A Dunaliella salina Extract Counteracts Skin Aging under Intense Solar Irradiation Thanks to Its Antiglycation and Anti-Inflammatory Properties
Abstract
:1. Introduction
2. Results
2.1. Dunaliella Salina Extract
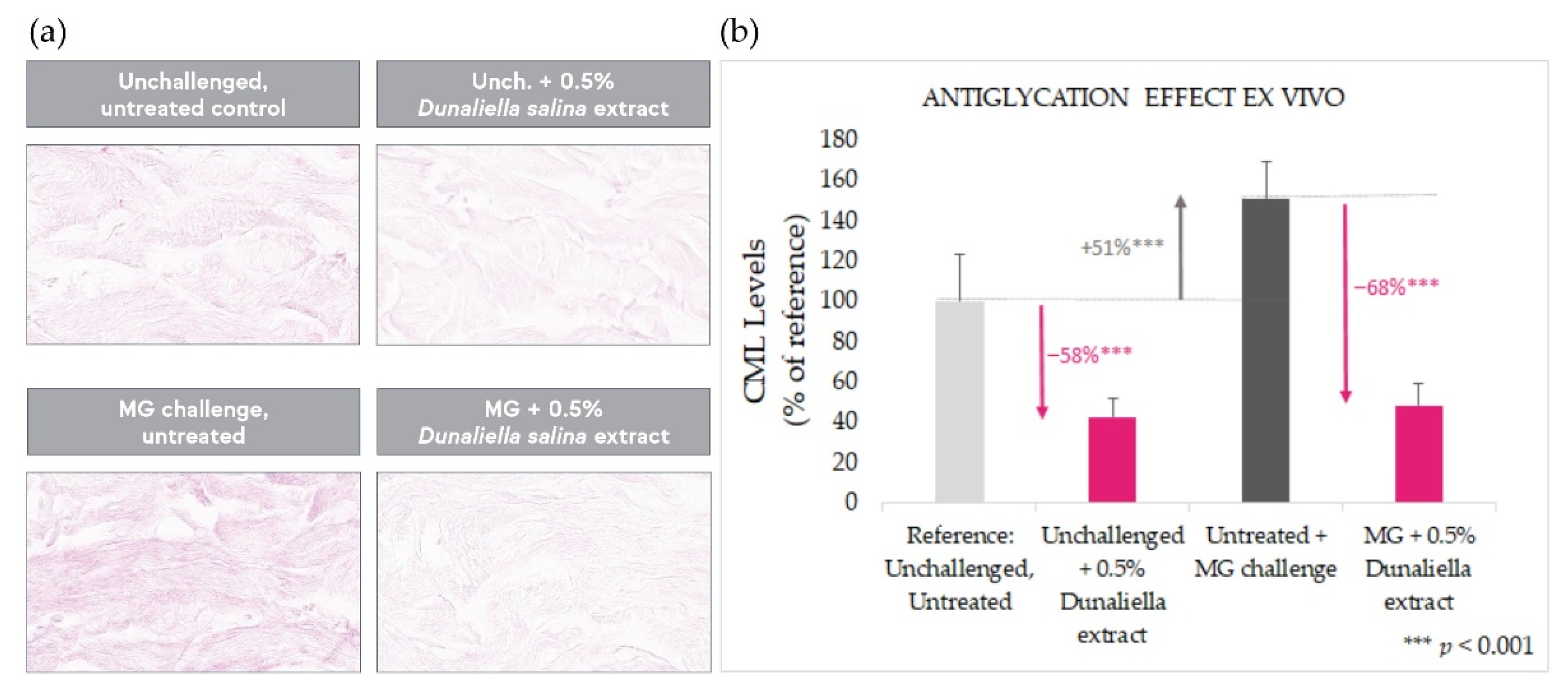
2.2. Antiglycation Effect in Skin Explants
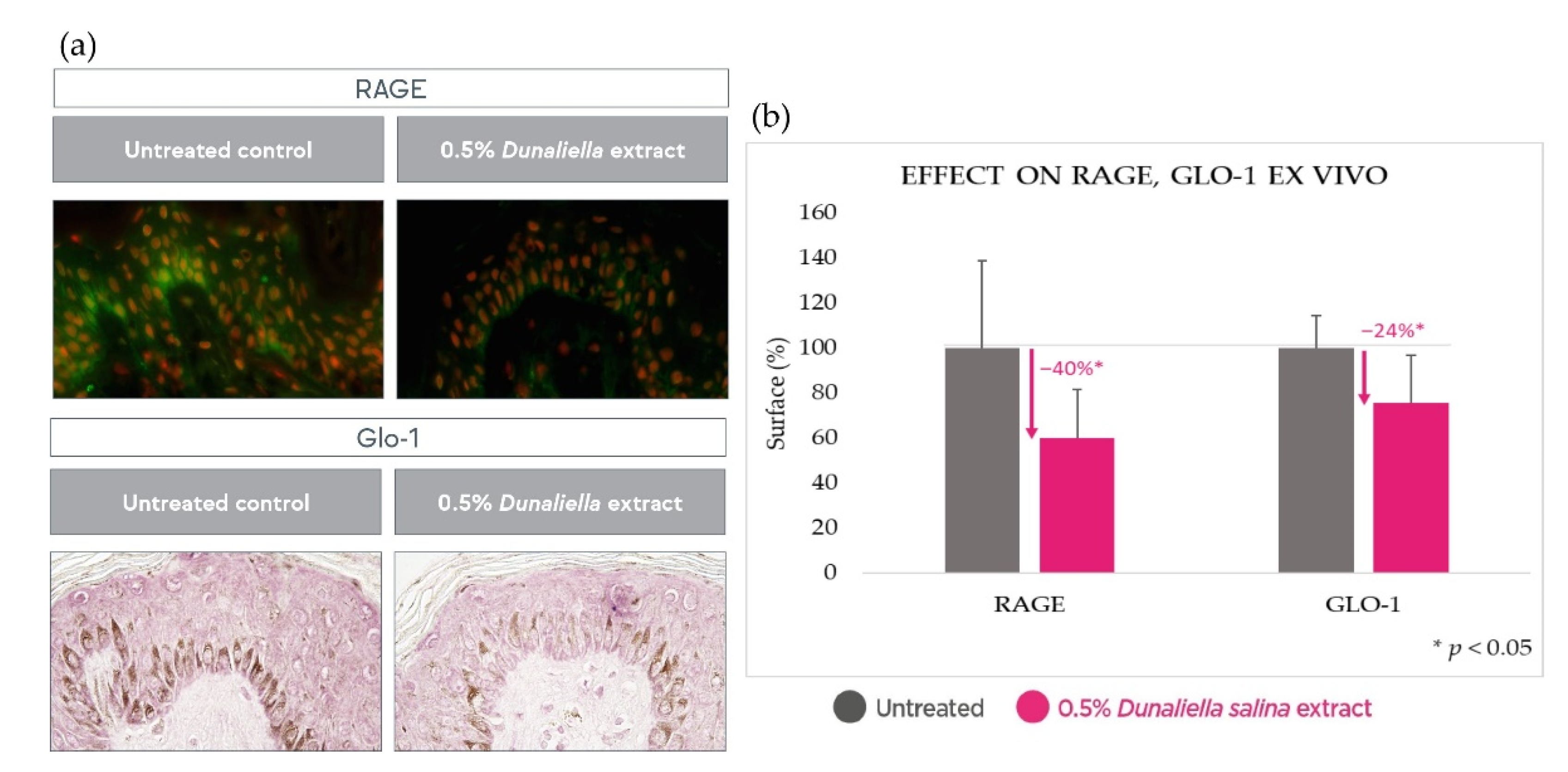
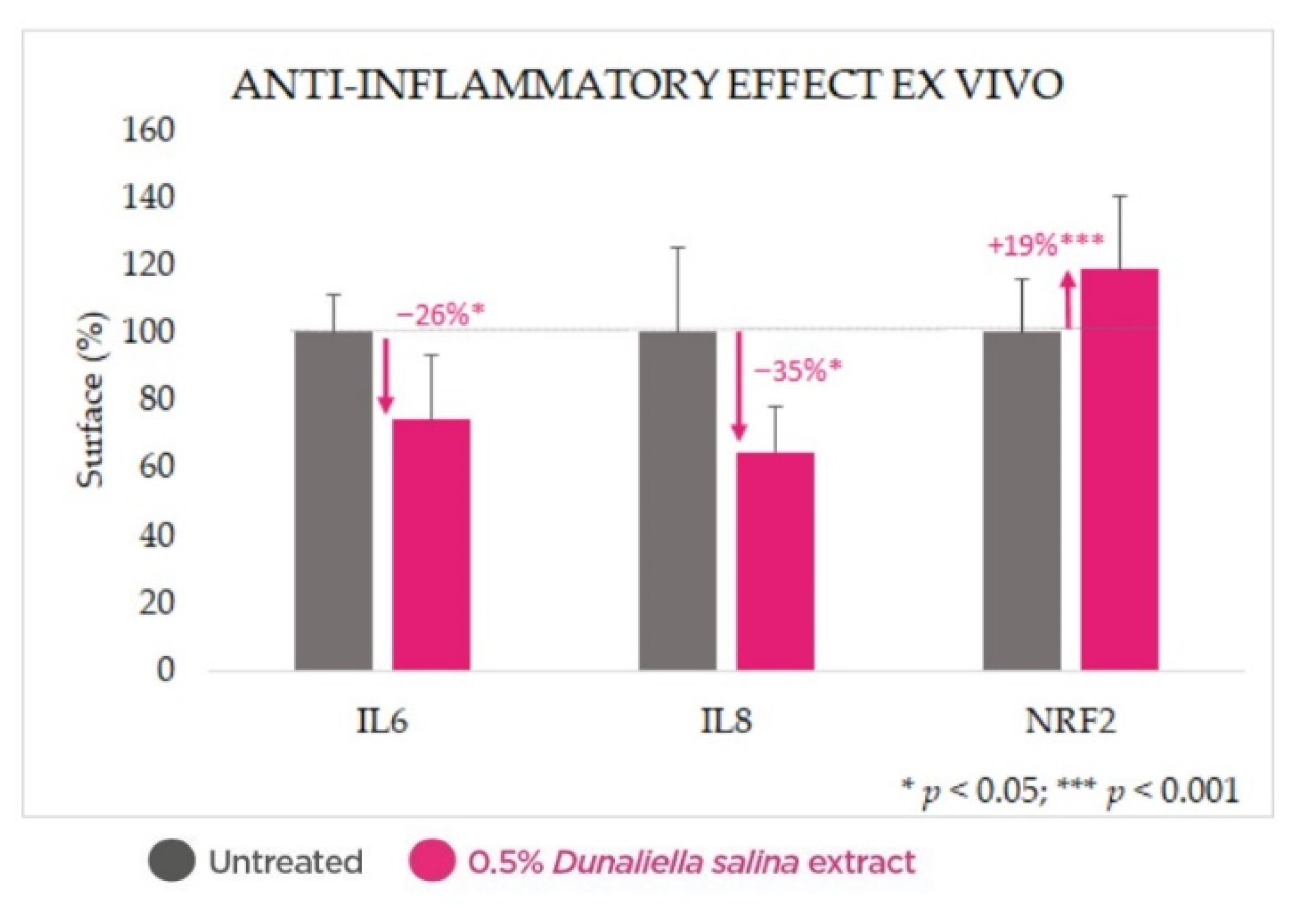
2.3. Anti-Inflammatory Effect in Skin Explants
2.4. Clinical Evaluation of Protective Effects under Intensive UV Irradiation
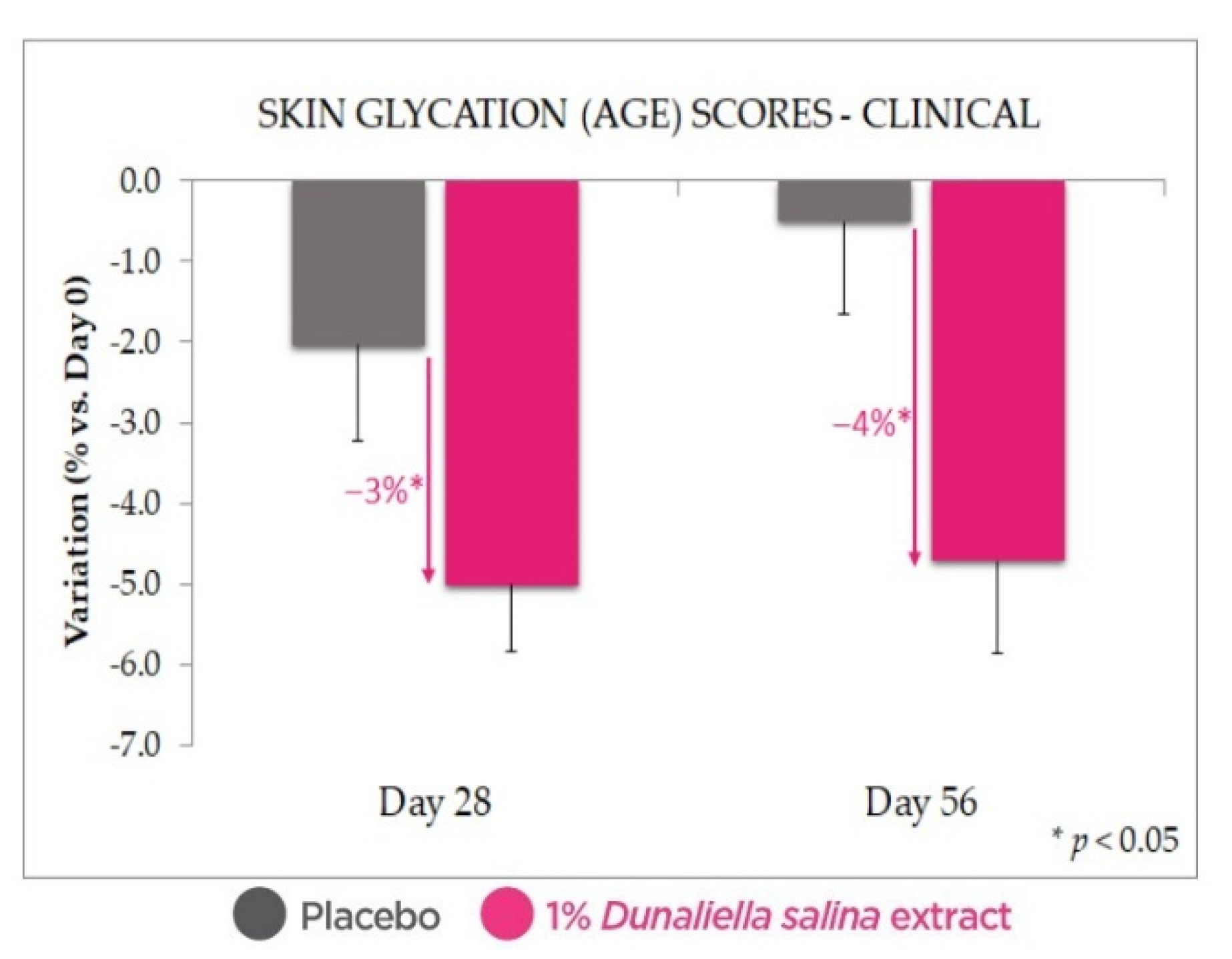
2.4.1. Antiglycation Effect
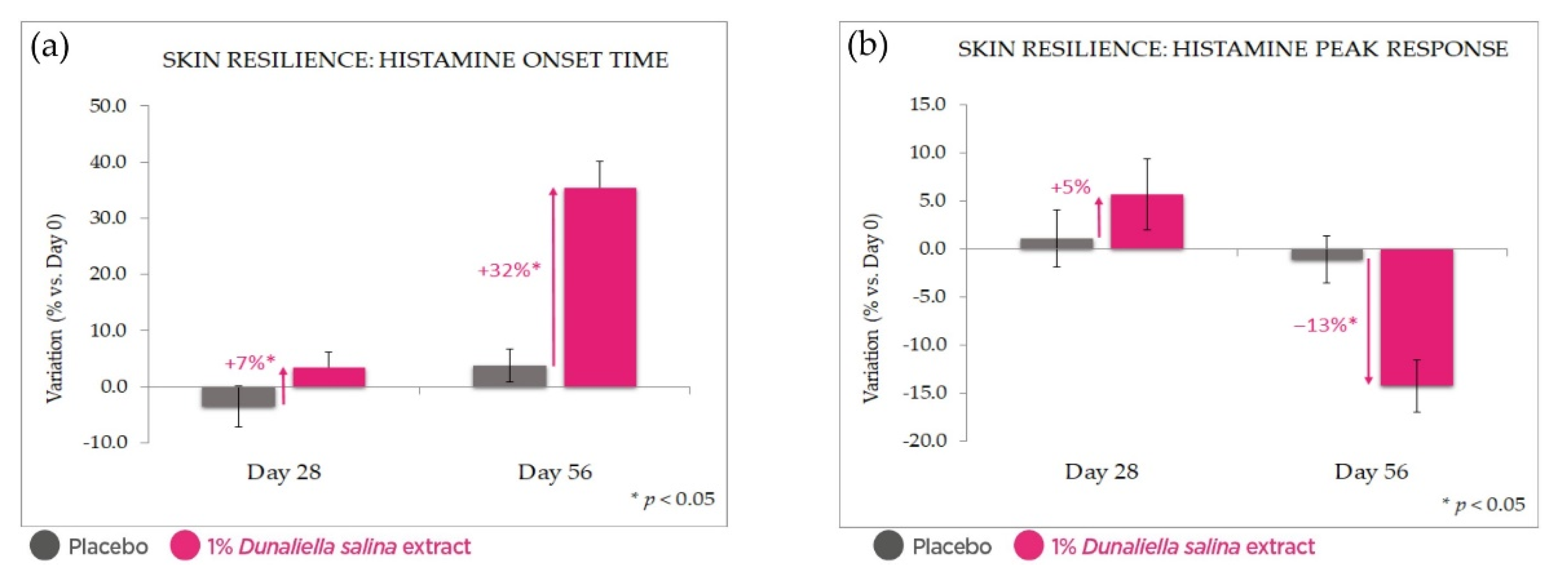
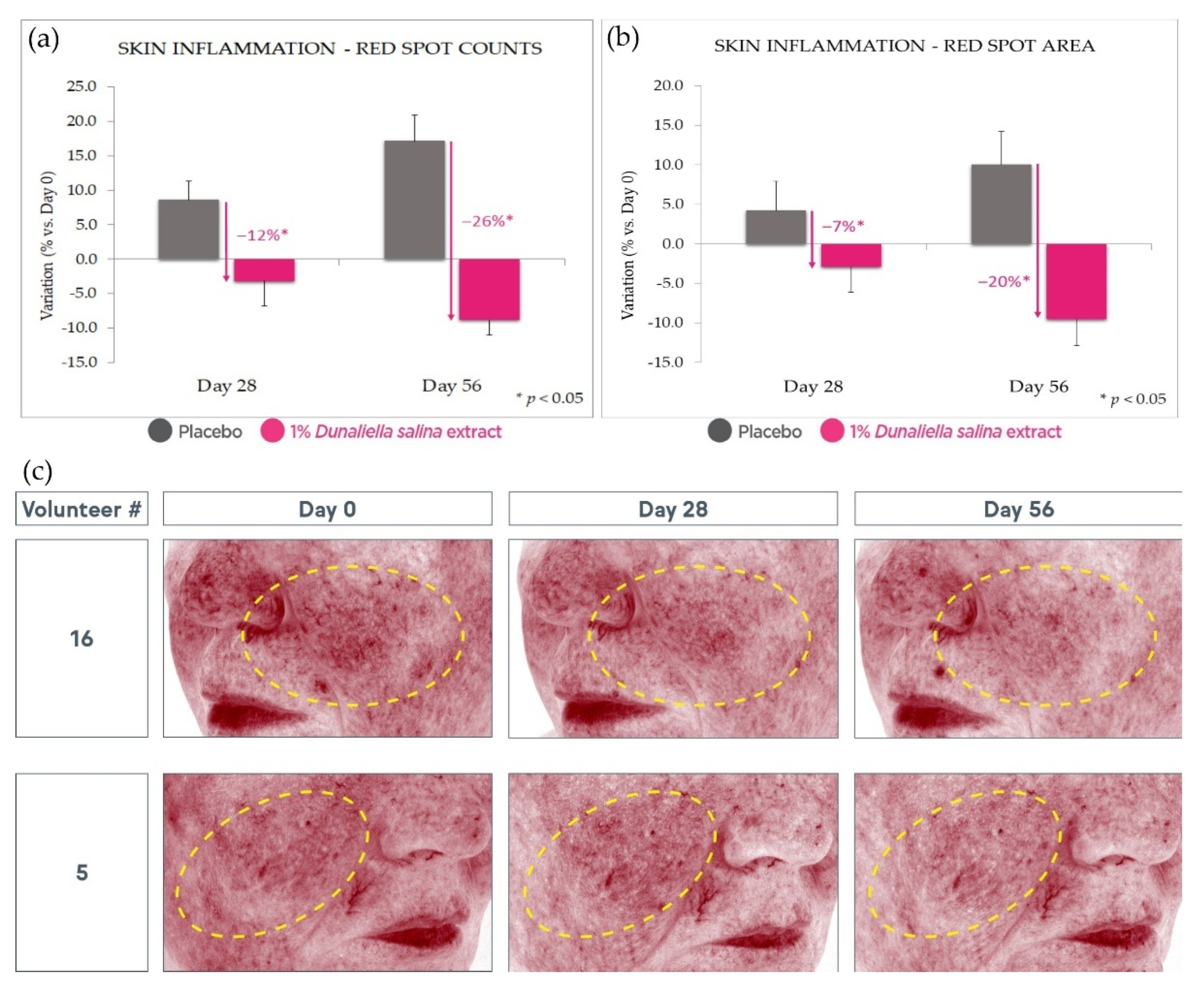
2.4.2. Anti-Inflammatory Effect
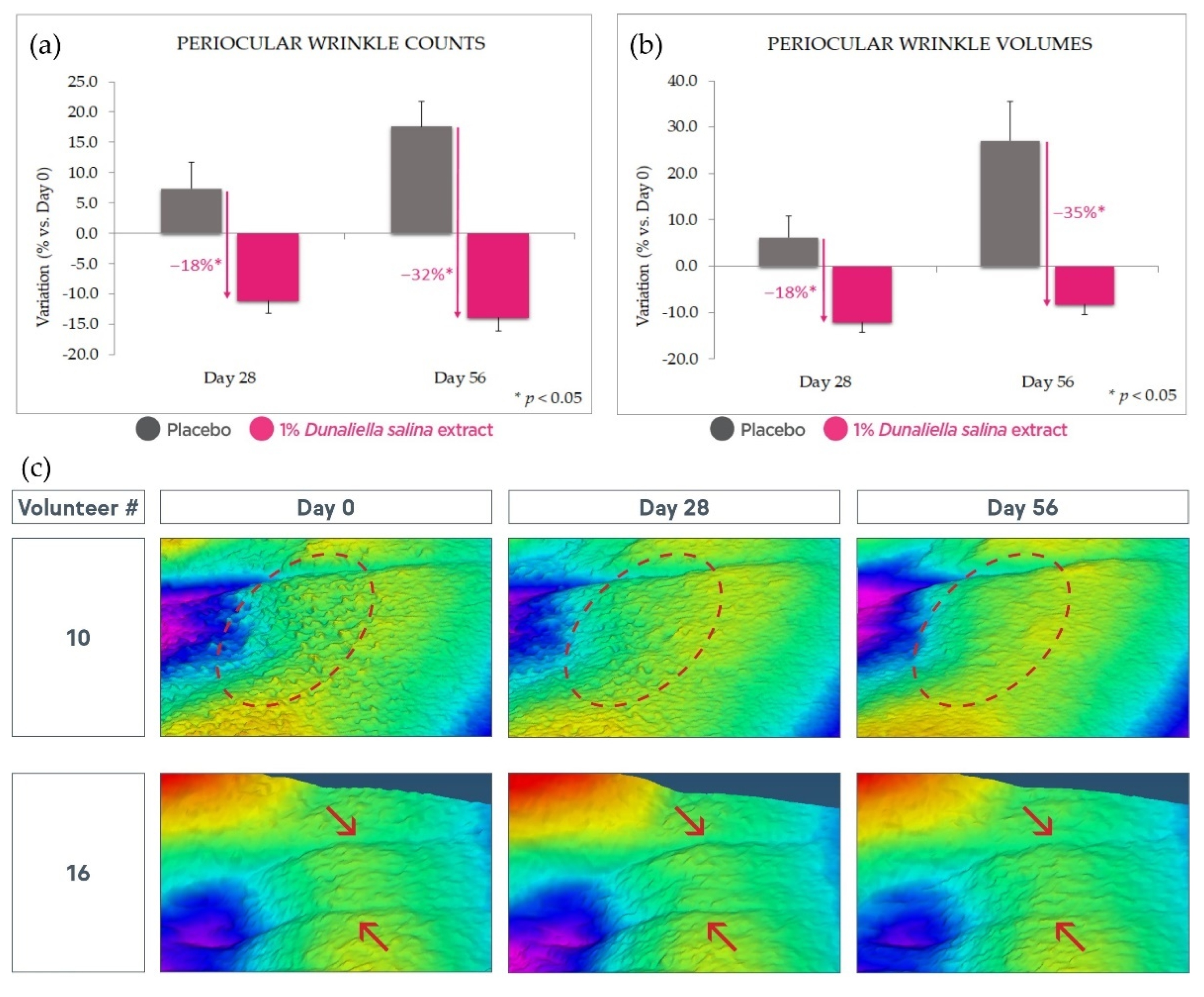
2.4.3. Antiaging Effect
3. Discussion and Conclusions
4. Materials and Methods
4.1. Extract
4.2. Antiglycation Effect of the Extract on Human Skin Explants
4.3. Anti-Inflammatory Effect of the Extract on Human Skin Explants
4.4. Clinical Evaluation of Protective and Antiaging Effects under Intense Solar Exposure
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, S.; Duan, E. Fighting against Skin Aging: The Way from Bench to Bedside. Cell Transplant. 2018, 27, 729–738. [Google Scholar] [CrossRef]
