Sdy-1 Executes Antitumor Activity in HepG2 and HeLa Cancer Cells by Inhibiting the Wnt/β-Catenin Signaling Pathway
Abstract
:1. Introduction
2. Results
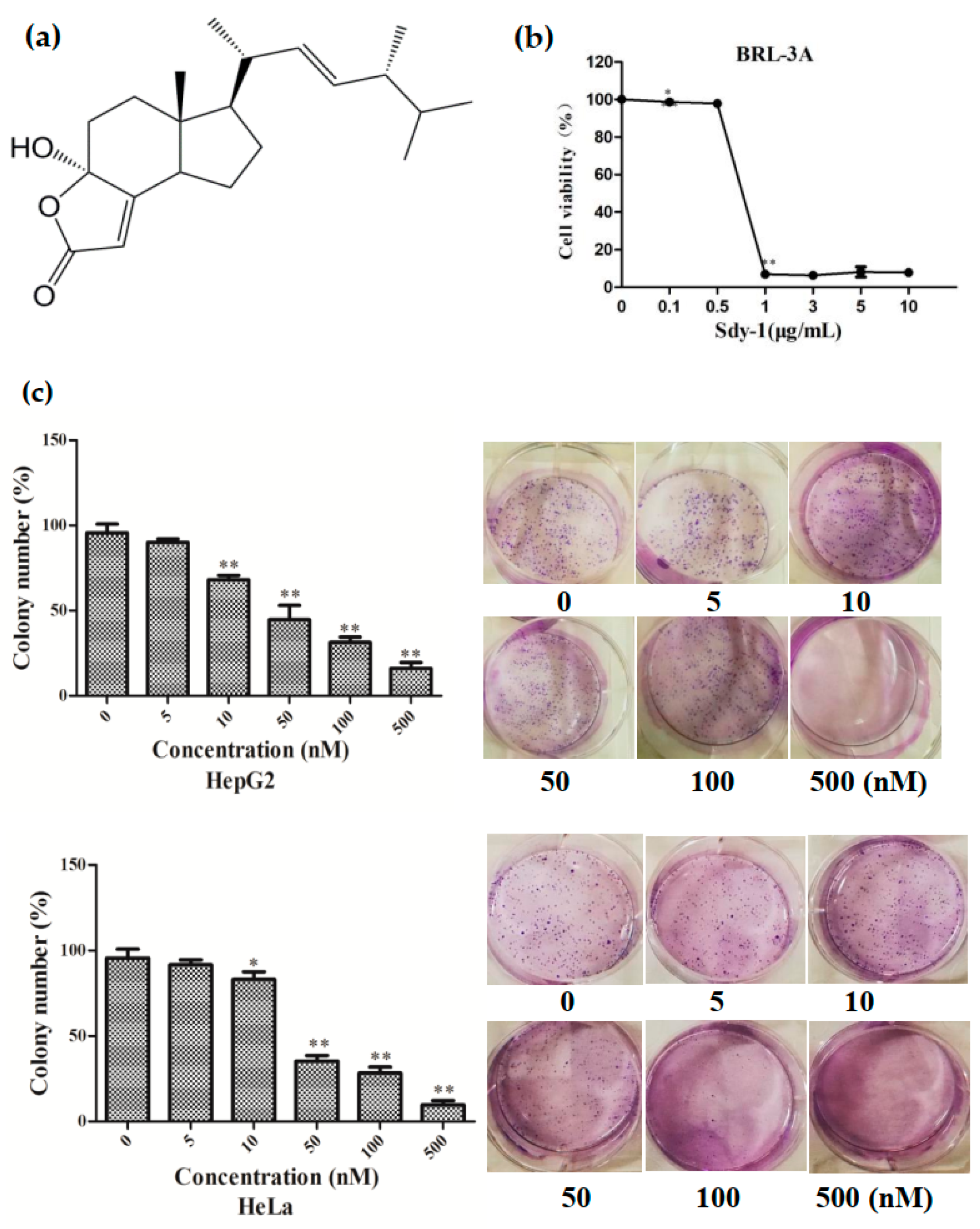
2.1. Sdy-1 Inhibits HepG2 and HeLa Cell Proliferation In Vitro
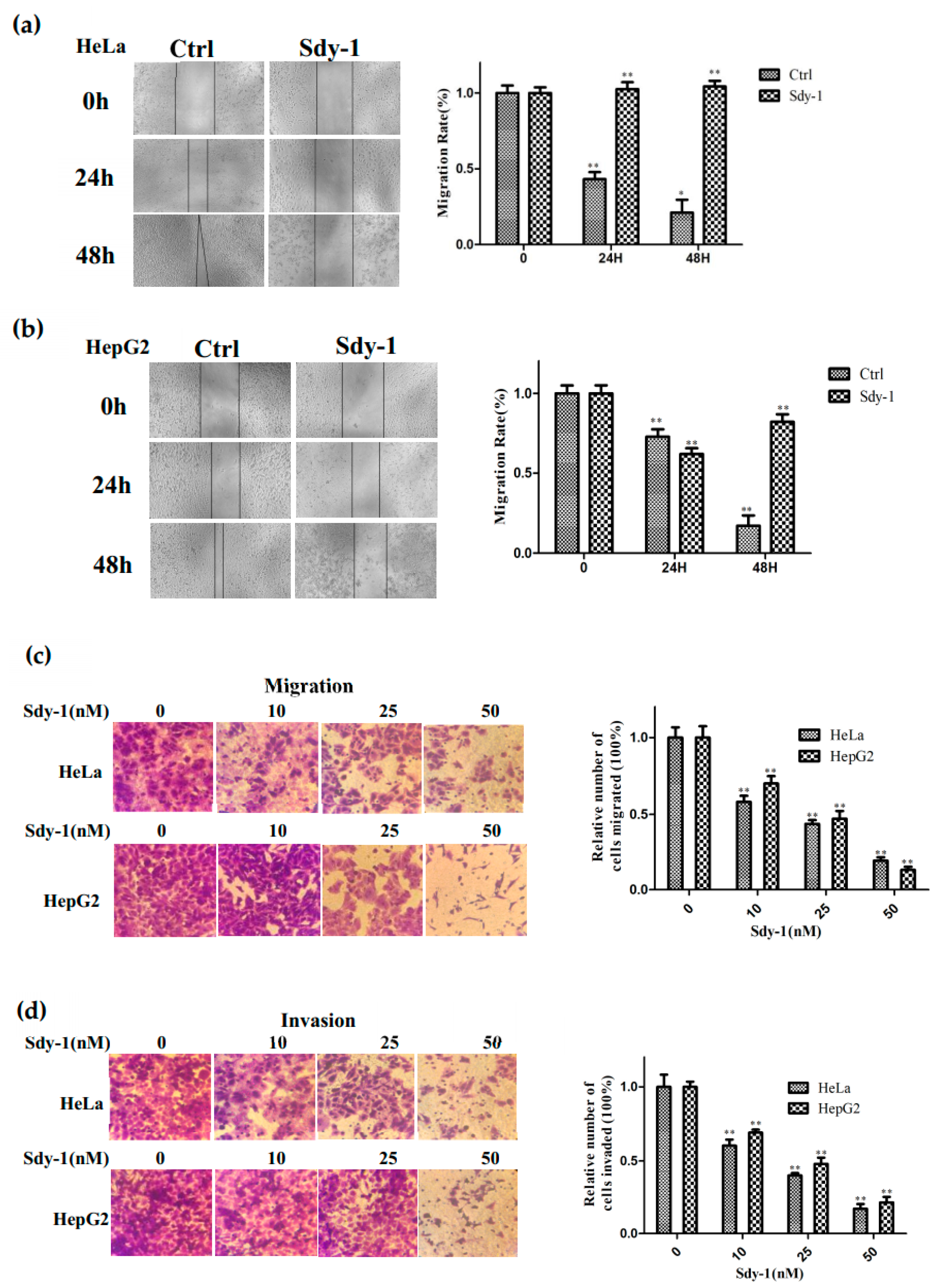
2.2. Sdy-1 Inhibits Migration and Invasion Progress of HepG2 and HeLa Cells
2.3. Sdy-1 Induces Cellular Apoptosis
2.4. Sdy-1 Induces G1 Arrest in HepG2 Cells
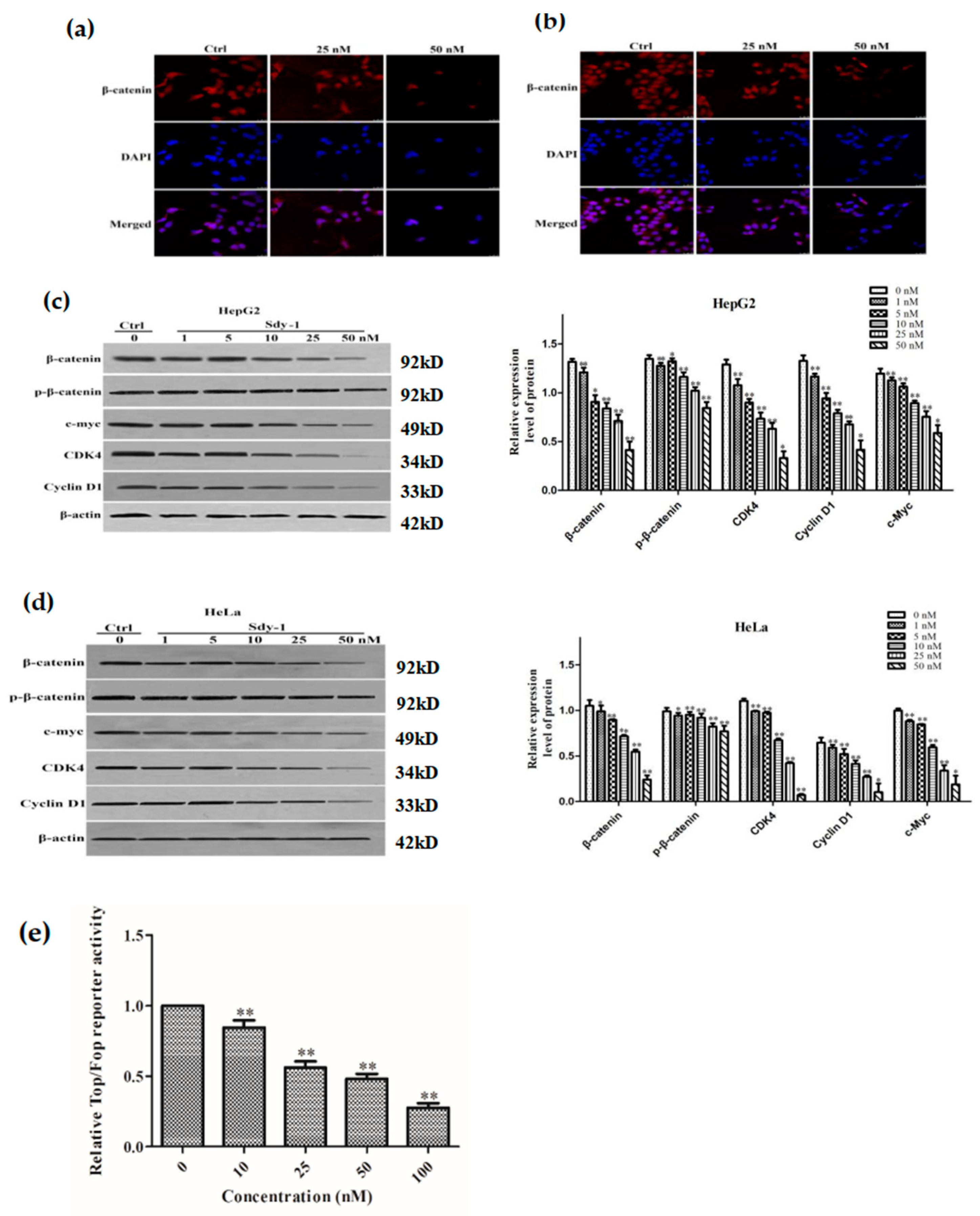
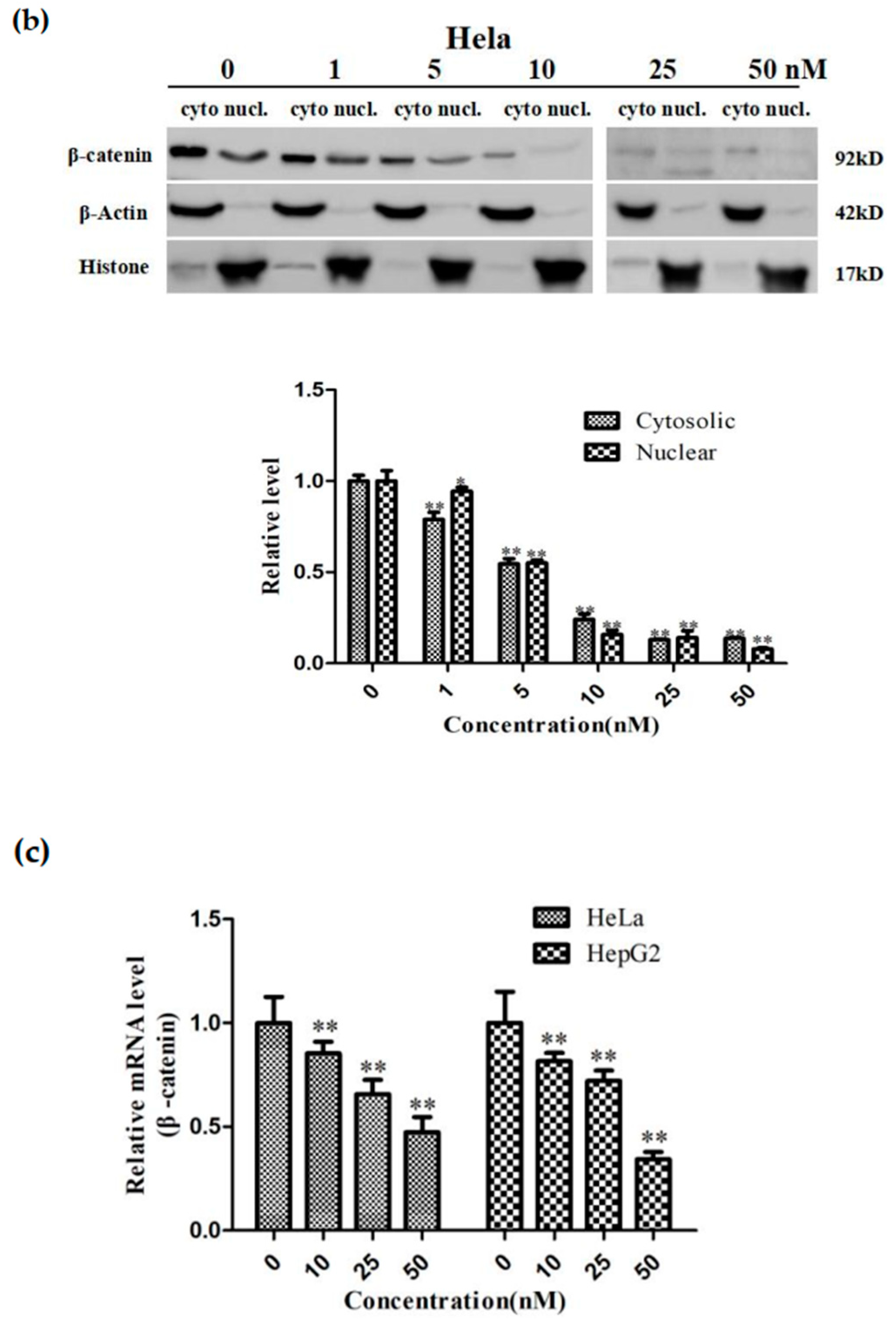
2.5. Sdy-1 Changes the Level of β-Catenin in the Cells
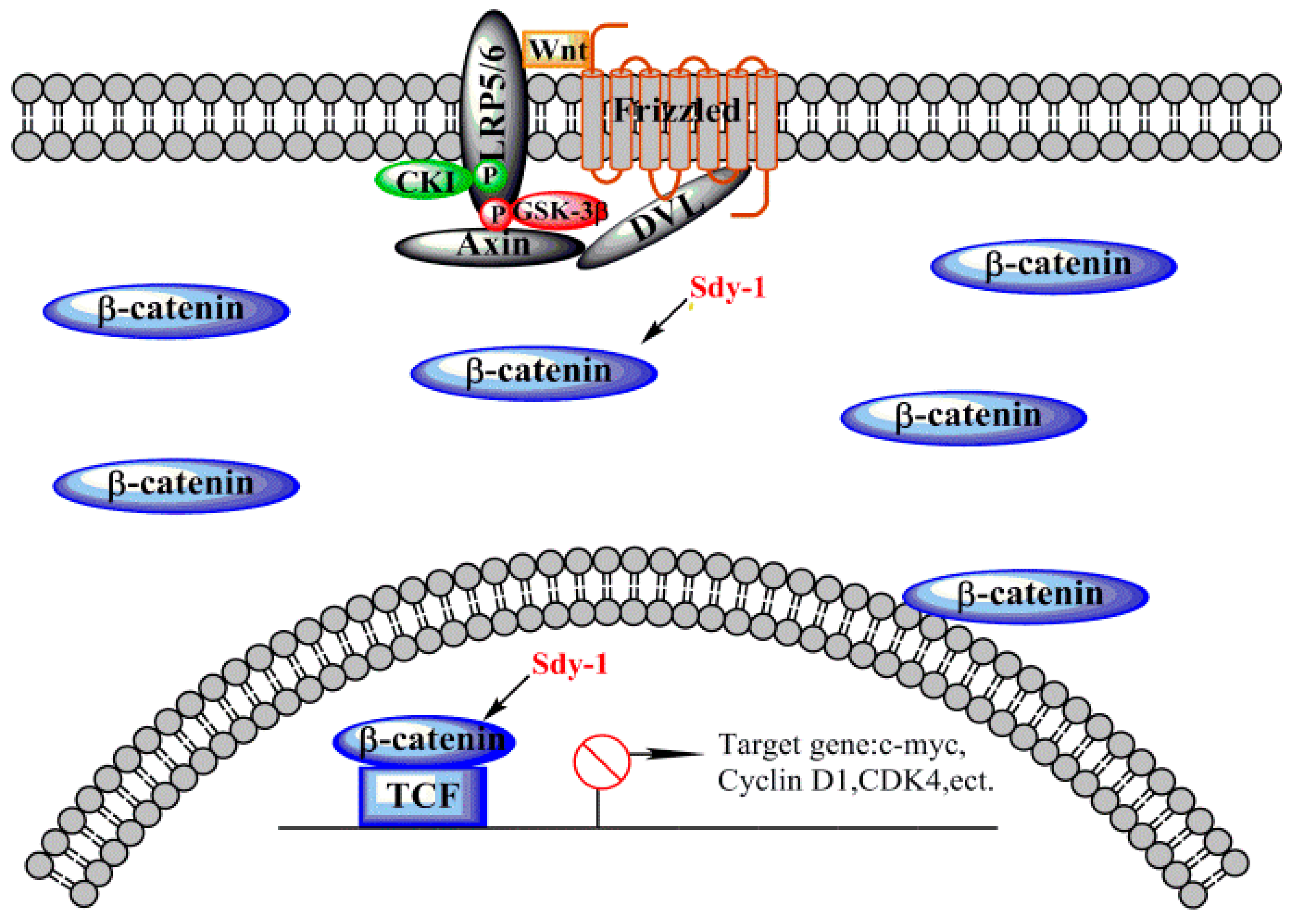
2.6. Sdy-1 Inhibits the Wnt Signaling Pathway
2.7. Sdy-1 Inhibits Transcription of the β-Catenin Gene in Cells
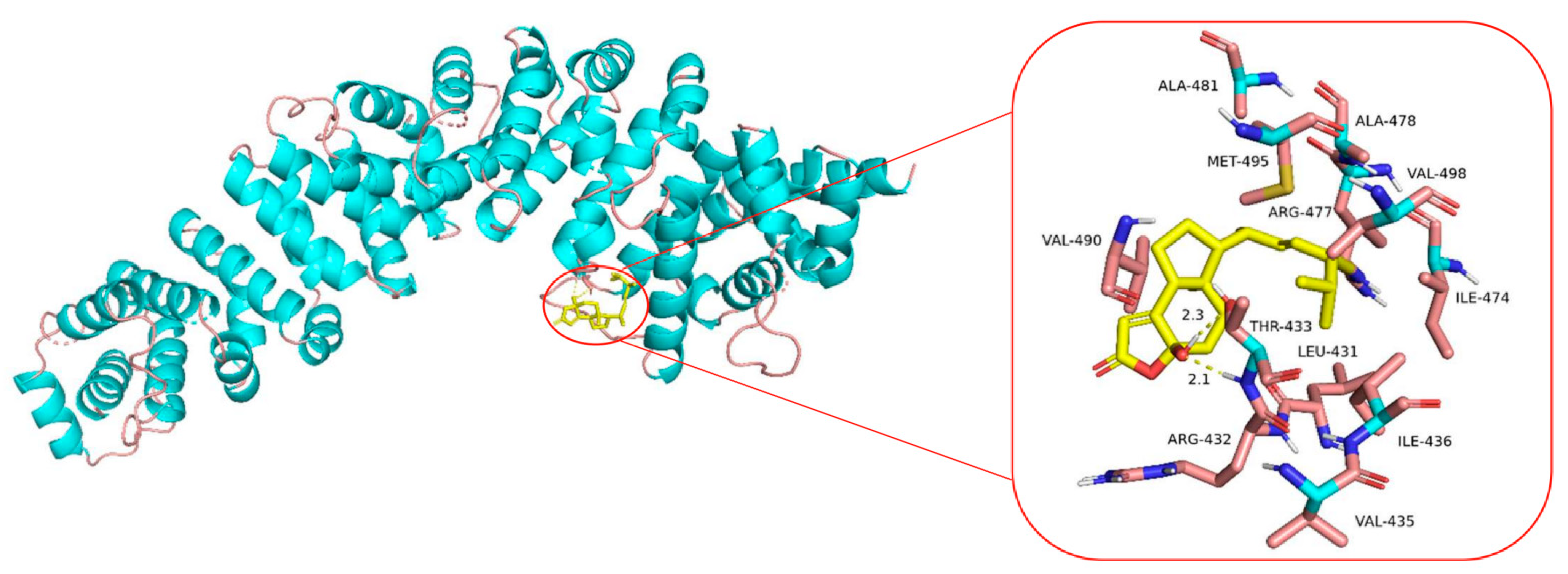
2.8. Molecular Docking Analysis of the Binding Interaction of Sdy-1 with β-Catenin
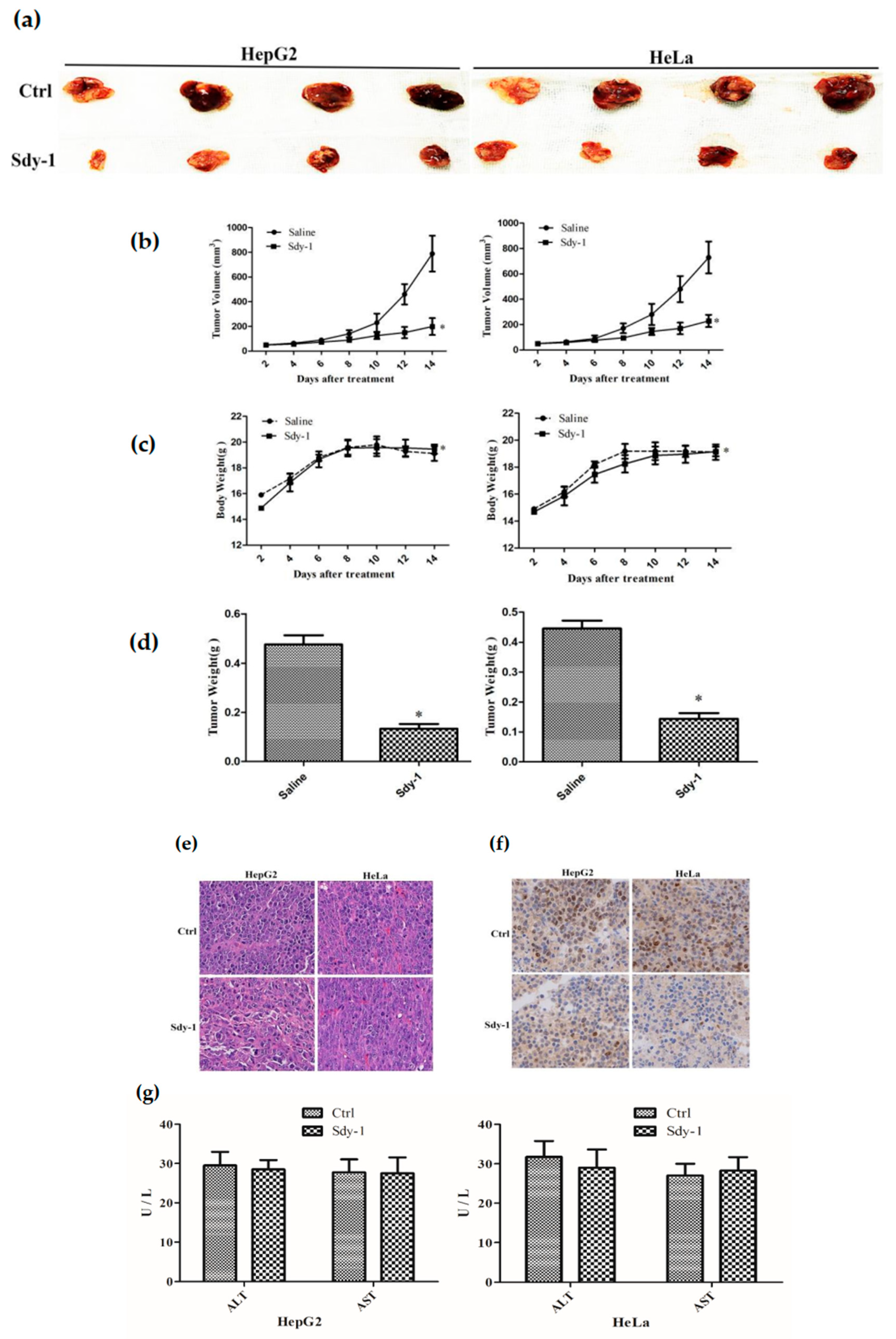
2.9. Sdy-1 Inhibits Xenograft Tumor Model
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Culture
4.3. MTT Assay
4.4. Colony Formation Assay
4.5. Scratch-Wound Assay
4.6. Transwell Assays
4.7. Analysis of Cell Apoptosis
4.8. Cell Cycle Analysis
4.9. Luciferase Reporter Gene Assay
4.10. Immunocytochemical Staining
4.11. Western Blot Analysis
4.12. q-PCR Analysis
4.13. Molecular Docking Analyses
4.14. Nude Mouse Xenograft Model
4.15. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Nusse, R.; Clevers, H. Wnt/β-catenin signaling, disease, and emerging therapeutic modalities. Cell 2017, 169, 985–999. [Google Scholar] [CrossRef]
