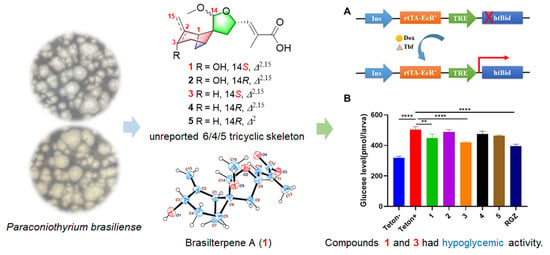
Brasilterpenes A–E, Bergamotane Sesquiterpenoid Derivatives with Hypoglycemic Activity from the Deep Sea-Derived Fungus Paraconiothyrium brasiliense HDN15-135
Abstract
:1. Introduction
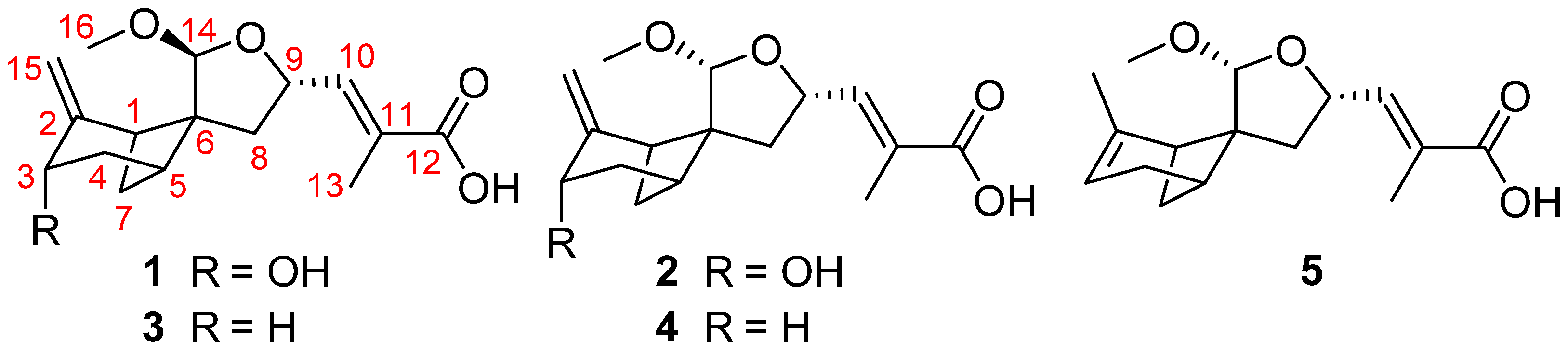
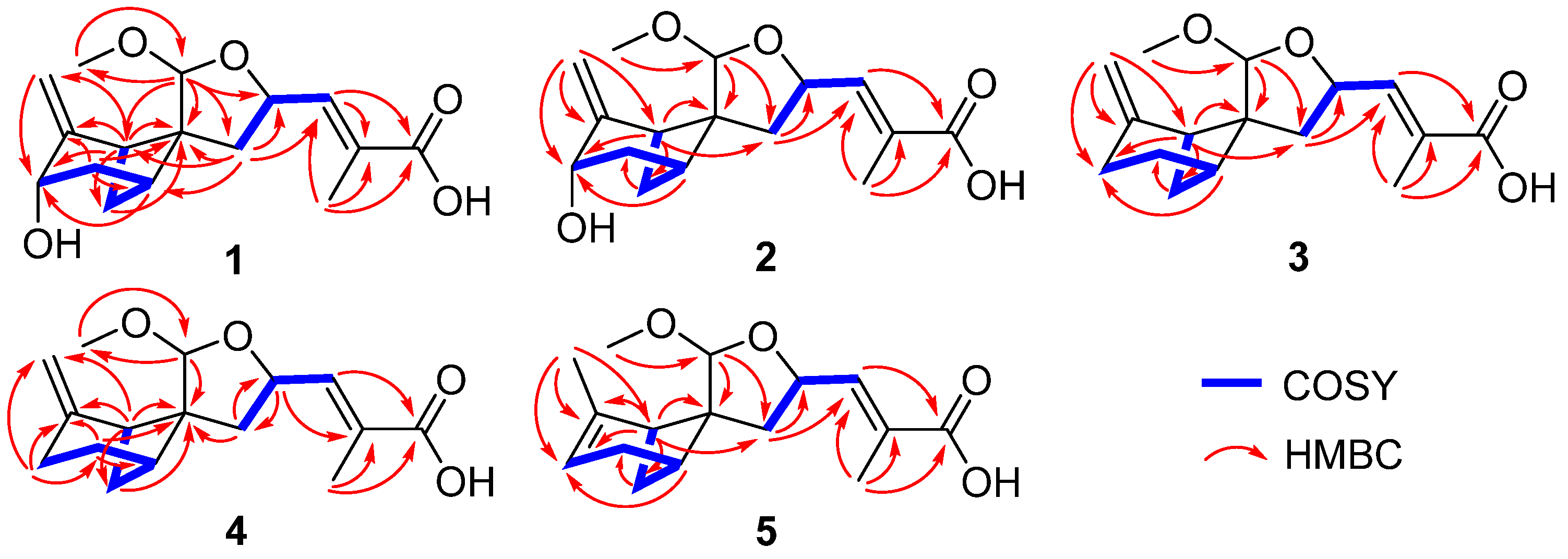
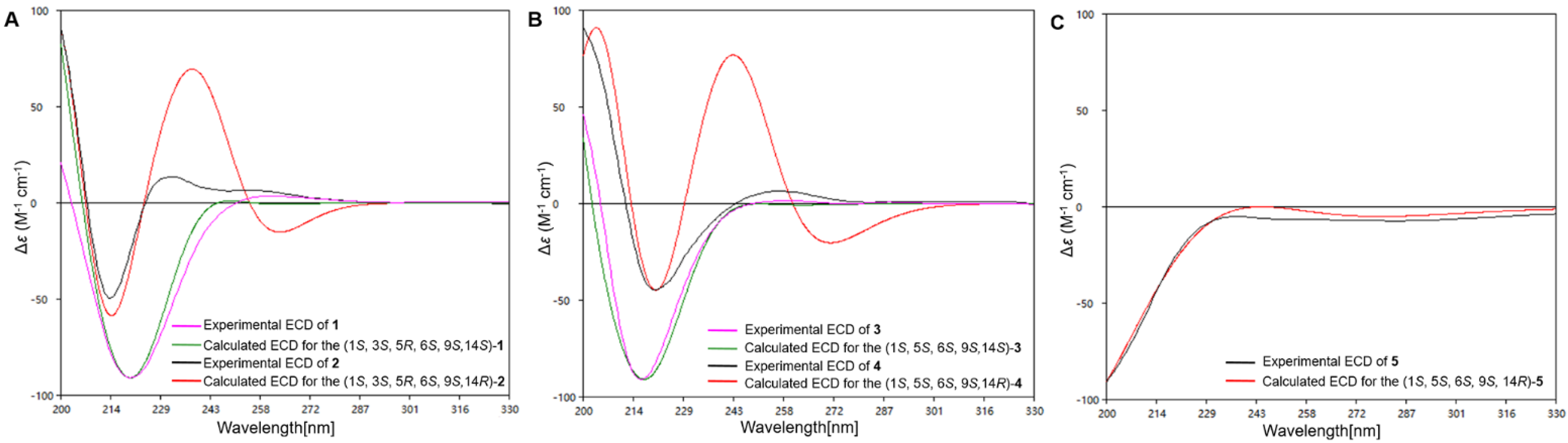
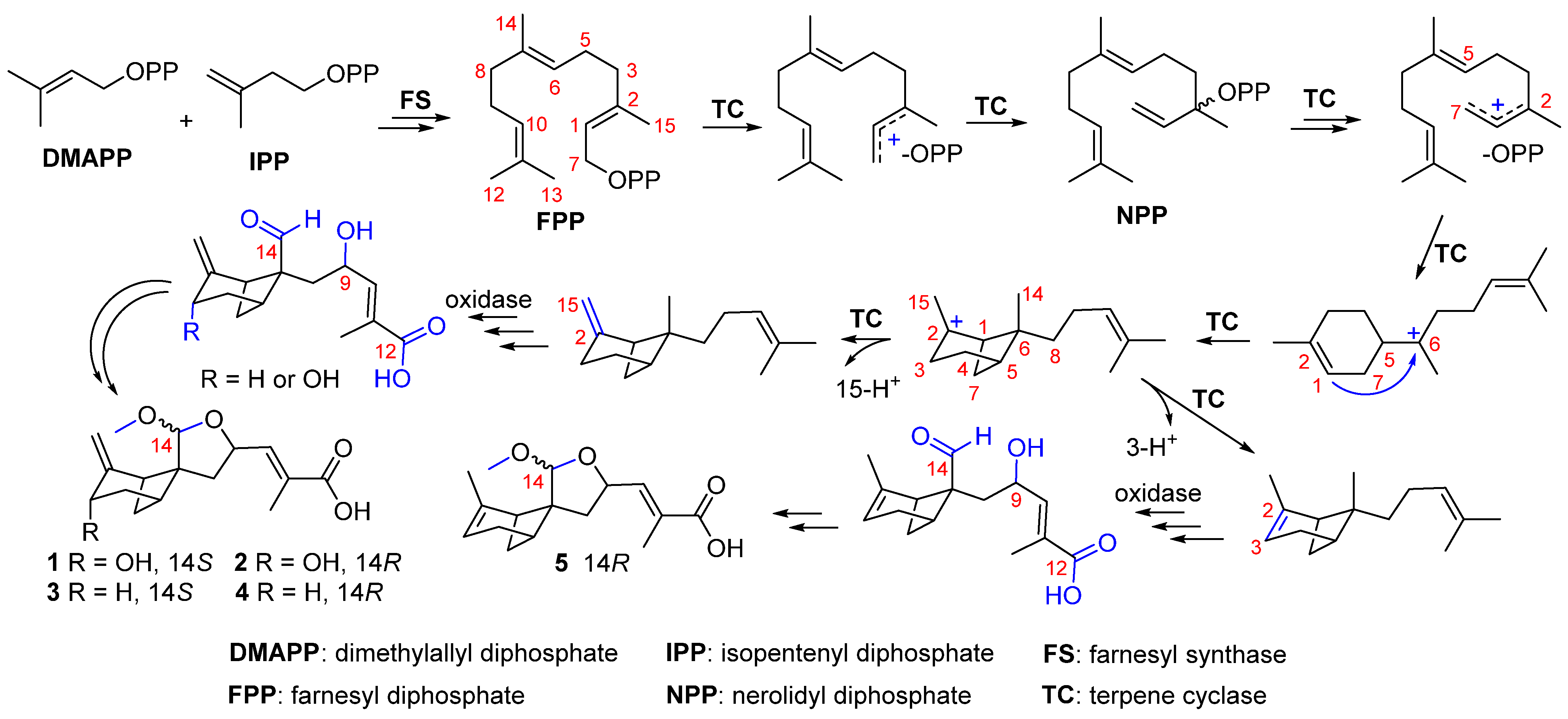
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Fungal Material
3.3. Fermentation, Extraction, and Isolation
- Brasilterpene A (1): colorless crystal; +16.7 (c 0.27, MeOH); UV (MeOH) λmax (log ε) 206 (2.15), 220 (2.11) nm; IR (KBr) νmax 3421, 2934, 1699, 1653, 1025 cm−1; ECD (4.5 mM, MeOH) λmax (Δε) 200 (+3.81), 220 (−17.01), 258 (+0.98) nm; 1H and 13C NMR data, Table 1; HRESIMS m/z [M − H]− 293.1393 (calcd for C16H21O5, 293.1394).
- Brasilterpene B (2): colorless oil; −30.2 (c 0.23, MeOH); UV (MeOH) λmax (log ε) 206 (2.01), 220 (2.36) nm; IR (KBr) νmax 3412, 2933, 1699, 1652, 1024, 993 cm−1; ECD (3.8 mM, MeOH) λmax (Δε) 200 (+12.53), 220 (−7.05), 234 (+2.48) nm; 1H and 13C NMR data, Table 1; HRESIMS m/z [M − H]− 293.1391 (calcd for C16H21O5, 293.1394).
- Brasilterpene C (3): colorless oil; +11.9 (c 0.33, MeOH); UV (MeOH) λmax (log ε) 206 (2.51), 217 (2.50) nm; IR (KBr) νmax 3394, 2937, 1699, 1653, 1207, 1020 cm−1; ECD (6.0 mM, MeOH) λmax (Δε) 200 (+14.98), 217 (−31.0), 255 (+0.45) nm; 1H and 13C NMR data, Table 3; HRESIMS m/z [M − H]− 277.1441 (calcd for C16H21O4, 277.1445).
- Brasilterpene D (4): colorless oil; −58.2 (c 0.37, MeOH); UV (MeOH) λmax (log ε) 210 (2.06), 223 (2.29) nm; IR (KBr) νmax 3402, 2937, 1699, 1207, 992 cm−1; ECD (5.4 mM, MeOH) λmax (Δε) 200 (+21.87), 217 (−10.78), 258 (+2.01) nm; 1H and 13C NMR data, Table 3; HRESIMS m/z [M − H]− 277.1442 (calcd for C16H21O4, 277.1445).
- Brasilterpene E (5): colorless oil; −104.2 (c 0.19, MeOH); UV (MeOH) λmax (log ε) 221 (3.12) nm; IR (KBr) νmax 3388, 2936, 1696, 1207, 1093, 1037, 992 cm−1; ECD (4.2 mM, MeOH) λmax (Δε) 200 (−14.97), 237 (−0.82), 280 (−1.20) nm; 1H and 13C NMR data, Table 4; HRESIMS m/z [M − H]− 277.1444 (calcd for C16H21O4, 277.1445).
