Marine Demospongiae: A Challenging Treasure of Bioactive Compounds
Abstract
1. Introduction
1.1. Natural Products from Marine Organisms
1.2. Description of the Class Demospongiae
1.3. Demospongiae as Sources of Beneficial Compounds
2. Biotechnological Activities of Compounds Isolated from Demospongiae or Their Associated Microorganisms
2.1. Cytotoxic Activity
2.2. Antibacterial and Antiviral Activities
2.3. Antifouling Activity
2.4. Other Miscellaneous Activities
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bergmann, W.; Feeney, R.J. The isolation of a new thymine pentoside from Sponges. J. Am. Chem. Soc. 1950, 72, 2809–2810. [Google Scholar] [CrossRef]
- Bergmann, W.; Feeney, R.J. Contributions to the study of marine products. XXXII. the nucleosides of sponges. I. J. Org. Chem. 1951, 16, 981–987. [Google Scholar] [CrossRef]
- Carté, B.K. Potential of marine biomedical natural products research and medical applications. Oxf. J. 1996, 46, 271–286. [Google Scholar]
- Burkholder, P.R.; Pfister, R.M.; Leitz, F.H. Production of a pyrrole antibiotic by a marine bacterium. Appl. Microbiol. 1966, 14, 649–653. [Google Scholar] [CrossRef] [PubMed]
- Proksch, P.; Edrada, R.A.; Ebel, R. Drugs from the seas—Current status and microbiological implications. Appl. Microbiol. Biotechnol. 2002, 59, 125–134. [Google Scholar] [CrossRef]
- Weinheimer, A.J.; Spraggins, R.L. The occurrence of two new prostaglandin derivatives (15-epi-PGA2 and its acetate, methyl ester) in the Gorgonian Plexaura Homomalla. Tetrahedron Lett. 1969, 10, 5185–5188. [Google Scholar] [CrossRef]
- Ireland, C.; Copp, B.; Foster, M.; McDonald, L.; Radisky, D.; Swersey, J. Biomedical potential of Marine natural products. In Pharmaceutical and Bioactive Natural Products; Springer: Boston, MA, USA, 1993; pp. 1–43. [Google Scholar]
- Gordon, E.M.; Barrett, R.W.; Dower, W.J.; Fodor, S.P.A.; Gallop, M.A. Applications of combinatorial technologies to drug discovery. 2. Combinatorial organic synthesis, library screening strategies, and future directions. J. Med. Chem. 1994, 37, 1385–1401. [Google Scholar] [CrossRef]
- Alonso, D.; Khalil, Z.; Satkunanthan, N.; Livett, B. Drugs from the Sea: Conotoxins as drug leads for neuropathic pain and other neurological conditions. Mini-Rev. Med. Chem. 2003, 3, 785–787. [Google Scholar] [CrossRef]
- Twelves, C.; Cortes, J.; Vahdat, L.; Wanders, J.; Akerele, C.; Kaufman, P. Phase III trials of eribulin mesylate (E7389) in extensively pretreated patients with locally recurrent or metastatic breast cancer. Clin. Breast Cancer 2010, 10, 160–163. [Google Scholar] [CrossRef]
- Munekata, P.E.S.; Pateiro, M.; Conte-Junior, C.A.; Domínguez, R.; Nawaz, A.; Walayat, N.; Fierro, E.M.; Lorenzo, J.M. Marine alkaloids: Compounds with in vivo activity and chemical synthesis. Mar. Drugs 2021, 19, 374. [Google Scholar] [CrossRef]
- Hu, G.P.; Yuan, J.; Sun, L.; She, Z.G.; Wu, J.H.; Lan, X.J.; Zhu, X.; Lin, Y.C.; Chen, S.P. Statistical research on marine natural products based on data obtained between 1985 and 2008. Mar. Drugs 2011, 9, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, D.J. Marine natural products. Nat. Prod. Rep. 2002, 19, 1–49. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Copp, B.R.; Munro, M.H.G.; Northcote, P.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2006, 23, 26–78. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, D. Marine natural products. Nat. Prod. Rep. 2000, 17, 7–55. [Google Scholar] [CrossRef]
- Mehbub, M.F.; Lei, J.; Franco, C.; Zhang, W. Marine sponge derived natural products between 2001 and 2010: Trends and opportunities for discovery of bioactives. Mar. Drugs 2014, 12, 4539–4577. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Munro, M.H.G.; Northcote, P.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2010, 27, 165–237. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Munro, M.H.G.; Northcote, P.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2011, 28, 196–268. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2012, 29, 144–222. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2013, 30, 237–323. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2014, 31, 160–258. [Google Scholar] [CrossRef]
- Lyu, C.; Chen, T.; Qiang, B.; Liu, N.; Wang, H.; Zhang, L.; Liu, Z. CMNPD: A comprehensive marine natural products database towards facilitating drug discovery from the ocean. Nucleic Acids Res. 2021, 49, D509–D515. [Google Scholar] [CrossRef] [PubMed]
- Laport, M.; Santos, O.; Muricy, G. Marine sponges: Potential sources of new antimicrobial drugs. Curr. Pharm. Biotechnol. 2009, 10, 86–105. [Google Scholar] [CrossRef] [PubMed]
- Sagar, S.; Kaur, M.; Minneman, K.P. Antiviral lead compounds from marine sponges. Mar. Drugs 2010, 8, 2619–2638. [Google Scholar] [CrossRef] [PubMed]
- Nagle, D.; Zhou, Y.; Mora, F.; Mohammed, K.; Kim, Y. Mechanism targeted discovery of antitumor Marine natural products. Curr. Med. Chem. 2004, 11, 1725–1756. [Google Scholar] [CrossRef] [PubMed]
