Fucoidan (Undaria pinnatifida)/Polydopamine Composite-Modified Surface Promotes Osteogenic Potential of Periodontal Ligament Stem Cells
Abstract
:1. Introduction
2. Results
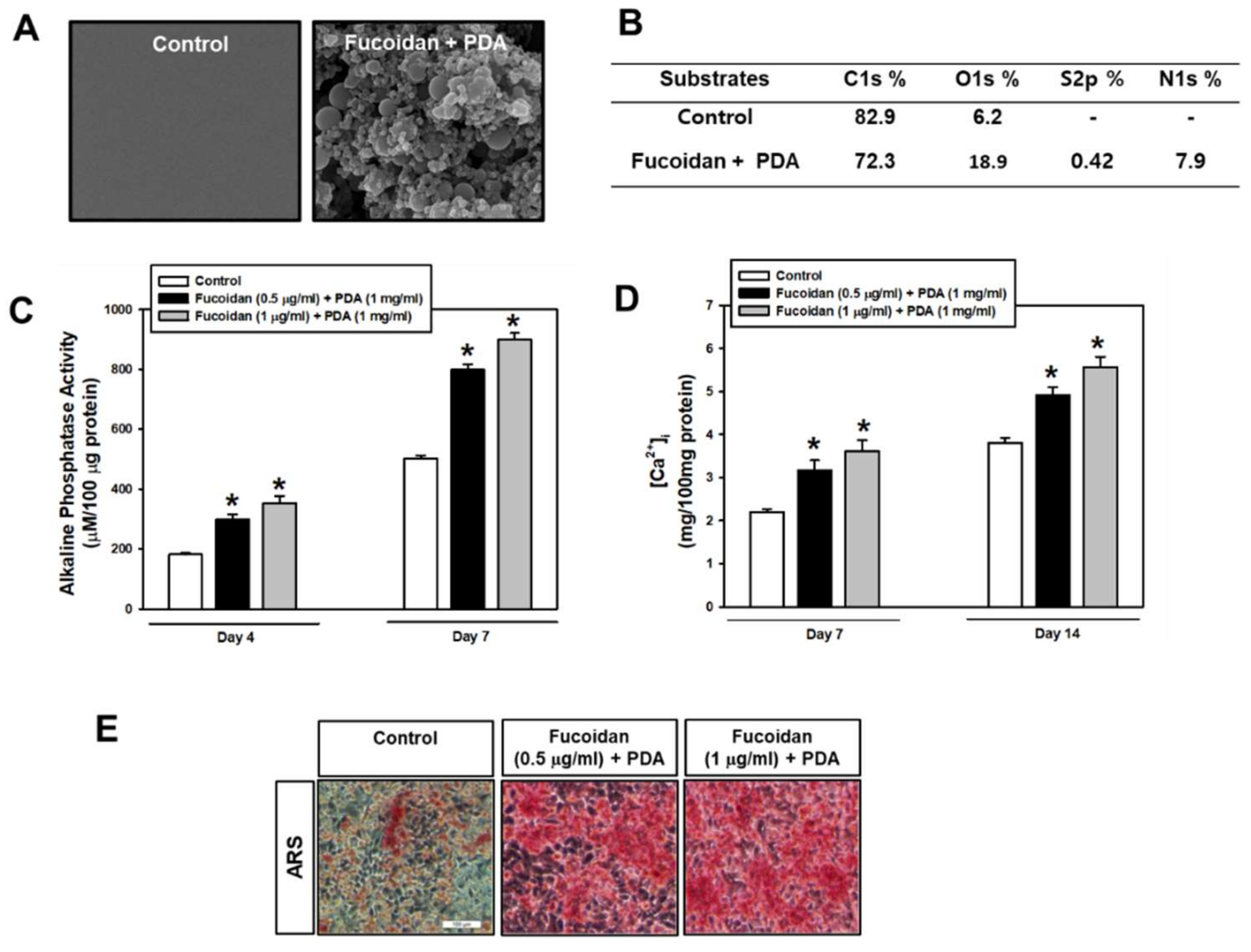
2.1. Fucoidan/PDA-Coated Surface Encourages the Osteogenic Differentiation of PDLSCs
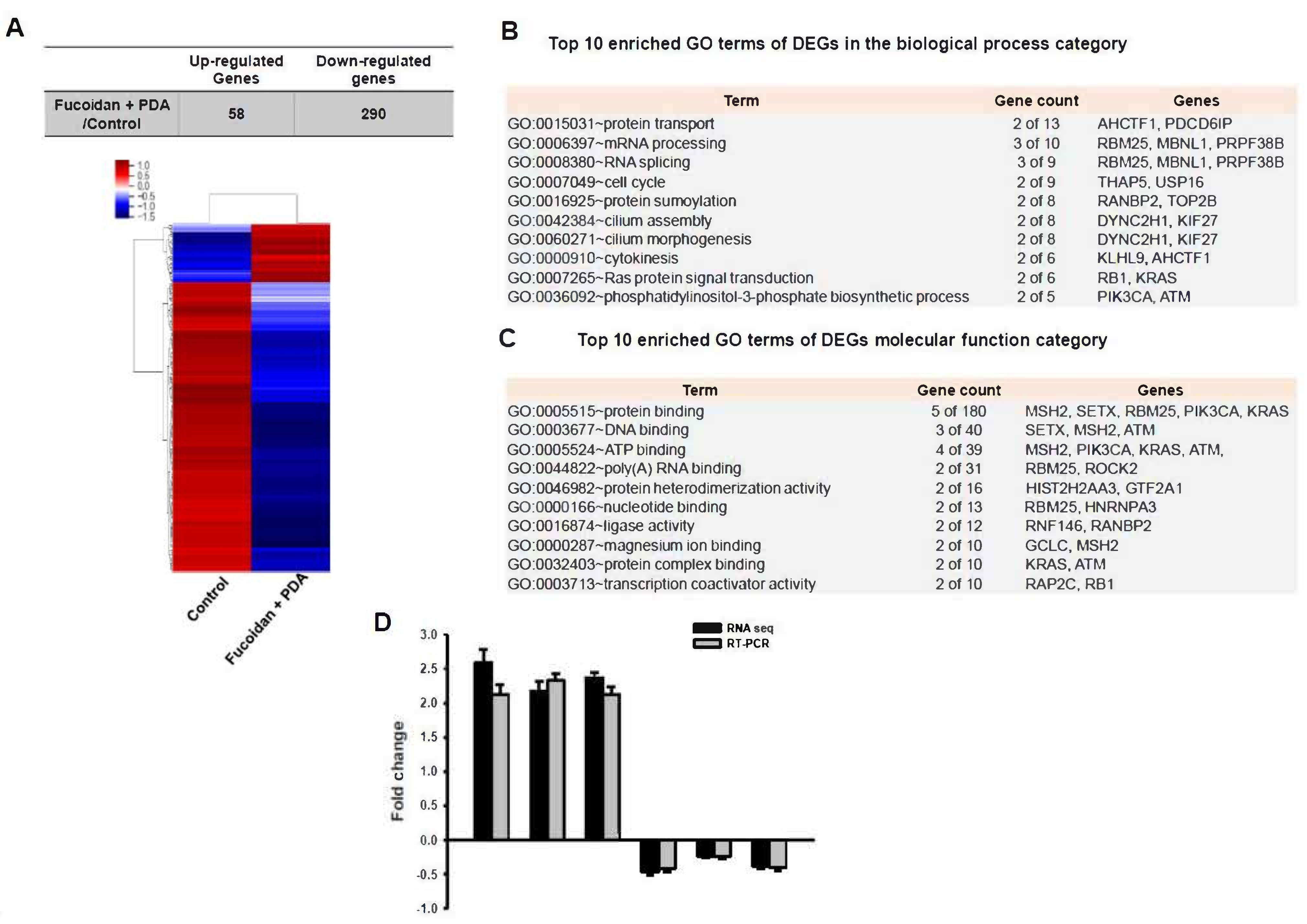
2.2. Identification of Differentially Expressed Genes (DEGs)
2.3. KEGG Pathway Analysis
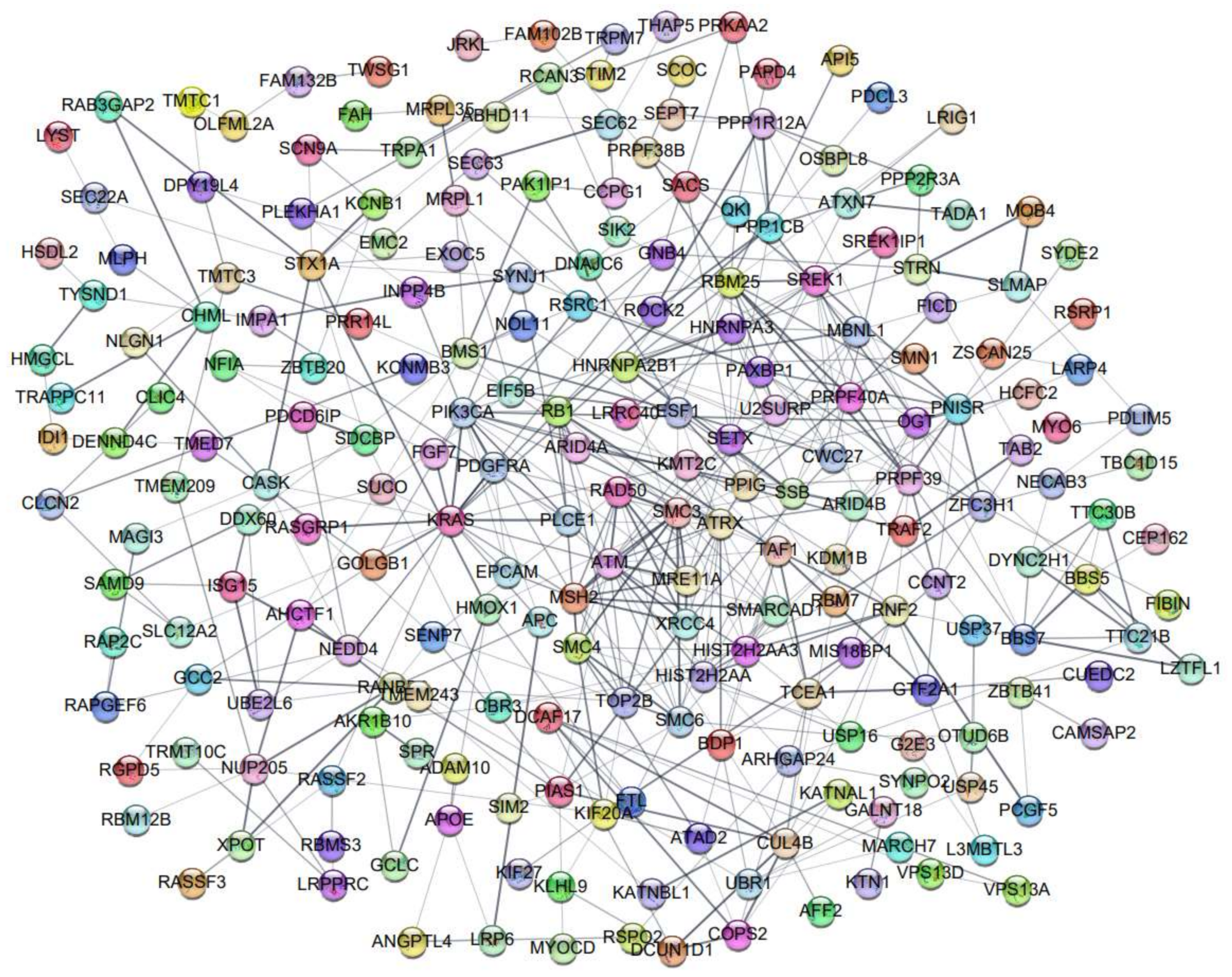
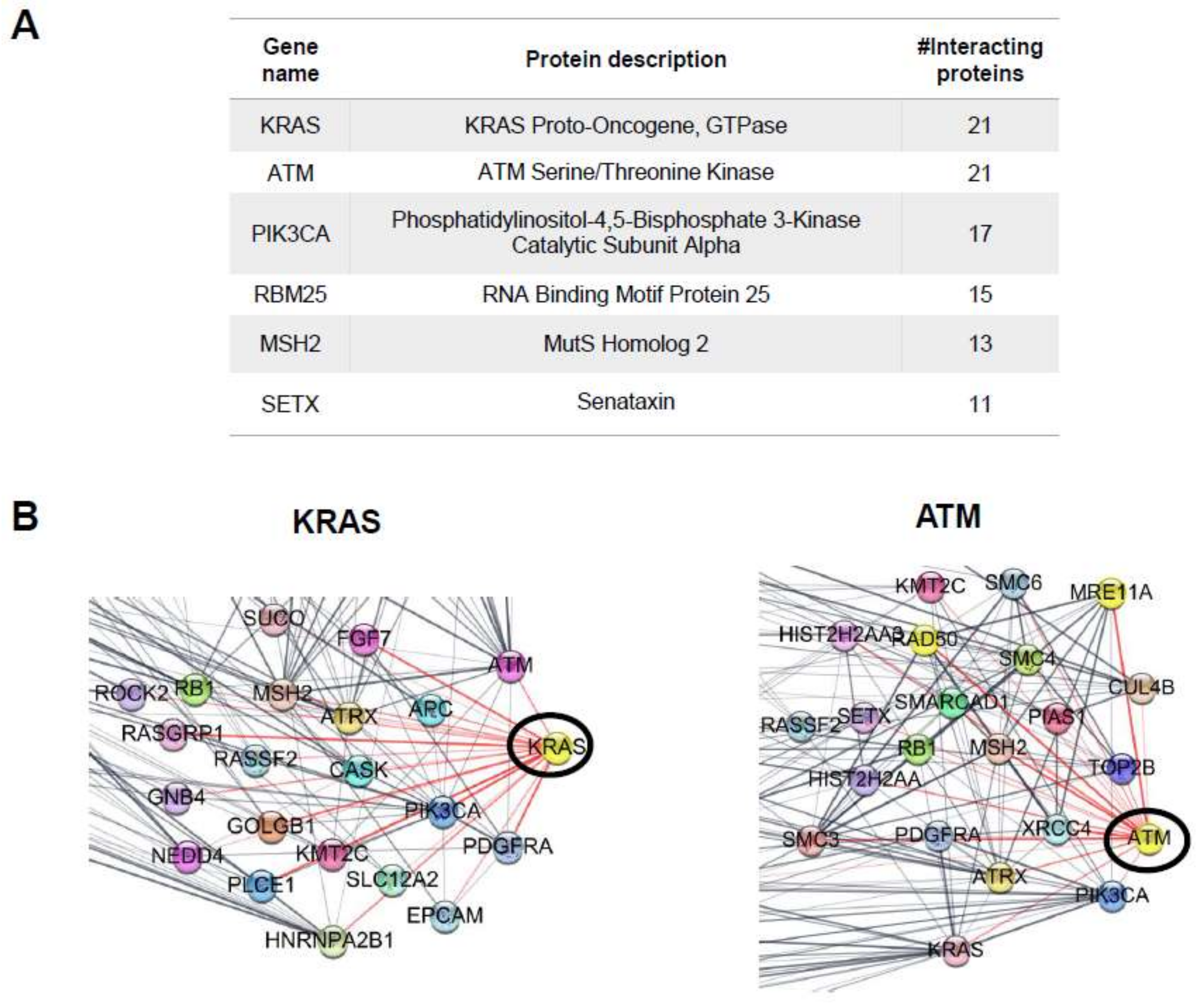
2.4. Protein–Protein Interaction (PPI) Network
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Periodontal Ligament Stem Cell Culture
4.3. Preparation of the Fucoidan/PDA Composite Substrate
4.4. Characterization of the Fucoidan/PDA-Coated Surface
4.5. Alkaline Phosphatase (ALP) Activity
4.6. Intracellular Calcium Quantification Assay
4.7. Alizarin Red S Staining
4.8. RNA Extraction and Real-Time Reverse Transcriptase–Polymerase Chain Reaction (RT-PCR)
4.9. Western Blot Analysis
4.10. Library Preparation and Sequencing
4.11. Data Analysis
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Incesu, E.; Yanikoglu, N. Evaluation of the effect of different polishing systems on the surface roughness of dental ceramics. J. Prosthet. Dent. 2020, 124, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Oladapo, B.I.; Ismail, S.O.; Bowoto, O.K.; Omigbodun, F.T.; Olawumi, M.A.; Muhammad, M.A. Lattice design and 3D-printing of PEEK with Ca10(OH)(PO4)3 and in-vitro bio-composite for bone implant. Int. J. Biol. Macromol. 2020, 165, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Min, J.; Choi, K.Y.; Dreaden, E.; Padera, R.F.; Braatz, R.D.; Spector, M.; Hammond, P.T. Designer Dual Therapy Nanolayered Implant Coatings Eradicate Biofilms and Accelerate Bone Tissue Repair. ACS Nano 2016, 10, 4441–4450. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Yang, P.; Qin, W.; Maitz, M.; Zhou, S.; Huang, N. The effect of coimmobilizing heparin and fibronectin on titanium on hemocompatibility and endothelialization. Biomaterials 2011, 32, 4691–4703. [Google Scholar] [CrossRef]
- Wang, X.; Yan, C.; Ye, K.; He, Y.; Li, Z.; Ding, J. Effect of RGD nanospacing on differentiation of stem cells. Biomaterials 2013, 34, 2865–2874. [Google Scholar] [CrossRef]
- Li, J.; Zhang, K.; Xu, Y.; Chen, J.; Yang, P.; Zhao, Y.; Zhao, A.; Huang, N. A novel coculture model of HUVECs and HUASMCs by hyaluronic acid micropattern on titanium surface. J. Biomed. Mater. Res. Part A 2014, 102, 1950–1960. [Google Scholar] [CrossRef]
