Ecotoxicological Impact of the Marine Toxin Palytoxin on the Micro-Crustacean Artemia franciscana
Abstract
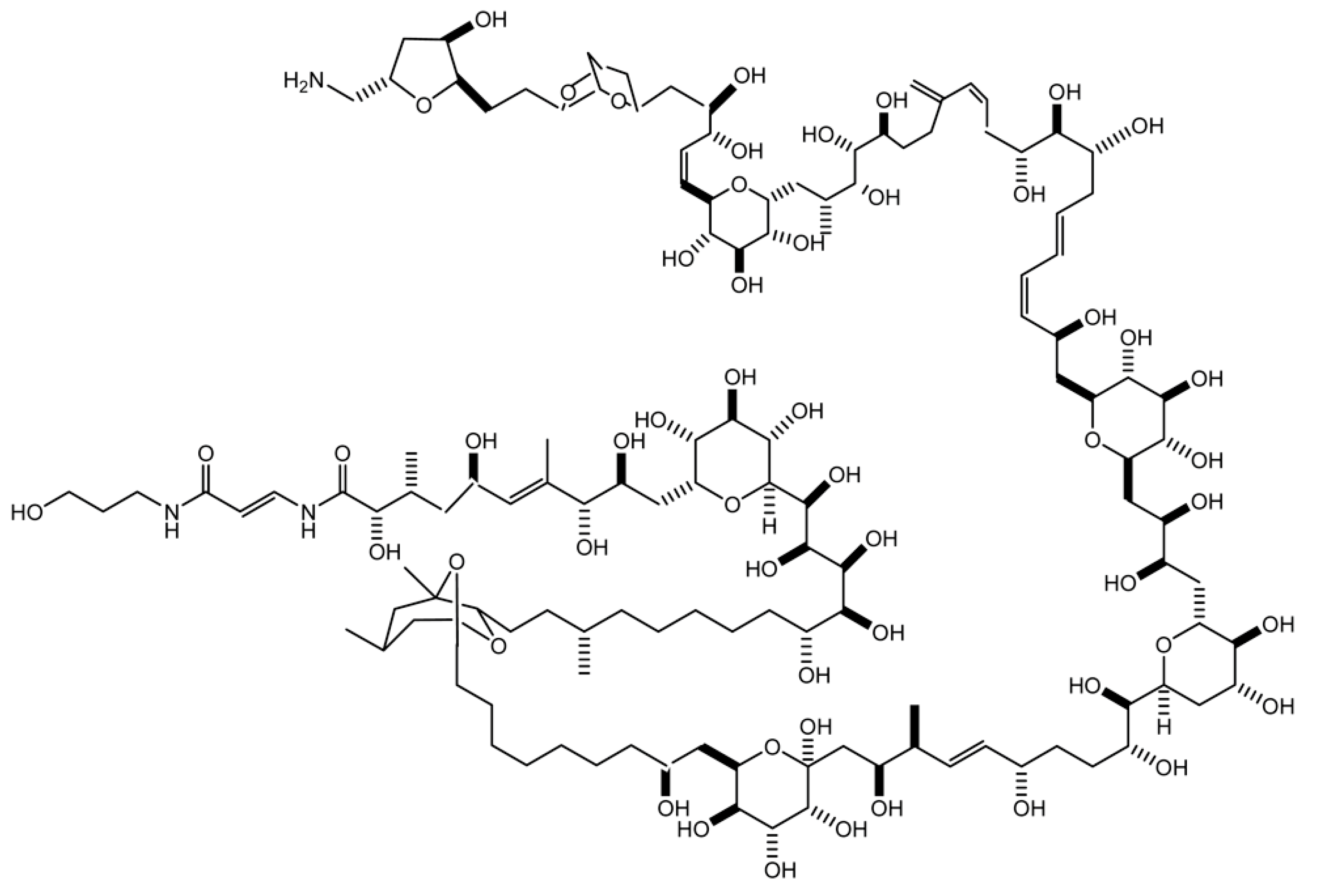
:1. Introduction
2. Results
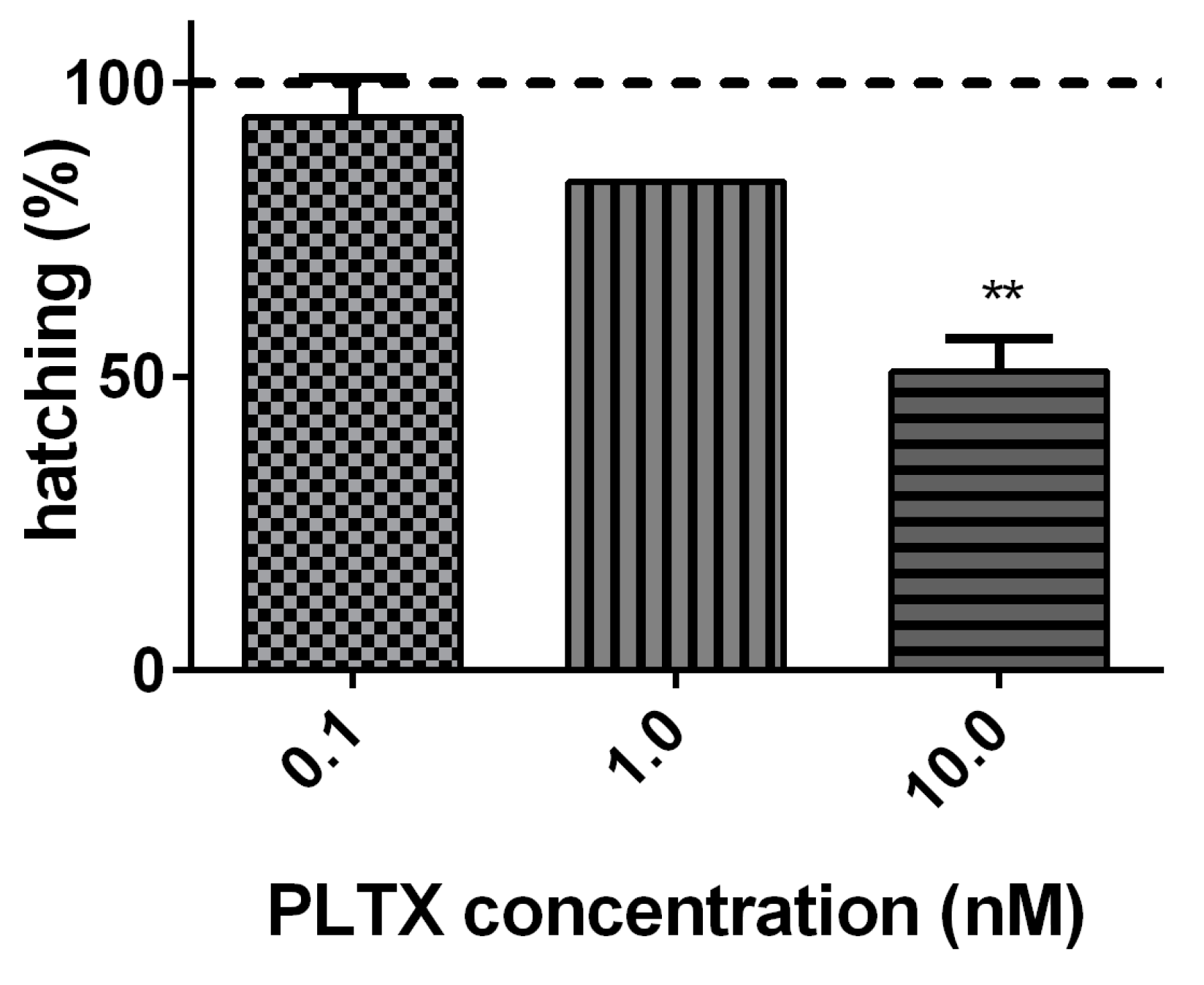
2.1. Effects of PLTX on A. franciscana Cysts Hatching
2.2. Effects of PLTX on A. franciscana Mortality
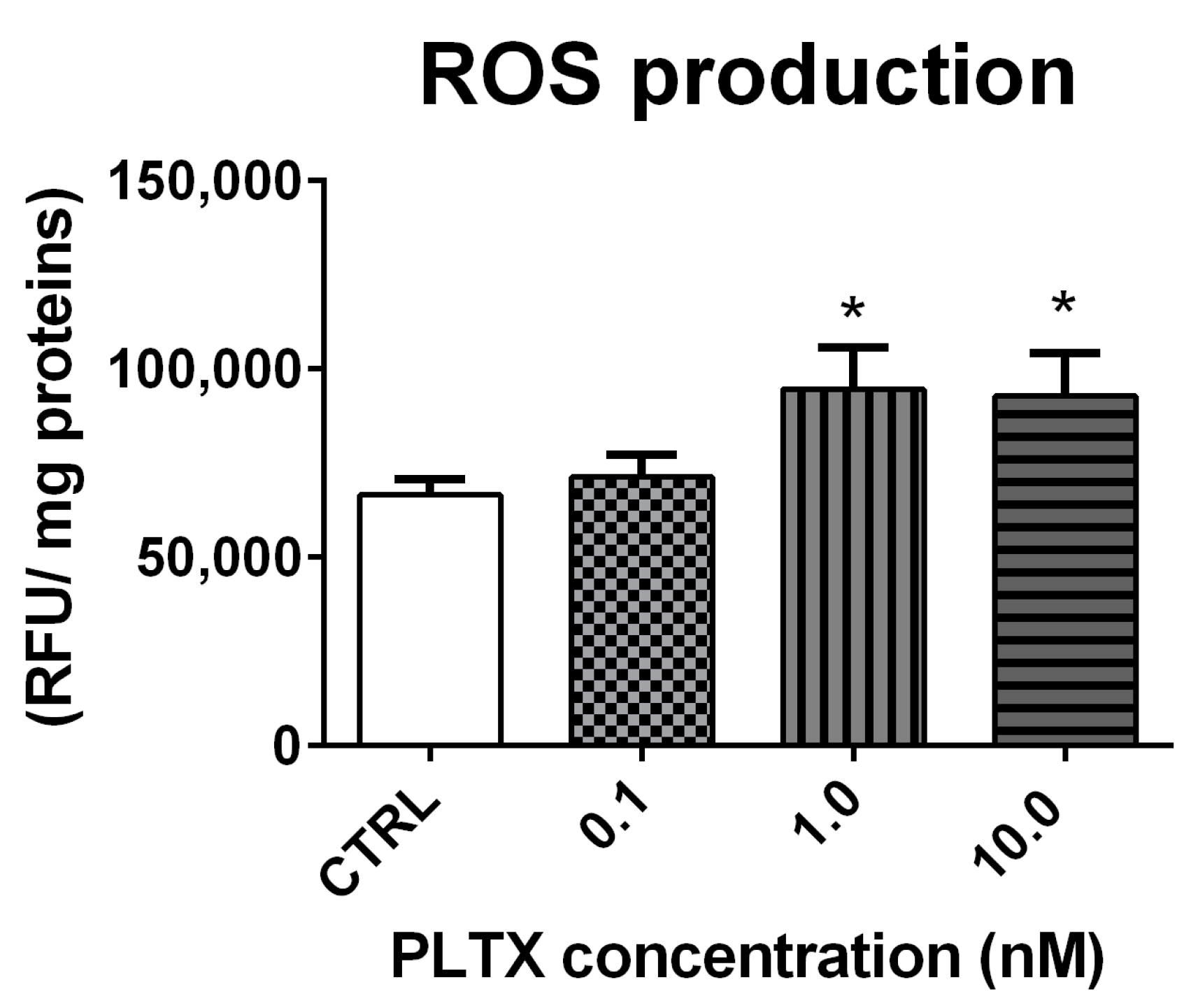
2.3. Effect of PLTX on ROS Production in A. franciscana Adults
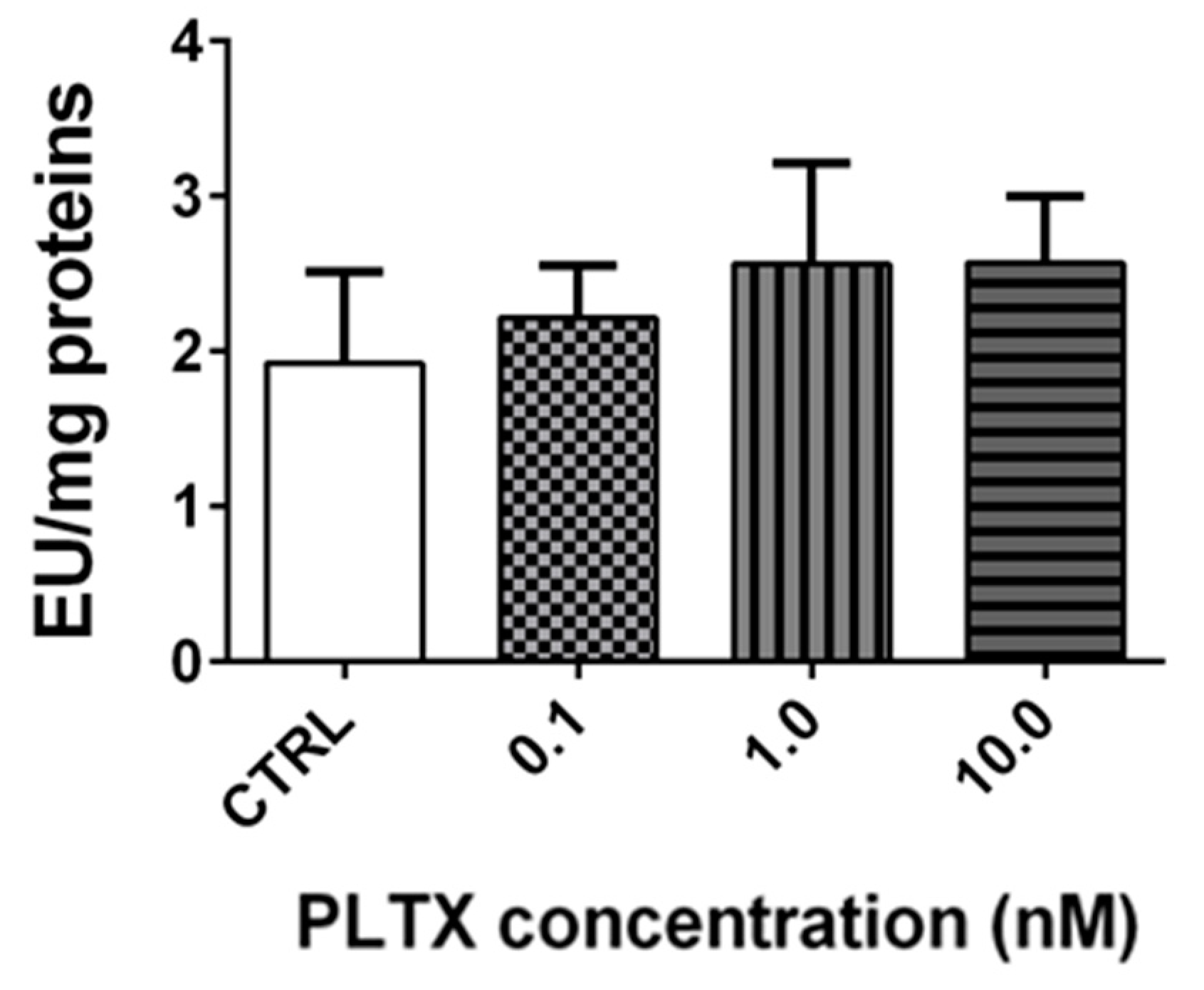
2.4. Effect of PLTX on Antioxidant Enzymes Activity in A. franciscana Adults
2.5. Effect of PLTX on Cholinesterase Activity in A. franciscana Adults
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Artemia franciscana
5.2. Palytoxin and Experimental Design
5.3. Hatching Assay
5.4. Mortality Assay
5.5. Reactive Oxygen Species Quantitation
5.6. Preparation of A. franciscana Samples for the Quantitation of the Enzymes’ Activity
5.7. Glutathione S-Transferase Activity
5.8. Catalase Activity
5.9. Peroxidase Activity
5.10. Superoxide Dismutase Activity
5.11. Cholinesterase Activity
5.12. Enzymes’ Activity Data Analysis
5.13. Protein Content
5.14. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Moore, R.E.; Scheuer, P.J. Palytoxin: A New Marine Toxin from a Coelenterate. Science 1971, 172, 495–498. [Google Scholar] [CrossRef]
- Rossini, G.P.; Bigiani, A. Palytoxin Action on the Na+,K+-ATPase and the Disruption of Ion Equilibria in Biological Systems. Toxicon 2011, 57, 429–439. [Google Scholar] [CrossRef]
- Gleibs, S.; Mebs, D.; Werding, B. Studies on the Origin and Distribution of Palytoxin in a Caribbean Coral Reef. Toxicon Off. J. Int. Soc. Toxinology 1995, 33, 1531–1537. [Google Scholar] [CrossRef]
- Usami, M.; Satake, M.; Ishida, S.; Inoue, A.; Kan, Y.; Yasumoto, T. Palytoxin Analogs from the Dinoflagellate Ostreopsis Siamensis. J. Am. Chem. Soc. 1995, 117, 5389–5390. [Google Scholar] [CrossRef]
- Kerbrat, A.S.; Amzil, Z.; Pawlowiez, R.; Golubic, S.; Sibat, M.; Darius, H.T.; Chinain, M.; Laurent, D. First Evidence of Palytoxin and 42-Hydroxy-Palytoxin in the Marine Cyanobacterium Trichodesmium. Mar. Drugs 2011, 9, 543–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Louzao, M.C.; Espiña, B.; Cagide, E.; Ares, I.R.; Alfonso, A.; Vieytes, M.R.; Botana, L.M. Cytotoxic Effect of Palytoxin on Mussel. Toxicon Off. J. Int. Soc. Toxinology 2010, 56, 842–847. [Google Scholar] [CrossRef] [PubMed]
