Antimicrobial and Immunoregulatory Activities of TS40, a Derived Peptide of a TFPI-2 Homologue from Black Rockfish (Sebastes schlegelii)
Abstract
1. Introduction
2. Results
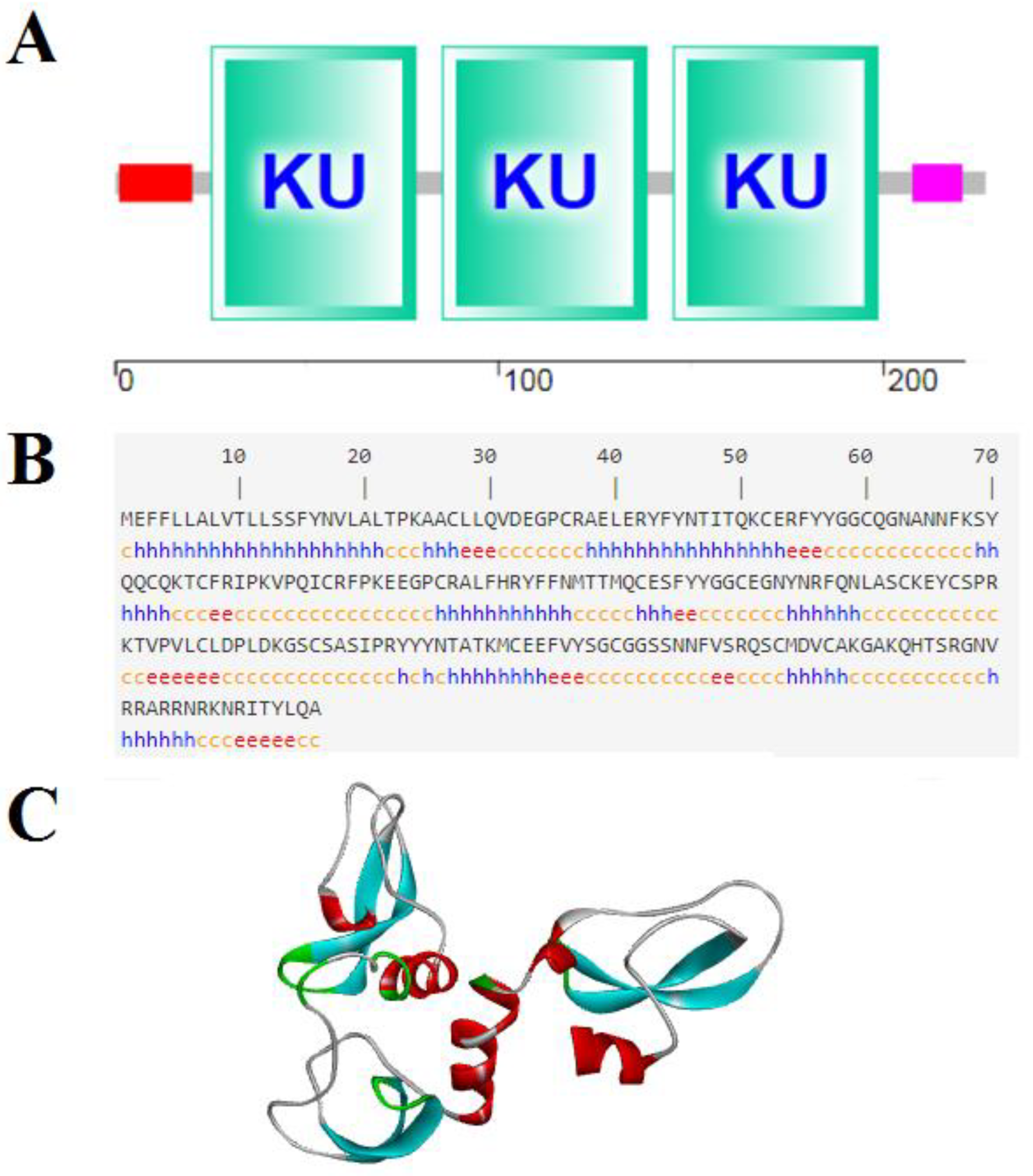
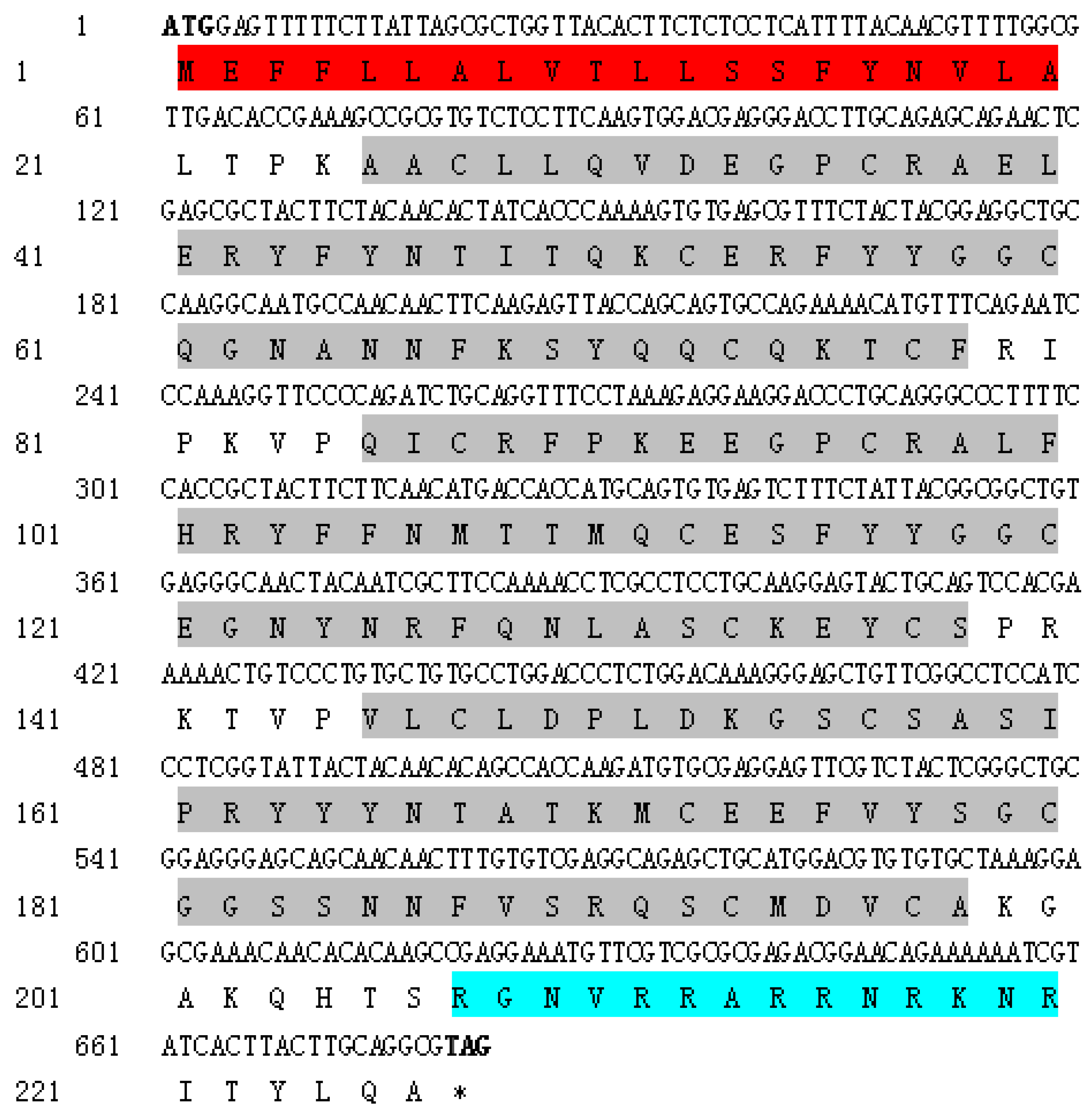
2.1. Sequence Characterization of SsTFPI-2
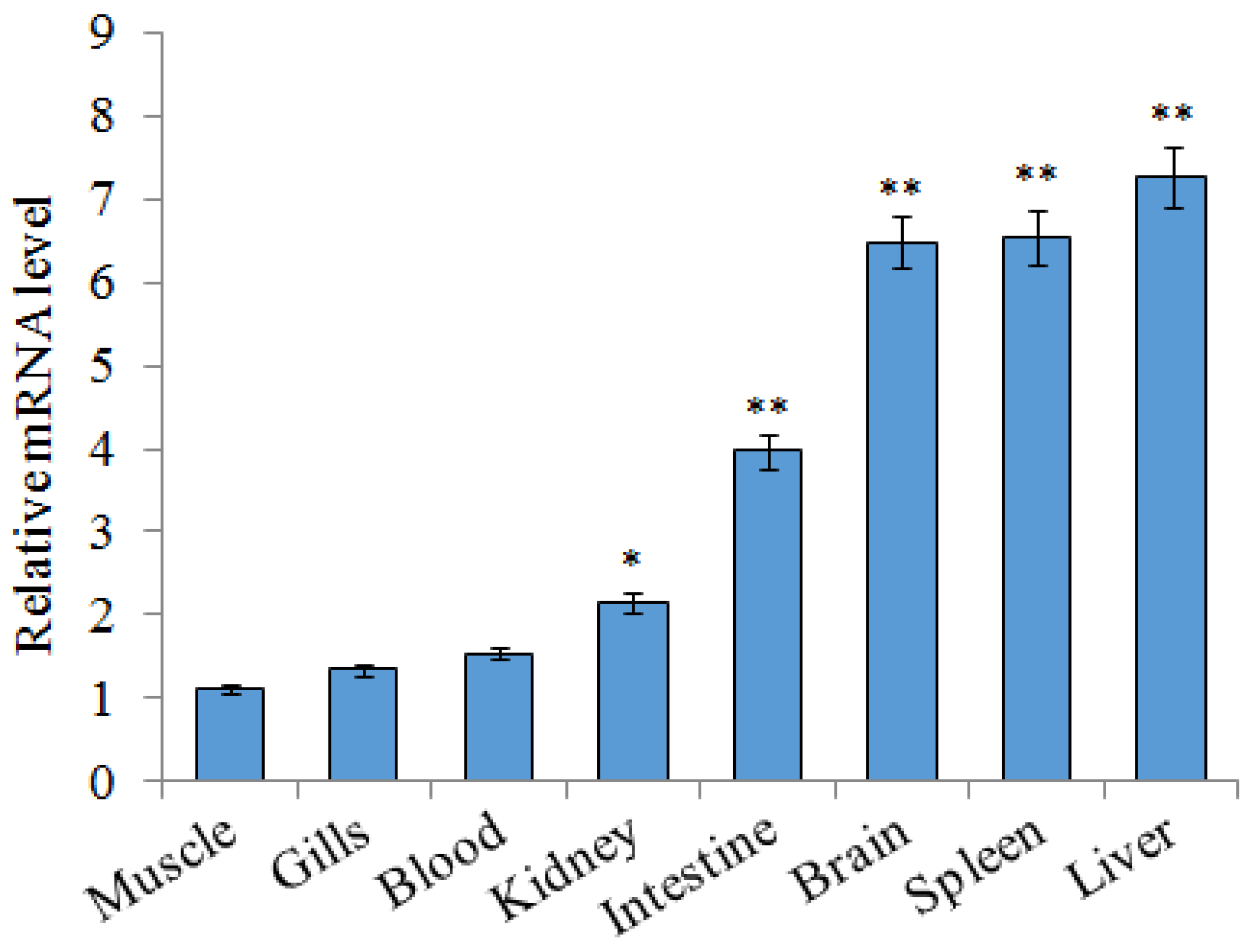
2.2. Expression of SsTFPI-2 under Normal Physiological Conditions
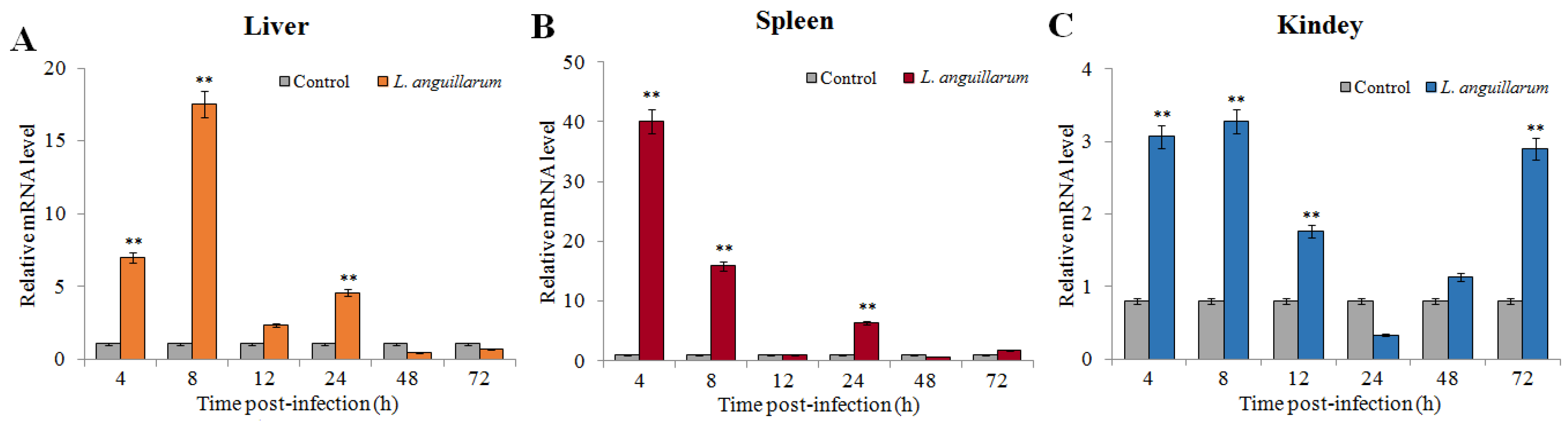
2.3. Expression of SsTFPI-2 under a Bacterial Challenge
2.4. Determination of Peptide Sequence
2.5. Antibacterial Activity of TS40
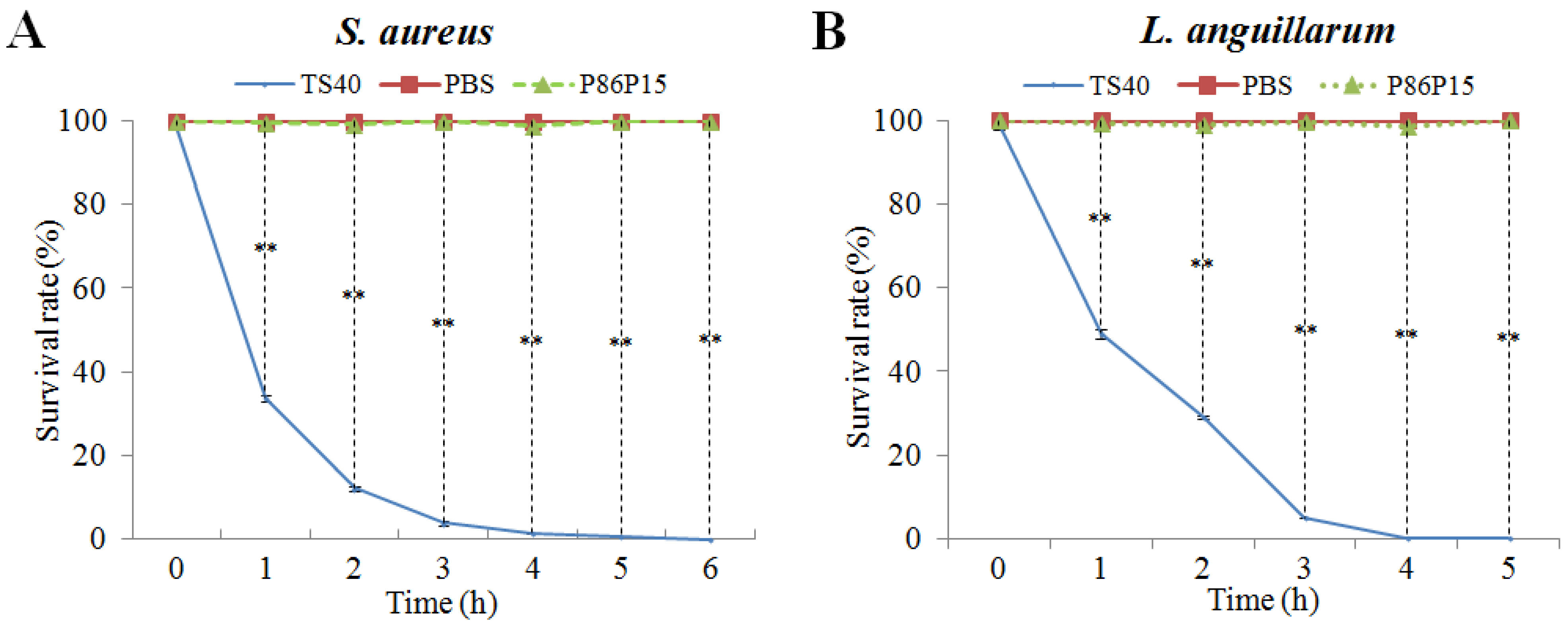
2.6. The Killing Kinetics of TS40
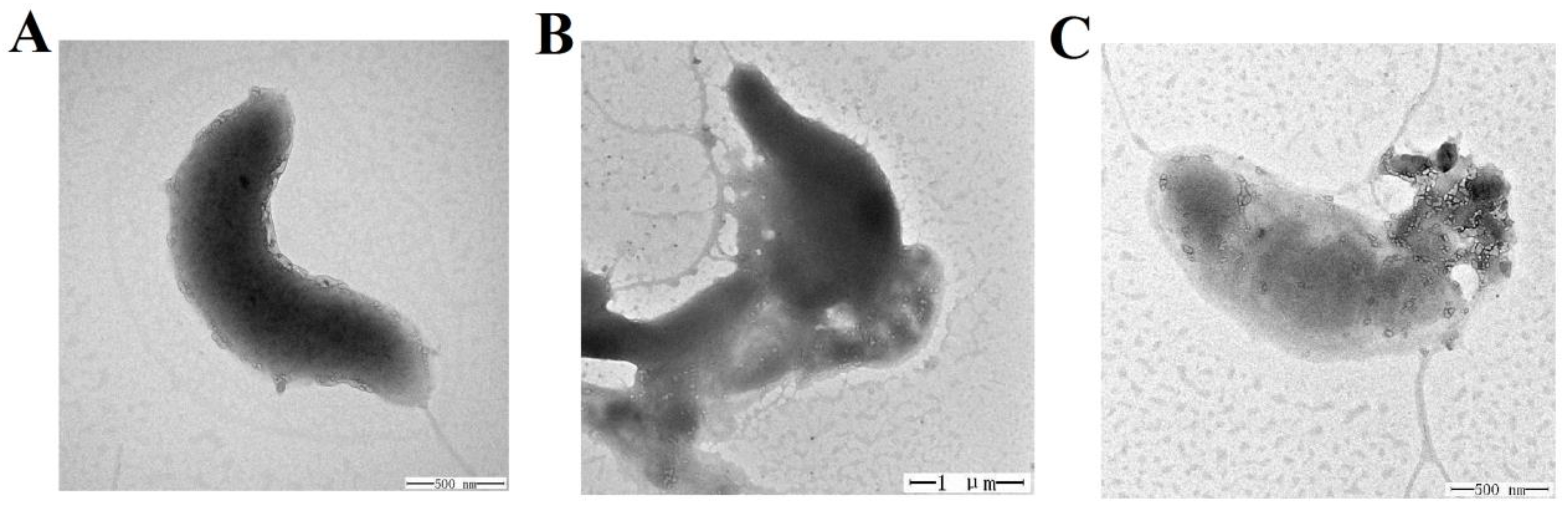
2.7. Effect of TS40 on Target Bacterial Morphology
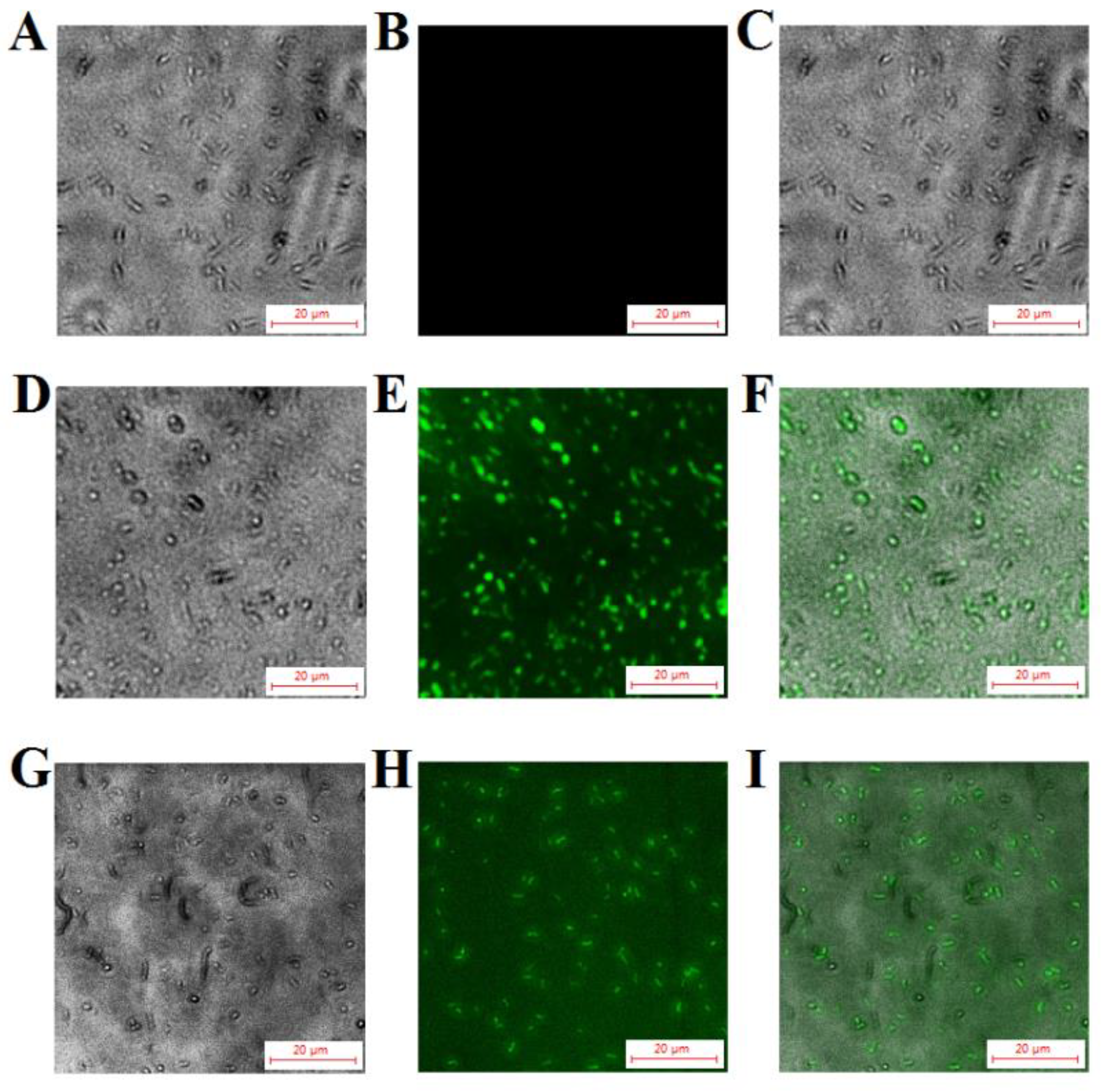
2.8. Localization of TS40 in Target Bacteria
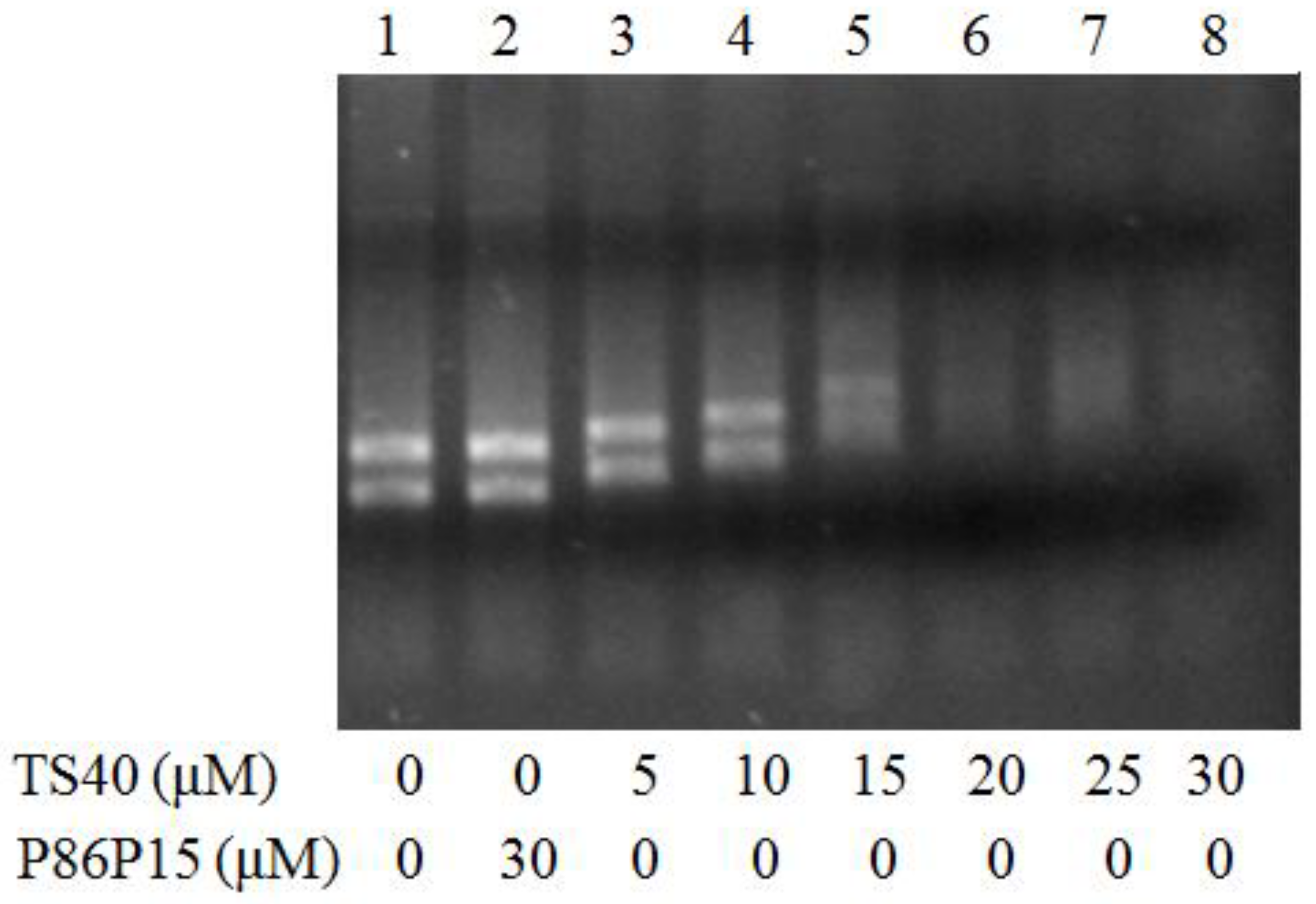
2.9. Effects of TS40 on Bacterial Genomic DNA
2.10. Effect of TS40 on Bacterial Total RNA
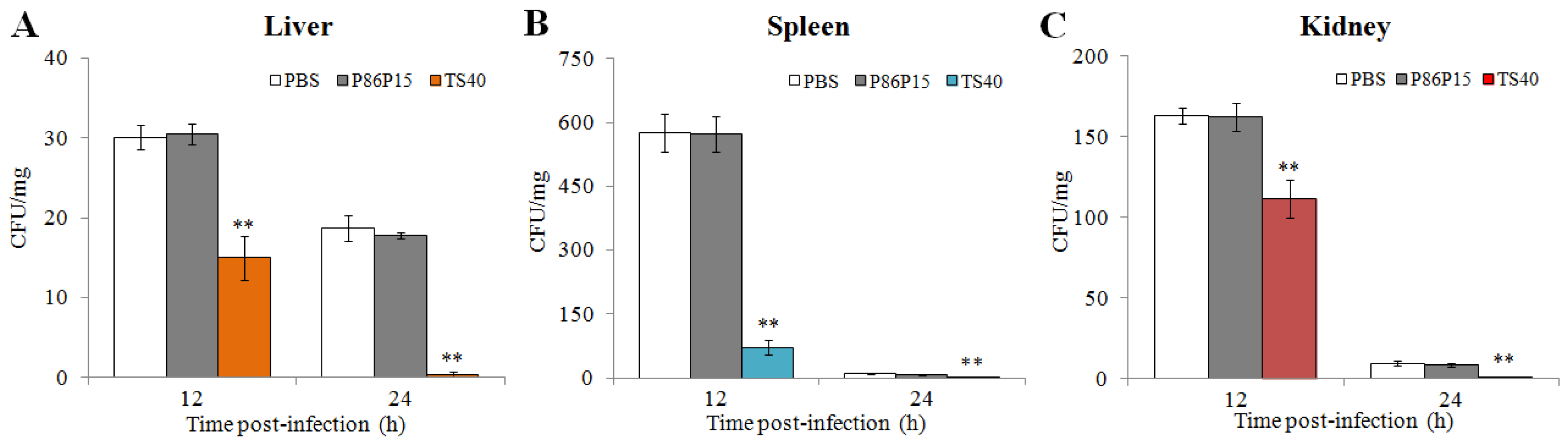
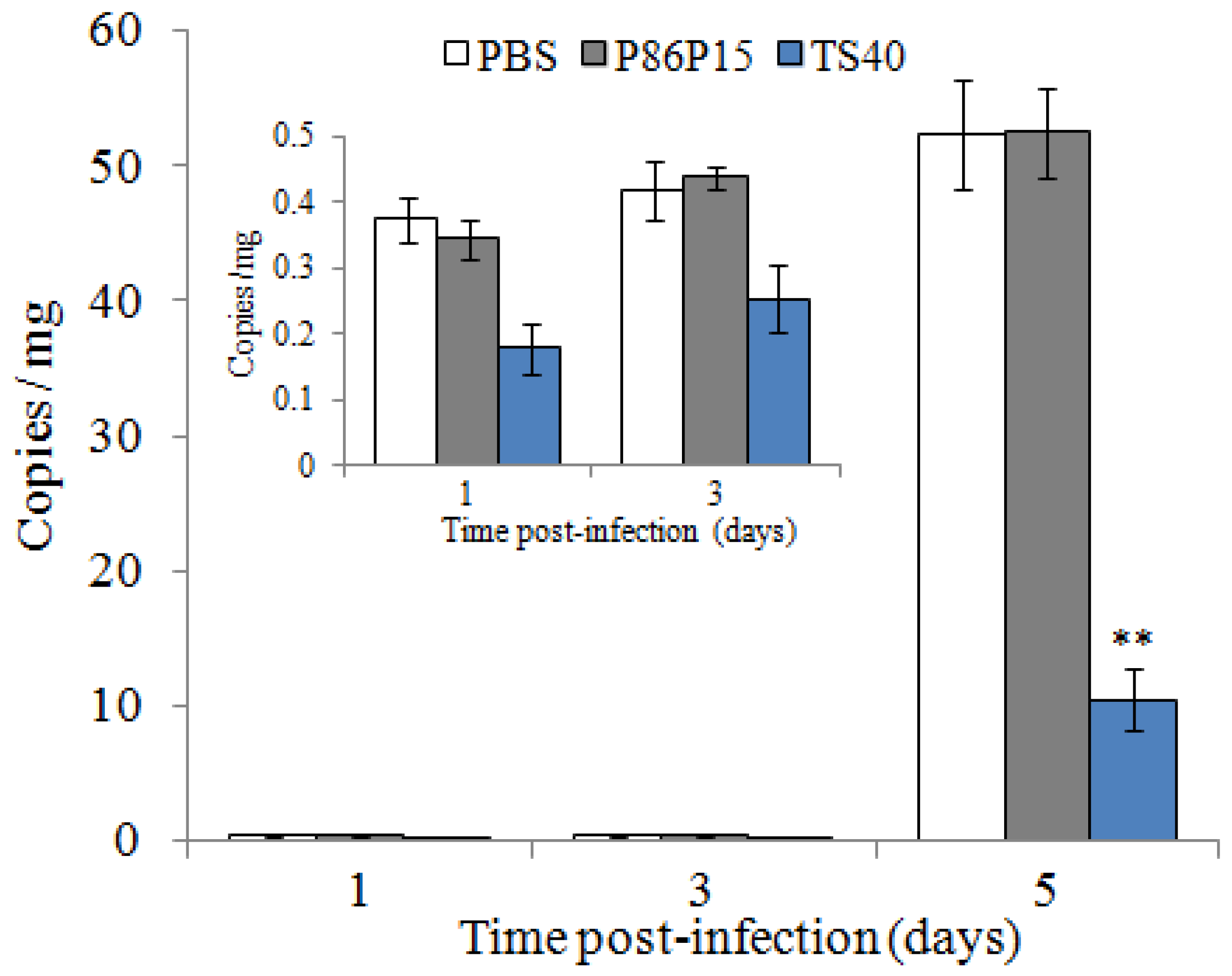
2.11. Effect of TS40 on Pathogens Infection
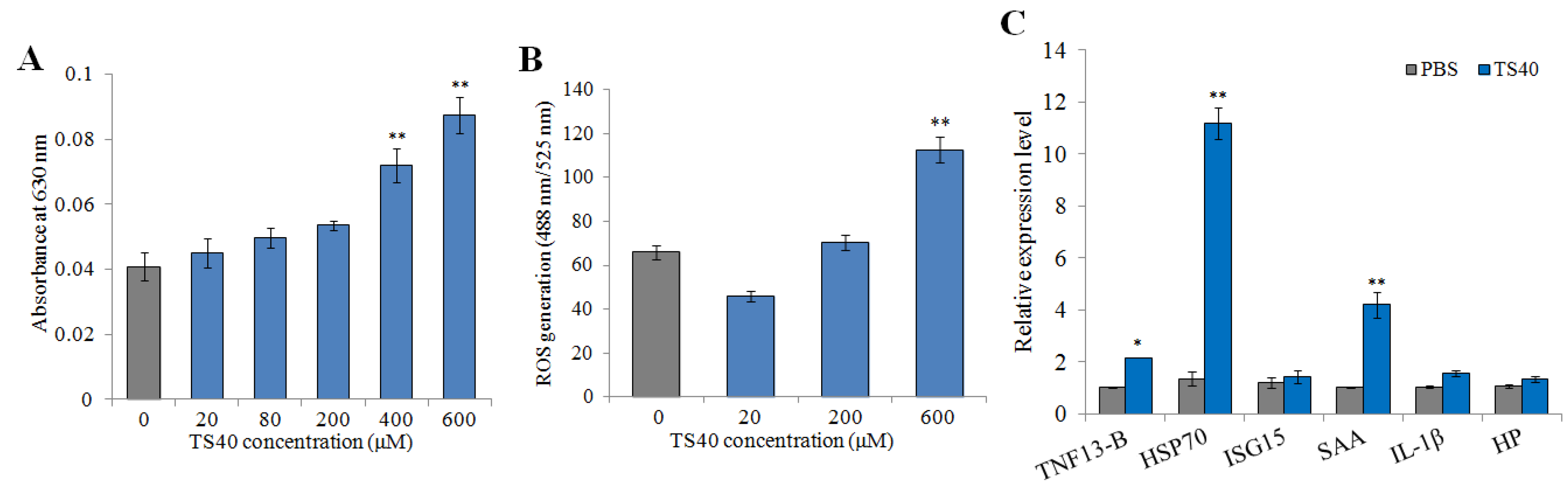
2.12. Effect of TS40 on Macrophages
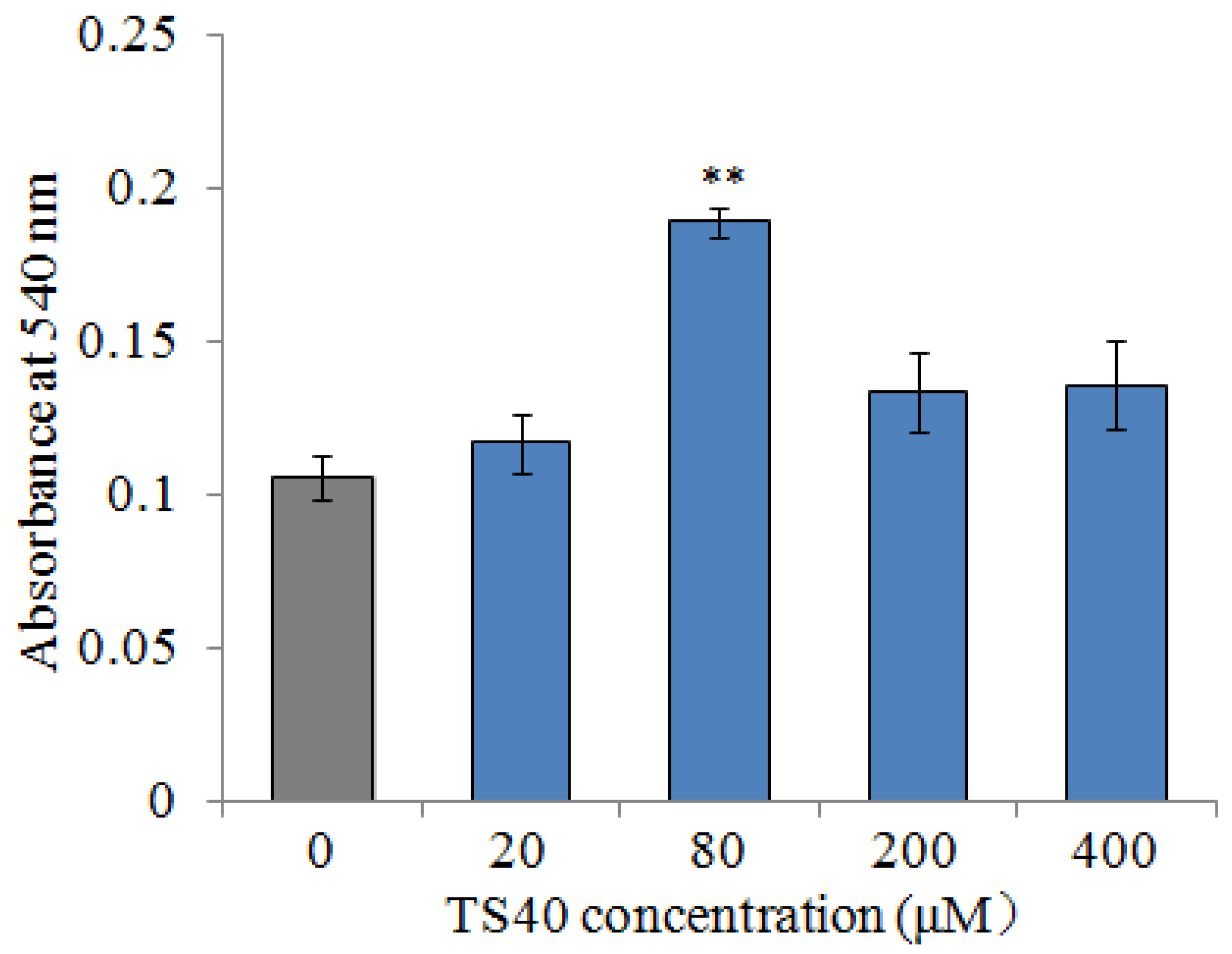
2.13. Effect of TS40 on the Proliferation of Peripheral Blood Leukocytes
3. Discussion
4. Materials and Methods
4.1. Experimental Animal and Sample Collection
4.2. Bacteria
4.3. Expression of SsTFPI-2 in Fish Tissues under Normal Physiological Conditions
4.4. Expression of SsTFPI-2 upon Bacterial Infection
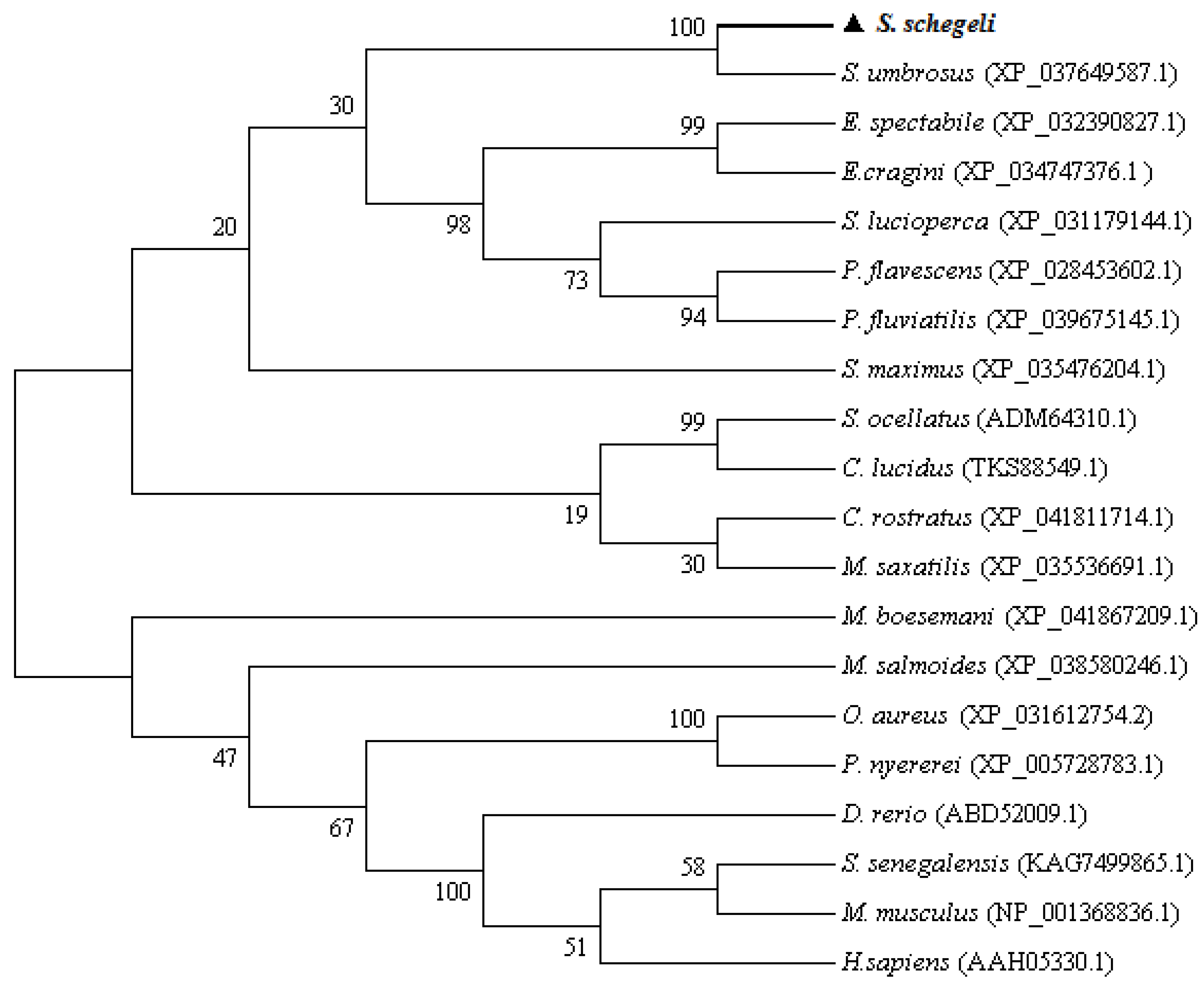
4.5. Bioinformatics Analysis
4.6. Peptides
4.7. Antibacterial Spectrum
4.8. Antibacterial Activity Assay
4.9. Killing Kinetics Assay
4.10. TEM Assay
4.11. Fluorescence Microscopy
4.12. Effect of TS40 on Genomic DNA
4.13. Effect of TS40 on Total RNA
4.14. In Vivo Study on Pathogens Infection
4.15. Effect of TS40 on Macrophages
4.15.1. Determination of Respiratory Burst
4.15.2. Detection of Reactive Oxygen Species (ROS)
4.15.3. Expression Analysis of Immune-Related Genes
4.16. Effect of TS40 on Peripheral Blood Leukocytes Proliferation
4.17. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Appendix A
Appendix B

Appendix C
References
- Zhao, L.M.; Zuo, Y.F.; Huang, L.X. Research Progress on Control of Disease in Aquaculture. J. Anhui Agric. Sci. 2018, 46, 18–21+52. [Google Scholar]
