Marine Natural Products from the Russian Pacific as Sources of Drugs for Neurodegenerative Diseases
Abstract
1. Introduction: The Russian Pacific
2. Neurodegenerative Diseases: Problems and Pharmacotherapeutic Targets
2.1. Pharmacotherapeutic Targets
2.1.1. Amyloid-β and the Regulatory Enzymes
2.1.2. Tau Protein and Hyperphosphorylation of Tau
2.1.3. Glutamatergic System and Glutamatergic Neurotransmission
2.1.4. Cholinergic System and Dysfunction
2.1.5. Neuroinflammation
2.1.6. Oxidative Stress
2.1.7. α-Synuclein
2.1.8. Monoaminoxidase B
2.1.9. Adenosine Receptors
2.1.10. The JNK Pathway
2.1.11. Autophagy
2.1.12. Matrix Metalloproteinases
2.1.13. Neurotrophic Factors
2.1.14. Peroxisome Proliferator-Activated Receptor Gamma Coactivator-1 Alpha (PGC-1α)
2.1.15. The Microbiota-Gut-Brain Axis
3. Marine Natural Products from the Russian Pacific for NDD Treatment and Prevention
3.1. Sea Lipids from the Russian Pacific: Pharmacology and Biotechnology
3.1.1. Polyunsaturated Fatty Acids (PUFAs) and Their Derivatives
3.1.2. Sphingolipids
- -
- 1-O-[(N-acetyl-α-D-neuramynosyl)-(2→8)-(N-acetyl-α-D-neuraminosyl)-(2→3)-β-D-galactopyranosyl-(1→4)-β-D-glucopyranosyl]-ceramide from the starfish Luidia maculata [275];
- -
- 1-O-α-L-arabinofuranosyl-(1→3)-α-D-galactopyranosyl-(1→4)-(N-acetyl-α-D-neuraminosyl)-(2→6)-β-D-galactofuranosyl-(1→3)-[α-L-arabinofuranosyl-(1→4)]-α-D-galactopyranosyl-(1→4)-(N-acetyl-α-D-neuraminosyl)-(2→3)-β-D-galactopyranosyl-(1→4)-β-D-glucopyranoside of ceramide composed of heterogeneous (2S,3S,4R)-phytosphingosine (iso-C-17-phytosphingosine as the major component) and (2R)-2-hydroxy fatty acid units (docosanoic acid as the major component) from the starfish Patiria (=Asterina) pectinifera [276];
- -
- 8-O-methyl-(N-glycolyl-α-D-neuraminosyl)-(2→11)-(N-glycolyl-α-D-neuraminosyl)-(2→11)-(N-glycolyl-α-D-neuraminosyl)-(2→3)-β-D-galactopyranosyl-(1→4)-β-D-glucopyranoside of a ceramide composed of phytosphingosines and 2-hydroxy n-fatty acids from the starfish Linckia laevigata [277];
- -
- α-NeuAc-(2→4)-α-NeuAc-(2→3)-β-Gal-(1→8)-α-NeuAc-(2→3)-β-GalNAc- (1→3)-β-Gal-(1→4)-β-Glc-(1→1)-Cer from the sea cucumber Apostichopus (=Stichopus) japonicus [278];
- -
- three gangliosides from the sea cucumber Stichopus chloronotus [279];
- -
3.2. Sea Sterols and Oxysterols
3.3. Bioactive Compounds of Marine Algae
3.3.1. Laminarans
3.3.2. Fucoidans
3.3.3. Fucoxanthin
3.4. Echinochrome
3.5. Asterosaponines
3.6. Marine Alkaloids
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Shuntov, V.; Ivanov, O. Climate changes and current state of biota in the russian waters of the far-eastern seas. Izv. TINRO 2019, 197, 83–107. [Google Scholar] [CrossRef]
- Adrianov, A.V. The environmental safety of Russia’s Far Eastern seas. Her. Russ. Acad. Sci. 2011, 81, 25–30. [Google Scholar] [CrossRef]
- Ivanov, O.; Shuntov, V. Importance of fish species in the bottom and near-bottoml biotopes of the Far-Eastern Seas and Pacific waters of Russia. Izv. TINRO 2022, 202, 268–282. [Google Scholar] [CrossRef]
- Volvenko, I. Инфopмациoннoе oбеспечение кoмплексных исследoваний вoдных биopесуpсoв севеpo-западнoй Пацифики. Часть 1. Кoнцепция, пpедыстopия, началo pеализации. Тpуды ВНИРO 2015, 156, 38–66. [Google Scholar]
- Shuntov, V.; Volvenko, I. Generalized assessments of composition, quantitative distribution and biomass of benthic macrofauna on the shelf and slope in the North-West Pacific. Izv. TINRO 2015, 182, 3–22. [Google Scholar] [CrossRef]
- Sirenko, B.I. Biological diversity of invertebrates in Far Eastern seas of Russia. In Proceedings of the Bridges of the Science between North America and Russian Far East 45-th Arctic Science Conference, Anchorage, Alaska, 25–27 August 1994; p. 28. [Google Scholar]
- Шунтoв, В.П. БИOЛOГИЯ ДАЛЬНЕВOCТOЧНЫХ МOРЕЙ РOCCИИ; Тихooкеанский научнo-исследoвательский pыбoхoзяйственный центp (ТИНРO-Центp): Vladivostok, Russia, 2016; ISBN 978-5-89131-123-7. [Google Scholar]
- Adrianov, A.V. Strategies and methodology of marine diodiversity studies. Russ. J. Mar. Biol. 2004, 30, S17–S21. [Google Scholar] [CrossRef]
- Ivanov, O.; Sukhanov, V. Some aspects of biogeography with reference to zoning of the Far- Eastern Seas of Russia and adjacent waters of the Pacific Ocean. Izv. TINRO 2015, 183, 3–26. [Google Scholar] [CrossRef]
- Brandt, A.; Elsner, N.; Brenke, N.; Golovan, O.; Malyutina, M.V.; Riehl, T.; Schwabe, E.; Würzberg, L. Epifauna of the Sea of Japan collected via a new epibenthic sledge equipped with camera and environmental sensor systems. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2013, 86, 43–55. [Google Scholar] [CrossRef]
- Malyutina, M.V.; Brandt, A. Introduction to SoJaBio (Sea of Japan Biodiversity Studies) Introduction. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2013, 86, 1–9. [Google Scholar] [CrossRef]
- Brandt, A.; Malyutina, M.V. The German-Russian deep-sea expedition KuramBio (Kurile Kamchatka biodiversity studies) on board of the RV Sonne in 2012 following the footsteps of the legendary expeditions with RV Vityaz Introduction. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2015, 111, 1–9. [Google Scholar] [CrossRef]
- Brandt, A.; Elsner, N.O.; Malyutina, M.V.; Brenke, N.; Golovan, O.A.; Lavrenteva, A.V.; Riehl, T. Abyssal macrofauna of the Kuril–Kamchatka Trench area (Northwest Pacific) collected by means of a camera–epibenthic sledge. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2015, 111, 175–187. [Google Scholar] [CrossRef]
- Blagodatski, A.; Cherepanov, V.; Koval, A.; Kharlamenko, V.I.; Khotimchenko, Y.S.; Katanaev, V.L. High-throughput targeted screening in triple-negative breast cancer cells identifies Wnt-inhibiting activities in Pacific brittle stars. Sci. Rep. 2017, 7, 11964. [Google Scholar] [CrossRef]
- Brandt, A.; Alalykina, I.; Fukumori, H.; Golovan, O.; Kniesz, K.; Lavrenteva, A.; Lörz, A.N.; Malyutina, M.; Philipps-Bussau, K.; Stransky, B. First insights into macrofaunal composition from the SokhoBio expedition (Sea of Okhotsk, Bussol Strait and northern slope of the Kuril-Kamchatka Trench). Deep. Sea Res. Part II Top. Stud. Oceanogr. 2018, 154, 106–120. [Google Scholar] [CrossRef]
- Malyutina, M.V.; Chernyshev, A.V.; Brandt, A. Introduction to the SokhoBio (Sea of Okhotsk Biodiversity Studies) expedition 2015. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2018, 154, 1–9. [Google Scholar] [CrossRef]
- Adrianov, A.V.; Maiorova, A.S. Meristoderes okhotensis sp. nov.—The first deepwater representative of kinorhynchs in the Sea of Okhotsk (Kinorhyncha: Cyclorhagida). Deep. Sea Res. Part II Top. Stud. Oceanogr. 2018, 154, 99–105. [Google Scholar] [CrossRef]
- Maiorova, A.S.; Adrianov, A.V. Deep-sea spoon worms (Echiura) from the Sea of Okhotsk and the adjacent slope of the Kuril-Kamchatka Trench. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2018, 154, 177–186. [Google Scholar] [CrossRef]
- Maiorova, A.; Adrianov, A. Biodiversity of echiurans (Echiura) of the Kuril-Kamchatka Trench area. Prog. Oceanogr. 2019, 180, 102216. [Google Scholar] [CrossRef]
- Dmitrenok, P.S. Editorial to the Special Issue: Dedicated to the 55th Anniversary of G.B. Elyakov Pacific Institute of Bioorganic Chemistry of the Far Eastern Branch of the Russian Academy of Sciences. Molecules 2021, 26, 4971. [Google Scholar] [CrossRef]
- Katanaev, V.L.; Di Falco, S.; Khotimchenko, Y. The Anticancer Drug Discovery Potential of Marine Invertebrates from Russian Pacific. Mar. Drugs 2019, 17, 474. [Google Scholar] [CrossRef]
- Beart, P.M.; Robinson, M.; Rattray, M.; Maragakis, N.J. Neurodegenerative diseases: Pathology, mechanisms, and potential therapeutic targets. In Advances in Neurobiology; Springer: Cham, Switzerland, 2017; p. 542. [Google Scholar]
- Doria, M.; Maugest, L.; Moreau, T.; Lizard, G.; Vejux, A. Contribution of cholesterol and oxysterols to the pathophysiology of Parkinson’s disease. Free Radic. Biol. Med. 2016, 101, 393–400. [Google Scholar] [CrossRef]
- Teijido, O.; Cacabelos, R. Pharmacoepigenomic Interventions as Novel Potential Treatments for Alzheimer’s and Parkinson’s Diseases. Int. J. Mol. Sci. 2018, 19, 3199. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, A.; Andleeb, A.; Waris, T.S.; Bazzar, M.; Moradi, A.R.; Awan, N.R.; Yar, M. Neurodegenerative diseases and effective drug delivery: A review of challenges and novel therapeutics. J. Control. Release 2021, 330, 1152–1167. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Zhang, Z.; Ang, L.C. Clinicopathological overlap of neurodegenerative diseases: A comprehensive review. J. Clin. Neurosci. 2020, 78, 30–33. [Google Scholar] [CrossRef]
