Would Antarctic Marine Benthos Survive Alien Species Invasions? What Chemical Ecology May Tell Us
Abstract
1. Introduction
2. Results
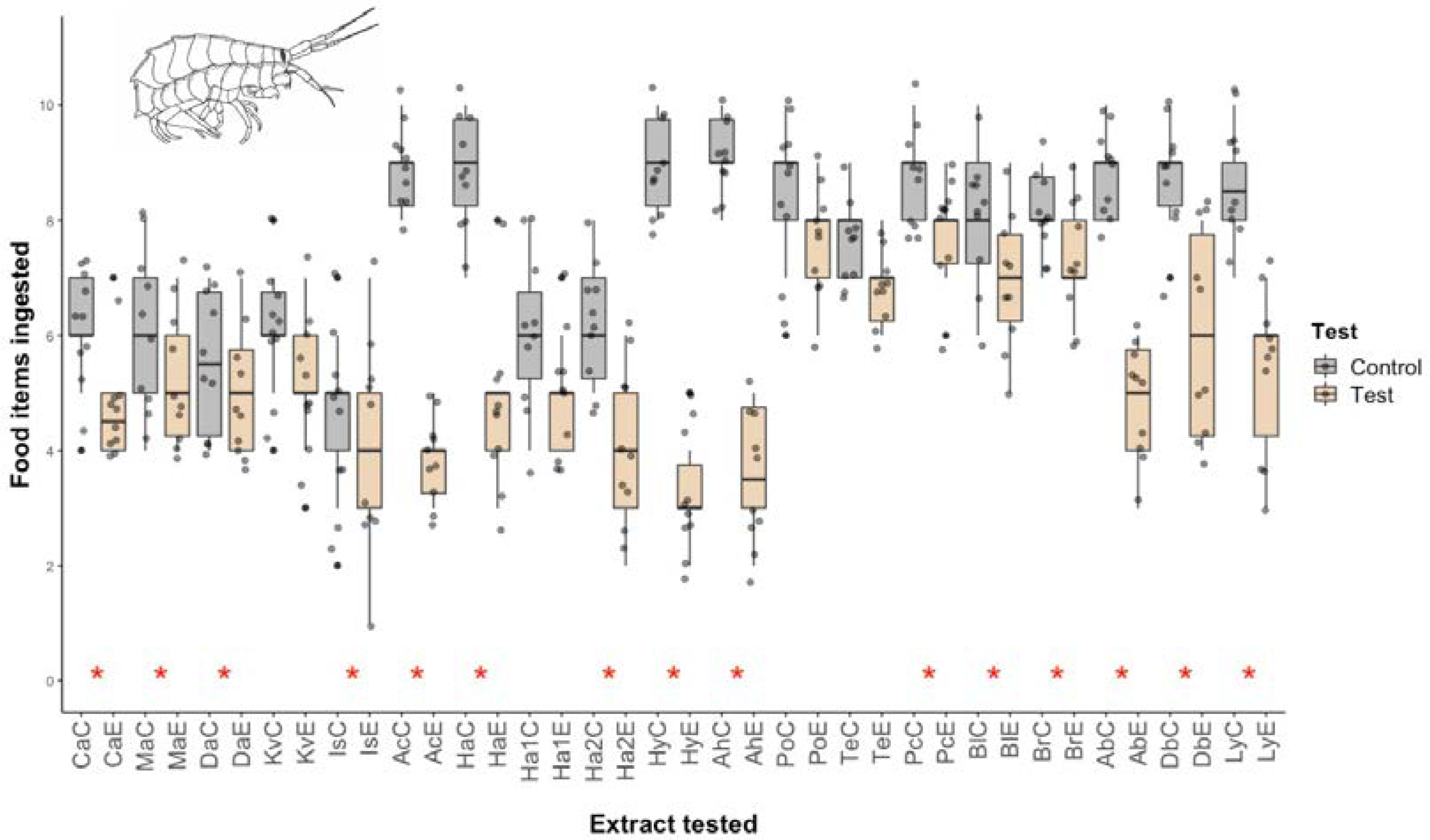
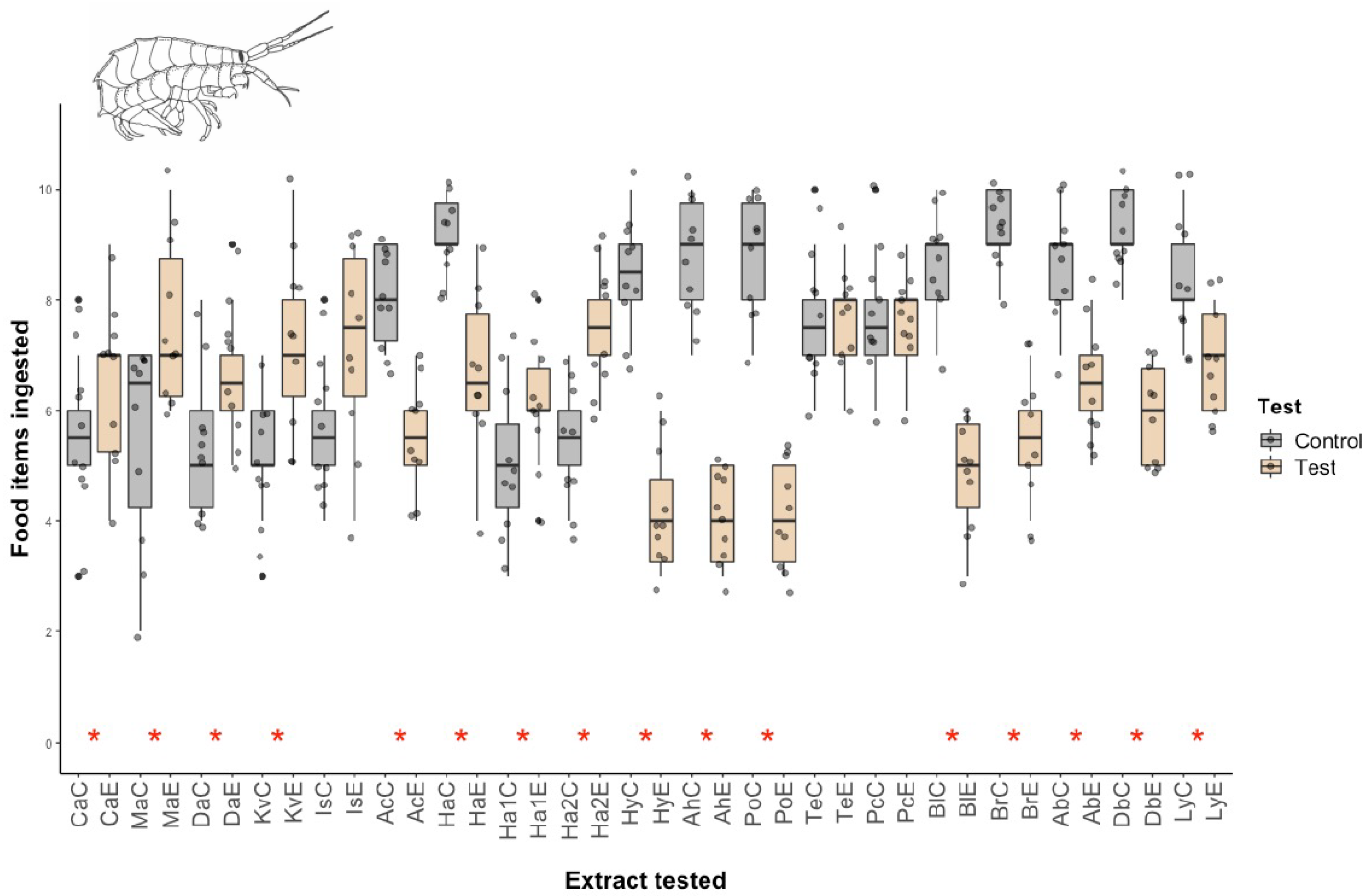
2.1. Micropredation Experiments
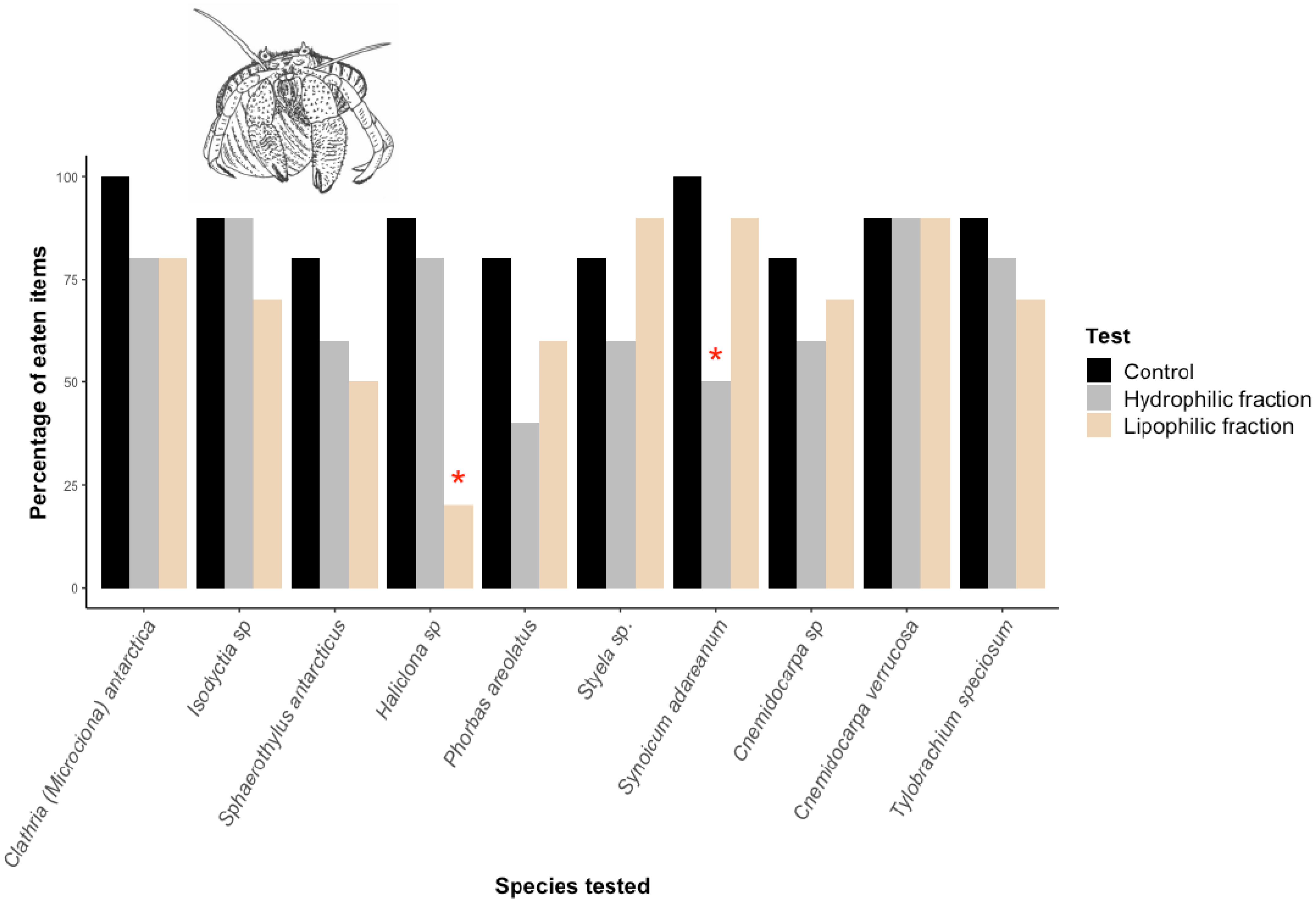
2.2. Macropredation Experiments
3. Discussion
3.1. Predation Experiments
3.2. Marine Natural Products
3.3. Climate Change and Alien Species
4. Materials and Methods
4.1. Sample Collection and Extraction of Antarctic Macroinvertebrates
4.2. Micropredation Experiments
4.3. Macropredation Experiments
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arntz, W.; Gutt, J.; Klages, M. Antarctic marine biodiversity. In Antarctic Communities: Species, Structure and Survival; Battaglia, B., Valencia, J., Walton, D.W.H., Eds.; Cambridge University Press: Cambridge, UK, 1997; pp. 3–14. [Google Scholar]
- Gutt, J. Some ‘driving forces’ structuring communities of the sublittoral Antarctic macrobenthos. Ant. Sci. 2000, 12, 297–313. [Google Scholar] [CrossRef]
- Dayton, P.K.; Robilliard, G.A.; Paine, R.T.; Dayton, L.B. Biological accommodation in the benthic community at McMurdo Sound, Antarctica. Ecol. Monogr. 1974, 44, 105–128. [Google Scholar] [CrossRef]
- Arntz, W.; Brey, T.; Gallardo, V.A. Antarctic zoobenthos. Ocean. Mar. Biol. 1994, 32, 241–304. [Google Scholar]
- Orejas, C.; Gili, J.M.; Arntz, W.E.; Ros, J.D.; López, P.J.; Teixido, N.; Filipe, P. Benthic suspension feeders, key players in Antarctic marine ecosystems? Contrib. Sci. 2000, 1, 299–311. [Google Scholar]
- Clarke, A.; Johnston, N.M. Antarctic marine benthic diversity. Ocean. Mar. Biol. 2003, 41, 47–114. [Google Scholar]
- Figuerola, B.; Avila, C.; Cristobo, J.; Vázquez, J.; Núñez-Pons, L.; Ballesteros, M.; Taboada, S. Chemical interactions in Antarctic marine benthic ecosystems. In Marine Ecosystems; Cruzado, A., Ed.; INTECH Open Access Publisher: Rijeka, Croatia, 2012; pp. 105–126. [Google Scholar]
- Angulo-Preckler, C.; Castro-Fernandez, P.; Martín-Martín, R.; Figuerola, B.; Avila, C. Chemical ecology in the Southern Ocean. In Life in Extreme Environments: Insights in Biological Capability; Di Prisco, G., Edwards, H., Elster, J., Huiskes, A., Eds.; Cambridge University Press: Cambridge, UK, 2020; pp. 251–278. [Google Scholar] [CrossRef]
- McClintock, J.B. Trophic biology of Antarctic shallow-water echinoderms. Mar. Ecol. Prog. Ser. 1994, 111, 191–202. [Google Scholar] [CrossRef]
- Bowden, D.A.; Clarke, A.; Peck, L.S.; Barnes, D.K.A. Antarctic sessile marine benthos: Colonisation and growth on artificial substrata over 3 years. Mar. Ecol. Prog. Ser. 2006, 316, 1–16. [Google Scholar] [CrossRef]
- Taboada, S.; Núñez-Pons, L.; Avila, C. Feeding repellence of Antarctic and sub-Antarctic benthic invertebrates against the omnivorous sea star Odontaster validus. Polar Biol. 2013, 36, 13–25. [Google Scholar] [CrossRef]
- Amsler, C.D.; Moeller, C.B.; McClintock, J.B.; Iken, K.B.; Baker, B.J. Chemical defenses against diatom fouling in Antarctic marine sponges. Biofouling 2000, 16, 29–45. [Google Scholar] [CrossRef]
- Avila, C.; Taboada, S.; Núñez-Pons, L. Antarctic marine chemical ecology: What is next? Mar. Ecol. 2008, 29, 1–71. [Google Scholar] [CrossRef]
- Peters, K.J.; Amsler, C.D.; McClintock, J.B.; Soest, R.W.M.; Baker, B.J. Palatability and chemical defenses of sponges from the western Antarctic Peninsula. Mar. Ecol. Prog. Ser. 2009, 385, 77–85. [Google Scholar] [CrossRef]
- Núñez-Pons, L.; Forestieri, R.; Nieto, R.M.; Varela, M.; Nappo, M.; Rodríguez, J.; Jiménez, C.; Castelluccio, F.; Carbone, M.; Ramos-Espla, A.; et al. Chemical defenses of tunicates of the genus Aplidium from the Weddell Sea (Antarctica). Polar Biol. 2010, 33, 1319–1329. [Google Scholar] [CrossRef]
- Núñez-Pons, L.; Rodríguez-Arias, M.; Gómez-Garreta, A.; Ribera-Siguán, A.; Avila, C. Feeding deterrency in Antarctic marine organisms: Bioassays with the omnivore amphipod Cheirimedon femoratus. Mar. Ecol. Prog. Ser. 2012, 462, 163–174. [Google Scholar] [CrossRef]
- Koplovitz, G.; McClintock, J.B. An evaluation of chemical and physical defenses against fish predation in a suite of seagrass-associated ascidians. J. Exp. Mar. Biol. Ecol. 2011, 407, 48–53. [Google Scholar] [CrossRef]
- Núñez-Pons, L.; Carbone, M.; Vázquez, J.; Rodríguez, J.; Nieto, R.M.; Varela, M.M.; Gavagnin, M.; Avila, C. Natural products from Antarctic colonial ascidians of the genera Aplidium and Synoicum: Variability and defensive role. Mar. Drugs 2012, 10, 1741–1764. [Google Scholar] [CrossRef]