- Martinez-Ferre, A.; Navarro-Garberi, M.; Bueno, C.; Martinez, S. Wnt signal specifies the intrathalamic limit and its organizer properties by regulating shh induction in the alar plate. J. Neurosci. 2013, 33, 3967–3980. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Li, B.; Zhou, L.; Yu, S.; Su, Z.; Song, J.; Sun, Q.; Sha, O.; Wang, X.; Jiang, W. Prodigiosin inhibits Wnt/β-catenin signaling and exerts anticancer activity in breast cancer cells. Proc. Natl. Acad. Sci. USA 2016, 113, 13150–13155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bisson, J.A.; Mills, B.; Paul, H.J.; Zwake, T.P.; David, C.E. Wnt5a and Wnt11 inhibit the canonical Wnt pathway and promote cardiac progenitor development via the Caspase-dependent degradation of AKT. Dev. Biol. 2015, 398, 80–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greer, E.R.; Chao, A.T.; Bejsovec, A. Pebble/ECT2 RhoGEF negatively regulates the Wingless/Wnt signaling pathway. Development 2013, 140, 4937–4946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiurillo, M.A. Role of the Wnt/β-catenin pathway in gastric cancer: An in-depth literature review. World J. Exp. Med. 2015, 5, 84–102. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Tan, Y.T.; Yang, Y.; Gao, J.; Li, H.L.; Zhen, W.; Lan, Q.; Rothman, N.; Shu, X.O.; Xiang, Y.B. Pre-diagnostic urinary 15-F2t -isoprostane level and liver cancer risk: Results from the Shanghai Women’s and Men’s Health Studies. Int. J. Cancer. 2018, 143, 1896–1903. [Google Scholar] [CrossRef] [Green Version]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Zheng, R.; Baade, P.D.; Zhang, S.; Zeng, H.; Bary, F.; Jemal, A.; Yu, X.Q.; He, J. Cancer statistics in China, 2015. CA Cancer J. Clin. 2016, 66, 115–132. [Google Scholar] [CrossRef] [Green Version]
