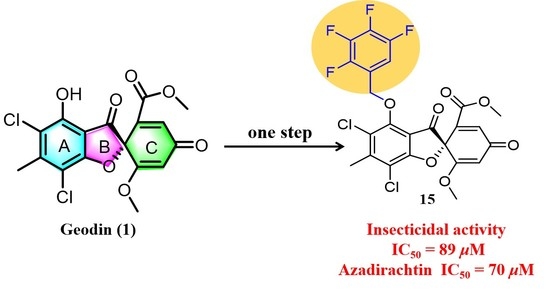
Design, Semisynthesis, Insecticidal and Antibacterial Activities of a Series of Marine-Derived Geodin Derivatives and Their Preliminary Structure–Activity Relationships
Abstract
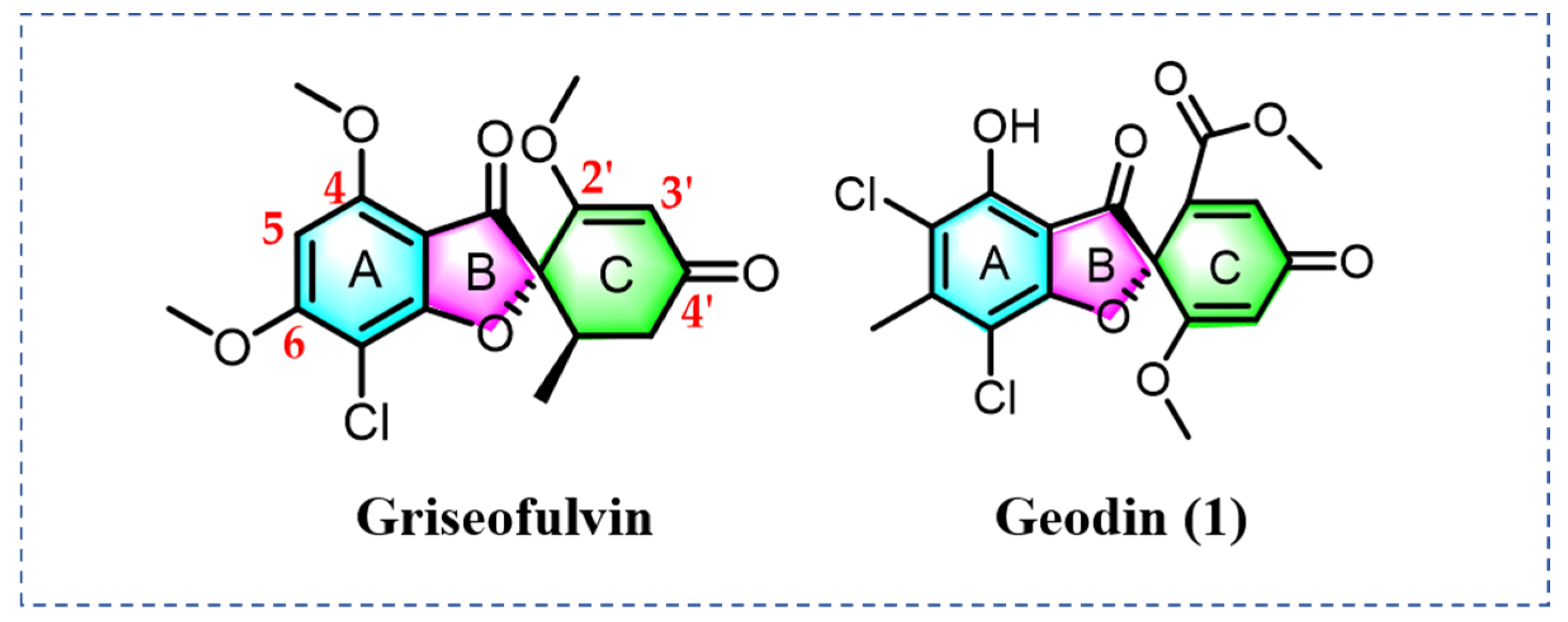
:1. Introduction
2. Results and Discussion
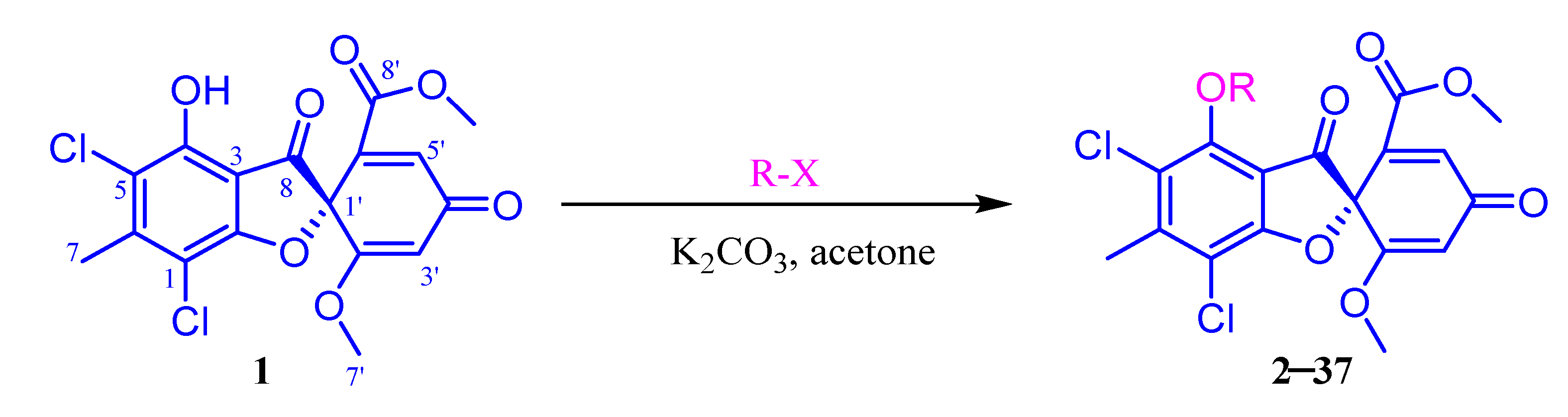
2.1. Chemistry
2.2. Insecticidal Activity against Helicoverpa armigera Hübner
2.3. Antibacterial Activity
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Biological Material
3.3. Extraction and Isolation
Geodin (1)
3.4. General Synthetic Methods for Compounds 2–37
3.4.1. Characterization Data of Compounds 2–37
Methyl(R)-4-((2-bromobenzyl)oxy)-5,7-dichloro-6′-methoxy-6-methyl-3,4′-dioxo-3H-spiro[benzofuran-2,1′-cyclohexane]-2′,5′-diene-2′-carboxylate (2)
Methyl(R)-4-((3-bromobenzyl)oxy)-5,7-dichloro-6′-methoxy-6-methyl-3,4′-dioxo-3Hspiro [benzofuran-2, 1′-cyclohexane]-2′,5′-diene-2′-carboxylate (3)
Methyl(R)-4-((4-bromobenzyl)oxy)-5,7-dichloro-6′-methoxy-6-methyl-3,4′-dioxo-3H-spiro[benzofuran-2,1′-cyclohexane]-2′,5′-diene-2′-carboxylate (4)
Methyl(R)-5, 7-dichloro-4-((2-chlorobenzyl) oxy)-6′-methoxy-6-methyl-3, 4′-dioxo-3H-spiro [benzofuran-2, 1′-cyclohexane]-2′, 5′-diene-2′-carboxylate (5)
Methyl(R)-5, 7-dichloro-4-((3-chlorobenzyl) oxy)-6′-methoxy-6-methyl-3, 4′-dioxo-3H-spiro [benzofuran-2, 1′-cyclohexane]-2′, 5′-diene-2′-carboxylate (6)
Methyl(R)-5,7-dichloro-4-((4-chlorobenzyl)oxy)-6′-methoxy-6-methyl-3,4′-dioxo-3H-spiro[benzofuran-2,1′-cyclohexane]-2′,5′-diene-2′-carboxylate (7)
Methyl(R)-5, 7-dichloro-4-((2, 6-dichlorobenzyl) oxy)-6′-methoxy-6-methyl-3, 4′-dioxo-3H-spiro [benzofuran-2, 1′-cyclohexane]-2′, 5′-diene-2′-carboxylate (8)
Methyl(R)-5, 7-dichloro-4-((3, 4-dichlorobenzyl) oxy)-6′-methoxy-6-methyl-3, 4′-dioxo-3H-spiro [benzofuran-2, 1′-cyclohexane]-2′, 5′-diene-2′-carboxylate (9)
Methyl(R)-5, 7-dichloro-4-((3-iodobenzyl) oxy)-6′-methoxy-6-methyl-3, 4′-dioxo-3H-spiro [benzofuran-2, 1′-cyclohexane]-2′, 5′-diene-2′-carboxylate (10)
Methyl(R)-5, 7-dichloro-4-((4-iodobenzyl) oxy)-6′-methoxy-6-methyl-3, 4′-dioxo-3H-spiro [benzofuran-2, 1′-cyclohexane]-2′, 5′-diene-2′-carboxylate (11)
Methyl(R)-5, 7-dichloro-4-((2-fluorobenzyl) oxy)-6′-methoxy-6-methyl-3, 4′-dioxo-3H-spiro [benzofuran-2, 1′-cyclohexane]-2′, 5′-diene-2′-carboxylate (12)