- Wang, J.X.; Wei, R.B.; Song, R. Novel antibacterial peptides isolated from the maillard reaction products of half-fin anchovy (Setipinna taty) hydrolysates/glucose and their mode of action in Escherichia coli. Mar. Drugs 2019, 17, 47. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.; Yi, Y.H.; Liang, L.F.; Shi, Q. High throughput identification of antimicrobial peptides from fish gastrointestinal microbiota. Toxins 2017, 9, 266. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.W.; Hou, L.; Chen, B.; Fan, D.Q.; Chen, Y.C.; Yang, Y.; Wang, K.J. A truncated Sph12-38 with potent antimicrobial activity showing resistance against bacterial challenge in Oryzias melastigma. Fish Shellfish Immunol. 2017, 67, 561–570. [Google Scholar] [CrossRef]
- Haney, E.F.; Mansour, S.C.; Hancock, R.E.W. Antimicrobial peptides: An introduction. Methods Mol. Biol. 2017, 1548, 3–22. [Google Scholar]
- Brogden, K.A. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005, 3, 238–250. [Google Scholar] [CrossRef]
- Gueguen, Y.; Bernard, R.; Julie, F.; Paulina, S.; Delphine, D.; Franck, V.; Philippe, B.; Evelyne, B. Oyster hemocytes express a proline-rich peptide displaying synergistic antimicrobial activity with a defensin. Mol. Immunol. 2009, 46, 516–522. [Google Scholar] [CrossRef]
- Mine, E.; Zülal, K. Antimicrobial Peptides (AMPs): A Promising Class of Antimicrobial Compounds. J. Appl. Microbiol. 2022, 132, 1573–1596. [Google Scholar]
- Anju, A.; Smitha, C.K.; Preetha, K.; Boobal, R.; Rosamma, P. Molecular characterization, recombinant expression and bioactivity profile of an antimicrobial peptide, Ssarasin from the Indian mud crab, Scylla serrata. Fish Shellfish Immunol. 2019, 88, 352–358. [Google Scholar] [CrossRef]
- Zhao, X.P.; He, S.W.; Yue, B.; Wang, G.H.; Zhang, M. Molecular characterization, expression analysis, and bactericidal activity of the derivative peptides of TFPI-1 and TFPI-2 in half-smooth tongue sole, Cynoglossus semilaevis. Fish Shellfish Immunol. 2016, 58, 563–571. [Google Scholar] [CrossRef]
- Sprecher, C.A.; Kisiel, W.; Mathewes, S.; Foster, D.C. Molecular cloning, expression, and partial characterization of a second human tissue-factor pathway inhibitor. Proc. Natl. Acad. Sci. USA 1994, 91, 3353–3357. [Google Scholar] [CrossRef] [PubMed]
- Ashikaga, K.; Kobayashi, T.; Kimura, M.; Owada, S.; Sasaki, S.; Iwasa, A.; Furukawa, K.; Motomura, S.; Okumura, K. Effects of amiodarone on electrical and structural remodeling induced in a canine rapid pacing-induced persistent atrial fibrillation model. Eur. J. Pharmacol. 2006, 536, 148–153. [Google Scholar] [CrossRef]