- Sia, D.; Villanueva, A.; Friedman, S.L.; Llovet, J.M. Liver Cancer Cell of Origin, Molecular Class, and Effects on Patient Prognosis. Gastroenterology 2016, 152, 745–761. [Google Scholar] [CrossRef] [Green Version]
- Spector, L.G.; Birch, J. The epidemiology of hepatoblastoma. Pediatr. Blood Cancer 2012, 59, 776–779. [Google Scholar] [CrossRef]
- Ikeda, H.; Nakamura, Y. Trends in incidence of childhood malignant solid tumors in Japan: Estimation based on hospital-based registration. J. Pediatr. Surg. 2015, 50, 1506–1512. [Google Scholar] [CrossRef] [PubMed]
- Zsiros, J.; Brugieres, L.; Brock, P.; Roebuck, D.; Maibach, R.; Zimmermann, A.; Childs, M.; Pariente, D.; Lathier, V.; Otte, J.B.; et al. Dose-dense cisplatin-based chemotherapy and surgery for children with high-risk hepatoblastoma (SIOPEL-4): A prospective, single-arm, feasibility study. Lancet Oncol. 2013, 14, 834–842. [Google Scholar] [CrossRef] [Green Version]
- Kremer, N.; Walther, A.E.; Tiao, G.M. Management of hepatoblastoma: An update. Curr. Opin. Pediatr. 2014, 26, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Apte, U.; Micsenyi, A.; Kotsagrelos, E.; Luo, J.H.; Ranganathan, S.; Monga, D.K.; Bell, A.; Michalopoulos, G.K.; Monga, S.P.S. Epidermal growth factor receptor: A novel target of the Wnt/β-catenin pathway in liver. Gastroenterology 2005, 129, 285–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cairo, S.; Armengol, C.; De Reynies, A.; Wei, Y.; Thomas, E.; Renard, C.; Goga, A.; Balakrishnan, A.; Semeraro, M.; Gresh, L.; et al. Hepatic stem-like phenotype and interplay of Wnt/β-catenin and Myc signaling in aggressive childhood liver cancer. Cancer Cell 2008, 14, 471–484. [Google Scholar] [CrossRef]
