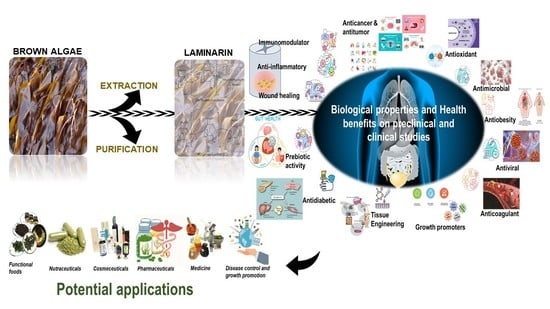
Biological Properties and Health-Promoting Functions of Laminarin: A Comprehensive Review of Preclinical and Clinical Studies
Abstract
1. Introduction
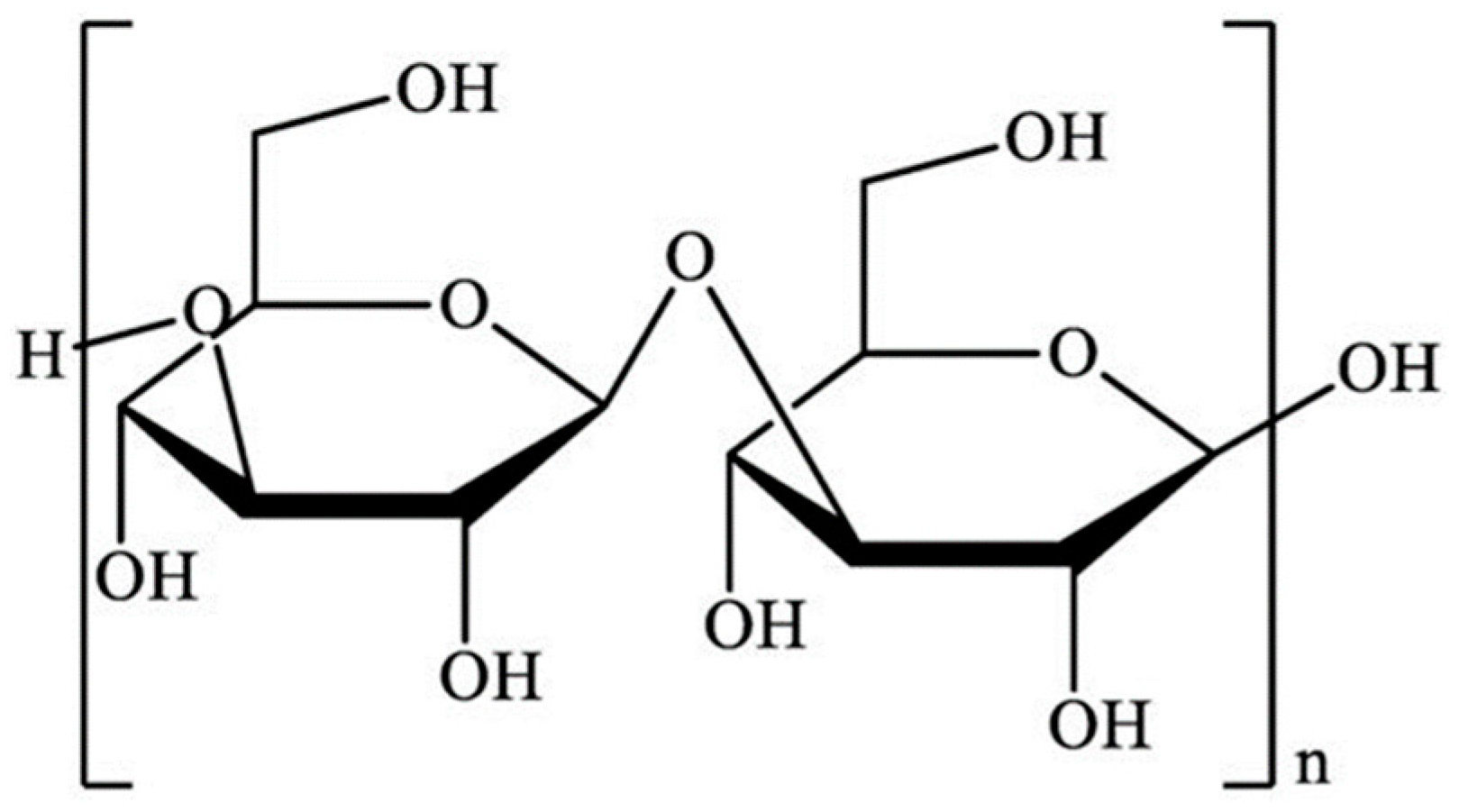
2. Structure and Molecular Characteristics of Laminarin
3. Biological Effects of Laminarin
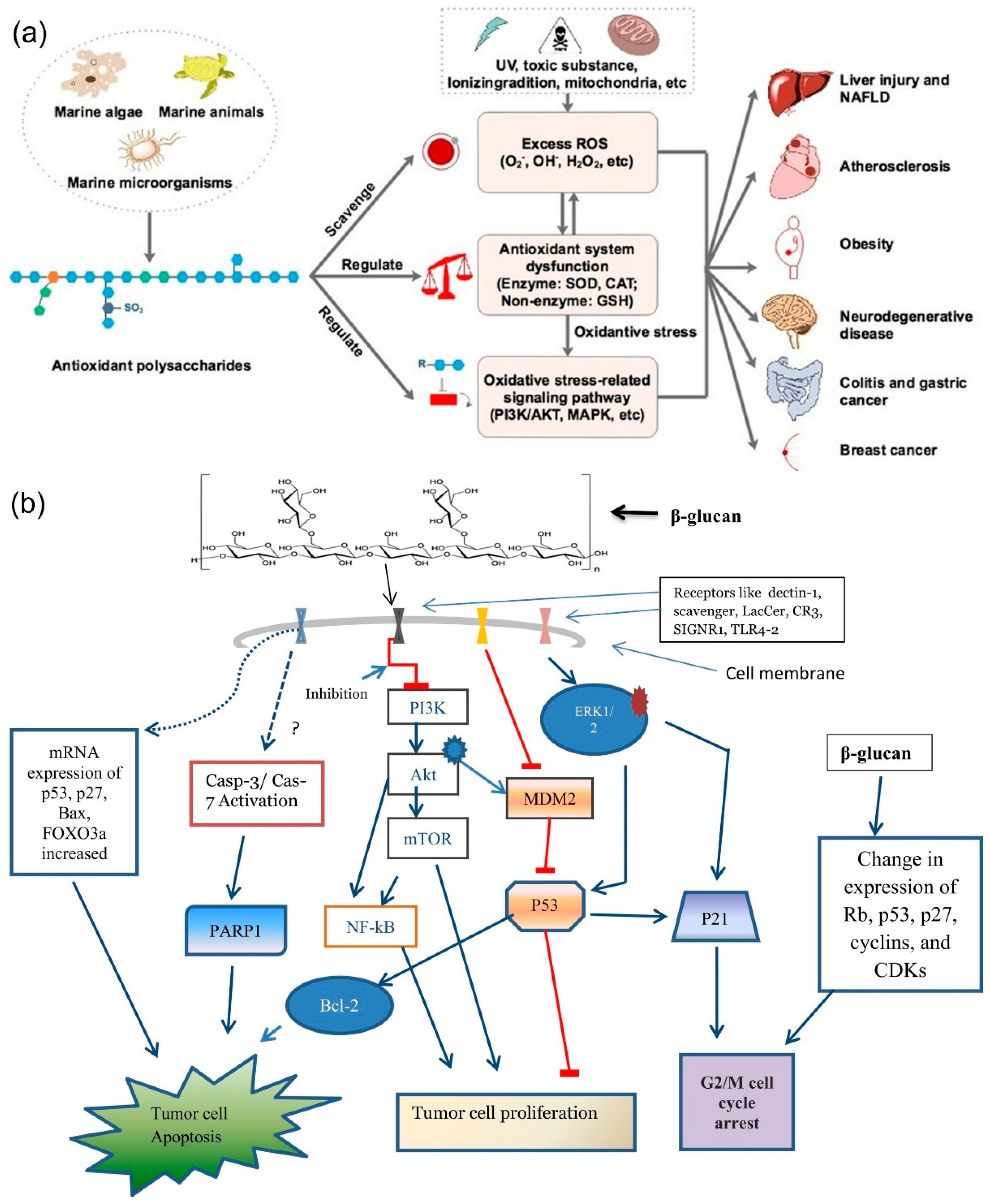
3.1. Antioxidant
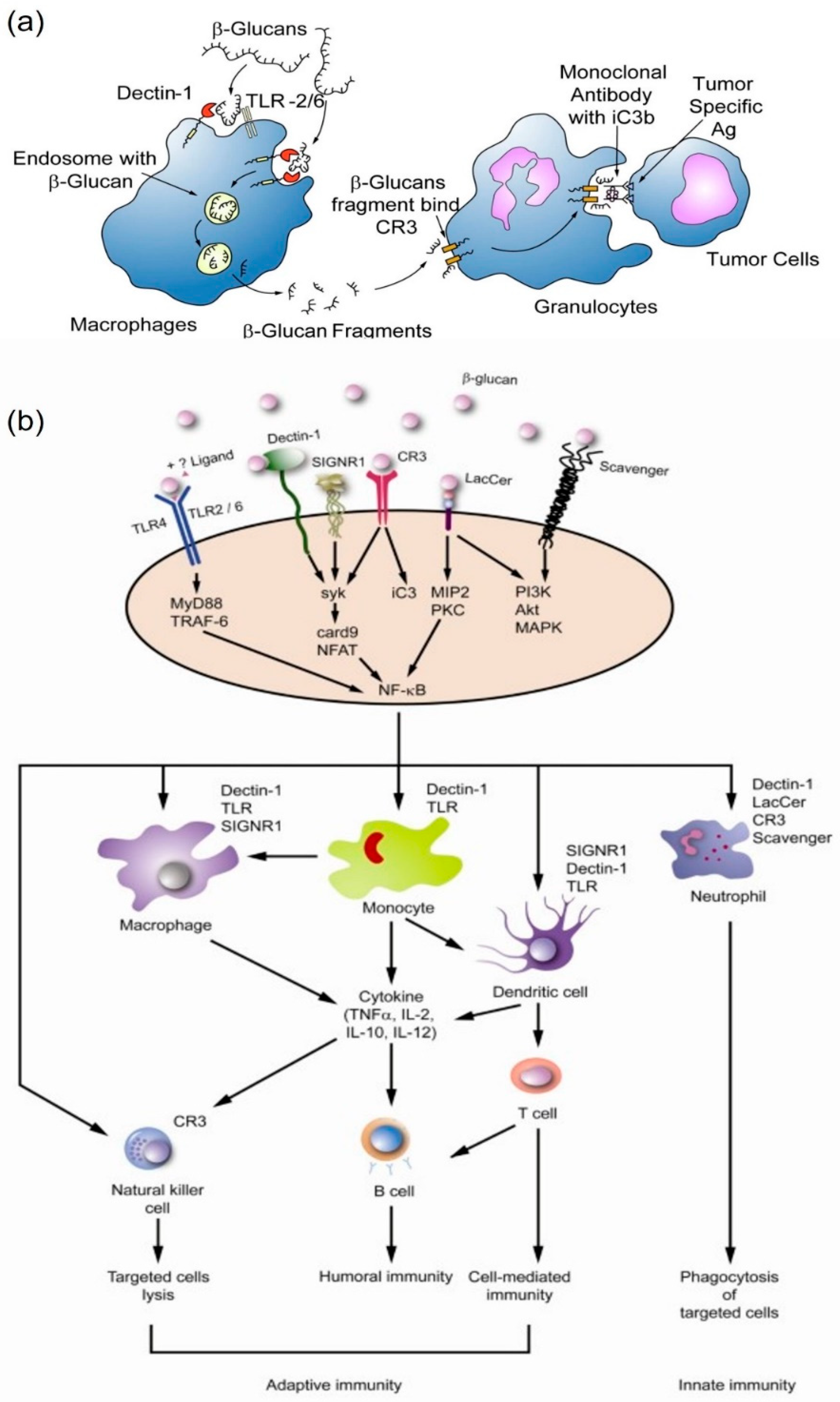
3.2. β-Glucan Related Receptors
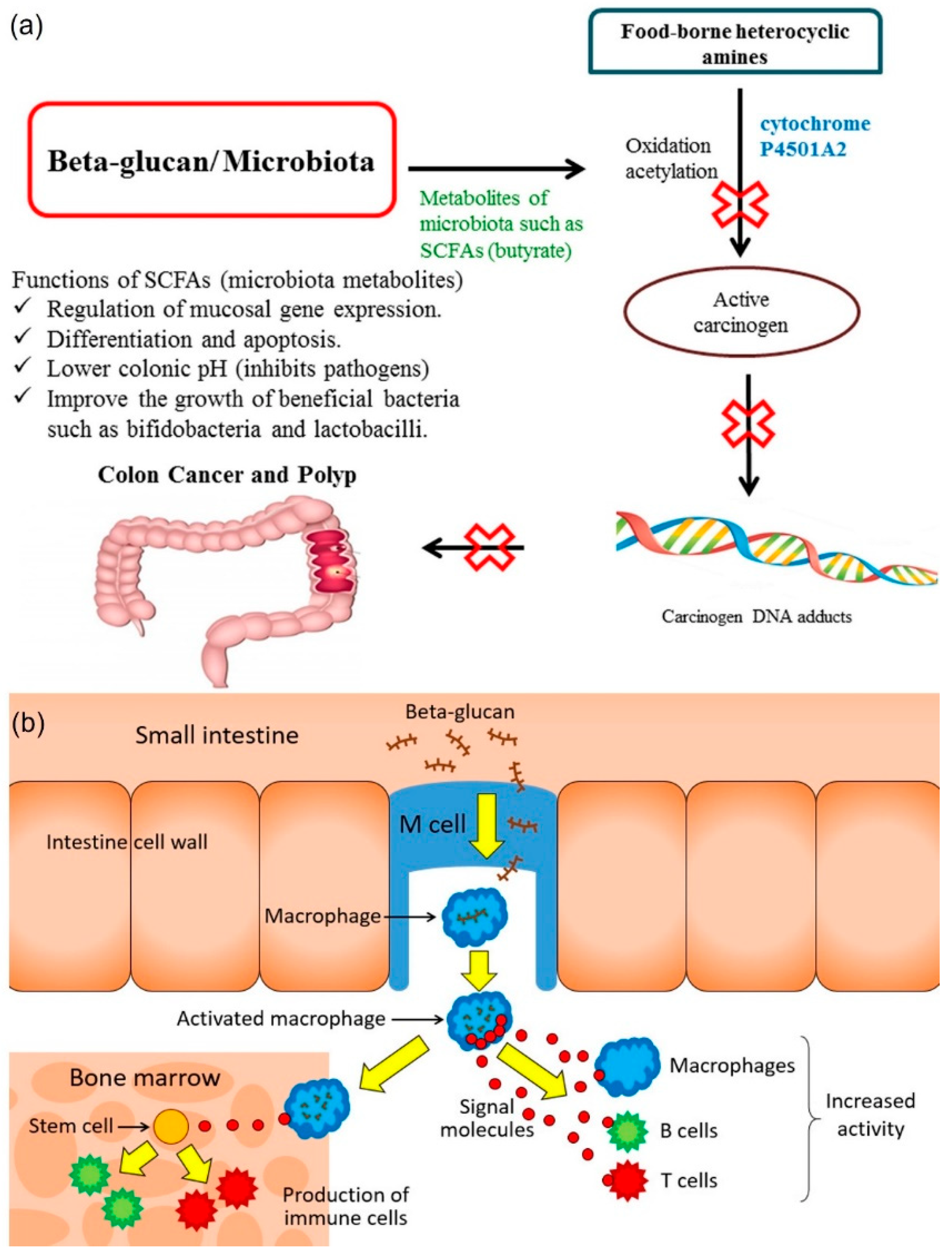
3.3. Immunomodulatory
3.4. Wound Healing
3.5. Obesity
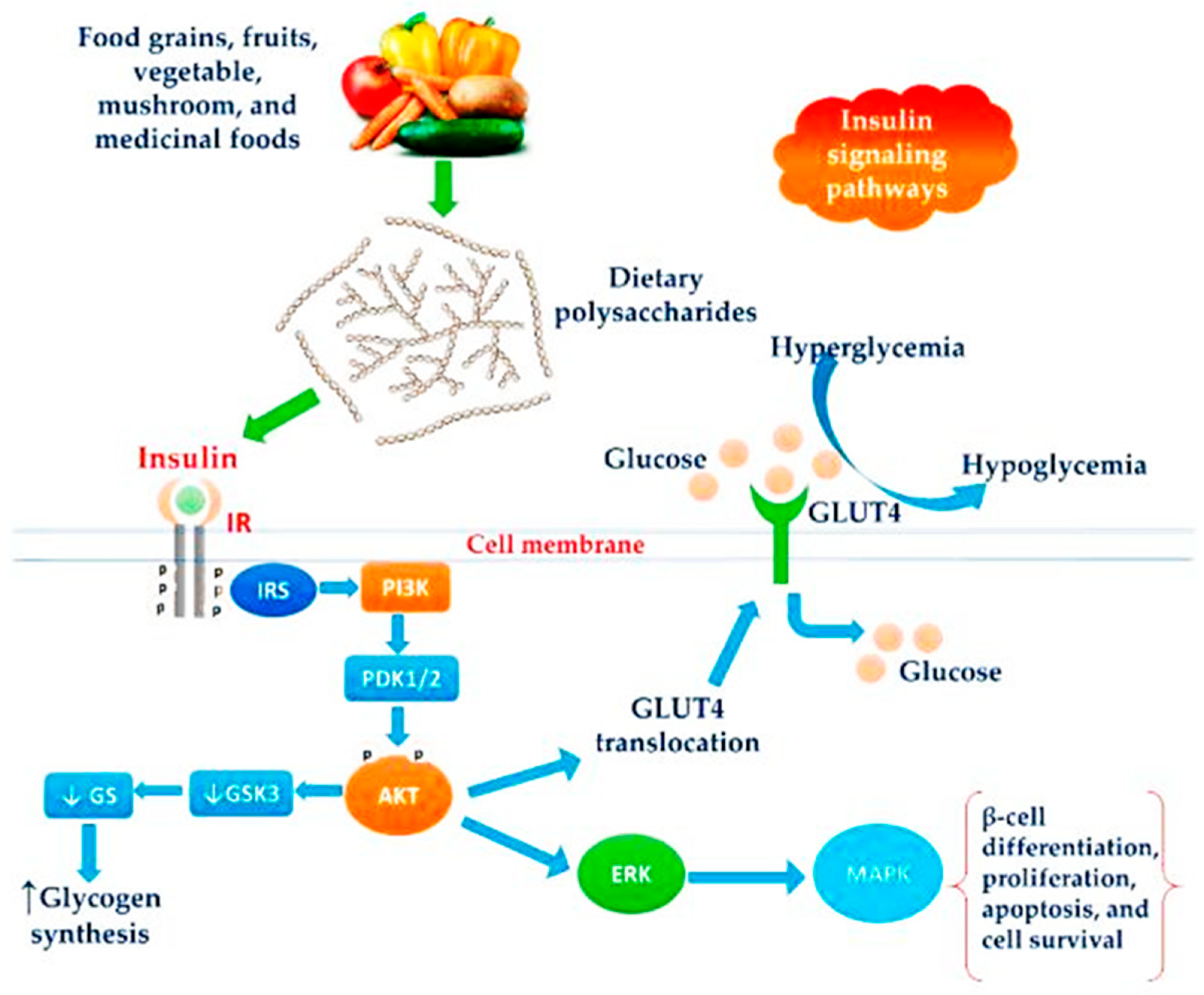
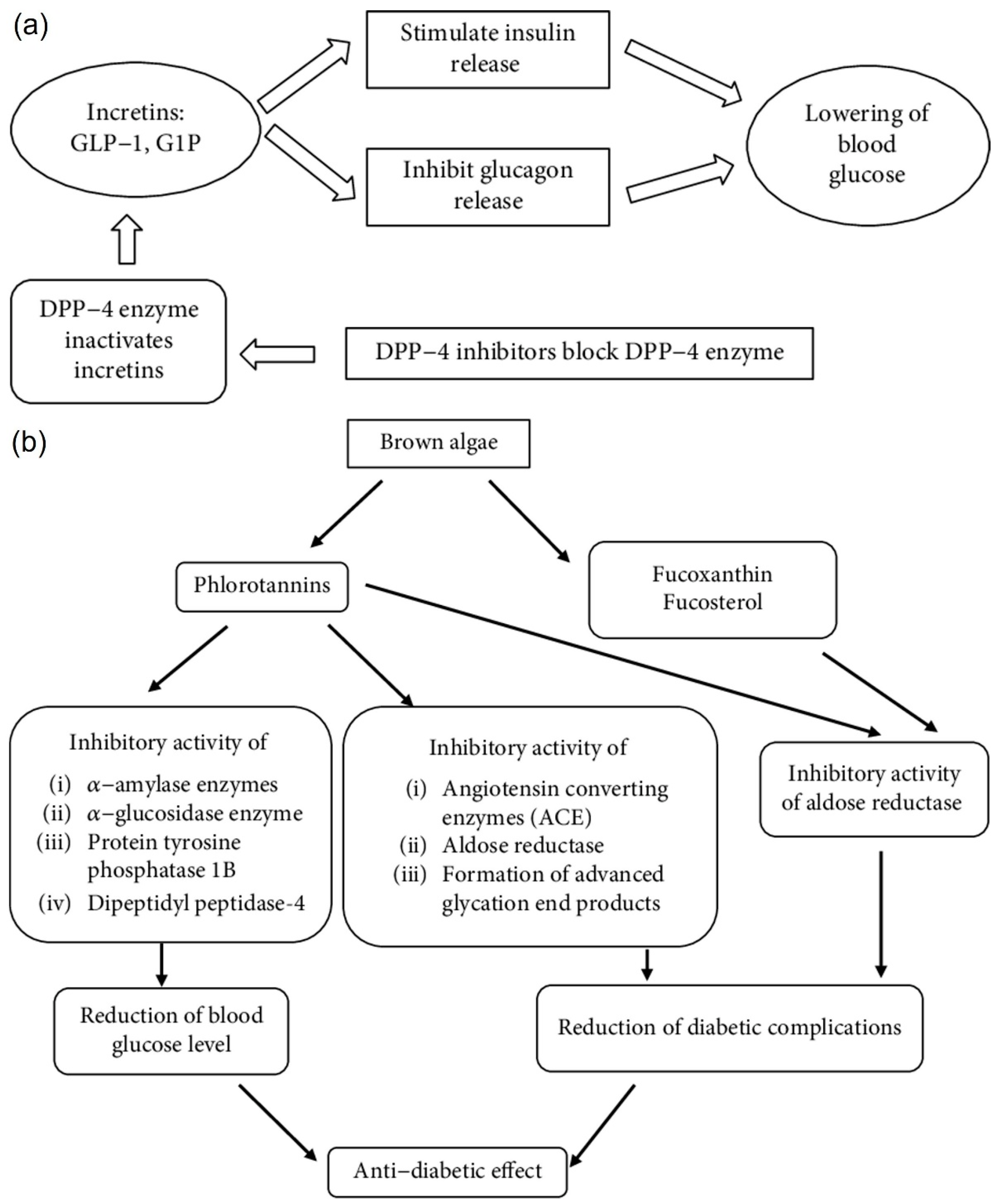
3.6. Diabetes
3.7. Cancer
4. Therapeutic Effects of Laminarin as a Bioactive Agent
4.1. Antioxidant Activity
4.2. Immunomodulatory Activity
4.3. Wound Healing
4.4. Anti-Obesity Activity
4.5. Anti-Diabetic Activity
4.6. Antitumor Activity
5. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alba, K.; Kontogiorgos, V. Seaweed polysaccharides (agar, alginate carrageenan). In Encyclopedia of Food Chemistry; Elsevier: Amsterdam, The Netherlands, 2018; pp. 240–250. [Google Scholar]
- Nigam, S.; Singh, R.; Bhardwaj, S.K.; Sami, R.; Nikolova, M.P.; Chavali, M.; Sinha, S. Perspective on the Therapeutic Applications of Algal Polysaccharides. J. Polym. Environ. 2022, 30, 785–809. [Google Scholar] [CrossRef] [PubMed]
- Zargarzadeh, M.; Amaral, A.J.; Custódio, C.A.; Mano, J.F. Biomedical applications of laminarin. Carbohydr. Polym. 2020, 232, 115774. [Google Scholar] [CrossRef] [PubMed]
- Usman, A.; Khalid, S.; Usman, A.; Hussain, Z.; Wang, Y. Algal polysaccharides, novel application, and outlook. In Algae Based Polymers, Blends, and Composites; Elsevier: Amsterdam, The Netherlands, 2017; pp. 115–153. [Google Scholar]
- Xu, S.-Y.; Huang, X.; Cheong, K.-L. Recent advances in marine algae polysaccharides: Isolation, structure, and activities. Mar. Drugs 2017, 15, 388. [Google Scholar] [CrossRef] [PubMed]
- Gabbia, D.; De Martin, S. Brown seaweeds for the management of metabolic syndrome and associated diseases. Molecules 2020, 25, 4182. [Google Scholar] [CrossRef] [PubMed]
- Jönsson, M.; Allahgholi, L.; Sardari, R.R.; Hreggviðsson, G.O.; Nordberg Karlsson, E. Extraction and modification of macroalgal polysaccharides for current and next-generation applications. Molecules 2020, 25, 930. [Google Scholar] [CrossRef]
- Ganesan, A.R.; Tiwari, U.; Rajauria, G. Seaweed nutraceuticals and their therapeutic role in disease prevention. Food Sci. Hum. Wellness 2019, 8, 252–263. [Google Scholar] [CrossRef]
- Wang, X.; Sun, G.; Feng, T.; Zhang, J.; Huang, X.; Wang, T.; Xie, Z.; Chu, X.; Yang, J.; Wang, H. Sodium oligomannate therapeutically remodels gut microbiota and suppresses gut bacterial amino acids-shaped neuroinflammation to inhibit Alzheimer’s disease progression. Cell Res. 2019, 29, 787–803. [Google Scholar] [CrossRef]
- Cotas, J.; Pacheco, D.; Gonçalves, A.M.; Silva, P.; Carvalho, L.G.; Pereira, L. Seaweeds’ nutraceutical and biomedical potential in cancer therapy: A concise review. J. Cancer Metastasis Treat. 2021, 7, 13. [Google Scholar] [CrossRef]
- Mourelle, M.L.; Gómez, C.P.; Legido, J.L. Role of Algal Derived Compounds in Pharmaceuticals and Cosmetics. In Recent Advances in Micro and Macroalgal Processing: Food and Health Perspectives; Wiley-Blackwell: Hoboken, NJ, USA, 2021; pp. 537–603. [Google Scholar]
- Dhahri, M.; Alghrably, M.; Mohammed, H.A.; Badshah, S.L.; Noreen, N.; Mouffouk, F.; Rayyan, S.; Qureshi, K.A.; Mahmood, D.; Lachowicz, J.I. Natural Polysaccharides as Preventive and Therapeutic Horizon for Neurodegenerative Diseases. Pharmaceutics 2022, 14, 1. [Google Scholar] [CrossRef]
- Otero, P.; Carpena, M.; Garcia-Oliveira, P.; Echave, J.; Soria-Lopez, A.; Garcia-Perez, P.; Fraga-Corral, M.; Cao, H.; Nie, S.; Xiao, J. Seaweed polysaccharides: Emerging extraction technologies, chemical modifications and bioactive properties. Crit. Rev. Food Sci. Nutr. 2021. [Google Scholar] [CrossRef]
- Bae, H.; Song, G.; Lee, J.-Y.; Hong, T.; Chang, M.-J.; Lim, W. Laminarin-derived from brown algae suppresses the growth of ovarian cancer cells via mitochondrial dysfunction and ER stress. Mar. Drugs 2020, 18, 152. [Google Scholar] [CrossRef]
- Bishehsari, F.; Engen, P.A.; Preite, N.Z.; Tuncil, Y.E.; Naqib, A.; Shaikh, M.; Rossi, M.; Wilber, S.; Green, S.J.; Hamaker, B.R. Dietary fiber treatment corrects the composition of gut microbiota, promotes SCFA production, and suppresses colon carcinogenesis. Genes 2018, 9, 102. [Google Scholar] [CrossRef]
- Haase, S.; Haghikia, A.; Gold, R.; Linker, R.A. Dietary fatty acids and susceptibility to multiple sclerosis. Mult. Scler. J. 2018, 24, 12–16. [Google Scholar] [CrossRef]
- Sellimi, S.; Maalej, H.; Rekik, D.M.; Benslima, A.; Ksouda, G.; Hamdi, M.; Sahnoun, Z.; Li, S.; Nasri, M.; Hajji, M. Antioxidant, antibacterial and in vivo wound healing properties of laminaran purified from Cystoseira barbata seaweed. Int. J. Biol. Macromol. 2018, 119, 633–644. [Google Scholar] [CrossRef]
- Rengasamy, K.R.; Mahomoodally, M.F.; Aumeeruddy, M.Z.; Zengin, G.; Xiao, J.; Kim, D.H. Bioactive compounds in seaweeds: An overview of their biological properties and safety. Food Chem. Toxicol. 2020, 135, 111013. [Google Scholar] [CrossRef]
- Wang, H.-M.D.; Li, X.-C.; Lee, D.-J.; Chang, J.-S. Potential biomedical applications of marine algae. Bioresour. Technol. 2017, 244, 1407–1415. [Google Scholar] [CrossRef]
- Kumar, Y.; Tarafdar, A.; Badgujar, P.C. Seaweed as a source of natural antioxidants: Therapeutic activity and food applications. J. Food Qual. 2021, 1–17. [Google Scholar] [CrossRef]
- Menshova, R.V.; Ermakova, S.P.; Anastyuk, S.D.; Isakov, V.V.; Dubrovskaya, Y.V.; Kusaykin, M.I.; Um, B.-H.; Zvyagintseva, T.N. Structure, enzymatic transformation and anticancer activity of branched high molecular weight laminaran from brown alga Eisenia bicyclis. Carbohydr. Polym. 2014, 99, 101–109. [Google Scholar] [CrossRef]