- Gomes, N.G.M.; Dasari, R.; Chandra, S.; Kiss, R.; Kornienko, A. Marine invertebrate metabolites with anticancer activities: Solutions to the “supply problem”. Mar. Drugs 2016, 14, 98. [Google Scholar] [CrossRef]
- Morrow, C.; Cárdenas, P. Proposal for a revised classification of the Demospongiae (Porifera). Front. Zool. 2015, 12, 1–27. [Google Scholar] [CrossRef]
- Borchiellini, C.; Manuel, M.; Alivon, E.; Boury-Esnault, N.; Vacelet, J.; Le Parco, Y. Sponge paraphyly and the origin of Metazoa. J. Evol. Biol. 2001, 14, 171–179. [Google Scholar] [CrossRef]
- Hooper, J.N.A.; Van Soest, R.W.M. Systema Porifera. A guide to the classification of sponges. In Systema Porifera; Springer: Boston, MA, USA, 2002; pp. 1–7. [Google Scholar]
- Beaulieu, S.E. Life on glass houses: Sponge stalk communities in the deep sea. Mar. Biol. 2001, 138, 803–817. [Google Scholar] [CrossRef]
- Beaulieu, S.E. Colonization of habitat islands in the deep sea: Recruitment to glass sponge stalks. Deep Sea Res. Part I Oceanogr. Res. Pap. 2001, 48, 1121–1137. [Google Scholar] [CrossRef]
- Hill, M.; Hill, A.; Lopez, N.; Harriott, O. Sponge-specific bacterial symbionts in the Caribbean sponge, Chondrilla nucula (Demospongiae, Chondrosida). Mar. Biol. 2006, 148, 1221–1230. [Google Scholar] [CrossRef]
- Taylor, M.W.; Radax, R.; Steger, D.; Wagner, M. Sponge-associated microorganisms: Evolution, ecology, and biotechnological potential. Microbiol. Mol. Biol. Rev. 2007, 71, 295–347. [Google Scholar] [CrossRef] [PubMed]
- Kautsar, S.A.; Blin, K.; Shaw, S.; Navarro-Muñoz, J.C.; Terlouw, B.R.; Van Der Hooft, J.J.J.; Van Santen, J.A.; Tracanna, V.; Suarez Duran, H.G.; Pascal Andreu, V.; et al. MIBiG 2.0: A repository for biosynthetic gene clusters of known function. Nucleic Acids Res. 2020, 48, D454–D458. [Google Scholar] [CrossRef] [PubMed]
- Medema, M.H.; de Rond, T.; Moore, B.S. Mining genomes to illuminate the specialized chemistry of life. Nat. Rev. Genet. 2021, 22, 553–571. [Google Scholar] [CrossRef] [PubMed]
- Dewick, P. Secondary metabolism: The building blocks and construction mechanisms. In Medicinal Natural Products: A Biosynthetic Approach; John Wiley & Sons, Ltd.: Hobokon, NJ, USA, 2002; pp. 7–38. [Google Scholar]
- Ghisalberti, E. Detection and isolation of bioactive natural products. In Bioactive Natural Products: Detection, Isolation and Structural Detection; CRS Press: London, UK, 2008; pp. 11–76. [Google Scholar]
- Wang, G. Diversity and biotechnological potential of the sponge-associated microbial consortia. J. Ind. Microbiol. Biotechnol. 2006, 33, 545–551. [Google Scholar] [CrossRef]
- Albarano, L.; Esposito, R.; Ruocco, N.; Costantini, M. Genome mining as new challenge in natural products discovery. Mar. Drugs 2020, 18, 199. [Google Scholar] [CrossRef]
- Nagai, H.; Kim, Y.H. Cancer prevention from the perspective of global cancer burden patterns. J. Thorac. Dis. 2017, 9, 448–451. [Google Scholar] [CrossRef]
- Khan, S.; Al-Fadhli, A.A.; Tilvi, S. Discovery of cytotoxic natural products from Red Sea sponges: Structure and synthesis. Eur. J. Med. Chem. 2021, 220, 113491. [Google Scholar] [CrossRef]
- Di, L.; Mannelli, C.; Esposito, F.P.; Sangiovanni, E.; Pagano, E.; Mannucci, C.; Polini, B.; Ghelardini, C.; Agli, M.D.; Izzo, A.A.; et al. Pharmacological activities of extracts and compounds isolated from mediterranean sponge sources. Pharmaceuticals 2021, 14, 1329. [Google Scholar]
- Mioso, R.; Marante, F.J.T.; Bezerra, R.D.S.; Borges, F.V.P.; Santos, B.V.D.O.; De Laguna, I.H.B. Cytotoxic compounds derived from marine sponges. A review (2010–2012). Molecules 2017, 22, 8. [Google Scholar] [CrossRef]
- Tsujii, S.; Rinehart, K.L.; Gunasekera, S.P.; Cross, S.S.; Lui, M.S.; Pomponi, S.A.; Cristina Diaz, M.; Kashman, Y. Topsentin, Bromotopsentin, and Dihydrodeoxybromotopsentin: Antiviral and antitumor bis(Indolyl)imidazoles from Caribbean deep-sea sponges of the Family Halichondriidae. Structural and synthetic studies. J. Org. Chem. 1988, 53, 5446–5453. [Google Scholar] [CrossRef]
- De Rosa, S.; De Stefano, S.; Zavodnik, N. Cacospongionolide: A new antitumoral sesterterpene, from the marine sponge Cacospongia Mollior. J. Org. Chem. 1988, 53, 5020–5023. [Google Scholar] [CrossRef]
- Cimino, G.; De Stefano, S.; Scognamiglio, G.; Sodano, G.; Trivellone, E. Sarains: A new class of alkaloids from the marine sponge Reniera sarai. Bull. Soc. Chim. Belg. 1986, 95, 783–800. [Google Scholar] [CrossRef]
- Caprioli, V.; Cimino, G.; De Giulio, A.; Madaio, A.; Scognamiglio, G.; Trivellone, E. Selected biological activities of saraines. Comp. Biochem. Physiol.—Part B Biochem. 1992, 103, 293–296. [Google Scholar] [CrossRef]
- Rady, H.M.; Hassan, A.Z.; Salem, S.M.; Mohamed, T.K.; Esmaiel, N.N.; Ez-El-Arab, M.A.; Ibrahim, M.A.; Fouda, F.K. Induction of apoptosis and cell cycle arrest by Negombata magnifica sponge in hepatocellular carcinoma. Med. Chem. Res. 2016, 25, 456–465. [Google Scholar] [CrossRef]
- Ka, E.; El-naggar, H.A.; Ibrahim, H.A.H.; Mansour, A.B.; Fekry, M.A.S. Biological activities of some marine sponge extracts from Aqaba Gulf, Red Sea, Egypt. Int. J. Fish. Aquat. Stud. 2017, 5, 652–659. [Google Scholar]
