]
- Tae, H.K.; Jin, H.L.; Chung, K.S.; Hee, D.H.; Byung, C.S.; Pai, S.I.; Hung, C.F.; Trimble, C.; Lim, J.S.; Tae, W.K.; et al. Epigallocatechin-3-Gallate Enhances CD8+ T Cell-Mediated Antitumor Immunity Induced by DNA Vaccination. Cancer Res. 2007, 67, 802–811. [Google Scholar] [CrossRef]
- Mantena, S.K.; Roy, A.M.; Katiyar, S.K. Epigallocatechin-3-Gallate Inhibits Photocarcinogenesis Through Inhibition of Angiogenic Factors and Activation of CD8+ T Cells in Tumors. Photochem. Photobiol. 2005, 81, 1174. [Google Scholar] [CrossRef]
- Hsieh, D.S.; Wang, H.; Tan, S.W.; Huang, Y.H.; Tsai, C.Y.; Yeh, M.K.; Wu, C.J. The Treatment of Bladder Cancer in a Mouse Model by Epigallocatechin-3-Gallate-Gold Nanoparticles. Biomaterials 2011, 32, 7633–7640. [Google Scholar] [CrossRef]
- Rawangkan, A.; Wongsirisin, P.; Namiki, K.; Iida, K.; Kobayashi, Y.; Shimizu, Y.; Fujiki, H.; Suganuma, M. Green Tea Catechin Is an Alternative Immune Checkpoint Inhibitor That Inhibits PD-L1 Expression and Lung Tumor Growth. Molecules 2018, 23, 2071. [Google Scholar] [CrossRef]
- Belguendouz, L.; Frémont, L.; Gozzelino, M.-T. Interaction of Transresveratrol with Plasma Lipoproteins. Biochem. Pharmacol. 1998, 55, 811–816. [Google Scholar] [CrossRef]
- Kisková, T.; Kassayová, M. Resveratrol Action on Lipid Metabolism in Cancer. Int. J. Mol. Sci. 2019, 20, 2704. [Google Scholar] [CrossRef]
- Pace-Asciak, C.R.; Hahn, S.; Diamandis, E.P.; Soleas, G.; Goldberg, D.M. The Red Wine Phenolics Trans-Resveratrol and Quercetin Block Human Platelet Aggregation and Eicosanoid Synthesis: Implications for Protection against Coronary Heart Disease. Clin. Chim. Acta 1995, 235, 207–219. [Google Scholar] [CrossRef]
- Rotondo, S.; Rajtar, G.; Manarini, S.; Celardo, A.; Rotilio, D.; de Gaetano, G.; Evangelista, V.; Cerletti, C. Effect of Trans-Resveratrol, a Natural Polyphenolic Compound, on Human Polymorphonuclear Leukocyte Function. Br. J. Pharmacol. 1998, 123, 1691–1699. [Google Scholar] [CrossRef]
- Kimura, Y.; Okuda, H.; Kubo, M. Effects of Stilbenes Isolated from Medicinal Plants on Arachidonate Metabolism and Degranulation in Human Polymorphonuclear Leukocytes. J. Ethnopharmacol. 1995, 45, 131–139. [Google Scholar] [CrossRef]
- Belguendouz, L.; Fremont, L.; Hard, A. Resveratrol Inhibits Metal Ion-Dependent and Independent Peroxidation of Porcine Low-Density Lipoproteins. Biochem Pharmacol. 1997, 53, 1347–1355. [Google Scholar] [CrossRef]
- Man-Ying Chan, M.; Mattiacci, J.A.; Hwang, H.S.; Shah, A.; Fong, D. Synergy between Ethanol and Grape Polyphenols, Quercetin, and Resveratrol, in the Inhibition of the Inducible Nitric Oxide Synthase Pathway. Biochem. Pharmacol. 2000, 60, 1539–1548. [Google Scholar] [CrossRef]
- Jang, M.; Cai, L.; Udeani, G.O.; Slowing, K.V.; Thomas, C.F.; Beecher, C.W.W.; Fong, H.H.S.; Farnsworth, N.R.; Kinghorn, A.D.; Mehta, R.G.; et al. Cancer Chemopreventive Activity of Resveratrol, a Natural Product Derived from Grapes. Science 1997, 275, 218–220. [Google Scholar] [CrossRef]
- Mgbonyebi, O.P.; Russo, J.; Russo, I.H. Antiproliferative Effect of Synthetic Resveratrol on Human Breast Epithelial Cells. Int. J. Oncol. 1998, 12, 865–874. [Google Scholar] [CrossRef]
- Lu, R.; Serrero, G. Resveratrol, a Natural Product Derived from Grape, Exhibits Antiestrogenic Activity and Inhibits the Growth of Human Breast Cancer Cells. J. Cell Physiol. 1999, 179, 297–304. [Google Scholar] [CrossRef]
- Hsieh, T.-C.; Wu, J.M. Differential Effects on Growth, Cell Cycle Arrest, and Induction of Apoptosis by Resveratrol in Human Prostate Cancer Cell Lines. Exp Cell Res. 1999, 249, 109–115. [Google Scholar] [CrossRef]