- Núñez-Pons, L.; Avila, C. Natural products mediating ecological interactions in Antarctic benthic communities: A mini-review of the known molecules. Nat. Prod. Rep. 2015, 32, 1114–1130. [Google Scholar] [CrossRef]
- Lebar, M.D.; Heimbegner, J.L.; Baker, B.J. Cold-water marine natural products. Nat. Prod. Rep. 2007, 24, 774–797. [Google Scholar] [CrossRef]
- Núñez-Pons, L.; Avila, C. Deterrent activities in the crude lipophilic fractions of Antarctic benthic organisms: Chemical defences against keystone predators. Polar Res. 2014, 33, 21624. [Google Scholar] [CrossRef]
- Avila, C. Biological and chemical diversity in Antarctica: From new species to new natural products. Biodiversity 2016, 17, 5–11. [Google Scholar] [CrossRef]
- Avila, C. Ecological and pharmacological activities of Antarctic marine natural products. Planta Med. 2016, 82, 767–774. [Google Scholar] [CrossRef]
- Soldatou, S.; Baker, B.J. Cold-water marine natural products, 2006 to 2016. Nat. Prod. Rep. 2017, 34, 585–626. [Google Scholar] [CrossRef] [PubMed]
- Avila, C. Chemical war in marine animal forests: Natural products and chemical interactions. In Perspectives on the Marine Animal Forests of the World; Rossi, S., Bramanti, L., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 239–307. [Google Scholar]
- Avila, C.; Angulo-Preckler, C. A minireview on biodiscovery in Antarctic marine benthic invertebrates. Front. Mar. Sci. 2021, 8, 86477. [Google Scholar] [CrossRef]
- Carroll, A.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2022, 39, 1122–1171. [Google Scholar] [CrossRef] [PubMed]
- Avila, C. Natural products of opisthobranch molluscs: A biological review. Oceanogr. Mar. Biol. Annu. Rev. 1995, 33, 487–559. [Google Scholar]
- Avila, C. Molluscan natural products as biological models: Chemical ecology, histology, and laboratory culture. In Molluscs; Cimino, G., Gavagnin, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 1–23. [Google Scholar]
- McClintock, J.B.; Baker, B.J. A review of the chemical ecology of Antarctic marine invertebrates. Amer. Zool. 1997, 37, 329–342. [Google Scholar] [CrossRef]
- McClintock, J.B.; Baker, B.J. Marine Chemical Ecology; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- McClintock, J.B.; Amsler, C.D.; Baker, B.J. Overview of the chemical ecology of benthic marine invertebrates along the western Antarctic Peninsula. Integr. Comp. Biol. 2010, 50, 967–980. [Google Scholar] [CrossRef]
- Núñez-Pons, L.; Carbone, M.; Paris, D.; Melck, D.; Ríos, P.; Cristobo, J.; Castelluccio, F.; Gavagnin, M.; Avila, C. Chemo-ecological studies on hexactinellid sponges from the Southern Ocean. Die Nat. 2012, 99, 353–368. [Google Scholar] [CrossRef] [PubMed]
- Figuerola, B.; Núñez-Pons, L.; Moles, J.; Avila, C. Feeding repellence in Antarctic bryozoans. Die Nat. 2013, 100, 1069–1081. [Google Scholar] [CrossRef]
- Figuerola, B.; Núñez-Pons, L.; Monleón-Getino, T.; Avila, C. Chemo-ecological interactions in Antarctic bryozoans. Polar Biol. 2014, 37, 1017–1030. [Google Scholar] [CrossRef]
- Avila, C.; Núñez-Pons, L.; Moles, J. From the tropics to the poles: Chemical defensive strategies in sea slugs (Mollusca: Heterobranchia). In Chemical Ecology: The Ecological Impacts of Marine Natural Products; Puglisi, M.P., Becerro, M.A., Eds.; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar] [CrossRef]
- Von Salm, J.L.; Schoenrock, K.M.; McClintock, J.B.; Amsler, C.D.; Baker, B.J. The status of marine chemical ecology in Antarctica: Form and function of unique high-latitude chemistry. In Chemical Ecology: The Ecological Impacts of Marine Natural Products; Puglisi, M.P., Becerro, M.A., Eds.; CRC. Press: Boca Raton, FL, USA, 2018; pp. 27–69. [Google Scholar]
- Avila, C. Terpenoids in marine heterobranch molluscs. Mar. Drugs 2020, 18, 162. [Google Scholar] [CrossRef]
- Thatje, S.; Arntz, W.E. Antarctic reptant decapods: More than a myth? Polar Biol. 2004, 27, 195–201. [Google Scholar] [CrossRef]
- Aronson, R.B.; Thatje, S.; Clarke, A.; Peck, L.S.; Blake, D.B.; Wilga, C.D.; Seibel, B.A. Climate change and invasibility of the Antarctic benthos. Annu. Rev. Ecol. Evol. Syst. 2007, 38, 129–154. [Google Scholar] [CrossRef]
- Figuerola, B.; Angulo-Preckler, C.; Núñez-Pons, L.; Moles, J.; Sala-Comorera, L.; García-Aljaro, C.; Blanch, A.R.; Avila, C. Experimental evidence of chemical defence mechanisms in Antarctic bryozoans. Mar. Environ. Res. 2017, 129, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Núñez-Pons, L.; Carbone, M.; Vázquez, J.; Gavagnin, M.; Avila, C. Lipophilic Defenses from Alcyonium Soft Corals of Antarctica. J. Chem. Ecol. 2013, 39, 675–685. [Google Scholar] [CrossRef]