- Ichihashi, M.; Yagi, M.; Nomoto, K.; Yonei, Y. Glycation Stress and Photo-Aging in Skin. Anti-Aging Med. 2011, 8, 23–29. [Google Scholar] [CrossRef] [Green Version]
- Gkogkolou, P.; Böhm, M. Advanced glycation end products. Derm. Endocrinol. 2012, 4, 259–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lohwasser, C.; Neureiter, D.; Weigle, B.; Kirchner, T.; Schuppan, D. The receptor for advanced glycation end products is highly expressed in the skin and upregulated by advanced glycation end products and tumor necrosis factor-alpha. J. Investig. Dermatol. 2006, 126, 291–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicholl, I.D.; Stitt, A.W.; Moore, J.E.; Ritchie, A.J.; Archer, D.B.; Bucala, R. Increased levels of advanced glycation endproducts in the lenses and blood vessels of cigarette smokers. Mol. Med. 1998, 4, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, T.; Cai, W.; Peppa, M.; Dardaine, V.; Baliga, B.S.; Uribarri, J.; Vlassara, H. Advanced glycoxidation end products in commonly consumed foods. J. Am. Diet Assoc. 2004, 104, 1287–1291. [Google Scholar] [CrossRef] [PubMed]
- Uribarri, J.; Cai, W.; Peppa, M.; Goodman, S.; Ferrucci, L.; Striker, G. Circulating glycotoxins and dietary advanced glycation endproducts: Two links to inflammatory response, oxidative stress, and aging. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2007, 62, 427–433. [Google Scholar] [CrossRef] [Green Version]
- Baynes, J.W. The Maillard hypothesis on aging: Time to focus on DNA. Ann. N. Y. Acad. Sci. 2002, 959, 360–367. [Google Scholar] [CrossRef]
- Paul, R.G.; Bailey, A.J. Glycation of collagen: The basis of its central role in the late complications of ageing and diabetes. Int. J. Biochem. Cell Biol. 1996, 28, 1297–1310. [Google Scholar] [CrossRef]
- DeGroot, J. The AGE of the matrix: Chemistry, consequence and cure. Curr. Opin. Pharmacol. 2004, 4, 301–305. [Google Scholar] [CrossRef]
- Mizutari, K.; Ono, T.; Ikeda, K.; Kayashima, K.; Horiuchi, S. Photo-enhanced modification of human skin elastin in actinic elastosis by N(epsilon)-(carboxymethyl)lysine, one of the glycoxidation products of the Maillard reaction. J. Investig. Dermatol. 1997, 108, 797–802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, P.; Ren, M.; Yang, C.; Hu, Y.X.; Ran, J.M.; Yan, L. Involvement of RAGE, MAPK and NFκB pathways in AGEs-induced MMP-9 activation in HaCaT keratinocytes. Exp. Dermatol. 2012, 21, 123–129. [Google Scholar] [CrossRef]