- Schneider, Y.; Vincent, F.; Duranton, B.Ã.; Badolo, L.; Gosse, F.; Bergmann, C.; Seiler, N.; Raul, F. Anti-Proliferative Effect of Resveratrol, a Natural Component of Grapes and Wine, on Human Colonic Cancer Cells. Cancer Lett. 2000, 158, 85–91. [Google Scholar] [CrossRef]
- Gao, X.; Xu, Y.X.; Janakiraman, N.; Chapman, R.A.; Gautam, S.C. Immunomodulatory Activity of Resveratrol: Suppression of Lymphocyte Proliferation, Development of Cell-Mediated Cytotoxicity, and Cytokine Production. Biochem. Pharmacol. 2001, 62, 1299–1308. [Google Scholar] [CrossRef]
- Bergman, M.; Levin, G.S.; Bessler, H.; Djaldetti, M.; Salman, H. Resveratrol Affects the Cross Talk between Immune and Colon Cancer Cells. Biomed. Pharmacother. 2013, 67, 43–47. [Google Scholar] [CrossRef]
- Majdalawieh, A.F.; Carr, R.I. In Vitro Investigation of the Potential Immunomodulatory and Anti-Cancer Activities of Black Pepper (Piper Nigrum) and Cardamom (Elettaria Cardamomum). J. Med. Food 2010, 13, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Surh, Y.-J. Anti-Tumor Promoting Potential of Selected Spice Ingredients with Antioxidative and Anti-Inflammatory Activities: A Short Review. Food Chem. Toxicol. 2002, 40, 1091–1097. [Google Scholar] [CrossRef]
- Kaefer, C.M.; Milner, J.A. The Role of Herbs and Spices in Cancer Prevention. J. Nutr. Biochem. 2008, 19, 347–361. [Google Scholar] [CrossRef] [PubMed]
- Pathak, N.; Khandelwal, S. Cytoprotective and Immunomodulating Properties of Piperine on Murine Splenocytes: An in Vitro Study. Eur. J. Pharmacol. 2007, 576, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Duessel, S.; Heuertz, R.M.; Ezekiel, U.R. Growth Inhibition of Human Colon Cancer Cells by Plant Compounds. Am. Soc. Clin. Lab. Sci. 2008, 21, 151–157. [Google Scholar]
- Menon, L.G.; Kuttan, R.; Kuttan, G. Effect of Rasayanas in the Inhibition of Lung Metastasis Induced by B16F-10 Melanoma Cells. J. Exp. Clin. Cancer Res. 1997, 16, 365–368. [Google Scholar]
- Selvendiran, K.; Mumtaz Banu, S.; Sakthisekaran, D. Oral Supplementation of Piperine Leads to Altered Phase II Enzymes and Reduced DNA Damage and DNA-Protein Cross Links in Benzo(a)Pyrene Induced Experimental Lung Carcinogenesis. Mol Cell Biochem. 2005, 268, 141–147. [Google Scholar] [CrossRef]
- Selvendiran, K.; Thirunavukkarasu, C.; Prince, J.; Singh, V.; Padmavathi, R.; Sakthisekaran, D. Chemopreventive Effect of Piperine on Mitochondrial TCA Cycle and Phase-I and Glutathione-Metabolizing Enzymes in Benzo(a)Pyrene Induced Lung Carcinogenesis in Swiss Albino Mice. Mol. Cell Biochem. 2005, 271, 101–106. [Google Scholar] [CrossRef]
- Krishnakumar, N.; Manoharan, S.; Palaniappan, P.R.; Venkatachalam, P.; Manohar, M.G.A. Chemopreventive Efficacy of Piperine in 7,12-Dimethyl Benz [a] Anthracene (DMBA)-Induced Hamster Buccal Pouch Carcinogenesis: An FT-IR Study. Food Chem. Toxicol. 2009, 47, 2813–2820. [Google Scholar] [CrossRef]
- Manoharan, S.; Balakrishnan, S.; Menon, V.P.; Alias, L.M.; Reena, A.R. Chemopreventive Efficacy of Curcumin and Piperine during 7,12-Dimethylbenz [a]Anthracene-Induced Hamster Buccal Pouch Carcinogenesis. Singapore Med. J. 2009, 50, 139. [Google Scholar]
- Block, R.M.; Lewis, R.D.; Shea@, J.B.; Fawley, J.; Richmond, V. Cell-Mediated Immune Response to Dog Pulp Tissue Altered by Eugenol within the Root Canal. Oral Surg. Oral Med. Oral Pathol. 1978, 45, 452–463. [Google Scholar] [CrossRef]
- Van Duuren, B.L.; Sivak, A.; Segal, A.; Orris, L.; Langseth, L. The Tumor-Promoting Agents of Tobacco Leaf and Tobacco Smoke Condensate. J Natl Cancer Inst. 1966, 37, 519–526. [Google Scholar] [PubMed]
- Van Duuren, B.L.; Goldschmidt, B.M. Cocarcinogenic and Tumor-Promoting Agents in Tobacco Carcinogenesis. J. Natl. Cancer Inst. 1976, 56, 1237–1242. [Google Scholar] [CrossRef] [PubMed]