- Yang, Z.; Tu, Q.; Wang, J.; Huang, N. The role of heparin binding surfaces in the direction of endothelial and smooth muscle cell fate and re-endothelialization. Biomaterials 2012, 33, 6615–6625. [Google Scholar] [CrossRef]
- Jeon, O.; Powell, C.; Solorio, L.D.; Krebs, M.D.; Alsberg, E. Affinity-based growth factor delivery using biodegradable, photocrosslinked heparin-alginate hydrogels. J. Control. Release 2011, 154, 258–266. [Google Scholar] [CrossRef] [Green Version]
- Ying, H.; Zhou, J.; Wang, M.; Su, D.; Ma, Q.; Lv, G.; Chen, J. In situ formed collagen-hyaluronic acid hydrogel as biomimetic dressing for promoting spontaneous wound healing. Mater. Sci. Eng. C 2019, 101, 487–498. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Q.; Zhang, Z.; Song, H.; Li, P. Potential antioxidant and anticoagulant capacity of low molecular weight fucoidan fractions extracted from Laminaria japonica. Int. J. Biol. Macromol. 2010, 46, 6–12. [Google Scholar] [CrossRef]
- Jeong, Y.; Thuy, L.T.; Ki, S.H.; Ko, S.; Kim, S.; Cho, W.K.; Choi, J.S.; Kang, S.M. Multipurpose Antifouling Coating of Solid Surfaces with the Marine-Derived Polymer Fucoidan. Macromol. Biosci. 2018, 18, e1800137. [Google Scholar] [CrossRef]
- Tian, T.; Chang, H.; He, K.; Ni, Y.; Li, C.; Hou, M.; Chen, L.; Xu, Z.; Chen, B.; Ji, M. Fucoidan from seaweed Fucus vesiculosus inhibits 2,4-dinitrochlorobenzene-induced atopic dermatitis. Int. Immunopharmacol. 2019, 75, 105823. [Google Scholar] [CrossRef]
- Vishchuk, O.S.; Ermakova, S.P.; Zvyagintseva, T.N. Sulfated polysaccharides from brown seaweeds Saccharina japonica and Undaria pinnatifida: Isolation, structural characteristics, and antitumor activity. Carbohydr. Res. 2011, 346, 2769–2776. [Google Scholar] [CrossRef]
- Kalimuthu, S.; Kim, S.K. Fucoidan, A Sulfated Polysaccharides from Brown Algae as Therapeutic Target for Cancer; Springer International Publishing: Cham, Switzerland, 2015; pp. 145–164. ISBN 978-3-319-07144-2. [Google Scholar]
- Pajovich, H.T.; Banerjee, I.A. Biomineralization of Fucoidan-Peptide Blends and Their Potential Applications in Bone Tissue Regeneration. J. Funct. Biomater. 2017, 8, 41. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.S.; Kang, H.J.; Park, J.Y.; Lee, J. Fucoidan promotes osteoblast differentiation via JNK-and ERK-dependent BMP2–Smad 1/5/8 signaling in human mesenchymal stem cells. Exp. Mol. Med. 2015, 47, e128. [Google Scholar] [CrossRef]