- Tinto, W.F.; Lough, A.J.; McLean, S.; Reynolds, W.F.; Yu, M.; Chan, W.R. Geodiamolides H and I, further cyclodepsipeptides from the marine sponge Geodia sp. Tetrahedron 1998, 54, 4451–4458. [Google Scholar] [CrossRef]
- Costantini, S.; Romano, G.; Rusolo, F.; Capone, F.; Guerriero, E.; Colonna, G.; Ianora, A.; Ciliberto, G.; Costantini, M. Anti-inflammatory effects of a methanol extract from the marine sponge Geodia cydonium on the human breast cancer MCF-7 cell line. Mediat. Inflamm. 2015, 2015, 204975. [Google Scholar] [CrossRef]
- Costantini, S.; Guerriero, E.; Teta, R.; Capone, F.; Caso, A.; Sorice, A.; Romano, G.; Ianora, A.; Ruocco, N.; Budillon, A.; et al. Evaluating the effects of an organic extract from the mediterranean sponge Geodia cydonium on human breast cancer cell lines. Int. J. Mol. Sci. 2017, 18, 2112. [Google Scholar] [CrossRef]
- Hawas, U.W.; Abou El-Kassem, L.T.; Abdelfattah, M.S.; Elmallah, M.I.Y.; Eid, M.A.G.; Monier, M.; Marimuthu, N. Cytotoxic activity of alkyl benzoate and fatty acids from the red sea sponge Hyrtios erectus. Nat. Prod. Res. 2018, 32, 1369–1374. [Google Scholar] [CrossRef]
- Rifai, S.; Fassouane, A.; Pinho, P.M.; Kijjoa, A.; Nazareth, N.; Nascimento, M.S.J.; Herz, W. Cytotoxicity and inhibition of lymphocyte proliferation of fasciculatin, a linear furanosesterterpene isolated from Ircinia variabilis collected from the Atlantic Coast of Morocco. Mar. Drugs 2005, 3, 15–21. [Google Scholar] [CrossRef]
- Ferretti, C.; Marengo, B.; De Ciucis, C.; Nitti, M.; Pronzato, M.A.; Marinari, U.M.; Pronzato, R.; Manconi, R.; Domenicotti, C. Effects of Agelas oroides and Petrosia ficiformis crude extracts on human neuroblastoma cell survival. Int. J. Oncol. 2007, 30, 161–169. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ferretti, C.; Vacca, S.; De Ciucis, C.; Marengo, B.; Duckworth, A.R.; Manconi, R.; Pronzato, R.; Domenicotti, C. Growth dynamics and bioactivity variation of the Mediterranean demosponges Agelas oroides (Agelasida, Agelasidae) and Petrosia ficiformis (Haplosclerida, Petrosiidae). Mar. Ecol. 2009, 30, 327–336. [Google Scholar] [CrossRef]
- Di Bari, G.; Gentile, E.; Latronico, T.; Corriero, G.; Fasano, A.; Marzano, C.N.; Liuzzi, G.M. Comparative analysis of protein profiles of aqueous extracts from marine sponges and assessment of cytotoxicity on different mammalian cell types. Environ. Toxicol. Pharmacol. 2014, 38, 1007–1015. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.H.; Woo, J.H.; Rho, J.R.; Choi, J.H. Anticancer activity of gukulenin a isolated from the marine sponge Phorbas gukhulensis in vitro and in vivo. Mar. Drugs 2019, 17, 126. [Google Scholar] [CrossRef]
- Matsumoto, R.; Fujii, Y.; Kawsar, S.M.A.; Kanaly, R.A.; Yasumitsu, H.; Koide, Y.; Hasan, I.; Iwahara, C.; Ogawa, Y.; Im, C.H.; et al. Cytotoxicity and glycan-binding properties of an 18 kDa lectin isolated from the marine sponge Halichondria okadai. Toxins 2012, 4, 323–338. [Google Scholar] [CrossRef]
- Agrawal, S.; Adholeya, A.; Deshmukh, S.K. The pharmacological potential of non-ribosomal peptides from marine sponge and tunicates. Front. Pharmacol. 2016, 7, 333. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Z.; Wang, H. Cytotoxic natural products from marine sponge-derived microorganisms. Mar. Drugs 2017, 15, 68. [Google Scholar] [CrossRef]
- Pagliara, P.; Caroppo, C. Cytotoxic and antimitotic activities in aqueous extracts of eight cyanobacterial strains isolated from the marine sponge Petrosia ficiformis. Toxicon 2011, 57, 889–896. [Google Scholar] [CrossRef]
- Pagliara, P.; Barca, A.; Verri, T.; Caroppo, C. The marine sponge Petrosia ficiformis harbors different cyanobacteria strains with potential biotechnological application. J. Mar. Sci. Eng. 2020, 8, 638. [Google Scholar] [CrossRef]
- Cheng, C.; Othman, E.M.; Stopper, H.; Edrada-Ebel, R.A.; Hentschel, U.; Abdelmohsen, U.R. Isolation of petrocidin a, a new cytotoxic cyclic dipeptide from the marine sponge-derived bacterium Streptomyces sp. SBT348. Mar. Drugs 2017, 15, 383. [Google Scholar] [CrossRef]
- Cheng, C.; Othman, E.M.; Fekete, A.; Krischke, M.; Stopper, H.; Edrada-, R.; Mueller, M.J.; Hentschel, U.; Abdelmohsen, U.R.; Cheng, C.; et al. Strepoxazine A, a new cyctotoxic phenoxazin from the marine sponge- derived bacterium Streptomyces sp. SBT345. Tetrahedron Lett. 2016, 57, 4196–4199. [Google Scholar] [CrossRef]
- Handayani, D.; Rasyid, W.; Rustini, Z.E.; Hertiani, T. Cytotoxic activity screening of fungal extracts derived from the west sumatran marine sponge Haliclona fascigera to several human cell lines: Hela, WiDr, T47D and Vero. J. Appl. Pharm. Sci. 2018, 8, 055–058. [Google Scholar] [CrossRef]
- Skropeta, D.; Pastro, N.; Zivanovic, A. Kinase inhibitors from marine sponges. Mar. Drugs 2011, 9, 2131–2154. [Google Scholar] [CrossRef] [PubMed]
- Alvi, K.A.; Jaspars, M.; Crews, P.; Strulovici, B.; Oto, E. Penazetidine A, an alkaloid inhibitor of protein kinase C. Bioorgan. Med. Chem. Lett. 1994, 4, 2447–2450. [Google Scholar] [CrossRef]