- Kadam, S.U.; Álvarez, C.; Tiwari, B.K.; O’Donnell, C.P. Extraction of biomolecules from seaweeds. In Seaweed Sustainability; Elsevier: Amsterdam, The Netherlands, 2015; pp. 243–269. [Google Scholar]
- Charoensiddhi, S.; Abraham, R.E.; Su, P.; Zhang, W. Seaweed and seaweed-derived metabolites as prebiotics. Adv. Food Nutr. Res. 2020, 91, 97–156. [Google Scholar]
- Kalasariya, H.S.; Yadav, V.K.; Yadav, K.K.; Tirth, V.; Algahtani, A.; Islam, S.; Gupta, N.; Jeon, B.-H. Seaweed-based molecules and their potential biological activities: An eco-sustainable cosmetics. Molecules 2021, 26, 5313. [Google Scholar] [CrossRef]
- Nguyen, S.G.; Kim, J.; Guevarra, R.B.; Lee, J.-H.; Kim, E.; Kim, S.-I.; Unno, T. Laminarin favorably modulates gut microbiota in mice fed a high-fat diet. Food Funct. 2016, 7, 4193–4201. [Google Scholar] [CrossRef] [PubMed]
- Kadam, S.U.; Tiwari, B.K.; O’Donnell, C.P. Extraction, structure and biofunctional activities of laminarin from brown algae. Int. J. Food Sci. Technol. 2015, 50, 24–31. [Google Scholar] [CrossRef]
- Kadam, S.U.; Álvarez, C.; Tiwari, B.K.; O’Donnell, C.P. Processing of seaweeds. In Seaweed Sustainability; Elsevier: Amsterdam, The Netherlands, 2015; pp. 61–78. [Google Scholar]
- Sweeney, T.; Collins, C.; Reilly, P.; Pierce, K.; Ryan, M.; O’Doherty, J. Effect of purified β-glucans derived from Laminaria digitata, Laminaria hyperborea and Saccharomyces cerevisiae on piglet performance, selected bacterial populations, volatile fatty acids and pro-inflammatory cytokines in the gastrointestinal tract of pigs. Br. J. Nutr. 2012, 108, 1226–1234. [Google Scholar] [CrossRef] [PubMed]
- George, J.; Thabitha, A.; Vignesh, N.; Manigandan, V.; Saravanan, R.; Daradkeh, G.; Qoronfleh, M.W. Antiskin Cancer and Antioxidant Activities of Formulated Agar from Brown Seaweed Laminaria digitata (Hudson) in Dimethyl Benzanthracene-Induced Swiss Albino Mice. Int. J. Polym. Sci. 2021, 9930777. [Google Scholar] [CrossRef]
- Song, K.; Xu, L.; Zhang, W.; Cai, Y.; Jang, B.; Oh, J.; Jin, J.-O. Laminarin promotes anti-cancer immunity by the maturation of dendritic cells. Oncotarget 2017, 8, 38554. [Google Scholar] [CrossRef]
- Yang, L.; Wang, L.; Zhu, C.; Wu, J.; Yuan, Y.; Yu, L.; Xu, Y.; Xu, J.; Wang, T.; Liao, Z. Laminarin counteracts diet-induced obesity associated with glucagon-like peptide-1 secretion. Oncotarget 2017, 8, 99470. [Google Scholar] [CrossRef]
- O’Shea, C.; O’Doherty, J.; Callanan, J.; Doyle, D.; Thornton, K.; Sweeney, T. The effect of algal polysaccharides laminarin and fucoidan on colonic pathology, cytokine gene expression and Enterobacteriaceae in a dextran sodium sulfate-challenged porcine model. J. Nutr. Sci. 2016, 5, e15. [Google Scholar] [CrossRef]
- Zaharudin, N.; Tullin, M.; Pekmez, C.T.; Sloth, J.J.; Rasmussen, R.R.; Dragsted, L.O. Effects of brown seaweeds on postprandial glucose, insulin and appetite in humans—A randomized, 3-way, blinded, cross-over meal study. Clin. Nutr. 2021, 40, 830–838. [Google Scholar] [CrossRef]
- Calderwood, D.; Rafferty, E.; Fitzgerald, C.; Stoilova, V.; Wylie, A.; Gilmore, B.F.; Castaneda, F.; Israel, A.; Maggs, C.A.; Green, B.D. Profiling the activity of edible European macroalgae towards pharmacological targets for type 2 diabetes mellitus. Appl. Phycol. 2021, 2, 10–21. [Google Scholar] [CrossRef]
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Carlson, J.L.; Erickson, J.M.; Lloyd, B.B.; Slavin, J.L. Health effects and sources of prebiotic dietary fiber. Curr. Dev. Nutr. 2018, 2, nzy005. [Google Scholar] [CrossRef]
- Lynch, M.B.; Sweeney, T.; Callan, J.J.; O’Sullivan, J.T.; O’Doherty, J.V. The effect of dietary Laminaria-derived laminarin and fucoidan on nutrient digestibility, nitrogen utilisation, intestinal microflora and volatile fatty acid concentration in pigs. J. Sci. Food Agric. 2010, 90, 430–437. [Google Scholar] [CrossRef]
- Odunsi, S.T.; Vázquez-Roque, M.I.; Camilleri, M.; Papathanasopoulos, A.; Clark, M.M.; Wodrich, L.; Lempke, M.; McKinzie, S.; Ryks, M.; Burton, D. Effect of alginate on satiation, appetite, gastric function, and selected gut satiety hormones in overweight and obesity. Obesity 2010, 18, 1579–1584. [Google Scholar] [CrossRef]
- Neyrinck, A.M.; Mouson, A.; Delzenne, N.M. Dietary supplementation with laminarin, a fermentable marine β (1–3) glucan, protects against hepatotoxicity induced by LPS in rat by modulating immune response in the hepatic tissue. Int. Immunopharmacol. 2007, 7, 1497–1506. [Google Scholar] [CrossRef]
- An, E.-K.; Hwang, J.; Kim, S.-J.; Park, H.-B.; Zhang, W.; Ryu, J.-H.; You, S.; Jin, J.-O. Comparison of the immune activation capacities of fucoidan and laminarin extracted from Laminaria japonica. Int. J. Biol. Macromol. 2022, 208, 230–242. [Google Scholar] [CrossRef]
- Gunathilaka, T.L.; Samarakoon, K.; Ranasinghe, P.; Peiris, L.D.C. Antidiabetic potential of marine brown algae—A mini review. J. Diabetes Res. 2020, 1230218. [Google Scholar] [CrossRef]
- Hentati, F.; Tounsi, L.; Djomdi, D.; Pierre, G.; Delattre, C.; Ursu, A.V.; Fendri, I.; Abdelkafi, S.; Michaud, P. Bioactive Polysaccharides from Seaweeds. Molecules 2020, 25, 3152. [Google Scholar] [CrossRef]
- Jayapala, N.; Perumal, M.K.; Baskaran, R.; Vallikannan, B. Pharmacological Importance of Bioactive Molecules of Seaweeds. In Sustainable Global Resources of Seaweeds; Springer: Berlin/Heidelberg, Germany, 2022; Volume 2, pp. 597–613. [Google Scholar]
- Ganesan, K.; Xu, B. Anti-Diabetic Effects and Mechanisms of Dietary Polysaccharides. Molecules 2019, 24, 2556. [Google Scholar] [CrossRef]
- Dobrinčić, A.; Balbino, S.; Zorić, Z.; Pedisić, S.; Bursać Kovačević, D.; Elez Garofulić, I.; Dragović-Uzelac, V. Advanced technologies for the extraction of marine brown algal polysaccharides. Mar. Drugs 2020, 18, 168. [Google Scholar] [CrossRef]
- García-Vaquero, M.; Rajauria, G.; O’Doherty, J.V.; Sweeney, T. Polysaccharides from macroalgae: Recent advances, innovative technologies and challenges in extraction and purification. Food Res. Int. 2017, 99, 1011–1020. [Google Scholar] [CrossRef]