- Rao, C.; Mohanam, S.; Puppala, A.; Rao, J. Regulation of ProMMP-1 and ProMMP-3 activation by tissue factor pathway inhibitor-2/matrix-associated serine protease inhibitor. Biochem. Biophys. Res. Commun. 1999, 255, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yue, B.; Wang, G.H.; Liu, Y.; Zhou, S.; Cheng, S.F.; Li, N.Q. TC38, a teleost TFPI-2 peptide that kills bacteria via penetration of the cell membrane and interaction with nucleic acids. Fish Shellfish Immunol. 2017, 64, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Chand, H.S.; Foster, D.C.; Kisiel, W. Structure, function and biology of tissue factor pathway inhibitor-2. Thromb. Haemost. 2005, 94, 1122–1130. [Google Scholar] [CrossRef]
- Zhong, R.; Huang, R.; Song, S. Role of Tissue Factor Pathway Inhibitor-2 in Ovarian Tumor Migration and Invasion. Chin.-Ger. J. Clin. Oncol. 2005, 4, 53–55. [Google Scholar] [CrossRef]
- Doshi, S.N.; Marmur, J.D. Evolving role of tissue factor and its pathway inhibitor. Crit. Care Med. 2002, 30, S241–S250. [Google Scholar] [CrossRef]
- Papareddy, P.; Kalle, M.; Sorensen, O.E.; Malmsten, M.; Morgelin, M.; Schmidtchen, A. The TFPI-2 derived peptide EDC34 improves outcome of gram-negative sepsis. PLoS Pathog. 2017, 9, e1003803. [Google Scholar] [CrossRef]
- Kasetty, G.; Smeds, E.; Holmberg, E.; Wrange, L.; Adikesavan, S.; Papareddy, P. Vertebrate TFPI-2 C-terminal peptides exert therapeutic applications against Gram-negative infections. BMC Microbiol. 2016, 16, 129. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Wang, L.N.; Zhou, W.H.; Wang, H.J.; Zhang, J.; Deng, S.S.; Li, W.H.; Li, H.W.; Mao, Z.H.; Ma, D. Tissue factor pathway inhibitor-2: A novel gene involved in zebrafish central nervous system development. Dev. Biol. 2013, 381, 38–49. [Google Scholar] [CrossRef][Green Version]
- He, S.W.; Wang, J.J.; Du, X.; Yue, B.; Wang, G.H.; Zhou, S.; Xie, B.; Zhang, M. A teleost TFPI-2 peptide that possesses a broad antibacterial spectrum and immune-stimulatory properties. Fish Shellfish Immunol. 2018, 82, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.H.; Xie, B.; Su, Y.L.; Gu, Q.Q.; Hao, D.F.; Liu, H.M.; Wang, C.B.; Hu, Y.H.; Zhang, M. Expression analysis of tissue factor pathway inhibitors TFPI-1 and TFPI-2 in Paralichthys olivaceus and antibacterial and anticancer activity of derived peptides. Vet. Res. 2021, 52, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Li, L.M.; Wen, H.S.; Zhang, Y.C. Relationships analysis between the individual fecundity and biological indicator in Sebastes schlegelii. Mod. Agric. Sci. Technol. 2015, 22, 265–267+277. [Google Scholar]