- Núñez-Pons, L.; Avila, C. Defensive metabolites from Antarctic invertebrates: Does energetic content interfere with feeding repellence? Mar. Drugs 2014, 12, 3770–3791. [Google Scholar] [CrossRef]
- Angulo-Preckler, C.; San Miguel, O.; García-Aljaro, C.; Avila, C. Antibacterial defenses and palatability of shallow-water Antarctic sponges. Hydrobiologia 2018, 806, 123–138. [Google Scholar] [CrossRef]
- Moles, J.; Núñez-Pons, L.; Taboada, S.; Figuerola, B.; Cristobo, J.; Avila, C. Anti-predatory chemical defences in Antarctic benthic fauna. Mar. Biol. 2015, 162, 1813–1821. [Google Scholar] [CrossRef]
- McClintock, J.B. Toxicity of shallow-water Antarctic echinoderms. Polar Biol. 1989, 9, 461–465. [Google Scholar] [CrossRef]
- McClintock, J.B.; Janssen, J. Pteropod abduction as a chemical defence in a pelagic Antarctic amphipod. Nature 1990, 346, 462–464. [Google Scholar] [CrossRef]
- Baker, B.J.; Kopitzke, R.W.; Hamann, M.; McClintock, J.B. Chemical ecology of Antarctic sponges in McMurdo Sound, Antarctica. Antarct. J. Rev. 1993, 28, 132–133. [Google Scholar]
- Becerro, M.A.; Thacker, R.W.; Turon, X.; Uriz, M.J.; Paul, V.J. Biogeography of sponge chemical ecology: Comparisons of tropical and temperate defenses. Oecologia 2003, 135, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Burns, E.E.; Ifrach, I.; Carmeli, S.; Pawlik, J.R.; Ilan, M. Comparison of anti-predatory defenses of Red Sea and Caribbean sponges. I. Chemical defense. Mar. Ecol. Prog. Ser. 2003, 252, 105–114. [Google Scholar] [CrossRef]
- Obermuller, B.E.; Morley, S.A.; Barnes, D.K.A.; Peck, L.S. Seasonal physiology and ecology of Antarctic marine benthic predators and scavengers. Mar. Ecol. Prog. Ser. 2010, 415, 109–126. [Google Scholar] [CrossRef]
- Peters, K.J.; Amsler, C.D.; McClintock, J.B.; Baker, B.J. Potential chemical defenses of Antarctic sponges against sympatric microorganisms. Polar Biol. 2010, 33, 649–658. [Google Scholar] [CrossRef]
- Meredith, M.P.; King, J.C. Rapid climate change in the ocean west of the Antarctic Peninsula during the second half of the 20th century. Geophys. Res. Lett. 2005, 32, L19604. [Google Scholar] [CrossRef]
- Turner, J.; Lu, H.; White, I.; King, J.C.; Phillips, T.; Hosking, J.S.; Bracegirdle, T.J.; Marshall, G.J.; Mulvaney, R.; Deb, P. Absence of 21st century warming on Antarctic Peninsula consistent with natural variability. Nature 2016, 535, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Clarke, A.; Barnes, D.K.A.; Hodgson, D.A. How isolated is Antarctica? Trends Ecol. Evol. 2005, 20, 1–3. [Google Scholar] [CrossRef]
- Figuerola, B.; Gordon, D.P.; Polonio, V.; Cristobo, J.; Avila, C. Cheilostome bryozoan diversity from the southwest Atlantic region: Is Antarctica really isolated? J. Sea Res. 2014, 85, 1–17. [Google Scholar] [CrossRef]
- Avila, C.; Angulo-Preckler, C.; Martín-Martín, R.P.; Figuerola, B.; Griffiths, H.J.; Waller, C.L. Invasive marine species discovered on non–native kelp rafts in the warmest Antarctic island. Sci. Rep. 2020, 10, 1–9. [Google Scholar]
- Aronson, R.B.; Frederich, M.; Price, R.; Thatje, S. Prospects for the return of shell-crushing crabs to Antarctica. J. Biogeogr. 2015, 42, 1–7. [Google Scholar] [CrossRef]
- López-Farrán, Z.; Guillaumot, C.; Vargas-Chacoff, L.; Paschke, K.; Dulière, V.; Danis, B.; Poulin, E.; Saucède, T.; Waters, J.; Gérard, K. Is the southern crab Halicarcinus planatus (Fabricius, 1775) the next invader of Antarctica? Glob. Chang. Biol. 2021, 27, 3487–3504. [Google Scholar] [CrossRef] [PubMed]
- Pyšek, P.; Hulme, P.E.; Simberloff, D.; Bacher, S.; Blackburn, T.M.; Carlton, J.T.; Dawson, W.; Essl, F.; Foxcroft, L.C.; Genovesi, P.; et al. Scientists’ warning on invasive alien species. Biol. Rev. 2020, 95, 1511–1534. [Google Scholar] [CrossRef]
- Thatje, S.; Fuentes, V. First record of anomuran and brachyuran larvae (Crustacea: Decapoda) from Antarctic waters. Polar Biol. 2003, 26, 279–282. [Google Scholar] [CrossRef]
- Thatje, S.; Schnack-Schiel, S.; Arntz, W.E. Developmental trade-offs in Subantarctic meroplankton communities and the enigma of low decapod diversity in high southern latitudes. Mar. Ecol. Prog. Ser. 2003, 260, 195–207. [Google Scholar] [CrossRef]
- Thatje, S.; Anger, K.; Calcagno, J.A.; Lovrich, G.A.; Pörtner, H.O.; Arntz, W.E. Challenging the cold: Crabs reconquer the Antarctic. Ecology 2005, 86, 619–625. [Google Scholar] [CrossRef]
- García Raso, J.E.; Manjón-Cabeza, M.E.; Ramos, A.; Olaso, I. New record of Lithodidae (Crustacea, Decapoda, Anomura) from the Antarctic (Bellingshausen Sea). Polar Biol. 2005, 28, 642–646. [Google Scholar] [CrossRef]