- Sugimoto, H. The new approach in development of anti-Alzheimer’s disease drugs via the cholinergic hypothesis. Chem. Biol. Interact. 2008, 175, 204–208. [Google Scholar] [CrossRef]
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.E.; Lang, A.E. Parkinson disease. Nat. Rev. Dis. Primers 2017, 3, 17013. [Google Scholar] [CrossRef]
- Nguyen, P.H.; Ramamoorthy, A.; Sahoo, B.R.; Zheng, J.; Faller, P.; Straub, J.E.; Dominguez, L.; Shea, J.E.; Dokholyan, N.V.; De Simone, A.; et al. Amyloid Oligomers: A Joint Experimental/Computational Perspective on Alzheimer’s Disease, Parkinson’s Disease, Type II Diabetes, and Amyotrophic Lateral Sclerosis. Chem. Rev. 2021, 121, 2545–2647. [Google Scholar] [CrossRef]
- Hamley, I.W. The amyloid beta peptide: A chemist’s perspective. Role in Alzheimer’s and fibrillization. Chem. Rev. 2012, 112, 5147–5192. [Google Scholar] [CrossRef]
- Kulshreshtha, A.; Piplani, P. Current pharmacotherapy and putative disease-modifying therapy for Alzheimer’s disease. Neurol. Sci. 2016, 37, 1403–1435. [Google Scholar] [CrossRef]
- Hampel, H.; Vassar, R.; De Strooper, B.; Hardy, J.; Willem, M.; Singh, N.; Zhou, J.; Yan, R.; Vanmechelen, E.; De Vos, A.; et al. The β-Secretase BACE1 in Alzheimer’s Disease. Biol. Psychiatry 2021, 89, 745–756. [Google Scholar] [CrossRef]
- Lin, Y.; Yao, Y.-J.; Liang, X.-C.; Shi, Y.; Ni, Y.-N.; Wu, Y.-T.; Yang, J.-X. Osthole suppresses BACE-1 expression by up-regulating miR-9 in Alzheimer’ s disease. Chin. Pharmacol. Bull. 2019, 35, 524–529. [Google Scholar]
- Chami, L.; Checler, F. BACE1 is at the crossroad of a toxic vicious cycle involving cellular stress and β-amyloid production in Alzheimer’s disease. Mol. Neurodegener. 2012, 7, 52. [Google Scholar] [CrossRef] [PubMed]
- Haass, C.; Selkoe, D.J. Soluble protein oligomers in neurodegeneration: Lessons from the Alzheimer’s amyloid beta-peptide. Nat. Rev. Mol. Cell Biol. 2007, 8, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Genereux, J.C.; Wiseman, R.L. Endoplasmic reticulum quality control and systemic amyloid disease: Impacting protein stability from the inside out. IUBMB Life 2015, 67, 404–413. [Google Scholar] [CrossRef]
- Pagani, L.; Eckert, A. Amyloid-Beta interaction with mitochondria. Int. J. Alzheimer’s Dis. 2011, 2011, 925050. [Google Scholar] [CrossRef]
- Sanders, O.D.; Rajagopal, L.; Rajagopal, J.A. The oxidatively damaged DNA and amyloid-β oligomer hypothesis of Alzheimer’s disease. Free Radic. Biol. Med. 2022, 179, 403–412. [Google Scholar] [CrossRef]
- Schmitt, U.; Hiemke, C.; Fahrenholz, F.; Schroeder, A. Over-expression of two different forms of the alpha-secretase ADAM10 affects learning and memory in mice. Behav. Brain Res. 2006, 175, 278–284. [Google Scholar] [CrossRef]
- Bell, K.F.; Zheng, L.; Fahrenholz, F.; Cuello, A.C. ADAM-10 over-expression increases cortical synaptogenesis. Neurobiol. Aging 2008, 29, 554–565. [Google Scholar] [CrossRef]
- Hur, J.-Y. γ-Secretase in Alzheimer’s disease. Exp. Mol. Med. 2022, 54, 433–446. [Google Scholar] [CrossRef]
- McDade, E.; Voytyuk, I.; Aisen, P.; Bateman, R.J.; Carrillo, M.C.; De Strooper, B.; Haass, C.; Reiman, E.M.; Sperling, R.; Tariot, P.N.; et al. The case for low-level BACE1 inhibition for the prevention of Alzheimer disease. Nat. Rev. Neurol. 2021, 17, 703–714. [Google Scholar] [CrossRef]
- Muralidar, S.; Ambi, S.V.; Sekaran, S.; Thirumalai, D.; Palaniappan, B. Role of tau protein in Alzheimer’s disease: The prime pathological player. Int. J. Biol. Macromol. 2020, 163, 1599–1617. [Google Scholar] [CrossRef]
- Kimura, T.; Ishiguro, K.; Hisanaga, S. Physiological and pathological phosphorylation of tau by Cdk5. Front. Mol. Neurosci. 2014, 7, 65. [Google Scholar] [CrossRef] [PubMed]
- Perea, J.R.; Bolós, M.; Avila, J. Microglia in Alzheimer’s Disease in the Context of Tau Pathology. Biomolecules 2020, 10, 1439. [Google Scholar] [CrossRef] [PubMed]
- Hanger, D.P.; Anderton, B.H.; Noble, W. Tau phosphorylation: The therapeutic challenge for neurodegenerative disease. Trends Mol. Med. 2009, 15, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.X.; Liu, F.; Grundke-Iqbal, I.; Iqbal, K. Post-translational modifications of tau protein in Alzheimer’s disease. J. Neural Transm. 2005, 112, 813–838. [Google Scholar] [CrossRef] [PubMed]
- Medina, M.; Garrido, J.J.; Wandosell, F.G. Modulation of GSK-3 as a Therapeutic Strategy on Tau Pathologies. Front. Mol. Neurosci. 2011, 4, 24. [Google Scholar] [CrossRef]
- Dolan, P.J.; Johnson, G.V. The role of tau kinases in Alzheimer’s disease. Curr. Opin. Drug Discov. Devel. 2010, 13, 595–603. [Google Scholar]
- Li, D.W.; Liu, Z.Q.; Chen, W.; Yao, M.; Li, G.R. Association of glycogen synthase kinase-3β with Parkinson’s disease (review). Mol. Med. Rep. 2014, 9, 2043–2050. [Google Scholar] [CrossRef]
- Llorens-Martín, M.; Jurado, J.; Hernández, F.; Avila, J. GSK-3β, a pivotal kinase in Alzheimer disease. Front. Mol. Neurosci. 2014, 7, 46. [Google Scholar]
- Fernández-Nogales, M.; Hernández, F.; Miguez, A.; Alberch, J.; Ginés, S.; Pérez-Navarro, E.; Lucas, J.J. Decreased glycogen synthase kinase-3 levels and activity contribute to Huntington’s disease. Hum. Mol. Genet. 2015, 24, 5040–5052. [Google Scholar] [CrossRef]
- Kickstein, E.; Krauss, S.; Thornhill, P.; Rutschow, D.; Zeller, R.; Sharkey, J.; Williamson, R.; Fuchs, M.; Köhler, A.; Glossmann, H.; et al. Biguanide metformin acts on tau phosphorylation via mTOR/protein phosphatase 2A (PP2A) signaling. Proc. Natl. Acad. Sci. USA 2010, 107, 21830–21835. [Google Scholar] [CrossRef]
- Wegiel, J.; Gong, C.-X.; Hwang, Y.-W. The role of DYRK1A in neurodegenerative diseases. FEBS J. 2011, 278, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Ryoo, S.R.; Cho, H.J.; Lee, H.W.; Jeong, H.K.; Radnaabazar, C.; Kim, Y.S.; Kim, M.J.; Son, M.Y.; Seo, H.; Chung, S.H.; et al. Dual-specificity tyrosine(Y)-phosphorylation regulated kinase 1A-mediated phosphorylation of amyloid precursor protein: Evidence for a functional link between Down syndrome and Alzheimer’s disease. J. Neurochem. 2008, 104, 1333–1344. [Google Scholar] [CrossRef]
- Ryu, Y.S.; Park, S.Y.; Jung, M.S.; Yoon, S.H.; Kwen, M.Y.; Lee, S.Y.; Choi, S.H.; Radnaabazar, C.; Kim, M.K.; Kim, H.; et al. Dyrk1A-mediated phosphorylation of Presenilin 1: A functional link between Down syndrome and Alzheimer’s disease. J. Neurochem. 2010, 115, 574–584. [Google Scholar] [CrossRef] [PubMed]
- Kimura, R.; Kamino, K.; Yamamoto, M.; Nuripa, A.; Kida, T.; Kazui, H.; Hashimoto, R.; Tanaka, T.; Kudo, T.; Yamagata, H.; et al. The DYRK1A gene, encoded in chromosome 21 Down syndrome critical region, bridges between beta-amyloid production and tau phosphorylation in Alzheimer disease. Hum. Mol. Genet. 2007, 16, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Pathak, A.; Rohilla, A.; Gupta, T.; Akhtar, M.J.; Haider, M.R.; Sharma, K.; Haider, K.; Yar, M.S. DYRK1A kinase inhibition with emphasis on neurodegeneration: A comprehensive evolution story-cum-perspective. Eur. J. Med. Chem. 2018, 158, 559–592. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Foster, J.B.; Lin, C.L. Glutamate transporter EAAT2: Regulation, function, and potential as a therapeutic target for neurological and psychiatric disease. Cell Mol. Life Sci. 2015, 72, 3489–3506. [Google Scholar] [CrossRef]
- Conway, M.E. Alzheimer’s disease: Targeting the glutamatergic system. Biogerontology 2020, 21, 257–274. [Google Scholar] [CrossRef]
- Andersen, J.V.; Markussen, K.H.; Jakobsen, E.; Schousboe, A.; Waagepetersen, H.S.; Rosenberg, P.A.; Aldana, B.I. Glutamate metabolism and recycling at the excitatory synapse in health and neurodegeneration. Neuropharmacology 2021, 196, 108719. [Google Scholar] [CrossRef]
- Di Domenico, F.; Tramutola, A.; Butterfield, D.A. Role of 4-hydroxy-2-nonenal (HNE) in the pathogenesis of alzheimer disease and other selected age-related neurodegenerative disorders. Free Radic. Biol. Med. 2017, 111, 253–261. [Google Scholar] [CrossRef]
- Zhao, X.; Kotilinek, L.A.; Smith, B.; Hlynialuk, C.; Zahs, K.; Ramsden, M.; Cleary, J.; Ashe, K.H. Caspase-2 cleavage of tau reversibly impairs memory. Nat. Med. 2016, 22, 1268–1276. [Google Scholar] [CrossRef]
