Novel Antioxidant Peptides from Crassostrea Hongkongensis Improve Photo-Oxidation in UV-Induced HaCaT Cells
Abstract
:1. Introduction
2. Results
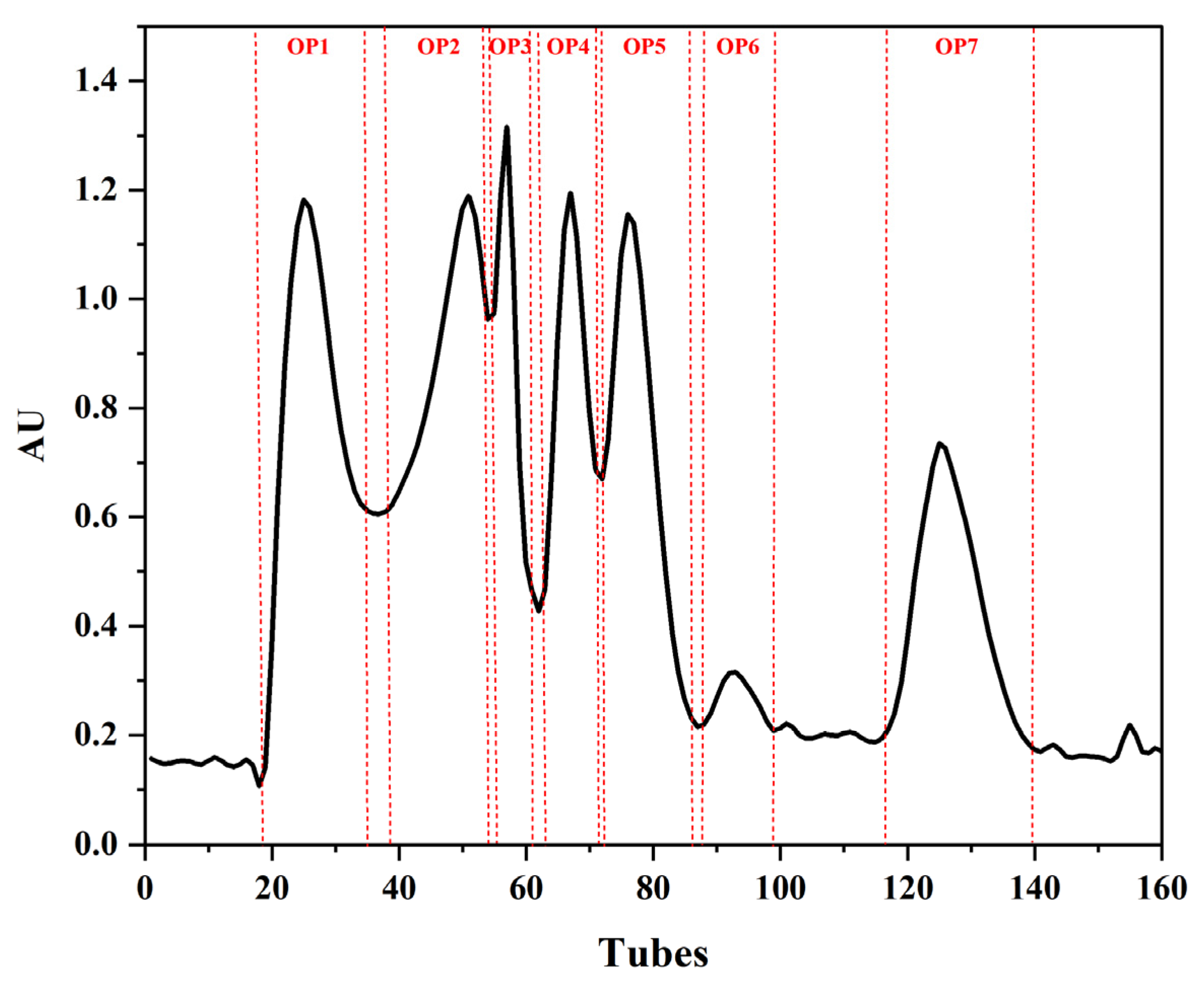
2.1. Isolation and Screening of Active Fractions from OUP (3~5 kDa)
2.2. Cell Viability
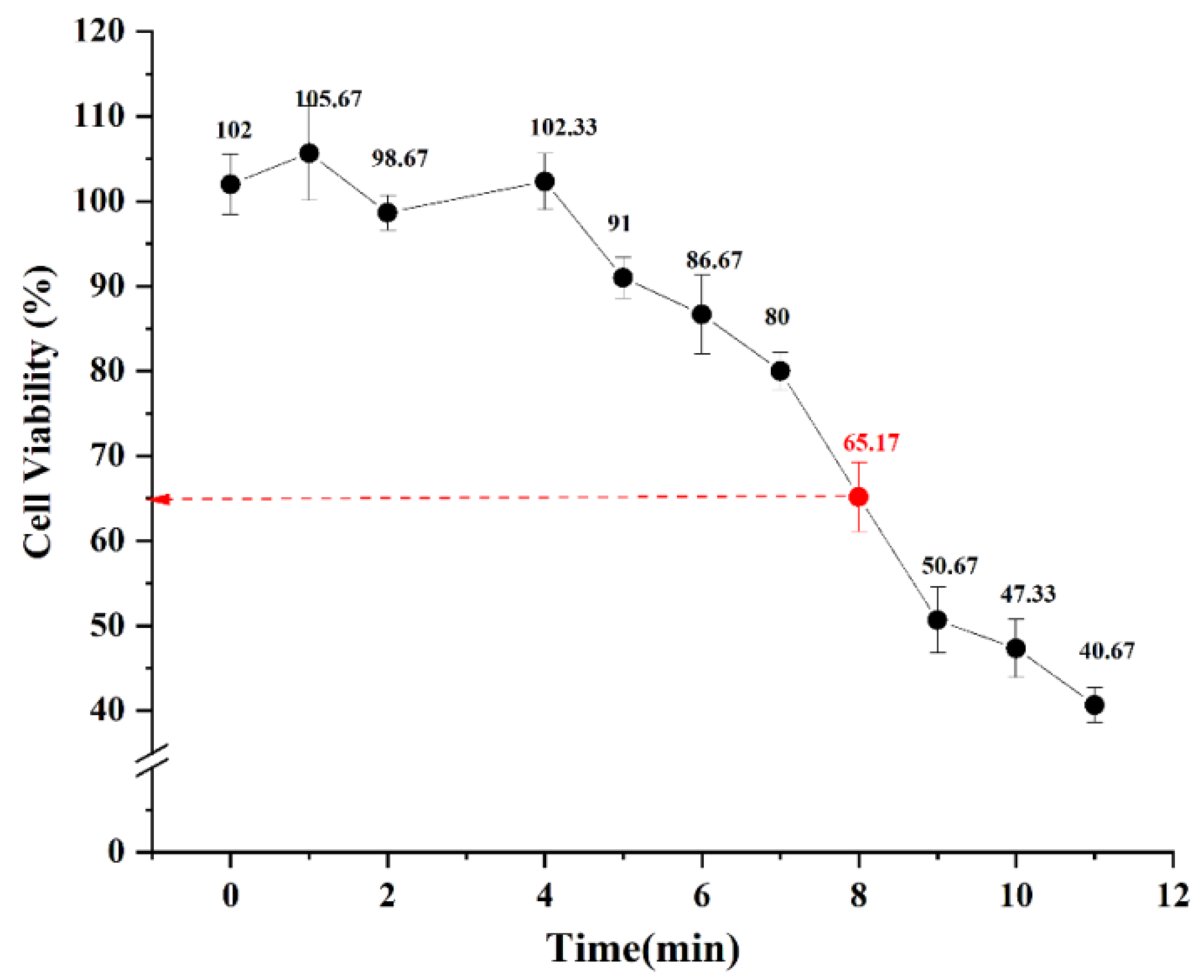
2.2.1. Viability of HaCaT Cells after UV-Irradiation
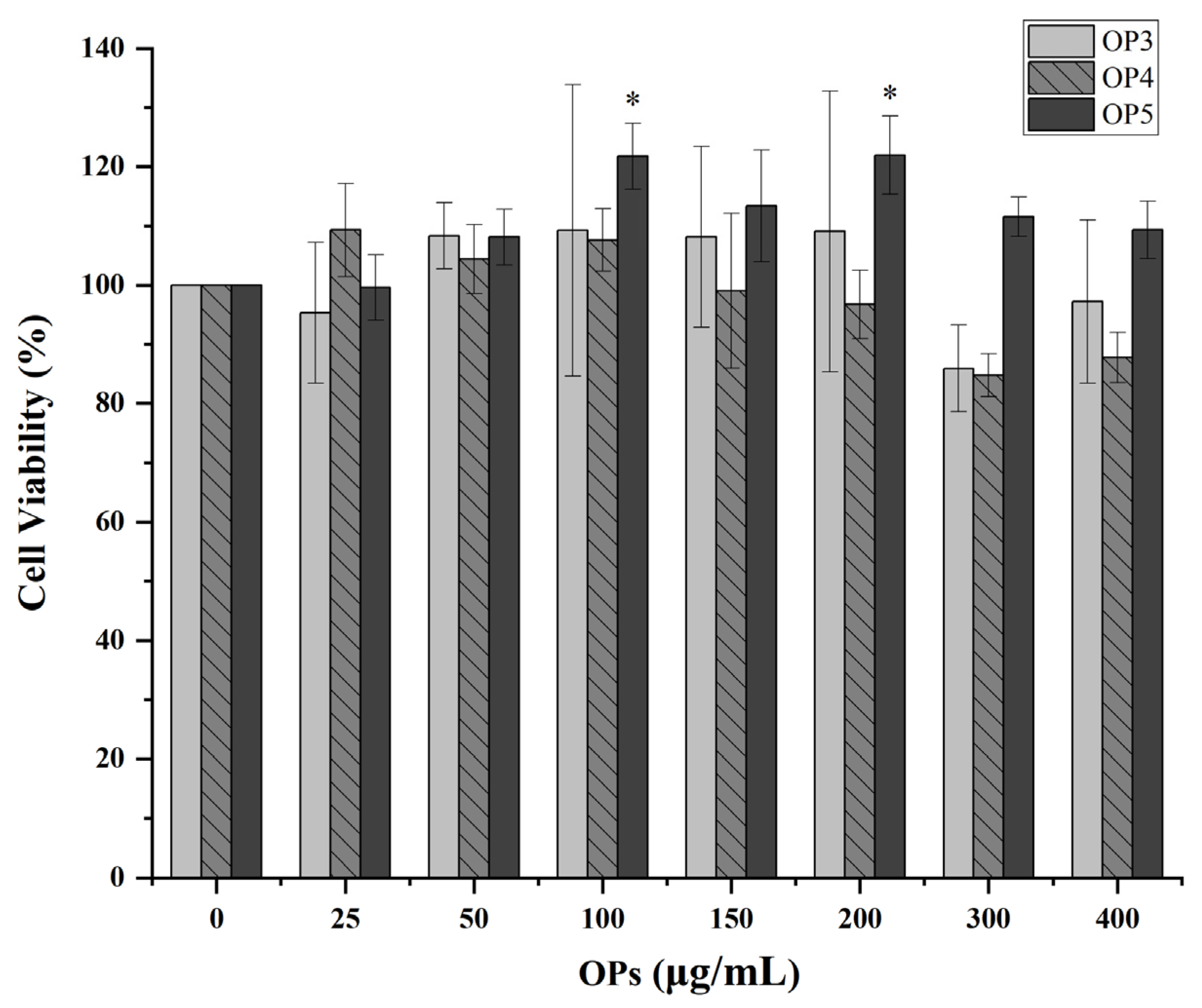
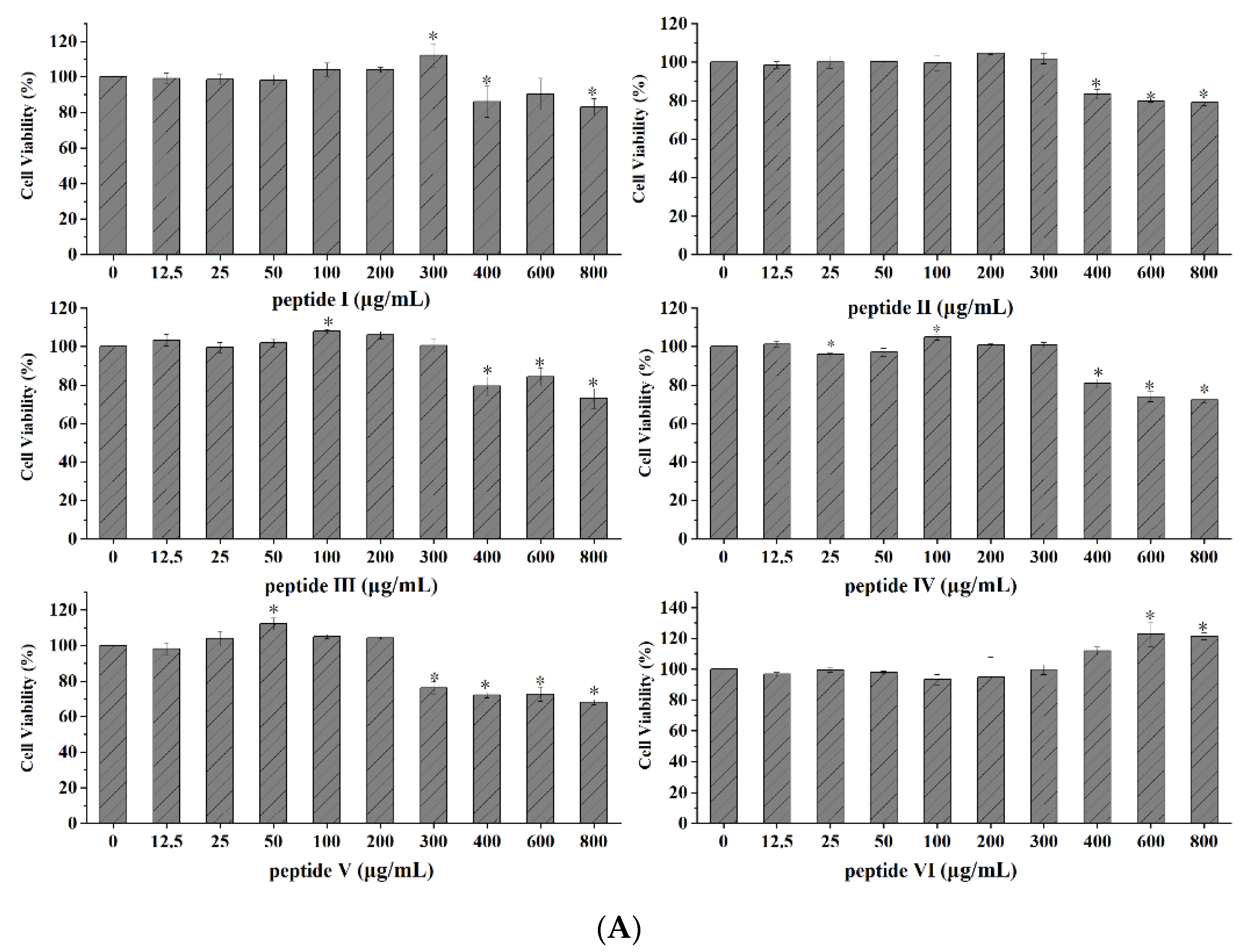
2.2.2. Evaluation of OPs Cytotoxicity
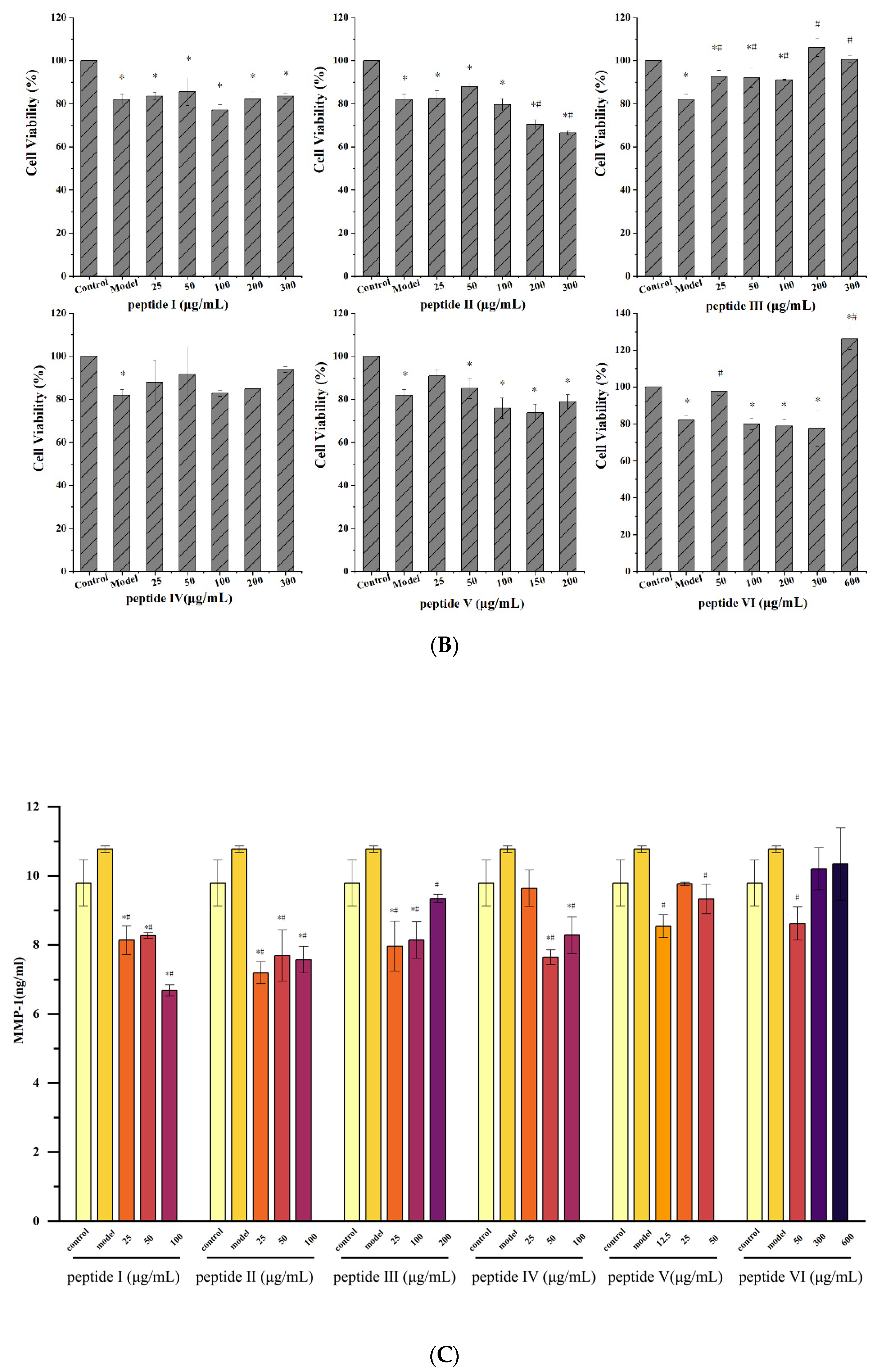
2.2.3. Effect of UV-Irradiation on HaCaT Cell Viability after Pretreatment of OPs
2.3. Inhibition of Reactive Oxygen Species (ROS) Generation by OPs
2.4. Effects of OPs on Malondialdehyde (MDA) Level and Superoxide Dismutase (SOD) Activity
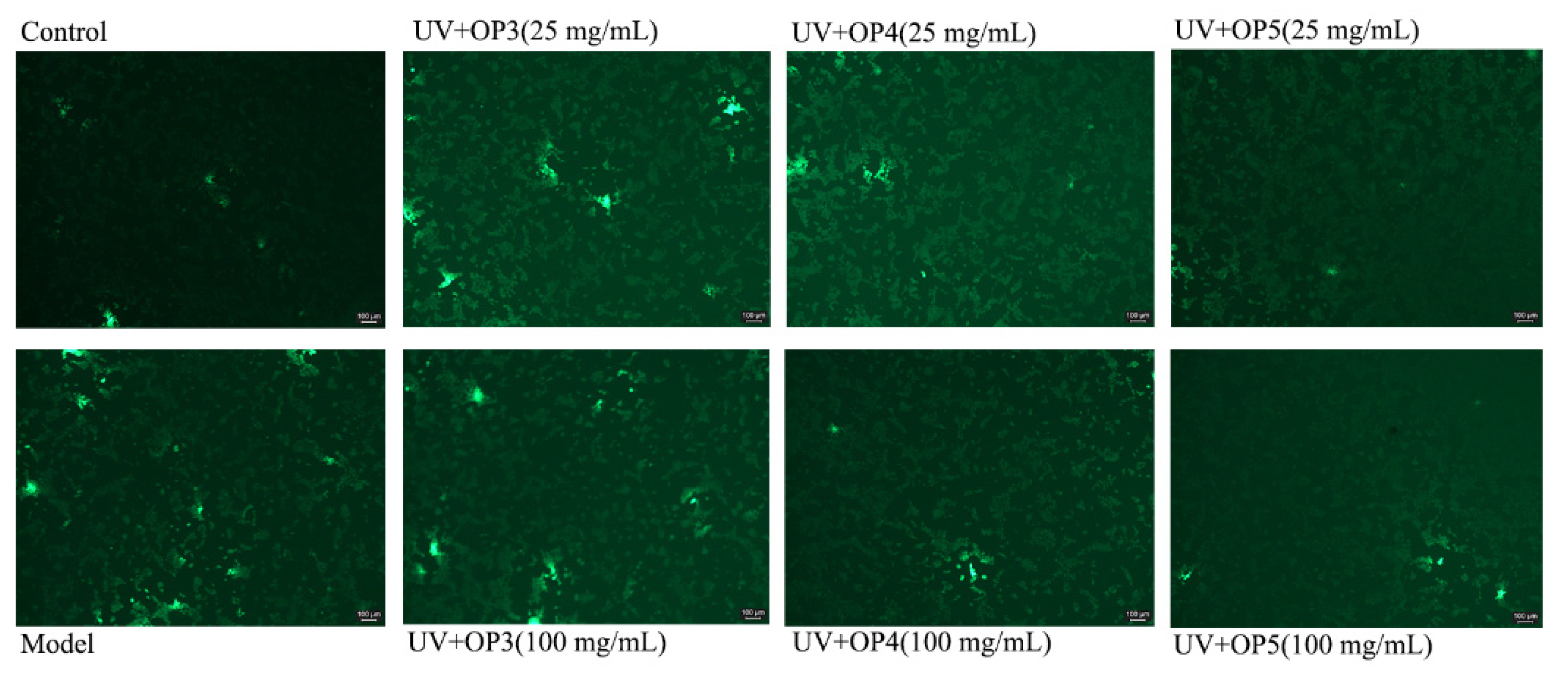
2.5. β-Galactosidase (SA-β-Gal) Staining
2.6. Effect of Pretreatment for OPs on the Expression of Aminoterminal Propeptide of Type I Procollagen (PINP)
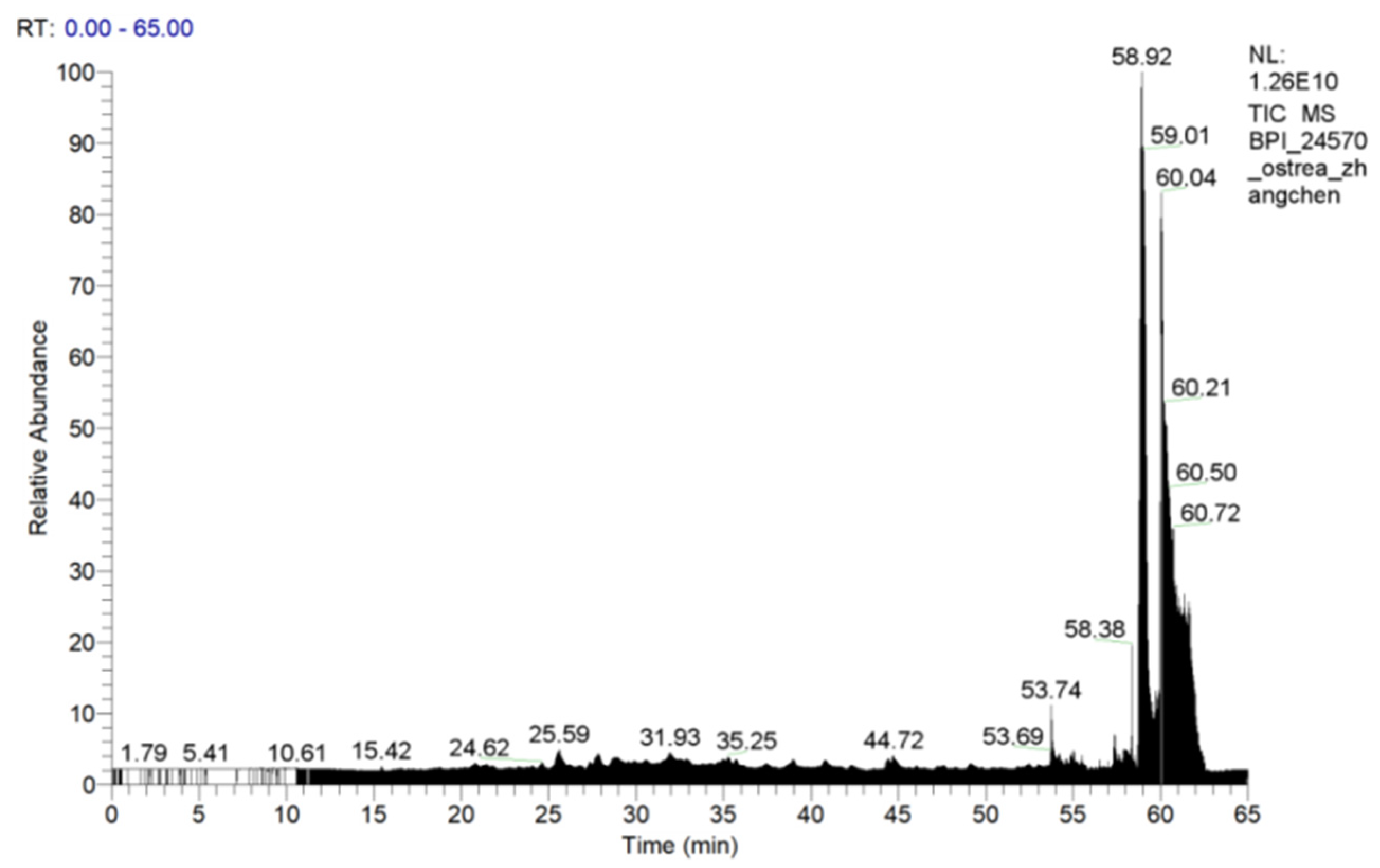
2.7. Purification of OP5 by RT-HPLC
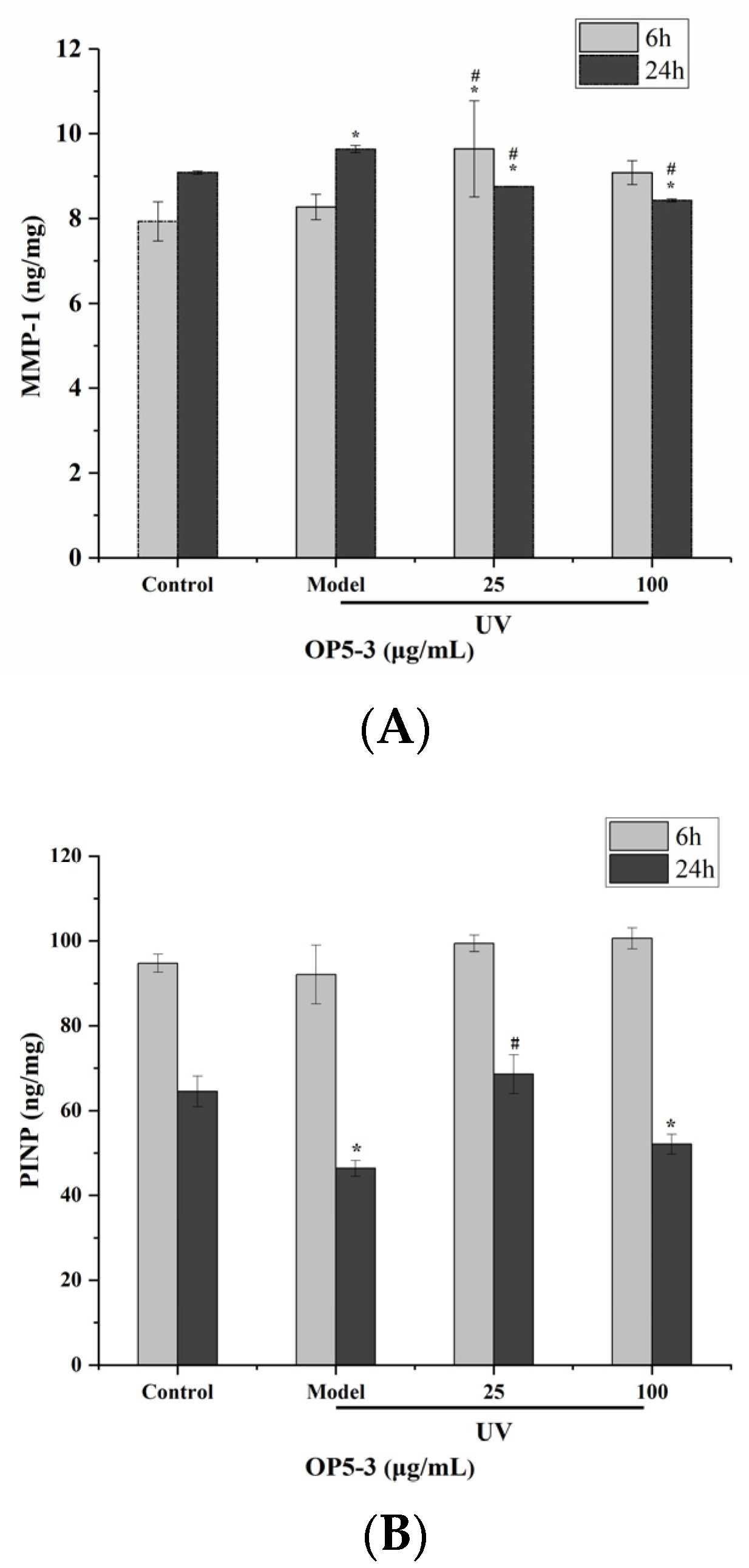
2.8. Effect of Pretreatment with OP5-3 on the Expressions of PINP and MMP-1
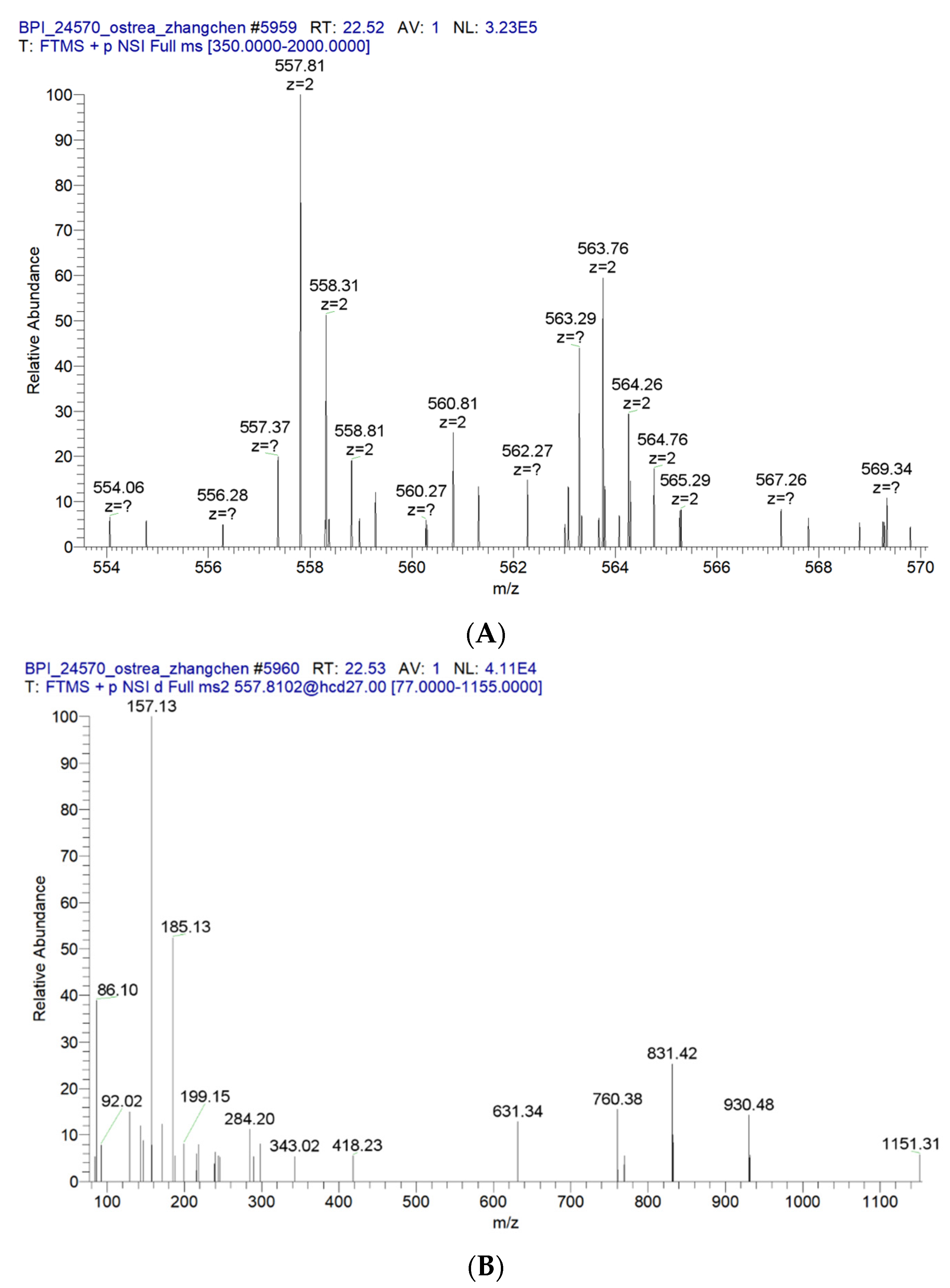
2.9. The Main Peptide Sequences of OP5-3 Were Identified by Mass Spectroscopy
2.10. Verification of the Anti-Photoaging Activity of Synthetic Peptides in HaCaT Cells
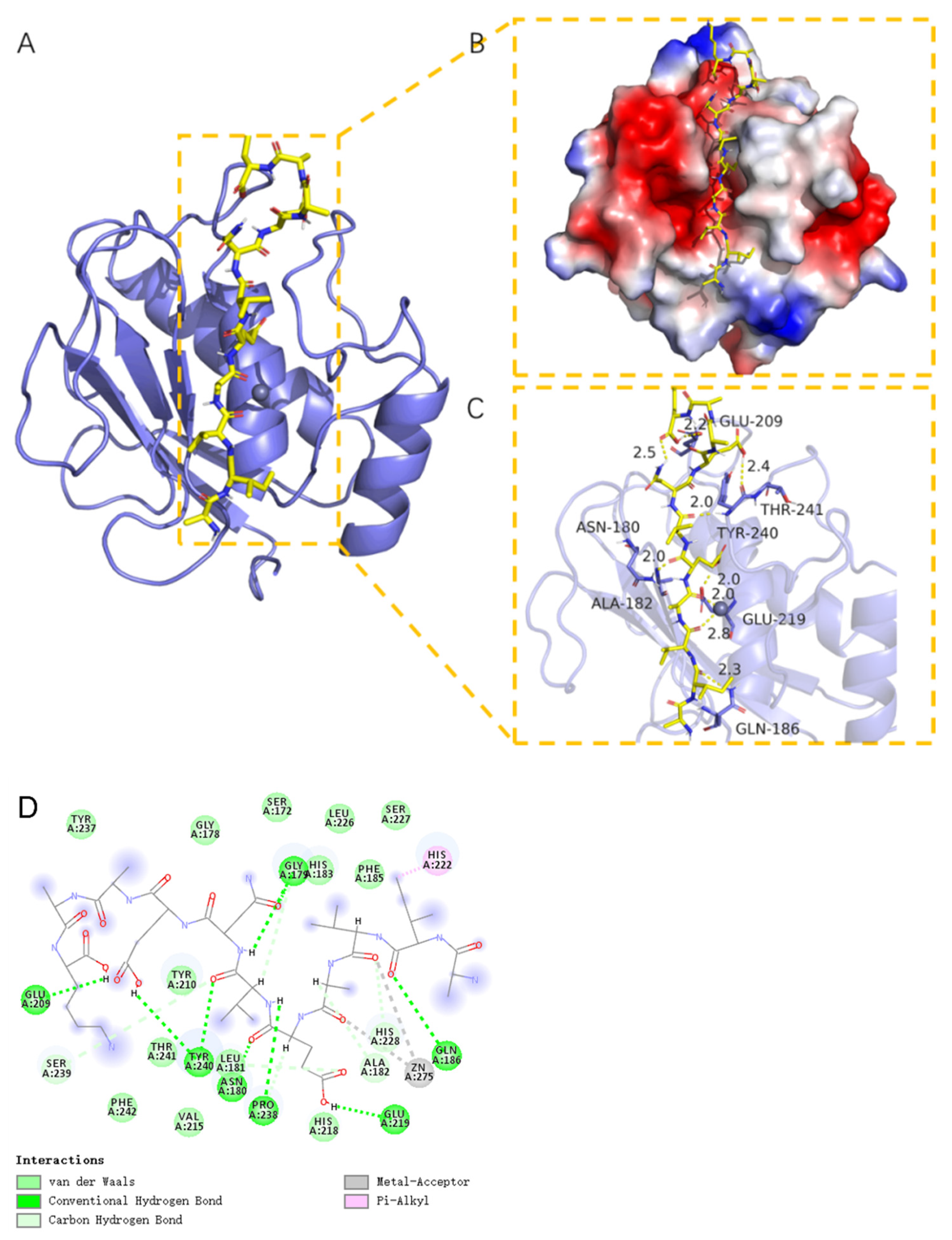
2.11. Molecular Docking Analysis of MMP-1 with AIVAEVNEAAK
2.12. ESI-MS and MS/MS Spectrum of the Peptide AIVAEVNEAAK
3. Discussion
4. Materials and Methods
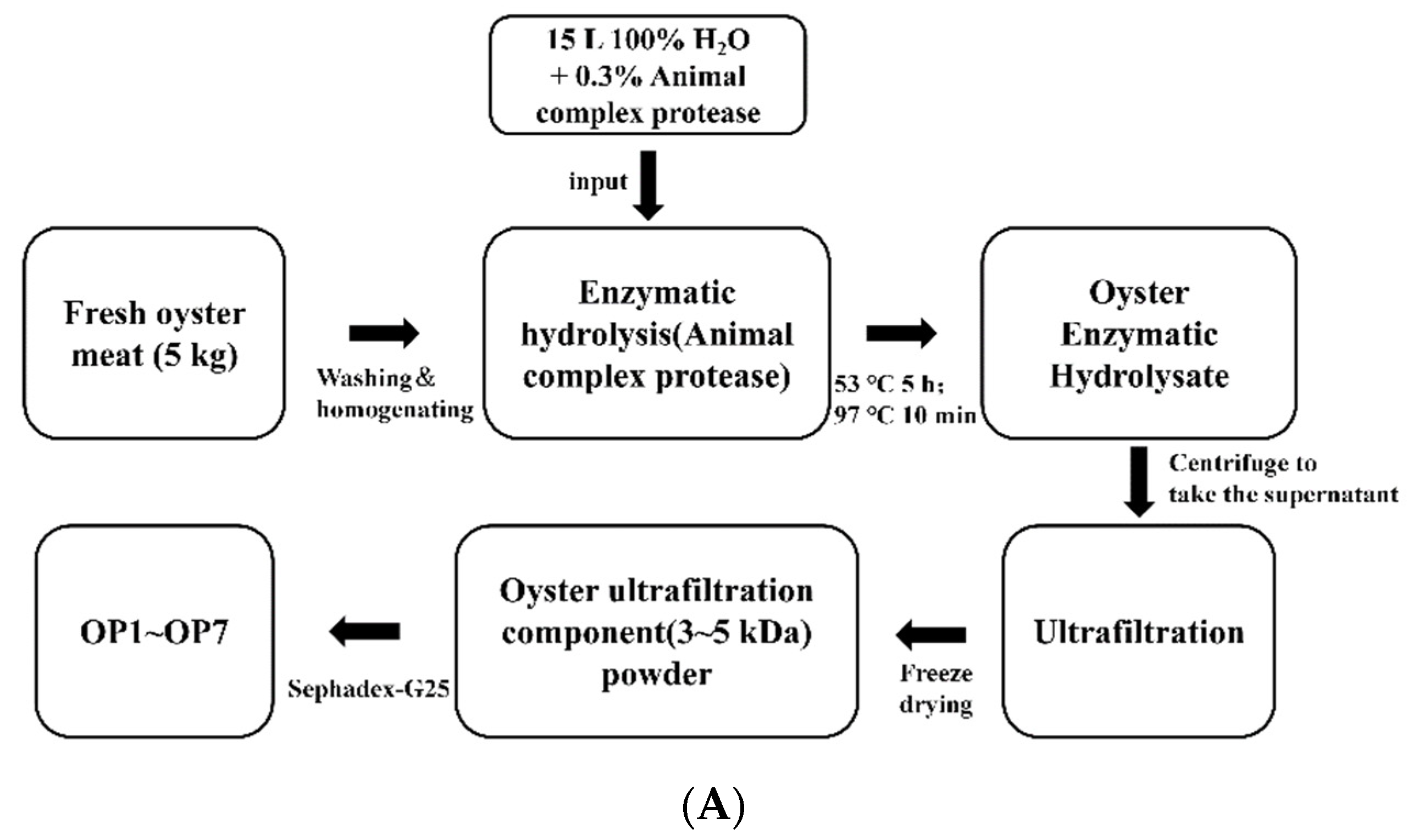
4.1. Preparation of Oyster Peptides
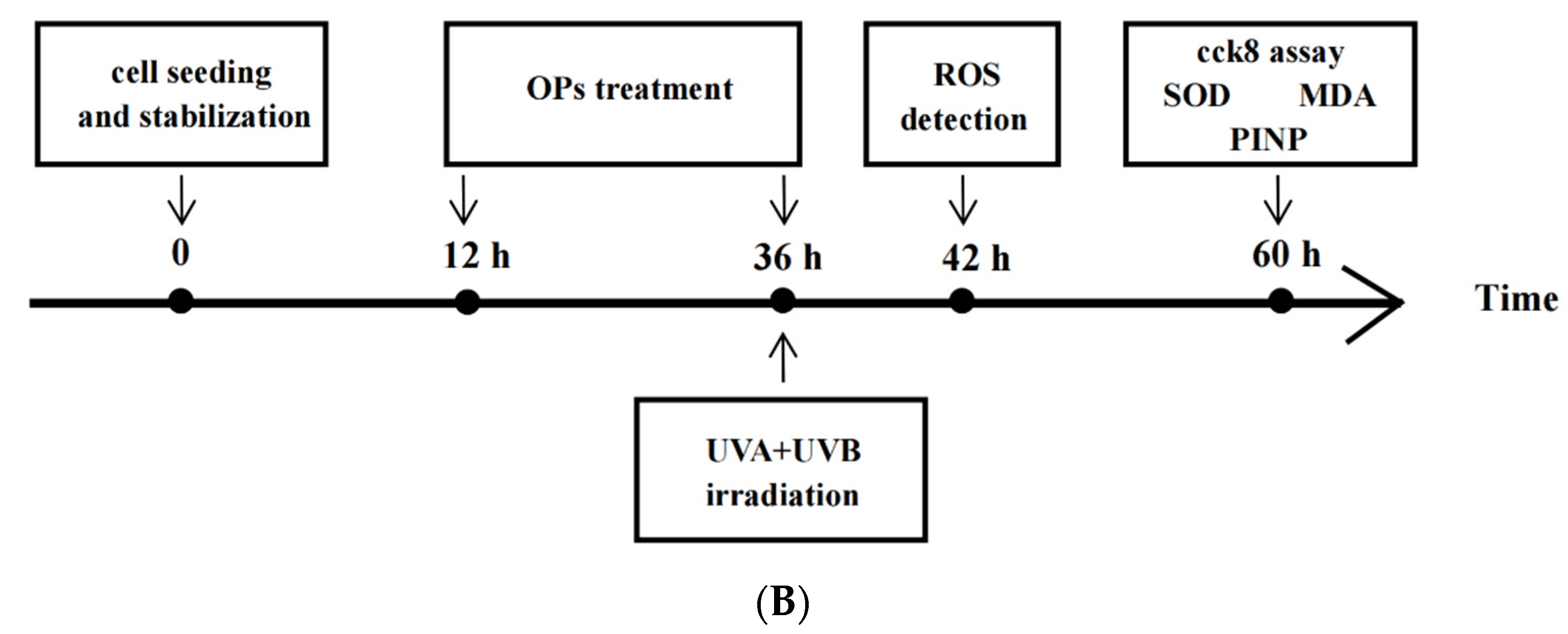