3.4. X-ray Crystallographic Analysis of Brasilterpene A (1)
3.5. Computational Section
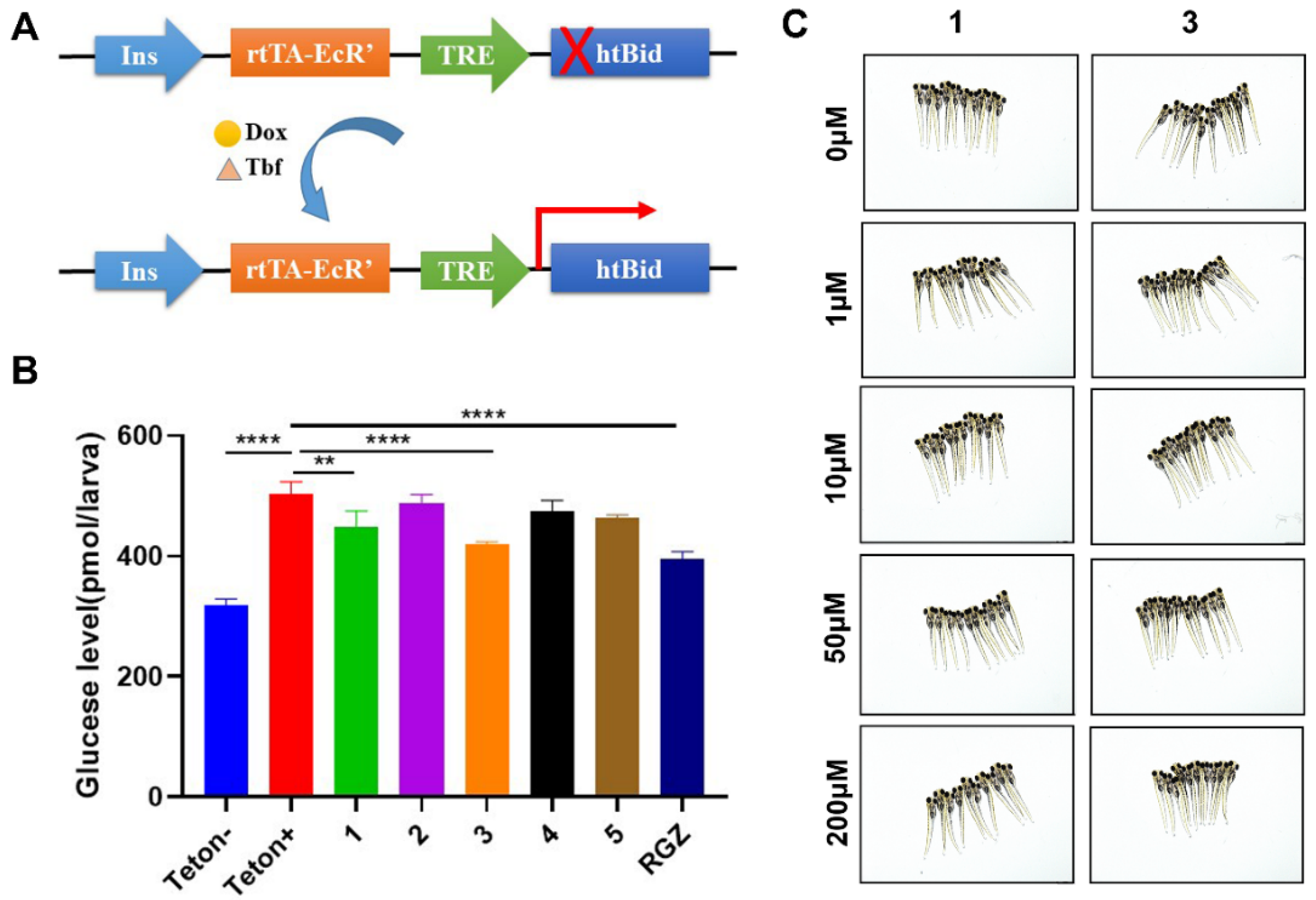
3.6. Assays of Free Blood Glucose in Hyperglycemic Zebrafish
3.7. Evaluation of Toxicological Effect in Zebrafish Larvae
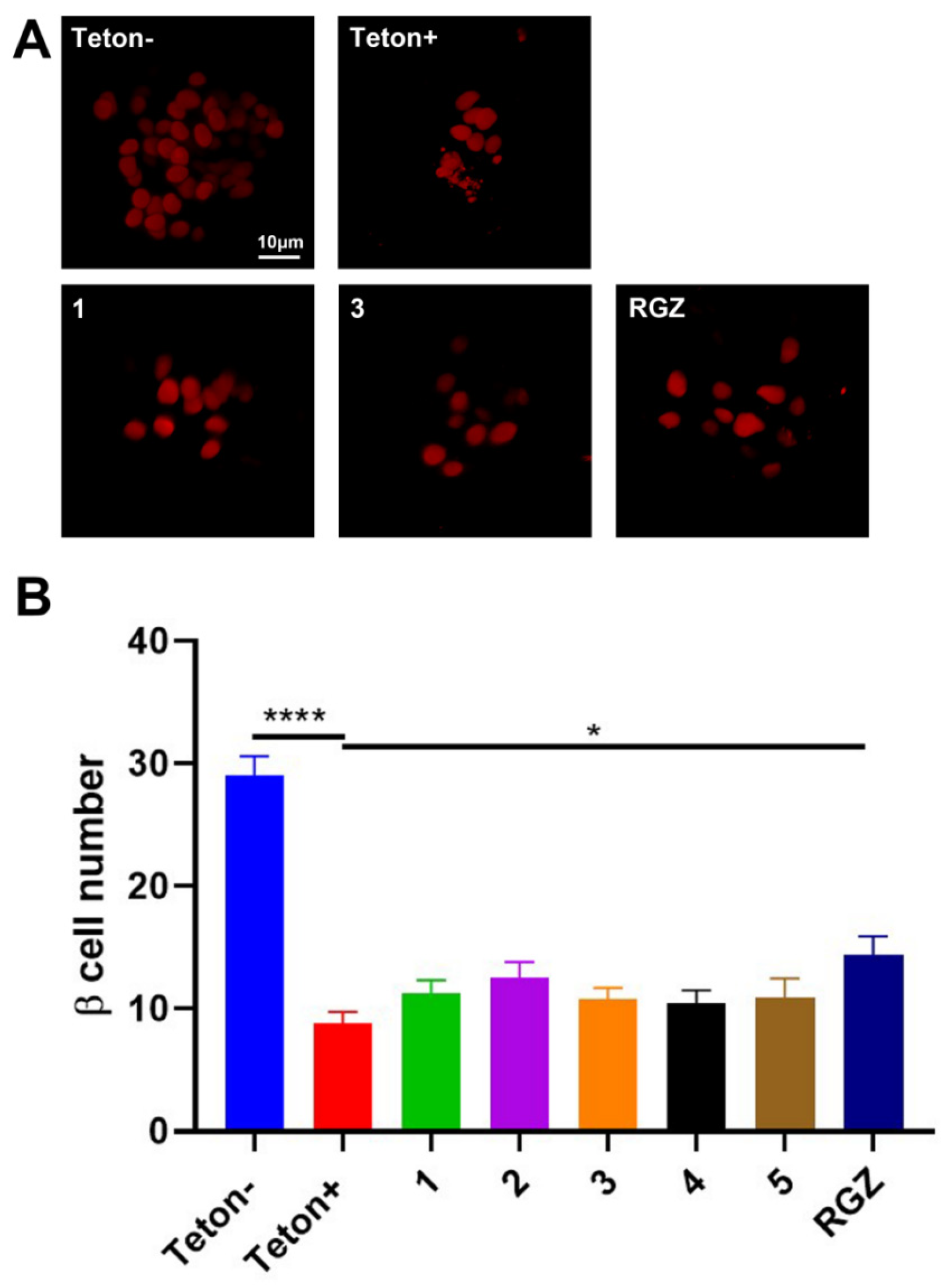
3.8. Assays of the Hypoglycemic Mechanism of Compounds 1 and 3
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef] [PubMed]
- Fralick, M.; Jenkins, A.J.; Khunti, K.; Mbanya, J.C.; Mohan, V.; Schmidt, M.I. Global accessibility of therapeutics for diabetes mellitus. Nat. Rev. Endocrinol. 2022, 18, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2019, 36, 122–173. [Google Scholar] [CrossRef] [Green Version]
- Elissawy, A.M.; El-Shazly, M.; Ebada, S.S.; Sing, A.B.; Proksch, P. Bioactive terpenes from marine-derived fungi. Mar. Drugs 2015, 13, 1966–1992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, M.; Wu, Z.; Guo, H.; Liu, L.; Chen, S. A review of terpenes from marine-derived fungi: 2015–2019. Mar. Drugs 2020, 18, 321. [Google Scholar] [CrossRef]
- Gozari, M.; Alborz, M.; El-Seedi, H.R.; Jassbi, A.R. Chemistry, biosynthesis and biological activity of terpenoids and meroterpenoids in bacteria and fungi isolated from different marine habitats. Eur. J. Med. Chem. 2021, 210, 112957. [Google Scholar] [CrossRef]
- Liu, S.Z.; Tang, X.X.; He, F.M.; Jia, J.X.; Hu, H.; Xie, B.Y.; Li, M.Y.; Qiu, Y.K. Two new compounds from a mangrove sediment-derived fungus Penicillium polonicum H175. Nat. Prod. Res. 2022, 36, 2370–2378. [Google Scholar] [CrossRef]
- Jia, J.; Kang, Q.; Liu, S.; Song, Y.; Wong, F.S.; Qiu, Y.; Li, M. Artemether and aspterric acid induce pancreatic alpha cells to transdifferentiate into beta cells in zebrafish. Br. J. Pharmacol. 2022, 179, 1962–1977. [Google Scholar] [CrossRef]
- Guo, W.; Zhang, Z.; Zhu, T.; Gu, Q.; Li, D. Penicyclones A–E, antibacterial polyketides from the deep-sea-derived fungus Penicillium sp. F23-2. J. Nat. Prod. 2015, 78, 2699–2703. [Google Scholar] [CrossRef]
- Zhang, Z.; He, X.; Wu, G.; Liu, C.; Lu, C.; Gu, Q.; Che, Q.; Zhu, T.; Zhang, G.; Li, D. Aniline-tetramic acids from the deep-sea-derived fungus Cladosporium sphaerospermum L3P3 cultured with the HDAC Inhibitor SAHA. J. Nat. Prod. 2018, 81, 1651–1657. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, Y.; Niu, S.; Liu, X.; Che, Y. Cytotoxic cleistanthane and cassane diterpenoids from the entomogenous fungus Paraconiothyrium hawaiiense. J. Nat. Prod. 2014, 77, 1513–1518. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Gao, H.; Chen, X.; Cai, X.; Yang, L.; Guo, L.; Yao, X.; Che, Y. Brasilamides A–D: Sesquiterpenoids from the plant endophytic fungus Paraconiothyrium brasiliense. Eur. J. Org. Chem. 2010, 2010, 3302–3306. [Google Scholar] [CrossRef]