- Sukumaran, K.; Unnikrishnan, M.C.; Kuttan, R. Inhjbition of Tumour Promotion in Mice by Eugenol. Indian J. Physiol. Pharmacol. 1994, 38, 306–308. [Google Scholar]
- Ghosh, R.; Nadiminty, N.; Fitzpatrick, J.E.; Alworth, W.L.; Slaga, T.J.; Kumar, A.P. Eugenol Causes Melanoma Growth Suppression through Inhibition of E2F1 Transcriptional Activity. J. Biol. Chem. 2005, 280, 5812–5819. [Google Scholar] [CrossRef]
- Edwards, R.L.; Luis, P.B.; Varuzza, P.V.; Joseph, A.I.; Presley, S.H.; Chaturvedi, R.; Schneider, C. The Anti-Inflammatory Activity of Curcumin Is Mediated by Its Oxidative Metabolites. J. Biol. Chem. 2017, 292, 21243–21252. [Google Scholar] [CrossRef]
- Galet, C.; Gollapudi, K.; Stepanian, S.; Byrd, J.B.; Henning, S.M.; Grogan, T.; Elashoff, D.; Heber, D.; Said, J.; Cohen, P.; et al. Effect of a Low-Fat Fish Oil Diet on Proinflammatory Eicosanoids and Cell-Cycle Progression Score in Men Undergoing Radical Prostatectomy. Cancer Prev. Res. 2014, 7, 97–104. [Google Scholar] [CrossRef]
- Panahi, Y.; Darvishi, B.; Ghanei, M.; Jowzi, N.; Beiraghdar, F.; Varnamkhasti, B.S. Molecular Mechanisms of Curcumins Suppressing Effects on Tumorigenesis, Angiogenesis and Metastasis, Focusing on NF-ΚB Pathway. Cytokine Growth Factor Rev. 2016, 28, 21–29. [Google Scholar] [CrossRef]
- Wilken, R.; Veena, M.S.; Wang, M.B.; Srivatsan, E.S. Curcumin: A Review of Anti-Cancer Properties and Therapeutic Activity in Head and Neck Squamous Cell Carcinoma. Mol. Cancer 2011, 10, 1–19. [Google Scholar] [CrossRef]
- Chandra, D.; Jahangir, A.; Cornelis, F.; Rombauts, K.; Meheus, L.; Jorcyk, C.L.; Gravekamp, C. Cryoablation and Meriva Have Strong Therapeutic Effect on Triple-Negative Breast Cancer. OncoImmunology 2016, 5, e1049802. [Google Scholar] [CrossRef]
- Nahar, P.P.; Slitt, A.L.; Seeram, N.P. Anti-Inflammatory Effects of Novel Standardized Solid Lipid Curcumin Formulations. J. Med. Food 2015, 18, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.I.; Kim, S.W.; Jung, I.D.; Lee, J.S.; Chang, J.H.; Lee, C.M.; Chun, S.H.; Yoon, M.S.; Kim, G.T.; Ryu, S.W.; et al. Curcumin Suppresses the Induction of Indoleamine 2,3-Dioxygenase by Blocking the Janus-Activated Kinase-Protein Kinase Cδ-STAT1 Signaling Pathway in Interferon-γ-Stimulated Murine Dendritic Cells. J. Biol. Chem. 2009, 284, 3700–3708. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.O.; Li, C.W.; Xia, W.; Cha, J.H.; Chan, L.C.; Wu, Y.; Chang, S.S.; Lin, W.C.; Hsu, J.M.; Hsu, Y.H.; et al. Deubiquitination and Stabilization of PD-L1 by CSN5. Cancer Cell 2016, 30, 925–939. [Google Scholar] [CrossRef] [PubMed]
- Ireson, C.R.; Jones, D.J.L.; Orr, S.; Coughtrie, M.W.H.; Boocock, D.J.; Williams, M.L.; Farmer, P.B.; Steward, W.P.; Gescher, A.J. Metabolism of the Cancer Chemopreventive Agent Curcumin in Human and Rat Intestine. Cancer Epidemiol. Prev. Biomark. 2002, 11, 105–111. [Google Scholar]
- Moradali, M.F.; Mostafavi, H.; Ghods, S.; Hedjaroude, G.A. Immunomodulating and Anticancer Agents in the Realm of Macromycetes Fungi (Macrofungi). Int. Immunopharmacol. 2007, 7, 701–724. [Google Scholar] [CrossRef]
- Wasser, S. Medicinal Mushrooms as a Source of Antitumor and Immunomodulating Polysaccharides. Appl. Microbiol. Biotechnol. 2002, 60, 258–274. [Google Scholar]
- Wachtel-Galor, S.; Yuen, J.; Buswell, J.A.; Benzie, I.F.F. Ganoderma lucidum (Lingzhi or Reishi): A Medicinal Mushroom. In Herbal Medicine: Biomolecular and Clinical Aspects, 2nd ed.; Benzie, I.F.F., Wachtel-Galor, S., Eds.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2011. Available online: https://www.ncbi.nlm.nih.gov/books/NBK92757/ (accessed on 13 April 2022)Chapter 9.
