Antihypertensive Effects of IGTGIPGIW Peptide Purified from Hippocampus abdominalis: p-eNOS and p-AKT Stimulation in EA.hy926 Cells and Lowering of Blood Pressure in SHR Model
Abstract
:1. Introduction
2. Results
2.1. Non-Cytotoxicity of IGTGIPGIW in EA.hy926 Cells
2.2. Effects of IGTGIPGIW on NO Generation in EA.hy926 Cells
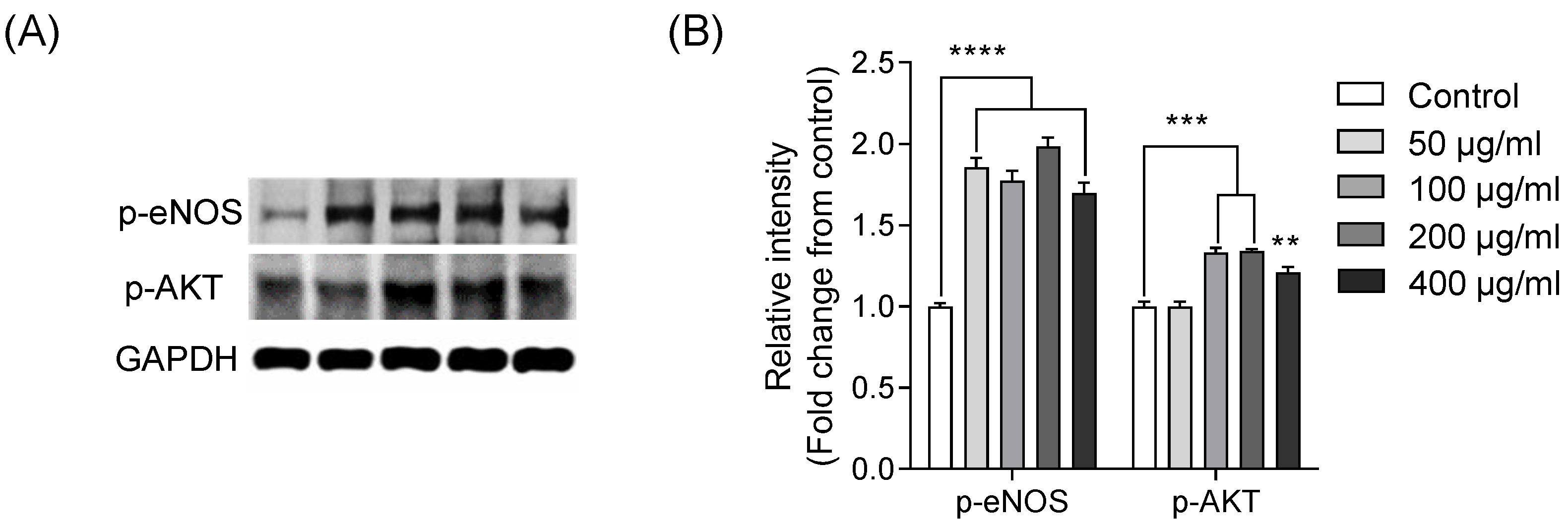
2.3. Vasorelaxation Mediated via p-AKT and p-eNOS Pathways in EA.hy926 Cells Treated with IGTGIPGIW
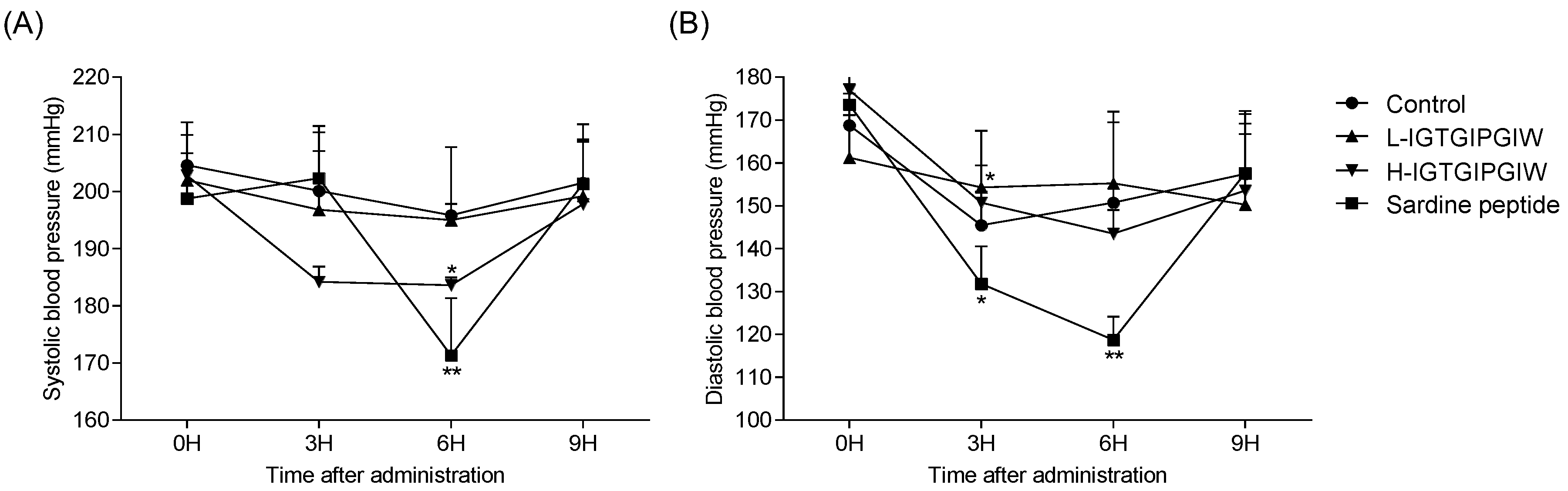
2.4. Effects of IGTGIPGIW on SBP and DBP of SHRs
2.5. Chromatographic Analysis of the Purified IGTGIPGIW Peptide
3. Discussion
4. Materials and Methods
4.1. Materials and Chemicals
4.2. Purification of IGTGIPGIW from Alcalase HydrolysaSte of H. abdominalis
4.3. Cell Culture and Cytotoxicity Analysis
4.4. Amino Acid Sequence Analysis
4.5. Detection of NO Production in Endothelial Cells
4.6. Western Blot Assay
4.7. Experimental Animals
4.8. Measurement of SBP and DBP
4.9. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, R.; Suh, I.; Singh, V.; Chaithiraphan, S.; Laothavorn, P.; Sy, R.; Babilonia, N.; Rahman, A.; Sheikh, S.; Tomlinson, B. Hypertension and stroke in Asia: Prevalence, control and strategies in developing countries for prevention. J. Hum. Hypertens. 2000, 14, 749–763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whelton, P.; He, J.; Muntner, P. Prevalence, awareness, treatment and control of hypertension in North America, North Africa and Asia. J. Hum. Hypertens. 2004, 18, 545–551. [Google Scholar] [CrossRef] [Green Version]
- Wolf-Maier, K.; Cooper, R.S.; Banegas, J.R.; Giampaoli, S.; Hense, H.-W.; Joffres, M.; Kastarinen, M.; Poulter, N.; Primatesta, P.; Rodríguez-Artalejo, F. Hypertension prevalence and blood pressure levels in 6 European countries, Canada, and the United States. JAMA 2003, 289, 2363–2369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mrowka, R. Recent Advances in Hypertension Research; Wiley Online Library: Hoboken, NJ, USA, 2019; Volume 226, p. e13295. [Google Scholar]
- Mills, K.T.; Stefanescu, A.; He, J. The global epidemiology of hypertension. Nat. Rev. Nephrol. 2020, 16, 223–237. [Google Scholar] [CrossRef]
- Chrysaidou, K.; Chainoglou, A.; Karava, V.; Dotis, J.; Printza, N.; Stabouli, S. Secondary hypertension in children and adolescents: Novel insights. Curr. Hypertens. Rev. 2020, 16, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Flynn, J.T. The Hypertensive Adolescent. Clin. J. Am. Soc. Nephrol. 2019, 14, 1074–1076. [Google Scholar] [CrossRef] [PubMed]
- Sabri, M.; Gheissari, A.; Mansourian, M.; Mohammadifard, N.; Sarrafzadegan, N. Essential hypertension in children, a growing worldwide problem. J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci. 2019, 24, 109. [Google Scholar]