- Liu, L.; Chen, X.; Li, D.; Zhang, Y.; Li, L.; Guo, L.; Cao, Y.; Che, Y. Bisabolane sesquiterpenoids from the plant endophytic fungus Paraconiothyrium brasiliense. J. Nat. Prod. 2015, 78, 746–753. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, K.-i.; Tomida, J.; Hirai, T.; Kawamura, Y.; Inoue, M. Paraconiothins A–J: Sesquiterpenoids from the endophytic fungus Paraconiothyrium brasiliense ECN258. J. Nat. Prod. 2019, 82, 3347–3356. [Google Scholar] [CrossRef] [PubMed]
- Breeden, D.C.; Young, T.E.; Coates, R.M.; Juvik, J.A. Identification and bioassay of kairomones for Helicoverpa zea. J. Chem. Ecol. 1996, 22, 513–539. [Google Scholar] [CrossRef] [PubMed]
- Chandra, A.; Misra, L.N.; Thakur, R.S. Tanavulgarol, an oxygenated sesquiterpene with an uncommon skeleton from Tanacetum vulgare. Phytochemistry 1987, 26, 3077–3078. [Google Scholar] [CrossRef]
- Kulkarni, K.S.; Paknikar, S.K.; Vaidya, A.S.; Kelkar, G.R.; Bates, R.B.; Bhattacharyya, S.C. Structure of β-bergamotene. Tetrahedron Lett. 1963, 4, 505–511. [Google Scholar] [CrossRef]
- Liu, M.; Hu, Z.X.; Luo, Y.Q.; Zhou, M.; Wang, W.G.; Li, X.N.; Du, X.; Pu, J.X.; Sun, H.D. Two new compounds from Schisandra propinqua var. propinqua. Nat. Prod. Bioprospect. 2017, 7, 257–262. [Google Scholar] [CrossRef] [Green Version]
- Misra, L.N.; Ahmad, A. An oxygenated tetrahydrobergamotene from the essential oil of Dracocephalum nutans. Planta Med. 1992, 58, 478–479. [Google Scholar] [CrossRef]
- Oh, H.; Gloer, J.B.; Shearer, C.A. Massarinolins A–C: New bioactive sesquiterpenoids from the aquatic fungus Massarina tunicata. J. Nat. Prod. 1999, 62, 497–501. [Google Scholar] [CrossRef]
- Wang, Y.; Li, D.H.; Li, Z.L.; Sun, Y.J.; Hua, H.M.; Liu, T.; Bai, J. Terpenoids from the marine-derived fungus Aspergillus fumigatus YK-7. Molecules 2016, 21, 31. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Jiang, N.; Guo, C.; Gao, J.-M. A new bergamotane sesquiterpenoid from the rhizomes of Amomum villosum var. xanthioides. Nat. Prod. Res. 2021, 35, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.Z.; Zhao, K.; Chen, H.P.; Bai, X.; Zhang, L.; Liu, J.K. Terpenoids from the mushroom-associated fungus Montagnula donacina. Phytochemistry 2018, 147, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Ren, F.; Che, Y.; Liu, G.; Liu, L. New bergamotane sesquiterpenoids from the plant endophytic fungus Paraconiothyrium brasiliense. Molecules 2015, 20, 14611–14620. [Google Scholar] [CrossRef] [Green Version]
