1. Introduction
Tuberculosis, caused by
Mycobacterium tuberculosis, remains a major infectious disease worldwide. In 2020, the World Health Organization (WHO) had recorded an estimated 10 million cases of tuberculosis and 1.5 million deaths globally [
1]. The current standard 6-month regimen, known as Directly Observed Therapy Short-course (DOTS), was first introduced by the WHO. The regimen administers isoniazid, rifampicin, ethambutol, and pyrazinamide for 2 months, followed by isoniazid and rifampicin for 4 months, and is highly effective against the drug-susceptible
M. tuberculosis [
2]. However, the regimen is long and complex, and its failure leads to relapse and the emergence of drug-resistant varieties, namely multidrug-resistant (MDR) and extensively drug-resistant (XDR) ones. One of the major reasons for the extended chemotherapeutic regimen is the ability of
Mycobacterium tuberculosis to remain in a dormant state [
3]. Furthermore, only three new anti-tuberculosis drugs, bedaquiline (TMC207), delamanid (OPC-67683), and pretomanid (PA-824), have been developed and approved in the past 50 years. In addition, there already exist several cases that are resistant to both bedaquiline and delamanid treatment [
4,
5]. Therefore, the discovery of new lead compounds targeting the mycobacteria would be urgently required to overcome and avoid drug resistance.
The marine environment is a rich source of drug leads, owing to its chemical and biological diversity. Bioactive compounds from marine organisms are also useful tools for identifying novel drug targets. Due to such biodiversity, more than 15 marine-derived drugs have already been approved, and more than 30 compounds are currently in clinical trials [
6]. To date, we have explored marine medicinal resources for anti-mycobacterial substances using the fast-growing
Mycobacterium smegmatis and the slow-growing
Mycobacterium bovis BCG that are highly homologous to
Mycobacterium tuberculosis. Moreover, we induced the dormancy response, such as isoniazid tolerance, using hypoxic culture conditions in
M. smegmatis and
M. bovis BCG, and used it for screening the anti-dormant mycobacterial substances. Previously, we had isolated some aaptamine-class alkaloids from the Indonesian marine sponge
Aaptos sp. as anti-dormant mycobacterial substances [
7,
8]. Among them, 3-(phenethylamino)demethyl(oxy)aaptamine (PDOA,
1) was found to exhibit a potent anti-microbial activity on
M. bovis BCG under both actively growing aerobic conditions and dormancy-inducing hypoxic conditions. We succeeded in the total synthesis of compound
1 and clarified the effectiveness of the latter against
M. tuberculosis, including the clinically isolated MDR and XDR strains [
8]. Therefore, PDOA may be a new lead compound for an anti-tuberculosis drug. However, the detailed mode of action and target molecule of compound
1 remain unclear.
A number of methods for identifying target molecules have been reported so far. Among them, an analysis method of target molecules utilizing the synthesized probe molecule is one of the useful methods. For instance, nybomycin, isolated as an anti-dormant mycobacterial substance from marine-derived
Streptomyces sp., was shown to bind to the mycobacterial genome [
9]. Dictyoceratin-C was found to target RNA polymerase II-associated protein 3 (RPAP3) and inhibit the growth of cancer cells selectively under hypoxic conditions [
10]. These target molecules were identified using the synthetic probe molecule of nybomycin and dictyoceratin-C.
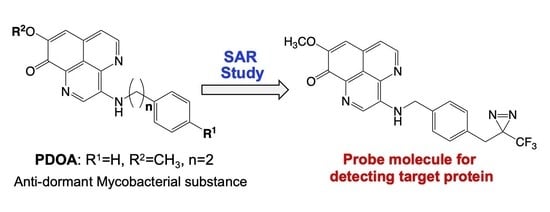
In this paper, the structure–activity relationship of compound 1 was investigated in order to create a probe molecule for detecting its target. We then evaluate the function of the compound having a photoaffinity group and exhibiting antimicrobial activity as a probe molecule.
