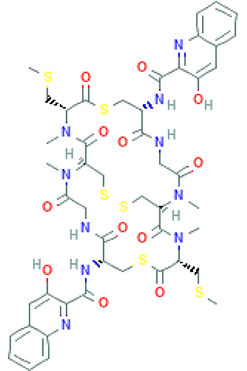
Abstract
This review highlights the underexplored potential and promises of marine bioactive peptides (MBPs) with unique structural, physicochemical, and biological activities to fight against the current and future human pathologies. A particular focus is given to the marine environment as a significant source to obtain or extract high-value MBPs from touched/untouched sources. For instance, marine microorganisms, including microalgae, bacteria, fungi, and marine polysaccharides, are considered prolific sources of amino acids at large, and peptides/polypeptides in particular, with fundamental structural sequence and functional entities of a carboxyl group, amine, hydrogen, and a variety of R groups. Thus, MBPs with tunable features, both structural and functional entities, along with bioactive traits of clinical and therapeutic value, are of ultimate interest to reinforce biomedical settings in the 21st century. On the other front, as the largest biome globally, the marine biome is the so-called “epitome of untouched or underexploited natural resources” and a considerable source with significant potentialities. Therefore, considering their biological and biomedical importance, researchers around the globe are redirecting and/or regaining their interests in valorizing the marine biome-based MBPs. This review focuses on the widespread bioactivities of MBPs, FDA-approved MBPs in the market, sustainable development goals (SDGs), and legislation to valorize marine biome to underlying the impact role of bioactive elements with the related pathways. Finally, a detailed overview of current challenges, conclusions, and future perspectives is also given to satisfy the stimulating demands of the pharmaceutical sector of the modern world.
1. Introduction
MBPs have gained significant research interests as robust alternatives to synthetic counterparts, becoming less effective with high drug-resistant issues. Therefore, naturally occurring sources with medicinal and pharmacological potentialities are ideal, owing to the ease in accessibility, free availability, natural abundance, being carbon neutral, and having fewer side effects than chemical-based synthetic formulations. Moreover, the growing technological advancement, scientific awareness, legislative authorities, and a broader variability of technological and analytical endorsement all support the exploitation of treasure of natural and underexploited sources from the marine biome [1,2]. In this context, the utilization of sustainable sources and the development of green technologies could be the key to going green. The value of “going green” has clearly transformed the pursuit of sustainable, recyclable, and socioeconomic friendlier products to fulfill the economic pressure by considering the high cost-effective ratio benefit. Therefore, the divergence from nonrenewable to renewable natural resources is fetching the center of interest in biomedical establishments. Technological legislation is a driving force and should be considered with care to develop green strategies for the cleaner obtainment of high-value biologically active products [1]. Several approaches are in practice to develop a state-of-the-art bio-based platform for various technological applications in bio- and non-bioindustries of the modern world. Green biotechnology has a noteworthy potential, of course in combination with 12 principles of green chemistry, to eradicate the generation of wasteful protection and deprotection steps [3]. The combination of green chemistry principles and modern biotechnology along with the employment of natural resources is urgently required to establish a sustainable future production and exploitation of the above-mentioned products.
MBPs are characteristically accessible from numerous natural sources, including marine biome, though in different extents with unique functionalities. Among several marine-derived compounds, MBPs are greener alternatives and have been broadly considered and utilized as pharmaceutically resourceful ingredients for numerous health determinations. Therefore, MBPs are being used to develop novel formulations in the biomedical, pharmaceutical, cosmeceutical, and nutraceutical industries. Owing to their advantageous features, such as robust affinity, functional reactivity, specificity, selectivity, etc., MBPs offer practical and positive replacements, compared with their synthetic counterpart formulations and chemical drugs [4,5]. Regardless of advantageous features, MBPs have been considered from diverse standpoints, i.e., based on (1) taxonomic sources, (2) biosynthesis pathways, (3) source of extraction, (4) ring and linear structures, (5) active fractions and compositional variations, (6) biologically active precursor molecules, etc. [2]. Irrespective of the category type, MBPs are protein-based specific fragments that solely depend on the activity based on the composition and amino acid sequence in that particular fragment [6]. However, the MBP activity indeed depends on the extraction and purification technique [2,7,8]. Thus far, an array of production, extraction, and purification approaches have been developed and exploited for MBPs [9,10,11]. From the above examples, an appropriately established methodology plays a significant role in the quality and quantity of the end-product of interests, specifically MBPs. It is also imperative to consider innumerable manipulating factors that can influence the complete performance of the entire isolation, extraction, and purification process and the overall product yield. For example, the source material’s physiochemical composition, matrix characteristics, material’s pretreatment considerations, solvent grade, type and concentration, reaction pH and temperature, pressure, reaction time, etc., are key influencing factors that should be considered before designing and running the experimental protocols [9]. The process efficacy of the entire extraction process and the end-product relies on (1) input parameters, (2) consideration of the nature of the substrate materials from the source, (3) interplay between the procedure and the substrate materials, and (4) chemistry of MBPs [2].
Considering the above critiques and opportunities herein, we sought to highlight the value, challenges, and opportunities that exist in MBPs with potent bioactivities and therapeutic potentialities. Following a brief introduction, a standardized initial methodological literature screening and inclusion/exclusion criteria were adopted to justify the scientific and literature theme. The next section concerns the marine biome and its potent MBPs with superior performance. The latter half of the paper addresses the generalized overview of bioactivities with biomedical and pharmaceutical potentialities to understand the fundamental impact and role of bioactive elements with related pathways. The FDA-approved MBPs in the market and MBPs with clinical trials, as well as sustainable development goals (SDGs) and legislation to valorize marine biome, are discussed. Finally, a detailed overview of current challenges, conclusions, and future perspectives is given to satisfy the stimulating demands of the pharmaceutical sector of the modern world.
2. Review of Methodological Approach: Inclusion/Exclusion Criteria
The summarized review contents and given examples are well justified by using a standardized methodological approach that is based on screening the initial literature and inclusion/exclusion criteria. In this study, inclusion/exclusion criteria were also considered important to justify the most recent and relevant literature as per the conceptualized theme of the research. Following the initial screening step, the studies meticulously corresponding with the title theme were included in this discussion unless otherwise excluded to avoid the literature redundancy. Careful literature search queries were made in the Scopus and PubMed literature databases using any or many of the following terms: bioactive compounds from marine sources, bioactive peptides from marine sources, and biomedical applications of marine peptides. The active search filters were article title, abstract, and keywords. The literature screening results attained from the Scopus database are shown in Figure 1. The literature search queries were executed on 21 March 2021 and 25 April 2021 at Scopus, while for PubMed database search, the simultaneous search queries were performed on 21 March 2021 and 25 April 2021 at PubMed. Table 1 summarizes the literature screening results attained from the PubMed database.