- Granéli, E.; Vidyarathna, N.K.; Funari, E.; Cumaranatunga, P.R.T.; Scenati, R. Can Increases in Temperature Stimulate Blooms of the Toxic Benthic Dinoflagellate Ostreopsis Ovata? Harmful Algae 2011, 10, 165–172. [Google Scholar] [CrossRef]
- Larsson, M.E.; Laczka, O.F.; Suthers, I.M.; Ajani, P.A.; Doblin, M.A. Hitchhiking in the East Australian Current: Rafting as a Dispersal Mechanism for Harmful Epibenthic Dinoflagellates. Mar. Ecol. Prog. Ser. 2018, 596, 49–60. [Google Scholar] [CrossRef] [Green Version]
- Casabianca, S.; Capellacci, S.; Giacobbe, M.G.; Dell’Aversano, C.; Tartaglione, L.; Varriale, F.; Narizzano, R.; Risso, F.; Moretto, P.; Dagnino, A.; et al. Plastic-Associated Harmful Microalgal Assemblages in Marine Environment. Environ. Pollut. Barking Essex 1987 2019, 244, 617–626. [Google Scholar] [CrossRef]
- Masó, M.; Garcés, E.; Pagès, F.; Camp, J. Drifting Plastic Debris as a Potential Vector for Dispersing Harmful Algal Bloom (HAB) Species. Sci. Mar. 2003, 67, 107–111. [Google Scholar] [CrossRef] [Green Version]
- Accoroni, S.; Totti, C. The Toxic Benthic Dinoflagellates of the Genus Ostreopsis in Temperate Areas: A Review. Adv. Oceanogr. Limnol. 2016, 7, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Faimali, M.; Giussani, V.; Piazza, V.; Garaventa, F.; Corrà, C.; Asnaghi, V.; Privitera, D.; Gallus, L.; Cattaneo-Vietti, R.; Mangialajo, L.; et al. Toxic Effects of Harmful Benthic Dinoflagellate Ostreopsis Ovata on Invertebrate and Vertebrate Marine Organisms. Mar. Environ. Res. 2012, 76, 97–107. [Google Scholar] [CrossRef]
- Gorbi, S.; Bocchetti, R.; Binelli, A.; Bacchiocchi, S.; Orletti, R.; Nanetti, L.; Raffaelli, F.; Vignini, A.; Accoroni, S.; Totti, C.; et al. Biological Effects of Palytoxin-like Compounds from Ostreopsis Cf. Ovata: A Multibiomarkers Approach with Mussels Mytilus Galloprovincialis. Chemosphere 2012, 89, 623–632. [Google Scholar] [CrossRef]