2. Results and Discussion
2.1. Synthesis of PDOA Analogues and Probe Molecules
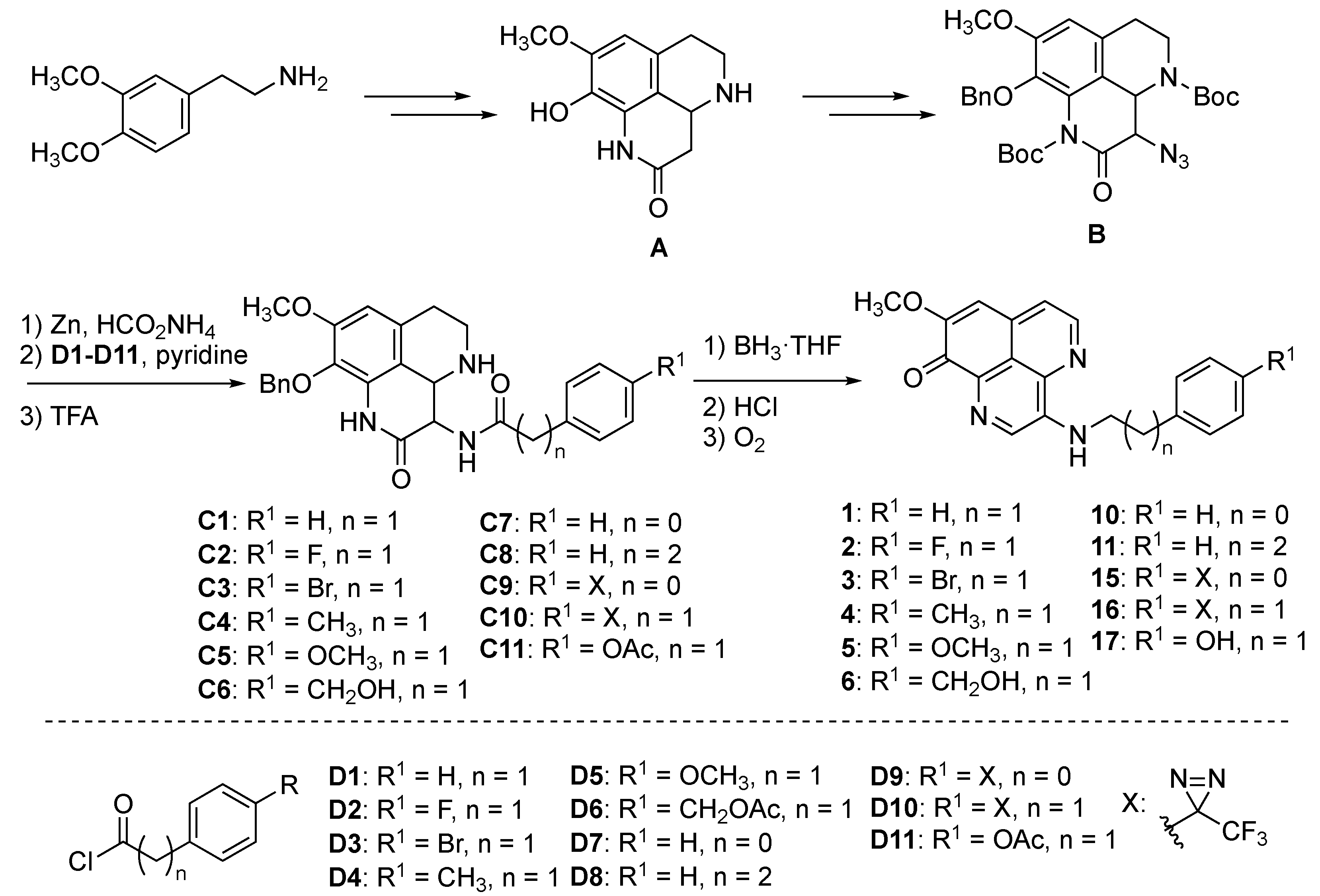
First, we conducted the synthesis of PDOA analogues by modifying its side chain. Previously, we had accomplished the total synthesis of
1, the outline of which is depicted in
Scheme 1 [
8]. Starting with a known tricyclic intermediate
A [
11], an azide group was installed at C-3 position of
A to produce an intermediate
B. Then, the phenethylamino side chain was attached through reduction of the azide group and subsequent acylation using acyl chloride
D1 (intermediate
C1: R
1 = H, n = 1); the final one-pot sequential transformations, i.e., reduction of amide moieties by BH
3/hydrolysis of the resulting amine-borane complex/oxidative aromatization using O
2, produced the desired compound
1. Applying the above synthetic method with the corresponding acyl chlorides
D2–
D8, we could prepare the objective PDOA analogues (
2–
6,
10,
11) (
Figures S1–S14). Compounds
15 and
16, having a trifluoromethyl diazirine group for the photoaffinity labeling of the target protein, were also synthesized in the same manner as above (
Figures S15–S18).