- Thatje, S.; Hall, S.; Hauton, C.; Held, C.; Tyler, P. Encounter of lithodid crab Paralomis birsteini on the continental slope of Antarctica, sampled by ROV. Polar Biol. 2008, 31, 1143–1148. [Google Scholar] [CrossRef]
- Aronson, R.B.; Smith, K.E.; Vos, S.C.; McClintock, J.B.; Amsler, M.O.; Moksnes, P.O.; Schiferl, J.C. No barrier to emergence of bathyal king crabs on the Antarctic shelf. Proc. Natl. Acad. Sci. USA 2015, 112, 12997–13002. [Google Scholar] [CrossRef]
- Smith, K.E.; Aronson, R.B.; Thatje, S.; Lovrich, G.A.; Amsler, M.O.; Steffel, B.; McClintock, J.B. Biology of the king crab Paralomis birsteini on the continental slope off the western Antarctic Peninsula. Polar Biol. 2017, 40, 2313–2322. [Google Scholar] [CrossRef]
- Hellberg, M.E.; Aronson, R.B.; Smith, K.E.; Duhon, M.I.; Ahyong, S.T.; Lovrich, G.A.; Thatje, S.; McClintock, J.B. Population expansion of an Antarctic king crab? Front. Biogeogr. 2019, 11, e4316. [Google Scholar] [CrossRef]
- Britayev, T.; Rzhavsky, A.; Pavlova, L.; Dvoretskij, A. Studies on impact of the alien Red King Crab (Paralithodes camtschaticus) on the shallow water benthic communities of the Barents Sea. J. Appl. Ichthyol. 2010, 26, 66–73. [Google Scholar] [CrossRef]
- Boudreau, S.A.; Worm, B. Ecological role of large benthic decapods in marine ecosystems: A review. Mar. Ecol. Prog. Ser. 2012, 469, 195–213. [Google Scholar] [CrossRef]
- Fuhrmann, M.; Pedersen, T.; Nilssen, E. Trophic niche of the invasive red king crab (Paralithodes camtschaticus) in a benthic food web. Mar. Ecol. Prog. Ser. 2017, 565, 113–129. [Google Scholar] [CrossRef]
- Smith, K.E.; Aronson, R.B.; Steffel, B.V.; Amsler, M.O.; Thatje, S.; Singh, H.; Anderson, J.; Brothers, C.J.; Brown, A.; Ellis, D.S.; et al. Climate change and the threat of novel marine predators in Antarctica. Ecosphere 2017, 8, e02017. [Google Scholar] [CrossRef]
- Thatje, S.; Smith, K.E.; McClintock, J.B.; Aronson, R.B. From deep to shallow seas: Antarctic king crab on the move. Ecology 2020, 101, e03125. [Google Scholar] [CrossRef]
- Thatje, S.; Hall, S. The effect of temperature on the evolution of per offspring investment in a globally distributed family of marine invertebrates (Crustacea: Decapoda: Lithodidae). Mar. Biol. 2016, 163, 1–9. [Google Scholar] [CrossRef]
- Frederich, M.; Sartoris, F.; Pörtner, H.O. Distribution patterns of decapod crustaceans in polar areas: A result of magnesium regulation? Polar Biol. 2001, 24, 719–723. [Google Scholar] [CrossRef]
- Smith, C.R.; Grange, L.J.; Honig, D.L.; Naudts, L.; Huber, B.; Guidi, L.; Domack, E. A large population of king crabs in Palmer Deep on the west Antarctic Peninsula shelf and potential invasive impacts. Proc. R. Soc. B Biol. Sci. 2012, 279, 1017–1026. [Google Scholar] [CrossRef]
- Thatje, S. The future fate of the Antarctic marine biota? Trends Ecol. Evol. 2005, 20, 418–419. [Google Scholar] [CrossRef][Green Version]
- Hall, S.; Thatje, S. Global bottlenecks in the distribution of marine Crustacea: Temperature constraints in the family Lithodidae. J. Biogeogr. 2009, 36, 2125–2135. [Google Scholar] [CrossRef]
- Hall, S.; Thatje, S. Temperature-driven biogeography of the deep-sea family Lithodidae (Crustacea: Decapoda: Anomura) in the Southern Ocean. Polar Biol. 2011, 34, 363–370. [Google Scholar] [CrossRef]
- Griffiths, H.; Whittle, R.J.; Roberts, S.J.; Belchier, M.; Linse, K. Antarctic crabs: Invasion or endurance? PLoS ONE 2013, 8, e6698. [Google Scholar] [CrossRef]
- De Broyer, C.; Jazdzewski, K. Biodiversity of the Southern Ocean: Towards a new synthesis for the Amphipoda (Crustacea). Boll. Mus. Civ. Stor. Nat. Verona 1996, 20, 547–568. [Google Scholar]
- Dauby, P.; Scailteur, Y.; De Broyer, C. Trophic diversity within eastern Weddell Sea amphipod community. Hydrobiologia 2001, 443, 69–86. [Google Scholar] [CrossRef]
- De Broyer, C.; Scailteur, Y.; Chapelle, G.; Rauschert, M. Diversity of epibenthic habitats of gammaridean amphipods in the eastern Weddell Sea. Polar Biol. 2001, 24, 744–753. [Google Scholar] [CrossRef]
- Huang, Y.M.; Amsler, M.O.; McClintock, J.B.; Amsler, C.D.; Baker, B.J. Patterns of gammaridean amphipod abundance and species composition associated with dominant subtidal macroalgae from the western Antarctic Peninsula. Polar Biol. 2007, 30, 1417–1430. [Google Scholar] [CrossRef]
- De Broyer, C.; Lowry, J.K.; Jazdzewski, K.; Robert, H. Part 1. Catalogue of the Gammaridean and Corophiidean Amphipoda (Crustacea) of the Southern Ocean with distribution and ecological data. In Census of Antarctic Marine Life: Synopsis of the Amphipoda of the Southern Ocean; De Broyer, C., Ed.; Institut Royal des Sciences Naturelles de Belgique: Brussels, Belgium, 2007; Volume 77, pp. 1–325. [Google Scholar]