- Patil, A.D.; Freyer, A.J.; Killmer, L.; Hofmann, G.; Johnson, R.K. Z-axinohydantoin and debromo-z-axinohydantoin from the sponge Stylotella aurantium: Inhibitors of protein kinase C. Nat. Prod. Lett. 1997, 9, 201–207. [Google Scholar] [CrossRef]
- Piel, J. Metabolites from symbiotic bacteria. Nat. Prod. Rep. 2009, 26, 338–362. [Google Scholar] [CrossRef]
- Graça, A.P.; Viana, F.; Bondoso, J.; Correia, M.I.; Gomes, L.; Humanes, M.; Reis, A.; Xavier, J.R.; Gaspar, H.; Lage, O.M. The antimicrobial activity of heterotrophic bacteria isolated from the marine sponge Erylus deficiens (Astrophorida, Geodiidae). Front. Microbiol. 2015, 6, 389. [Google Scholar] [CrossRef]
- Krylova, D.D.; Aleshina, G.M.; Kokryakov, V.N.; Ereskovsky, A.V. Antimicrobial properties of mesohylar granular cells of Halisarca Dujardini Johnston, 1842 (Demospongiae, Halisarcida). Boll. Mus. Ist. Biol. Univ. Genova 2003, 68, 399–404. [Google Scholar]
- Morales, T.; Cubero, J.; Lanz, Z.; Gomez-Guinan, Y.; Segnini-Bravo, M. Actividad antimicrobiana de extractos organicos aislados de Aplysina fistularis (Demospongiae: Aplysinidae). Rev. Biol. Trop. 2000, 48, 199–206. [Google Scholar]
- Kubota, T.; Ishiguro, Y.; Takahashi-Nakaguchi, A.; Fromont, J.; Gonoi, T.; Kobayashi, J. Manzamenones L-N, new dimeric fatty-acid derivatives from an Okinawan marine sponge Plakortis sp. Bioorganic Med. Chem. Lett. 2013, 23, 244–247. [Google Scholar] [CrossRef]
- Mohammed, R.; Peng, J.; Kelly, M.; Yousaf, M.; Winn, E.; Odde, S.; Bie, Z.; Xie, A.; Doerksen, R.J.; Hamann, M.T. Polyketide-peroxides from a species of Jamaican plakortis (Porifera: Demospongiae). Aust. J. Chem. 2010, 63, 877–885. [Google Scholar] [CrossRef]
- Parama Cita, Y.; Kamal Muzaki, F.; Radjasa, O.K.; Sudarmono, P. Screening of antimicrobial activity of sponges extract from Pasir Putih, East Java (Indonesia). J. Mar. Sci. Res. Dev. 2017, 7, 2. [Google Scholar] [CrossRef]
- Chander, M.; Vijayachari, P. Antimicrobial and antioxidant potentials of marine sponges of South Andaman, India. Bangladesh J. Pharmacol. 2018, 13, 13–15. [Google Scholar] [CrossRef]
- Avilés, E.; Rodríguez, A. Monamphilectine a, a potent antimalarial β-lactam from a marine sponge Hymeniacidon sp: Isolation, structure, semisythesis, and bioactivity. Org. Lett. 2010, 12, 5290–5293. [Google Scholar] [CrossRef]
- Touati, I.; Chaieb, K.; Bakhrouf, A.; Gaddour, K. Screening of antimicrobial activity of marine sponge extracts collected from Tunisian coast. J. Mycol. Med. 2007, 17, 183–187. [Google Scholar] [CrossRef]
- Sun, S.; Canning, C.B.; Bhargava, K.; Sun, X.; Zhu, W.; Zhou, N.; Zhang, Y.; Zhou, K. Polybrominated diphenyl ethers with potent and broad spectrum antimicrobial activity from the marine sponge Dysidea. Bioorgan. Med. Chem. Lett. 2015, 25, 2181–2183. [Google Scholar] [CrossRef]
- Kosgahakumbura, K.N.M.L.; Hettiarachchi, C.; Jayasinghe, R.; Cárdenas, P.; Gunasekera, S. Isolation of cysteine-rich peptides from the deep-sea marine sponge Stryphnus fortis and determination of its antimicrobial effect. In Proceedings of the International conference on Frontiers in Chemical Technology, Colombo, Sri Lanka, 20–22 July 2020; p. 55. [Google Scholar]
- Giraldes, B.W.; Goodwin, C.; Al-Fardi, N.A.A.; Engmann, A.; Leitão, A.; Ahmed, A.A.; Ahmed, K.O.; Abdulkader, H.A.; Al-Korbi, H.A.; Al Easa, H.S.S.; et al. Two new sponge species (Demospongiae: Chalinidae and Suberitidae) isolated from hyperarid mangroves of Qatar with notes on their potential antibacterial bioactivity. PLoS ONE 2020, 15, e0232205. [Google Scholar] [CrossRef]
- Guimarães, T.D.R.; Quiroz, C.G.; Rigotto, C.; De Oliveira, S.Q.; De Almeida, M.T.R.; Bianco, É.M.; Moritz, M.I.G.; Carraro, J.L.; Palermo, J.A.; Cabrera, G.; et al. Anti HSV-1 activity of halistanol sulfate and halistanol sulfate C isolated from Brazilian marine sponge Petromica citrina (Demospongiae). Mar. Drugs 2013, 11, 4176–4192. [Google Scholar] [CrossRef]
- Jayatilake, G.S.; Thornton, M.P.; Leonard, A.C.; Grimwade, J.E.; Baker, B.J. Metabolites from an antarctic sponge-associated bacterium, Pseudomonas aeruginosa. J. Nat. Prod. 1996, 59, 293–296. [Google Scholar] [CrossRef]
- Mitova, M.; Tommonaro, G.; De Rosa, S. A novel cyclopeptide from a bacterium associated with the marine sponge Ircinia muscarum. J. Biosci. 2003, 58, 740–745. [Google Scholar] [CrossRef]
- Suzumura, K.; Yoko, T.; Funatsu, M.; Nagai, K.; Tanaka, K.; Zhang, H.; Suzuki, K. YM-266183 and YM-266184, novel thiopeptide antibiotics produced by Bacillus cereus isolated from a marine sponge. J. Antibiot. 2003, 56, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Chen, H.; Han, X.; Lin, W.; Yan, X. Antimicrobial screening and active compound isolation from marine bacterium NJ6-3-1 associated with the sponge Hymeniacidon perleve. World J. Microbiol. Biotechnol. 2005, 21, 201–206. [Google Scholar] [CrossRef]
- Odekina, P.A.; Agbo, M.O.; Omeje, E.O. Antimicrobial and antioxidant activities of novel marine bacteria (Bacillus 2011SOCCUF3) Isolated from marine Ssponge (Spongia officinalis). Pharm. Sci. 2020, 26, 82–87. [Google Scholar] [CrossRef]