- Gao, Y.; Zhou, S.H.; Chen, G.; Dai, X.; Ye, J. A Phase III Study of a Ganoderma Lucidum (Curt. Fr.) P. Karst. Extract (Ganopoly) in Patients with Advanced Cancer. Int. J. Med. Mushroom 2002, 4, 207–214. [Google Scholar]
- Burdock, G.A. Review of the Biological Properties and Toxicity of Bee Propolis (Propolis). Food Chem. Toxicol. 1998, 36, 347–363. [Google Scholar] [CrossRef]
- Castaldo, S.; Capasso, F. Propolis, an Old Remedy Used in Modern Medicine. Fitoterapia 2002, 73 (Suppl. 1), S1–S6. [Google Scholar] [CrossRef]
- Borrelli, F.; Maffia, P.; Pinto, L.; Ianaro, A.; Russo, A.; Capasso, F.; Ialenti, A. Phytochemical Compounds Involved in the Anti-Inflammatory Effect of Propolis Extract. Fitoterapia 2002, 73 (Suppl. 1), S53–S63. [Google Scholar] [CrossRef]
- Oršolić, N.; Bašić, I. Immunomodulation by Water-Soluble Derivative of Propolis: A Factor of Antitumor Reactivity. J. Ethnopharmacol. 2003, 84, 265–273. [Google Scholar] [CrossRef]
- Marcucci, M.C.; Ferreres, F.; Custódio, A.R.; Ferreira, M.M.C.; Bankovad, V.S.; García-Viguera, C.; Bretz, W.A. Evalution of Phenolic Compounds in Brazilian Propolis from Different Geographic Regions. Z Naturforsch C J. Biosci. 2000, 55, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Cole, N.; Sou, P.W.; Ngo, A.; Tsang, K.H.; Severino, J.A.J.; Arun, S.J.; Duke, C.C.; Reeve, V.E. Topical “sydney” Propolis Protects against UV-Radiation-Induced Inflammation, Lipid Peroxidation and Immune Suppression in Mouse Skin. Int. Arch. Allergy Immunol. 2010, 152, 87–97. [Google Scholar] [CrossRef]
- Bankova, V. Recent Trends and Important Developments in Propolis Research. Evid. -Based Complementary Altern. Med. 2005, 2, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.L.; Liang, W.H.; Lee, Y.J.; Chuang, S.K.; Tseng, T.H. Antitumor Progression Potential of Caffeic Acid Phenethyl Ester Involving P75NTR in C6 Glioma Cells. Chem.-Biol. Interact. 2010, 188, 607–615. [Google Scholar] [CrossRef]
- Ang, E.S.M.; Pavlos, N.J.; Chai, L.Y.; Qi, M.; Cheng, T.S.; Steer, J.H.; Joyce, D.A.; Zheng, M.H.; Xu, J. Caffeic Acid Phenethyl Ester, an Active Component of Honeybee Propolis Attenuates Osteoclastogenesis and Bone Resorption via the Suppression of RANKL-Induced NF-ΚB and NFAT Activity. J. Cell. Physiol. 2009, 221, 642–649. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, J.K.; Kim, H.S.; Chung, S.T.; Eom, J.H.; Kim, K.A.; Chung, S.J.; Paik, S.Y.; Oh, H.Y. Immunomodulatory Effect of Caffeic Acid Phenethyl Ester in Balb/c Mice. Int. Immunopharmacol. 2004, 4, 429–436. [Google Scholar] [CrossRef]
- Huang, M.-T.; Ma, W.; Yen, P.; Xie, J.-G.; Han, J.; Frenkel, K.; Grunberger, D.; Conney, H.-A. Inhibitory Effects of Caffeic Acid Phenethyl Ester (CAPE) on 12-0-Tetradecanoylphorbol-13-Acetate-Induced Tumor Promotion in Mouse Skin and the Synthesis of DNA, RNA and Protein in HeLa Cells. Carcinogenesis 1996, 17, 761–765. [Google Scholar] [CrossRef]
- Natarajan, K.; Singh, S.; Burke, T.R.; GRUNBERGERt, D.; Aggarwal, B.B. Caffeic Acid Phenethyl Ester Is a Potent and Specific Inhibitor of Activation of Nuclear Transcription Factor NF-KB (Tumor Necrosis Factor/Okadaic Acid/Ceramide/Phorbol Ester/Hydrogen Peroxide). Proc. Natl. Acad. Sci. USA 1996, 93, 9090–9095. [Google Scholar] [CrossRef]