- Amsler, M.O.; McClintock, J.B.; Amsler, C.D.; Angus, R.A.; Baker, B.J. An evaluation of sponge-associated amphipods from the Antarctic Peninsula. Ant. Sci. 2009, 21, 579–589. [Google Scholar] [CrossRef]
- Di Franco, D.; Linse, K.; Griffiths, H.J.; Haas, C.; Saeedi, H.; Brandt, A. Abundance and distributional patterns of benthic Peracarid Crustaceans from the Atlantic sector of the Southern Ocean and Weddell Sea. Front. Mar. Sci. 2020, 7, 554663. [Google Scholar] [CrossRef]
- Koplovitz, G.; McClintock, J.B.; Amsler, C.D.; Baker, B.J. Palatability and chemical anti-predatory defenses in common ascidians from the Antarctic Peninsula. Aquat. Biol. 2009, 7, 81–92. [Google Scholar] [CrossRef]
- Hazlett, B.A. The behavioral ecology of hermit crabs. Ann. Rev. Ecol. Syst. 1981, 12, 1–22. [Google Scholar] [CrossRef]
- Ribeiro, S.M.; Bianco, É.M.; Rogers, R.; Teixeira, V.L.; Pereira, R.C. Chemical defense of Hymeniacidon heliophila (Porifera: Halichondrida) against tropical predators. Braz. J. Oceanogr. 2010, 58, 315–321. [Google Scholar] [CrossRef]
- Waddell, B.; Pawlik, J.R. Defenses of Caribbean sponges against invertebrate predators. I. Assays with hermit crabs. Mar. Ecol. Prog. Ser. 2000, 195, 125–132. [Google Scholar] [CrossRef]
- Comoglio, L.I.; Amin, O.A. Feeding habits of the false southern king crab Paralomis granulosa (Lithodidae) in the Beagle Channel, Tierra del Fuego, Argentina. Sci. Mar. 1999, 63, 361–366. [Google Scholar] [CrossRef]
- Cohen, A.N.; Carlton, J.T.; Fountain, M.C. Introduction, dispersal and potential impacts of the green crab Carcinus maenas in San Francisco Bay, California. Mar. Biol. 1995, 122, 225–237. [Google Scholar] [CrossRef]
- Grosholz, E.D.; Ruiz, G.M. Predicting the impact of introduced marine species: Lessons from the multiple invasions of the European green crab Carcinus maenas. Biol. Conserv. 1996, 78, 59–66. [Google Scholar] [CrossRef]
- Carlton, J.T.; Cohen, A.N. Episodic global dispersal in shallow water marine organisms: The case history of the European shore crabs Carcinus maenas and C. aestuarii. J. Biogeogr. 2003, 30, 1809–1820. [Google Scholar] [CrossRef]
- Sotka, E.E.; Forbey, J.; Horn, M.; Poore, A.G.B.; Raubenheimer, D.; Whalen, K.E. The emerging role of pharmacology in understanding consumer-prey interactions in marine and freshwater systems. Integr. Comp. Biol. 2009, 49, 291–313. [Google Scholar] [CrossRef]
- Pawlik, J.R.; Albizati, K.F.; Faulkner, D.J. Evidence of a defensive role for limatulone, a novel triterpene from the intertidal limpet Collisella limatula. Mar. Ecol. Prog. Ser. 1986, 30, 251–260. [Google Scholar] [CrossRef]
- Kubanek, J.; Pawlik, J.R.; Eve, T.M.; Fenical, W. Triterpene glycosides defend the caribbean reef sponge Erylus formosus from predatory fishes. Mar. Ecol. Prog. Ser. 2000, 207, 69–77. [Google Scholar] [CrossRef]
- Pawlik, J.R. Antipredatory defensive roles of natural products from marine invertebrates. In Handbook of Marine Natural Products; Fattorusso, E., Gerwick, W.H., Taglilatela-Scarfati, G., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; p. 1452. [Google Scholar]
- Jayatilake, G.S.; Baker, B.J.; McClintock, J.B. Rhapsamine, a cytotoxin from the Antarctic sponge Leucetta leptorhapsis. Tetrahedron Lett. 1997, 38, 7507–7510. [Google Scholar] [CrossRef]
- Vetter, W.; Janussen, D. Halogenated natural products in five species of Antarctic sponges: Compounds with POP-like properties? Environ. Sci. Technol. 2005, 39, 3889–3895. [Google Scholar] [CrossRef] [PubMed]
- Trimurtulu, G.; Faulkner, D.J.; Perry, N.B.; Ettouati, L.; Litaudon, M.; Blunt, J.; Munro, M.H.G.; Jameson, G.B. Alkaloids from the antarctic sponge Kirkpatrickia varialosa. Part 2: Variolin A and N(30)-methyl tetrahydrovariolin B. Tetrahedron 1994, 50, 3993–4000. [Google Scholar] [CrossRef]
- Jayatilake, G.S.; Baker, B.J.; McClintock, J.B. Isolation and identification of a stilbene derivative from the Antarctic sponge Kirkpatrickia variolosa. J. Nat. Prod. 1995, 58, 1958–1960. [Google Scholar] [CrossRef]
- Perry, N.B.; Ettouati, L.; Litaudon, M.; Blunt, J.; Munro, M.H.G.; Parkin, S.; Hope, H. Alkaloids from the antarctic sponge Kirkpatrickia variolosa. Part 1: Variolin B, a new antitumour and antiviral compound. Tetrahedron 1994, 50, 3987–3992. [Google Scholar] [CrossRef]
- MarinLit Search on the Genus Axinella. Available online: https://marinlit.rsc.org (accessed on 13 July 2022).