- Armengol, C.; Cairo, S.; Fabre, M. Wnt signaling and hepatocarcinogenesis: The hepatoblastoma model. Int. J. Biochem. Cell Biol. 2011, 43, 265–270. [Google Scholar] [CrossRef]
- de Sanjosé, S.; Serrano, B.; Castellsagué, X.; Brotons, M.; Muñoz, J.; Bruni, L.; Bosch, F.X. Human papillomavirus (HPV) and related cancers in the Global Alliance for Vaccines and Immunization (GAVI) countries. A WHO/ICO HPV Information Centre Report. Vaccine 2012, 30, D1–D83. [Google Scholar]
- Dutta, S.; Biswas, N.; Muhkherjee, G. Evaluation of socio-demographic factors for non-compliance to treatment in locally advanced cases of cancer cervix in a Rural Medical College Hospital in India. Indian J. Palliat. Care 2013, 19, 158–165. [Google Scholar] [CrossRef]
- Uren, A.; Fallen, S.; Yuan, H.; Usubutun, A.; Kucukali, T.; Schlegel, R.; Toretsky, J.A. Activation of the canonical Wnt pathway during genital keratinocyte transformation: A model for cervical cancer progression. Cancer Res. 2005, 65, 6199–6206. [Google Scholar] [CrossRef] [Green Version]
- Shinohara, A.; Yokoyama, Y.; Wan, X.; Takahashi, Y.; Mori, Y.; Takami, T.; Shimokawa, K.; Tamaya, T. Cytoplasmic/nuclear expression without mutation of exon 3 of the β-catenin gene is frequent in the development of the neoplasm of the uterine cervix. Gynecol. Oncol. 2001, 82, 450–455. [Google Scholar] [CrossRef]
- Xu, J. Bioactive natural products derived from mangrove-associated microbes. RSC Adv. 2014, 5, 841–892. [Google Scholar] [CrossRef]