Methyl(R)-5, 7 -dichloro-4-((3, 5-difluorobenzyl) oxy)-6′-methoxy-6-methyl-3, 4′-dioxo-3H-spiro [benzofuran-2, 1′-cyclohexane]-2′, 5′-diene-2′-carboxylate (13)
Methyl(R)-5, 7-dichloro-4-((3, 4-difluorobenzyl) oxy)-6′-methoxy-6-methyl-3, 4′-dioxo-3H-spiro [benzofuran-2, 1′-cyclohexane]-2′, 5′-diene-2′-carboxylate (14)
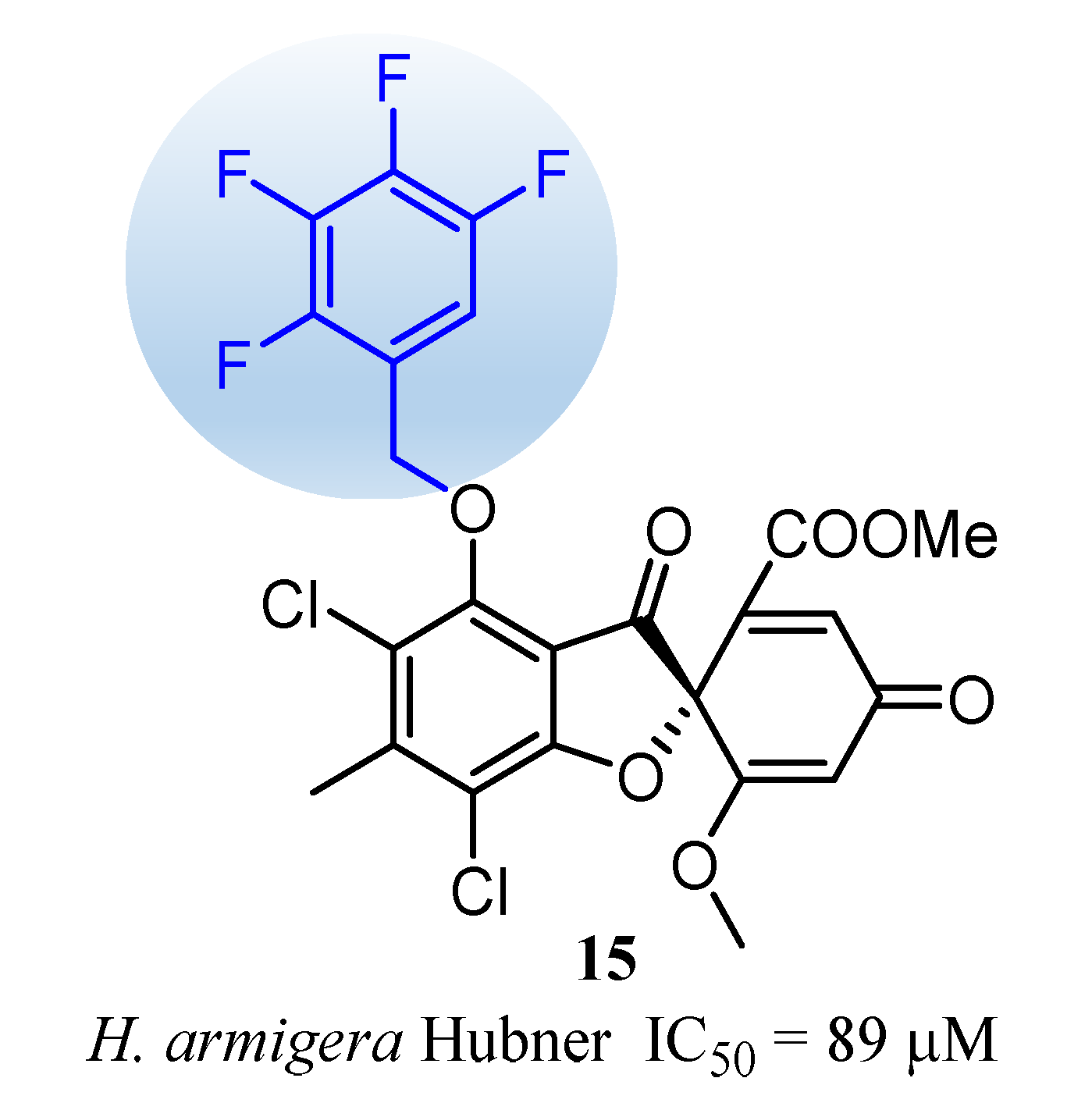
Methyl(R)-5, 7-dichloro-6′-methoxy-6-methyl-3, 4′-dioxo-4-((2, 3, 4, 5-tetrafluorobenzyl) oxy)-3H-spiro[benzofuran-2, 1′-cyclohexane]-2′, 5′-diene-2′-carboxylate (15)
Methyl(R)-5,7-dichloro-6′-methoxy-6-methyl-3, 4′-dioxo-4-((2, 3, 4-trifluorobenzyl)oxy)-3H-spiro [benzofuran-2, 1′-cyclohexane]-2′, 5′-diene-2′-carboxylate (16)
Methyl(R)-5,7-dichloro-6′-methoxy-6-methyl-3, 4′-dioxo-4-((perfluorophenyl) methoxy)-3H-spiro[benzofuran-2,1′-cyclohexane]-2′,5′-diene-2′-carboxylate (17)
Methyl(R)-5, 7-dichloro-6′-methoxy-6-methyl-3, 4′-dioxo-4-((2-(trifluoromethyl) benzyl) oxy)-3H-spiro[benzofuran-2,1′-cyclohexane]-2′, 5′-diene-2′-carboxylate (18)
Methyl(R)-5,7-dichloro-6′-methoxy-6-methyl-3,4′-dioxo-4-((3-(trifluoromethyl)benzyl) oxy)-3H-spiro[benzofuran-2, 1′-cyclohexane]-2′, 5′-diene-2′-carboxylate (19)
Methyl(R)-5,7-dichloro-6′-methoxy-6-methyl-3, 4′-dioxo-4-((4-(trifluoromethyl) benzyl) oxy)-3H-spiro[benzofuran-2, 1′-cyclohexane]-2′, 5′-diene-2′-carboxylate (20)
Methyl(R)-4-((3, 5-bis(trifluoromethyl)benzyl)oxy)-5, 7-dichloro-6′-methoxy-6-methyl-3, 4′-dioxo-3H-spiro[benzofuran-2, 1′-cyclohexane]-2′, 5′-diene-2′-carboxylate (21)
Methyl(R)-5,7-dichloro-6′-methoxy-6-methyl-4-((3-nitrobenzyl)oxy)-3,4′-dioxo-3H-spiro[benzofuran-2,1′-cyclohexane]-2′,5′-diene-2′-carboxylate (22)
Methyl(R)-5,7-dichloro-6′-methoxy-6-methyl-4-((2-methylbenzyl)oxy)-3,4′-dioxo-3H-spiro[benzofuran-2,1′-cyclohexane]-2′,5′-diene-2′-carboxylate (23)
Methyl(R)-5,7-dichloro-6′-methoxy-6-methyl-4-((3-methylbenzyl)oxy)-3,4′-dioxo-3H-spiro[benzofuran-2,1′-cyclohexane]-2′,5′-diene-2′-carboxylate (24)
Methyl(R)-5,7-dichloro-6′-methoxy-6-methyl-4-((4-methylbenzyl)oxy)-3,4′-dioxo-3H-spiro [benzofuran-2,1′-cyclohexane]-2′,5′-diene-2′-carboxylate (25)
Methyl(R)-4-((4-(tert-butyl)benzyl) oxy)-5, 7-dichloro-6′-methoxy-6-methyl-3, 4′-dioxo-3H-spiro [benzofuran-2, 1′-cyclohexane]-2′, 5′-diene-2′-carboxylate (26)
Methyl(R)-4-([1,1′-biphenyl]-4-ylmethoxy)-5,7-dichloro-6′-methoxy-6-methyl-3,4′-dioxo-3H-spiro[benzofuran-2,1′-cyclohexane]-2′,5′-diene-2′-carboxylate (27)