- McClintock, J.B. Investigation of the relationship between invertebrate predation and biochemical composition, energy content, spicule armament and toxicity of benthic sponges at McMurdo Sound, Antarctica. Mar. Biol. 1987, 94, 479–487. [Google Scholar] [CrossRef]
- Riccio, G.; Nuzzo, G.; Zazo, G.; Coppola, D.; Senese, G.; Romano, L.; Costantini, M.; Ruocco, N.; Bertolino, M.; Fontana, A.; et al. Bioactivity screening of Antarctic sponges reveals anticancer activity and potential cell death via ferroptosis by mycalols. Mar. Drugs 2021, 19, 459. [Google Scholar] [CrossRef] [PubMed]
- MarinLit Search on the Genus Haliclona. Available online: https://marinlit.rsc.org (accessed on 13 July 2022).
- Cutignano, A.; Nuzzo, G.; D’Angelo, D.; Borbone, E.; Fusco, A.; Fontana, A. Mycalol: A natural lipid with promising cytotoxic properties against human anaplastic thyroid carcinoma cells. Angew. Chemie Int. Ed. 2013, 52, 9256–9260. [Google Scholar] [CrossRef]
- MarinLit Search on the Genus Mycale. Available online: https://marinlit.rsc.org (accessed on 13 July 2022).
- McClintock, J.B.; Baker, B.J.; Amsler, C.D.; Barlow, T.L. Chemotactic tube-foot responses of the spongivorous sea star Perknaster fuscus to organic extracts of sponges from McMurdo Sound, Antarctica. Antarct. Sci. 2000, 12, 41–46. [Google Scholar] [CrossRef]
- Moon, B.; Baker, B.J.; McClintock, J.B. Purine and nucleoside metabolites from the Antarctic sponge Isodictya erinacea. J. Nat. Prod. 1998, 61, 116–118. [Google Scholar] [CrossRef]
- Moon, B.; Park, Y.C.; McClintock, J.B.; Baker, B.J. Structure and bioactivity of erebusinone, a pigment from the Antarctic sponge Isodictya erinacea. Tetrahedron 2000, 56, 9057–9062. [Google Scholar] [CrossRef]
- Park, Y.C. Chemical investigation of three Antarctic marine sponges. Ph.D. Thesis, University of South Florida, Tampa, FL, USA, 2004. [Google Scholar]
- Bory, A.; Shilling, A.J.; Allen, J.; Azhari, A.; Roth, A.; Shaw, L.N.; Kyle, D.E.; Adams, J.H.; Amsler, C.D.; McClintock, J.B.; et al. Bioactivity of spongian diterpenoid scaffolds from the Antarctic sponge Dendrilla antarctica. Mar. Drugs 2020, 18, 327. [Google Scholar] [CrossRef] [PubMed]
- Shilling, A.J.; Witowski, C.G.; Maschek, J.A.; Azhari, A.; Vesely, B.; Kyle, D.E.; Amsler, C.D.; McClintock, J.B.; Baker, B.J. Spongian diterpenoids derived from the Antarctic sponge Dendrilla antarctica are potent inhibitors of the Leishmania parasite. J. Nat. Prod. 2020, 83, 1553–1562. [Google Scholar] [CrossRef]
- Prieto, I.M.; Paola, A.; Pérez, M.; García, M.; Blustein, G.; Schejter, L.; Palermo, J.A. Antifouling diterpenoids from the sponge Dendrilla antarctica. Chem. Biodivers. 2021, 19, e202100618. [Google Scholar] [CrossRef]
- Molinski, T.F.; Faulkner, D.J. Metabolites of the Antarctic sponge Dendrilla membranosa. J. Org. Chem. 1987, 52, 296–298. [Google Scholar] [CrossRef]
- Molinski, T.F.; Faulkner, D.J. An antibacterial pigment from the sponge Dendrilla membranosa. Tetrahedron Lett. 1988, 29, 2137–2138. [Google Scholar] [CrossRef]
- Fontana, A.; Scognamiglio, G.; Cimino, G. Dendrinolide, a new degraded diterpenoid from the Antarctic sponge Dendrilla membranosa. J. Nat. Prod. 1997, 60, 475–477. [Google Scholar] [CrossRef]
- Baker, B.J.; Kopitzke, R.W.; Yoshida, W.Y.; McClintock, J.B. Chemical and ecological studies of the Antarctic sponge Dendrilla membranosa. J. Nat. Prod. 1995, 58, 1459–1462. [Google Scholar] [CrossRef]
- Ankisetty, S.; Amsler, C.D.; McClintock, J.B.; Baker, B.J. Further membranolide diterpenes from the Antarctic sponge Dendrilla membranosa. J. Nat. Prod. 2004, 67, 1172–1174. [Google Scholar] [CrossRef]
- Witowski, C.W. Investigation of bioactive metabolites from the Antarctic sponge Dendrilla membranosa and marine microorganisms. Ph.D. Thesis, University of South Florida, Tampa, FL, USA, 2015. [Google Scholar]
- Von Salm, J.L.; Witowski, C.G.; Fleeman, R.M.; McClintock, J.B.; Amsler, C.D.; Shaw, L.N.; Baker, B.J. Darwinolide, a new diterpene scaffold that inhibits methicillin resistant Staphylococcus aureus biofilm from the Antarctic sponge Dendrilla membranosa. Org. Lett. 2016, 18, 2596–2599. [Google Scholar] [CrossRef]
- Ciaglia, E.; Malfitano, A.M.; Laezza, C.; Fontana, A.; Nuzzo, G.; Cutignano, A.; Abate, M.; Pelin, M.; Sosa, S.; Bifulco, M.; et al. Immuno-modulatory and anti-inflammatory effects of Dihydrogracilin A, a terpene derived from the marine sponge Dendrilla membranosa. Int. J. Mol. Sci. 2017, 18, 1643. [Google Scholar] [CrossRef]
- Palermo, J.A.; Brasco, M.F.; Spagnuolo, C.; Seldes, A.M. Illudalane sesquiterpenoids from the soft coral Alcyonium paessleri: The first natural nitrate esters. J. Org. Chem. 2000, 65, 4482–4486. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Brasco, M.F.; Seldes, A.M.; Palermo, J.A. Paesslerins A and B: Novel tricyclic sesquiterpenoids from the soft coral Alcyonium paessleri. Org. Lett. 2001, 3, 1415–1417. [Google Scholar] [CrossRef] [PubMed]
- Manzo, E.; Ciavatta, M.L.; Nuzzo, G.; Gavagnin, M. Terpenoid content of the Antarctic soft coral Alcyonium antarcticum. Nat. Prod. Comm. 2009, 4, 1615–1619. [Google Scholar] [CrossRef]
- Carbone, M.; Núñez-Pons, L.; Castelluccio, F.; Avila, C.; Gavagnin, M. Illudalane sesquiterpenoids of the alcyopterosin series from the Antarctic marine soft coral Alcyonium grandis. J. Nat. Prod. 2009, 72, 1357–1360. [Google Scholar] [CrossRef]
- Cimino, G.; De Rosa, S.; De Stefano, S.; Sodano, G. Cholest-4-en-4,16β,18,22R-tetrol-3-one 16,18-diacetate A novel polyhydroxylated steroid from the hydroid Eudendrium sp. Tetrahedron Lett. 1980, 21, 3303–3304. [Google Scholar] [CrossRef]
- De Napoli, L.; Fattorusso, E.; Magno, S.; Mayol, L. Acyclic polyhalogenated monoterpenes from four marine hydroids. Biochem. Syst. Ecol. 1984, 12, 321–322. [Google Scholar] [CrossRef]
- Fattorusso, E.; Lanzotti, V.; Magno, S.; Novellino, E. Sterols of four Mediterranean hydroids. Biochem. Syst. Ecol. 1985, 13, 167–168. [Google Scholar] [CrossRef]
- Fattorusso, E.; Lanzotti, V.; Magno, S.; Novellino, E. Two new polyoxygenated sterols from the marine hydroid Eudendrium glomeratum. J. Nat. Prod. 1985, 48, 784–787. [Google Scholar] [CrossRef]
- Fattorusso, E.; Lanzotti, V.; Magno, S.; Novellino, E. Cholest-5-ene-2.alpha.,3.alpha.,7.beta.,15.beta.,18-pentol 2,7,15,18-tetraacetate, a novel highly hydroxylated sterol from the marine hydroid Eudendrium glomeratum. J. Org. Chem. 1985, 50, 2868–2870. [Google Scholar] [CrossRef]
- Aiello, A.; Fattorusso, E.; Magno, S.; Mayol, L. Brominaed β-carbolines from the marine hydroid Aglaophenia pluma Linnaeus. Tetrahedron 1987, 43, 5929–5932. [Google Scholar] [CrossRef]
- Aiello, A.; Fattorusso, E.; Magno, S. Isolation and structure elucidation of two new polyhydroxylated sterols from the Mediterranean hydroid Eudendrium glomeratum. J. Nat. Prod. 1987, 50, 191–194. [Google Scholar] [CrossRef]
- Stachowicz, J.J.; Lindquist, N. Hydroid defenses against predators: The importance of secondary metabolites versus nematocysts. Oecologia 2000, 124, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Heine, J.N.; McClintock, J.B.; Slattery, M.; Weston, J. Energetic composition, biomass, and chemical defense in the common antarctic nemertean Parborlasia corrugatus Mclntosh. J. Exp. Mar. Biol. Ecol. 1991, 153, 15–25. [Google Scholar] [CrossRef]
- Berne, S.; Sepcić, K.; Križaj, I.; Kem, W.R.; McClintock, J.B.; Turk, T. Isolation and characterisation of a cytolytic protein from mucus secretions of the Antarctic heteronemertine Parborlasia corrugatus. Toxicon 2003, 41, 483–491. [Google Scholar] [CrossRef]
- Göransson, U.; Jacobsson, E.; Strand, M.; Andersson, H.S. The toxins of nemertean worms. Toxins 2019, 11, 120. [Google Scholar] [CrossRef]
- MarinLit Search on Polychaeta. Available online: https://marinlit.rsc.org (accessed on 13 July 2022).