- Esposito, Z.; Belli, L.; Toniolo, S.; Sancesario, G.; Bianconi, C.; Martorana, A. Amyloid β, glutamate, excitotoxicity in Alzheimer’s disease: Are we on the right track? CNS Neurosci. Ther. 2013, 19, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Di Meo, S.; Reed, T.T.; Venditti, P.; Victor, V.M. Role of ROS and RNS Sources in Physiological and Pathological Conditions. Oxid. Med. Cell. Longev. 2016, 2016, 1245049. [Google Scholar] [CrossRef] [PubMed]
- Bordji, K.; Becerril-Ortega, J.; Nicole, O.; Buisson, A. Activation of extrasynaptic, but not synaptic, NMDA receptors modifies amyloid precursor protein expression pattern and increases amyloid-ß production. J. Neurosci. 2010, 30, 15927–15942. [Google Scholar] [CrossRef] [PubMed]
- Bukke, V.N.; Archana, M.; Villani, R.; Romano, A.D.; Wawrzyniak, A.; Balawender, K.; Orkisz, S.; Beggiato, S.; Serviddio, G.; Cassano, T. The Dual Role of Glutamatergic Neurotransmission in Alzheimer’s Disease: From Pathophysiology to Pharmacotherapy. Int. J. Mol. Sci. 2020, 21, 7452. [Google Scholar] [CrossRef]
- Kullmann, D.M.; Lamsa, K.P. Long-term synaptic plasticity in hippocampal interneurons. Nat. Rev. Neurosci. 2007, 8, 687–699. [Google Scholar] [CrossRef]
- Brito-Moreira, J.; Paula-Lima, A.C.; Bomfim, T.R.; Oliveira, F.B.; Sepúlveda, F.J.; De Mello, F.G.; Aguayo, L.G.; Panizzutti, R.; Ferreira, S.T. Aβ oligomers induce glutamate release from hippocampal neurons. Curr. Alzheimer. Res. 2011, 8, 552–562. [Google Scholar] [CrossRef]
- Talantova, M.; Sanz-Blasco, S.; Zhang, X.; Xia, P.; Akhtar, M.W.; Okamoto, S.; Dziewczapolski, G.; Nakamura, T.; Cao, G.; Pratt, A.E.; et al. Aβ induces astrocytic glutamate release, extrasynaptic NMDA receptor activation, and synaptic loss. Proc. Natl. Acad. Sci. USA 2013, 110, E2518–E2527. [Google Scholar] [CrossRef]
- Wang, Q.; Walsh, D.M.; Rowan, M.J.; Selkoe, D.J.; Anwyl, R. Block of long-term potentiation by naturally secreted and synthetic amyloid beta-peptide in hippocampal slices is mediated via activation of the kinases c-Jun N-terminal kinase, cyclin-dependent kinase 5, and p38 mitogen-activated protein kinase as well as metabotropic glutamate receptor type 5. J. Neurosci. 2004, 24, 3370–3378. [Google Scholar]
- Calvo-Rodriguez, M.; Hou, S.S.; Snyder, A.C.; Kharitonova, E.K.; Russ, A.N.; Das, S.; Fan, Z.; Muzikansky, A.; Garcia-Alloza, M.; Serrano-Pozo, A.; et al. Increased mitochondrial calcium levels associated with neuronal death in a mouse model of Alzheimer’s disease. Nat. Commun. 2020, 11, 2146. [Google Scholar] [CrossRef]
- Parsons, C.G.; Stöffler, A.; Danysz, W. Memantine: A NMDA receptor antagonist that improves memory by restoration of homeostasis in the glutamatergic system--too little activation is bad, too much is even worse. Neuropharmacology 2007, 53, 699–723. [Google Scholar] [CrossRef]
- Knight, R.; Khondoker, M.; Magill, N.; Stewart, R.; Landau, S. A Systematic Review and Meta-Analysis of the Effectiveness of Acetylcholinesterase Inhibitors and Memantine in Treating the Cognitive Symptoms of Dementia. Dement. Geriatr. Cogn. Disord. 2018, 45, 131–151. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Reddy, P.H. Role of Glutamate and NMDA Receptors in Alzheimer’s Disease. J. Alzheimer’s Dis. 2017, 57, 1041–1048. [Google Scholar] [CrossRef]
- Anggono, V.; Huganir, R.L. Regulation of AMPA receptor trafficking and synaptic plasticity. Curr. Opin. Neurobiol. 2012, 22, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Guntupalli, S.; Widagdo, J.; Anggono, V. Amyloid-β-Induced Dysregulation of AMPA Receptor Trafficking. Neural. Plast. 2016, 2016, 3204519. [Google Scholar] [CrossRef]
- Renner, M.; Lacor, P.N.; Velasco, P.T.; Xu, J.; Contractor, A.; Klein, W.L.; Triller, A. Deleterious effects of amyloid beta oligomers acting as an extracellular scaffold for mGluR5. Neuron 2010, 66, 739–754. [Google Scholar] [CrossRef]
- Hamilton, A.; Esseltine, J.L.; DeVries, R.A.; Cregan, S.P.; Ferguson, S.S. Metabotropic glutamate receptor 5 knockout reduces cognitive impairment and pathogenesis in a mouse model of Alzheimer’s disease. Mol. Brain 2014, 7, 40. [Google Scholar] [CrossRef] [PubMed]
- Berent-Spillson, A.; Russell, J.W. Metabotropic glutamate receptor 3 protects neurons from glucose-induced oxidative injury by increasing intracellular glutathione concentration. J. Neurochem. 2007, 101, 342–354. [Google Scholar] [CrossRef]
- Tichauer, J.E.; von Bernhardi, R. Transforming growth factor-β stimulates β amyloid uptake by microglia through Smad3-dependent mechanisms. J. Neurosci. Res. 2012, 90, 1970–1980. [Google Scholar] [CrossRef]
- Caraci, F.; Gulisano, W.; Guida, C.A.; Impellizzeri, A.A.; Drago, F.; Puzzo, D.; Palmeri, A. A key role for TGF-β1 in hippocampal synaptic plasticity and memory. Sci. Rep. 2015, 5, 11252. [Google Scholar] [CrossRef]
- Durand, D.; Carniglia, L.; Beauquis, J.; Caruso, C.; Saravia, F.; Lasaga, M. Astroglial mGlu3 receptors promote alpha-secretase-mediated amyloid precursor protein cleavage. Neuropharmacology 2014, 79, 180–189. [Google Scholar] [CrossRef]
- Goeldner, C.; Ballard, T.M.; Knoflach, F.; Wichmann, J.; Gatti, S.; Umbricht, D. Cognitive impairment in major depression and the mGlu2 receptor as a therapeutic target. Neuropharmacology 2013, 64, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.L.; Jones, F.; Kubota, E.S.; Pocock, J.M. Stimulation of microglial metabotropic glutamate receptor mGlu2 triggers tumor necrosis factor alpha-induced neurotoxicity in concert with microglial-derived Fas ligand. J. Neurosci. 2005, 25, 2952–2964. [Google Scholar] [CrossRef] [PubMed]
- Bruno, V.; Caraci, F.; Copani, A.; Matrisciano, F.; Nicoletti, F.; Battaglia, G. The impact of metabotropic glutamate receptors into active neurodegenerative processes: A “dark side” in the development of new symptomatic treatments for neurologic and psychiatric disorders. Neuropharmacology 2017, 115, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, A.; Vasefi, M.; Vander Tuin, C.; McQuaid, R.J.; Anisman, H.; Ferguson, S.S. Chronic Pharmacological mGluR5 Inhibition Prevents Cognitive Impairment and Reduces Pathogenesis in an Alzheimer Disease Mouse Model. Cell. Rep. 2016, 15, 1859–1865. [Google Scholar] [CrossRef]
- Haas, L.T.; Salazar, S.V.; Smith, L.M.; Zhao, H.R.; Cox, T.O.; Herber, C.S.; Degnan, A.P.; Balakrishnan, A.; Macor, J.E.; Albright, C.F.; et al. Silent Allosteric Modulation of mGluR5 Maintains Glutamate Signaling while Rescuing Alzheimer’s Mouse Phenotypes. Cell. Rep. 2017, 20, 76–88. [Google Scholar] [CrossRef]
- Shrivastava, A.N.; Kowalewski, J.M.; Renner, M.; Bousset, L.; Koulakoff, A.; Melki, R.; Giaume, C.; Triller, A. β-amyloid and ATP-induced diffusional trapping of astrocyte and neuronal metabotropic glutamate type-5 receptors. Glia 2013, 61, 1673–1686. [Google Scholar] [CrossRef]
- Kim, S.H.; Fraser, P.E.; Westaway, D.; St George-Hyslop, P.H.; Ehrlich, M.E.; Gandy, S. Group II metabotropic glutamate receptor stimulation triggers production and release of Alzheimer’s amyloid(beta)42 from isolated intact nerve terminals. J. Neurosci. 2010, 30, 3870–3875. [Google Scholar] [CrossRef]
- Caraci, F.; Molinaro, G.; Battaglia, G.; Giuffrida, M.L.; Riozzi, B.; Traficante, A.; Bruno, V.; Cannella, M.; Merlo, S.; Wang, X.; et al. Targeting group II metabotropic glutamate (mGlu) receptors for the treatment of psychosis associated with Alzheimer’s disease: Selective activation of mGlu2 receptors amplifies beta-amyloid toxicity in cultured neurons, whereas dual activation of mGlu2 and mGlu3 receptors is neuroprotective. Mol. Pharmacol. 2011, 79, 618–626. [Google Scholar]
- Pitsikas, N.; Kaffe, E.; Markou, A. The metabotropic glutamate 2/3 receptor antagonist LY341495 differentially affects recognition memory in rats. Behav. Brain Res. 2012, 230, 374–379. [Google Scholar] [CrossRef]
- Shimazaki, T.; Kaku, A.; Chaki, S. Blockade of the metabotropic glutamate 2/3 receptors enhances social memory via the AMPA receptor in rats. Eur. J. Pharmacol. 2007, 575, 94–97. [Google Scholar] [CrossRef]
- Cheng, Y.J.; Lin, C.H.; Lane, H.Y. Involvement of Cholinergic, Adrenergic, and Glutamatergic Network Modulation with Cognitive Dysfunction in Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 2283. [Google Scholar] [CrossRef] [PubMed]
- Haam, J.; Yakel, J.L. Cholinergic modulation of the hippocampal region and memory function. J. Neurochem. 2017, 142 (Suppl. S2), 111–121. [Google Scholar] [CrossRef] [PubMed]