- Ohmes, J.; Xiao, Y.; Wang, F.; Mikkelsen, M.D.; Nguyen, T.T.; Schmidt, H.; Seekamp, A.; Meyer, A.S.; Fuchs, S. Effect of Enzymatically Extracted Fucoidans on Angiogenesis and Osteogenesis in Primary Cell Culture Systems Mimicking Bone Tissue Environment. Mar. Drugs 2020, 18, 481. [Google Scholar] [CrossRef]
- Park, S.-J.; Lee, K.W.; Lim, D.-S.; Lee, S. The Sulfated Polysaccharide Fucoidan Stimulates Osteogenic Differentiation of Human Adipose-Derived Stem Cells. Stem Cells Dev. 2012, 21, 2204–2211. [Google Scholar] [CrossRef]
- Lee, J.S.; Jin, G.H.; Yeo, M.G.; Jang, C.H.; Lee, H.; Kim, G.H. Fabrication of electrospun biocomposites comprising polycaprolactone/fucoidan for tissue regeneration. Carbohydr. Polym. 2012, 90, 181–188. [Google Scholar] [CrossRef]
- Huang, Y.-C.; Liu, T.-J. Mobilization of mesenchymal stem cells by stromal cell-derived factor-1 released from chitosan/tripolyphosphate/fucoidan nanoparticles. Acta Biomater. 2012, 8, 1048–1056. [Google Scholar] [CrossRef]
- Lowe, B.; Venkatesan, J.; Anil, S.; Shim, M.S.; Kim, S.K. Preparation and characterization of chitosan-natural nanohydroxyapatite-fucoidan nanocomposites for bone tissue engineering. Int. J. Biol. Macromol. 2016, 93, 1479–1487. [Google Scholar] [CrossRef]
- Bhutani, U.; Laha, A.; Mitra, K.; Majumdar, S. Sodium alginate and gelatin hydrogels: Viscosity effect on hydrophobic drug release. Mater. Lett. 2016, 164, 76–79. [Google Scholar] [CrossRef]
- Voron’koa, N.G.; Derkacha, S.R.; Kuchinaa, Y.A.; Sokolana, N.I.; Kuranovaa, L.K.; Obluchinskaya, E.D. Influence of added gelatin on the rheological properties of a Fucus vesiculosus extract. Food Biosci. 2019, 29, 1–8. [Google Scholar] [CrossRef]
- Mou, C.; Yang, Y.; Bai, Y.; Yuan, P.; Wang, Y.; Zhang, L. Hyaluronic acid and polydopamine functionalized phase change nanoparticles for ultrasound imaging-guided photothermal-chemotherapy. J. Mater. Chem. B 2019, 7, 1246–1257. [Google Scholar] [CrossRef]
- Luo, C.; Liu, W.; Luo, B.; Tian, J.; Wen, W.; Liu, M.; Zhou, C. Antibacterial activity and cytocompatibility of chitooligosaccharide-modified polyurethane membrane via polydopamine adhesive layer. Carbohydr. Polym. 2017, 156, 235–243. [Google Scholar] [CrossRef]