- Angulo-Preckler, C.; Cid, C.; Oliva, F.; Avila, C. Antifouling activity in some benthic Antarctic invertebrates by in situ experiments at Deception Island, Antarctica. Mar. Environ. Res. 2015, 105, 30–38. [Google Scholar] [CrossRef]
- Figuerola, B.; Avila, C. The phylum Bryozoa as a promising source of anticancer drugs. Mar. Drugs 2019, 17, 477. [Google Scholar] [CrossRef]
- Ciavatta, M.L.; Lefranc, F.; Vieira, L.M.; Kiss, R.; Carbone, M.; van Otterlo, W.A.L.; Lopanik, N.B.; Waeschenbach, A. The Phylum Bryozoa: From Biology to Biomedical Potential. Mar. Drugs 2020, 18, 200. [Google Scholar] [CrossRef]
- Paul, V.J. Ecological Roles of Marine Natural Products; Comstock Publications Association: Ithaca, NY, USA, 1992. [Google Scholar]
- Sharp, J.H.; Winson, M.K.; Porter, J.S. Bryozoan metabolites: An ecological perspective. Nat. Prod. Rep. 2007, 24, 659–673. [Google Scholar] [CrossRef]
- Ivanchina, N.V.; Kicha, A.A.; Kalinovsky, A.I.; Dmitrenok, P.S.; Dmitrenok, A.S.; Chaikina, E.L.; Stonik, V.A.; Gavagnin, M.; Cimino, G. Polar steroidal compounds from the Far Eastern starfish Henricia leviuscula. J. Nat. Prod. 2006, 69, 224–228. [Google Scholar] [CrossRef]
- Ivanchina, N.V.; Kicha, A.A.; Stonik, V.A. Steroid glycosides from marine organisms. Steroids 2011, 76, 425–454. [Google Scholar] [CrossRef] [PubMed]
- Franco, L.H.; Joffé, E.B.; Puricelli, L.; Tatian, M.; Seldes, A.M.; Palermo, J.A. Indole alkaloids from the tunicate Aplidium meridianum. J. Nat. Prod. 1998, 61, 1130–1132. [Google Scholar] [CrossRef] [PubMed]
- Diyabalanage, T.; Amsler, C.D.; McClintock, J.B.; Baker, B.J. Palmerolide A, a cytotoxic macrolide from the Antarctic tunicate Synoicum adareanum. J. Amer. Chem. Soc. 2006, 128, 5630–5631. [Google Scholar] [CrossRef] [PubMed]
- Miyata, Y.; Diyabalanage, T.; Amsler, C.D.; McClintock, J.B.; Valeriote, F.A.; Baker, B.J. Ecdysteroids from the Antarctic tunicate Synoicum adareanum. J. Nat. Prod. 2007, 70, 1859–1864. [Google Scholar] [CrossRef] [PubMed]
- Reyes, F.; Fernández, R.; Rodríguez, A.; Francesch, A.; Taboada, S.; Avila, C.; Cuevas, C. Aplicyanins A-F, new cytotoxic bromoindole derivatives from the marine tunicate Aplidium cyaneum. Tetrahedron 2008, 64, 5119–5123. [Google Scholar] [CrossRef]
- Appleton, D.R.; Chuen, C.S.; Berridge, M.V.; Webb, V.L.; Copp, B.R. Rossinones, A, B, biologically active meroterpenoids from the Antarctic Ascidian, Aplidium species. J. Org. Chem. 2009, 74, 9195–9198. [Google Scholar] [CrossRef]
- Lebar, M.D.; Baker, B.J. Synthesis and structure reassessment of Psammopemmin A. Austral. J. Chem. 2010, 63, 862–866. [Google Scholar] [CrossRef]
- Noguez, J.H.; Diyabalanage, T.K.; Miyata, Y.; Xie, X.S.; Valeriote, F.A.; Amsler, C.D.; McClintock, J.B.; Baker, B.J. Palmerolide macrolides from the Antarctic tunicate Synoicum adareanum. Bioorg. Med. Chem. 2011, 19, 6608–6614. [Google Scholar] [CrossRef]
- Carbone, M.; Núñez-Pons, L.; Paone, M.; Castelluccio, F.; Avila, C.; Gavagnin, M. Rossinone-related meroterpenes from the Antarctic ascidian Aplidium fuegiense. Tetrahedron 2012, 68, 3541–3544. [Google Scholar] [CrossRef]
- Núñez-Pons, L.; Nieto, R.M.; Avila, C.; Jiménez, C.; Rodríguez, J. Mass spectrometry detection of minor new meridianins from the Antarctic colonial ascidians Aplidium falklandicum and Aplidium meridianum. J. Mass Spectrom. 2015, 50, 103–111. [Google Scholar] [CrossRef]
- Barnes, D.K.A.; Griffiths, H.J.; Kaiser, S. Geographic range shift responses to climate change by Antarctic benthos: Where we should look. Mar. Ecol. Prog. Ser. 2009, 393, 13–26. [Google Scholar] [CrossRef]
- Griffiths, H.J.; Meijers, A.; Bracegirdle, T. More losers than winners in a century of future Southern Ocean seafloor warming. Nat. Clim. Chang. 2017, 7, 749–754. [Google Scholar] [CrossRef]
- Peck, L.S. Antarctic marine biodiversity: Adaptations, environments and responses to Change. Oceanogr. Mar. Biol. 2018, 56, 105–236. [Google Scholar]
- Hu, N.; Bourdeau, P.E.; Harlos, C.; Liiu, Y.; Hollander, J. Meta-analysis reveals variance in tolerance to climate change across marine trophic levels. Sci. Total Environ. 2022, 827, 154244. [Google Scholar] [CrossRef]
- Gudimov, A.V.; Gudimova, E.N.; Pavlova, L.V. Effect of the red king crab Paralithodes camtschaticus on the Murmansk coastal macrobenthos: The first estimates using sea urchins of the genus Strongylocentrotus as an example. Dokl. Biol. Sci. 2003, 393, 539–541. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, L.L.; Nilssen, E.M. The invasive history, impact and management of the red king crab Paralithodes camtschaticus off the coast of Norway. In In the Wrong Place—Alien Marine Crustaceans: Distribution, Biology and Impacts; Galil, B.S., Clark, P.F., Carlton, J.T., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; Volume 6, pp. 521–536. [Google Scholar]
- Oug, K.; Cochrane, S.K.J.; Sundet, J.H.; Norling, K.; Nilsson, H.C. Effects of the invasive red king crab (Paralithodes camtschaticus) on soft-bottom fauna in Varangerfjorden, northern Norway. Mar. Biodivers. 2011, 41, 467–479. [Google Scholar] [CrossRef]
- Falk-Petersen, J.; Renaud, P.; Anisimova, N. Establishment and ecosystem effects of the alien invasive red king crab (Paralithodes camtschaticus) in the Barents Sea–a review. ICES J. Mar. Sci. 2011, 68, 479–488. [Google Scholar] [CrossRef]
- Avila, C.; Iken, K.; Fontana, A.; Cimino, G. Chemical ecology of the Antarctic nudibranch Bathydoris hodgsoni Eliot 1907: Defensive role and origin of its natural products. J. Exp. Mar. Biol. Ecol. 2000, 252, 27–44. [Google Scholar] [CrossRef]
- Iken, K.; Avila, C.; Fontana, A.; Gavagnin, M. Chemical ecology and origin of defensive compounds in the Antarctic nudibranch Austrodoris kerguelenensis (Opisthobranchia: Gastropoda). Mar. Biol. 2002, 141, 101–109. [Google Scholar]
- Spherification Kit by the Cook Ferran Adrià. Available online: www.albertyferranadria.com/eng/texturas.html (accessed on 20 July 2022).