- Lee, E.J.; Kim, J.Y.; Oh, S.H. Advanced glycation end products (AGEs) promote melanogenesis through receptor for AGEs. Sci. Rep. 2016, 6, 27848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hollenbach, M. The Role of Glyoxalase-I (Glo-I), Advanced Glycation Endproducts (AGEs), and Their Receptor (RAGE) in Chronic Liver Disease and Hepatocellular Carcinoma (HCC). Int. J. Mol. Sci. 2017, 18, 2466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohmori, S.; Mori, M.; Shiraha, K.; Kawase, M. Biosynthesis and degradation of methylglyoxal in animals. Prog. Clin. Biol. Res. 1989, 290, 397–412. [Google Scholar] [PubMed]
- Thornalley, P.J. The glyoxalase system in health and disease. Mol. Asp. Med. 1993, 14, 287–371. [Google Scholar] [CrossRef]
- Thornalley, P.J. The glyoxalase system: New developments towards functional characterization of a metabolic pathway fundamental to biological life. Biochem. J. 1990, 269, 1–11. [Google Scholar] [CrossRef]
- Odjakova, M.; Popova, E.; Al Sharif, M.; Mironova, R. Plant-Derived Agents with Anti-Glycation Activity. In Glycosylation; Petrescu, S., Ed.; IntechOpen: London, UK, 2012; pp. 223–256. [Google Scholar]