- Hubé, F.; Reverdiau, P.; Iochmann, S.; Gruel, Y. Computer model of the interaction of human TFPI-2 Kunitz-type serine protease inhibitor with human plasmin. Thromb. Res. 2003, 111, 197–198. [Google Scholar] [CrossRef][Green Version]
- Wang, G.L.; Huang, W.H.; Li, W.; Chen, S.Y.; Chen, W.B.; Zhou, Y.C.; Peng, P.; Gu, W. TFPI-2 suppresses breast cancer cell proliferation and invasion through regulation of ERK signaling and interaction with actinin-4 and myosin-9. Sci. Rep. 2018, 8, 14402. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Xiao, Z.Z.; Sun, L. Identification and analysis of the tissue factor pathway inhibitor 2 of Sciaenops ocellatus. Fish Shellfish Immunol. 2011, 30, 209–214. [Google Scholar] [CrossRef]
- Petersen, L.C.; Sprecher, C.A.; Foster, D.C.; Blumberg, H.; Hamamoto, T.; Kisiel, W. Inhibitory properties of a novel human Kunitz-type protease inhibitor homologous to tissue factor pathway inhibitor. Biochemistry 1995, 35, 266–272. [Google Scholar] [CrossRef]
- Zhang, M.; Sun, L. The tissue factor pathway inhibitor 1 of Sciaenops ocellatus possesses antimicrobial activity and is involved in the immune response against bacterial infection. Dev. Comp. Immunol. 2011, 35, 247–252. [Google Scholar] [CrossRef]
- Schirm, S.; Liu, X.; Jennings, L.L.; Jedrzejewski, P.; Dai, Y.M.; Hardy, S. Fragmented tissue factor pathway inhibitor (TFPI) and TFPI C-terminal peptides eliminate serum-resistant Escherichia coli from blood cultures. J. Infect. Dis. 2009, 199, 1807–1815. [Google Scholar] [CrossRef]
- Wang, H.R.; Hu, Y.H.; Zhang, W.W.; Sun, L. Construction of an attenuated pseudomonas fluorescens strain and evaluation of its potential as a cross-protective vaccine. Vaccine 2009, 27, 4047–4055. [Google Scholar] [CrossRef]
- Papareddy, P.; Kalle, M.; Kasetty, G.; Morgelin, M.; Rydengard, V.; Albiger, B.; Lundqvist, K.; Malmsten, M.; Schmidtchen, A. C-terminal peptides of tissue factor pathway inhibitor are novel host defense molecules. J. Biol. Chem. 2010, 285, 28387–28398. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.Z.; Fu, X.Z.; Li, N.Q.; Lin, Q.; Liu, L.H.; Wu, S.Q. Molecular characterization and expression pattern of tumor suppressor protein p53 in mandarin fish, Siniperca chuatsi following virus challenge. Fish Shellfish Immunol. 2016, 51, 392–400. [Google Scholar] [CrossRef] [PubMed]
- He, S.W.; Zhang, J.; Li, N.Q.; Zhou, S.; Yue, B.; Zhang, M. A TFPI-1 peptide that induces degradation of bacterial nucleic acids, and inhibits bacterial and viral infection in half-smooth tongue sole, Cynoglossus semilaevis. Fish Shellfish Immunol. 2017, 60, 466–473. [Google Scholar] [CrossRef]