- Dineley, K.T.; Pandya, A.A.; Yakel, J.L. Nicotinic ACh receptors as therapeutic targets in CNS disorders. Trends Pharmacol. Sci. 2015, 36, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Francis, P.T.; Palmer, A.M.; Snape, M.; Wilcock, G.K. The cholinergic hypothesis of Alzheimer’s disease: A review of progress. J. Neurol. Neurosurg. Psychiatry 1999, 66, 137–147. [Google Scholar] [CrossRef]
- Perry, E.K.; Gibson, P.H.; Blessed, G.; Perry, R.H.; Tomlinson, B.E. Neurotransmitter enzyme abnormalities in senile dementia. Choline acetyltransferase and glutamic acid decarboxylase activities in necropsy brain tissue. J. Neurol. Sci. 1977, 34, 247–265. [Google Scholar] [CrossRef]
- Davis, K.L.; Thal, L.J.; Gamzu, E.R.; Davis, C.S.; Woolson, R.F.; Gracon, S.I.; Drachman, D.A.; Schneider, L.S.; Whitehouse, P.J.; Hoover, T.M.; et al. A double-blind, placebo-controlled multicenter study of tacrine for Alzheimer’s disease. The Tacrine Collaborative Study Group. N. Engl. J. Med. 1992, 327, 1253–1259. [Google Scholar] [CrossRef]
- Bohnen, N.I.; Albin, R.L. The cholinergic system and Parkinson disease. Behav. Brain Res. 2011, 221, 564–573. [Google Scholar] [CrossRef]
- Colović, M.B.; Krstić, D.Z.; Lazarević-Pašti, T.D.; Bondžić, A.M.; Vasić, V.M. Acetylcholinesterase inhibitors: Pharmacology and toxicology. Curr. Neuropharmacol. 2013, 11, 315–335. [Google Scholar] [CrossRef]
- Hansen, R.A.; Gartlehner, G.; Webb, A.P.; Morgan, L.C.; Moore, C.G.; Jonas, D.E. Efficacy and safety of donepezil, galantamine, and rivastigmine for the treatment of Alzheimer’s disease: A systematic review and meta-analysis. Clin. Interv. Aging 2008, 3, 211–225. [Google Scholar]
- Feng, B.; Li, X.; Xia, J.; Wu, S. Discovery of novel isoflavone derivatives as AChE/BuChE dual-targeted inhibitors: Synthesis, biological evaluation and molecular modelling. J. Enzyme Inhib. Med. Chem. 2017, 32, 968–977. [Google Scholar] [CrossRef]
- Greig, N.H.; Utsuki, T.; Ingram, D.K.; Wang, Y.; Pepeu, G.; Scali, C.; Yu, Q.S.; Mamczarz, J.; Holloway, H.W.; Giordano, T.; et al. Selective butyrylcholinesterase inhibition elevates brain acetylcholine, augments learning and lowers Alzheimer beta-amyloid peptide in rodent. Proc. Natl. Acad. Sci. USA 2005, 102, 17213–17218. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Singh, B. A review on cholinesterase inhibitors for Alzheimer’s disease. Arch. Pharmacal Res. 2013, 36, 375–399. [Google Scholar] [CrossRef] [PubMed]
- Andrade, S.; Ramalho, M.J.; Loureiro, J.A.; Pereira, M.D.C. Natural Compounds for Alzheimer’s Disease Therapy: A Systematic Review of Preclinical and Clinical Studies. Int. J. Mol. Sci. 2019, 20, 2313. [Google Scholar] [CrossRef]
- Alonso, E.; Vale, C.; Vieytes, M.R.; Laferla, F.M.; Giménez-Llort, L.; Botana, L.M. The Cholinergic Antagonist Gymnodimine Improves Aβ and Tau Neuropathology in an in Vitro Model of Alzheimer Disease. Cell. Physiol. Biochem. 2011, 27, 783–794. [Google Scholar] [CrossRef] [PubMed]
- Moodie, L.W.K.; Sepčić, K.; Turk, T.; FrangeŽ, R.; Svenson, J. Natural cholinesterase inhibitors from marine organisms. Nat. Prod. Rep. 2019, 36, 1053–1092. [Google Scholar] [CrossRef]
- Ning, H.; Huang, B.; Tae, H.S.; Liu, Z.; Yu, S.; Li, L.; Zhang, L.; Adams, D.J.; Guo, C.; Dai, Q. α-Conotoxin Bt1.8 from Conus betulinus selectively inhibits α6/α3β2β3 and α3β2 nicotinic acetylcholine receptor subtypes. J. Neurochem. 2021, 159, 90–100. [Google Scholar] [CrossRef]
- Schain, M.; Kreisl, W.C. Neuroinflammation in Neurodegenerative Disorders-a Review. Curr. Neurol. Neurosci. Rep. 2017, 17, 25. [Google Scholar] [CrossRef]
- Salter, M.W.; Stevens, B. Microglia emerge as central players in brain disease. Nat. Med. 2017, 23, 1018–1027. [Google Scholar] [CrossRef]
- Wang, W.Y.; Tan, M.S.; Yu, J.T.; Tan, L. Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Ann. Transl. Med. 2015, 3, 136. [Google Scholar]
- Prinz, M.; Priller, J.; Sisodia, S.S.; Ransohoff, R.M. Heterogeneity of CNS myeloid cells and their roles in neurodegeneration. Nat. Neurosci. 2011, 14, 1227–1235. [Google Scholar] [CrossRef]
- Colonna, M.; Butovsky, O. Microglia Function in the Central Nervous System During Health and Neurodegeneration. Annu. Rev. Immunol. 2017, 35, 441–468. [Google Scholar] [CrossRef]
- Kamei, J.; Mizoguchi, H.; Narita, M.; Tseng, L.F. Therapeutic potential of PKC inhibitors in painful diabetic neuropathy. Expert Opin. Investig. Drugs 2001, 10, 1653–1664. [Google Scholar] [CrossRef] [PubMed]
- Coultrap, S.J.; Vest, R.S.; Ashpole, N.M.; Hudmon, A.; Bayer, K.U. CaMKII in cerebral ischemia. Acta Pharmacol. Sin. 2011, 32, 861–872. [Google Scholar] [CrossRef]
- Crown, E.D. The role of mitogen activated protein kinase signaling in microglia and neurons in the initiation and maintenance of chronic pain. Exp. Neurol. 2012, 234, 330–339. [Google Scholar] [CrossRef]
- Wang, G.; Pan, J.; Chen, S.D. Kinases and kinase signaling pathways: Potential therapeutic targets in Parkinson’s disease. Prog. Neurobiol. 2012, 98, 207–221. [Google Scholar] [CrossRef] [PubMed]
- Tell, V.; Hilgeroth, A. Recent developments of protein kinase inhibitors as potential AD therapeutics. Front. Cell. Neurosci. 2013, 7, 189. [Google Scholar] [CrossRef] [PubMed]
- Leng, F.; Edison, P. Neuroinflammation and microglial activation in Alzheimer disease: Where do we go from here? Nat. Rev. Neurol. 2021, 17, 157–172. [Google Scholar] [CrossRef]
- Liao, Y.; Qi, X.L.; Cao, Y.; Yu, W.F.; Ravid, R.; Winblad, B.; Pei, J.J.; Guan, Z.Z. Elevations in the Levels of NF-κB and Inflammatory Chemotactic Factors in the Brains with Alzheimer’s Disease—One Mechanism May Involve α3 Nicotinic Acetylcholine Receptor. Curr. Alzheimer Res. 2016, 13, 1290–1301. [Google Scholar] [CrossRef]
- Chen, C.H.; Zhou, W.; Liu, S.; Deng, Y.; Cai, F.; Tone, M.; Tone, Y.; Tong, Y.; Song, W. Increased NF-κB signalling up-regulates BACE1 expression and its therapeutic potential in Alzheimer’s disease. Int. J. Neuropsychopharmacol. 2012, 15, 77–90. [Google Scholar] [CrossRef]
- Yu, M.; Li, H.; Liu, Q.; Liu, F.; Tang, L.; Li, C.; Yuan, Y.; Zhan, Y.; Xu, W.; Li, W.; et al. Nuclear factor p65 interacts with Keap1 to repress the Nrf2-ARE pathway. Cell. Signal. 2011, 23, 883–892. [Google Scholar] [CrossRef]
- Sandberg, M.; Patil, J.; D’Angelo, B.; Weber, S.G.; Mallard, C. NRF2-regulation in brain health and disease: Implication of cerebral inflammation. Neuropharmacology 2014, 79, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Dolatshahi, M.; Ranjbar Hameghavandi, M.H.; Sabahi, M.; Rostamkhani, S. Nuclear factor-kappa B (NF-κB) in pathophysiology of Parkinson disease: Diverse patterns and mechanisms contributing to neurodegeneration. Eur. J. Neurosci. 2021, 54, 4101–4123. [Google Scholar] [CrossRef] [PubMed]
- Sukhorukov, V.S.; Mudzhiri, N.M.; Voronkova, A.S.; Baranich, T.I.; Glinkina, V.V.; Illarioshkin, S.N. Mitochondrial Disorders in Alzheimer’s Disease. Biochemistry 2021, 86, 667–679. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Behl, T.; Sharma, L.; Aelya, L.; Bungau, S. Mitochondrial Dysfunction in Huntington’s Disease: Pathogenesis and Therapeutic Opportunities. Curr. Drug Targets 2021, 22, 1637–1667. [Google Scholar] [CrossRef]
- Mandal, P.K.; Roy, R.G.; Samkaria, A. Oxidative Stress: Glutathione and Its Potential to Protect Methionine-35 of Aβ Peptide from Oxidation. ACS Omega 2022, 7, 27052–27061. [Google Scholar] [CrossRef]
- Liu, Z.Q. Enhancing Antioxidant Effect against Peroxyl Radical-Induced Oxidation of DNA: Linking with Ferrocene Moiety! Chem. Rec. 2019, 19, 2385–2397. [Google Scholar] [CrossRef]
- Kao, Y.-C.; Ho, P.-C.; Tu, Y.-K.; Jou, I.-M.; Tsai, K.-J. Lipids and Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 1505. [Google Scholar] [CrossRef]
- Song, K.; Li, Y.; Zhang, H.; An, N.; Wei, Y.; Wang, L.; Tian, C.; Yuan, M.; Sun, Y.; Xing, Y.; et al. Oxidative Stress-Mediated Blood-Brain Barrier (BBB) Disruption in Neurological Diseases. Oxidative Med. Cell. Longev. 2020, 2020, 4356386. [Google Scholar] [CrossRef]
- Anwar, M.M. Oxidative stress-A direct bridge to central nervous system homeostatic dysfunction and Alzheimer’s disease. Cell Biochem. Funct. 2022, 40, 17–27. [Google Scholar] [CrossRef]
- Dionísio, P.A.; Amaral, J.D.; Rodrigues, C.M.P. Oxidative stress and regulated cell death in Parkinson’s disease. Ageing Res. Rev. 2021, 67, 101263. [Google Scholar] [CrossRef]
- Hemerková, P.; Vališ, M. Role of Oxidative Stress in the Pathogenesis of Amyotrophic Lateral Sclerosis: Antioxidant Metalloenzymes and Therapeutic Strategies. Biomolecules 2021, 11, 437. [Google Scholar] [CrossRef] [PubMed]