- Kimura, Y.; Matsumoto, T.; Nakajima, H.; Hamasaki, T.; Matsuda, Y. Dihydroampullicin, a new plant growth regulators produced by the Ampulliferina-like fungus sp. No. 27. Biosci. Biotechnol. Biochem. 1993, 57, 687–688. [Google Scholar] [CrossRef] [Green Version]
- Kimura, Y.; Nakajima, H.; Hamasaki, T. Pinthunamide, a new tricyclic sesquiterpene amide produced by a fungus, Ampullifernia sp. Tetrahedron Lett. 1989, 30, 1267–1270. [Google Scholar] [CrossRef]
- Kimura, Y.; Nakajima, H.; Hamasaki, T.; Matsumoto, T.; Matsuda, Y.; Tsuneda, A. Ampullicin and isoampullicin, new metabolites from an Ampulliferina-like fungus sp. No. 27. Agric. Biol. Chem. 1990, 54, 813–814. [Google Scholar] [CrossRef]
- Massias, M.; Rebuffat, S.; Molho, L.; Chiaroni, A.; Riche, C.; Bodo, B. Expansolides A and B: Tetracyclic sesquiterpene lactones from Penicillium expansum. J. Am. Chem. Soc. 1990, 112, 8112–8115. [Google Scholar] [CrossRef]
- Ying, Y.M.; Fang, C.A.; Yao, F.Q.; Yu, Y.; Shen, Y.; Hou, Z.N.; Wang, Z.; Zhang, W.; Shan, W.G.; Zhan, Z.J. Bergamotane sesquiterpenes with alpha-glucosidase inhibitory activity from the plant pathogenic fungus Penicillium expansum. Chem. Biodivers. 2017, 14, e1600184. [Google Scholar] [CrossRef]
- Zhang, L.H.; Feng, B.M.; Chen, G.; Li, S.G.; Sun, Y.; Wu, H.H.; Bai, J.; Hua, H.M.; Wang, H.F.; Pei, Y.H. Sporulaminals A and B: A pair of unusual epimeric spiroaminal derivatives from a marine-derived fungus Paraconiothyrium sporulosum YK-03. RSC Adv. 2016, 6, 42361–42366. [Google Scholar] [CrossRef]
- Grimblat, N.; Kaufman, T.S.; Sarotti, A.M. Computational chemistry driven solution to rubriflordilactone B. Org. Lett. 2016, 18, 6420–6423. [Google Scholar] [CrossRef] [PubMed]
- Grimblat, N.; Zanardi, M.M.; Sarotti, A.M. Beyond DP4: An improved probability for the stereochemical assignment of isomeric compounds using quantum chemical calculations of NMR shifts. J. Org. Chem. 2015, 80, 12526–12534. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.C.; Chooi, Y.H.; Dhingra, S.; Xu, W.; Calvo, A.M.; Tang, Y. The fumagillin biosynthetic gene cluster in Aspergillus fumigatus encodes a cryptic terpene cyclase involved in the formation of β-trans-bergamotene. J. Am. Chem. Soc. 2013, 135, 4616–4619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Maddison, L.A.; Page McCaw, P.; Chen, W. Overnutrition induces beta-cell differentiation through prolonged activation of beta-cells in zebrafish larvae. Am. J. Physiol. Endocrinol. Metab. 2014, 306, 799–807. [Google Scholar] [CrossRef] [Green Version]