- Ge, J.; Gu, K.; Sun, K.; Wang, X.; Yao, S.; Mo, X.; Long, S.; Lan, T.; Qin, C. Preparation and Swelling Behaviors of High-Strength Hemicellulose-g-Polydopamine Composite Hydrogels. Materials 2021, 14, 186. [Google Scholar] [CrossRef]
- Zhang, M.; Huang, Y.; Pan, W.; Tong, X.; Zeng, Q.; Su, T.; Qi, X.; Shen, J. Polydopamine-incorporated dextran hydrogel drug carrier with tailorable structure for wound healing. Carbohydr. Polym. 2021, 253, 117213. [Google Scholar] [CrossRef]
- Lee, M.; Kim, Y.; Ryu, J.H.; Kim, K.; Han, Y.-M.; Lee, H. Long-term, feeder-free maintenance of human embryonic stem cells by mussel-inspired adhesive heparin and collagen type I. Acta Biomater. 2016, 32, 138–148. [Google Scholar] [CrossRef]
- Wang, M.; Deng, Y.; Zhou, P.; Luo, Z.; Li, Q.; Xie, B.; Zhang, X.; Chen, T.; Pei, D.; Tang, Z.; et al. In Vitro Culture and Directed Osteogenic Differentiation of Human Pluripotent Stem Cells on Peptides-Decorated Two-Dimensional Microenvironment. ACS Appl. Mater. Interfaces 2015, 7, 4560–4572. [Google Scholar] [CrossRef]
- Li, Y.-M.; Wu, J.-Y.; Jiang, J.; Dong, S.-K.; Chen, Y.-S.; He, H.-Y.; Liu, C.-S.; Zhao, J.-Z. Chondroitin sulfate-polydopamine modified polyethylene terephthalate with extracellular matrix-mimetic immunoregulatory functions for osseointegration. J. Mater. Chem. B 2019, 7, 7756–7770. [Google Scholar] [CrossRef]
- Carnes, D.L.; Maeder, C.L.; Graves, D.T. Cells with Osteoblastic Phenotypes Can Be Explanted From Human Gingiva and Periodontal Ligament. J. Periodontol. 1997, 68, 701–707. [Google Scholar] [CrossRef]
- Cho, H.; Tarafder, S.; Fogge, M.; Kao, K.; Lee, C.H. Periodontal ligament stem/progenitor cells with protein-releasing scaffolds for cementum formation and integration on dentin surface. Connect. Tissue Res. 2016, 57, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Kl, V.; Ryana, H.; Dalvi, P.J. Autologous periodontal stem cell assistance in periodontal regeneration technique (SAI-PRT) in the treatment of periodontal intrabony defects: A case report with one-year follow-up. J. Dent. Res. Dent. Clin. Dent. Prospect. 2017, 11, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-S.; Kim, E.; Han, S.; Kang, K.L.; Heo, J.S. Evaluating the oxysterol combination of 22(S)-hydroxycholesterol and 20(S)-hydroxycholesterol in periodontal regeneration using periodontal ligament stem cells and alveolar bone healing models. Stem Cell Res. Ther. 2017, 8, 276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, F.; Akiyama, K.; Liu, Y.; Yamaza, T.; Wang, T.-M.; Chen, J.-H.; Wang, B.B.; Huang, G.T.-J.; Wang, S.; Shi, S. Utility of PDL progenitors for in vivo tissue regeneration: A report of 3 cases. Oral Dis. 2010, 16, 20–28. [Google Scholar] [CrossRef]