- Cho, C.-H.; Lu, Y.-A.; Kim, M.-Y.; Jeon, Y.-J.; Lee, S.-H. Therapeutic Potential of Seaweed-Derived Bioactive Compounds for Cardiovascular Disease Treatment. Appl. Sci. 2022, 12, 1025. [Google Scholar] [CrossRef]
- Rioux, L.-E.; Turgeon, S.L. Seaweed carbohydrates. In Seaweed Sustainability; Elsevier: Amsterdam, The Netherlands, 2015; pp. 141–192. [Google Scholar]
- Chudasama, N.A.; Sequeira, R.A.; Moradiya, K.; Prasad, K. Seaweed polysaccharide-based products and materials: An assessment on their production from a sustainability point of view. Molecules 2021, 26, 2608. [Google Scholar] [CrossRef] [PubMed]
- Patil, N.P.; Le, V.; Sligar, A.D.; Mei, L.; Chavarria, D.; Yang, E.Y.; Baker, A.B. Algal polysaccharides as therapeutic agents for atherosclerosis. Front. Cardiovasc. Med. 2018, 5, 153. [Google Scholar] [CrossRef] [PubMed]
- Stone, B.A. Chemistry of β-glucans. In Chemistry, Biochemistry, and Biology of 1-3 Beta Glucans and Related Polysaccharides; Elsevier: Amsterdam, The Netherlands, 2009; pp. 5–46. [Google Scholar]
- Rajauria, G.; Ravindran, R.; Garcia-Vaquero, M.; Rai, D.K.; Sweeney, T.; O’Doherty, J. Molecular characteristics and antioxidant activity of laminarin extracted from the seaweed species Laminaria hyperborea, using hydrothermal-assisted extraction and a multi-step purification procedure. Food Hydrocoll. 2021, 112, 106332. [Google Scholar] [CrossRef]
- Garcia-Vaquero, M.; Rajauria, G.; Tiwari, B.; Sweeney, T.; O’Doherty, J. Extraction and yield optimisation of fucose, glucans and associated antioxidant activities from Laminaria digitata by applying response surface methodology to high intensity ultrasound-assisted extraction. Mar. Drugs 2018, 16, 257. [Google Scholar] [CrossRef]
- Kadam, S.U.; O’Donnell, C.P.; Rai, D.K.; Hossain, M.B.; Burgess, C.M.; Walsh, D.; Tiwari, B.K. Laminarin from Irish brown seaweeds Ascophyllum nodosum and Laminaria hyperborea: Ultrasound assisted extraction, characterization and bioactivity. Mar. Drugs 2015, 13, 4270–4280. [Google Scholar] [CrossRef]
- Imbs, T.I.; Ermakova, S.P.; Malyarenko, O.S.; Isakov, V.V.; Zvyagintseva, T.N. Structural elucidation of polysaccharide fractions from the brown alga Coccophora langsdorfii and in vitro investigation of their anticancer activity. Carbohydr. Polym. 2016, 135, 162–168. [Google Scholar] [CrossRef]
- Sterner, M.; Gröndahl, F. Extraction of laminarin from Saccharina latissima seaweed using cross-flow filtration. J. Appl. Phycol. 2021, 33, 1825–1844. [Google Scholar] [CrossRef]
- Ji, C.-F.; Ji, Y.-B.; Meng, D.-Y. Sulfated modification and anti-tumor activity of laminarin. Exp. Ther. Med. 2013, 6, 1259–1264. [Google Scholar] [CrossRef]
- Shanmugam, M.; Mody, K. Heparinoid-active sulphated polysaccharides from marine algae as potential blood anticoagulant agents. Curr. Sci. 2000, 79, 1672–1683. [Google Scholar]
- Choi, J.-i.; Kim, H.-J.; Lee, J.-W. Structural feature and antioxidant activity of low molecular weight laminarin degraded by gamma irradiation. Food Chem. 2011, 129, 520–523. [Google Scholar] [CrossRef]
- Déléris, P.; Nazih, H.; Bard, J.-M. Seaweeds in human health. In Seaweed in Health and Disease Prevention; Academic Press: Cambridge, MA, USA, 2016; pp. 319–367. [Google Scholar]
- Miao, H.Q.; Elkin, M.; Aingorn, E.; Ishai-Michaeli, R.; Stein, C.A.; Vlodavsky, I. Inhibition of heparanase activity and tumor metastasis by laminarin sulfate and synthetic phosphorothioate oligodeoxynucleotides. Int. J. Cancer 1999, 83, 424–431. [Google Scholar] [CrossRef]
- Rattigan, R.; O’Doherty, J.V.; Vigors, S.; Ryan, M.T.; Sebastiano, R.S.; Callanan, J.J.; Thornton, K.; Rajauria, G.; Margassery, L.M.; Dobson, A.D. The effects of the marine-derived polysaccharides laminarin and chitosan on aspects of colonic health in pigs challenged with dextran sodium sulphate. Mar. Drugs 2020, 18, 262. [Google Scholar] [CrossRef]
- Rattigan, R.; Sweeney, T.; Maher, S.; Thornton, K.; Rajauria, G.; O’Doherty, J. Laminarin-rich extract improves growth performance, small intestinal morphology, gene expression of nutrient transporters and the large intestinal microbial composition of piglets during the critical post-weaning period. Br. J. Nutr. 2020, 123, 255–263. [Google Scholar] [CrossRef]
- Vigors, S.; O’Doherty, J.V.; Rattigan, R.; McDonnell, M.J.; Rajauria, G.; Sweeney, T. Effect of a laminarin rich macroalgal extract on the caecal and colonic microbiota in the post-weaned pig. Mar. Drugs 2020, 18, 157. [Google Scholar] [CrossRef]
- Smith, A.; O’doherty, J.; Reilly, P.; Ryan, M.; Bahar, B.; Sweeney, T. The effects of laminarin derived from Laminaria digitata on measurements of gut health: Selected bacterial populations, intestinal fermentation, mucin gene expression and cytokine gene expression in the pig. Br. J. Nutr. 2011, 105, 669–677. [Google Scholar] [CrossRef]
- O’Doherty, J.; Dillon, S.; Figat, S.; Callan, J.; Sweeney, T. The effects of lactose inclusion and seaweed extract derived from Laminaria spp. on performance, digestibility of diet components and microbial populations in newly weaned pigs. Anim. Feed. Sci. Technol. 2010, 157, 173–180. [Google Scholar] [CrossRef]
- Gahan, D.; Lynch, M.; Callan, J.; O’sullivan, J.; O’Doherty, J. Performance of weanling piglets offered low-, medium-or high-lactose diets supplemented with a seaweed extract from Laminaria spp. Animal 2009, 3, 24–31. [Google Scholar] [CrossRef]
- Devillé, C.; Damas, J.; Forget, P.; Dandrifosse, G.; Peulen, O. Laminarin in the dietary fibre concept. J. Sci. Food Agric. 2004, 84, 1030–1038. [Google Scholar] [CrossRef]
- Rajauria, G. In-vitro antioxidant properties of lipophilic antioxidant compounds from 3 brown seaweed. Antioxidants 2019, 8, 596. [Google Scholar] [CrossRef]
- Rajauria, G.; Foley, B.; Abu-Ghannam, N. Characterization of dietary fucoxanthin from Himanthalia elongata brown seaweed. Food Res. Int. 2017, 99, 995–1001. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yuan, W.; Sharma-Shivappa, R.; van Zanten, J. Antioxidant activity of phlorotannins from brown algae. Int. J. Agric. Biol. Eng. 2017, 10, 184–191. [Google Scholar] [CrossRef]