- Chen, F. High cell density culture of microalgae in heterotrophic growth. Trends Biotechnol. 1996, 14, 421–426. [Google Scholar] [CrossRef]
- Polle, J.E.W.; Tran, D.; Ben-Amotz, A. History, Distribution, and Habitats of Algae of the Genus Dunaliella Teodoresco (Chlorophyceae). In The Alga Dunaliella; Ben-Amotz, A., Polle, J.E.W., Rao, D.V.S., Eds.; CRC Press: New York, NY, USA, 2009; pp. 1–14. [Google Scholar]
- Oren, A. A hundred years of Dunaliella research: 1905–2005. Saline Syst. 2005, 1, 2. [Google Scholar] [CrossRef] [Green Version]
- Ben-Amotz, A.; Lers, A.; Avron, M. Stereoisomers of β-Carotene and Phytoene in the Alga Dunaliella bardawil. Plant Physiol. 1988, 86, 1286–1291. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.; Liu, J.; Zeng, X.; Huangfu, J.; Jiang, Y.; Wang, M.; Chen, F. Protective actions of microalgae against endogenous and exogenous advanced glycation endproducts (AGEs) in human retinal pigment epithelial cells. Food Funct. 2011, 2, 251–258. [Google Scholar] [CrossRef]
- Sun, Z.; Peng, X.; Liu, J.; Fan, K.W.; Wang, M.; Chen, F. Inhibitory effect of microalgal extracts on the formation of advanced glycation endproducts (AGEs). Food Chem. 2010, 120, 261–267. [Google Scholar] [CrossRef]