- Xu, J. Biomolecules Produced by Mangrove-Associated Microbes. Curr. Med. Chem. 2011, 18, 5224–5266. [Google Scholar] [CrossRef] [PubMed]
- Subban, K.; Subramani, R.; Johnpaul, M. A novel antibacterial and antifungal phenolic compound from the endophytic fungus Pestalotiopsis mangiferae. Nat. Prod. Res. 2013, 27, 1445–1449. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.F.; Jia, O.Y.; Wang, S.J.; Zhu, Q. A new bioactive diterpenoid from Pestalotiopsis adusta, an endophytic fungus from clerodendrum canescens. Nat. Prod. Res. 2016, 30, 2642–2647. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Ebada, S.S.; Proksch, P. Pestalotiops is a highly creative genus: Chemistry and bioactivity of secondary metabolites. Fungal Divers. 2010, 44, 15–31. [Google Scholar] [CrossRef]
- Xu, J.; Yang, X.; Lin, Q. Chemistry and biology of Pestalotiopsis-derived natural products. Fungal Divers. 2014, 66, 37–68. [Google Scholar] [CrossRef]
- Zhou, J.; Li, G.; Deng, Q.; Yang, X.; Xu, J. Cytotoxic constituents from the mangrove endophytic Pestalotiopsis sp. induce G0/G1 cell cycle arrest and apoptosis in human cancer cells. Nat. Prod. Res. 2018, 32, 2968–2972. [Google Scholar] [CrossRef]
- De Riccardis, F.; Spinella, A.; Izzo, I.; Giordano, A.; Sodano, G. Synthesis of (17R)-17-methylincisterol, a highly degraded marine steroid. Tetrahedron Lett. 1995, 36, 4303–4306. [Google Scholar] [CrossRef]
- Tsai, W.J.; Yang, S.C.; Huang, Y.L.; Chen, C.C.; Chuang, K.A.; Kuo, Y.C. 4-Hydroxy-17-methylincisterol from Agaricus blazei decreased cytokine production and cell proliferation in human peripheral blood mononuclear cells via Inhibition of NF-AT and NF-κB activation. Evid. Based Compl. Alt. 2013, 2013, 435916. [Google Scholar] [CrossRef] [Green Version]