- Spaas, J.; van Veggel, L.; Schepers, M.; Tiane, A.; van Horssen, J.; Wilson, D.M., 3rd; Moya, P.R.; Piccart, E.; Hellings, N.; Eijnde, B.O.; et al. Oxidative stress and impaired oligodendrocyte precursor cell differentiation in neurological disorders. Cell. Mol. Life Sci. 2021, 78, 4615–4637. [Google Scholar] [CrossRef] [PubMed]
- Amato, A.; Terzo, S.; Mulè, F. Natural Compounds as Beneficial Antioxidant Agents in Neurodegenerative Disorders: A Focus on Alzheimer’s Disease. Antioxidants 2019, 8, 608. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.Y.; Leem, E.; Lee, J.M.; Kim, S.R. Control of Reactive Oxygen Species for the Prevention of Parkinson’s Disease: The Possible Application of Flavonoids. Antioxidants 2020, 9, 583. [Google Scholar] [CrossRef] [PubMed]
- Sinyor, B.; Mineo, J.; Ochner, C. Alzheimer’s Disease, Inflammation, and the Role of Antioxidants. J. Alzheimer’s Dis. Rep. 2020, 4, 175–183. [Google Scholar] [CrossRef]
- Van der Schyf, C.J. Rational drug discovery design approaches for treating Parkinson’s disease. Expert Opin. Drug Discov. 2015, 10, 713–741. [Google Scholar] [CrossRef]
- Choi, D.Y.; Choi, H. Natural products from marine organisms with neuroprotective activity in the experimental models of Alzheimer’s disease, Parkinson’s disease and ischemic brain stroke: Their molecular targets and action mechanisms. Arch. Pharmacal Res. 2015, 38, 139–170. [Google Scholar] [CrossRef]
- Ramsay, R.R.; Albreht, A. Kinetics, mechanism, and inhibition of monoamine oxidase. J. Neural Transm. 2018, 125, 1659–1683. [Google Scholar] [CrossRef]
- Youdim, M.B.; Edmondson, D.; Tipton, K.F. The therapeutic potential of monoamine oxidase inhibitors. Nat. Rev. Neurosci. 2006, 7, 295–309. [Google Scholar] [CrossRef]
- Schedin-Weiss, S.; Inoue, M.; Hromadkova, L.; Teranishi, Y.; Yamamoto, N.G.; Wiehager, B.; Bogdanovic, N.; Winblad, B.; Sandebring-Matton, A.; Frykman, S.; et al. Monoamine oxidase B is elevated in Alzheimer disease neurons, is associated with γ-secretase and regulates neuronal amyloid β-peptide levels. Alzheimer’s Res. Ther. 2017, 9, 57. [Google Scholar] [CrossRef]
- Lee, H.W.; Choi, H.; Nam, S.J.; Fenical, W.; Kim, H. Potent Inhibition of Monoamine Oxidase B by a Piloquinone from Marine-Derived Streptomyces sp. CNQ-027. J. Microbiol. Biotechnol. 2017, 27, 785–790. [Google Scholar] [CrossRef]
- Oh, J.M.; Lee, C.; Nam, S.J.; Kim, H. Chromenone Derivatives as Monoamine Oxidase Inhibitors from Marine-Derived MAR4 Clade Streptomyces sp. CNQ-031. J. Microbiol. Biotechnol. 2021, 31, 1022–1027. [Google Scholar] [CrossRef] [PubMed]
- Franco, R.; Navarro, G. Adenosine A(2A) Receptor Antagonists in Neurodegenerative Diseases: Huge Potential and Huge Challenges. Front. Psychiatry 2018, 9, 68. [Google Scholar] [CrossRef]
- Shook, B.C.; Jackson, P.F. Adenosine A(2A) Receptor Antagonists and Parkinson’s Disease. ACS Chem. Neurosci. 2011, 2, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Lonikar, N.; Choudhari, P.; Bhusnuare, O. Insilico analysis of marine indole alkaloids for design of adenosine A2A receptor antagonist. J. Biomol. Struct. Dyn. 2021, 39, 3515–3522. [Google Scholar] [CrossRef] [PubMed]
- Nijboer, C.H.; van der Kooij, M.A.; van Bel, F.; Ohl, F.; Heijnen, C.J.; Kavelaars, A. Inhibition of the JNK/AP-1 pathway reduces neuronal death and improves behavioral outcome after neonatal hypoxic-ischemic brain injury. Brain Behav. Immun. 2010, 24, 812–821. [Google Scholar] [CrossRef] [PubMed]
- Schellino, R.; Boido, M.; Vercelli, A. JNK Signaling Pathway Involvement in Spinal Cord Neuron Development and Death. Cells 2019, 8, 1576. [Google Scholar] [CrossRef] [PubMed]
- Kumagae, Y.; Zhang, Y.; Kim, O.J.; Miller, C.A. Human c-Jun N-terminal kinase expression and activation in the nervous system. Brain Res. Mol. Brain Res. 1999, 67, 10–17. [Google Scholar] [CrossRef]
- Wu, H.; Wei, S.; Huang, Y.; Chen, L.; Wang, Y.; Wu, X.; Zhang, Z.; Pei, Y.; Wang, D. Aβ monomer induces phosphorylation of Tau at Ser-214 through β2AR-PKA-JNK signaling pathway. FASEB J. 2020, 34, 5092–5105. [Google Scholar] [CrossRef]
- Shoji, M.; Iwakami, N.; Takeuchi, S.; Waragai, M.; Suzuki, M.; Kanazawa, I.; Lippa, C.F.; Ono, S.; Okazawa, H. JNK activation is associated with intracellular beta-amyloid accumulation. Brain Res. Mol. Brain Res. 2000, 85, 221–233. [Google Scholar] [CrossRef]
- Ghasemi, R.; Zarifkar, A.; Rastegar, K.; Maghsoudi, N.; Moosavi, M. Repeated intra-hippocampal injection of beta-amyloid 25–35 induces a reproducible impairment of learning and memory: Considering caspase-3 and MAPKs activity. Eur. J. Pharmacol. 2014, 726, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Chen, Z.; Han, X.; Wu, H.; Yu, Y.; Wu, J.; Liu, S.; Hou, Y. Progesterone attenuates Aβ(25-35)-induced neuronal toxicity via JNK inactivation and progesterone receptor membrane component 1-dependent inhibition of mitochondrial apoptotic pathway. J. Steroid. Biochem. Mol. Biol. 2015, 154, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Pallàs, M.; Camins, A.; Smith, M.; Perry, G.; Lee, H.-g.; Casadesus, G. From Aging to Alzheimer’s Disease: Unveiling “The Switch” with the Senescence-Accelerated Mouse Model (SAMP8). J. Alzheimer’s Dis. JAD 2009, 15, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Orejana, L.; Barros-Miñones, L.; Aguirre, N.; Puerta, E. Implication of JNK pathway on tau pathology and cognitive decline in a senescence-accelerated mouse model. Exp. Gerontol. 2013, 48, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Vela, S.; Sainz, N.; Moreno-Aliaga, M.J.; Solas, M.; Ramirez, M.J. DHA Selectively Protects SAMP-8-Associated Cognitive Deficits Through Inhibition of JNK. Mol. Neurobiol. 2019, 56, 1618–1627. [Google Scholar] [CrossRef]
- Yarza, R.; Vela, S.; Solas, M.; Ramirez, M.J. c-Jun N-terminal Kinase (JNK) Signaling as a Therapeutic Target for Alzheimer’s Disease. Front. Pharmacol. 2015, 6, 321. [Google Scholar] [CrossRef] [PubMed]
- Barthet, V.J.A.; Ryan, K.M. Autophagy in Neurodegeneration: Can’t Digest It, Spit It Out! Trends Cell Biol. 2018, 28, 171–173. [Google Scholar] [CrossRef]
- Watanabe, Y.; Taguchi, K.; Tanaka, M. Ubiquitin, Autophagy and Neurodegenerative Diseases. Cells 2020, 9, 2022. [Google Scholar] [CrossRef]
- Okamoto, K.; Mizuno, Y.; Fujita, Y. Bunina bodies in amyotrophic lateral sclerosis. Neuropathology 2008, 28, 109–115. [Google Scholar] [CrossRef]
- Hau, A.M.; Greenwood, J.A.; Löhr, C.V.; Serrill, J.D.; Proteau, P.J.; Ganley, I.G.; McPhail, K.L.; Ishmael, J.E. Coibamide A induces mTOR-independent autophagy and cell death in human glioblastoma cells. PLoS ONE 2013, 8, e65250. [Google Scholar] [CrossRef]
- Kanno, S.; Yomogida, S.; Tomizawa, A.; Yamazaki, H.; Ukai, K.; Mangindaan, R.E.; Namikoshi, M.; Ishikawa, M. Papuamine causes autophagy following the reduction of cell survival through mitochondrial damage and JNK activation in MCF-7 human breast cancer cells. Int. J. Oncol. 2013, 43, 1413–1419. [Google Scholar] [CrossRef] [PubMed]
- White, J.A.; Banerjee, R.; Gunawardena, S. Axonal Transport and Neurodegeneration: How Marine Drugs Can Be Used for the Development of Therapeutics. Mar. Drugs 2016, 14, 102. [Google Scholar] [CrossRef] [PubMed]
- Djajadikerta, A.; Keshri, S.; Pavel, M.; Prestil, R.; Ryan, L.; Rubinsztein, D.C. Autophagy Induction as a Therapeutic Strategy for Neurodegenerative Diseases. J. Mol. Biol. 2020, 432, 2799–2821. [Google Scholar] [CrossRef]
- Rosenberg, G.A. Matrix metalloproteinases and their multiple roles in neurodegenerative diseases. Lancet Neurol. 2009, 8, 205–216. [Google Scholar] [CrossRef]
- Agrawal, S.M.; Lau, L.; Yong, V.W. MMPs in the central nervous system: Where the good guys go bad. Semin. Cell Dev. Biol. 2008, 19, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Gentile, E.; Liuzzi, G.M. Marine pharmacology: Therapeutic targeting of matrix metalloproteinases in neuroinflammation. Drug Discov. Today 2017, 22, 299–313. [Google Scholar] [CrossRef] [PubMed]
- Vandooren, J.; Van Damme, J.; Opdenakker, G. Chapter 9—On the Structure and functions of gelatinase B/Matrix metalloproteinase-9 in neuroinflammation. In Progress in Brain Research; Dityatev, A., Wehrle-Haller, B., Pitkänen, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 214, pp. 193–206. [Google Scholar]