- Von Oppen-Bezalel, L.; Shaish, A. Application of the Colorless Carotenoids, Phytoene, and Phytofluene in Cosmetics, Wellness, Nutrition, and Therapeutics. In The Alga Dunaliella; Ben-Amotz, A., Polle, J.E.W., Rao, D.V.S., Eds.; CRC Press: New York, NY, USA, 2009; pp. 423–444. [Google Scholar]
- Gad, H.A.; Roberts, A.; Hamzi, S.H.; Gad, H.A.; Touiss, I.; Altyar, A.E.; Kensara, O.A.; Ashour, M.L. Jojoba Oil: An Updated Comprehensive Review on Chemistry, Pharmaceutical Uses, and Toxicity. Polymers 2021, 13, 1711. [Google Scholar] [CrossRef]
- Melendez-Martinez, A.J.; Stinco, C.M.; Liu, C.; Wang, X.-D. A simple HPLC method for the comprehensive analysis of cis/trans (Z/E) geometrical isomers of carotenoids for nutritional studies. Food Chem. 2013, 138, 1341–1350. [Google Scholar] [CrossRef]
- Meléndez-Martínez, A.J.; Mapelli-Brahm, P.; Benítez-González, A.; Stinco, C.M. A comprehensive review on the colorless carotenoids phytoene and phytofluene. Arch. Biochem. Biophys. 2015, 572, 188–200. [Google Scholar] [CrossRef] [Green Version]