- Neves, R.A.F.; Contins, M.; Nascimento, S.M. Effects of the Toxic Benthic Dinoflagellate Ostreopsis Cf. Ovata on Fertilization and Early Development of the Sea Urchin Lytechinus Variegatus. Mar. Environ. Res. 2018, 135, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Pagliara, P.; Caroppo, C. Toxicity Assessment of Amphidinium Carterae, Coolia Cfr. Monotis and Ostreopsis Cfr. Ovata (Dinophyta) Isolated from the Northern Ionian Sea (Mediterranean Sea). Toxicon 2012, 60, 1203–1214. [Google Scholar] [CrossRef] [PubMed]
- Privitera, D.; Giussani, V.; Isola, G.; Faimali, M.; Piazza, V.; Garaventa, F.; Asnaghi, V.; Cantamessa, E.; Cattaneo-Vietti, R.; Chiantore, M. Toxic Effects of Ostreopsis Ovata on Larvae and Juveniles of Paracentrotus Lividus. Harmful Algae 2012, 18, 16–23. [Google Scholar] [CrossRef]
- Gorbi, S.; Avio, G.C.; Benedetti, M.; Totti, C.; Accoroni, S.; Pichierri, S.; Bacchiocchi, S.; Orletti, R.; Graziosi, T.; Regoli, F. Effects of Harmful Dinoflagellate Ostreopsis Cf. Ovata Exposure on Immunological, Histological and Oxidative Responses of Mussels Mytilus Galloprovincialis. Fish Shellfish Immunol. 2013, 35, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Cen, J.; Cui, L.; Duan, Y.; Zhang, H.; Lin, Y.; Zheng, J.; Lu, S. Effects of Palytoxins Extracted from Ostreopsis Ovata on the Oxidative Stress and Immune Responses in Pacific White Shrimp Litopenaeus Vannamei. Fish Shellfish Immunol. 2019, 95, 670–678. [Google Scholar] [CrossRef]
- Pezzolesi, L.; Guerrini, F.; Ciminiello, P.; Dell’Aversano, C.; Iacovo, E.D.; Fattorusso, E.; Forino, M.; Tartaglione, L.; Pistocchi, R. Influence of Temperature and Salinity on Ostreopsis Cf. Ovata Growth and Evaluation of Toxin Content through HR LC-MS and Biological Assays. Water Res. 2012, 46, 82–92. [Google Scholar] [CrossRef]
- Neves, R.A.F.; Fernandes, T.; dos Santos, L.N.; Nascimento, S.M. Toxicity of Benthic Dinoflagellates on Grazing, Behavior and Survival of the Brine Shrimp Artemia Salina. PLoS ONE 2017, 12, e0175168. [Google Scholar] [CrossRef] [Green Version]
- Rhodes, L.; Adamson, J.; Suzuki, T.; Briggs, L.; Garthwaite, I. Toxic Marine Epiphytic Dinoflagellates, Ostreopsis Siamensis and Coolia Monotis (Dinophyceae), in New Zealand. N. Z. J. Mar. Freshw. Res. 2000, 34, 371–383. [Google Scholar] [CrossRef]
- Anne-Sophie, P.; Julie, R.; Laurence, G.-G.; Sophie, M.; Eva, T.; Thomas, O.P.; Rodolphe, L.; Stéphane, G. Effects of the Toxic Dinoflagellate Ostreopsis Cf. Ovata on Survival, Feeding and Reproduction of a Phytal Harpacticoid Copepod. J. Exp. Mar. Biol. Ecol. 2019, 516, 103–113. [Google Scholar] [CrossRef]