- Behl, T.; Kaur, G.; Sehgal, A.; Bhardwaj, S.; Singh, S.; Buhas, C.; Judea-Pusta, C.; Uivarosan, D.; Munteanu, M.A.; Bungau, S. Multifaceted Role of Matrix Metalloproteinases in Neurodegenerative Diseases: Pathophysiological and Therapeutic Perspectives. Int. J. Mol. Sci. 2021, 22, 1413. [Google Scholar] [CrossRef]
- Yang, Y.; Estrada, E.Y.; Thompson, J.F.; Liu, W.; Rosenberg, G.A. Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J. Cereb. Blood Flow Metab. 2007, 27, 697–709. [Google Scholar] [CrossRef]
- Chelluboina, B.; Klopfenstein, J.D.; Pinson, D.M.; Wang, D.Z.; Vemuganti, R.; Veeravalli, K.K. Matrix Metalloproteinase-12 Induces Blood-Brain Barrier Damage After Focal Cerebral Ischemia. Stroke 2015, 46, 3523–3531. [Google Scholar] [CrossRef]
- Deb, S.; Gottschall, P.E. Increased production of matrix metalloproteinases in enriched astrocyte and mixed hippocampal cultures treated with beta-amyloid peptides. J. Neurochem. 1996, 66, 1641–1647. [Google Scholar] [CrossRef]
- Backstrom, J.R.; Lim, G.P.; Cullen, M.J.; Tökés, Z.A. Matrix metalloproteinase-9 (MMP-9) is synthesized in neurons of the human hippocampus and is capable of degrading the amyloid-beta peptide (1-40). J. Neurosci. 1996, 16, 7910–7919. [Google Scholar] [CrossRef] [PubMed]
- Lorenzl, S.; Albers, D.S.; Relkin, N.; Ngyuen, T.; Hilgenberg, S.L.; Chirichigno, J.; Cudkowicz, M.E.; Beal, M.F. Increased plasma levels of matrix metalloproteinase-9 in patients with Alzheimer’s disease. Neurochem. Int. 2003, 43, 191–196. [Google Scholar] [CrossRef]
- Yoshiyama, Y.; Asahina, M.; Hattori, T. Selective distribution of matrix metalloproteinase-3 (MMP-3) in Alzheimer’s disease brain. Acta Neuropathol. 2000, 99, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, D.J. Biochemistry and molecular biology of amyloid beta-protein and the mechanism of Alzheimer’s disease. Handb. Clin. Neurol. 2008, 89, 245–260. [Google Scholar] [PubMed]
- Ringland, C.; Schweig, J.E.; Eisenbaum, M.; Paris, D.; Ait-Ghezala, G.; Mullan, M.; Crawford, F.; Abdullah, L.; Bachmeier, C. MMP9 modulation improves specific neurobehavioral deficits in a mouse model of Alzheimer’s disease. BMC Neurosci. 2021, 22, 39. [Google Scholar] [CrossRef]
- Choi, D.H.; Kim, Y.J.; Kim, Y.G.; Joh, T.H.; Beal, M.F.; Kim, Y.S. Role of matrix metalloproteinase 3-mediated alpha-synuclein cleavage in dopaminergic cell death. J. Biol. Chem. 2011, 286, 14168–14177. [Google Scholar] [CrossRef]
- Annese, V.; Herrero, M.; Pentima, M.; Gómez López, A.; Lombardi, L.; Ros, C.; de Pablos, V.; Fernandez, E.; De Stefano, M.E. Metalloproteinase-9 contributes to inflammatory glia activation and nigro-striatal pathway degeneration in both mouse and monkey models of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced Parkinsonism. Brain Struct. Funct. 2014, 220, 703–727. [Google Scholar] [CrossRef]
- Liu, C.Z.; Guo, D.S.; Ma, J.J.; Dong, L.R.; Chang, Q.Q.; Yang, H.Q.; Liang, K.K.; Li, X.H.; Yang, D.W.; Fan, Y.Y.; et al. Correlation of matrix metalloproteinase 3 and matrix metalloproteinase 9 levels with non-motor symptoms in patients with Parkinson’s disease. Front. Aging Neurosci. 2022, 14, 889257. [Google Scholar] [CrossRef]
- Nasrolahi, A.; Mahmoudi, J.; Akbarzadeh, A.; Karimipour, M.; Sadigh-Eteghad, S.; Salehi, R.; Farhoudi, M. Neurotrophic factors hold promise for the future of Parkinson’s disease treatment: Is there a light at the end of the tunnel? Rev. Neurosci. 2018, 29, 475–489. [Google Scholar] [CrossRef]
- Nasrolahi, A.; Javaherforooshzadeh, F.; Jafarzadeh-Gharehziaaddin, M.; Mahmoudi, J.; Asl, K.D.; Shabani, Z. Therapeutic potential of neurotrophic factors in Alzheimer’s Disease. Mol. Biol. Rep. 2022, 49, 2345–2357. [Google Scholar] [CrossRef]
- O’Bryant, S.E.; Hobson, V.; Hall, J.R.; Waring, S.C.; Chan, W.; Massman, P.; Lacritz, L.; Cullum, C.M.; Diaz-Arrastia, R. Brain-derived neurotrophic factor levels in Alzheimer’s disease. J. Alzheimer’s Dis. 2009, 17, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Lübke, J.H.; Idoon, F.; Mohasel-Roodi, M.; Alipour, F.; Hami, J.; Ehteshampour, A.; Mostafaee, H.; Sadeghi, A. Neurotrophic factors in Alzheimer’s disease: Pathogenesis and therapy. Acta Neurobiol. Exp. 2021, 81, 314–327. [Google Scholar]
- Forlenza, O.V.; Miranda, A.S.; Guimar, I.; Talib, L.L.; Diniz, B.S.; Gattaz, W.F.; Teixeira, A.L. Decreased Neurotrophic Support is Associated with Cognitive Decline in Non-Demented Subjects. J. Alzheimer’s Dis. 2015, 46, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Deister, C.; Schmidt, C.E. Optimizing neurotrophic factor combinations for neurite outgrowth. J. Neural. Eng. 2006, 3, 172–179. [Google Scholar] [CrossRef]
- Budni, J.; Bellettini-Santos, T.; Mina, F.; Garcez, M.L.; Zugno, A.I. The involvement of BDNF, NGF and GDNF in aging and Alzheimer’s disease. Aging Dis. 2015, 6, 331–341. [Google Scholar] [PubMed]
- Cheng, H.; Fu, Y.S.; Guo, J.W. Ability of GDNF to diminish free radical production leads to protection against kainate-induced excitotoxicity in hippocampus. Hippocampus 2004, 14, 77–86. [Google Scholar] [CrossRef]
- Krashia, P.; Nobili, A.; D’Amelio, M. Unifying Hypothesis of Dopamine Neuron Loss in Neurodegenerative Diseases: Focusing on Alzheimer’s Disease. Front. Mol. Neurosci. 2019, 12, 123. [Google Scholar] [CrossRef]
- Austin, S.; St-Pierre, J. PGC1α and mitochondrial metabolism--emerging concepts and relevance in ageing and neurodegenerative disorders. J. Cell Sci. 2012, 125, 4963–4971. [Google Scholar] [CrossRef]
- McGill, J.K.; Beal, M.F. PGC-1alpha, a new therapeutic target in Huntington’s disease? Cell 2006, 127, 465–468. [Google Scholar] [CrossRef]
- St-Pierre, J.; Drori, S.; Uldry, M.; Silvaggi, J.M.; Rhee, J.; Jäger, S.; Handschin, C.; Zheng, K.; Lin, J.; Yang, W.; et al. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell 2006, 127, 397–408. [Google Scholar] [CrossRef]
- Zheng, B.; Liao, Z.; Locascio, J.J.; Lesniak, K.A.; Roderick, S.S.; Watt, M.L.; Eklund, A.C.; Zhang-James, Y.; Kim, P.D.; Hauser, M.A.; et al. PGC-1α, a potential therapeutic target for early intervention in Parkinson’s disease. Sci. Transl. Med. 2010, 2, 52ra73. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Lei, Y.H.; Zhou, J.P.; Hou, Y.Y.; Wan, Z.; Wang, H.L.; Meng, H. Role of PGC-1α in Mitochondrial Quality Control in Neurodegenerative Diseases. Neurochem. Res. 2019, 44, 2031–2043. [Google Scholar] [CrossRef] [PubMed]
- Zuccoli, G.S.; Carregari, V.C. Mitochondrial Dysregulation and the Influence in Neurodegenerative Diseases. Adv. Exp. Med. Biol. 2022, 1382, 109–118. [Google Scholar]
- Jamwal, S.; Blackburn, J.K.; Elsworth, J.D. PPARγ/PGC1α signaling as a potential therapeutic target for mitochondrial biogenesis in neurodegenerative disorders. Pharmacol. Ther. 2021, 219, 107705. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, S.; Yarmohammadi, A.; Yarmohammadi, M.; Farzaei, M.H.; Echeverria, J. Marine Natural Products: Promising Candidates in the Modulation of Gut-Brain Axis towards Neuroprotection. Mar. Drugs 2021, 19, 165. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.V.; Foster, K.R. Why does the microbiome affect behaviour? Nat. Rev. Microbiol. 2018, 16, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Heiss, C.N.; Olofsson, L.E. The role of the gut microbiota in development, function and disorders of the central nervous system and the enteric nervous system. J. Neuroendocrinol. 2019, 31, e12684. [Google Scholar] [CrossRef]
- Vogt, N.M.; Kerby, R.L.; Dill-McFarland, K.A.; Harding, S.J.; Merluzzi, A.P.; Johnson, S.C.; Carlsson, C.M.; Asthana, S.; Zetterberg, H.; Blennow, K.; et al. Gut microbiome alterations in Alzheimer’s disease. Sci. Rep. 2017, 7, 13537. [Google Scholar] [CrossRef]
- Liu, P.; Wu, L.; Peng, G.; Han, Y.; Tang, R.; Ge, J.; Zhang, L.; Jia, L.; Yue, S.; Zhou, K.; et al. Altered microbiomes distinguish Alzheimer’s disease from amnestic mild cognitive impairment and health in a Chinese cohort. Brain Behav. Immun. 2019, 80, 633–643. [Google Scholar] [CrossRef]
- Cattaneo, A.; Cattane, N.; Galluzzi, S.; Provasi, S.; Lopizzo, N.; Festari, C.; Ferrari, C.; Guerra, U.P.; Paghera, B.; Muscio, C.; et al. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol. Aging 2017, 49, 60–68. [Google Scholar] [CrossRef]
- Nguyen, T.T.T.; Fujimura, Y.; Mimura, I.; Fujii, Y.; Nguyen, N.L.; Arakawa, K.; Morita, H. Cultivable butyrate-producing bacteria of elderly Japanese diagnosed with Alzheimer’s disease. J. Microbiol. 2018, 56, 760–771. [Google Scholar] [CrossRef] [PubMed]