- Cheng, D.; Liang, B.; Li, M.; Jin, M. Influence of laminarin polysaccahrides on oxidative damage. Int. J. Biol. Macromol. 2011, 48, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Liang, S.; Yao, X.-R.; Jin, Y.-X.; Shen, X.-H.; Yuan, B.; Zhang, J.-B.; Kim, N.-H. Laminarin improves developmental competence of porcine early-stage embryos by inhibiting oxidative stress. Theriogenology 2018, 115, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Chan, G.C.-F.; Chan, W.K.; Sze, D.M.-Y. The effects of β-glucan on human immune and cancer cells. J. Hematol. Oncol. 2009, 2, 1–11. [Google Scholar] [CrossRef]
- Cognigni, V.; Ranallo, N.; Tronconi, F.; Morgese, F.; Berardi, R. Potential benefit of β-glucans as adjuvant therapy in immuno-oncology: A review. Explor. Target. Antitumor Ther. 2021, 2, 122–138. [Google Scholar] [CrossRef]
- Usoltseva, R.V.; Belik, A.A.; Kusaykin, M.I.; Malyarenko, O.S.; Zvyagintseva, T.N.; Ermakova, S.P. Laminarans and 1,3-β-D-glucanases. Int. J. Biol. Macromol. 2020, 163, 1010–1025. [Google Scholar] [CrossRef]
- Majtan, J.; Jesenak, M. β-Glucans: Multi-functional modulator of wound healing. Molecules 2018, 23, 806. [Google Scholar] [CrossRef]
- Graiff, A.; Ruth, W.; Kragl, U.; Karsten, U. Chemical characterization and quantification of the brown algal storage compound laminarin—A new methodological approach. J. Appl. Phycol. 2016, 28, 533–543. [Google Scholar] [CrossRef]
- Praveen, M.A.; Parvathy, K.K.; Balasubramanian, P.; Jayabalan, R. An overview of extraction and purification techniques of seaweed dietary fibers for immunomodulation on gut microbiota. Trends Food Sci. Technol. 2019, 92, 46–64. [Google Scholar] [CrossRef]
- Sweeney, T.; Meredith, H.; Vigors, S.; McDonnell, M.J.; Ryan, M.; Thornton, K.; O’Doherty, J.V. Extracts of laminarin and laminarin/fucoidan from the marine macroalgal species Laminaria digitata improved growth rate and intestinal structure in young chicks but does not influence Campylobacter jejuni colonisation. Anim. Feed. Sci. Technol. 2017, 232, 71–79. [Google Scholar] [CrossRef]
- Ryan, M.T.; Collins, C.B.; O’Doherty, J.; Sweeney, T. Effects of dietary β-glucans supplementation on cytokine expression in porcine liver. J. Anim. Sci. 2012, 90, 40–42. [Google Scholar] [CrossRef]
- Strain, C.R.; Collins, K.C.; Naughton, V.; McSorley, E.M.; Stanton, C.; Smyth, T.J.; Soler-Vila, A.; Rea, M.C.; Ross, P.R.; Cherry, P. Effects of a polysaccharide-rich extract derived from Irish-sourced Laminaria digitata on the composition and metabolic activity of the human gut microbiota using an in vitro colonic model. Eur. J. Nutr. 2020, 59, 309–325. [Google Scholar] [CrossRef]
- Ostrzenski, A. Resectoscopic cervical trauma minimized by inserting Laminaria digitata preoperatively. Int. J. Fertil. Menopausal Stud. 1994, 39, 111–113. [Google Scholar]
- Rinaudo, M. Main properties and current applications of some polysaccharides as biomaterials. Polym. Int. 2008, 57, 397–430. [Google Scholar] [CrossRef]
- Déjean, G.; Tamura, K.; Cabrera, A.; Jain, N.; Pudlo, N.A.; Pereira, G.; Viborg, A.H.; Van Petegem, F.; Martens, E.C.; Brumer, H. Synergy between cell surface glycosidases and glycan-binding proteins dictates the utilization of specific beta (1, 3)-glucans by human gut Bacteroides. mBio 2020, 11, e00095-20. [Google Scholar] [CrossRef]
- Wasko, N.J.; Nichols, F.; Clark, R.B. Multiple sclerosis, the microbiome, TLR2, and the hygiene hypothesis. Autoimmun. Rev. 2020, 19, 102430. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Sandhu, K.; Peterson, V.; Dinan, T.G. The gut microbiome in neurological disorders. Lancet Neurol. 2020, 19, 179–194. [Google Scholar] [CrossRef]
- Deville, C.; Gharbi, M.; Dandrifosse, G.; Peulen, O. Study on the effects of laminarin, a polysaccharide from seaweed, on gut characteristics. J. Sci. Food Agric. 2007, 87, 1717–1725. [Google Scholar] [CrossRef]
- Takei, M.N.; Kuda, T.; Taniguchi, M.; Nakamura, S.; Hajime, T.; Kimura, B. Detection and isolation of low molecular weight alginate-and laminaran-susceptible gut indigenous bacteria from ICR mice. Carbohydr. Polym. 2020, 238, 116205. [Google Scholar] [CrossRef]
- Cui, Y.; Zhu, L.; Li, Y.; Jiang, S.; Sun, Q.; Xie, E.; Chen, H.; Zhao, Z.; Qiao, W.; Xu, J. Structure of a laminarin-type β-(1→ 3)-glucan from brown algae Sargassum henslowianum and its potential on regulating gut microbiota. Carbohydr. Polym. 2021, 255, 117389. [Google Scholar] [CrossRef] [PubMed]
- Remya, R.; Rajasree, S.R.; Suman, T.; Aranganathan, L.; Gayathri, S.; Gobalakrishnan, M.; Karthih, M. Laminarin based AgNPs using brown seaweed Turbinaria ornata and its induction of apoptosis in human retinoblastoma Y79 cancer cell lines. Mater. Res. Express 2018, 5, 035403. [Google Scholar] [CrossRef]
- Ermakova, S.; Men’shova, R.; Vishchuk, O.; Kim, S.-M.; Um, B.-H.; Isakov, V.; Zvyagintseva, T. Water-soluble polysaccharides from the brown alga Eisenia bicyclis: Structural characteristics and antitumor activity. Algal Res. 2013, 2, 51–58. [Google Scholar] [CrossRef]
- Zhu, X.; Zhu, R.; Jian, Z.; Yu, H. Laminarin enhances the activity of natural killer cells in immunosuppressed mice. Cent. -Eur. J. Immunol. 2019, 44, 357. [Google Scholar] [CrossRef] [PubMed]
- Usoltseva, R.V.; Anastyuk, S.D.; Shevchenko, N.M.; Zvyagintseva, T.N.; Ermakova, S.P. The comparison of structure and anticancer activity in vitro of polysaccharides from brown algae Alaria marginata and A. angusta. Carbohydr. Polym. 2016, 153, 258–265. [Google Scholar] [CrossRef]
- Malyarenko, O.S.; Usoltseva, R.V.; Shevchenko, N.M.; Isakov, V.V.; Zvyagintseva, T.N.; Ermakova, S.P. In vitro anticancer activity of the laminarans from Far Eastern brown seaweeds and their sulfated derivatives. J. Appl. Phycol. 2017, 29, 543–553. [Google Scholar] [CrossRef]
- Wani, S.M.; Gani, A.; Mir, S.A.; Masoodi, F.A.; Khanday, F.A. β-Glucan: A dual regulator of apoptosis and cell proliferation. Int. J. Biol. Macromol. 2021, 182, 1229–1237. [Google Scholar] [CrossRef]