4.2. Cell Culture
4.3. UV-Irradiation of Cells
4.4. Cell Viability Assay
4.5. Determination of SOD, MDA, PINP, and MMP-1 Intracellular or Extracellular
4.6. Evaluation of Intracellular ROS Levels
4.7. SA-β-Gal Staining
4.8. Reversed-Phase High-Performance Liquid Chromatography (RP-HPLC) Analysis and Mass Spectroscopy
4.9. Solid-Phase Synthesis of Peptides
4.10. Molecular Docking
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yaar, M.; Gilchrest, B.A. Photoageing: Mechanism, prevention and therapy. Br. J. Dermatol. 2007, 157, 874–887. [Google Scholar] [CrossRef] [PubMed]
- Sorrentino, J.A.; Krishnamurthy, J.; Tilley, S.; Alb, J.G., Jr.; Burd, C.E.; Sharpless, N.E. p16INK4a reporter mice reveal age-promoting effects of environmental toxicants. J. Clin. Investig. 2014, 124, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Schuch, A.P.; Moreno, N.C.; Schuch, N.J.; Menck, C.F.M.; Garcia, C.C.M. Sunlight damage to cellular DNA: Focus on oxidatively generated lesions. Free Radic. Biol. Med. 2017, 107, 110–124. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Matsumura, H.; Kato, T.; Ichinose, S.; Takada, A.; Namiki, T.; Asakawa, K.; Morinaga, H.; Mohri, Y.; De Arcangelis, A.; et al. Stem cell competition orchestrates skin homeostasis and ageing. Nature 2019, 568, 344–350. [Google Scholar] [CrossRef]
- De Laat, A.T.J.; van der, A.R.J.; Allaart, M.A.F.; van Weele, M.; Benitez, G.C.; Casiccia, C.; Paes Leme, N.M.; Quel, E.; Salvador, J.; Wolfram, E. Extreme sunbathing: Three weeks of small total O3columns and high UV radiation over the southern tip of South America during the 2009 Antarctic O3hole season. Geophys. Res. Lett. 2010, 37. [Google Scholar] [CrossRef] [Green Version]