- Phadale, R.; Kumar, M.S. Characterization of an antimicrobial and antioxidant compound from a marine bacterium Gsa10 associated with the sponge Halichondria glabrata. J. Microbiol. Biotechnol. Food Sci. 2018, 7, 651–658. [Google Scholar] [CrossRef]
- Mohan, G.; Thangappanpillai, A.K.; Ramasamy, B. Antimicrobial activities of secondary metabolites and phylogenetic study of sponge endosymbiotic bacteria, Bacillus sp. at Agatti Island, Lakshadweep Archipelago. Biotechnol. Rep. 2016, 11, 44–52. [Google Scholar] [CrossRef]
- Chelossi, E.; Milanese, M.; Milano, A.; Pronzato, R.; Riccardi, G. Characterisation and antimicrobial activity of epibiotic bacteria from Petrosia ficiformis (Porifera, Demospongiae). J. Exp. Mar. Biol. Ecol. 2004, 309, 21–33. [Google Scholar] [CrossRef]
- Koch, M.J.; Hesketh-Best, P.J.; Smerdon, G.; Warburton, P.J.; Howell, K.; Upton, M. Impact of growth media and pressure on the diversity and antimicrobial activity of isolates from two species of hexactinellid sponge. Microbiology 2021, 167, 001123. [Google Scholar] [CrossRef]
- Flemer, B.; Kennedy, J.; Margassery, L.M.; Morrissey, J.P.; O’Gara, F.; Dobson, A.D.W. Diversity and antimicrobial activities of microbes from two Irish marine sponges, Suberites carnosus and Leucosolenia sp. J. Appl. Microbiol. 2012, 112, 289–301. [Google Scholar] [CrossRef]
- O’ Halloran, J.A.; Barbosa, T.M.; Morrissey, J.P.; Kennedy, J.; O’ Gara, F.; Dobson, A.D.W. Diversity and antimicrobial activity of Pseudovibrio spp. from Irish marine sponges. J. Appl. Microbiol. 2011, 110, 1495–1508. [Google Scholar] [CrossRef]
- Krishnan, P. Antimicrobial activity of Ircinia sp.; a marine sponge and its associated bacteria from Andaman Coast. Adv. Anim. Vet. Sci. 2014, 2, 37–41. [Google Scholar] [CrossRef]
- Pejin, B.; Talevski, A.; Ciric, A.; Glamoclija, J.; Nikolic, M.; Talevski, T.; Sokovic, M. In vitro evaluation of antimicrobial activity of the freshwater sponge Ochridaspongia rotunda (Arndt, 1937). Nat. Prod. Res. 2014, 28, 1489–1494. [Google Scholar] [CrossRef] [PubMed]
- Hole, W. Marine Fouling and its Prevention; US Naval Institute: Annapolis, MD, USA, 1952. [Google Scholar]
- Ortlepp, S.; Sjögren, M.; Dahlström, M.; Weber, H.; Ebel, R.; Edrada, R.A.; Thoms, C.; Schupp, P.; Bohlin, L.; Proksch, P. Antifouling activity of bromotyrosine-derived sponge metabolites and synthetic analogues. Mar. Biotechnol. 2007, 9, 776–785. [Google Scholar] [CrossRef] [PubMed]
- Campos, P.E.; Pichon, E.; Moriou, C.; Clerc, P.; Trépos, R.; Frederich, M.; De Voogd, N.; Hellio, C.; Gauvin-Bialecki, A.; Al-Mourabit, A. New antimalarial and antimicrobial tryptamine derivatives from the marine sponge Fascaplysinopsis reticulata. Mar. Drugs 2019, 17, 167. [Google Scholar] [CrossRef]
- Sjögren, M.; Goransson, U.; Johnson, A.; Dahlstrom, M.; Anderson, R.; Bergman, J.; Jonsson, P.; Bohlin, L. Antifouling activity of a dibrominated cyclopeptide from the marine sponge Geodia barretti. J. Nat. Prod. 2004, 67, 368–372. [Google Scholar] [CrossRef] [PubMed]
- Tsoukatou, M.; Hellio, C.; Vagias, C.; Harvala, C.; Roussis, V. Chemical defense and antifouling activity of three Mediterranean sponges of the genus Ircinia. Z. Fur Naturforsch.-Sect. C J. Biosci. 2002, 57, 161–171. [Google Scholar] [CrossRef]
- Lee, K.K.; Liu, P.C.; Chuang, W.H. Pathogenesis of gastroenteritis caused by Vibrio carchariae in cultured marine fish. Mar. Biotechnol. 2002, 4, 267–277. [Google Scholar] [CrossRef]
- Nicolas, J.; Basuyaux, O.; Mazurie, J.; Thébault, A. Vibrio carchariae, a pathogen of the abalone Haliotis tuberculata. Dis. Aquat. Organ. 2002, 50, 35–43. [Google Scholar] [CrossRef]
- Limna Mol, V.P.; Raveendran, T.V.; Parameswaran, P.S. Antifouling activity exhibited by secondary metabolites of the marine sponge, Haliclona exigua (Kirkpatrick). Int. Biodeterior. Biodegrad. 2009, 63, 67–72. [Google Scholar] [CrossRef]
- Sears, M.A.; Gerhart, D.J.; Rittschof, D. Antifouling agents from marine sponge Lissodendoryx isodictyalis carter. J. Chem. Ecol. 1990, 16, 791–799. [Google Scholar] [CrossRef]
- Okino, T.; Yoshimura, E.; Hirota, H.; Fusetani, N. Antifouling kalihinenes from the marine sponge Acanthella cavernosa. Tetrahedron Lett. 1995, 36, 8637–8640. [Google Scholar] [CrossRef]
- Qian, P.Y.; Dobretsov, S.; Dahms, H.U.; Pawlik, J. Antifouling activity and microbial diversity of two congeneric sponges Callyspongia spp. from Hong Kong and the Bahamas. Mar. Ecol. Prog. Ser. 2006, 324, 151–165. [Google Scholar] [CrossRef]
- Ribeiro, S.; Rogers, R.; Rubem, A.; Da Gama, B.; Muricy, G.; Pereira, R. Antifouling activity of twelve demosponges from Brazil. Braz. J. Biol. 2013, 73, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Sera, Y.; Adachi, K.; Fujii, K.; Shizuri, Y. A new antifouling hexapeptide from a Palauan sponge, Haliclona sp. J. Nat. Prod. 2003, 66, 719–721. [Google Scholar] [CrossRef] [PubMed]
- World Malaria Report. 2020. Available online: https://www.who.int/publications/i/item/9789240015791 (accessed on 1 January 2022).