- Falkner, B. The childhood role in development of primary hypertension. Am. J. Hypertens. 2018, 31, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Kjeldsen, S.E. Hypertension and cardiovascular risk: General aspects. Pharmacol. Res. 2018, 129, 95–99. [Google Scholar] [CrossRef]
- Christiansen, S.C.; Schatz, M.; Yang, S.-J.; Ngor, E.; Chen, W.; Zuraw, B.L. Hypertension and asthma: A comorbid relationship. J. Allergy Clin. Immunol. Pract. 2016, 4, 76–81. [Google Scholar] [CrossRef]
- Long, A.N.; Dagogo-Jack, S. Comorbidities of diabetes and hypertension: Mechanisms and approach to target organ protection. J. Clin. Hypertens. 2011, 13, 244–251. [Google Scholar] [CrossRef] [Green Version]
- Petrie, J.R.; Guzik, T.J.; Touyz, R.M. Diabetes, hypertension, and cardiovascular disease: Clinical insights and vascular mechanisms. Can. J. Cardiol. 2018, 34, 575–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugerman, H.J.; Wolfe, L.G.; Sica, D.A.; Clore, J.N. Diabetes and hypertension in severe obesity and effects of gastric bypass-induced weight loss. Ann. Surg. 2003, 237, 751. [Google Scholar] [CrossRef] [PubMed]
- Iwakiri, Y.; Groszmann, R.J. Pathophysiology of portal hypertension. Liver Biol. Pathobiol. 2020, 21, 659–669. [Google Scholar]
- Simonetto, D.A.; Liu, M.; Kamath, P.S. Portal hypertension and related complications: Diagnosis and management. Mayo Clin. Proc. 2019, 94, 714–726. [Google Scholar] [CrossRef] [Green Version]
- Lehrer, S.; Stone, J.; Lapinski, R.; Lockwood, C.J.; Schachter, B.S.; Berkowitz, R.; Berkowitz, G.S. Association between pregnancy-induced hypertension and asthma during pregnancy. Am. J. Obstet. Gynecol. 1993, 168, 1463–1466. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.-K.; Wijesekara, I. Development and biological activities of marine-derived bioactive peptides: A review. J. Funct. Foods 2010, 2, 1–9. [Google Scholar] [CrossRef]
- Ngo, D.-H.; Vo, T.-S.; Ngo, D.-N.; Wijesekara, I.; Kim, S.-K. Biological activities and potential health benefits of bioactive peptides derived from marine organisms. Int. J. Biol. Macromol. 2012, 51, 378–383. [Google Scholar] [CrossRef]
- Pujiastuti, D.Y.; Ghoyatul Amin, M.N.; Alamsjah, M.A.; Hsu, J.-L. Marine organisms as potential sources of bioactive peptides that inhibit the activity of angiotensin I-converting enzyme: A review. Molecules 2019, 24, 2541. [Google Scholar] [CrossRef] [Green Version]
- Abachi, S.; Bazinet, L.; Beaulieu, L. Antihypertensive and angiotensin-I-converting enzyme (ACE)-inhibitory peptides from fish as potential cardioprotective compounds. Mar. Drugs 2019, 17, 613. [Google Scholar] [CrossRef] [Green Version]