- Kostyuk, V.; Potapovich, A.; Albuhaydar, A.R.; Mayer, W.; De Luca, C.; Korkina, L. Natural Substances for Prevention of Skin Photoaging: Screening Systems in the Development of Sunscreen and Rejuvenation Cosmetics. Rejuvenation Res. 2018, 21, 91–101. [Google Scholar] [CrossRef]
- Vayalil, P.K.; Mittal, A.; Hara, Y.; Elmets, C.A.; Katiyar, S.K. Green tea polyphenols prevent ultraviolet light-induced oxidative damage and matrix metalloproteinases expression in mouse skin. J. Investig. Dermatol. 2004, 122, 1480–1487. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; He, M. The role of a new insulin-like peptide in the pearl oyster Pinctada fucata martensii. Sci. Rep. 2020, 10, 433. [Google Scholar] [CrossRef] [Green Version]
- Siregar, A.S.; Nyiramana, M.M.; Kim, E.J.; Shin, E.J.; Woo, M.S.; Kim, J.M.; Kim, J.H.; Lee, D.K.; Hahm, J.R.; Kim, H.J.; et al. Dipeptide YA is Responsible for the Positive Effect of Oyster Hydrolysates on Alcohol Metabolism in Single Ethanol Binge Rodent Models. Mar. Drugs 2020, 18, 512. [Google Scholar] [CrossRef]
- Li, Y.; Qiu, W.; Zhang, Z.; Han, X.; Bu, G.; Meng, F.; Kong, F.; Cao, X.; Huang, A.; Feng, Z.; et al. Oral oyster polypeptides protect ovary against d-galactose-induced premature ovarian failure in C57BL/6 mice. J. Sci. Food Agric. 2020, 100, 92–101. [Google Scholar] [CrossRef]
- Zhang, X.; Peng, Z.; Zheng, H.; Zhang, C.; Lin, H.; Qin, X. The Potential Protective Effect and Possible Mechanism of Peptides from Oyster (Crassostrea hongkongensis) Hydrolysate on Triptolide-Induced Testis Injury in Male Mice. Mar. Drugs 2021, 19, 566. [Google Scholar] [CrossRef]