- Guppy, R.; Ackbarali, C.; Ibrahim, D. Toxicity of Crude Organic Extracts from the Zoanthid Palythoa Caribaeorum: A Biogeography Approach. Toxicon 2019, 167, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Varò, I.; Navarro, J.C.; Amat, F.; Guilhermino, L. Characterisation of Cholinesterases and Evaluation of the Inhibitory Potential of Chlorpyrifos and Dichlorvos to Artemia Salina and Artemia Parthenogenetica. Chemosphere 2002, 48, 563–569. [Google Scholar] [CrossRef]
- Persoone, G.; Wells, P.G. Artemia in Aquatic Toxicology: A Review. Artemia Res. Its Appl. 1987, 1, 259–275. [Google Scholar]
- Nunes, B.S.; Carvalho, F.D.; Guilhermino, L.M.; Van Stappen, G. Use of the Genus Artemia in Ecotoxicity Testing. Environ. Pollut. Barking Essex 1987 2006, 144, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Mackie, J.A.; Geller, J. Experimental Parameters Affecting Quantitative PCR of Artemia Franciscana: A Model for a Marine Zooplanktonic Target in Natural Plankton Samples. Limnol. Oceanogr. Methods 2010, 8, 337–347. [Google Scholar] [CrossRef]
- Rodd, A.L.; Creighton, M.A.; Vaslet, C.A.; Rangel-Mendez, J.R.; Hurt, R.H.; Kane, A.B. Effects of Surface-Engineered Nanoparticle-Based Dispersants for Marine Oil Spills on the Model Organism Artemia Franciscana. Environ. Sci. Technol. 2014, 48, 6419–6427. [Google Scholar] [CrossRef] [PubMed]
- Abatzopoulos, T.J.; Beardmore, J.; Clegg, J.S.; Sorgeloos, P. (Eds.) Artemia: Basic and Applied Biology; Biology of Aquatic Organisms; Springer: Dordrecht, The Netherlands, 2002; ISBN 978-1-4020-0746-0. [Google Scholar]
- Triantaphyllidis, G.; Abatzopoulos, T.; Sorgeloos, P. Review of the Biogeography of the Genus Artemia (Crustacea, Anostraca). J. Biogeogr. 1998, 25, 213–226. [Google Scholar] [CrossRef]
- Cooper, S.D.; Winkler, D.W.; Lenz, P.H. The Effect of Grebe Predation on a Brine Shrimp Population. J. Anim. Ecol. 1984, 53, 51–64. [Google Scholar] [CrossRef]
- Soto-Jiménez, M.F.; Arellano-Fiore, C.; Rocha-Velarde, R.; Jara-Marini, M.E.; Ruelas-Inzunza, J.; Voltolina, D.; Frías-Espericueta, M.G.; Quintero-Alvarez, J.M.; Páez-Osuna, F. Biological Responses of a Simulated Marine Food Chain to Lead Addition. Environ. Toxicol. Chem. 2011, 30, 1611–1617. [Google Scholar] [CrossRef]