- Knopf, F.; Schnabel, K.; Haase, C.; Pfeifer, K.; Anastassiadis, K.; Weidinger, G. Dually inducible TetON systems for tissue-specific conditional gene expression in zebrafish. Proc. Natl. Acad. Sci. USA 2010, 107, 19933–19938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spartan’14; Wavefunction Inc.: Irvine, CA, USA, 2013.
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09, Revision A.1; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Bruhn, T.; Hemberger, Y.; Schaumlöffel, A.; Bringmann, G. SpecDis, Version 1.53; University of Wuerzburg: Wuerzburg, Germany, 2011. [Google Scholar]
- Li, M.; Page-McCaw, P.; Chen, W. FGF1 mediates overnutrition-induced compensatory β-cell differentiation. Diabetes 2016, 65, 96–109. [Google Scholar] [CrossRef] [Green Version]



| No. | 1 a | 2 b | ||
|---|---|---|---|---|
| Δc, Type | δH (J in Hz) | δc, Type | δH (J in Hz) | |
| 1 | 51.1, CH | 2.61, t (5.6) | 47.5, CH | 2.74, t (5.6) |
| 2 | 153.8, C | 154.3, C | ||
| 3 | 64.8, CH | 4.28, m | 64.6, CH | 4.33, m |
| 4 | 35.9, CH2 | 2.38, m | 36.0, CH2 | 2.34, m |
| 1.64, dt (13.7, 3.2) | 1.70, dt (13.9, 3.4) | |||
| 5 | 38.7, CH | 2.23, m | 38.44, CH | 2.24, m |
| 6 | 54.2, C | 54.4, C | ||
| 7 | 29.1, CH2 | 2.17, m | 31.0, CH2 | 2.26, m |
| 1.55, d (9.0) | 1.40, d (8.2) | |||
| 8 | 40.8, CH2 | 2.42, dd (12.7, 8.9) | 38.42, CH2 | 2.46, dd (12.0, 6.8) |
| 1.97, dd (12.7, 5.0) | 1.80, dd (12.0, 9.4) | |||
| 9 | 73.0, CH | 4.81, m | 73.6, CH | 4.83, m |
| 10 | 142.1, CH | 6.60, dd (7.9, 1.4) | 143.1, CH | 6.50, dd (8.2, 1.4) |
| 11 | 128.3, C | 127.9, C | ||
| 12 | 168.6, C | 168.7, C | ||
| 13 | 12.7, CH3 | 1.77, d (1.4) | 12.4, CH3 | 1.76, d (1.4) |
| 14 | 105.7, CH | 4.37, s | 104.9, CH | 4.48, s |
| 15 | 110.7, CH2 | 5.03, t (1.6) | 108.7, CH2 | 4.94, t (1.9) |
| 4.80, t (1.6) | 4.71, t (1.9) | |||
| 16 | 54.0, CH3 | 3.20, s | 54.3, CH3 | 3.16, s |
| OH-3 | 4.90, d (4.7) | 4.91, d (5.4) | ||
| COOH-12 | 12.40, s | 12.29, s | ||
| Data Type | 1a | 1b | |
|---|---|---|---|
| sDP4+a | 1H | 0.00% | 100.00% |
| 13C | 0.89% | 99.11% | |
| 1H + 13C | 0.00% | 100.00% | |
| uDP4+b | 1H | 0.00% | 100.00% |
| 13C | 4.59% | 95.41% | |
| 1H + 13C | 0.00% | 100.00% | |
| DP4+ | 1H | 0.00% | 100.00% |
| 13C | 0.04% | 99.96% | |
| 1H + 13C | 0.00% | 100.00% |
| No. | 3 | 4 | ||
|---|---|---|---|---|
| δc, Type | δH (J in Hz) | δc, Type | δH (J in Hz) | |
| 1 | 51.4, CH | 2.55, t (5.6) | 47.8, CH | 2.72, t (5.4) |
| 2 | 149.9, C | 149.7, C | ||
| 3 | 23.2, CH2 | 2.53, m | 23.0, CH2 | 2.55, m |
| 2.31, m | 2.37, m | |||
| 4 | 23.4, CH2 | 2.00, m | 23.3, CH2 | 1.87, m |
| 1.76, m | ||||
| 5 | 38.8, CH | 2.28, m | 38.4, CH | 2.24, m |
| 6 | 54.5, C | 54.7, C | ||