Methyl(R)-5,7-dichloro-4-((2′-cyano-[1,1′-biphenyl]-4-yl)methoxy)-6′-methoxy-6-methyl-3, 4′-dioxo-3H-spiro [benzofuran-2, 1′-cyclohexane]-2′, 5′-diene-2′-carboxylate (28)
Methyl(R)-5, 7-dichloro-4-((2-cyanobenzyl) oxy)-6′-methoxy-6-methyl-3, 4′-dioxo-3H-spiro [benzofuran-2, 1′-cyclohexane]-2′, 5′-diene-2′-carboxylate (29)
Methyl(R)-5, 7-dichloro-4-((4-cyanobenzyl) oxy)-6′-methoxy-6-methyl-3, 4′-dioxo-3H-spiro [benzofuran-2, 1′-cyclohexane]-2′, 5′-diene-2′-carboxylate (30)
Methyl(R)-5,7-dichloro-4-((2-chloro-4-fluorobenzyl)oxy)-6′-methoxy-6-methyl-3, 4′-dioxo-3H-spiro [benzofuran-2, 1′-cyclohexane]-2′, 5′-diene-2′-carboxylate (31)
Methyl(R)-5, 7-dichloro-4-((3-chloro-4-fluorobenzyl) oxy)-6′-methoxy-6-methyl-3, 4′-dioxo-3H-spiro [benzofuran-2, 1′-cyclohexane]-2′, 5′-diene-2′-carboxylate (32)
Methyl(R)-5, 7-dichloro-4-((3-chloro-2-fluorobenzyl) oxy)-6′-methoxy-6-methyl-3, 4′-dioxo-3H-spiro [benzofuran-2, 1′-cyclohexane]-2′, 5′-diene-2′-carboxylate (33)
Methyl(R)-5, 7-dichloro-4-((4-chloro-2-fluorobenzyl) oxy)-6′-methoxy-6-methyl-3, 4′-dioxo-3H-spiro [benzofuran-2, 1′-cyclohexane]-2′, 5′-diene-2′-carboxylate (34)
Methyl(R)-4-((4-bromo-2-fluorobenzyl) oxy)-5,7-dichloro-6′-methoxy-6-methyl-3, 4′-dioxo-3H-spiro [benzofuran-2, 1′-cyclohexane]-2′, 5′-diene-2′-carboxylate (35)
Methyl(R)-5, 7-dichloro-4-((2-cyano-5-fluorobenzyl) oxy)-6′-methoxy-6-methyl-3, 4′-dioxo-3H-spiro [benzofuran-2, 1′-cyclohexane]-2′, 5′-diene-2′-carboxylate (36)
Methyl(R)-5,7-dichloro-4-ethoxy-6′-methoxy-6-methyl-3,4′-dioxo3Hspiro[benzofuran-2,1′-cyclohexane]-2′,5′-diene-2′-carboxylate (37)
3.5. Insecticidal Activity
3.6. Antibacterial Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Yao, S.Y.; Yang, Y.; Xue, Y.Y.; Zhao, W.L.; Liu, X.G.; Du, M.F.; Yin, X.M.; Guan, R.B.; Wei, J.Z.; An, S.H. New insights on the effects of spinosad on the development of Helicoverpa armigera. Ecotox. Environ. Saf. 2021, 221, 112452. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, V.; Hajizadeh, J.; Zibaee, A.; Sendi, J.J. Effect of polygonum persicaria (polygonales: Polygonaceae) extracted agglutinin on life table and antioxidant responses in Helicoverpa armigera (Lepidoptera: Noctuidae) larvae. J. Econ. Entomol. 