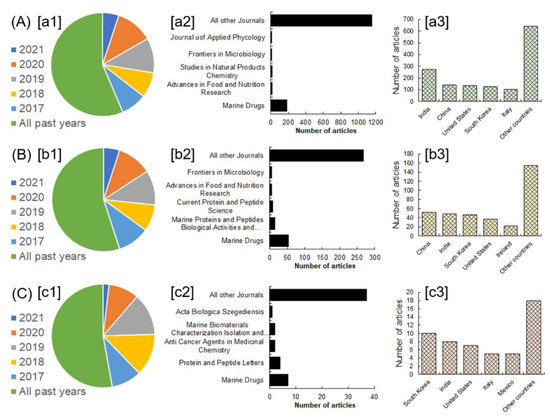
Figure 1.
The literature screening results attained from the Scopus database. The letters A–C characterize the search terms: (A) bioactive compounds from marine sources; (B) bioactive peptides from marine sources; (C) biomedical applications of marine peptides. The letters [a1]–[c1] correspond to the number of articles from all years in that specific category of search term, [b2]–[c2] represents the number of articles published in different journals, and [a3]–[c3] represents the number of articles based on territory. Data were extracted from https://www.scopus.com, access on 25 April 2021.

Table 1.
The literature screening results attained from the PubMed database. Data were extracted from https://pubmed.ncbi.nlm.nih.gov/, access on 25 April 2021.
3. Marine Biome—A Rich Source of MBPs
The marine biome represents the aquatic region and is considered the prime aquatic biome in the world with a plethora of untouched or underexploited sources of high-value interests, having around 500,000 to 10 million marine species [12]. Owing to the difficulty in reaching the lower/deeper zones of the marine biome, several MBPs have yet to be explored to identify and characterize marine biome; thus, marine biome constitutes a rich source of novel compounds with superior performance. As a major component of the biosphere, the aquatic biome covers almost 70% of the earth’s surface [13]. The aquatic biome is broadly divided into two central regions—namely, (1) freshwater regions and (2) marine regions. The freshwater regions of the aquatic biome comprise ponds/lakes, streams/rivers, and wetlands, while the marine region comprises neritic, oceanic, and benthic biomes. Other marine biome zones include intertidal, estuaries, and coral reefs. These alone have the highest biodiversity of all marine biomes. Considering all marine biomes, marine-originated species embrace about half of the total global biodiversity, with a considerable potential to extract products of interest. Thus, several marine biome sources have been broadly discovered and utilized to obtain novel bioactive compounds, e.g., MBPs. The aquatic organisms are classified into three basic categories: (1) plankton, including phytoplankton (bacteria and algae) and zooplankton (tiny animals that feed on phytoplankton); (2) nekton (invertebrates such as shrimp and vertebrates such as fish); (3) benthos (sponges, clams, sea stars, etc.).
Owing to this massive biodiversity, the marine biome is proved to be a highly advantageous and acceptable natural source that can fulfill the growing ecological and socioeconomic demands. Moreover, marine-based natural resources and/or their extracted bioproducts are easily accessible, have high bioactive efficacy, and exert no/fewer side effects [2], compared with the similar representative from synthetic formulations. In the marine biome, the complex habitat environment and the extreme living conditions, such as ultraviolet light exposure, variation in saltiness, thermal conditions of the environment, and limited/excessive nutrient availability, are the contributory aspects to produce MBPs. In consequence, the marine resources including microorganisms such as microalgae survive these extreme environments by rapidly acclimatizing to the new and surrounding atmosphere [14]. Such quick environmental adaptions further assist them to produce more stable and highly effective metabolites, which are biologically active and not common in other similar synthetic counterparts. Owing to their high protein content, microalgae and other marine sources are considered potential sources to produce both elementary proteins and therapeutic peptides [15]. Furthermore, seeing the enormous marine biome potential for industrial sections, the principle of “going green” has scrutinized this substitute search to eco-friendly, ecological, and sustainable materials with comparable socioeconomic benefits. The expansion of distinguishing practices or approaches to improve the cutting-edge platforms also supports the green agenda. The synergistic use of marine-derived natural resources, along with green and modern biotechnology, must be considered to unveil a sustainable production of high-value-added products with highly requisite features [2].
4. Marine Bioactive Peptides (MBPs)
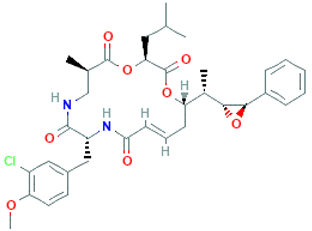
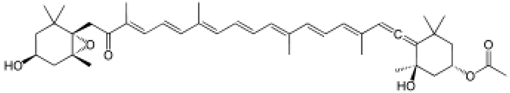
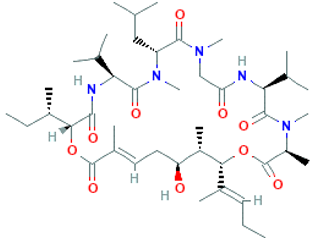
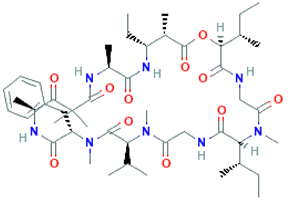
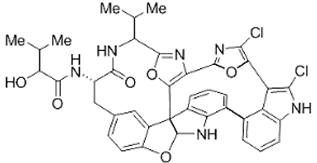
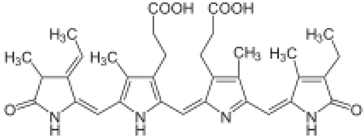
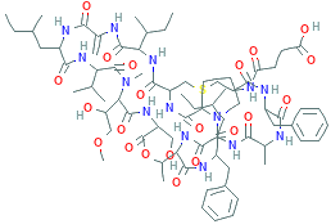
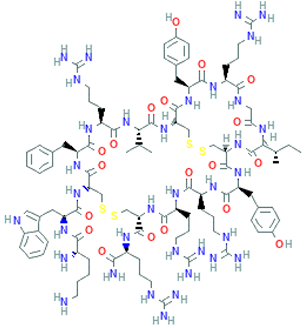