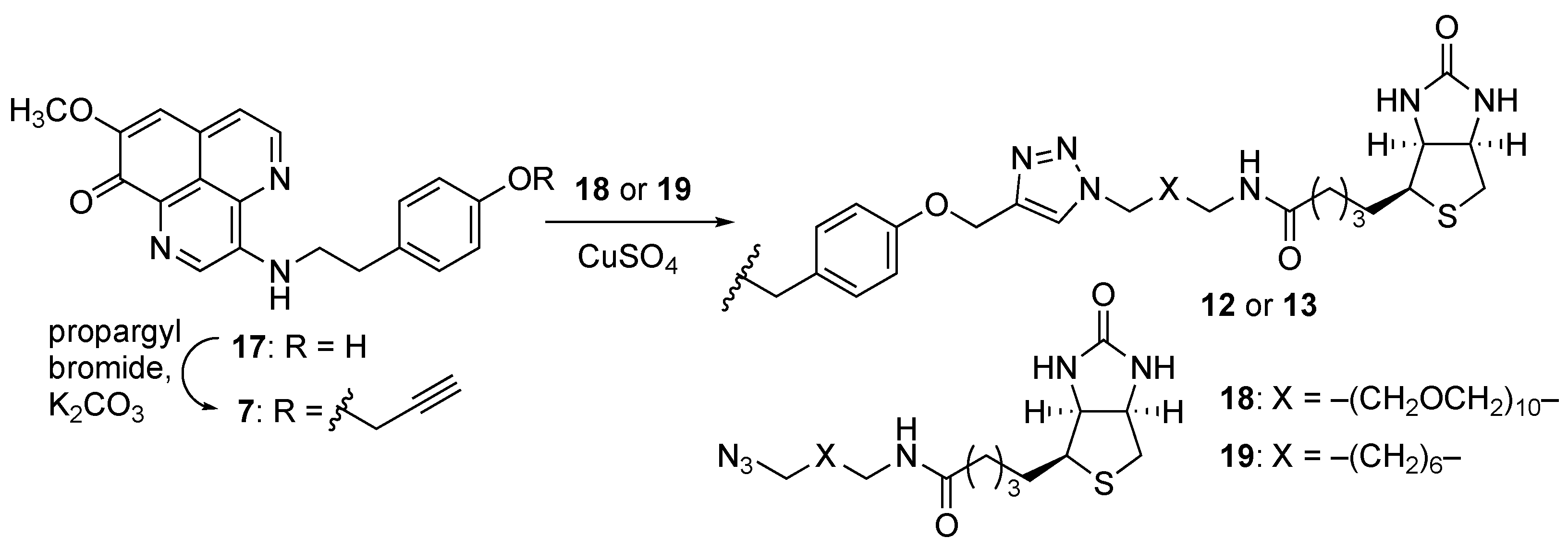
The biotinylated probes
12 and
13 were prepared as shown in
Scheme 2. The compound
17 with the 4-hydroxyphenethyl side chain was prepared through the same procedure as above by using the acyl chloride
D11, and then reacted with propargyl bromide to produce an
O-propargyl analogue
7 (
Figures S19–S22). Thereafter, Cu(I)-catalyzed Huisgen reaction with polyethylene glycol (PEG)- or alkyl chain-tethered biotin
18 or
19 produced the compounds
12 or
13, respectively (
Figures S23 and S24).