- Zhong, Q.; Wei, B.; Wang, S.; Ke, S.; Chen, J.; Zhang, H.; Wang, H. The antioxidant activity of polysaccharides derived from marine organisms: An overview. Mar. Drugs 2019, 17, 674. [Google Scholar] [CrossRef]
- Vannucci, L.; Krizan, J.; Sima, P.; Stakheev, D.; Caja, F.; Rajsiglova, L.; Horak, V.; Saieh, M. Immunostimulatory properties and antitumor activities of glucans. Int. J. Oncol. 2013, 43, 357–364. [Google Scholar] [CrossRef]
- Jayachandran, M.; Chen, J.; Chung, S.S.M.; Xu, B. A critical review on the impacts of β-glucans on gut microbiota and human health. J. Nutr. Biochem. 2018, 61, 101–110. [Google Scholar] [CrossRef]
- Younis, N.S.; Mohamed, M.E.; El Semary, N.A. Green synthesis of silver nanoparticles by the Cyanobacteria synechocystis sp.: Characterization, antimicrobial and diabetic wound-healing actions. Mar. Drugs 2022, 20, 56. [Google Scholar] [CrossRef]
- Ouyang, Y.; Qiu, Y.; Liu, Y.; Zhu, R.; Chen, Y.; El-Seedi, H.R.; Chen, X.; Zhao, C. Cancer-fighting potentials of algal polysaccharides as nutraceuticals. Food Res. Int. 2021, 147, 110522. [Google Scholar] [CrossRef]
- Wan, Y.; Xu, X.; Gilbert, R.G.; Sullivan, M.A. A Review on the Structure and Anti-Diabetic (Type 2) Functions of β-Glucans. Foods 2021, 11, 57. [Google Scholar] [CrossRef]
- Unnikrishnan, P.; Suthindhiran, K.; Jayasri, M. Antidiabetic potential of marine algae by inhibiting key metabolic enzymes. Front. Life Sci. 2015, 8, 148–159. [Google Scholar] [CrossRef]
- Ghosh, T.; Bhayani, K.; Paliwal, C.; Maurya, R.; Chokshi, K.; Pancha, I.; Mishra, S. Cyanobacterial pigments as natural anti-hyperglycemic agents: An in vitro study. Front. Mar. Sci. 2016, 3, 146. [Google Scholar] [CrossRef]
- Shalaby, N.M.; Abd-Alla, H.I.; Aly, H.F.; Albalawy, M.A.; Shaker, K.H.; Bouajila, J. Preliminary in vitro and in vivo evaluation of antidiabetic activity of Ducrosia anethifolia Boiss. and its linear furanocoumarins. BioMed Res. Int. 2014, 831841. [Google Scholar]
- Paliwal, C.; Nesamma, A.A.; Jutur, P.P. Industrial scope with high-value biomolecules from microalgae. In Sustainable Downstream Processing of Microalgae for Industrial Application; CRC Press: Boca Raton, FL, USA, 2019; pp. 83–98. [Google Scholar]

| Bioactive Algal Polysaccharides and Their Sources with Yield | Bioactivity Potential | Therapeutic and Nutraceutical Applications | Ref. |
|---|---|---|---|
| Laminarin Laminaria digitata (51.8%) Saccharina latissima (19 ± 2.6%) S. japonica Alaria angusta Undaria pinnatifida (3.2 ± 0.9%) Dictyopteris delicatula Dictyota menstrualis L. saccharina Ecklonia cava Ascophyllum nodosum Sargassum spp. (13.47%) | Antitumor Antimicrobial Wound healing Immunomodulatory Antioxidant activity Antibacterial and antifungal Modulatory effects on skin cells Hydro-gelling properties Anti-inflammatory Chemoprotective Neuroprotective Antiviral Antiallergic Antipruritic Hepatoprotective Anti-cholesterol Antidiabetic | Active ingredients Photoprotection Antiaging products Antioxidant Skin-benefiting activities Growth regulation Lipolytic activity Tissue engineering Cancer therapies Biofuel production Food industry Antimicrobial Health care and cosmetic uses Boosts immunity Prevents inflammation Biofertiliser | [3,10] [14] [15,19] [7,17,20] [11,21,22,23] [24,25] [26,27] [28] [13,22] [29] [30,31,32] [33] [34,35,36] [37] [38] [27,39,40] [41,42] [43,44] |
| Fucoidan S. japonica (1.26%), A. angusta, U. pinnatifida (1.5 ± 0.3%), Dictyopteris spp., L. saccharina E. cava, A. nodosum, Cladosiphon okamuranus, Fucus vesiculosus (18.22%), L. japonica, F. evanescens (4.44% (4.7%, CaCl2; 3.02% (5.11%, Ethanol)) Dictyota menstrualis, S. polycystum, D. delicatula, Turbinaria conoides, S. latissimi, Spatoglossum asperum Cystoseira sedoides Coccophora langsdorfii | Anti-tumor activity Anti-cancer activity Anti-viral activity Immunomodulatory activity Wound healing activity Anti-angiogenic activity Anti-allergy activity Anticoagulant activity Anti-diabetic activity Anti-hypolipidemic Anti-hyperglycemic activity Antioxidant activity Cognitive protective activity Anti-angiogenic activity Antimicrobial activity Anti-obesity activity Anti-inflammatory activity Anticancer | Immunomodulatory effect Tumor destruction Improved body weight and fasting blood glucose Anti-proliferative Antimetastatic Hypotriglyceridemic effects Anti-inflammatory response Neuroprotective effects Increase nitric oxide production. Activation of PI3K/Akt/eNOS-dependent pathways. Anti-inflammatory Anticancer | [10,35] [4,5] [5,24] [5] [7,19,36] [13] [25,45,46] [24] [41,42,47] [6] [5] [5,20] [8,23] [7] [22] [45] [6,10] [5] |
| Alginate L. digitata (51.8%), A. nodosum, L. hyperborea, Macrocystis pyrifera, S. muticum (13.47%) S. binderi (54%) U. pinnatifida (23.6 ± 1.2%) | Anti-obesity activity Antiviral Activity Anticancer Activities Antidiabetic Activities Antioxidant Activity Anti-inflammatory Anti-microbial Anti-coagulant Anti-ageing Anti-obesity | Overweight and Obesity Inhibition of α-amylase, pancreatic lipase and pepsin. Applications such as feed stabilizer, paper and welding rod coatings, and dye thickener in textile printing Drug delivery Pharmaceutical applications | [38] [2,24] [4,10,45] [18,47] [13] [48,45] [22,35] [42,49] [19,25] |
| Ulvan Ulva pertusa Monostroma nitidum and U. pertusa U. pertusa Monostroma spp. M. nitidum Chlorella Pyrenoidosa U. intestinalis U. prolifera U. lactuca Ulva and Enteromorpha spp. | Antioxidant activity Immunomodulating activity Antihyperlipidemic activity Antidiabetic activity Anticoagulant activity Anticancer activity Neuroprotective activity Immunostimulation Anti-inflammatory, Antiviral, Anticoagulant Gel-forming, skin protective and antioxidant properties | Influence plant immunity Triggering plant defense in several different plants Treatment of gastric ulcers Preventive/Therapautic Skin aging products | [7,20] [13] [35] [47] [19] [10] [12] [12] [24] [42] [36,25] |