The amino-acid-based organic substances joined by covalent bonds (e.g., amide or peptide bonds) are termed bioactive peptides (BPs). Based on the natural source, some peptides exist freely. However, the majority of the peptides are surrounded or encoded with respective parent protein molecules. In later cases, the pretreatments in the presence of related enzymes, such as proteolytic enzymes, facilitate the release of BPs in an efficient manner [2,16,17]. Such pretreatments cause the hydrolysis of the cell walls or cell membranes, as applied. Thus, the deployment of highly effective and suitable pretreatment supports the retrieval of intracellularly captured bioactive constituents, which are mainly not easy to extract via conventional extraction procedures [2]. Following enzyme facilitated pretreatment, once the encrypted peptides are released from their respective source materials, the amino acid composition and sequence govern the activity [17]. However, owing to the similar peptide length that ranges between 2 and 20 amino acids, most of the BPs share their structural and physicochemical features [18]. The broad bioactivity spectra of MBPs, such as antimicrobial, antiviral, anticancer, anticoagulant, antidiabetic, cardioprotective, immunomodulatory, neuroregenerative, appetite suppressing, etc. [19,20,21,22,23,24], have attracted the attention of biomedical, pharmaceutical, and nutraceutical sectors, with the hope that they can be used as a new frontier to diagnose, treat, or prevent various pathologies in human. Based on the evidence derived from the literature, some of the MBPs and/or their representative biologically active byproducts have gained considerable commercial values and seized the pharmaceutical market. From the perspective of the market status and share, ziconotide and brentuximab vedotin are two important representatives of MBPs and peptide derivatives, and both have successfully reached the market. The premier, Ziconotide (Prialt®), was obtained from a marine cone snail. It was the first peptide from the marine biome approved by the Food and Drug Administration (FDA) USA in 2004 to exploit and use against analgesics [25]. Later in 2011, FDA also approved another marine-derived drug, Adcetris®, to manipulate and use against cancer. Since then, numerous other MBPs have been evaluated for various phases of clinical related trials in the United States and Europe [13]. Figure 2 shows various marine sources along with their representative products or byproducts that have been either accepted or granted to enter the clinical trials. The commercial value of these therapeutic protein-based products was around USD 174.7 Billion in 2015. With a recent hike in interests, it is anticipated that this value will reach/cross USD 266.6 Billion in 2021 [26]. Several other marine-based peptides with different bioactivities and applications are summarized in Table 2.
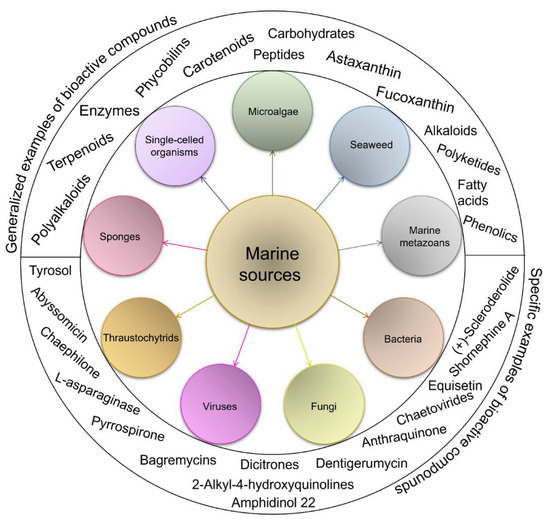
Figure 2.
Various marine sources, along with their representative products or byproducts that have been either accepted or granted to enter the clinical trials.

Table 2.
Marine-based peptides (MBPs) with different bioactivities and applications.
5. Bioactivities—An Overview
As mentioned earlier, the marine biome having millions of macro- and microspecies (both animal and plants) is becoming highly significant [49,50]. This is also because the marine is considered a prolific source of structurally and functionally diverse MBPs at large, and MBPs in particular. The pharmacological activities, such as antimicrobial, antiviral, anticancer, anticoagulant, antidiabetic, cardioprotective, immunomodulatory, neuroregenerative, appetite suppressing, and many other MBPs, have been described in the literature [14,15,16,17,18,19,51,52]. Anticancer, anti-inflammatory, immunomodulating, antioxidant, hepatoprotective, and neuroprotective activities of phycobiliproteins from cyanobacteria and red algae are reported [15,53]. According to one estimate, the marine biome offers around 8000 species of red algae, which are highly enriched with several MBPs. An array of cyanobacterial-based MBPs, e.g., polypeptide or a hybrid of polyketide–polypeptide, are obtained via polyketide synthase and nonribosomal peptide synthase [54] and comprise exceptional characteristics, e.g., amino and/or hydroxy acids, heteroaromatic ring systems, and extended polyketide-derived units [55]. Likewise, several other BP-based functional entities, e.g., microcystin (a cyclic peptide), majusculamide 8 (depsipeptide), etc. have been isolated and/or extracted from marine-based blue-green algae [56,57]. Ghalamara et al. [58] proposed and extracted bioactive peptides-enriched and bioactive fractions from codfish blood, which shows notable antimicrobial properties and inhibits Escherichia coli growth [58].
Even though marine organisms at large, and microalgae, in particular, are highly enriched with diverse MBPs, their application as antimicrobials against human pathogens and diseases in aquaculture is still in its early stages [59]. With ever-rising heavy drug resistance and/or emergence/reemergence of new resistant microbial infections, there is a dire need to develop robust classes of natural antibiotics in combination with MBPs to treat the emerging/reemerging bacterial pathogens [60]. Diverse natural compounds with potent antimicrobial properties have been described in algae that belong to a wide range of chemical classes, including fatty acids, indoles, acetogenins, phenols, terpenes, and volatile, halogenated hydrocarbons [61]. Macro- and microalgae have grown to produce MBPs to pledge pathogenic bacteria [62], which are considered ubiquitous to their environment. Under the current scenario, effective treatment for a multi-drug-resistant S. aureus has become a challenge and extremely worrying concern [63]. Therefore, an alternative, novel antimicrobial agent is highly demanded.