- Siregar, A.S.; Nyiramana, M.M.; Kim, E.J.; Cho, S.B.; Woo, M.S.; Lee, D.K.; Hong, S.G.; Han, J.; Kang, S.S.; Kim, D.R.; et al. Oyster-Derived Tyr-Ala (YA) Peptide Prevents Lipopolysaccharide/D-Galactosamine-Induced Acute Liver Failure by Suppressing Inflammatory, Apoptotic, Ferroptotic, and Pyroptotic Signals. Mar. Drugs 2021, 19, 614. [Google Scholar] [CrossRef]
- Fuda, H.; Watanabe, M.; Hui, S.P.; Joko, S.; Okabe, H.; Jin, S.; Takeda, S.; Miki, E.; Watanabe, T.; Chiba, H. Anti-apoptotic effects of novel phenolic antioxidant isolated from the Pacific oyster (Crassostrea gigas) on cultured human hepatocytes under oxidative stress. Food Chem. 2015, 176, 226–233. [Google Scholar] [CrossRef]
- Wang, Q.; Li, W.; He, Y.; Ren, D.; Kow, F.; Song, L.; Yu, X. Novel antioxidative peptides from the protein hydrolysate of oysters (Crassostrea talienwhanensis). Food Chem. 2014, 145, 991–996. [Google Scholar] [CrossRef]
- Wu, S.; Huang, X. Preparation and antioxidant activities of oligosaccharides from Crassostrea gigas. Food Chem. 2017, 216, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Xiang, N.; Zhao, C.; Diao, X.; Han, Q.; Zhou, H. Dynamic responses of antioxidant enzymes in pearl oyster Pinctada martensii exposed to di(2-ethylhexyl) phthalate (DEHP). Environ. Toxicol. Pharmacol. 2017, 54, 184–190. [Google Scholar] [CrossRef]
- Qian, B.; Zhao, X.; Yang, Y.; Tian, C. Antioxidant and anti-inflammatory peptide fraction from oyster soft tissue by enzymatic hydrolysis. Food Sci. Nutr. 2020, 8, 3947–3956. [Google Scholar] [CrossRef]
- Xiang, X.W.; Zheng, H.Z.; Wang, R.; Chen, H.; Xiao, J.X.; Zheng, B.; Liu, S.L.; Ding, Y.T. Ameliorative Effects of Peptides Derived from Oyster (Crassostrea gigas) on Immunomodulatory Function and Gut Microbiota Structure in Cyclophosphamide-Treated Mice. Mar. Drugs 2021, 19, 456. [Google Scholar] [CrossRef]