- He, S.W.; Wang, G.H.; Yue, B.; Zhou, S.; Zhang, M. TO17: A teleost antimicrobial peptide that induces degradation of bacterial nucleic acids and inhibits bacterial infection in red drum, Sciaenops ocellatus. Fish Shellfish Immunol. 2018, 72, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.L.; Wang, G.H.; Wang, J.J.; Xie, B.; Gu, Q.Q.; Hao, D.F.; Liu, H.M.; Zhang, M. TC26, a teleost TFPI-1 derived antibacterial peptide that induces degradation of bacterial nucleic acids and inhibits bacterial infection in vivo. Fish Shellfish Immunol. 2020, 98, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Malanovic, N.; Lohner, K. Antimicrobial peptides targeting gram-Positive Bacteria. Pharmaceuticals 2016, 9, 59. [Google Scholar] [CrossRef]
- Friedrich, C.L.; Moyles, D.; Beveridge, T.J.; Hancock, R.E.W. Antibacterial action of structurally diverse cationic peptides on gram-positive bacteria. Antimicrob. Agents Chemother. 2000, 44, 2086–2092. [Google Scholar] [CrossRef]
- Baindara, P.; Singh, N.; Ranjan, M.; Nallabelli, N.; Chaudhry, V.; Pathania, G.L.; Sharma, N.; Kumar, A.; Patil, P.B.; Korpole, S. Laterosporulin10: A novel defensin like Class IId bacteriocin from Brevibacillus sp. strain SKDU10 with inhibitory activity against microbial pathogens. Microbiology 2016, 162, 1286–1299. [Google Scholar] [CrossRef]
- Gu, Q.Q.; He, S.W.; Liu, L.H.; Wang, G.H.; Hao, D.F.; Liu, H.M.; Wang, C.B.; Li, C.; Zhang, M.; Li, N.Q. A teleost bactericidal permeability-increasing protein-derived peptide that possesses a broad antibacterial spectrum and inhibits bacterial infection as well as human colon cancer cells growth. Dev. Comp. Immunol. 2021, 118, 103995. [Google Scholar] [CrossRef]
- Cudic, M.; Lockatell, C.V.; Johnson, D.E.; Otvos, L. In vitro and in vivo activity of an antibacterial peptide analog against uropathogens. Peptides 2003, 24, 807–820. [Google Scholar] [CrossRef]
- Fernandez-Lope, S.; Kim, H.S.; Choi, E.C.; Delgado, M.; Granja, J.R.; Khasanov, A.; Kraehenbuehl, K.; Long, G.; Weinberger, D.A.; Wilcoxen, K.M.; et al. Antibacterial agents based on the cyclic D, L-alpha-peptide architecture. Nature 2001, 412, 452–455. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, J.N.; Zhang, B.; Liu, H.R.; Song, W.; He, J.; Lv, D.C.; Wang, S.Y.; Xu, X.G. Activity of antibacterial protein from maggots against Staphylococcus aureus in vitro and in vivo. Int. J. Mol. Med. 2013, 31, 1159–1165. [Google Scholar] [CrossRef]