2018, 111, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.C.; Parry, H.; Tay, T.W.; Reynolds, R.D.; Chapman, W.J. Movement ecology of pest Helicoverpa: Implications for ongoing spread. Annu. Rev. Entomol. 2019, 64, 277–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, X.L.; Wang, X.F.; Xu, K.; Li, W.; Chen, D.; Zhang, P. Characterization of a new insecticidal anthraquinone derivative from an endophyte of Acremonium vitellinum against Helicoverpa armigera. J. Agric. Food Chem. 2020, 68, 11480–11487. [Google Scholar] [CrossRef]
- Bilal, M.; Freed, S.; Ashraf, Z.M.; Syed Muhammad Zaka, M.S.; Khan, B.M. Activity of acetylcholinesterase and acid and alkaline phosphatases in different insecticide-treated Helicoverpa armigera (Hübner). Environ. Sci. Pollut. Res. 2018, 25, 22903–22910. [Google Scholar] [CrossRef]
- Newman, J.D.; Cragg, M.G. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Wang, T.N.; Li, L.; Zhou, Y.N.; Lu, A.D.; Li, H.Y.; Chen, J.X.; Duan, Z.Y.; Wang, Q.M. Structural simplification of marine natural products: Discovery of hamacanthin derivatives containing indole and piperazinone as novel antiviral and anti-phytopathogenic-fungus agents. J. Agric. Food Chem. 2021, 69, 10093–10103. [Google Scholar] [CrossRef]
- Li, L.; Zou, J.Y.; Xu, C.J.; You, S.Y.; Li, Y.Q.; Wang, Q.M. Synthesis and anti-tobacco mosaic virus/fungicidal/insecticidal/antitumor bioactivities of natural product hemigossypol and its derivatives. J. Agric. Food Chem. 2021, 69, 1224–1233. [Google Scholar] [CrossRef]
- Shao, C.L.; Wu, H.X.; Wang, C.Y.; Liu, Q.A.; Xu, Y.; Wei, M.Y.; Qian, P.Y.; Gu, Y.C.; Zheng, C.J.; She, Z.G.; et al. Potent antifouling resorcylic acid lactones from the gorgonian-derived fungus Cochliobolus lunatus. J. Nat. Prod. 2011, 74, 629–633. [Google Scholar] [CrossRef]
- Jia, Y.L.; Wei, M.Y.; Chen, H.Y.; Guan, F.F.; Wang, C.Y.; Shao, C.L. (+)- and (−)-Pestaloxazine A, a pair of antiviral enantiomeric alkaloid dimers with a symmetric spiro[oxazinane-piperazinedione] skeleton from Pestalotiopsis sp. Org. Lett. 2015, 17, 4216–4219. [Google Scholar] [CrossRef]
- Hou, X.M.; Li, Y.Y.; Shi, Y.W.; Fang, Y.W.; Chao, R.; Gu, Y.C.; Wang, C.Y.; Shao, C.L. Integrating molecular networking and 1h nmr to target the isolation of chrysogeamides from a library of marine-derived Penicillium fungi. J. Org. Chem. 2019, 84, 1228–1237. [Google Scholar] [CrossRef]