- Campagnuolo, C.; Fattorusso, E.; Romano, A.; Taglialatela-Scafati, O.; Basilico, N.; Parapini, S.; Taramelli, D. Antimalarial polyketide cycloperoxides from the marine sponge Plakortis simplex. Eur. J. Org. Chem. 2005, 6, 5077–5083. [Google Scholar] [CrossRef]
- Campos, P.E.; Wolfender, J.L.; Queiroz, E.F.; Marcourt, L.; Al-Mourabit, A.; Frederich, M.; Bordignon, A.; De Voogd, N.; Illien, B.; Gauvin-Bialecki, A. Unguiculin A and ptilomycalins E-H, antimalarial guanidine alkaloids from the marine sponge Monanchora unguiculata. J. Nat. Prod. 2017, 80, 1404–1410. [Google Scholar] [CrossRef]
- Inbaneson, S.J.; Ravikumar, S. In vitro antiplasmodial activity of marine sponge Hyattella intestinalis associated bacteria against Plasmodium falciparum. Asian Pac. J. Trop. Biomed. 2011, 1, S100–S104. [Google Scholar] [CrossRef]
- Le Pape, P.; Zidane, M.; Abdala, H.; Moré, M.T. A glycoprotein isolated from the sponge, Pachymatisma johnstonii, has anti-leishmanial activity. Cell Biol. Int. 2000, 24, 51–56. [Google Scholar] [CrossRef]
- Dube, A.; Singh, N.; Saxena, A.; Lakshmi, V. Antileishmanial potential of a marine sponge, Haliclona exigua (Kirkpatrick) against experimental visceral leishmaniasis. Parasitol. Res. 2007, 101, 317–324. [Google Scholar] [CrossRef]
- Shady, N.H.; Fouad, M.A.; Ahmed, S.; Pimentel-Elardo, S.M.; Nodwell, J.R.; Kamel, M.S.; Abdelmohsen, U.R. A new antitrypanosomal alkaloid from the Red Sea marine sponge Hyrtios sp. J. Antibiot. 2018, 71, 1036–1039. [Google Scholar] [CrossRef]
- El-Hossary, E.M.; Cheng, C.; Hamed, M.M.; El-Sayed Hamed, A.N.; Ohlsen, K.; Hentschel, U.; Abdelmohsen, U.R. Antifungal potential of Marine natural products. Eur. J. Med. Chem. 2017, 126, 631. [Google Scholar] [CrossRef]
- Naglik, J.R.; Richardson, J.P.; Moyes, D.L. Candida albicans pathogenicity and epithelial immunity. PLoS Pathog. 2014, 10, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Rifai, S.; Fassouane, A.; Kijjoa, A.; Van Soest, R. Antimicrobial activity of untenospongin b, a metabolite from the marine sponge Hippospongia communis collected from the atlantic coast of Morocco. Mar. Drugs 2004, 2, 147–153. [Google Scholar] [CrossRef]
- Kibungu, W.C.; Fri, J.; Clarke, A.M.; Otigbu, A.; Akum Njom, H. Seasonal variation in antimicrobial activity of crude extracts of Psammaplysilla sp. 1 from Phillips Reef, South Africa. Int. J. Microbiol. 2021, 2021, 7568493. [Google Scholar] [CrossRef] [PubMed]
- Pozzolini, M.; Millo, E.; Oliveri, C.; Mirata, S.; Salis, A.; Damonte, G.; Arkel, M.; Scarfì, S. Elicited ROS scavenging activity, photoprotective, and wound-healing properties of collagen-derived peptides from the marine sponge Chondrosia reniformis. Mar. Drugs 2018, 16, 465. [Google Scholar] [CrossRef]
- Pozzolini, M.; Tassara, E.; Dodero, A.; Castellano, M.; Vicini, S.; Ferrando, S.; Aicardi, S.; Cavallo, D.; Bertolino, M.; Petrenko, I.; et al. Potential biomedical applications of collagen filaments derived from the marine demosponges Ircinia oros (Schmidt, 1864) and sarcotragus foetidus (Schmidt, 1862). Mar. Drugs 2021, 19, 563. [Google Scholar] [CrossRef]

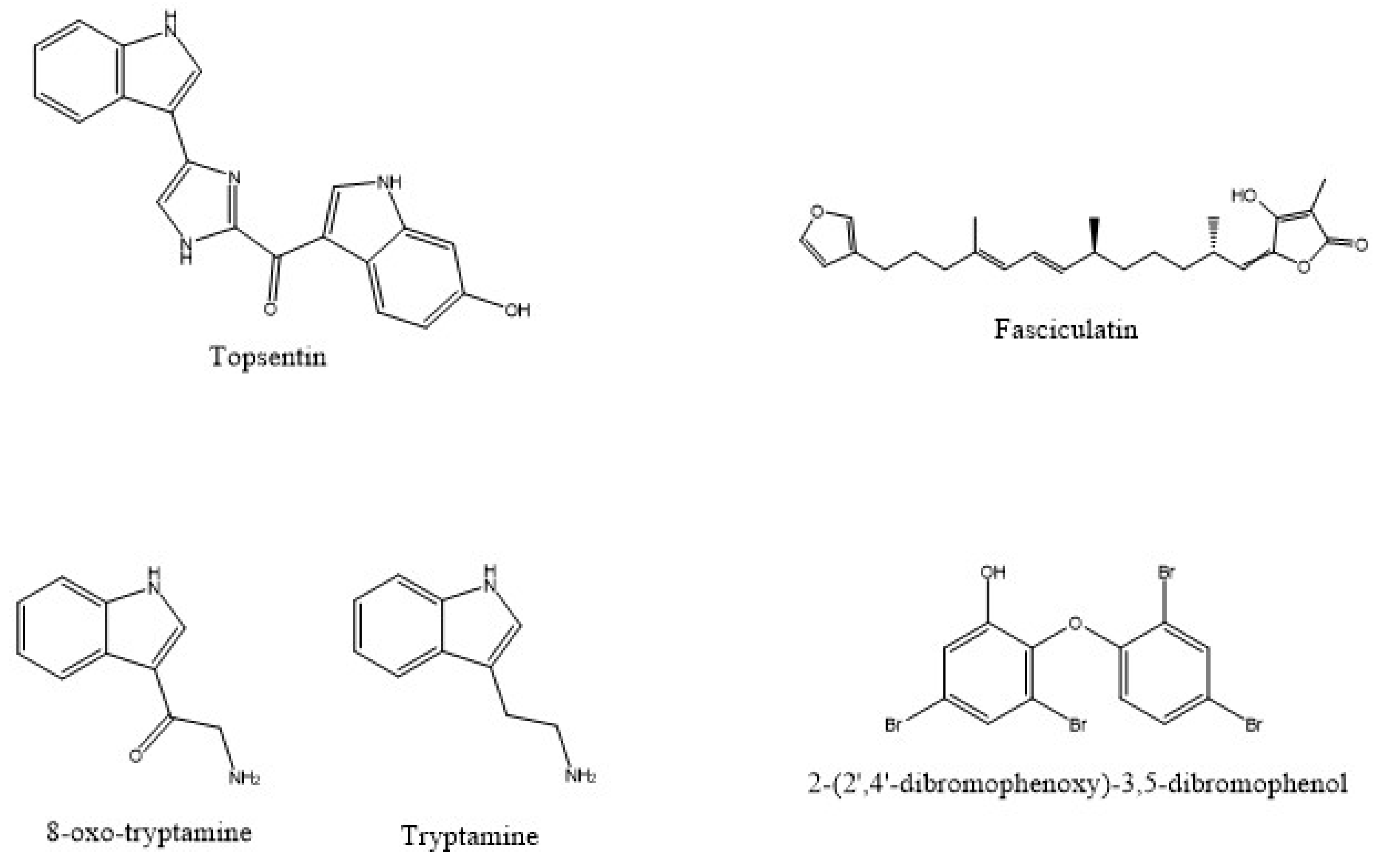

| Source | Associated Organisms | Extract/Compound | Cell Line/Organism Tested | Reference |
|---|---|---|---|---|
| Spongosorites sp. and S. ruetzleri | Topsentin and bromotopsentin | P388, HCT8, A549, T47 | [44] | |
| C. mollior | Cacospongiolide | Shrimp | [45] | |
| R. sarai | Saraine A | A. salina | [47] | |
| N. magnifica | Petroleum ether and total methanolic extracts | HepG2 | [48] | |
| N. magnifica | Aqueous ethanol extract | CACO-2 and MCF-7 | [49] | |
| Geodia sp. | Geodiamolide H 3 | HOP 92, SF-268, OV Car-4, A498, UO-31, MDA-MB-231, HS 578T | [50] | |
| G. cydonium | Methanolic extract | MCF-7, MDA-MB231, MDA-MB468 | [52] | |
| H. erectus | Oxysterol and 4′-methylheptyl benzoate | MCF-7 and HepG2 | [53] | |
| I. variabilis | Fasciculatin | MCF-7, NCI-H460 and SF-268 | [54] | |
| A. oroides and P. ficiformis | Methanolic extract | LAN5 and SK-N-BE(2)-C | [55] | |