- Cao, L.Y.; Dai, C.; Li, Z.J. Antibacterial activity and mechanism of a scorpion venom peptide derivative in vitro and in vivo. PLoS ONE 2012, 7, e40135. [Google Scholar] [CrossRef] [PubMed]
- Masso-Silva, A.J.; Diamond, G. Antimicrobial peptides from fish. Pharmaceuticals 2014, 7, 265–310. [Google Scholar] [CrossRef] [PubMed]
- Wan, M.; van der Does, A.M.; Tang, X.; Lindbom, L.; Agerberth, B.; Haeggstrom, J.Z. Antimicrobial peptide LL-37 promotes bacterial phagocytosis by human macrophages. J. Leukoc. Biol. 2014, 95, 971–981. [Google Scholar] [CrossRef]
- Zheng, Y.; Niyonsaba, F.; Ushio, H.; Nagaoka, I.; Ikeda, S.; Okumura, K.; Ogawa, H. Cathelicidin LL-37 induces the generation of reactive oxygen species and release of human alpha-defensins from neutrophils. Br. J. Dermatol. 2007, 157, 1124–1131. [Google Scholar] [CrossRef]
- Mohammadi, M.; Hasan-Abad, A.M.; Dehghani, P.; Nabipour, I.; Roozbehani, M.; Hemphill, A.; Taherzadeh, M.; Mohaghegh, M.A.; Fouladvand, M. Dicentracin-Like from Asian sea bass fish and Moronecidine-Like from Hippocampus comes: Two candidate antimicrobial peptides against Leishmanina major infection. Int. J. Pept. Res. Ther. 2021, 27, 168–778. [Google Scholar] [CrossRef]
- You, F.T.; Ren, W.H.; Hou, H.H.; Pei, L.L.; He, Z.J. Molecular cloning, expression, bioinformatics analysis and bioactivity characterization of TNF13B (BAFF) gene in bat (Vespertilio superans Thomas). Int. Immunopharmacol. 2012, 12, 433–440. [Google Scholar] [CrossRef]
- Revathy, K.S.; Umasuthan, N.; Whang, I.; Lee, Y.; Lee, S.; Oh, M.J.; Jung, S.J.; Choi, C.Y.; Park, C.J.; Park, H.J.; et al. A novel acute phase reactant, serum amyloid A-like 1, from Oplegnathus fasciatus: Genomic and molecular characterization and transcriptional expression analysis. Dev. Comp. Immunol. 2012, 37, 294–305. [Google Scholar] [CrossRef]
- Abdel-Tawwab, M.; Khalil, R.H.; Diab, A.M.; Khallaf, M.A.; Abdel-Razek, N.; Abdel-Latif, H.M.R.; Khalifa, E. Dietary garlic and chitosan enhanced the antioxidant capacity, immunity, and modulated the transcription of HSP70 and Cytokine genes in Zearalenone-intoxicated European seabass. Fish Shellfish Immunol. 2021, 113, 35–41. [Google Scholar] [CrossRef]
- Jia, R.; Du, J.L.; Cao, L.P.; Xu, P.; Wang, J.H.; Liu, Y.J.; Yin, G.J.; Jiang, H.J.; Bo, A.X. Effects of peptides from swine blood on activities of immune cells in Ctenopharynodon idellus in vitro. J. Soc. Agric. 2014, 45, 1084–1088. [Google Scholar]