- Hwang, D.; Kang, M.J.; Jo, M.J.; Seo, Y.B.; Park, N.G.; Kim, C.D. Anti-Inflammatory Activity of beta-thymosin Peptide Derived from Pacific Oyster (Crassostrea gigas) on NO and PGE(2) Production by Down-Regulating NF-kappaB in LPS-Induced RAW264.7 Macrophage Cells. Mar. Drugs 2019, 17, 129. [Google Scholar] [CrossRef] [Green Version]
- Sotiropoulou, G.; Zingkou, E.; Pampalakis, G. Redirecting drug repositioning to discover innovative cosmeceuticals. Exp. Dermatol. 2021, 30, 628–644. [Google Scholar] [CrossRef]
- Han, J.H.; Bang, J.S.; Choi, Y.J.; Choung, S.Y. Anti-melanogenic effects of oyster hydrolysate in UVB-irradiated C57BL/6J mice and B16F10 melanoma cells via downregulation of cAMP signaling pathway. J. Ethnopharmacol. 2019, 229, 137–144. [Google Scholar] [CrossRef]
- Peng, Z.; Chen, B.; Zheng, Q.; Zhu, G.; Cao, W.; Qin, X.; Zhang, C. Ameliorative Effects of Peptides from the Oyster (Crassostrea hongkongensis) Protein Hydrolysates against UVB-Induced Skin Photodamage in Mice. Mar. Drugs 2020, 18, 288. [Google Scholar] [CrossRef]
- Buonocore, G.; Perrone, S.; Tataranno, M.L. Oxygen toxicity: Chemistry and biology of reactive oxygen species. Semin. Fetal Neonatal Med. 2010, 15, 186–190. [Google Scholar] [CrossRef]
- Kammeyer, A.; Luiten, R.M. Oxidation events and skin aging. Ageing Res. Rev. 2015, 21, 16–29. [Google Scholar] [CrossRef]
- Williams, D.E.; Andersen, R.J. Biologically active marine natural products and their molecular targets discovered using a chemical genetics approach. Nat. Prod. Rep. 2020, 37, 617–633. [Google Scholar] [CrossRef]