- Oh, J.-Y.; Kim, E.-A.; Lee, H.; Kim, H.-S.; Lee, J.-S.; Jeon, Y.-J. Antihypertensive effect of surimi prepared from olive flounder (Paralichthys olivaceus) by angiotensin-I converting enzyme (ACE) inhibitory activity and characterization of ACE inhibitory peptides. Process Biochem. 2019, 80, 164–170. [Google Scholar] [CrossRef]
- Yathisha, U.G.; Bhat, I.; Karunasagar, I.; Mamatha, B.S. Antihypertensive activity of fish protein hydrolysates and its peptides. Crit. Rev. Food Sci. Nutr. 2019, 59, 2363–2374. [Google Scholar]
- Abuine, R.; Rathnayake, A.U.; Byun, H.-G. Biological activity of peptides purified from fish skin hydrolysates. Fish. Aquat. Sci. 2019, 22, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.-H.; Chen, C.-A.; Tsai, J.-S.; Chen, G.-W. Preparation and identification of novel antihypertensive peptides from the in vitro gastrointestinal digestion of marine cobia skin hydrolysates. Nutrients 2019, 11, 1351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nasri, M. Bioactive Peptides from Fish Collagen Byproducts: A Review. In Byproducts from Agriculture and Fisheries: Adding Value for Food, Feed, Pharma, and Fuels; John Wiley & Sons: Hoboken, NJ, USA, 2019; pp. 309–333. [Google Scholar]
- Ghalamara, S.; Silva, S.; Brazinha, C.; Pintado, M. Valorization of fish by-products: Purification of bioactive peptides from codfish blood and sardine cooking wastewaters by membrane processing. Membranes 2020, 10, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shukla, S. Therapeutic Importance of Peptides from Marine Source: A Mini Review; NISCAIR-CSIR: New Delhi, India, 2016; pp. 1422–1431. [Google Scholar]
- Kim, H.-S.; Je, J.-G.; Ryu, B.; Kang, N.; Shanura Fernando, I.; Jayawardena, T.U.; Asanka Sanjeewa, K.; Oh, J.-Y.; Lee, T.-G.; Jeon, Y.-J. Antioxidant and angiotensin-I converting enzyme inhibitory peptides from Hippocampus abdominalis. Eur. Food Res. Technol. 2019, 245, 479–487. [Google Scholar] [CrossRef]
- Marrazzo, F.; Larson, G.; Lama, T.T.S.; Droghi, M.T.; Joyce, M.; Ichinose, F.; Watkins, M.T.; Stowell, C.; Crowley, J.; Berra, L. Inhaled nitric oxide prevents systemic and pulmonary vasoconstriction due to hemoglobin-based oxygen carrier infusion: A case report. J. Crit. Care 2019, 51, 213–216. [Google Scholar] [CrossRef]
- Taguchi, K.; Matsumoto, T.; Kamata, K.; Kobayashi, T. Akt/eNOS pathway activation in endothelium-dependent relaxation is preserved in aortas from female, but not from male, type 2 diabetic mice. Pharmacol. Res. 2012, 65, 56–65. [Google Scholar] [CrossRef]
- Fuchs, F.D.; Whelton, P.K. High blood pressure and cardiovascular disease. Hypertension 2020, 75, 285–292. [Google Scholar] [CrossRef]