Another biotinylated probe
14 was synthesized through the modification of substituent R
2 of
1 (
Scheme 3). The cleavage of the methyl ether at C-8 position of
1 using BBr
3 produced the 8-OH analogue
8 (
Figures S25 and S26). The subsequent propargylation and Huisgen reaction with PEG-tethered biotin
12 produced the desired probe molecule
14 (
Figure S29).
2.2. Antimicrobial Activity of Compounds on M. bovis BCG
In order to understand the structure–activity relationship of 3-(phenethylamino)demethyl(oxy)aaptamine (PDOA,
1) as an anti-dormant mycobacterial substance, we evaluated the antimicrobial activity of synthesized analogues (
2–
16) against
M. bovis BCG under both active growing aerobic conditions and dormancy-inducing hypoxic conditions (
Figure 1 and
Table 1).
The antimicrobial activity of compounds that were modified in the substituent R1, to halogen (2 and 3), electron-donating group (4, 5 and 7), or hydroxymethyl group (6), was evaluated next. The results show that the halogen and electron-donating groups do not affect the activity and rather retain almost the same antimicrobial activity as of the natural product. Interestingly, the activity of compound 3, with Br in R1 position, slightly increased against M. bovis BCG, under hypoxic conditions. On the other hand, the antimicrobial activity was reduced in case of the analogue 6 with the hydroxymethyl group. We next examined the effect of the length of the methylene chain. The antimicrobial activity of compound 10, in which the length of methylene chain was shortened from two to one, was decreased. The activity of compound 11 with three methylene chains was slightly decreased against M. bovis BCG in aerobic conditions; however, there was no significant difference with that of the natural product. We further investigated the effect of the substituent R2 on the antimicrobial activity. The results show the modification of R2 to have a significant effect on the activity; the latter was significantly reduced in compounds 8, 9, and 14. Therefore, we decided to utilize the R1 position to prepare the probe molecule for 1. Accordingly, we first synthesized the biotinylated probes (12 and 13) by utilizing compound 7, and we investigated the antimicrobial activity. Unfortunately, the compounds did not show activity up to 200 µM. Thus, we next synthesized the compounds 15 and 16, in which the trifluoromethyl diazirine group was introduced in R1 position, and we evaluated their antimicrobial activity. Both compounds showed the MIC values of 1.5 µM, same as that of the natural product, against M. bovis BCG under hypoxic conditions, whereas the activities of compounds 15 and 16 against M. bovis BCG under aerobic conditions slightly decreased, with MIC values of 6.25 and 12.5 µM, respectively. Unexpectedly, compound 15, having one methylene chain, showed more potent activity compared to compound 16 having the same methylene length as the natural product; the activity of compound 10 was reduced compared to that of the natural product (1).