- Heijtz, R.D.; Wang, S.; Anuar, F.; Qian, Y.; Björkholm, B.; Samuelsson, A.; Hibberd, M.L.; Forssberg, H.; Pettersson, S. Normal gut microbiota modulates brain development and behavior. Proc. Natl. Acad. Sci. USA 2011, 108, 3047–3052. [Google Scholar] [CrossRef] [PubMed]
- Nishino, R.; Mikami, K.; Takahashi, H.; Tomonaga, S.; Furuse, M.; Hiramoto, T.; Aiba, Y.; Koga, Y.; Sudo, N. Commensal microbiota modulate murine behaviors in a strictly contamination-free environment confirmed by culture-based methods. Neurogastroenterol. Motil. 2013, 25, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Bu, X.L.; Yao, X.Q.; Jiao, S.S.; Zeng, F.; Liu, Y.H.; Xiang, Y.; Liang, C.R.; Wang, Q.H.; Wang, X.; Cao, H.Y.; et al. A study on the association between infectious burden and Alzheimer’s disease. Eur. J. Neurol. 2015, 22, 1519–1525. [Google Scholar] [CrossRef] [PubMed]
- Doulberis, M.; Kotronis, G.; Gialamprinou, D.; Polyzos, S.A.; Papaefthymiou, A.; Katsinelos, P.; Kountouras, J. Alzheimer’s disease and gastrointestinal microbiota; impact of Helicobacter pylori infection involvement. Int. J. Neurosci. 2021, 131, 289–301. [Google Scholar] [CrossRef]
- Wang, X.L.; Zeng, J.; Yang, Y.; Xiong, Y.; Zhang, Z.H.; Qiu, M.; Yan, X.; Sun, X.Y.; Tuo, Q.Z.; Liu, R.; et al. Helicobacter pylori filtrate induces Alzheimer-like tau hyperphosphorylation by activating glycogen synthase kinase-3β. J. Alzheimer’s Dis. 2015, 43, 153–165. [Google Scholar] [CrossRef]
- Zhao, Y.; Jaber, V.; Lukiw, W.J. Secretory Products of the Human GI Tract Microbiome and Their Potential Impact on Alzheimer’s Disease (AD): Detection of Lipopolysaccharide (LPS) in AD Hippocampus. Front. Cell. Infect. Microbiol. 2017, 7, 318. [Google Scholar] [CrossRef]
- Minter, M.R.; Zhang, C.; Leone, V.; Ringus, D.L.; Zhang, X.; Oyler-Castrillo, P.; Musch, M.W.; Liao, F.; Ward, J.F.; Holtzman, D.M.; et al. Antibiotic-induced perturbations in gut microbial diversity influences neuro-inflammation and amyloidosis in a murine model of Alzheimer’s disease. Sci. Rep. 2016, 6, 30028. [Google Scholar] [CrossRef]
- Kim, M.S.; Kim, Y.; Choi, H.; Kim, W.; Park, S.; Lee, D.; Kim, D.K.; Kim, H.J.; Choi, H.; Hyun, D.W.; et al. Transfer of a healthy microbiota reduces amyloid and tau pathology in an Alzheimer’s disease animal model. Gut 2020, 69, 283–294. [Google Scholar] [CrossRef]
- Bonfili, L.; Cecarini, V.; Berardi, S.; Scarpona, S.; Suchodolski, J.S.; Nasuti, C.; Fiorini, D.; Boarelli, M.C.; Rossi, G.; Eleuteri, A.M. Microbiota modulation counteracts Alzheimer’s disease progression influencing neuronal proteolysis and gut hormones plasma levels. Sci. Rep. 2017, 7, 2426. [Google Scholar] [CrossRef] [PubMed]
- Abraham, D.; Feher, J.; Scuderi, G.L.; Szabo, D.; Dobolyi, A.; Cservenak, M.; Juhasz, J.; Ligeti, B.; Pongor, S.; Gomez-Cabrera, M.C.; et al. Exercise and probiotics attenuate the development of Alzheimer’s disease in transgenic mice: Role of microbiome. Exp. Gerontol. 2019, 115, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Yang, X.; Yang, J.; Lai, G.; Yong, T.; Tang, X.; Shuai, O.; Zhou, G.; Xie, Y.; Wu, Q. Prebiotic Effect of Fructooligosaccharides from Morinda officinalis on Alzheimer’s Disease in Rodent Models by Targeting the Microbiota-Gut-Brain Axis. Front. Aging Neurosci. 2017, 9, 403. [Google Scholar] [CrossRef]
- Den, H.; Dong, X.; Chen, M.; Zou, Z. Efficacy of probiotics on cognition, and biomarkers of inflammation and oxidative stress in adults with Alzheimer’s disease or mild cognitive impairment—A meta-analysis of randomized controlled trials. Aging 2020, 12, 4010–4039. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.H.; Song, X.X.; Liu, X.L.; Chen, S.D.; Tang, H.D. Inflammatory pathways in Alzheimer’s disease mediated by gut microbiota. Ageing Res. Rev. 2021, 68, 101317. [Google Scholar] [CrossRef]
- Lin, J.; Yu, J.; Zhao, J.; Zhang, K.; Zheng, J.; Wang, J.; Huang, C.; Zhang, J.; Yan, X.; Gerwick, W.H.; et al. Fucoxanthin, a Marine Carotenoid, Attenuates β-Amyloid Oligomer-Induced Neurotoxicity Possibly via Regulating the PI3K/Akt and the ERK Pathways in SH-SY5Y Cells. Oxid. Med. Cell. Longev. 2017, 2017, 6792543. [Google Scholar] [CrossRef]
- Latyshev, N.A.; Kasyanov, S.P.; Kharlamenko, V.I.; Svetashev, V.I. Lipids and of fatty acids of edible crabs of the north-western Pacific. Food Chem. 2009, 116, 657–661. [Google Scholar] [CrossRef]
- Braverman, N.E.; Moser, A.B. Functions of plasmalogen lipids in health and disease. Biochim. Biophys. Acta 2012, 1822, 1442–1452. [Google Scholar] [CrossRef]
- Wood, P.L.; Smith, T.; Lane, N.; Khan, M.A.; Ehrmantraut, G.; Goodenowe, D.B. Oral bioavailability of the ether lipid plasmalogen precursor, PPI-1011, in the rabbit: A new therapeutic strategy for Alzheimer’s disease. Lipids Health Dis. 2011, 10, 227. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Liu, C. Ether Glycerophospholipids and Their Potential as Therapeutic Agents. Curr. Org. Chem. 2013, 17, 802–811. [Google Scholar] [CrossRef]
- Deniau, A.L.; Mosset, P.; Pédrono, F.; Mitre, R.; Le Bot, D.; Legrand, A.B. Multiple beneficial health effects of natural alkylglycerols from shark liver oil. Mar. Drugs 2010, 8, 2175–2184. [Google Scholar] [CrossRef]
- Bakes, M.J.; Nichols, P.D. Lipid, fatty acid and squalene composition of liver oil from six species of deep-sea sharks collected in southern australian waters. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 1995, 110, 267–275. [Google Scholar] [CrossRef]
- Hayashi, K. Content and Composition of Glyceryl Ethers in the Pyloric Ceca and Ovaries of the Starfish Distolasterias nippon, Asterina pectinifera, and Lysastrosoma anthosticta. Fish. Sci. 1998, 64, 852–853. [Google Scholar] [CrossRef][Green Version]
- Imbs, A.; Demidkova, D.; Dautova, T. Lipids and fatty acids of cold-water soft corals and hydrocorals: A comparison with tropical species and implications for coral nutrition. Mar. Biol. 2016, 163, 202. [Google Scholar] [CrossRef]
- Phleger, C.F.; Nichols, P.D.; Virtue, P. Lipids and buoyancy in Southern ocean pteropods. Lipids 1997, 32, 1093–1100. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, C.D.; Haraldsson, G.G. Ether lipids. Chem. Phys. Lipids 2011, 164, 315–340. [Google Scholar] [CrossRef]
- Latyshev, N.A.; Ermakova, S.P.; Ermolenko, E.V.; Imbs, A.B.; Kasyanov, S.P.; Sultanov, R.M. 1-O-alkylglycerols from the hepatopancreas of the crab Paralithodes camtschaticus, liver of the squid Berryteuthis magister, and liver of the skate Bathyraja parmifera, and their anticancer activity on human melanoma cells. J. Food Biochem. 2019, 43, e12828. [Google Scholar] [CrossRef]
- Lee, D.-S.; Cho, H.-A.; Yoon, N.-Y.; Kim, Y.-K.; Lim, C.-W.; Shim, K.-B. Biochemical Composition of Muscle from Tanaka’s Eelpout Lycodes tanakae, Magistrate Armhook Squid Berryteuthis magister, and Ocean Sunfish Mola mola, Caught in the East Sea, Korea. Fish. Aquat. Sci. 2012, 15, 99–105. [Google Scholar] [CrossRef][Green Version]
- Hayashi, K.; Okawa, Y.; Kawasaki, K.-i. Liver Lipids of Gonatid Squid Berryteuthis magister: A Rich Source of Alkyl Glyceryl Ethers. Nippon. Suisan Gakkaishi 1985, 51, 1523–1526. [Google Scholar] [CrossRef]
- Ermolenko, E.; Latyshev, N.; Sultanov, R.; Kasyanov, S. Technological approach of 1-O-alkyl-sn-glycerols separation from Berryteuthis magister squid liver oil. J. Food Sci. Technol. 2016, 53, 1722–1726. [Google Scholar] [CrossRef]
- Sultanov, R.; Ermolenko, E.; Poleschuk, T.; Denisenko, Y.; Kasyanov, S. Action of alkyl glycerol ethers and n-3 polyunsaturated fatty acids diet on hematological parameters of blood and liver plasmalogen level in aged rats. J. Food Sci. 2021, 86, 2727–2735. [Google Scholar] [CrossRef]
- Tyrtyshnaia, A.A.; Manzhulo, I.V.; Sultanov, R.M.; Ermolenko, E.V. Adult hippocampal neurogenesis in neuropathic pain and alkyl glycerol ethers treatment. Acta Histochem. 2017, 119, 812–821. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Karageorgou, D.; Katapodis, P.; Sharma, A.; Rova, U.; Christakopoulos, P.; Matsakas, L. Bioprospecting of thraustochytrids for omega-3 fatty acids: A sustainable approach to reduce dependency on animal sources. Trends Food Sci. Technol. 2021, 115, 433–444. [Google Scholar] [CrossRef]
- Huang, T.L. Omega-3 fatty acids, cognitive decline, and Alzheimer’s disease: A critical review and evaluation of the literature. J. Alzheimer’s Dis. 2010, 21, 673–690. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Chugh, V.; Gupta, A.K. Essential fatty acids as functional components of foods—A review. J. Food Sci. Technol. 2014, 51, 2289–2303. [Google Scholar] [CrossRef]