- Borzecki, A.M.; Wong, A.T.; Hickey, E.C.; Ash, A.S.; Berlowitz, D.R. Identifying hypertension-related comorbidities from administrative data: What’s the optimal approach? Am. J. Med. Qual. 2004, 19, 201–206. [Google Scholar] [CrossRef]
- Fang, L.; Karakiulakis, G.; Roth, M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir. Med. 2020, 8, e21. [Google Scholar] [CrossRef]
- Carey, R.M.; Whelton, P.K.; Hypertension Guideline Writing Committee. Prevention, detection, evaluation, and management of high blood pressure in adults: Synopsis of the 2017 American College of Cardiology/American Heart Association Hypertension Guideline. Ann. Intern. Med. 2018, 168, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Teske, P.R.; Cherry, M.I.; Matthee, C.A. The evolutionary history of seahorses (Syngnathidae: Hippocampus): Molecular data suggest a West Pacific origin and two invasions of the Atlantic Ocean. Mol. Phylogenet. Evol. 2004, 30, 273–286. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Ryu, B.-M.; Qian, Z.-J. A review-biology, aquaculture and medical use of seahorse. Hippocampus spp. Annu. Res. Rev. Biol. 2017, 1–12. [Google Scholar]
- Chen, L.; Wang, X.; Huang, B. The genus Hippocampus—A review on traditional medicinal uses, chemical constituents and pharmacological properties. J. Ethnopharmacol. 2015, 162, 104–111. [Google Scholar] [CrossRef]
- Zhang, Y.; Ryu, B.; Cui, Y.; Li, C.; Zhou, C.; Hong, P.; Lee, B.; Qian, Z.-J. A peptide isolated from Hippocampus abdominalis improves exercise performance and exerts anti-fatigue effects via AMPK/PGC-1α pathway in mice. J. Funct. Foods 2019, 61, 103489. [Google Scholar] [CrossRef]
- Molagoda, I.M.N.; Choi, Y.H.; Lee, S.; Sung, J.; Lee, C.R.; Lee, H.G.; Lim, J.; Lee, K.-J.; Jeon, Y.-J.; Ma, J. Ethanolic extract of Hippocampus abdominalis exerts anti-melanogenic effects in B16F10 melanoma cells and zebrafish larvae by activating the ERK signaling pathway. Cosmetics 2019, 7, 1. [Google Scholar] [CrossRef] [Green Version]
- Choo, C.; Liew, H. Exploitation and trade in seahorses in Peninsular Malaysia. Malay. Nat. J. 2005, 57, 57–66. [Google Scholar]
- Martin-Smith, K.M.; Vincent, A.C. Exploitation and trade of Australian seahorses, pipehorses, sea dragons and pipefishes (family Syngnathidae). Oryx 2006, 40, 141–151. [Google Scholar] [CrossRef] [Green Version]
- Rosa, I.L.; Oliveira, T.P.; Osório, F.M.; Moraes, L.E.; Castro, A.L.; Barros, G.M.; Alves, R. Fisheries and trade of seahorses in Brazil: Historical perspective, current trends, and future directions. Biodivers. Conserv. 2011, 20, 1951–1971. [Google Scholar] [CrossRef]