- Hou, X.M.; Liang, T.M.; Guo, Z.Y.; Wang, C.Y.; Shao, C.L. Discovery, absolute assignments, and total synthesis of asperversiamides A–C and their potent activity against Mycobacterium marinum. Chem. Commun. 2019, 55, 1104–1107. [Google Scholar] [CrossRef]
- Chao, R.; Hou, X.M.; Xu, W.F.; Hai, Y.; Wei, M.Y.; Wang, C.Y.; Gu, Y.C.; Shao, C.L. Targeted isolation of asperheptatides from a coral-derived fungus using LC-MS/MS-based molecular networking and antitubercular activities of modified cinnamate derivatives. J. Nat. Prod. 2021, 84, 11–19. [Google Scholar] [CrossRef]
- Hai, Y.; Wei, M.Y.; Wang, C.Y.; Gu, Y.C.; Shao, C.L. The intriguing chemistry and biology of sulfur-containing natural products from marine microorganisms (1987–2020). Mar. Life Sci. Technol. 2021, 3, 488–518. [Google Scholar] [CrossRef]
- Xu, W.F.; Wu, N.N.; Wu, Y.W.; Qi, Y.X.; Wei, M.Y.; Pineda, M.L.; Ng, G.M.; Spadafora, C.; Zheng, J.Y.; Lu, L.; et al. Structure modification, antialgal, antiplasmodial, and toxic evaluations of a series of new marine-derived 14-membered resorcylic acid lactone derivatives. Mar. Life Sci. Technol. 2021. [Google Scholar] [CrossRef]
- Rønnest, H.M.; Rebacz, B.; Markworth, L.; Terp, H.A.; Larsen, O.T.; Krämer, A.; Clausen, H.M. Synthesis and Structure-activity relationship of griseofulvin analogues as inhibitors of centrosomal clustering in cancer cells. J. Med. Chem. 2009, 52, 3342–3347. [Google Scholar] [CrossRef]
- Gulab, S.; Mou, X.F.; Fang, Y.W.; Liang, T.M.; Wei, M.Y.; Chen, G.Y.; Shao, C.L. Secondary metabolites isolated from the soft coral-derived fungus Aspergillus sp. from the South China Sea. Chem. Nat. Compd. 2018, 54, 547–549. [Google Scholar]
- Rinderknecht, H.; Ward, J.L.; Bergel, F.; Morrison, A.L. Studies on antibiotic II Bacteriological activity and posiible mode of action of certain nonnitrogenouse natural and synthetic antibiotics. J. Biochem. 1947, 41, 463–469. [Google Scholar] [CrossRef]
- Sato, S.; Okusa, N.; Ogawa, A.; Ikenoue, T.; Seki, T.; Tsuji, T. Identification and preliminary SAR studies of (+) geodin as a glucose uptake stimulator for rat adipocytes. J. Antibiot. 2005, 58, 583–589. [Google Scholar] [CrossRef] [Green Version]
- Shinohara, C.; Chikanishi, T.; Nakashima, S.; Hashimoto, A.; Hamanaka, A.; Endo, A.; Hasumi, K. Enhancement of Fibrinolytic Activity of Vascular Endothelial Cells by Chaetoglobosin A, Crinipellin B, Geodin and Triticone B. J. Antibiot. 2000, 53, 262–268. [Google Scholar] [CrossRef] [Green Version]