- Ingarao, C.; Pagliani, T. First Report of an Ostreopsis Ovata Bloom on Abruzzo Coast (W Adriatic) Associated with Human Respiratory Intoxication. Harmful Algae News 2014, 48, 2–3. [Google Scholar]
- Sansoni, G.; Borghini, B.; Camici, G.; Casotti, M.; Righini, P.; Rustighi, C. Fioriture algali di Ostreopsis ovata (Gonyaulacales: Dinophyceae): Un problema emergente. Biol. Ambient. 2003, 17, 17–23. [Google Scholar]
- Guerrini, F.; Pezzolesi, L.; Feller, A.; Riccardi, M.; Ciminiello, P.; Dell’Aversano, C.; Tartaglione, L.; Iacovo, E.D.; Fattorusso, E.; Forino, M.; et al. Comparative Growth and Toxin Profile of Cultured Ostreopsis Ovata from the Tyrrhenian and Adriatic Seas. Toxicon 2010, 55, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Rotini, A.; Manfra, L.; Canepa, S.; Tornambè, A.; Migliore, L. Can Artemia Hatching Assay Be a (Sensitive) Alternative Tool to Acute Toxicity Test? Bull. Environ. Contam. Toxicol. 2015, 95, 745–751. [Google Scholar] [CrossRef]
- Libralato, G.; Prato, E.; Migliore, L.; Cicero, A.M.; Manfra, L. A Review of Toxicity Testing Protocols and Endpoints with Artemia spp. Ecol. Indic. 2016, 69, 35–49. [Google Scholar] [CrossRef] [Green Version]
- Sorgeloos, P.; Remiche-Van Der Wielen, C.; Persoone, G. The Use of Artemia Nauplii for Toxicity Tests—A Critical Analysis. Ecotoxicol. Environ. Saf. 1978, 2, 249–255. [Google Scholar] [CrossRef]
- Toğulga, M. The Short-Term Toxicity of Two Toxicants to Artemia Nauplii. Turk. J. Zool. 1998, 22, 259–266. [Google Scholar]
- Rajasree, S.R.R.; Kumar, V.G.; Abraham, L.S.; Manoharan, N. Assessment on the Toxicity of Engineered Nanoparticles on the Lifestages of Marine Aquatic Invertebrate Artemia Salina. Int. J. Nanosci. 2011, 10, 1153–1159. [Google Scholar] [CrossRef]
- Cavion, F.; Fusco, L.; Sosa, S.; Manfrin, C.; Alonso, B.; Zurutuza, A.; Loggia, R.D.; Tubaro, A.; Prato, M.; Pelin, M. Ecotoxicological Impact of Graphene Oxide: Toxic Effects on the Model Organism Artemia Franciscana. Environ. Sci. Nano 2020, 7, 3605–3615. [Google Scholar] [CrossRef]