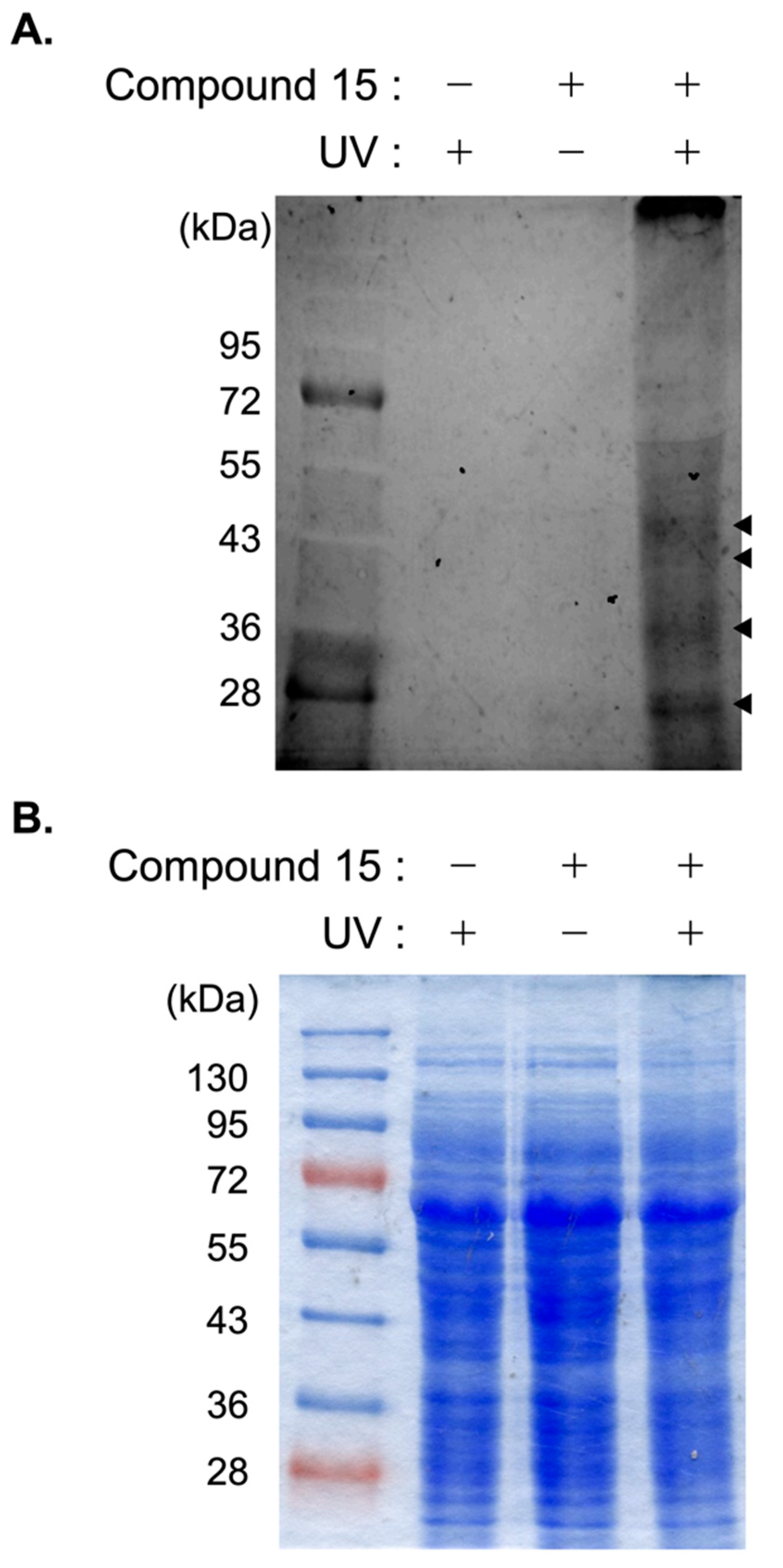
2.3. Functional Evaluation of Compound 15 as a Probe Molecule
The aaptamine-class alkaloids are known to be fluorescent [
12,
13]. Moreover, compound
15, with trifluoromethyl diazirine moiety at R
1, retained antimicrobial activity against
M. bovis BCG under both aerobic and hypoxic conditions with MIC values of 6.25 and 1.56 µM, respectively. Therefore, we speculated that the target protein of compound
1 could be clarified by preparing a covalent bond between compound
15 and target proteins, followed by the detection of fluorescence from compound
15. We evaluated the function of compound
15 as a probe molecule for detecting the target protein of compound
1. As shown in
Figure 2, three lanes on the acrylamide gel were loaded with the same amounts of protein. No visible band was detected in the fluorescence image under the condition of absence of compound
15 or absence of UV irradiation. However, compound
15 was found to covalently bind to particular proteins under UV-irradiated conditions, which could be detected as four major bands (
Figure 2). Although further studies would be necessary for determining the appropriate experimental conditions, the current result strongly suggested compound
15 as a probe molecule that can identify the target protein of compound
1.