- Zou, X.Z.; Qiu, Z.X. Syntheses of 4-methoxymethylbenzyl permethrinates containing fluorine and their insecticidal activity. J. Fluor. Chem. 2002, 116, 173–179. [Google Scholar] [CrossRef]
- Chen, Y.W.; Li, Y.X.; Pan, L.; Liu, J.B.; Wan, Y.Y.; Chen, W.; Xiong, L.X.; Yang, N.; Song, H.B. Synthesis, insecticidal activities and SAR of novel phthalamides targeting calcium channel. Bioorgan. Med. Chem. 2014, 22, 6366–6379. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Gao, L.; Li, H.G.; Sun, P.W.; Meng, F.F.; Zhang, Y.; Xie, Y.T.; Sun, B.Q.; Zhou, S.; Ma, Y.; et al. Synthesis, insecticidal activities, and structure–activity relationship of phenylpyrazole derivatives containing a fluoro-substituted benzene moiety. J. Agric. Food Chem. 2020, 68, 11282–11289. [Google Scholar] [CrossRef]
- Guo, Y.; Yan, Y.Y.; Yu, X.; Wang, Y.; Zhi, X.Y.; Hu, Y.; Xu, H. Synthesis and insecticidal activity of some novel fraxinellone-based esters. J. Agric. Food Chem. 2012, 60, 7016–7021. [Google Scholar] [CrossRef]
- Wu, Q.L.; Cai, J.L.; Zhao, F.H.; Zhou, Z.Y.; Yang, D.Y.; Qin, Z.H. Synthesis and insecticidal activity of the fluorinated galegine analogues. Nat. Prod. Res. 2021, 35, 5773–5777. [Google Scholar] [CrossRef]
- Bai, M.; Zheng, C.J.; Huang, G.L.; Mei, R.Q.; Wang, B.; Luo, Y.P.; Zheng, C.; Niu, Z.G.; Chen, G.Y. Bioactive meroterpenoids and isocoumarins from the mangrove-derived fungus Penicillium sp. TGM112. J. Nat. Prod. 2019, 82, 1155–1164. [Google Scholar] [CrossRef] [PubMed]
- Fromtling, R.A.; Galgiani, J.N.; Pfaller, M.A.; Espinel-Ingroff, A.; Bartizal, K.F.; Bartlett, M.S.; Body, B.A.; Frey, C.; Hall, G.; Roberts, G.D.; et al. Multicenter evaluation of a broth macrodilution antifungal susceptibility test for yeasts. Antimicrob. Agents Chemother. 1993, 37, 39–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
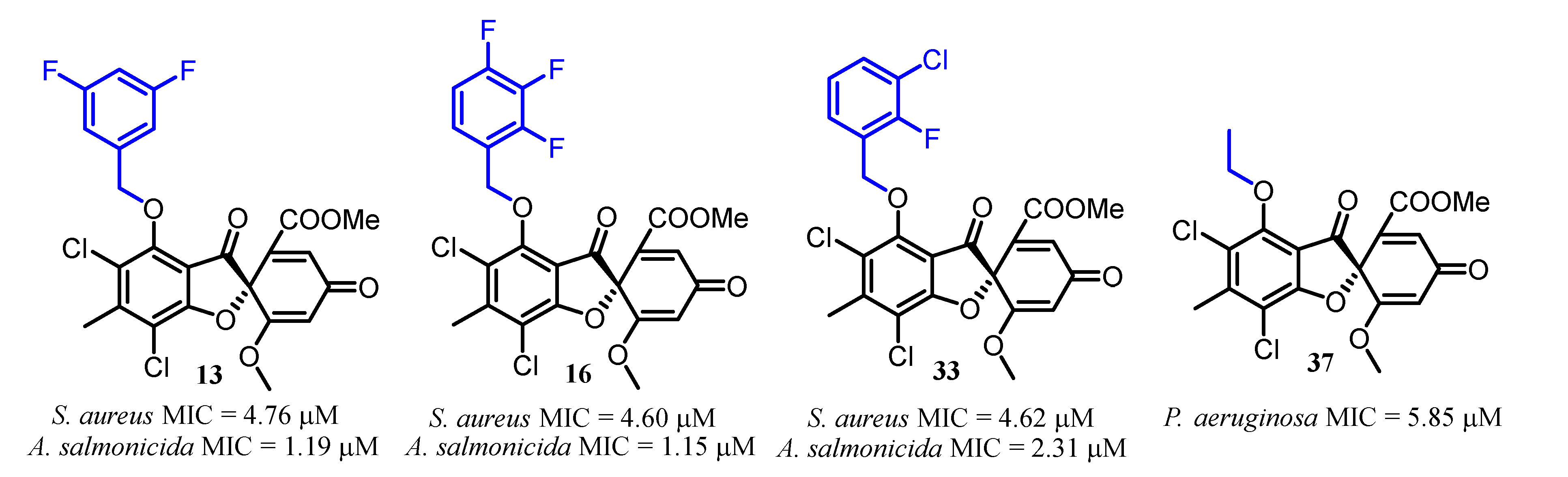
| No. | R | No. | R | No. | R |
|---|---|---|---|---|---|
| 2 | 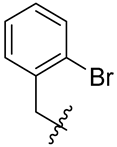 | 14 | 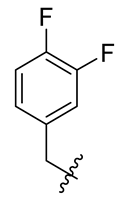 | 26 | 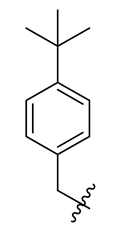 |
| 3 | 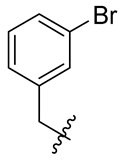 | 15 | 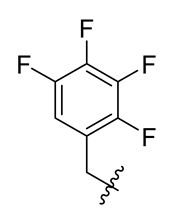 | 27 | 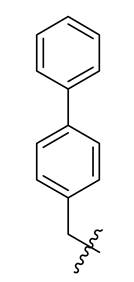 |