- Pelin, M.; Zanette, C.; De Bortoli, M.; Sosa, S.; Loggia, R.D.; Tubaro, A.; Florio, C. Effects of the Marine Toxin Palytoxin on Human Skin Keratinocytes: Role of Ionic Imbalance. Toxicology 2011, 282, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Pelin, M.; Ponti, C.; Sosa, S.; Gibellini, D.; Florio, C.; Tubaro, A. Oxidative Stress Induced by Palytoxin in Human Keratinocytes Is Mediated by a H+-Dependent Mitochondrial Pathway. Toxicol. Appl. Pharmacol. 2013, 266, 1–8. [Google Scholar] [CrossRef]
- Pelin, M.; Sosa, S.; Pacor, S.; Tubaro, A.; Florio, C. The Marine Toxin Palytoxin Induces Necrotic Death in HaCaT Cells through a Rapid Mitochondrial Damage. Toxicol. Lett. 2014, 229, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Schieber, M.; Chandel, N.S. ROS Function in Redox Signaling and Oxidative Stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ott, M.; Gogvadze, V.; Orrenius, S.; Zhivotovsky, B. Mitochondria, Oxidative Stress and Cell Death. Apoptosis Int. J. Program. Cell Death 2007, 12, 913–922. [Google Scholar] [CrossRef] [PubMed]
- Boyland, E.; Chasseaud, L.F. The Role of Glutathione and Glutathione S-Transferases in Mercapturic Acid Biosynthesis. In Advances in Enzymology—and Related Areas of Molecular Biology; Nord, F.F., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006; pp. 173–219. ISBN 978-0-470-12277-8. [Google Scholar]
- Papadopoulos, A.I.; Lazaridou, E.; Mauridou, G.; Touraki, M. Glutathione S-Transferase in the Branchiopod Artemia Salina. Mar. Biol. 2004, 144, 295–301. [Google Scholar] [CrossRef]
- Livingstone, D.R. Organic Xenobiotic Metabolism in Marine Invertebrates. In Advances in Comparative and Environmental Physiology: Volume 7; Houlihan, D.F., Livingstone, D.R., Lee, R.F., Eds.; Springer: Berlin, Heidelberg, 1991; pp. 45–185. ISBN 978-3-642-75897-3. [Google Scholar]
- Barata, C.; Navarro, J.C.; Varo, I.; Riva, M.C.; Arun, S.; Porte, C. Changes in Antioxidant Enzyme Activities, Fatty Acid Composition and Lipid Peroxidation in Daphnia Magna during the Aging Process. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2005, 140, 81–90. [Google Scholar] [CrossRef]
- Nunes, B.; Carvalho, F.; Guilhermino, L. Effects of Widely Used Pharmaceuticals and a Detergent on Oxidative Stress Biomarkers of the Crustacean Artemia Parthenogenetica. Chemosphere 2006, 62, 581–594. [Google Scholar] [CrossRef]