- Zhang, G.; Fang, X.; Guo, X.; Li, L.; Luo, R.; Xu, F.; Yang, P.; Zhang, L.; Wang, X.; Qi, H.; et al. The oyster genome reveals stress adaptation and complexity of shell formation. Nature 2012, 490, 49–54. [Google Scholar] [CrossRef] [Green Version]
- Zarkovic, N. Roles and Functions of ROS and RNS in Cellular Physiology and Pathology. Cells 2020, 9, 767. [Google Scholar] [CrossRef] [Green Version]
- Munoz-Espin, D.; Serrano, M. Cellular senescence: From physiology to pathology. Nat. Rev. Mol. Cell Biol. 2014, 15, 482–496. [Google Scholar] [CrossRef]
- McHugh, D.; Gil, J. Senescence and aging: Causes, consequences, and therapeutic avenues. J. Cell Biol. 2018, 217, 65–77. [Google Scholar] [CrossRef]
- Tchkonia, T.; Kirkland, J.L. Aging, Cell Senescence, and Chronic Disease: Emerging Therapeutic Strategies. JAMA 2018, 320, 1319–1320. [Google Scholar] [CrossRef]
- Calcinotto, A.; Kohli, J.; Zagato, E.; Pellegrini, L.; Demaria, M.; Alimonti, A. Cellular Senescence: Aging, Cancer, and Injury. Physiol. Rev. 2019, 99, 1047–1078. [Google Scholar] [CrossRef] [PubMed]
- Saleh, T.; Tyutyunyk-Massey, L.; Murray, G.F.; Alotaibi, M.R.; Kawale, A.S.; Elsayed, Z.; Henderson, S.C.; Yakovlev, V.; Elmore, L.W.; Toor, A.; et al. Tumor cell escape from therapy-induced senescence. Biochem. Pharmacol. 2019, 162, 202–212. [Google Scholar] [CrossRef]
- Shmulevich, R.; Krizhanovsky, V. Cell Senescence, DNA Damage, and Metabolism. Antioxid. Redox Signal. 2021, 34, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Segura, A.; Nehme, J.; Demaria, M. Hallmarks of Cellular Senescence. Trends Cell Biol. 2018, 28, 436–453. [Google Scholar] [CrossRef]