- Roos, T.U.; Heiss, E.H.; Schwaiberger, A.V.; Schachner, D.; Sroka, I.M.; Oberan, T.; Vollmar, A.M.; Dirsch, V.M. Caffeic Acid Phenethyl Ester Inhibits PDGF-Induced Proliferation of Vascular Smooth Muscle Cells via Activation of P38 MAPK, HIF-1α, and Heme Oxygenase-1. J. Nat. Prod. 2011, 74, 352–356. [Google Scholar] [CrossRef]
- Basini, G.; Baioni, L.; Bussolati, S.; Grasselli, F.; Daquino, C.; Spatafora, C.; Tringali, C. Antiangiogenic Properties of an Unusual Benzo[k,l]Xanthene Lignan Derived from CAPE (Caffeic Acid Phenethyl Ester). Investig. New Drugs 2012, 30, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Márquez, N.; Sancho, R.; Macho, A.; Calzado, M.A.; Fiebich, B.L.; Muñoz, E. Caffeic Acid Phenethyl Ester Inhibits T-Cell Activation by Targeting Both Nuclear Factor of Activated T-Cells and NF-ΚB Transcription Factors. J. Pharmacol. Exp. Ther. 2004, 308, 993–1001. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.C.; Lin, Y.L.; Liang, Y.C.; Yang, Y.H.; Lee, J.H.; Yu, H.H.; Wu, W.M.; Chiang, B.L. The Effect of Caffeic Acid Phenethyl Ester on the Functions of Human Monocyte-Derived Dendritic Cells. BMC Immunol. 2009, 10, 1–13. [Google Scholar] [CrossRef]
- Wang, L.C.; Chu, K.H.; Liang, Y.C.; Lin, Y.L.; Chiang, B.L. Caffeic Acid Phenethyl Ester Inhibits Nuclear Factor-ΚB and Protein Kinase B Signalling Pathways and Induces Caspase-3 Expression in Primary Human CD4+ T Cells. Clin. Exp. Immunol. 2010, 160, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.-Z.; Lin, J.; Grunberger, D.; Fisher2, P.B. Growth Suppression and Toxicity Induced by Caffeic Acid Phenethyl Ester (CAPE) in Type 5 Adenovirus-Transformed Rat Embryo Cells Correlate Directly with Transformation Progression. Cancer Res 1994, 54, 1865–1870. [Google Scholar] [PubMed]
- Chena, J.-H.; Shaoa, Y.; Huang, M.; Chinb, C.-K.; Hoa, C.-T. Inhibitory Effect of Caffeic Acid Phenethyl Ester on Human Leukemia HL-60 Cells. Cancer Lett. 1996, 108, 211–214. [Google Scholar] [CrossRef]
- Lee, Y.-J.; Liao, P.-H.; Chen, W.-K.; Yang, C.-C. Preferential Cytotoxicity of Caffeic Acid Phenethyl Ester Analogues on Oral Cancer Cells. Cancer Lett. 2000, 153, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Paulino, N.; Abreu, S.R.L.; Uto, Y.; Koyama, D.; Nagasawa, H.; Hori, H.; Dirsch, V.M.; Vollmar, A.M.; Scremin, A.; Bretz, W.A. Anti-Inflammatory Effects of a Bioavailable Compound, Artepillin C, in Brazilian Propolis. Eur. J. Pharmacol. 2008, 587, 296–301. [Google Scholar] [CrossRef]
- von Holtz, R.L.; Fink, C.S.; Awad, A.B. β-Sitosterol Activates the Sphingomyelin Cycle and Induces Apoptosis in LNCaP Human Prostate Cancer Cells. Nutr. Cancer 1998, 32, 8–12. [Google Scholar] [CrossRef]
- Galasso, C.; Nuzzo, G.; Brunet, C.; Ianora, A.; Sardo, A.; Fontana, A.; Sansone, C. The Marine Dinoflagellate Alexandrium Minutum Activates a Mitophagic Pathway in Human Lung Cancer Cells. Mar. Drugs 2018, 16, 502. [Google Scholar] [CrossRef]
- D’Ippolito, G.; Cutignano, A.; Tucci, S.; Romano, G.; Cimino, G.; Fontana, A. Biosynthetic intermediates and stereochemical aspects of aldehyde biosynthesis in the marine diatom Thalassiosira rotula. Phytochemistry 2006, 67, 314–322. [Google Scholar] [CrossRef]
- Kim, S.K.; Thomas, N.V.; Li, X. Anticancer Compounds from Marine Macroalgae and Their Application as Medicinal Foods. In Advances in Food and Nutrition Research; Academic Press Inc.: Cambridge, MA, USA, 2011; Volume 64, pp. 213–224. [Google Scholar]