| T. aurantium, T. citrina, H.perlevis, I. variabilis, C. nucula, A. aerophoba and S. spinosulus | Aqueous extract | THP-1, CaCo-2 and BHK-21 | [57] | |
| P. gukhulensis | Gukulenin A | A2780, SKOV3, OVCAR-3 and TOV-21G | [58] | |
| H. okadai | Lectin | Jurkat leukemia T and K562 | [59] | |
| P. ficiformis | Synechoccus sp. red and blue-green types, Cyanobium sp., Leptolyngbya cfr. Minuta, Leptolyngbya crf. ectocarpii, Leptolyngbya sp. 1, 2 and 3 | Aqueous extract | A. salina and P. lividus | [62] |
| P. ficiformis | Cyanobium sp., Synechoccus sp., Pseudoanabaena sp. 1, 2, L. ectocarpi, Halomicronema cf. metazoicum, H. metazoicum | Aqueous cell supernatans | HeLa, SH-SY5Y and B-104-1-1 | [63] |
| P. ficiformis | Streptomyces sp. SBT348 | Petrocidin A | HL-60 and HT-29 | [64] |
| A. oroides | Streptomyces sp. SBT345 | Strepoxazine A | HL-60 | [65] |
| H. fascigera | Trichophyton sp. | Ethil acetate extract | WiDr, T47D and HeLa | [66] |
| P. sollasi | Penazetidine A | PKC | [68] | |
| S. aurantium | Hymenialdisines 4 and 5 | PKC | [69] |
| Source | Associated Organisms | Isolated Compound | Cell Line/Organism Tested | Reference |
|---|---|---|---|---|
| H. dujardini | Eosinophilic amoebocytes (EA) fraction | E. coli and L. monocytogenes | [72] | |
| A. fistularis | Ethyl acetate extract | S. aureus | [73] | |
| Plakortis sp. | Manzamenones M and N | E. coli, S. aureus and C. neoformans | [74] | |
| P. angulospiculatus | Plakortide N and F | C. neoformans | [75] | |
| X. testudinaria | Methanol extract | S. aureus, E.coli, K. Pneumoniae, S. tiphy, P. aeruginosa MDR and S. aureus MRSA | [76] | |
| X. testudinaria | Methanol extract | S. epidermidis | [77] | |
| Hymeniacidon sp. | 1 Monoamphilectine and 8,15-diisocyano-11(20)-amphilectene | M. tuberculosis (H37Rv) | [78] | |
| A. dormicons and A. orides | Ethyl acetate extract | S. epidermidis, S. aureus, M. luteus, E. feacalis, E. coli, P. Aeruginosa, S. thyphymerium and L. monocytogenes. | [79] | |
| D. granulosa | 2-(2’,4’-dibromophenoxy)-3,5-dibromophenol | K. pneumoniae | [80] | |
| S. fortis | Peptide C | S. aureus | [81] | |
| S. luna | Aqueous extractA and methanol extract B | S. aureus and E. faecalis | [82] | |
| H. perleve | P. piscicida (NJ6-3-1) and B. megaterium (NJ6-3-2) | Ethyl acetate extract | B. subtilis, S. aureus, E. coli, A. tumefaciens and S. cerevisiae | [87] |
| S. officinales | Bacillus 2011SOCCUF3 | Methanol extract | S. aureus, S. tiphy, P. aeruginosa and E. coli | [88] |
| H. glabrata | Bacillus amyloliquefaciens | Ethyl acetate extract | E. coli, P. aeruginosa, B. subtilis and S. aureus | [89] |
| D. fragilis | Bacillus sp. | Pyrrolo(1,2-a)pyrazine-1,4-dione,hexahydro | V. alginolyticus, V. vulnificus, V. parahaemolyticus, Flavobacterium sp., P. mirabilis, C. brackii, A. salmonicida and Edwardsiella sp. | [90] |
| E. deficiens | Proteobacteria, Actinobacteria and Firmicutes phyla | Aqueous extract | V. anguillarum | [71] |
| P. ficiformis | Rhodococcus sp. and Pseudomonas sp. | Bacterial isolates | S. aureus | [91] |
| P. carpenteri and Hertwigia sp. | B. altitudinis, Streptomyces sp., Brevundimonas sp., M. maritypicum and D. acidovorans | Bacterial isolates | S. aureus, E. coli and M. luteus | [92] |
| S. carnosus and Leucosolenia sp. | Proteobacteria, Actinobacteria, Firmicutes and Bacteroidetes phyla | Bacterial isolates | E. coli NCIMB 12212, B. subtilis IA40, S. aureus NCIMB 9518, K. marxianus CB86556 | [93] |
| P. boletiformis, A. dissimilis and H. simulans | Pseudovibrio spp. | Bacterial isolates | E. coli, S. Typhimurium, B. subtilis, S. aureus, S. aureus MRSA, S. aureus VISA, hVISA, C. perfringens, C. difficile, Y. enterocolitica, B. cereus, E. faecium, Enterococcus (VRE) and L. monocytogenes | [94] |
| Ircinia sp. | Vibrio sp., Aeromonas sp., Bacillus sp., Corynebacterium sp., Pseudomonas sp., Streptococcus sp., Enterococcus sp., Neisseria sp., Veillonella sp., Citrobacter sp. and Klebsiella sp. | Bacterial isolates | A. hydrophila, B. subtilis, E. durans, S. lentus, K. pneumoniae and R. solanacearum | [95] |