- Eleuterio, E.; Trubiani, O.; Sulpizio, M.; Di Giuseppe, F.; Pierdomenico, L.; Marchisio, M.; Giancola, R.; Giammaria, G.; Miscia, S. Proteome of human stem cells from periodontal ligament and dental pulp. PLoS ONE 2013, 8, e71101. [Google Scholar] [CrossRef] [Green Version]
- Manivasagan, P.; Hoang, G.; Moorthy, M.S.; Mondal, S.; Doan, V.H.M.; Kim, H.; Phan, T.T.V.; Nguyen, T.P.; Oh, J. Chitosan/fucoidan multilayer coating of gold nanorods as highly efficient near-infrared photothermal agents for cancer therapy. Carbohydr. Polym. 2019, 211, 360–369. [Google Scholar] [CrossRef]
- Kim, Y.W.; Baek, S.-H.; Lee, S.-H.; Kim, T.-H.; Kim, S.-Y. Fucoidan, a Sulfated Polysaccharide, Inhibits Osteoclast Differentiation and Function by Modulating RANKL Signaling. Int. J. Mol. Sci. 2014, 15, 18840–18855. [Google Scholar] [CrossRef] [Green Version]
- Venkatesan, J.; Bhatnagar, I.; Kim, S.K. Chitosan-alginate biocomposite containing fucoidan for bone tissue engineering. Mar. Drugs. 2014, 12, 300–316. [Google Scholar] [CrossRef]
- Wang, F.; Schmidt, H.; Pavleska, D.; Wermann, T.; Seekamp, A.; Fuchs, S. Crude Fucoidan Extracts Impair Angiogenesis in Models Relevant for Bone Regeneration and Osteosarcoma via Reduction of VEGF and SDF-1. Mar. Drugs 2017, 15, 186. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.-T.; Lu, T.-W.; Chen, C.-H.; Mi, F.-L. Development of genipin-crosslinked and fucoidan-adsorbed nano-hydroxyapatite/hydroxypropyl chitosan composite scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2019, 128, 973–984. [Google Scholar] [CrossRef]
- Case, L.B.; Waterman, C.M. Integration of actin dynamics and cell adhesion by a three-dimensional, mechanosensitive molecular clutch. Nat. Cell Biol. 2015, 17, 955–963. [Google Scholar] [CrossRef]
- Schwarz, U.S.; Gardel, M.L. United we stand–integrating the actin cytoskeleton and cell–matrix adhesions in cellular mechanotransduction. J. Cell Sci. 2012, 125, 3051–3060. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Sun, Q.; Wang, S.; Huo, B. Spreading Shape and Area Regulate the Osteogenesis of Mesenchymal Stem Cells. Tissue Eng. Regen. Med. 2019, 16, 573–583. [Google Scholar] [CrossRef]