- Zhao, X.P.; Liu, Y.; Wang, J.J.; Wang, G.H.; Wang, R.; Zhang, M. A high-mobility group box 1 that binds to DNA, enhances pro-inflammatory activity, and acts as an anti-infection molecule in black rockfish, Sebastes schlegelii. Fish Shellfish Immunol. 2016, 56, 402–409. [Google Scholar]
- Zhang, M.; Sun, L.; Hu, Y.H.; Xiao, Z.Z. Characterization of a megalocytivirus from cultured rock bream, Oplegnathus fasciatus (Temminck & Schlege) in China. Aquac. Res. 2012, 43, 556–564. [Google Scholar]
- Fu, X.; Li, N.; Lai, Y.; Luo, X.; Wang, Y.; Shi, C.; Huang, Z.; Wu, S.; Su, J. A novel fish cell line derived from the brain of Chinese perch Siniperca chuatsi: Development and characterization. J. Fish Biol. 2015, 86, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Nikolakopoulou, K.; Zarkadis, L.K. Molecular cloning and characterisation of two homologues of Mannose-Binding Lectin in rainbow trout. Fish Shellfish Immunol. 2006, 21, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Hu, Y.H.; Xiao, Z.Z.; Sun, Y.; Sun, L. Construction and analysis of experimental DNA vaccines against megalocytivirus. Fish Shellfish Immunol. 2012, 33, 1192–1198. [Google Scholar] [CrossRef]
- Zhang, M.; Cao, M.; Xiu, Y.J.; Fu, Q.; Yang, N.; Su, B.F.; Li, C. Identification of antimicrobial peptide genes in black rockfish Sebastes schlegelii and their responsive mechanisms to Edwardsiella tarda infection. Biology 2021, 10, 1015. [Google Scholar] [CrossRef]
- Zhou, S.; Gao, Z.X.; Zhang, M.; Liu, D.Y.; Zhao, X.P.; Liu, Y. Development of a quadruplex loop-mediated isothermal amplification assay for field detection of four Vibrio species associated with fish disease. Springerplus 2016, 5, 1104. [Google Scholar] [CrossRef]
- Secombes, C.; Ellis, A.; Hardie, L. Isolation of salmonid macrophages and analysis of their killing activity. Dis. Aquat. Organ. 1990, 25, 175–183. [Google Scholar]
- Wang, G.H.; Wang, J.J.; Yue, B.; Du, X.; Du, H.H.; Zhang, M.; Hu, Y.H. High mobility group box 2 of black rockfish Sebastes schlegelii: Gene cloning, immunoregulatory properties and antibacterial effect. Fish Shellfish Immunol. 2019, 84, 719–725. [Google Scholar] [CrossRef]
- Shim, K.J.; Jung, K.H.; Chung, M.K.; Choung, S.Y. Development of an in vitro environmental monitoring system by using immune cells. J. Health Sci. 2002, 48, 130–133. [Google Scholar] [CrossRef][Green Version]
- Li, Q.; Zhan, W.; Xing, J.; Sheng, X. Production, characterisation and applicability of monoclonal antibodies to immunoglobulin of Japanese flounder (Paralichthys olivaceus). Fish Shellfish Immunol. 2007, 23, 982–990. [Google Scholar] [CrossRef] [PubMed]

| Properties | SsTFPI-2 | TS40 |