- Park, H.-J.; Kim, J.-Y.; Kim, H.S.; Lee, S.-H.; Jang, J.S.; Lee, M.H. Synergistic effect of fruit–seed mixed juice on inhibition of angiotensin I-converting enzyme and activation of NO production in EA. hy926 cells. Food Sci. Biotechnol. 2019, 28, 881–893. [Google Scholar] [CrossRef] [Green Version]
- Sato, A.; Nishioka, S.; Kiuchi, M.; Imada, Y.; Makino, K.; Nakagawa, K.; Tanaka, R.; Matsumura, Y.; Ohkita, M. Grape Extract from Chardonnay Seeds Restores Deoxycorticosterone Acetate–Salt-Induced Endothelial Dysfunction and Hypertension in Rats. Biol. Pharm. Bull. 2020, 43, 59–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huan, Y.; Cohen, D.L.; Townsend, R.R. Pathophysiology of Hypertension in Chronic Kidney Disease. In Chronic Renal Disease; Elsevier: Amsterdam, The Netherlands, 2015; pp. 163–169. [Google Scholar]
- Pugh, D.; Gallacher, P.J.; Dhaun, N. Management of hypertension in chronic kidney disease. Drugs 2019, 79, 365–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahmdel, M.; Cho, S.M.; Jeon, Y.-J.; Lee, D.H. A Flounder Fish Peptide Shows Anti-Hypertensive Effects by Suppressing the Renin-Angiotensin-Aldosterone System and Endothelin-1. Protein Pept. Lett. 2021, 28, 831–840. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.-G.; Kim, H.-S.; An, H.; Baek, K.; Lee, J.M.; Yim, M.-J.; Ko, S.-C.; Kim, J.-Y.; Oh, G.-W.; Je, J.-G.; et al. Antihypertensive Effects of IGTGIPGIW Peptide Purified from Hippocampus abdominalis: p-eNOS and p-AKT Stimulation in EA.hy926 Cells and Lowering of Blood Pressure in SHR Model. Mar. Drugs 2022, 20, 354. https://doi.org/10.3390/md20060354
Lee H-G, Kim H-S, An H, Baek K, Lee JM, Yim M-J, Ko S-C, Kim J-Y, Oh G-W, Je J-G, et al. Antihypertensive Effects of IGTGIPGIW Peptide Purified from Hippocampus abdominalis: p-eNOS and p-AKT Stimulation in EA.hy926 Cells and Lowering of Blood Pressure in SHR Model. Marine Drugs. 2022; 20(6):354. https://doi.org/10.3390/md20060354
Chicago/Turabian StyleLee, Hyo-Geun, Hyun-Soo Kim, Hyesuck An, Kyunghwa Baek, Jeong Min Lee, Mi-Jin Yim, Seok-Chun Ko, Ji-Yul Kim, Gun-Woo Oh, Jun-Geon Je, and et al. 2022. "Antihypertensive Effects of IGTGIPGIW Peptide Purified from Hippocampus abdominalis: p-eNOS and p-AKT Stimulation in EA.hy926 Cells and Lowering of Blood Pressure in SHR Model" Marine Drugs 20, no. 6: 354. https://doi.org/10.3390/md20060354
APA StyleLee, H.-G., Kim, H.-S., An, H., Baek, K., Lee, J. M., Yim, M.-J., Ko, S.-C., Kim, J.-Y., Oh, G.-W., Je, J.-G., Lee, D.-S., & Jeon, Y.-J. (2022). Antihypertensive Effects of IGTGIPGIW Peptide Purified from Hippocampus abdominalis: p-eNOS and p-AKT Stimulation in EA.hy926 Cells and Lowering of Blood Pressure in SHR Model. Marine Drugs, 20(6), 354. https://doi.org/10.3390/md20060354