- Lu, J.; Fan, Y.; Gong, X.; Zhou, X.; Yi, C.; Zhang, Y.; Pan, J. The Lineage Specification of Mesenchymal Stem Cells Is Directed by the Rate of Fluid Shear Stress. J. Cell. Physiol. 2016, 231, 1752–1760. [Google Scholar] [CrossRef]
- Vishavkarma, R.; Raghavan, S.; Kuyyamudi, C.; Majumder, A.; Dhawan, J.; Pullarkat, P.A. Role of Actin Filaments in Correlating Nuclear Shape and Cell Spreading. PLoS ONE 2014, 9, e107895. [Google Scholar] [CrossRef] [Green Version]
- Han, N.; Jia, L.; Guo, L.; Su, Y.; Luo, Z.; Du, J.; Mei, S.; Liu, Y. Balanced oral pathogenic bacteria and probiotics promoted wound healing via maintaining mesenchymal stem cell homeostasis. Stem Cell Res. Ther. 2020, 11, 1–12. [Google Scholar] [CrossRef]
- Mun, H.; Jeon, T.J. Regulation of actin cytoskeleton by Rap1 binding to RacGEF1. Mol. Cells 2012, 34, 71–76. [Google Scholar] [CrossRef] [Green Version]
- Hoelzle, M.K.; Svitkina, T. The cytoskeletal mechanisms of cell–cell junction formation in endothelial cells. Mol. Biol. Cell 2012, 23, 310–323. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, J.; Li, Y.; Zhou, Y.; Cui, Y.; Yang, G.; Hong, Y. Rap1A Regulates Osteoblastic Differentiation via the ERK and p38 Mediated Signaling. PLoS ONE 2015, 10, e0143777. [Google Scholar] [CrossRef]
- Pan, B.L.; Tong, Z.W.; Li, S.D.; Wu, L.; Liao, J.L.; Yang, Y.X.; Li, H.H.; Dai, Y.J.; Li, J.E.; Pan, L. Decreased microRNA-182-5p helps alendronate promote osteoblast proliferation and differentiation in osteoporosis via the Rap1/MAPK pathway. Biosci. Rep. 2018, 38, BSR20180696. [Google Scholar] [CrossRef] [Green Version]
- Xu, D.; Wan, Y.; Li, Z.; Wang, C.; Zou, Q.; Du, C.; Wang, Y. Tailorable hierarchical structures of biomimetic hydroxyapatite micro/nano particles promoting endocytosis and osteogenic differentiation of stem cells. Biomater. Sci. 2020, 8, 3286–3300. [Google Scholar] [CrossRef] [PubMed]
- Balla, T. Phosphoinositides: Tiny Lipids with Giant Impact on Cell Regulation. Physiol. Rev. 2013, 93, 1019–1137. [Google Scholar] [CrossRef] [PubMed]
- Hemmings, B.A.; Restuccia, D.F. Pi3k-pkb/akt pathway. Cold Spring Harb. Perspect. Biol. 2012, 4, a011189. [Google Scholar]
- Mao, Y.S.; Yin, H.L. Regulation of the actin cytoskeleton by phosphatidylinositol 4-phosphate 5 kinases. Pflügers Arch. J. Physiol. 2007, 455, 5–18. [Google Scholar] [CrossRef]