|---|---|---|
| Number of amino acids | 226 | 40 |
| Total number of atoms | 3571 | 654 |
| Formula | C1141H1754N324O329S23 | C192H329N75O55S3 |
| Molecular weight (MW/Da) | 26,011.83 | 4664.37 |
| Theoretical isoelectric point (PI) | 9.14 | 11.79 |
| Grand average of hydropathicity (GRAVY) | −0.462 | −1.087 |
| Instability index (II) | 43.32 | 86.90 |
| Aliphatic index (AI) | 55.27 | 51.25 |
| Total number of negatively charged residues (ASP + GLU) | 16 | 1 |
| Total number of positively charged residues (ARG + LYS) | 31 | 11 |
| Strains | MIC (μM) | MBC (μM) |
|---|---|---|
| Listonella anguillarum | 25 | 50 |
| Vibrio parahaemolyticus | 400 | >800 |
| Staphylococcus aureus | 12.5 | 25 |
| Streptococcus agalactiae | 800 | >800 |
| Primers | Sequences (5′–3′) |
|---|---|
| SsTFPIRTF | TCCCAAAGGTTCCCCAGAT |
| SsTFPIRTR | CTCACAGCCGCCGTAATAGA |
| MCPRTF | CATCAGCCAGAGCACCCAG |
| MCPRTR | ACCTCACGCTCCTCACTTGTC |
| EF1α-F | AACCTGACCACTGAGGTGAAGTCTG |
| EF1α-R | TCCTTGACGGACACGTTCTTGATGTT |
| TNF13B-F | GGAAAACCTTCAGGAAAGAATACA |
| TNF13B-R | TGAGGCTCGTCTCCCACC |
| IL-1β-F | GCATCCGAGGCACAAATCC |
| IL-1β-R | ACACCCGCTCCACTCAACAG |
| HP-F | GGCAGGGAAAGAGGGAATAG |
| HP-R | GGAAGTGTGGATGGAGAAAAA |
| SAA-F | CTTCCCCGGTGAAGCCTTTA |
| SAA-R | CCATGCTCATTTGCTCTCTGAT |
| HSP70-F | CTGTTTGAAGCAATTGAGGGC |
| HSP70-R | CAGGAGTTTCTGGATTTTAGGGA |
| ISG15-F | CTACGGCCTGCAGCAAGGAGC |
| ISG15-R | CCCTGGTCTTGAAGTTGGCCA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Wang, G.; Hao, D.; Wang, C.; Zhang, M. Antimicrobial and Immunoregulatory Activities of TS40, a Derived Peptide of a TFPI-2 Homologue from Black Rockfish (Sebastes schlegelii). Mar. Drugs 2022, 20, 353. https://doi.org/10.3390/md20060353
Liu H, Wang G, Hao D, Wang C, Zhang M. Antimicrobial and Immunoregulatory Activities of TS40, a Derived Peptide of a TFPI-2 Homologue from Black Rockfish (Sebastes schlegelii). Marine Drugs. 2022; 20(6):353. https://doi.org/10.3390/md20060353
Chicago/Turabian StyleLiu, Hongmei, Guanghua Wang, Dongfang Hao, Changbiao Wang, and Min Zhang. 2022. "Antimicrobial and Immunoregulatory Activities of TS40, a Derived Peptide of a TFPI-2 Homologue from Black Rockfish (Sebastes schlegelii)" Marine Drugs 20, no. 6: 353. https://doi.org/10.3390/md20060353
APA StyleLiu, H., Wang, G., Hao, D., Wang, C., & Zhang, M. (2022). Antimicrobial and Immunoregulatory Activities of TS40, a Derived Peptide of a TFPI-2 Homologue from Black Rockfish (Sebastes schlegelii). Marine Drugs, 20(6), 353. https://doi.org/10.3390/md20060353