| 4 | 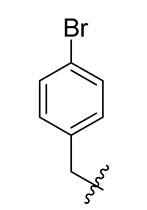 | 16 | 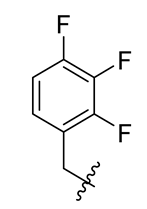 | 28 | 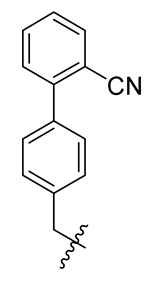 |
| 5 | 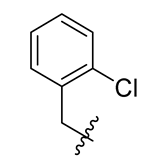 | 17 | 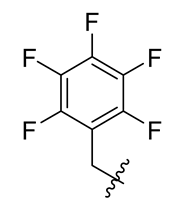 | 29 | 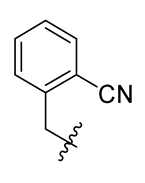 |
| 6 | 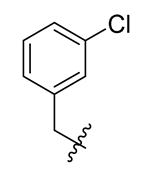 | 18 | 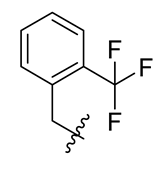 | 30 | 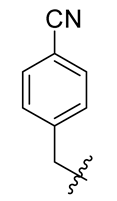 |
| 7 | 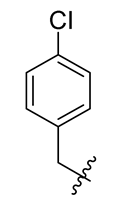 | 19 | 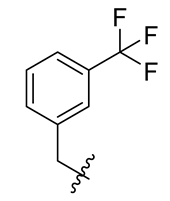 | 31 | 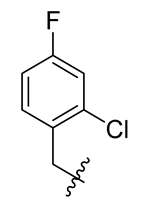 |
| 8 | 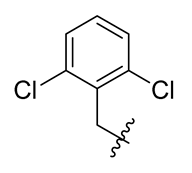 | 20 | 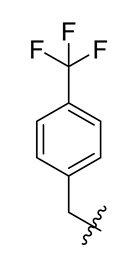 | 32 | 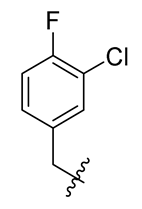 |
| 9 | 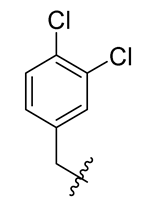 | 21 | 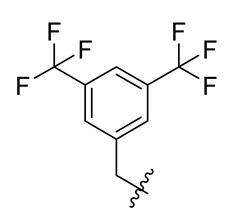 | 33 | 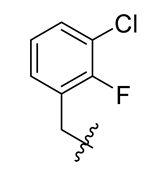 |
| 10 | 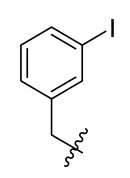 | 22 | 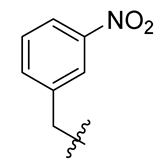 | 34 | 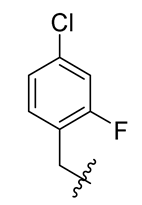 |
| 11 | 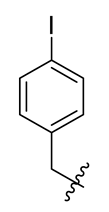 | 23 | 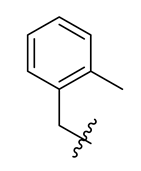 | 35 | 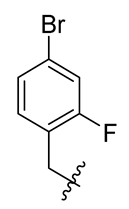 |
| 12 | 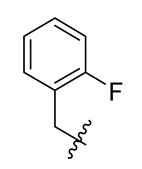 | 24 | 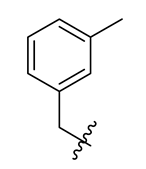 | 36 | 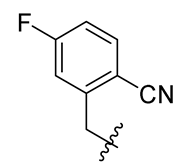 |
| 13 | 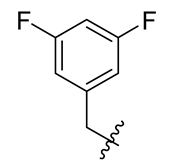 | 25 | 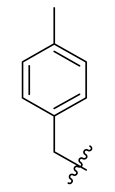 | 37 | 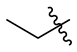 |
| No. | IC50 (μM) (μg/mL) | MIC (μM) | ||
|---|---|---|---|---|
| Insecticidal Activity | Antimicrobial Activity | |||
| H. armigera Hübner | S. aureus | A. salmonicida | P. aeruginosa | |
| 1 | 500 (200) | >50 | >50 | >50 |
| 2 | 176 (100) | >50 | 2.20 | >50 |
| 3 | 176 (100) | >50 | >50 | >50 |
| 4 | >350 (>200) | 17.60 | 8.80 | >50 |
| 5 | >381 (>200) | 4.77 | 4.77 | >50 |
| 6 | 190 (100) | 9.55 | 4.77 | >50 |
| 7 | 190 (100) | >50 | >50 | >50 |
| 8 | 179 (100) | 8.96 | 4.48 | >50 |
| 9 | 179 (100) | >50 | 4.48 | >50 |
| 10 | >325 (>200) | 16.25 | 4.06 | >50 |
| 11 | >325 (>200) | >50 | 8.13 | >50 |
| 12 | 197 (100) | 4.93 | 2.46 | >50 |
| 13 | 190 (100) | 4.76 | 1.19 | >50 |
| 14 | 190 (100) | 9.52 | 2.38 | >50 |
| 15 | 89 (50) | 8.91 | 2.23 | >50 |
| 16 | >368 (>200) | 4.60 | 1.15 | >50 |
| 17 | 172 (100) | 17.27 | >50 | >50 |
| 18 | 179 (100) | 8.97 | >50 | >50 |