- Maruyama, H.; Tamauchi, H.; Iizuka, M.; Nakano, T.; Maruyamá, H. The Role of NK Cells in Antitumor Activity of Dietary Fucoidan from Undaria Pinnatifida Sporophylls (Mekabu). Planta Med. 2006, 72, 1415–1417. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Sun, Y.P.; Xin, H.; Zhang, Y.; Li, Z.; Xu, Z. In Vivo Antitumor and Immunomodulation Activities of Different Molecular Weight Lambda-Carrageenans from Chondrus Ocellatus. Pharmacol. Res. 2004, 50, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, L.; Zhou, Y. Immunomodulation and Antitumor Activities of Different-Molecular-Weight Polysaccharides from Porphyridium Cruentum. Carbohydr. Polym. 2012, 87, 1206–1210. [Google Scholar] [CrossRef]
- Shi, W.; Lu, D.; Wu, C.; Li, M.; Ding, Z.; Li, Y.; Chen, B.; Lin, X.; Su, W.; Shao, X.; et al. Coibamide A Kills Cancer Cells through Inhibiting Autophagy. Biochem. Biophys. Res. Commun. 2021, 547, 52–58. [Google Scholar] [CrossRef]
- Kong, C.S.; Kim, J.A.; Yoon, N.Y.; Kim, S.K. Induction of Apoptosis by Phloroglucinol Derivative from Ecklonia Cava in MCF-7 Human Breast Cancer Cells. Food Chem. Toxicol. 2009, 47, 1653–1658. [Google Scholar] [CrossRef]
- Kurihara, H.; Koda, H.; Asami, S.; Kiso, Y.; Tanaka, T. Contribution of the Antioxidative Property of Astaxanthin to Its Protective Effect on the Promotion of Cancer Metastasis in Mice Treated with Restraint Stress. Life Sci. 2002, 70, 2509–2520. [Google Scholar] [CrossRef]
- Tanaka, T.; Morishita, Y.; Suzui, M.; Kojima, T.; Okumura, A.; Mori, H. Chemoprevention of Mouse Urinary Bladder Carcinogenesis by the Naturally Occurring Carotenoid Astaxanthin. Carcinogenesis 1994, 15, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Shnimizu, M.; Moriwaki, H. Cancer Chemoprevention by Carotenoids. Molecules 2012, 17, 3202–3242. [Google Scholar] [CrossRef]
- Tanaka, T.; Makita, H.; Ohnishi, M.; Mori, H.; Satoh, K.; Hara, A. Chemoprevention of rat oral carcinogenesis by naturally occurring xanthophylls, astaxanthin and canthaxanthin. Cancer Res. 1995, 55, 4059–4064. [Google Scholar]
- Chang, Y.-J.; Huang, J.-R.; Tsai, Y.-C.; Hung, J.-T.; Wu, D.; Fujio, M.; Wong, C.-H.; Yu, A.L. Potent Immune-Modulating and Anticancer Effects of NKT Cell Stimulatory Glycolipids. Proc. Natl. Acad. Sci. USA 2007, 104, 10299–10304. [Google Scholar] [CrossRef] [PubMed]
- Bae, E.A.; Seo, H.; Kim, I.K.; Jeon, I.; Kang, C.Y. Roles of NKT Cells in Cancer Immunotherapy. Arch. Pharmacol. Res. 2019, 42, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Manzo, E.; Cutignano, A.; Pagano, D.; Gallo, C.; Barra, G.; Nuzzo, G.; Sansone, C.; Ianora, A.; Urbanek, K.; Fenoglio, D.; et al. A New Marine-Derived Sulfoglycolipid Triggers Dendritic Cell Activation and Immune Adjuvant Response. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gallo, C.; Ferrera, F.; Castiglia, D.; Fontana, A. Identication of Sulfavant A as the First Synthetic TREM2 Ligand Discloses a Homeostatic Response of Dendritic Cells After Receptor Engagement. Res. Sq. 2022. [Google Scholar] [CrossRef]
- Rinehart, K.L.; Gloer, J.B.; Cook, J.C.; Mizsak, S.A.; Scahill, T.A. Structures of the Didemnins, Antiviral and Cytotoxic Depsipeptides from a Caribbean Tunicate. J. Am. Chem. Soc. 1981, 103, 1857–1859. [Google Scholar] [CrossRef]