- Ueguchi, Y.; Matsunami, K.; Otsuka, H.; Kondo, K. Constituents of cultivatedAgaricus blazei. J. Nat. Med. 2011, 65, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Mansoor, T.A.; Hong, J.; Lee, C.O.; Bae, S.J.; Im, K.S.; Jung, J.H. Cytotoxic sterol derivatives from a marine sponge homaxinella sp. J. Nat. Prod. 2005, 68, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, B.T.; Tamai, K.; He, X. Wnt/β-Catenin Signaling: Components, Mechanisms, and Diseases. Dev. Cell 2009, 17, 9–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Liang, F.; Chen, B.; Sun, Z.; Xue, T.; Yang, R.; Luo, D. Identification of demethylincisterol A3 as a selective inhibitor of protein tyrosine phosphatase Shp2. Eur. J. Pharmacol. 2017, 795, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Coad, J.; Ducatman, B.; Agazie, Y.M. SHP2 is up-regulated in breast cancer cells and in infiltrating ductal carcinoma of the breast, implying its involvement in breast oncogenesis. Histopathology 2008, 53, 389–402. [Google Scholar] [CrossRef] [PubMed]
- Sausgruber, N.; Coissieux, M.M.; Britschgi, A.; Wyckoff, J.; Aceto, N.; Leroy, C.; Stadler, M.B.; Voshol, H.; Bonenfant, D.; Bentires-Alj, M. Tyrosine phosphatase SHP2 increases cell motility in triple-negative breast cancer through the activation of SRC-family kinases. Oncogene 2015, 34, 2272–2278. [Google Scholar] [CrossRef]