- Viciano, E.; Monroig, Ó.; Barata, C.; Peña, C.; Navarro, J.C. Antioxidant Activity and Lipid Peroxidation in Artemia Nauplii Enriched with DHA-Rich Oil Emulsion and the Effect of Adding an External Antioxidant Based on Hydroxytyrosol. Aquac. Res. 2017, 48, 1006–1019. [Google Scholar] [CrossRef]
- Barata, C.; Varo, I.; Navarro, J.C.; Arun, S.; Porte, C. Antioxidant Enzyme Activities and Lipid Peroxidation in the Freshwater Cladoceran Daphnia Magna Exposed to Redox Cycling Compounds. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2005, 140, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Livingstone, D.R.; Martinez, P.G.; Michel, X.; Narbonne, J.F.; O’Hara, S.; Ribera, D.; Winston, G.W. Oxyradical Production as a Pollution-Mediated Mechanism of Toxicity in the Common Mussel, Mytilus Edulis L., and Other Molluscs. Funct. Ecol. 1990, 4, 415–424. [Google Scholar] [CrossRef]
- Stephensen, E.; Sturve, J.; Förlin, L. Effects of Redox Cycling Compounds on Glutathione Content and Activity of Glutathione-Related Enzymes in Rainbow Trout Liver. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2002, 133, 435–442. [Google Scholar] [CrossRef]
- Gonçalves-Soares, D.; Zanette, J.; Yunes, J.S.; Yepiz-Plascencia, G.M.; Bainy, A.C.D. Expression and Activity of Glutathione S-Transferases and Catalase in the Shrimp Litopenaeus Vannamei Inoculated with a Toxic Microcystis Aeruginosa Strain. Mar. Environ. Res. 2012, 75, 54–61. [Google Scholar] [CrossRef]
- Beattie, K.A.; Ressler, J.; Wiegand, C.; Krause, E.; Codd, G.A.; Steinberg, C.E.W.; Pflugmacher, S. Comparative Effects and Metabolism of Two Microcystins and Nodularin in the Brine Shrimp Artemia Salina. Aquat. Toxicol. Amst. Neth. 2003, 62, 219–226. [Google Scholar] [CrossRef]
- Olsen, T.; Ellerbeck, L.; Fisher, T.; Callaghan, A.; Crane, M. Variability in Acetylcholinesterase and Glutathione S-Transferase Activities in Chironomus Riparius Meigen Deployed in Situ at Uncontaminated Field Sites. Environ. Toxicol. Chem. 2001, 20, 1725–1732. [Google Scholar] [CrossRef] [PubMed]
- Ajuzie, C.C. Palatability and Fatality of the Dinoflagellate Prorocentrum Lima to Artemia Salina. J. Appl. Phycol. 2007, 19, 513–519. [Google Scholar] [CrossRef]