- Meléndez-Martínez, A.J.; Stinco, C.M.; Mapelli-Brahm, P. Skin Carotenoids in Public Health and Nutricosmetics: The Emerging Roles and Applications of the UV Radiation-Absorbing Colourless Carotenoids Phytoene and Phytofluene. Nutrients 2019, 11, 1093. [Google Scholar] [CrossRef] [Green Version]
- Companjen, A.; van der Wel, L.; Wei, L.; Laman, J.D.; Prens, E.P. A modified ex vivo skin organ culture system for functional studies. Arch. Dermatol. Res. 2001, 293, 184–190. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]

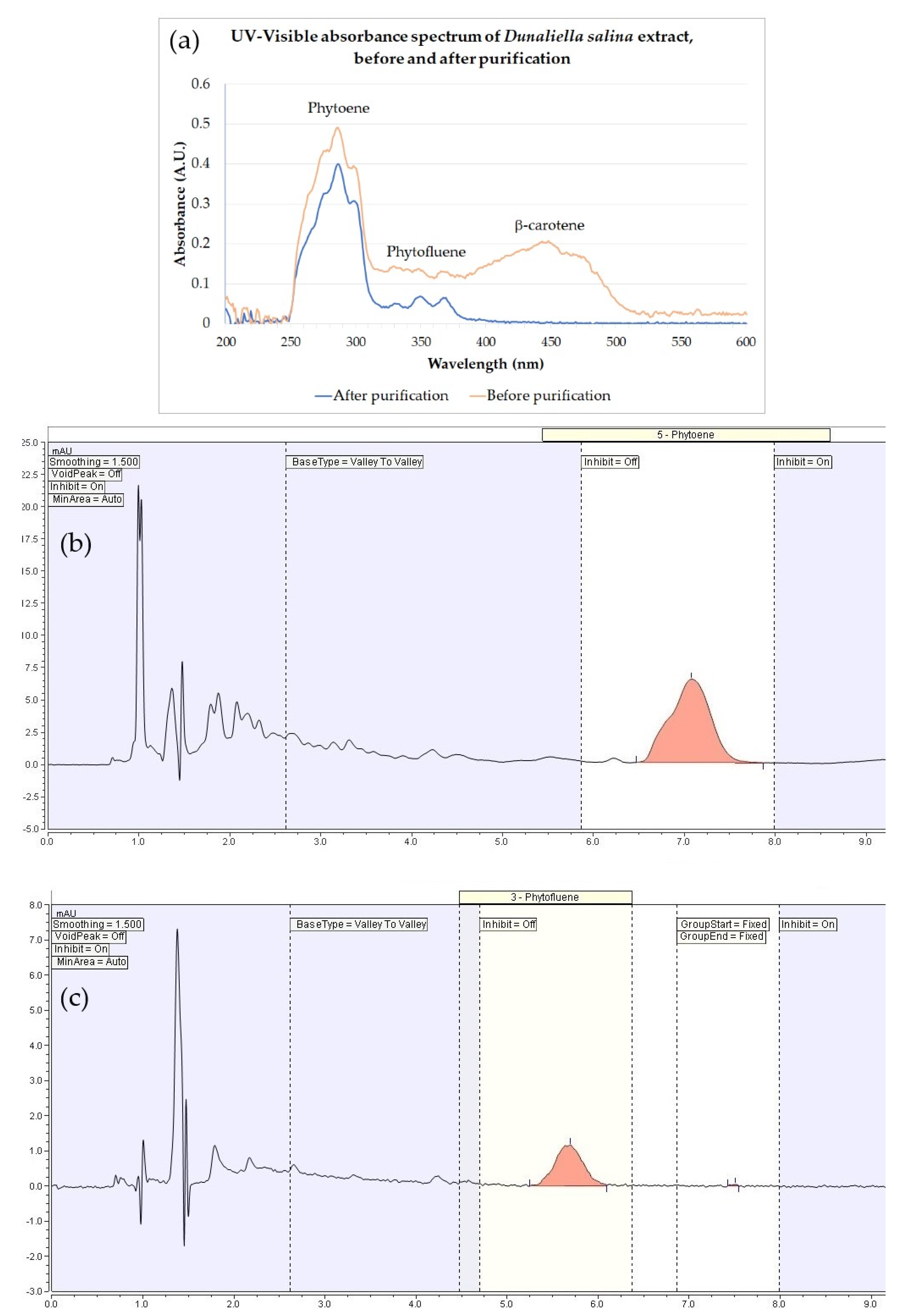
| Extract | L* | a* | b* |
|---|---|---|---|
| Before purification | 60.27 | 0.07 | 74.00 |
| After purification | 65.49 | −1.48 | 5.59 |
| Ingredient | % in Formula—Active | % in Formula—Placebo |
|---|---|---|
| Water | 85.85 | 85.85 |
| Butylene glycol | 4.00 | 4.00 |
| Dipropylene glycol | 1.00 | 1.00 |
| Hexylene glycol | 1.00 | 1.00 |
| Polysorbate 20 | 1.00 | 1.00 |
| Cyclomethicone | 4.00 | 4.00 |
| Dunaliella salina extract (in jojoba seed oil) | 1.00 | 0.00 |
| Jojoba seed oil | 0.00 | 1.00 |
| Carbomer | 0.80 | 0.80 |
| Triethanolamine | 0.70 | 0.70 |
| Phenoxyethanol | 0.40 | 0.40 |
| Methyl paraben | 0.15 | 0.15 |
| EDTA | 0.10 | 0.10 |