- Korkut, C.; Ataman, B.; Ramachandran, P.; Ashley, J.; Barria, R.; Gherbesi, N.; Budnik, V. Trans-synaptic transmission of vesicular Wnt signals through Evi/Wntless. Cell 2009, 139, 393–404. [Google Scholar] [CrossRef] [Green Version]
- Sato, T.; Van Es, J.H.; Snippert, H.J.; Stange, D.E.; Vries, R.G.; Van Den Born, M.; Barker, N.; Shroyer, N.F.; Van Den Wetering, M.; Clevers, H. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 2010, 469, 415–418. [Google Scholar] [CrossRef] [Green Version]
- Strand, M.; Micchelli, C.A. Quiescent gastric stem cells maintain the adult Drosophila stomach. Proc. Natl. Acad. Sci. USA 2011, 108, 17696–17701. [Google Scholar] [CrossRef] [Green Version]
- Zoni, E.; Van Der Pluijm, G.; Gray, P.C.; Kruithof-de Julio, M. Epithelial plasticity in cancer: Unmasking a micro RNA network for TGF-β-, Notch-, and Wnt-mediated EMT. J. Oncol. 2015, 15, 753–760. [Google Scholar] [CrossRef] [Green Version]
- Komiya, Y.; Habas, R. Wnt signal transduction pathways. Organogenesis 2008, 4, 68–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, W.; Kim, M.; Jho, E.H. Wnt/β-catenin signalling: From plasma membrane to nucleus. Biochem. J. 2013, 450, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Duchartre, Y.; Kim, Y.M.; Kahn, M. The Wnt signaling pathway in cancer. Crit. Rev. Oncol. Hemat. 2016, 99, 141–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gill, B.S.; Kumar, S. Ganoderic Acid A Targeting β-Catenin in Wnt Signaling Pathway: In Silico and In Vitro Study. Interdiscip. Sci. 2018, 10, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Kar, M.; Roy, S.; Padhi, S.; Kumar, A.; Thakur, S.; Akhter, Y.; Gatto, G.; Banerjee, B. Inhibition of CD44 sensitizes cisplatin-resistance and affects Wnt/β-catenin signaling in HNSCC cells. Int. J. Bio. Macromol. 2020, 149, 501–512. [Google Scholar] [CrossRef]