- Thell, K.; Hellinger, R.; Schabbauer, G.; Gruber, C.W. Immunosuppressive Peptides and Their Therapeutic Applications. Drug Discov. Today 2014, 19, 645–653. [Google Scholar] [CrossRef]
- Lee, J.; Currano, J.N.; Carroll, P.J.; Joullié, M.M. Didemnins, Tamandarins and Related Natural Products. Nat. Prod. Rep. 2012, 29, 404–424. [Google Scholar] [CrossRef]
- Tsukimoto, M.; Nagaoka, M.; Shishido, Y.; Fujimoto, J.; Nishisaka, F.; Matsumoto, S.; Harunari, E.; Imada, C.; Matsuzaki, T. Bacterial Production of the Tunicate-Derived Antitumor Cyclic Depsipeptide Didemnin B. J. Nat. Prod. 2011, 74, 11–2329. [Google Scholar] [CrossRef]
- Xu, Y.; Kersten, R.D.; Nam, S.J.; Lu, L.; Al-Suwailem, A.M.; Zheng, H.; Fenical, W.; Dorrestein, P.C.; Moore, B.S.; Qian, P.Y. Bacterial biosynthesis and maturation of the didemnin anti-cancer agents. J. Am. Chem. Soc. 2012, 134, 8625–8632. [Google Scholar] [CrossRef]
- Zhang, Q.T.; Liu, Z.D.; Wang, Z.; Wang, T.; Wang, N.; Wang, N.; Zhang, B.; Zhao, Y.F. Recent Advances in Small Peptides of Marine Origin in Cancer Therapy. Mar. Drugs 2021, 19, 115. [Google Scholar] [CrossRef]
- Delgado-Calle, J.; Kurihara, N.; Atkinson, E.G.; Nelson, J.; Miyagawa, K.; Galmarini, C.M.; Roodman, G.D.; Bellido, T. Aplidin (plitidepsin) is a novel anti-myeloma agent with potent anti-resorptive activity mediated by direct effects on osteoclasts. Oncotarget 2019, 10, 2709–2721. [Google Scholar] [CrossRef] [PubMed]
- White, K.M.; Rosales, R.; Yildiz, S.; Kehrer, T.; Miorin, L.; Moreno, E.; Jangra, S.; Uccellini, M.B.; Rathnasinghe, R.; Coughlan, L.; et al. Plitidepsin has potent preclinical efficacy against SARS-CoV-2 by targeting the host protein eEF1A. Science 2021, 371, 926–931. [Google Scholar] [CrossRef] [PubMed]
- Cooper, E.L.; Yao, D. Diving for Drugs: Tunicate Anticancer Compounds. Drug Discov. Today 2012, 17, 636–648. [Google Scholar] [CrossRef] [PubMed]
- Oda, T.; Kamoshita, K.; Maruyama, S.; Masuda, K.; Nishimoto, M.; Xu, J.; Ukai, K.; Mangindaan, R.E.P.; Namikoshi, M. Cytotoxicity of Lissoclibadins and Lissoclinotoxins, Isolated from a Tropical Ascidian Lissoclinum Cf. Badium, against Human Solid-Tumor-Derived Cell Lines. Biol. Pharm. Bull. 2007, 30, 385–387. [Google Scholar] [CrossRef][Green Version]
- Fedorov, S.N.; Radchenko, O.S.; Shubina, L.K.; Balaneva, N.N.; Bode, A.M.; Stonik, V.A.; Dong, Z. Evaluation of cancer-preventive activity and structure-activity relationships of 3-demethylubiquinone Q2, isolated from the ascidian Aplidium glabrum, and its synthetic analogs. Pharm. Res. 2006, 23, 70–81. [Google Scholar] [CrossRef]
- Nuzzo, G.; Gallo, C.; Crocetta, F.; Romano, L.; Barra, G.; Senese, G.; dell’Isola, M.; Carbone, D.; Tanduo, V.; Albiani, F.; et al. Identification of the Marine Alkaloid Lepadin A as Potential Inducer of Immunogenic Cell Death. Biomolecules 2022, 12, 246. [Google Scholar] [CrossRef]
- Journal, E.; Meenakshi, V.K.; Senthamarai, S.; Paripooranaselvi, M.; Gomathy, S.; Sankaravadivu, S.; Chamundeswari, K.P. Scholars Research Library In Vitro and in Vivo Antitumor and Immunomodulatory Studies of Microcosmus Exasperatus against DLA Bearing Mice. Eur. J. Appl. Eng. Sci. Res. 2013, 23, 18–25. Available online: http://scholarsresearchlibrary.com/archive.html (accessed on 13 April 2022).