Cancer is a multifaceted disease in which abnormal cells divide overpoweringly and abolish normal body tissue. Owing to its molecular variations and consequential cellular effects, it has expanded extensive courtesy in terms of public health. Cancer involves several types that cause adversative consequences, e.g., leading to the growth of a cell mass with the capability to penetrate adjacent normal tissues and move to new locations via a mechanism known as metastasis (Figure S1) [64,65]. Figure S2 shows a detailed metastasis to new locations in (A) lungs and (B) brain shown in Figure S1. MBPs can attack the cancerous cells membrane by the processes called either necrosis or apoptosis that may cause cell death. In necrosis, the peptides target the negatively charged molecules on the cancer cell membrane and cause cell lysis, while in the case of apoptosis (Figure 3), they cause disruption of the mitochondrial membrane [66,67,68]. Amphipathic peptides have shown notable bioactivities and can easily be obtained from various marine-based natural resources [5,69]. Amphipathic peptides can be used to strengthen the natural defense against invasive pathogens. The prospective therapeutic application of MBPs has been established because of their wide-ranging bioactivity spectrum and the likelihood of not inducing resistance [70]. Initiation of extrinsic apoptotic activity pathways and inhibition of angiogenesis is an excellent example of killing action of MBPs. Owing to their capability to work synergistically, most of the MBPs, expressly antitumor peptides, are considered highly active in combination with the old style, but in practice, they are chemotherapeutic agents [5].
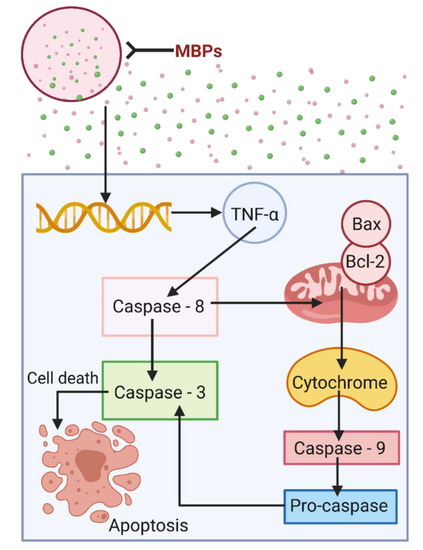
Figure 3.
Anticancer potentialities of MBPs to abolish cancer cells and avoid metastasis via apoptosis mechanism. Created with BioRender.com and extracted under premium membership.
Based on the structural characteristics, they are mainly categorized into (1) cysteine-rich and β-sheet peptides, e.g., α- and β -defensins; (2) α-helices comprising peptides, e.g., LL-37 cathelicidin, cecropins, magainins, etc.; (3) peptide structures that are rich in glycine, proline, tryptophan, arginine, histidine, etc.; (4) peptides comprising a single disulfide bond, e.g., bactenecin. Further examples in the literature regarding some particular peptides having anticancerous activities are given in Table 2. Microalgal peptides with antiatherosclerotic activity have also been recognized, as described by Fan et al. [71]. Atherogenesis is an artery wall syndrome that encompasses several progressions, including cell adhesion, migration, differentiation, proliferation, and cell interaction with the extracellular matrix till the formation of atherosclerotic plaques, controlled by a complex network/cascade of cytokines and growth regulatory peptides. Besides the above-mentioned model examples, several other biological activities, such as anticoagulant, antidiabetic, cardioprotective, immunomodulatory, neuroregenerative, appetite suppressing, and others have fascinated researchers and strengthened the marine pharmacology sector [72,73,74]. Oxidative stress is a foremost reason for inflammatory events implicated in many of the above-mentioned diseases (e.g., neurodegenerative, cardiovascular, cancer, diabetes, etc.) [72,74].
6. FDA-Approved MBPs in the Market and Clinical Trials
Owing to the unique structural and multifunctional attributes, an array of MBPs have attained FDA-approved status, as already verified with numerous bioactivities, such as antimicrobials (antibacterial, and antifungal), antiviral, anticancer, antioxidant, antihypertensive, anticoagulant, antithrombotic, immunomodulatory, cholesterol-lowering activities, etc. The market-available MBP-based products have been developed using a unique combination of pristine counterparts and compositional alternation, as appropriate for medicinal, nutraceutical, and pharmaceutical uses. A plethora of BPs have been extracted from marine sources, treated, and purified, though using different materials and methods. However, a small fraction of those BPs have been legalized to move for clinical phase assessment, and even a few have accomplished to reach in the market. To avoid literature redundancy, selected FDA-approved MBPs are summarized in Table 3 with detailed information. In contrast, several other marine-derived biologically active compounds/products, including Pliditepsin, PM00104, Kahalalide F, Hemiasterlin, Spisulosine, Pseudopterosin A, Salinosporamide A, Tetrodotoxin, Conotoxin G, Bryostatin 1, and Plinabulin, are under clinical trials (phases I–III) [21,22,75,76,77].

Table 3.
Selected FDA-approved MBPs.
7. Sustainable Development Goals (SDGs) and Legislation to Valorize Marine Biome
The sustainable development goals (SDGs) are blueprints that urge to attain better tomorrow and a more sustainable future. Moreover, a highly efficient transformation of innumerable marine resources into high-value entities, such as MBPs, as per SDGs, is of supreme interest. The potential of marine pharmacology pointedly subsidizes accomplishing 14 out of 17 of the United Nations (UN) SDGs (available online: https://sdgs.un.org/ -Last (accessed on 27 April 2021)). More specifically, with particular reference to SDG 14, “life below water” is a central theme for the sighting of biodiversity and the sustainable use of marine-based natural resources. Promotion of resource efficiency and technological development contribute to SDG 12 (Responsible Consumption and Production) and SDG 9 (Industry, Innovation, and Infrastructure). Therefore, in order to achieve SDG 3, with good health and well-being theme, the well-developed and effectively deployed policy frameworks and regulations or a combination of SDG measures are much needed, including the expansion of new products in the medical and pharmaceutical industries. The establishment of partnerships between governments, industry, civil society, and the scientific sector contributes to SDG 17 (Partnerships for the Goals). As confirmed most recently by Agenda 2030, the ecosystem-based approach is vital to “conserve and sustainably use the oceans, seas and marine resources for sustainable development” (SDG 14). At the sea-basin level, a close regional alliance of Member States within pertinent regional sea conventions helps synchronize the regional execution and valuation of ocean-related SDGs.
European Union (EU) legislation supports marine spatial planning (MSP) to balance the maritime economy while protecting and valorizing biodiversity. Several complementary policies have been regulated, i.e., the regulation of fisheries through the Common Fisheries Policy (CFP), EU Biodiversity Strategy to 2020, EU Regulation 1143/2014 on Invasive Alien Species, and the control of the input of nutrients and chemicals into waters through the Water Framework Directive (WFD), etc. The EU Marine Strategy Framework Directive, the environmental pillar of the EU maritime policy, introduced the principle of ecosystem-based marine spatial planning and provided a supportive framework for national initiatives toward spatial planning, designed for achieving good status for the environment. In summary, successful execution of the Marine Directive will be dynamic if the Integrated Maritime Policy is to be delivered as intended. To provide further insight on the current policy frameworks and regulations, some of the EU directives and legislation are summarized in Table S1.