- Shin, J.W.; Kwon, S.H.; Choi, J.Y.; Na, J.I.; Huh, C.H.; Choi, H.R.; Park, K.C. Molecular Mechanisms of Dermal Aging and Antiaging Approaches. Int. J. Mol. Sci. 2019, 20, 2126. [Google Scholar] [CrossRef] [Green Version]
- Van Doren, S.R. Matrix metalloproteinase interactions with collagen and elastin. Matrix Biol. 2015, 44–46, 224–231. [Google Scholar] [CrossRef]





| Group | Protein Content (%) | ABTS Free Radical Scavenging Rate (%) | DPPH Free Radical Scavenging Rate (%) | Hydroxyl Free Radicals Scavenging Rate (%) |
|---|---|---|---|---|
| OP1 | 20.20 | 3.00 d | 37.64 b | 59.89 d |
| OP2 | 15.07 | 1.97 d | 89.49 a | 74.31 a |
| OP3 | 11.86 | 11.89 c | 87.99 a | 68.14 b |
| OP4 | 19.98 | 26.53 b | 12.40 c | - |
| OP5 | 96.71 | 11.81 c | 7.39 c | 39.03 e |
| OP6 | 34.12 | 26.10 b | - | 62.96 c |
| OP7 | 16.37 | 36.61 a | 5.81 c | 60.07 d |
| Sequence | Amino Sequence of OPs | Molecular Mass/Da | Number of Amino Acids |
|---|---|---|---|
| 1 | AIVAEVNEAAK | 1113.6030 | 11 |
| 2 | IGGIGTVPVGR | 1024.6030 | 11 |
| 3 | TALAIDAIINQK | 1269.7292 | 12 |
| 4 | AGIDQAIAR | 913.4981 | 9 |
| 5 | VLVPTQEAVQK | 1210.6921 | 11 |
| 6 | NARNAHEIEIK | 1293.6789 | 11 |
| 7 | TITLEVEPSDTIENVK | 1786.9200 | 16 |
| 8 | GVAMNPVDHPHGGGEGR | 1685.7693 | 17 |
| 9 | YEDQIGIR | 992.4927 | 8 |
| 10 | LICIVPK | 841.5095 | 7 |
| 11 | VTDVEIAEVLSK | 1301.7078 | 12 |
| 12 | VLSLDLGALVAGAK | 1325.7918 | 14 |
| 13 | AAVEEGVVPGGGVALVR | 1578.8730 | 17 |
| 14 | MYLL->M<-EKQHNR | 1477.7169 | 11 |
| 15 | ->M<-YLL->M<-EKQHNR | 1493.7119 | 11 |
| 16 | LERGKLDPK | 1054.6135 | 9 |
| 17 | DDLVIGSPFASVK | 1346.7082 | 13 |
| Number | Amino Sequence | Hydropathicity/Hydrophobicity | Molecular Mass/Da | Number of Amino Acids |