- Pps APC, P. Antitumor and Immunomodulatory Activity of Phallusia Nigra Savigny, 1816 Against Ehrlich Ascites Carcinoma. Res. J. Pharm. Sci. 2012, 1, 7–12. [Google Scholar] [CrossRef]
- Janakiram, N.B.; Mohammed, A.; Rao, C.V. Sea Cucumbers Metabolites as Potent Anti-Cancer Agents. Mar. Drugs 2015, 13, 2909–2923. [Google Scholar] [CrossRef]
- Pettit, G.R.; Herald, C.L.; Doubek, D.L.; Herald, D.L.; Arnold, E.; Clardy, J. Isolation and structure of bryostatin-1. Am. Chem. Soc. 1982, 104, 24–6846. [Google Scholar] [CrossRef]
- Raghuvanshi, R.; Bharate, S.B. Preclinical and Clinical Studies on Bryostatins, A Class of Marine-Derived Protein Kinase C Modulators: A Mini-Review. Curr Top Med Chem. 2020, 20, 1124–1135. [Google Scholar] [CrossRef] [PubMed]
- Trenn, G.; Pettit, G.R.; Takayama, H.; Hu-Li, J.; Sitkovsky, M.V. Immunomodulating Properties of a Novel Series of Protein Kinase C Activators. The Bryostatins. J. Immunol. 1988, 140, 433–439. [Google Scholar] [PubMed]
- Hornung, R.L.; Pearson, J.W.; Beckwith, M.; Longo, D.L. Preclinical Evaluation of Bryostatin as an Anticancer Agent against Several Murine Tumor Cell Lines in Vitro versus in Vivo Activity. Cancer Res. 1992, 52, 101–107. [Google Scholar] [PubMed]
- Mary Varterasian, B.L.; Mohammad, R.M.; Eilender, D.S.; Hulburd, K.; Rodriguez, D.H.; Pemberton, P.A.; Pluda, J.M.; Dan, M.D.; Chen, B.D.; Al-Katib, A.M. Phase I Study of Bryostatin 1 in Patients with Relapsed. Non-Hodgkin’s Lymphoma and Chronic Lymphocytic Leukemia. J. Clin. Oncol. 1998, 16, 56–62. [Google Scholar] [CrossRef]
- Rinehart, K.L.; Holt, T.G.; Fregeau, N.L.; Stroh, J.G.; Keifer, P.A.; Sun, F.; Li, L.H.; Martin, D.G. Ecteinascidins 729, 743, 745, 759A, 759B, and 770: Potent antitumor agents from the Caribbean tunicate Ecteinascidia turbinata. J. Org. Chem. 1990, 55, 4512–4515. [Google Scholar] [CrossRef]
- Larsen, A.K.; Galmarini, C.M.; D’Incalci, M. Unique features of Trabectedin mechanism of action. Cancer Chemother. Pharmacol. 2016, 77, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Belgiovine, C.; Frapolli, R.; Liguori, M.; Digifico, E.; Colombo, F.S.; Meroni, M.; Allavena, P.; D’Incalci, M. Inhibition of tumor-associated macrophages by trabectedin improves the antitumor adaptive immunity in response to anti-PD-1 therapy. Eur. J. Immunol. 2021, 51, 2677–2686. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, P.; Zhang, R.; Ivan, C.; Galletti, G.; Clise-Dwyer, K.; Barbaglio, F.; Scarfò, L.; Aracil, M.; Klein, C.; Wierda, W.; et al. Trabectedin Reveals a Strategy of Immunomodulation in Chronic Lymphocytic Leukemia. Cancer Immunol. Res. 2019, 7, 2036–2051. [Google Scholar] [CrossRef]
- D’Ippolito, G.; Iadicicco, O.; Romano, G. Fontana A Detection of short-chain aldehydes in marine organisms: The diatom Thalassiosira rotula. Tetrahedron. Lett. 2002, 43, 6137–6140. [Google Scholar] [CrossRef]
- Cutignano, A.; D’Ippolito, G.; Romano, G.; Lamari, N.; Cimino, G.; Febbraio, F.; Nucci, R.; Fontana, A. Chloroplastic Glycolipids Fuel Aldehyde Biosynthesis in the Marine Diatom Thalassiosira rotula. ChemBioChem 2006, 7, 450–456. [Google Scholar] [CrossRef]
- D’Ippolito, G.; Romano, G.; Caruso, T.; Spinella, A.; Cimino, G.; Fontana, A. Production of octadienal in the marine diatom Skeletonema costatum. Org. Lett. 2003, 5, 885–887. [Google Scholar] [CrossRef] [PubMed]
- D’Ippolito, G.; Tucci, S.; Cutignano, A.; Giovanna, R.; Cimino, G.; Miralto, A.; Fontana, A. The role of complex lipids in the synthesis of bioactive aldehydes of the marine diatom Skeletonema costatum. Biochim. Biophys Acta–Mol. Cell Biol. Lipids 2004, 1686, 100–107. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nuzzo, G.; Senese, G.; Gallo, C.; Albiani, F.; Romano, L.; d’Ippolito, G.; Manzo, E.; Fontana, A. Antitumor Potential of Immunomodulatory Natural Products. Mar. Drugs 2022, 20, 386. https://doi.org/10.3390/md20060386
Nuzzo G, Senese G, Gallo C, Albiani F, Romano L, d’Ippolito G, Manzo E, Fontana A. Antitumor Potential of Immunomodulatory Natural Products. Marine Drugs. 2022; 20(6):386. https://doi.org/10.3390/md20060386
Chicago/Turabian StyleNuzzo, Genoveffa, Giuseppina Senese, Carmela Gallo, Federica Albiani, Lucia Romano, Giuliana d’Ippolito, Emiliano Manzo, and Angelo Fontana. 2022. "Antitumor Potential of Immunomodulatory Natural Products" Marine Drugs 20, no. 6: 386. https://doi.org/10.3390/md20060386
APA StyleNuzzo, G., Senese, G., Gallo, C., Albiani, F., Romano, L., d’Ippolito, G., Manzo, E., & Fontana, A. (2022). Antitumor Potential of Immunomodulatory Natural Products. Marine Drugs, 20(6), 386. https://doi.org/10.3390/md20060386