8. Conclusions, Current Challenges, and Future Considerations
In summary, marine biotechnology and pharmacology have infinite potential to formulate/synthesize new MBPs that show a dynamic role in bio- and non-bioindustries of the modern world. MBPs obtained by following green chemistry agenda principles using naturally existing sources from marine biomes such as microalgae have succeeding merits, including natural abundance, ease in availability throughout the year, materials renewability and sustainability, carbon-neutral aspects, re-processibility with a zero-waste SDG agenda, facile synthesis options with possibilities to scale up, net positive and high cost-effective ratio, no or minimal consumption of harsh chemicals/reagents, and no or less toxic contaminants/byproducts, etc. Nonetheless, colossal steps have already been reserved and taken in the past years; however, focused and genetically positioned research is necessary to further strengthen the marine pharmacology consideration for a better tomorrow.
Regardless of ever-growing scientific awareness and technological advancement, several challenges still exist to address valorize MBPs from marine origins. These include (1) reachability ease to the unexplored biodiversity, (2) standardized procedural isolation (regardless of the source variability), (3) cost-effective handling of the extracted products, (4) stability maintenance under standardized environment for any or many products at the same time (regardless of the compositional variability), (5) possible scale-up probability, (6) sustainable marketing and commercialization, all of which are vital challenges and should be considered with care. A considerable amount of information is available in the literature about numerous potential aspects of marine biotechnology and pharmacology. However, substantial critiques are still unresolved, which necessitate future studies. Despite contemporary scientific advancements in marine biotechnology, extensive research with verified employability of marine sources is needed in this line of research.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/md20030208/s1, Figure S1: Schematic representation of cancer metastasis to new locations. Cancer involves several types that cause adversative consequences, e.g., leading to the growth of a cell mass with the capability to penetrate and move to adjacent normal tissues. In metastasis, cancer cells break away from where they first formed (primary cancer site), travel through the blood stream, and form new tumors (metastatic tumors) in other parts of the body (secondary cancer site). Created with BioRender.com and extracted under premium membership. Figure S2: A detailed metastasis to new locations (A) lungs and (B) brain, with reference to the model locations marked in Figure S1. Created with BioRender.com and extracted under premium membership. Table S1: EU directives and legislation that support current policy frameworks and regulations in the field of marine environmental policy.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Consejo Nacional de Ciencia y Tecnología (CONACYT) Mexico is thankfully acknowledged for partially supporting this research under Sistema Nacional de Investigadores (SNI) program awarded to Ashutosh Sharma (CVU: 398262), Roberto Parra-Saldívar (CVU: 35753), and Hafiz M. N. Iqbal (CVU: 735340).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Centella, M.H.; Arévalo-Gallegos, A.; Parra-Saldivar, R.; Iqbal, H.M. Marine-derived bioactive compounds for value-added applications in bio-and non-bio sectors. J. Clean. Prod. 2017, 168, 1559–1565. [Google Scholar] [CrossRef]
- Sosa-Hernández, J.E.; Escobedo-Avellaneda, Z.; Iqbal, H.; Welti-Chanes, J. State-of-the-art extraction methodologies for bioactive compounds from algal biome to meet bio-economy challenges and opportunities. Molecules 2018, 23, 2953. [Google Scholar] [CrossRef] [PubMed]
- DeVierno Kreuder, A.; House-Knight, T.; Whitford, J.; Ponnusamy, E.; Miller, P.; Jesse, N.; Rodenborn, R.; Sayag, S.; Gebel, M.; Aped, I.; et al. A method for assessing greener alternatives between chemical products following the 12 principles of green chemistry. ACS Sustain. Chem. Eng. 2017, 5, 2927–2935. [Google Scholar] [CrossRef]
- Wang, X.; Yu, H.; Xing, R.; Li, P. Characterization, preparation, and purification of marine bioactive peptides. BioMed Res. Int. 2017, 2017, 9746720. [Google Scholar] [CrossRef]
- Li, D.; Liao, X.; Zhong, S.; Zhao, B.; Xu, S. Synthesis of Marine Cyclopeptide Galaxamide Analogues as Potential Anticancer Agents. Mar. Drugs 2022, 20, 158. [Google Scholar] [CrossRef]
- Tyagi, A.; Tuknait, A.; Anand, P.; Gupta, S.; Sharma, M.; Mathur, D.; Joshi, A.; Singh, S.; Gautam, A.; Raghava, G.P. CancerPPD: A database of anticancer peptides and proteins. Nucleic Acids Res. 2015, 43, D837–D843. [Google Scholar] [CrossRef]
- Delattre, C.; Pierre, G.; Laroche, C.; Michaud, P. Production, extraction and characterization of microalgal and cyanobacterial exopolysaccharides. Biotechnol. Adv. 2016, 34, 1159–1179. [Google Scholar] [CrossRef]
- Al-Dhafri, K.S.; Ching, C.L. Isolation and Characterization of Antimicrobial Peptides Isolated from Fagonia bruguieri. Appl. Biochem. Biotechnol. 2022, 1–14. [Google Scholar] [CrossRef]
- Hernández, Y.; Lobo, M.G.; González, M. Factors affecting sample extraction in the liquid chromatographic determination of organic acids in papaya and pineapple. Food Chem. 2009, 114, 734–741. [Google Scholar] [CrossRef]