|---|---|---|---|---|
| Peptide I | AIVAEVNEAAK | ----+-+--+ | 1113.6062 | 11 |
| Peptide II | IGGIGTVPVGR | -++-++-+-+ | 1024.6058 | 11 |
| Peptide III | TALAIDAIINQK | +----+---+++ | 1269.7335 | 12 |
| Peptide IV | VLVPTQEAVQK | ---++++--++ | 1210.6921 | 11 |
| Peptide V | GVAMNPVDHPHGGGEGR | +---++-++++++++++ | 1685.7718 | 17 |
| Peptide VI | LICIVPK | --+--++ | 841.5039 | 7 |
| Column | Trap Column: Acclaim PePmap 100, 75 μm × 2 cm, nanoviper, C18, 3 μm, 100 Å Analytical Column: C18 (L), 5 μm, 150 Å |
|---|---|
| Chromatographic Gradient | |
| Time | Phase B concentration (%) |
| 0 | 5 |
| 6 | 8 |
| 6.5 | 10 |
| 45 | 24 |
| 51 | 40 |
| 54 | 80 |
| 59 | 80 |
| 59.9 | 5 |
| 65 | 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Lv, J.; Qin, X.; Peng, Z.; Lin, H. Novel Antioxidant Peptides from Crassostrea Hongkongensis Improve Photo-Oxidation in UV-Induced HaCaT Cells. Mar. Drugs 2022, 20, 100. https://doi.org/10.3390/md20020100
Zhang C, Lv J, Qin X, Peng Z, Lin H. Novel Antioxidant Peptides from Crassostrea Hongkongensis Improve Photo-Oxidation in UV-Induced HaCaT Cells. Marine Drugs. 2022; 20(2):100. https://doi.org/10.3390/md20020100
Chicago/Turabian StyleZhang, Chen, Jiatong Lv, Xiaoming Qin, Zhilan Peng, and Haisheng Lin. 2022. "Novel Antioxidant Peptides from Crassostrea Hongkongensis Improve Photo-Oxidation in UV-Induced HaCaT Cells" Marine Drugs 20, no. 2: 100. https://doi.org/10.3390/md20020100
APA StyleZhang, C., Lv, J., Qin, X., Peng, Z., & Lin, H. (2022). Novel Antioxidant Peptides from Crassostrea Hongkongensis Improve Photo-Oxidation in UV-Induced HaCaT Cells. Marine Drugs, 20(2), 100. https://doi.org/10.3390/md20020100