| Carrageenan Kappaphycus alvarezii, and Eucheuma dendiculatum (50–55%) Chondrus crispus, Sarcothalia crispata E. cottonii (46.43%) Furcellaria lumbricalis and Coccotylus truncates (76.3%) Mastocarpus stellatus (28.65%) | Anticoagulant activity Antiviral activity Cholesterol-lowering activity Anti-tumor activity Immunomodulatory activity Antioxidant activity Anti-allergic activity | Enzymes hydrolyzing plant polysaccharides Food industry; mainly in dairy and meat applications Thickening, gelling, and stabilizing agent. Fertilizer Production of nanoengineered injectable hydrogels in tissue regeneration therapy | [7] [19] [49] [35] [10] [13] [18,20] [48,25] |
| Agar Gracilaria lemaneiformis (29.7 ± 1.9%) G. vermiculophylla (9.7–34.6%) G. cornea Gelidium amansii | Gelling and stabilizing properties Antioxidant Antidiabetic activity Neuroprotective and anti-neurodegenerative properties Scavenging properties, Antioxidant and gel-forming activity | Potential application in pharmaceutical industries Gelling agent Food application, including bakery, confectionery, dairy products, canned meat and fish products Preparation of bacteriological culture media Support for the three-dimensional cultures of human and animal cells Thickening agent | [7,48] [20] [47,35] [13] [18,42,49] [50,45] [36,25] |
| Source | Extraction, Purification, Yield and Characterisation | Study Design | Model, Administration Dose, Route and Period | Biological Activities | Effects/Outcome of Laminarin Extract or Fraction and Related Products | Ref. |
|---|---|---|---|---|---|---|
| Edible seaweed species including Laminaria digitata | 50 mL of boiling absolute ethanol (>99.5%) was added to 1.25 g of dried seaweed powder, then centrifuged after which the supernatant was removed. Ethanol extracts and water extracts of L. digitata were freeze dried. On experimental days, dried seaweed extracts were reconstituted in appropriate buffer for experimentation. | The in vivo anti-hyperglycemic activity. | C57/BL6 mice 500 mg/kg Orally 0–105 min | Hyperglycemic | Extracts of L. digitata strongly inhibited DPP-4 activity. Medicinal foods or biotherapeutics to tackle type 2 diabetes mellitus Targeted GLP-1 secretion, DPP-4 activity or alpha-glucosidase activity. | [34] |
| Laminaria digitata | The formulated agar in a brown seaweed, L. digitata was prepared. 10 g of processed seaweed sample was transferred to the beaker and mixed up with 100 mL of high-pure Milli-Q water then autoclaved at 121 °C for 1 h for extraction of water-soluble polysaccharides. The yield of formulated agar in a brown seaweed, L. digitata was found to be 40%. Characterized by FTIR, and SEM analysis. | The anti-skin cancer effect of formulated agar (FA) from L. digitata on dimethyl benzanthracene induced skin cancer mice. | Female Swiss albino mice 60–120 mg/mL Orally Four weeks | Anticancer Antioxidant Anti-skin cancer agent | FA from brown seaweed, L. digitata is a potent anti-skin cancer agent and antioxidant. Enhanced the antioxidant system | [29] |
| Laminaria digitata | Laminarin extracted from L. digitata (purity of 95%) was obtained from Sigma-Aldrich (St. Louis, MO, USA) and dissolved in sterile water before use. The response to laminarin treatment (0.1, 0.25, 0.5, 1, and 2 mg/mL) were used. | The anticancer effects of laminarin, a beta-1, 3-glucan derived from brown algae, have been reported in ovarian cancer (OC), colon cancer, leukemia, and melanoma. | A zebrafish xenograft model zebrafish embryos 0.5, 1, and 2 mg/mL Orally 24 h–72 h | Anticancer | Laminarin derived from brown algae had anticancer effects in human OC cells. Prevented tumor formation within the embryo yolks A novel OC suppressor. | [14] |
| Laminaria spp. | The laminarin-rich extract was obtained from Laminaria spp. using hydrothermal assisted extraction by applying optimized extraction conditions. The laminarin-rich extract was included in sufficient quantity to achieve a concentration of 200 ppm in the relevant treatment. The crude extract was partially purified in order to enhance the polysaccharide content by mixing the crude extract with pure ethanol followed by water and calcium chloride. | The effects of increasing dietary inclusion levels of laminarin in the first 14 d post-weaning on pig growth performance and weaning associated intestinal dysfunction. | Ninety-six healthy piglets 650 g/kg Lethal injection 28 days | Anti-obesity Gastrointestinal health | Laminarin-rich extract has potential to prevent pathogen proliferation. Enhanced the volatile fatty acid profile in the colon in a porcine model of colitis. | [62] |
| Laminaria spp. | The laminarin-rich extract was sourced from BioAtlantis Ltd (Tralee, Co. Kerry, Ireland). The extract was prepared by using water as an extraction solvent under optimum heating conditions. A single extraction was performed from Laminaria spp. to produce the extract. Appropriate quantity of laminarin as 100, 200 and 300 parts per million (ppm) was added. | The effects of dietary supplementation with laminarin on colonic health in pigs challenged with dextran sodium sulphate. | Forty-two healthy male pigs 200 ppm Orally 35 days | Anti-inflammatory | 300 ppm of laminarin from a laminarin-rich extract as a dietary supplement, improved performance and prevented post-weaning intestinal dysfunction | [63] |
| Laminaria spp. | The laminarin enriched extract was sourced from BioAtlantis Ltd. The laminarin rich extract was obtained from Laminaria spp. using hydrothermal assisted extraction by applying optimised extraction conditions. The crude extract was partially purified in order to enhance polysaccharide content. | The effects of supplementing the diet of newly weaned pigs with 300 ppm of a laminarin rich extract, on animal performance, volatile fatty acids, and the intestinal microbiota using 16S rRNA gene sequencing | Fifty-four weaned pigs 300 ppm Orally 28 days | Anti-obesity Gut health | 300 ppm of a laminarin rich macroalgal extract reduced post-weaning intestinal dysfunction in pigs. Promoted the proliferation of bacterial taxa Enhanced nutrient digestion by reducing the load of taxa | [64] |