- Hamayeli, H.; Hassanshahian, M.; Hesni, M.A. Identification of Bioactive Compounds and Evaluation of the Antimicrobial and Anti-biofilm Effect of Psammocinia sp. and Hyattella sp. Sponges from the Persian Gulf. Thalass. Int. J. Mar. Sci. 2021, 37, 357–366. [Google Scholar] [CrossRef]
- Lim, S.M.; Agatonovic-Kustrin, S.; Lim, F.T.; Ramasamy, K. High-performance thin layer chromatography-based phytochemical and bioactivity characterisation of anticancer endophytic fungal extracts derived from marine plants. J. Pharm. Biomed. Anal. 2021, 193, 113702. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Heimann, K.; Zhang, W. Protein Recovery from Underutilised Marine Bioresources for Product Development with Nutraceutical and Pharmaceutical Bioactivities. Mar. Drugs 2020, 18, 391. [Google Scholar] [CrossRef] [PubMed]
- Cheung RC, F.; Ng, T.B.; Wong, J.H. Marine peptides: Bioactivities and applications. Mar. Drugs 2015, 13, 4006–4043. [Google Scholar] [CrossRef] [PubMed]
- De Morais, M.G.; Vaz BD, S.; de Morais, E.G.; Costa, J.A.V. Biologically active metabolites synthesized by microalgae. BioMed Res. Int. 2015, 2015, 835761. [Google Scholar] [CrossRef]
- Talero, E.; García-Mauriño, S.; Ávila-Román, J.; Rodríguez-Luna, A.; Alcaide, A.; Motilva, V. Bioactive compounds isolated from microalgae in chronic inflammation and cancer. Mar. Drugs 2015, 13, 6152–6209. [Google Scholar] [CrossRef]
- Rosenthal, A.; Pyle, D.L.; Niranjan, K. Aqueous and enzymatic processes for edible oil extraction. Enzym. Microb. Technol. 1996, 19, 402–420. [Google Scholar] [CrossRef]
- Sánchez, A.; Vázquez, A. Bioactive peptides: A review. Food Qual. Saf. 2017, 1, 29–46. [Google Scholar] [CrossRef]
- Möller, N.P.; Scholz-Ahrens, K.E.; Roos, N.; Schrezenmeir, J. Bioactive peptides and proteins from foods: Indication for health effects. Eur. J. Nutr. 2008, 47, 171–182. [Google Scholar] [CrossRef]
- Chi, C.F.; Hu, F.Y.; Wang, B.; Li, T.; Ding, G.F. Antioxidant and anticancer peptides from the protein hydrolysate of blood clam (Tegillarca granosa) muscle. J. Funct. Foods 2015, 15, 301–313. [Google Scholar] [CrossRef]
- Jose, D.T.; Uma, C.; Sivagurunathan, P.; Aswini, B.; Dinesh, M.D. Extraction and antibacterial evaluation of marine AMPs against diabetic wound pathogens. J. Appl. Pharm. Sci. 2020, 10, 087–092. [Google Scholar]
- Ucak, I.; Afreen, M.; Montesano, D.; Carrillo, C.; Tomasevic, I.; Simal-Gandara, J.; Barba, F.J. Functional and bioactive properties of peptides derived from marine side streams. Mar. Drugs 2021, 19, 71. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.T.; Liu, Z.D.; Wang, Z.; Wang, T.; Wang, N.; Wang, N.; Zhang, B.; Zhao, Y.F. Recent Advances in Small Peptides of Marine Origin in Cancer Therapy. Mar. Drugs 2021, 19, 115. [Google Scholar] [CrossRef] [PubMed]
- Safronova, V.N.; Panteleev, P.V.; Sukhanov, S.V.; Toropygin, I.Y.; Bolosov, I.A.; Ovchinnikova, T.V. Mechanism of Action and Therapeutic Potential of the β-Hairpin Antimicrobial Peptide Capitellacin from the Marine Polychaeta Capitella teleta. Mar. Drugs 2022, 20, 167. [Google Scholar] [CrossRef]
- Zeng, Z.; Wang, Y.; Anwar, M.; Hu, Z.; Wang, C.; Lou, S.; Li, H. Molecular cloning and expression analysis of mytilin-like antimicrobial peptides from Asian green mussel Perna viridis. Fish Shellfish Immunol. 2022, 121, 239–244. [Google Scholar] [CrossRef]
- Olivera, B.M. Biodiversity-based Discovery and Exogenomics. J. Biol. Chem. 2006, 281, 31173–31177. [Google Scholar]
- Morlighem, J.É.R.; Radis-Baptista, G. The place for enzymes and biologically active peptides from marine organisms for application in industrial and pharmaceutical biotechnology. Curr. Protein Pept. Sci. 2019, 20, 334–355. [Google Scholar] [CrossRef]
- Messina, C.M.; Manuguerra, S.; Arena, R.; Renda, G.; Ficano, G.; Randazzo, M.; Fricano, S.; Sadok, S.; Santulli, A. In Vitro Bioactivity of Astaxanthin and Peptides from Hydrolisates of Shrimp (Parapenaeus longirostris) By-Products: From the Extraction Process to Biological Effect Evaluation, as Pilot Actions for the Strategy “From Waste to Profit”. Mar. Drugs 2021, 19, 216. [Google Scholar] [CrossRef]
- Dahiya, R.; Rampersad, S.; Ramnanansingh, T.G.; Kaur, K.; Kaur, R.; Mourya, R.; Chennupati, S.V.; Fairman, R.; Jalsa, N.K.; Sharma, A.; et al. Synthesis and Bioactivity of a Cyclopolypeptide from Caribbean Marine Sponge. Iran. J. Pharm. Res. IJPR 2020, 19, 156. [Google Scholar]
- Leisch, M.; Egle, A.; Greil, R. Plitidepsin: A potential new treatment for relapsed/refractory multiple myeloma. Future Oncol. 2019, 15, 109–120. [Google Scholar] [CrossRef]
- Nowruzi, B.; Blanco, S.; Nejadsattari, T. Chemical and molecular evidences for the poisoning of a duck by anatoxin-a, nodularin and cryptophycin at the coast of Lake Shoormast (Mazandaran province, Iran). Int. J. Algae 2018, 20, 359–376. [Google Scholar] [CrossRef]
- Nowruzi, B.; Haghighat, S.; Fahimi, H.; Mohammadi, E. Nostoc cyanobacteria species: A new and rich source of novel bioactive compounds with pharmaceutical potential. J. Pharm. Health Serv. Res. 2018, 9, 5–12. [Google Scholar] [CrossRef]