- Van den Bout, I.; Divecha, N. PIP5K-driven PtdIns(4,5)P2 synthesis: Regulation and cellular functions. J. Cell Sci. 2009, 122, 3837–3850. [Google Scholar] [CrossRef]
- Zhao, X.; Cui, P.; Hu, G.; Wang, C.; Jiang, L.; Zhao, J.; Xu, J.; Zhang, X. PIP5k1β controls bone homeostasis through modulating both osteoclast and osteoblast differentiation. J. Mol. Cell Biol. 2020, 12, 55–70. [Google Scholar] [CrossRef] [Green Version]
- Gong, W.; Zhang, N.; Cheng, G. Rehmannia glutinosa Libosch extracts prevent bone loss and architectural deterioration and enhance osteoblastic bone formation by regulating the IGF-1/PI3K/mTOR pathway in streptozotocin-induced diabetic rats. Int. J. Mol. Sci. 2019, 20, 3964. [Google Scholar] [CrossRef] [Green Version]
- Park, Y.-K.; Heo, S.-J.; Koak, J.-Y.; Park, G.-S.; Cho, T.-J.; Kim, S.-K.; Cho, J. Characterization and Differentiation of Circulating Blood Mesenchymal Stem Cells and the Role of Phosphatidylinositol 3-Kinase in Modulating the Adhesion. Int. J. Stem Cells 2019, 12, 265–278. [Google Scholar] [CrossRef]
- Lang, L.; Hou, Y.; Chen, Y.; Tu, G.; Tao, J.; Yang, D.; Xi, L.; Fu, L.; Sun, K.; Yin, J.; et al. ATM-Mediated Phosphorylation of Cortactin Involved in Actin Polymerization Promotes Breast Cancer Cells Migration and Invasion. Cell. Physiol. Biochem. 2018, 51, 2972–2988. [Google Scholar] [CrossRef]
- Richard, P.; Feng, S.; Tsai, Y.-L.; Li, W.; Rinchetti, P.; Muhith, U.; Irizarry-Cole, J.; Stolz, K.; Sanz, L.A.; Hartono, S.; et al. SETX (senataxin), the helicase mutated in AOA2 and ALS4, functions in autophagy regulation. Autophagy 2021, 17, 1889–1906. [Google Scholar] [CrossRef]
- Shim, N.Y.; Ryu, J.; Heo, J.S. Osteoinductive function of fucoidan on periodontal ligament stem cells: Role of PI3K/Akt and Wnt/β-catenin signaling pathways. Oral Dis. 2021. [Google Scholar] [CrossRef]
- Shim, N.; Heo, J. Performance of the Polydopamine-Graphene Oxide Composite Substrate in the Osteogenic Differentiation of Mouse Embryonic Stem Cells. Int. J. Mol. Sci. 2021, 22, 7323. [Google Scholar] [CrossRef]
- An, S.Y.; Lee, H.J.; Lee, S.C.; Heo, J.S. Supplement of nitric oxide through calcium carbonate-based nanoparticles contributes osteogenic differentiation of mouse embryonic stem cells. Tissue Cell 2020, 66, 101390. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [Green Version]