| Saccharina longicruris | Laminarin (TCI Shanghai, China) was dissolved in saline to prepared stock solution of 50 mg/mL. One g/kg of laminarin by intragastric administration and characterized by western blot assay. | The effect of laminarin on energy homeostasis, mice were orally administrated with laminarin to test food intake, fat deposition, and glucose homeostasis. | Homeostasis model C57/BL6 mice 50 mg/mL Orally Four weeks | Anti-obesity | Laminarin counteracts diet-induced obesity. Maintained glucose homeostasis. Promoted GLP-1 secretion via increasing intracellular calcium in enteroendocrine cells. | [31] |
| Laminaria digitata | Laminarin derived from L. digitata was purchased from Invivogen. The endotoxin levels in the purified laminarin were evaluated using a Limulus gametocyte lysate (LAL) assay kit (Lonza, Gampel, Switzerland). | The effects of laminarin on the maturation of dendritic cells and on the in vivo activation of anti-cancer immunity using homozygous transgenic mice (OT-I and II). | C57BL/6 mice, OT-I and OT-II TCR transgenic mice, and C57BL/6-Ly5.1 (CD45.1) congenic mice 25 mg/kg Intrasplenic B16-OVA injection 24 h | Immunomodulatory | The purified laminarin stimulated and inhibited tumor growth and metastasis Potential immune stimulatory molecule for use in cancer immunotherapy. | [30] |
| Laminaria spp. | The crude seaweed extract was derived from Laminaria spp. (BioAtlantis Ltd.). The laminarin content of the supplements and the feed samples was determined by spectrophotometry using a commercial assay kit (Megazyme Ireland Ltd., Bray, Ireland). | The potent anti-inflammatory activities of the algal polysaccharides laminarin and fucoidan in the gastrointestinal tract of pigs | Dextran sodium sulfate-challenged porcine model Thirty-five pigs 300 mg/kg Oral administration 56 days | Anti-inflammatory | Laminarin has potent anti-inflammatory activities in the gastrointestinal tract. Reduced Enterobacteriaceae in proximal colon digesta. Decreased in colonic IL-6 mRNA abundance for its inflammatory effect | [32] |
| Laminarin spp. | A high-fat diet (HFD) and 1% laminarin-supplemented water (HFL). Laminarin (Sigma) supplementation was terminated at the fourth week, followed by continuation HFD for an additional 2 weeks. | The anti-obesity effects of the potential prebiotic, laminarin, on mice, fed a high-fat diet. | Laminarin-supplemented high-fat diet (HFL) mice model Eighteen female BALB/c mice 45% (w/v) Orally 6 weeks | Anti-obesity effects Gut Health and microbiota | Laminarin (Sigma) reduced the adverse effects of a high-fat diet by shifting gut microbiota towards higher energy metabolism. Laminarin could be used to develop anti-obesity functional foods | [25] |
| Laminaria digitata, L. hyperborea and Saccharomyces cerevisiae | Purified laminarin from L. digitata and L. hyperborea (990 g laminarin/kg) was sourced from Bioatlantis Limited and extracted. A basal diet supplemented with 250 parts per million (ppm) laminarin from L. digitata. | The effects of purified laminarin derived from L. digitata, L. hyperborea and S. cerevisiae on piglet performance in selected bacterial populations and intestinal volatile fatty acid (VFA) production | Thirty-two pigs 250 mg/kg Orally 28 days | Anti-inflammatory | Purified laminarin may be acting via a different mechanism from the insoluble β-glucan. Identified as natural biomolecules with immunomodulatory activity | [28] |
| Laminaria digitata | Purified laminarin (990 g/kg laminarin) was sourced from Bioatlantis Ltd., and extracted.A basal diet supplemented with 300, and 600 parts per million (ppm) laminarin from L. digitata. | The optimum inclusion level of laminarin derived from L. digitata on selected microbial populations, intestinal fermentation, cytokine and mucin gene expression in the porcine ileum and colon | Twenty-one Pigs 990 g/kg Orally 26 days | Gastrointestinal health | Dietary inclusion of 300 ppm purified laminarin appears to be the optimum dose. Reduced enterobacteriaceae populations Enhanced IL-6 and IL-8 cytokine expression in response to an ex-vivo LPS challenge | [65] |
| Laminaria digitata | Seaweed extracts derived from L. digitata were included at 2.8 g/kg. The extract contained both laminarin (112 g/kg), fucoidan (89 g/kg) and ash (799 g/kg) and was sourced from Bioatlantis Ltd. | The interactions between two different lactose (L) levels and seaweed extract containing laminarin and fucoidan derived from Laminaria spp. on growth performance, coefficient of total tract apparent digestibility (CTTAD) and faecal microbial populations in the weanling pig. | Two hundred and forty pigs 112 g/kg Orally 25 days | Intestinal digestibility | The inclusion of a high dietary concentration of laminarin increased the CTTAD of diet components and decreased the counts of E. coli in the faeces. Improved performance of pigs after weaning | [66] |
| Laminaria hyperborea | The seaweed extract was extracted from Laminaria spp. The seaweed extract contained laminarin (112 g kg−1), fucoidan (89 g kg−1) and ash (799 g kg−1) and was sourced from BioAtlantis Ltd. | The effect of dietary Laminaria-derived laminarin and fucoidan on nutrient digestibility, nitrogen utilisation, intestinal microflora and volatile fatty acid concentration in pigs | Thirty finishing boars/pigs 112 g/kg Orally 14 days | Anti-obesity effects Dietary and gut health | Reduced intestinal Enterobacterium spp. and increased in Lactobacilli spp. The dietary laminarin may provide a dietary means to improve gut health in pigs | [37] |
| Laminaria spp. | The seaweed extract was extracted from Laminaria spp. Seaweeds extracts of 1, 2 and 4 g/kg were used. The seaweed extract was sourced from BioAtlantis Ltd. | The interaction between different levels of lactose and seaweed extract derived from Laminaria spp. on growth performance and nutrient digestibility of weanling pigs. | 384 piglets 1,2,4 g/kg Orally 21 days | Intestinal health | Pigs responded differently to the inclusion levels of seaweed extract at each level of lactose supplementation. | [67] |