- Zhu, Y.; Cheng, J.; Min, Z.; Yin, T.; Zhang, R.; Zhang, W.; Hu, L.; Cui, Z.; Gao, C.; Xu, S.; et al. Effects of fucoxanthin on autophagy and apoptosis in SGC-7901cells and the mechanism. J. Cell. Biochem. 2018, 119, 7274–7284. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Cui, Z.; Li, Y.H.; Hsu, W.H.; Lee, B.H. In vitro and in vivo anticancer activity of pardaxin against proliferation and growth of oral squamous cell carcinoma. Mar. Drugs 2016, 14, 2. [Google Scholar] [CrossRef] [PubMed]
- Hassana MA, K.; Azeminb, W.A.; Dharmaraja, S.; Mohdb, K.S. Hepcidin TH1-5 Induces Apoptosis and Activate Caspase-9 in MCF-7 Cells. J. Appl. Pharm. Sci. 2016, 6, 081–086. [Google Scholar] [CrossRef][Green Version]
- Chang, W.T.; Pan, C.Y.; Rajanbabu, V.; Cheng, C.W.; Chen, J.Y. Tilapia (Oreochromis mossambicus) antimicrobial peptide, hepcidin 1–5, shows antitumor activity in cancer cells. Peptides 2011, 32, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Y.; Lin, W.J.; Lin, T.L. A fish antimicrobial peptide, tilapia hepcidin TH2-3, shows potent antitumor activity against human fibrosarcoma cells. Peptides 2009, 30, 1636–1642. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.T.; Cheng, K.L.; Baruchello, R.; Rondanin, R.; Marchetti, P.; Simoni, D.; Lee, R.M.; Guh, J.H.; Hsu, L.C. Hemiasterlin derivative (R)(S)(S)-BF65 and Akt inhibitor MK-2206 synergistically inhibit SKOV3 ovarian cancer cell growth. Biochem. Pharmacol. 2016, 113, 12–23. [Google Scholar] [CrossRef]
- Sato, S.I.; Murata, A.; Orihara, T.; Shirakawa, T.; Suenaga, K.; Kigoshi, H.; Uesugi, M. Marine natural product aurilide activates the OPA1-mediated apoptosis by binding to prohibitin. Chem. Biol. 2011, 18, 131–139. [Google Scholar] [CrossRef]
- Han, B.; Gross, H.; Goeger, D.E.; Mooberry, S.L.; Gerwick, W.H. Aurilides B and C, Cancer Cell Toxins from a Papua New Guinea Collection of the Marine Cyanobacterium Lyngbya majuscula. J. Nat. Prod. 2006, 69, 572–575. [Google Scholar] [CrossRef]
- Suenaga, K.; Mutou, T.; Shibata, T.; Itoh, T.; Fujita, T.; Takada, N.; Hayamizu, K.; Takagi, M.; Irifune, T.; Kigoshi, H. Aurilide, a cytotoxic depsipeptide from the sea hare Dolabella auricularia: Isolation, structure determination, synthesis, and biological activity. Tetrahedron 2004, 60, 8509–8527. [Google Scholar] [CrossRef]
- Simmons, T.L.; Nogle, L.M.; Media, J.; Valeriote, F.A.; Mooberry, S.L.; Gerwick, W.H. Desmethoxymajusculamide C, a cyanobacterial depsipeptide with potent cytotoxicity in both cyclic and ring-opened forms. J. Nat. Prod. 2009, 72, 1011–1016. [Google Scholar] [CrossRef] [PubMed]
- Lachia, M.; Moody, C.J. The synthetic challenge of diazonamide A, a macrocyclic indole bis-oxazole marine natural product. Nat. Prod. Rep. 2008, 25, 227–253. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Ling, P.; Wang, Z.; Niu, R.; Hu, C.; Zhang, T.; Lin, X. A novel polypeptide from shark cartilage with potent anti-angiogenic activity. Cancer Biol. Ther. 2007, 6, 775–780. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Gao, M.H.; Zhang, X.C.; Chu, X.M. Molecular immune mechanism of C-phycocyanin from Spirulina platensis induces apoptosis in HeLa cells in vitro. Biotechnol. Appl. Biochem. 2006, 43, 155–164. [Google Scholar]
- Sewell, J.M.; Mayer, I.; Langdon, S.P.; Smyth, J.F.; Jodrell, D.I.; Guichard, S.M. The mechanism of action of Kahalalide F: Variable cell permeability in human hepatoma cell lines. Eur. J. Cancer 2005, 41, 1637–1644. [Google Scholar] [CrossRef]
- Edler, M.C.; Fernandez, A.M.; Lassota, P.; Ireland, C.M.; Barrows, L.R. Inhibition of tubulin polymerization by vitilevuamide, a bicyclic marine peptide, at a site distinct from colchicine, the vinca alkaloids, and dolastatin 10. Biochem. Pharmacol. 2002, 63, 707–715. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, X.; Hong, S.; Chen, J.; Liu, N.; Underhill, C.B.; Creswell, K.; Zhang, L. RGD-Tachyplesin inhibits tumor growth. Cancer Res. 2001, 61, 2434–2438. [Google Scholar]
- Erba, E.; Bergamaschi, D.; Ronzoni, S.; Faretta, M.; Taverna, S.; Bonfanti, M.; Catapano, C.V.; Faircloth, G.; Jimeno, J.; D’incalci, M. Mode of action of thiocoraline, a natural marine compound with anti-tumour activity. Br. J. Cancer 1999, 80, 971–980. [Google Scholar] [CrossRef]
- Li, Y.X.; Wijesekara, I.; Li, Y.; Kim, S.K. Phlorotannins as bioactive agents from brown algae. Process Biochem. 2011, 46, 2219–2224. [Google Scholar] [CrossRef]
- Valverde, F.; Romero-Campero, F.J.; León, R.; Guerrero, M.G.; Serrano, A. New challenges in microalgae biotechnology. Eur. J. Protistol. 2016, 55, 95–101. [Google Scholar] [CrossRef]
- De Sousa Santos AK, F.; da Fonseca, D.V.; Salgado PR, R.; Muniz, V.M.; de Arruda Torres, P.; Lira, N.S.; da Silva Dias, C.; de Morais Pordeus, L.C.; Barbosa-Filho, J.M.; de Almeida, R.N. Antinociceptive activity of Sargassum polyceratium and the isolation of its chemical components. Rev. Bras. Farmacogn. 2015, 25, 683–689. [Google Scholar]
- Arumugam, V.; Venkatesan, M.; Ramachandran, K.; Ramachandran, S.; Palanisamy, S.K.; Sundaresan, U. Purification, Characterization and Antibacterial Properties of Peptide from Marine Ascidian Didemnum sp. Int. J. Pept. Res. Ther. 2020, 26, 201–208. [Google Scholar] [CrossRef]