| TOTAL | 100.00 | 100.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Havas, F.; Krispin, S.; Cohen, M.; Loing, E.; Farge, M.; Suere, T.; Attia-Vigneau, J. A Dunaliella salina Extract Counteracts Skin Aging under Intense Solar Irradiation Thanks to Its Antiglycation and Anti-Inflammatory Properties. Mar. Drugs 2022, 20, 104. https://doi.org/10.3390/md20020104
Havas F, Krispin S, Cohen M, Loing E, Farge M, Suere T, Attia-Vigneau J. A Dunaliella salina Extract Counteracts Skin Aging under Intense Solar Irradiation Thanks to Its Antiglycation and Anti-Inflammatory Properties. Marine Drugs. 2022; 20(2):104. https://doi.org/10.3390/md20020104
Chicago/Turabian StyleHavas, Fabien, Shlomo Krispin, Moshe Cohen, Estelle Loing, Morgane Farge, Thierry Suere, and Joan Attia-Vigneau. 2022. "A Dunaliella salina Extract Counteracts Skin Aging under Intense Solar Irradiation Thanks to Its Antiglycation and Anti-Inflammatory Properties" Marine Drugs 20, no. 2: 104. https://doi.org/10.3390/md20020104
APA StyleHavas, F., Krispin, S., Cohen, M., Loing, E., Farge, M., Suere, T., & Attia-Vigneau, J. (2022). A Dunaliella salina Extract Counteracts Skin Aging under Intense Solar Irradiation Thanks to Its Antiglycation and Anti-Inflammatory Properties. Marine Drugs, 20(2), 104. https://doi.org/10.3390/md20020104