| 19 | >358 (>200) | 17.94 | 8.97 | >50 |
| 20 | 179 (100) | 17.94 | 4.49 | >50 |
| 21 | >319 (>200) | >50 | 4.00 | >50 |
| 22 | 187 (100) | >50 | >50 | >50 |
| 23 | 198 (100) | >50 | >50 | >50 |
| 24 | >397 (>200) | >50 | >50 | >50 |
| 25 | nt | >50 | >50 | >50 |
| 26 | 366 (200) | >50 | 2.29 | >50 |
| 27 | 176 (100) | >50 | >50 | >50 |
| 28 | 169 (100) | >50 | >50 | >50 |
| 29 | >388 (>200) | 9.72 | >50 | >50 |
| 30 | 194 (100) | 4.86 | 4.86 | >50 |
| 31 | >369 (>200) | 9.23 | 4.62 | >50 |
| 32 | 184 (100) | 9.23 | 9.23 | >50 |
| 33 | >369 (>200) | 4.62 | 2.31 | >50 |
| 34 | 369 (200) | 9.23 | >50 | >50 |
| 35 | 170 (100) | >50 | 4.27 | >50 |
| 36 | >375 (>200) | 18.97 | >50 | >50 |
| 37 | >468 (>200) | >50 | >50 | 5.85 |
| Azadirachtin | 70 (50) | nt | nt | nt |
| Sea-Nine 211 | nt | nt | 0.27 | 0.27 |
| Ciprofloxacin | nt | 0.16 | nt | nt |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chao, R.; Said, G.; Zhang, Q.; Qi, Y.-X.; Hu, J.; Zheng, C.-J.; Zheng, J.-Y.; Shao, C.-L.; Chen, G.-Y.; Wei, M.-Y. Design, Semisynthesis, Insecticidal and Antibacterial Activities of a Series of Marine-Derived Geodin Derivatives and Their Preliminary Structure–Activity Relationships. Mar. Drugs 2022, 20, 82. https://doi.org/10.3390/md20020082
Chao R, Said G, Zhang Q, Qi Y-X, Hu J, Zheng C-J, Zheng J-Y, Shao C-L, Chen G-Y, Wei M-Y. Design, Semisynthesis, Insecticidal and Antibacterial Activities of a Series of Marine-Derived Geodin Derivatives and Their Preliminary Structure–Activity Relationships. Marine Drugs. 2022; 20(2):82. https://doi.org/10.3390/md20020082
Chicago/Turabian StyleChao, Rong, Gulab Said, Qun Zhang, Yue-Xuan Qi, Jie Hu, Cai-Juan Zheng, Ji-Yong Zheng, Chang-Lun Shao, Guang-Ying Chen, and Mei-Yan Wei. 2022. "Design, Semisynthesis, Insecticidal and Antibacterial Activities of a Series of Marine-Derived Geodin Derivatives and Their Preliminary Structure–Activity Relationships" Marine Drugs 20, no. 2: 82. https://doi.org/10.3390/md20020082
APA StyleChao, R., Said, G., Zhang, Q., Qi, Y.-X., Hu, J., Zheng, C.-J., Zheng, J.-Y., Shao, C.-L., Chen, G.-Y., & Wei, M.-Y. (2022). Design, Semisynthesis, Insecticidal and Antibacterial Activities of a Series of Marine-Derived Geodin Derivatives and Their Preliminary Structure–Activity Relationships. Marine Drugs, 20(2), 82. https://doi.org/10.3390/md20020082