| O. rotunda | Methanol extract | B. cereus, E. Cloacae, E. Coli, L. Monocytogenes, M. Flavus, P. Aeruginosa, S. Typhimurium and S. aureus | [96] |
| Source | Extract/Compound | Pathogens Tested | Reference |
|---|---|---|---|
| I. variabilis, I. spinosula and I. oros | Aqueous, ethanol and dichloromethane extract | A. coffeaformis, P. tricornutum, C. Closterium, E. intestinalis, U. lactuca and S. muticum | [101] |
| F. reticulata | Tryptamine and 6-bromo-8,1’-dihydro-isoplysin A and 5,6-dibromo-8,1’-dihydro-isoplysin A | V.carchariae and V. natrigens | [99] |
| N. chaliniformis | Ethyl acetate and aqueous extracts | B. cereus, B. pumilus, B. megaterium, P. haloplanktis, P. chlororaphis, P. putida, P. aeruginosa and B. amphitrite | [104] |
| L. isodictyalis | Ethyl acetate extract | B. amphitrite | [105] |
| A. cavernosa | Kalihinenes X, Y and Z | B. amphitrite | [106] |
| Callyspongia spp. and C. plicifera | Dichloromethane extract | B. amphitrite and H. elegans | [107] |
| G. barretti | Barretin and 8,9-dihydrobarretin | B. improvisus | [100] |
| I. basta, P. purpurea and A. rhax | Bastadins 3, 4, 9, bastadin-16, hemibastadin-1, aplysamine-2, psammaplin A | B. improvisus | [98] |
| T. rubra, T. maza, H. heliophile and P. citrina | Acetone/dichloromethane extract | P. perna | [108] |
| Haliclona sp. | Two hexapeptides | M. edulis galloprovincialis | [109] |
| Source | Sponge Host | Extract/Compound | Activity | Reference |
|---|---|---|---|---|
| P. simplex | Plakortin and Plakortide Q | Antiplasmodial | [111] | |
| Hymeniacidon sp. | Monoamphilectine A | Antiplasmodial | [78] | |
| M. unguiculata | Ptilomycalin F and Fromiamycalin | Antiplasmodial | [112] | |
| F.reticulata | 8-oxo-tryptamine and (E)-6-bromo-20-demethyl-30-N-methylaplysinopsin and (Z)-6-bromo-20-demethyl-30-N-methylaplysinopsin | Antiplasmodial | [99] | |
| H. intestinalis | Bacterial colonies THB20 and THB34 | Ethyl acetate extract | Antiplasmodial | [113] |
| P. johnstonii | Pachymatismin | Antileishmanial | [114] | |
| H. exigua | Araguspongin C | Antileishmanial | [115] | |
| Hyrtios sp. | Hyrtiodoline A | Antitrypanosomial | [116] | |
| A. oroides | Ethanol extract | Antifungal | [79] | |
| H. communis | Untenospongin B | Antifungal | [119] | |
| S. carnosus and Leucosolenia sp. | Psedoalteromonas, Bacillus, Vibrio and Staphylococcus phyla | Isolated of bacteria | Antifungal | [93] |
| S. officinalis | Bacillus 2011SOCCUF3 | Methanol extract | Antifungal | [88] |
| N. exigua | Methanolic extract | Antifungal | [77] | |
| S. fortis | Peptide C | Antifungal | [81] | |
| P. angulospigulatus | Plakortide N and Plakortide F | Antifungal | [75] | |
| Plakortis sp. | Menzamenone M and Menzamenone N | Antifungal | [74] | |
| E. deficiens | Proteobacteria, Actinobacteria and Firmicutes phyla | Aqueous extract | Antifungal | [71] |
| Psammaplysilla sp. 1 | Ethyl acetate extract | Antifungal | [120] | |
| C. reniformis | Collagen extract | Wound-healing | [121] | |
| I. oros and S. foetidus | Collagen filaments | Wound-healing | [122] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esposito, R.; Federico, S.; Bertolino, M.; Zupo, V.; Costantini, M. Marine Demospongiae: A Challenging Treasure of Bioactive Compounds. Mar. Drugs 2022, 20, 244. https://doi.org/10.3390/md20040244
Esposito R, Federico S, Bertolino M, Zupo V, Costantini M. Marine Demospongiae: A Challenging Treasure of Bioactive Compounds. Marine Drugs. 2022; 20(4):244. https://doi.org/10.3390/md20040244
Chicago/Turabian StyleEsposito, Roberta, Serena Federico, Marco Bertolino, Valerio Zupo, and Maria Costantini. 2022. "Marine Demospongiae: A Challenging Treasure of Bioactive Compounds" Marine Drugs 20, no. 4: 244. https://doi.org/10.3390/md20040244
APA StyleEsposito, R., Federico, S., Bertolino, M., Zupo, V., & Costantini, M. (2022). Marine Demospongiae: A Challenging Treasure of Bioactive Compounds. Marine Drugs, 20(4), 244. https://doi.org/10.3390/md20040244