- Quinlan, A.R.; Hall, I.M. BEDTools: A flexible suite of utilities for comparing genomic features. Bioinformatics 2010, 26, 841–842. [Google Scholar] [CrossRef] [Green Version]
- Gentleman, R.C.; Carey, V.J.; Bates, D.M.; Bolstad, B.; Dettling, M.; Dudoit, S.; Ellis, B.; Gautier, L.; Ge, Y.; Gentry, J.; et al. Bioconductor: Open software development for computational biology and bioinformatics. Genome Biol. 2004, 5, R80. [Google Scholar] [CrossRef] [Green Version]




| Category | Term | Count | Genes |
|---|---|---|---|
| KEGG_PATHWAY | Pathways in cancer | 11 | RB1, PDGFRA, FGF7, APC, MSH1, PIK3CA, ROCK2, GNB4, TRAF2, KRAS |
| KEGG_PATHWAY | Regulation of actin cytoskeleton | 9 | APC, KRAS, ROCK2, ENAH, FGF7, PIK3CA, PDGFRA, PPP1CB, PPP1R12A |
| KEGG_PATHWAY | Rap1 signaling pathway | 7 | KRAS, RAPGEF6, FGF7, MAGI3, PIK3CA, PLCE1, PDGFRA |
| KEGG_PATHWAY | Oxytocin signaling pathway | 6 | PPP1CB, OXTR, PPP1R12A, ROCK2, KRAS |
| KEGG_PATHWAY | Inositol phosphate metabolism | 5 | INPP4B, SYNJ1, PIK3CA, IMPA1, PLCE1 |
| KEGG_PATHWAY | Melanoma | 5 | RB1, PDGFRA, FGF7, PIKC3A, KRAS |
| KEGG_PATHWAY | Phosphatidylinositol signaling system | 5 | INPP4B, SYNJ1, PIK3CA, IMPA1, PLCE1 |
| KEGG_PATHWAY | Mineral absorption | 4 | HMOX1, TRPM7, CLCN2, FTL |
| KEGG_PATHWAY | Colorectal cancer | 4 | APC, MSH2, PIK3CA, KRAS |
| KEGG_PATHWAY | Glioma | 4 | RB1, PDGFRA, PIK3CA, KRAS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwack, K.H.; Ji, J.Y.; Park, B.; Heo, J.S. Fucoidan (Undaria pinnatifida)/Polydopamine Composite-Modified Surface Promotes Osteogenic Potential of Periodontal Ligament Stem Cells. Mar. Drugs 2022, 20, 181. https://doi.org/10.3390/md20030181
Kwack KH, Ji JY, Park B, Heo JS. Fucoidan (Undaria pinnatifida)/Polydopamine Composite-Modified Surface Promotes Osteogenic Potential of Periodontal Ligament Stem Cells. Marine Drugs. 2022; 20(3):181. https://doi.org/10.3390/md20030181
Chicago/Turabian StyleKwack, Kyu Hwan, Ju Young Ji, Borami Park, and Jung Sun Heo. 2022. "Fucoidan (Undaria pinnatifida)/Polydopamine Composite-Modified Surface Promotes Osteogenic Potential of Periodontal Ligament Stem Cells" Marine Drugs 20, no. 3: 181. https://doi.org/10.3390/md20030181
APA StyleKwack, K. H., Ji, J. Y., Park, B., & Heo, J. S. (2022). Fucoidan (Undaria pinnatifida)/Polydopamine Composite-Modified Surface Promotes Osteogenic Potential of Periodontal Ligament Stem Cells. Marine Drugs, 20(3), 181. https://doi.org/10.3390/md20030181