- Cui, C.; Zhou, X.; Zhang, W.; Qu, Y.; Ke, X. Is β-Catenin a Druggable Target for Cancer Therapy? Trends Biochem. Sci. 2018, 43, 623–634. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock and AutoDockTools: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Y.; Cao, R.; Zhang, T.; Li, S.; Zhong, W. Design and synthesis of piperidine derivatives as novel human heat shock protein 70 inhibitors for the treatment of drug-resistant tumors. Eur. J. Med. Chem. 2015, 97, 19–31. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, M.; Zhou, D.; Wu, J.; Zhou, J.; Xu, J. Sdy-1 Executes Antitumor Activity in HepG2 and HeLa Cancer Cells by Inhibiting the Wnt/β-Catenin Signaling Pathway. Mar. Drugs 2022, 20, 125. https://doi.org/10.3390/md20020125
Sun M, Zhou D, Wu J, Zhou J, Xu J. Sdy-1 Executes Antitumor Activity in HepG2 and HeLa Cancer Cells by Inhibiting the Wnt/β-Catenin Signaling Pathway. Marine Drugs. 2022; 20(2):125. https://doi.org/10.3390/md20020125
Chicago/Turabian StyleSun, Mengyu, Dongdong Zhou, Jingwan Wu, Jing Zhou, and Jing Xu. 2022. "Sdy-1 Executes Antitumor Activity in HepG2 and HeLa Cancer Cells by Inhibiting the Wnt/β-Catenin Signaling Pathway" Marine Drugs 20, no. 2: 125. https://doi.org/10.3390/md20020125
APA StyleSun, M., Zhou, D., Wu, J., Zhou, J., & Xu, J. (2022). Sdy-1 Executes Antitumor Activity in HepG2 and HeLa Cancer Cells by Inhibiting the Wnt/β-Catenin Signaling Pathway. Marine Drugs, 20(2), 125. https://doi.org/10.3390/md20020125