- R. Available online: https://www.R-project.org/ (accessed on 20 July 2022).
- Wickham, R.H. Ggplot2 Package: Elegant Graphics for Data Analysis; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Hazlett, B.A. Stimuli involved in the feeding behavior of the hermit crab Clibanarius vittatus (Decapoda, Paguridea). Crustaceana 1968, 15, 305–311. [Google Scholar] [CrossRef]
- Sokal, R.R.; Rohlf, F.J. Biometry: The Principles and Practice of Statistics in Biological Research; Freeman, W.H., Ed.; W. H. Freeman: New York, NY, USA, 1995. [Google Scholar]
| Species | Wet Weight (g) | Dry Weight (g) | Liphophilic Extract (g) | Hydrophilic Extract (g) |
|---|---|---|---|---|
| PORIFERA | ||||
| Clathria (Microciona) antarctica (Topsent, 1916) 1 | 32.1 | 9.15 | 0.07 | 0.15 |
| Clathria sp. | 38.39 | 5.18 | 0.43 | 0.06 |
| Mycale (Oxymycale) acerata Kirkpatrick, 1907 | 17.37 | 2.73 | 0.47 | 0.03 |
| Dendrilla antarctica Topsent, 1905 | 35.52 | 1.34 | 0.24 | 0.10 |
| Kirkpatrickia variolosa (Kirkpatrick, 1907) | 67.41 | 15.34 | 0.48 | 0.29 |
| Isodyctia sp. 1 * | 10.56 | 1.26 | 0.45 | 0.08 |
| Isodyctia sp. 2 * | 41.13 | 5.42 | 0.28 | 0.12 |
| Axinella crinita Thiele, 1905 | 85.69 | 12.14 | 0.04 | 0.03 |
| Sphaerothylus antarcticus Kirkpatrick, 1907 | 53.43 | 12.47 | 0.22 | 0.04 |
| Haliclona sp. 1 * | 45.19 | 2.59 | 0.52 | 0.22 |
| Haliclona sp. 2 * | 19.54 | 1.30 | 0.28 | 0.16 |
| Haliclona sp. 3 * | 76.32 | 7.06 | 0.25 | 0.21 |
| Haliclona sp. 4 * | 124.76 | 8.43 | 0.46 | 0.15 |
| Phorbas areolatus (Thiele, 1905) 1 | 103.18 | 23.79 | 0.32 | 0.15 |
| CNIDARIA | ||||
| Hydroidea sp. 2 | 30.15 | 1.53 | 0.03 | 0.04 |
| Alcyonium haddoni Wright & Studer, 1889 | 17.01 | 1.09 | 0.01 | 0.05 |
| ANNELIDA | ||||
| Harmothoe sp. | 1.42 | 1.14 | 0.05 | 0.01 |
| Terebellidae sp. | 64.5 | 26.25 | 0.43 | 0.02 |
| NEMERTEA | ||||
| Parborlasia corrugatus (McIntosh, 1876) | 66.83 | 6.41 | 0.03 | 0.09 |
| BRYOZOA | ||||
| Bugula longissima Busk, 1884 | 21.96 | 1.37 | 0.06 | 0.06 |
| Cheilostomata sp. | 44.85 | 3.88 | 0.15 | 0.07 |
| ECHINODERMATA | ||||
| Abatus sp. | 21.57 | 6.27 | 0.05 | 0.04 |
| Diplasterias brucei (Koehler, 1907) 3 | 17.02 | 5.02 | 0.17 | 0.10 |
| Lysasterias sp. | 68.92 | 15.10 | 0.64 | 0.48 |
| TUNICATA | ||||
| Styela sp. | 84.91 | 1.63 | 0.07 | 0.08 |
| Cnemidocarpa sp. | 66.21 | 2.35 | 0.05 | 0.13 |
| Cnemidocarpa verrucosa (Lesson, 1830) | 71.52 | 2.46 | 0.07 | 0.12 |
| Synoicum adareanum (Herdman, 1902) | 78.53 | 2.67 | 0.04 | 0.19 |
| Tylobranchion speciosum Herdman, 1886 | 4.45 | 0.11 | 0.01 | 0.02 |
| Group/Activity (%) against: | Mediterranean Macropredators (D. arrosor)  | Antarctic Macropredators (O. validus)  | Mediterranean Micropredators (Amphipoda: Lyssianasidae)  | Antarctic Micropredators (C. femoratus)  |
|---|---|---|---|---|
| Porifera | 20 1 | 55.2 ± 26.9 11,21,33,44,45 | 100 1 | 100 ± 0 16,21,33 |
| Cnidaria | nt | 80 ± 19.4 11,21,42,45 | 100 1 | 100 ± 0 16,21,42 |
| Annelida | nt | 25 ± 35 11,45 | 50 1 | nt |
| Nemertea | nt | 50 ± 70.7 11,45 | 100 1 | nt |
| Bryozoa | nt | 49.7 ± 46.6 11,21,34,41,45 | 100 1 | 50 ± 57.7 16,21,34,41 |
| Echinodermata | nt | 62.5 ± 31.1 11,21,45 | 100 1 | 0 ± 0 16,21 |
| Tunicata | 20 1 | 93.3 ± 14.9 11,15,18,21,45 | nt | 100 ± 0 16,18,21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avila, C.; Buñuel, X.; Carmona, F.; Cotado, A.; Sacristán-Soriano, O.; Angulo-Preckler, C. Would Antarctic Marine Benthos Survive Alien Species Invasions? What Chemical Ecology May Tell Us. Mar. Drugs 2022, 20, 543. https://doi.org/10.3390/md20090543
Avila C, Buñuel X, Carmona F, Cotado A, Sacristán-Soriano O, Angulo-Preckler C. Would Antarctic Marine Benthos Survive Alien Species Invasions? What Chemical Ecology May Tell Us. Marine Drugs. 2022; 20(9):543. https://doi.org/10.3390/md20090543
Chicago/Turabian StyleAvila, Conxita, Xavier Buñuel, Francesc Carmona, Albert Cotado, Oriol Sacristán-Soriano, and Carlos Angulo-Preckler. 2022. "Would Antarctic Marine Benthos Survive Alien Species Invasions? What Chemical Ecology May Tell Us" Marine Drugs 20, no. 9: 543. https://doi.org/10.3390/md20090543
APA StyleAvila, C., Buñuel, X., Carmona, F., Cotado, A., Sacristán-Soriano, O., & Angulo-Preckler, C. (2022). Would Antarctic Marine Benthos Survive Alien Species Invasions? What Chemical Ecology May Tell Us. Marine Drugs, 20(9), 543. https://doi.org/10.3390/md20090543