3. Materials and Methods
3.1. General
The following instruments were used to obtain physical data: a JEOL ECA-500 (1H-NMR: 500 MHz, 13C-NMR: 125 MHz) spectrometer for 1H and 13C NMR data, using tetramethylsilane as an internal standard; a JASCO FT/IR-5300 infrared spectrometer for IR spectra; a Waters Q-Tof Ultima API mass spectrometer for ESI-TOF MS; and a Hitachi L-6000 pump equipped with Hitachi L-4000H UV detector for HPLC Silica gel (Kanto 40-100 μm, Nacalai COSMOSIL 75C18-OPN) and pre-coated thin layer chromatography (TLC) plates (Merck 60F254, Merck 60RP-18 WF254S) were used for column chromatography and TLC, respectively. Spots on the TLC plates were detected by spraying with an acidic p-anisaldehyde solution (p-anisaldehyde: 25 mL, c-H2SO4: 25 mL, AcOH: 5 mL, EtOH: 425 mL) or with a phosphomolybdic acid solution (phosphomolybdic acid: 25 g, EtOH: 500 mL) with subsequent heating. Unless otherwise noted, all reactions were performed under a N2 atmosphere. After the workup, the organic layers were dried over anhydrous Na2SO4. Chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA) or Kishida Chemical Co., Ltd. (Osaka, Japan).
3.2. Bacterial Culture
Mycobacterium bovis BCG Pasteur was grown in Middlebrook 7H9 broth (BD, Franklin lakes, NJ, USA) containing 10% OADC (BD), 0.5% glycerol, and 0.05% Tween 80, or on Middlebrook 7H10 agar (BD) containing 10% OADC and 0.5% glycerol.
3.3. Antimicrobial Activity of the Compounds under Aerobic and Hypoxic Conditions
The minimum inhibitory concentrations (MICs) against
M. bovis BCG Pasteur were determined using the established MTT method [
14]. The samples were dissolved in DMSO, and the activity of the samples was evaluated by preparing samples in 2-fold dilution series from 200 uM (final concentration). The mid-log phase of
M. bovis BCG (1 × 10
5 CFU/0.1 mL) was inoculated in a 96-well plate, and the serially diluted sample was added to the 96-well plate. In case of aerobic conditions, bacteria were incubated at 37 °C for 7 days. Alternatively, the hypoxic model was established based on the protocol of Rustad et al., with minor modifications [
15]. The mycobacterial bacilli were grown in Middlebrook 7H9 broth at 37 °C under a nitrogen atmosphere containing 0.2% oxygen until the optical density at 600 nm reached 0.8. Subsequently, the bacilli were inoculated in a 96-well plate at the same density under aerobic conditions and incubated at 37 °C under a nitrogen atmosphere containing 0.2% oxygen for 14 days. After incubation, an aliquot (50 µL) of MTT solution (0.5 mg/mL) was added to each well and incubated at 37 °C for an additional 12 h under aerobic or hypoxic condition. The optical density at 560 nm was then measured to determine the MIC value. The reproducibility of data was confirmed by three independent experiments.
3.4. Synthesis
3.4.1. General Procedure for the Synthesis of PDOA Analogues
Procedure A
HCO2NH4 (10 mmol) and Zn powder (2 mmol) were added to a solution of intermediate B (1 mmol) in CH2Cl2/MeOH (2:1, 10 mL), and the whole mixture was stirred for 5 h. The mixture was filtered through a pad of Celite, and the filtrate was concentrated in vacuo. The residue was dissolved in H2O and then basified with 28% aq. NH3. The whole mixture was subsequently extracted with CHCl3. The removal of solvent from the CHCl3 extract under reduced pressure gave a crude amine, which was used for the next reaction without further purification.