| 7 | 26.5, CH2 | 2.15, m | 28.1, CH2 | 2.22, m |
| 1.38, d (9.5) | 1.30, d (8.9) | |||
| 8 | 40.3, CH2 | 2.41, dd (12.5, 9.3) | 38.1, CH2 | 2.45, dd (11.9, 6.8) |
| 1.97, dd (12.6, 4.4) | 1.78, m, overlapped | |||
| 9 | 73.1, CH | 4.83, m | 73.6, CH | 4.84, m |
| 10 | 142.5, CH | 6.61, d (7.8) | 143.3, CH | 6.51, d (8.2) |
| 11 | 128.0, C | 127.7, C | ||
| 12 | 168.6, C | 168.7, C | ||
| 13 | 12.7, CH3 | 1.77, s | 12.4, CH3 | 1.77, s |
| 14 | 105.6, CH | 4.48, s | 104.7, CH | 4.52, s |
| 15 | 107.6, CH2 | 4.71, s | 106.9, CH2 | 4.61, s |
| 4.63, s | 4.58, s | |||
| 16 | 53.9, CH3 | 3.21, s | 54.4, CH3 | 3.18, s |
| COOH-12 | 12.40, s | 12.36, s | ||
| No. | 5 | No. | 5 | ||
|---|---|---|---|---|---|
| δc, Type | δH (J in Hz) | δc, Type | δH (J in Hz) | ||
| 1 | 42.8, CH | 2.12, m | 9 | 75.4, CH | 4.94, m |
| 2 | 145.6, C | 10 | 143.2, CH | 6.53, d (7.6) | |
| 3 | 114.7, CH | 5.15, m | 11 | 127.6, C | |
| 4 | 30.6, CH2 | 2.29, m | 12 | 168.9, C | |
| 2.25, m | 13 | 12.0, CH3 | 1.76, s | ||
| 5 | 38.4, CH | 2.43, d (3.9) | 14 | 105.5, CH | 4.42, s |
| 6 | 53.3, C | 15 | 22.5, CH3 | 1.66, m | |
| 7 | 29.9, CH2 | 2.15, m | 16 | 54.3, CH3 | 3.18, s |
| 1.18, d (8.1) | COOH-12 | 12.29, s | |||
| 8 | 38.2, CH2 | 2.35, dd (12.0, 6.8) | |||
| 1.77, dd (12.0, 9.9) | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Shi, Y.; Liu, Y.; Zhang, Y.; Wu, J.; Zhang, G.; Che, Q.; Zhu, T.; Li, M.; Li, D. Brasilterpenes A–E, Bergamotane Sesquiterpenoid Derivatives with Hypoglycemic Activity from the Deep Sea-Derived Fungus Paraconiothyrium brasiliense HDN15-135. Mar. Drugs 2022, 20, 338. https://doi.org/10.3390/md20050338
Wang W, Shi Y, Liu Y, Zhang Y, Wu J, Zhang G, Che Q, Zhu T, Li M, Li D. Brasilterpenes A–E, Bergamotane Sesquiterpenoid Derivatives with Hypoglycemic Activity from the Deep Sea-Derived Fungus Paraconiothyrium brasiliense HDN15-135. Marine Drugs. 2022; 20(5):338. https://doi.org/10.3390/md20050338
Chicago/Turabian StyleWang, Wenxue, Yeqin Shi, Yuanyuan Liu, Yundong Zhang, Jiajin Wu, Guojian Zhang, Qian Che, Tianjiao Zhu, Mingyu Li, and Dehai Li. 2022. "Brasilterpenes A–E, Bergamotane Sesquiterpenoid Derivatives with Hypoglycemic Activity from the Deep Sea-Derived Fungus Paraconiothyrium brasiliense HDN15-135" Marine Drugs 20, no. 5: 338. https://doi.org/10.3390/md20050338
APA StyleWang, W., Shi, Y., Liu, Y., Zhang, Y., Wu, J., Zhang, G., Che, Q., Zhu, T., Li, M., & Li, D. (2022). Brasilterpenes A–E, Bergamotane Sesquiterpenoid Derivatives with Hypoglycemic Activity from the Deep Sea-Derived Fungus Paraconiothyrium brasiliense HDN15-135. Marine Drugs, 20(5), 338. https://doi.org/10.3390/md20050338