- El Gamal, A.A. Biological importance of marine algae. Saudi Pharm. J. 2010, 18, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Mazard, S.; Penesyan, A.; Ostrowski, M.; Paulsen, I.T.; Egan, S. Tiny microbes with a big impact: The role of cyanobacteria and their metabolites in shaping our future. Mar. Drugs 2016, 14, 97. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.T.; Okino, T.; Gerwick, W.H. Bouillonamide: A mixed polyketide–peptide cytotoxin from the marine cyanobacterium Moorea bouillonii. Mar. Drugs 2013, 11, 3015–3024. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, J.F.; Niedzwiadek, B.; Menard, C.; Lau, B.P.; Lewis, D.; Kuper-Goodman, T.; Carbone, S.; Holmes, C. Comparison of liquid chromatography/mass spectrometry, ELISA, and phosphatase assay for the determination of microcystins in blue-green algae products. J. AOAC Int. 2001, 84, 1035–1044. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, G.N.E. Cytotoxicity and antibacterial activity of the blue green alga Microcystis aeruginosa extracts against human cancer cell lines and foodborne bacteria. Egypt. J. Chem. 2020, 63, 2–3. [Google Scholar] [CrossRef]
- Ghalamara, S.; Silva, S.; Brazinha, C.; Pintado, M. Valorization of fish by-products: Purification of bioactive peptides from codfish blood and sardine cooking wastewaters by membrane processing. Membranes 2020, 10, 44. [Google Scholar] [CrossRef]
- Falaise, C.; François, C.; Travers, M.A.; Morga, B.; Haure, J.; Tremblay, R.; Turcotte, F.; Pasetto, P.; Gastineau, R.; Hardivillier, Y.; et al. Antimicrobial compounds from eukaryotic microalgae against human pathogens and diseases in aquaculture. Mar. Drugs 2016, 14, 159. [Google Scholar] [CrossRef]
- Sukmarini, L. Drug Development from Peptide-derived Marine Natural Products. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Tangerang, Indonesia, 2021; Volume 1011, p. 012063. [Google Scholar]
- Plaza, M.; Santoyo, S.; Jaime, L.; Reina GG, B.; Herrero, M.; Señoráns, F.J.; Ibáñez, E. Screening for bioactive compounds from algae. J. Pharm. Biomed. Anal. 2010, 51, 450–455. [Google Scholar] [CrossRef]
- Bule, M.H.; Ahmed, I.; Maqbool, F.; Bilal, M.; Iqbal, H.M. Microalgae as a source of high-value bioactive compounds. Front. Biosci 2018, 10, 197–216. [Google Scholar]
- Winokur, P.; Chenoweth, C.E.; Rice, L.; Mehrad, B.; Lynch, J.P. Resistant pathogens: Emergence and control. In Ventilator-Associated Pneumonia; Springer: Boston, MA, USA, 2001; pp. 131–164. [Google Scholar]
- Zheng, L.H.; Wang, Y.J.; Sheng, J.; Wang, F.; Zheng, Y.; Lin, X.K.; Sun, M. Antitumor peptides from marine organisms. Mar. Drugs 2011, 9, 1840–1859. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Shi, Y.; Qian, F. Opportunities and delusions regarding drug delivery targeting pancreatic cancer-associated fibroblasts. Adv. Drug Deliv. Rev. 2021, 172, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.H.; Hou, C.Y.; Zhang, D.; Zhao, W.J.; Cong, Y.; Duan, Z.Y.; Qiao, Z.Y.; Wang, H. Enzyme-sensitive cytotoxic peptide–dendrimer conjugates enhance cell apoptosis and deep tumor penetration. Biomater. Sci. 2018, 6, 604–613. [Google Scholar] [CrossRef]
- Mirza, A.Z. Advancement in the development of heterocyclic nucleosides for the treatment of cancer-A review. Nucleosides Nucleotides Nucleic Acids 2019, 38, 836–857. [Google Scholar] [CrossRef]
- Böhmová, E.; Pola, R.; Pechar, M.; Parnica, J.; Machová, D.; Janoušková, O.; Etrych, T. Polymer Cancerostatics Containing Cell-Penetrating Peptides: Internalization Efficacy Depends on Peptide Type and Spacer Length. Pharmaceutics 2020, 12, 59. [Google Scholar] [CrossRef]
- Lobine, D.; Rengasamy, K.R.; Mahomoodally, M.F. Functional foods and bioactive ingredients harnessed from the ocean: Current status and future perspectives. Crit. Rev. Food Sci. Nutr. 2021, 1–30. [Google Scholar] [CrossRef]
- Reddy, P.A.; Jones, S.T.; Lewin, A.H.; Carroll, F. Synthesis of hemopressin peptides by classical solution phase fragment condensation. Int. J. Pept. 2012, 2012, 186034. [Google Scholar] [CrossRef]
- Fan, X.; Bai, L.; Zhu, L.; Yang, L.; Zhang, X. Marine algae-derived bioactive peptides for human nutrition and health. J. Agric. Food Chem. 2014, 62, 9211–9222. [Google Scholar] [CrossRef]
- Robertson, R.C.; Guihéneuf, F.; Bahar, B.; Schmid, M.; Stengel, D.B.; Fitzgerald, G.F.; Ross, R.P.; Stanton, C. The anti-inflammatory effect of algae-derived lipid extracts on lipopolysaccharide (LPS)-stimulated human THP-1 macrophages. Mar. Drugs 2015, 13, 5402–5424. [Google Scholar] [CrossRef]
- Bélanger, W.; Arnold, A.A.; Turcotte, F.; Saint-Louis, R.; Deschênes, J.S.; Genard, B.; Marcotte, I.; Tremblay, R. Extraction Improvement of the Bioactive Blue–Green Pigment “Marennine” from Diatom Haslea ostrearia’s Blue Water: A Solid-Phase Method Based on Graphitic Matrices. Mar. Drugs 2020, 18, 653. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Iqbal, H.M. Biologically active macromolecules: Extraction strategies, therapeutic potential and biomedical perspective. Int. J. Biol. Macromol. 2020, 151, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Anjum, K.; Abbas, S.Q.; Akhter, N.; Shagufta, B.I.; Shah SA, A.; Hassan SS, U. Emerging biopharmaceuticals from bioactive peptides derived from marine organisms. Chem. Biol. Drug Des. 2017, 90, 12–30. [Google Scholar] [CrossRef] [PubMed]
- Alves, C.; Silva, J.; Pinteus, S.; Gaspar, H.; Alpoim, M.C.; Botana, L.M.; Pedrosa, R. From marine origin to therapeutics: The antitumor potential of marine algae-derived compounds. Front. Pharmacol. 2018, 9, 777. [Google Scholar] [CrossRef] [PubMed]
- Ghareeb, M.A.; Tammam, M.A.; El-Demerdash, A.; Atanasov, A.G. Insights about clinically approved and Preclinically investigated marine natural products. Curr. Res. Biotechnol. 2020, 2, 88–102. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).