Pyridine (10 mmol) and acyl chloride D (1.1 mmol) were added to a solution of the above crude amine in CH2Cl2 (10 mL) and the whole mixture was stirred for 20 h at rt. Sat. NaHCO3 aq. was added to the mixture at 0 °C, and the whole mixture was extracted with CH2Cl2. The removal of solvent from the CH2Cl2 extract under reduced pressure gave a crude product, which was used for the next reaction without further purification.
TFA (4.0 mL, 50 mmol) was added to a solution of the above product in CH2Cl2 (10 mL) at 0 °C, and the whole mixture was stirred for 2 h at rt. The solvent was removed from the mixture under reduced pressure; the residue was dissolved in H2O and then basified with 28% aq. NH3. The whole mixture was extracted with CHCl3. The removal of solvent from the CHCl3 extract under reduced pressure gave a crude product, which was purified using a SiO2 column (CHCl3/MeOH = 20:1 containing 1% Et3N to 5:1 containing 1% Et3N) to give the corresponding intermediate C.
Procedure B
A BH3·THF complex (0.8 mL of a 1.0 M solution in THF, 0.8 mmol, 8.0 equiv.) was added to a solution of the intermediate C (0.1 mmol) in anhydrous THF (1 mL) and the whole mixture was stirred at 45 °C for 2 h. MeOH (2 drops) and 6 M aq. HCl were added to the mixture at rt, and the whole mixture was stirred under O2 atmosphere at 85 °C for 24 h. The mixture was washed with Et2O, and the H2O layer was basified with 4 M aq. NaOH. CHCl3 was added to the mixture, and the whole mixture was extracted with CHCl3-MeOH (10:1). The removal of solvent from the CHCl3 extract under reduced pressure gave a crude product, which was purified by SiO2 column (CHCl3/MeOH = 30:1 + 1% Et3N) and ODS column chromatography (70% MeOH) to produce the corresponding PDOA analogues.
3.5. Functional Evaluation of Compound 15 as a Probe Molecule
A culture of M. bovis BCG (500 mL, OD600 = 1.0) was centrifuged at 8000× g for 20 min. The mycelium was sonicated in 50 mM Tris-HCl (pH 7.4), supplemented with 0.05% Tween 80, and centrifuged (15,000× g, 30 min). The protein concentration of the supernatant was adjusted to 1 mg/mL and used for the functional evaluation of compound 15.
Compound 15 (50 nmol) was added to 1 mg/mL of lysate, and the mixture was incubated at 4 °C for 1 h. After UV irradiation (365 nm) for 10 min on ice, 150 µL of portion was mixed with 600 µL of cold acetone to precipitate the protein in lysate. After centrifugation (15,000× g, 60 min), the precipitant was mixed with 1× SDS sample buffer and subjected to SDS-PAGE. Based on the fluorescence of compound 15 (Ex 505 nm, Em 555 nm), compound-15-labeled proteins were detected by an Image Quant LAS4010 Scanner (GE Healthcare Life Sciences, Buckinghamshire, UK). To detect whole proteins, the gel was stained by Coomassie Brilliant Blue (CBB).
4. Conclusions
Compound 1 (PDOA) is considered to be a new lead compound for anti-tuberculosis drug, although its detailed mode of action and target molecule still remain unclear. Pull-down assays using synthesized probe molecules are highly useful methods for analyzing target molecules of bioactive natural products. To prepare the probe molecule for detecting the target protein of 1, we investigated the structure–activity relationship of 1 using the synthetic analogues 2–16. The tolerance of the functional group at R2 position was found to be low, and OCH3 at R2 position was revealed to be important for retaining the activity. On the other hand, the tolerance of the functional group at R1 position was higher than that at R2 position. Based on the information of the structure–activity relationship, we succeeded in creating compound 15 with a photoaffinity group that retained the antimicrobial activity of 1. We also investigated its function as a probe molecule using its fluorescence properties as an indicator; four bands, derived from the lysate of M. bovis BCG, could be detected using compound 15. Further studies, such as competition experiment with compound 1 to identify the target protein, are under progress.