- Migliore, L.; Civitareale, C.; Brambilla, G.; Delupis, G.D.D. Toxicity of Several Important Agricultural Antibiotics to Artemia. Water Res. 1997, 31, 1801–1806. [Google Scholar] [CrossRef]
- Zulkifli, S.Z.; Aziz, F.Z.A.; Ajis, S.Z.M.; Ismail, A. Nauplii of Brine Shrimp (Artemia Salina) as a Potential Toxicity Testing Organism for Heavy Metals Contamination. In Proceedings of the From Sources to Solution; Aris, A.Z., Tengku Ismail, T.H., Harun, R., Abdullah, A.M., Ishak, M.Y., Eds.; Springer: Singapore, 2014; pp. 233–237. [Google Scholar]
- Zhu, S.; Luo, F.; Chen, W.; Zhu, B.; Wang, G. Toxicity Evaluation of Graphene Oxide on Cysts and Three Larval Stages of Artemia Salina. Sci. Total Environ. 2017, 595, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Jemec, A.; Tisler, T.; Drobne, D.; Sepcić, K.; Jamnik, P.; Ros, M. Biochemical Biomarkers in Chronically Metal-Stressed Daphnids. Comp. Biochem. Physiol. Toxicol. Pharmacol. CBP 2008, 147, 61–68. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, Y.; Duo, L. Biochemical Toxicity, Lysosomal Membrane Stability and DNA Damage Induced by Graphene Oxide in Earthworms. Environ. Pollut. Barking Essex 1987 2021, 269, 116225. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cavion, F.; Pelin, M.; Ponti, C.; Della Loggia, R.; Tubaro, A.; Sosa, S. Ecotoxicological Impact of the Marine Toxin Palytoxin on the Micro-Crustacean Artemia franciscana. Mar. Drugs 2022, 20, 81. https://doi.org/10.3390/md20020081
Cavion F, Pelin M, Ponti C, Della Loggia R, Tubaro A, Sosa S. Ecotoxicological Impact of the Marine Toxin Palytoxin on the Micro-Crustacean Artemia franciscana. Marine Drugs. 2022; 20(2):81. https://doi.org/10.3390/md20020081
Chicago/Turabian StyleCavion, Federica, Marco Pelin, Cristina Ponti, Roberto Della Loggia, Aurelia Tubaro, and Silvio Sosa. 2022. "Ecotoxicological Impact of the Marine Toxin Palytoxin on the Micro-Crustacean Artemia franciscana" Marine Drugs 20, no. 2: 81. https://doi.org/10.3390/md20020081
APA StyleCavion, F., Pelin, M., Ponti, C., Della Loggia, R., Tubaro, A., & Sosa, S. (2022). Ecotoxicological Impact of the Marine Toxin Palytoxin on the Micro-Crustacean Artemia franciscana. Marine Drugs, 20(2), 81. https://doi.org/10.3390/md20020081