| Laminaria spp. | Laminarin (purity of 90%). was provided by Goëmar (St Malo, France). The molecular weight was 4500 to 5500 g/mol and the purity was 90%. laminarin treated rats (LAM) received the same diet containing 5 g/100 g laminarin for 4 days followed by a dietary treatment of 10 g/100 g LAM for 21 days. | The hypothesis stated that LAM, a β (1–3) polysaccharide extracted from brown algae, can modulate the response to systemic inflammation. | Male Wistar rats 5 g/100 g for 4 days followed by 10 g/100 g Intraperitoneal 21 days | Hepatoprotective | The effects could be due to a direct effect of purified laminarin on immune cells, or to an indirect effect through their dietary fibre properties. Decreased serum monocytes number, TNF-α and nitrite. | [39] |
| Laminaria saccharina | Brown seaweeds, L. saccharina (Algoplus, Roscoff, France) were collected. Laminarin was extracted from ground algae (15–25 g) by addition of absolute ethanol, hot H2SO4, and hot HCl. The various methods of extraction of laminarin was implemented by partial characterisation. | The various methods of extraction of laminarin were compared by partial characterization and, on the other hand, to study the fate of this polysaccharide and its effects in the gastrointestinal tract in order to determine its potential as a dietary fibre in human nutrition. | Wistar rats 15–25 g (w/v) Orally 18 days. | Anti-obesity effects Gut health | Laminarin can be considered a dietary fibre No increased in the intestinal transit and stool output. Laminarin resisted hydrolysis in the human upper gastrointestinal tract. | [68] |
| Source | Extraction, Purification, Yield and Characterisation | Study Design | Model, Administration Dose, Route and Period | Biological Activities | Effects/Outcome of Laminarin Extract or Fraction and Related Products | Ref. |
|---|---|---|---|---|---|---|
| Laminaria digitata and Undaria pinnatifida | The dried seaweed was soaked in 200 mL of water for 10 min, then rinsed and drained to remove excess water. Finally, they were cut into pieces and added with 0.5 g iodine enriched salt (6.5 mg iodine). The yield of dietary fibre (g/serving) about 1.8. | The effects of two brown edible seaweeds, L. digitata and U. pinnatifida, on postprandial glucose metabolism and appetite following a starch load in a human meal study as first trail. | A randomized, Three-way, blinded cross-over trial Twenty healthy participants 5 g (w/v) Orally 180 min | Hyperglycaemic | Concomitant ingestion of brown seaweeds may improve postprandial glycaemic and appetite control in healthy and normal weight adults, depending on the dose. Brown seaweeds inhibited the postprandial glucose and insulin response. | [33] |
| Laminaria digitata and Eisenia bicyclis | Laminarins from L. digitata and Eisenia bicyclis (Purity of 95%) were obtained from Sigma-Aldrich (St. Louis, MO, USA). Laminarin from L. digitata was reduced to laminaritol, to reduce background responses in the bicinchoninic acid (BCA) reducing sugar by enzyme kinetics assay. Purified by Crystallization, NMR, and electrophoresis and used for the human gut microbiota (HGM) study. | The symbiont Bacteroides uniform deploys a single, exemplar polysaccharide utilization locus (PUL) to access yeast β (1, 3)-glucan, brown seaweed β (1, 3)-glucan (laminarin), and cereal mixed-linkage β (1, 3)/β (1, 4)-glucan as first trial. | 2441 adults | Anti-obesity effects Human gut microbiota | Fine-grained knowledge of PUL function Metabolic network analysis and proactive manipulation of the HGM. Purified Laminarin provided a validated set of molecular markers to identify β (1, 3)-glucan utilization potential among members of the HGM. | [85] |
| Laminaria digitata | The purified laminarin (LAM) from the brown algae L. digitata were prepared. The basal diet supplemented with 250 ppm purified LAM; basal diet supplemented with a seaweed extracts. | The effects of supplementing the diet with seaweed extracts on growth performance, small intestinal morphology and function, immune response and Campylobacter jejuni colonisation following an experimental challenge in young chicks as first trial. | Hundred- and thirty-five-day-old male Ross chicks 250 ppm Orally 13 days | Intestinal health | Supplementation with laminarin improved growth rate, positively modified small intestinal architecture and Impacted the intestinal immune response. | [80] |
| Laminaria digitata | A group of 30 patients were dilated preoperatively with Laminaria tents, prepared from L. digitata. | The safety and efficacy of L. digitata was examined to dilate the cervix before resectoscopic surgery as randomized trial. | Thirty patients Randomized trial | Resectoscopic cervical trauma | More than 8-mm cervical dilatation | [83] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karuppusamy, S.; Rajauria, G.; Fitzpatrick, S.; Lyons, H.; McMahon, H.; Curtin, J.; Tiwari, B.K.; O’Donnell, C. Biological Properties and Health-Promoting Functions of Laminarin: A Comprehensive Review of Preclinical and Clinical Studies. Mar. Drugs 2022, 20, 772. https://doi.org/10.3390/md20120772
Karuppusamy S, Rajauria G, Fitzpatrick S, Lyons H, McMahon H, Curtin J, Tiwari BK, O’Donnell C. Biological Properties and Health-Promoting Functions of Laminarin: A Comprehensive Review of Preclinical and Clinical Studies. Marine Drugs. 2022; 20(12):772. https://doi.org/10.3390/md20120772
Chicago/Turabian StyleKaruppusamy, Shanmugapriya, Gaurav Rajauria, Stephen Fitzpatrick, Henry Lyons, Helena McMahon, James Curtin, Brijesh K. Tiwari, and Colm O’Donnell. 2022. "Biological Properties and Health-Promoting Functions of Laminarin: A Comprehensive Review of Preclinical and Clinical Studies" Marine Drugs 20, no. 12: 772. https://doi.org/10.3390/md20120772
APA StyleKaruppusamy, S., Rajauria, G., Fitzpatrick, S., Lyons, H., McMahon, H., Curtin, J., Tiwari, B. K., & O’Donnell, C. (2022). Biological Properties and Health-Promoting Functions of Laminarin: A Comprehensive Review of Preclinical and Clinical Studies. Marine Drugs, 20(12), 772. https://doi.org/10.3390/md20120772