- Tyrtyshnaia, A.A.; Manzhulo, I.V. The Effect of Omega-3 Polyunsaturated Fatty Acids on Neuroinflammation in the Hippocampus. Neurochem. J. 2018, 12, 168–179. [Google Scholar] [CrossRef]
- Manzhulo, I.V.; Ogurtsova, O.S.; Lamash, N.E.; Latyshev, N.A.; Kasyanov, S.P.; Dyuizen, I.V. Analgetic effect of docosahexaenoic acid is mediated by modulating the microglia activity in the dorsal root ganglia in a rat model of neuropathic pain. Acta Histochem. 2015, 117, 659–666. [Google Scholar] [CrossRef]
- Manzhulo, I.; Tyrtyshnaia, A.; Kipryushina, Y.; Dyuizen, I.; Ermolenko, E.; Manzhulo, O. Docosahexaenoic acid improves motor function in the model of spinal cord injury. Neurosci. Lett. 2018, 672, 6–14. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, H.; Pu, H.; Wang, G.; Li, W.; Leak, R.K.; Chen, J.; Liou, A.K.; Hu, X. n-3 PUFA supplementation benefits microglial responses to myelin pathology. Sci. Rep. 2014, 4, 7458. [Google Scholar] [CrossRef]
- Tyrtyshnaia, A.; Manzhulo, I.; Kipryushina, Y.; Ermolenko, E. Neuroinflammation and adult hippocampal neurogenesis in neuropathic pain and alkyl glycerol ethers treatment in aged mice. Int. J. Mol. Med. 2019, 43, 2153–2163. [Google Scholar] [CrossRef]
- Manzhulo, I.V.; Tyrtyshnaia, A.A.; Manzhulo, O.S.; Starinets, A.A.; Kasyanov, S.P.; Dyuizen, I.V. Neuroprotective Activity of Docosahexaenoic Acid in the Central and Peripheral Nervous System after Chronic Constriction Injury of the Sciatic Nerve. Neurochem. J. 2020, 14, 101–107. [Google Scholar] [CrossRef]
- Calderon, F.; Kim, H.Y. Docosahexaenoic acid promotes neurite growth in hippocampal neurons. J. Neurochem. 2004, 90, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Kevala, K.; Kim, J.; Moon, H.S.; Jun, S.B.; Lovinger, D.; Kim, H.Y. Docosahexaenoic acid promotes hippocampal neuronal development and synaptic function. J. Neurochem. 2009, 111, 510–521. [Google Scholar] [CrossRef]
- Kim, H.Y.; Moon, H.S.; Cao, D.; Lee, J.; Kevala, K.; Jun, S.B.; Lovinger, D.M.; Akbar, M.; Huang, B.X. N-Docosahexaenoylethanolamide promotes development of hippocampal neurons. Biochem. J. 2011, 435, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Spector, A.A. Synaptamide, endocannabinoid-like derivative of docosahexaenoic acid with cannabinoid-independent function. Prostaglandins Leukot Essent Fat. Acids 2013, 88, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Sonti, S.; Duclos, R.I., Jr.; Tolia, M.; Gatley, S.J. N-Docosahexaenoylethanolamine (synaptamide): Carbon-14 radiolabeling and metabolic studies. Chem. Phys. Lipids 2018, 210, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.A.; Katakura, M.; Kharebava, G.; Kevala, K.; Kim, H.Y. N-Docosahexaenoylethanolamine is a potent neurogenic factor for neural stem cell differentiation. J. Neurochem. 2013, 125, 869–884. [Google Scholar] [CrossRef] [PubMed]
- Kharebava, G.; Rashid, M.A.; Lee, J.W.; Sarkar, S.; Kevala, K.; Kim, H.Y. N-docosahexaenoylethanolamine regulates Hedgehog signaling and promotes growth of cortical axons. Biol. Open 2015, 4, 1660–1670. [Google Scholar] [CrossRef] [PubMed]
- Tyrtyshnaia, A.A.; Egorova, E.L.; Starinets, A.A.; Ponomarenko, A.I.; Ermolenko, E.V.; Manzhulo, I.V. N-Docosahexaenoylethanolamine Attenuates Neuroinflammation and Improves Hippocampal Neurogenesis in Rats with Sciatic Nerve Chronic Constriction Injury. Mar. Drugs 2020, 18, 516. [Google Scholar] [CrossRef]
- Tyrtyshnaia, A.; Bondar, A.; Konovalova, S.; Sultanov, R.; Manzhulo, I. N-Docosahexanoylethanolamine Reduces Microglial Activation and Improves Hippocampal Plasticity in a Murine Model of Neuroinflammation. Int. J. Mol. Sci. 2020, 21, 9703. [Google Scholar] [CrossRef]
- Alhouayek, M.; Bottemanne, P.; Makriyannis, A.; Muccioli, G.G. N-acylethanolamine-hydrolyzing acid amidase and fatty acid amide hydrolase inhibition differentially affect N-acylethanolamine levels and macrophage activation. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2017, 1862, 474–484. [Google Scholar] [CrossRef]
- Ponomarenko, A.I.; Tyrtyshnaia, A.A.; Pislyagin, E.A.; Dyuizen, I.V.; Sultanov, R.M.; Manzhulo, I.V. N-docosahexaenoylethanolamine reduces neuroinflammation and cognitive impairment after mild traumatic brain injury in rats. Sci. Rep. 2021, 11, 756. [Google Scholar] [CrossRef] [PubMed]
- Latyshev, N.A.; Ermolenko, E.V.; Kasyanov, S.P. Concentration and purification of polyunsaturated fatty acids from squid liver processing wastes. Eur. J. Lipid Sci. Technol. 2014, 116, 1608–1613. [Google Scholar] [CrossRef]
- Tyrtyshnaia, A.; Konovalova, S.; Bondar, A.; Ermolenko, E.; Sultanov, R.; Manzhulo, I. Anti-Inflammatory Activity of N-Docosahexaenoylethanolamine and N-Eicosapentaenoylethanolamine in a Mouse Model of Lipopolysaccharide-Induced Neuroinflammation. Int. J. Mol. Sci. 2021, 22, 10728. [Google Scholar] [CrossRef]
- Ye, B.; Wang, X.; Tao, N.; Zhu, Q.; Hua, C. Extraction of oil and lecithin from Pacific saury (cololabis saira) viscera by supercritical CO2. J. Chin. Inst. Food Sci. Technol. 2015, 15, 100–109. [Google Scholar]
- Zhang, J.; Tao, N.; Wang, M.; Shi, W.; Ye, B.; Wang, X.; Zhu, Q.; Hua, C. Characterization of phospholipids from Pacific saury (Cololabis saira) viscera and their neuroprotective activity. Food Biosci. 2018, 24, 120–126. [Google Scholar] [CrossRef]
- Xiao, M.; Xiang, W.; Chen, Y.; Peng, N.; Du, X.; Lu, S.; Zuo, Y.; Li, B.; Hu, Y.; Li, X. DHA Ameliorates Cognitive Ability, Reduces Amyloid Deposition, and Nerve Fiber Production in Alzheimer’s Disease. Front. Nutr. 2022, 9, 852433. [Google Scholar] [CrossRef]
- Hashimoto, M.; Hossain, S.; Shimada, T.; Sugioka, K.; Yamasaki, H.; Fujii, Y.; Ishibashi, Y.; Oka, J.; Shido, O. Docosahexaenoic acid provides protection from impairment of learning ability in Alzheimer’s disease model rats. J. Neurochem. 2002, 81, 1084–1091. [Google Scholar] [CrossRef] [PubMed]
- Andrieu, S.; Guyonnet, S.; Coley, N.; Cantet, C.; Bonnefoy, M.; Bordes, S.; Bories, L.; Cufi, M.N.; Dantoine, T.; Dartigues, J.F.; et al. Effect of long-term omega 3 polyunsaturated fatty acid supplementation with or without multidomain intervention on cognitive function in elderly adults with memory complaints (MAPT): A randomised, placebo-controlled trial. Lancet Neurol. 2017, 16, 377–389. [Google Scholar] [CrossRef]
- Delrieu, J.; Payoux, P.; Carrié, I.; Cantet, C.; Weiner, M.; Vellas, B.; Andrieu, S. Multidomain intervention and/or omega-3 in nondemented elderly subjects according to amyloid status. Alzheimer’s Dement. 2019, 15, 1392–1401. [Google Scholar] [CrossRef]
- Hooper, C.; Coley, N.; De Souto Barreto, P.; Payoux, P.; Salabert, A.S.; Andrieu, S.; Weiner, M.; Vellas, B. Cortical β-Amyloid in Older Adults Is Associated with Multidomain Interventions with and without Omega 3 Polyunsaturated Fatty Acid Supplementation. J. Prev. Alzheimer’s Dis. 2020, 7, 128–134. [Google Scholar] [CrossRef]
- Lin, P.Y.; Cheng, C.; Satyanarayanan, S.K.; Chiu, L.T.; Chien, Y.C.; Chuu, C.P.; Lan, T.H.; Su, K.P. Omega-3 fatty acids and blood-based biomarkers in Alzheimer’s disease and mild cognitive impairment: A randomized placebo-controlled trial. Brain Behav. Immun. 2022, 99, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Sonnino, S.; Prinetti, A. The role of sphingolipids in neuronal plasticity of the brain. J. Neurochem. 2016, 137, 485–488. [Google Scholar] [CrossRef] [PubMed]
- Gulbins, E.; Walter, S.; Becker, K.A.; Halmer, R.; Liu, Y.; Reichel, M.; Edwards, M.J.; Müller, C.P.; Fassbender, K.; Kornhuber, J. A central role for the acid sphingomyelinase/ceramide system in neurogenesis and major depression. J. Neurochem. 2015, 134, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Olsen, A.S.B.; Færgeman, N.J. Sphingolipids: Membrane microdomains in brain development, function and neurological diseases. Open Biol. 2017, 7, 170069. [Google Scholar] [CrossRef] [PubMed]
- Itokazu, Y.; Wang, J.; Yu, R.K. Gangliosides in Nerve Cell Specification. Prog. Mol. Biol. Transl. Sci. 2018, 156, 241–263. [Google Scholar]
- Crivelli, S.M.; Giovagnoni, C.; Visseren, L.; Scheithauer, A.L.; de Wit, N.; den Hoedt, S.; Losen, M.; Mulder, M.T.; Walter, J.; de Vries, H.E.; et al. Sphingolipids in Alzheimer’s disease, how can we target them? Adv. Drug Deliv. Rev. 2020, 159, 214–231. [Google Scholar] [CrossRef]
- Jung, J.S.; Shin, K.O.; Lee, Y.M.; Shin, J.A.; Park, E.M.; Jeong, J.; Kim, D.H.; Choi, J.W.; Kim, H.S. Anti-inflammatory mechanism of exogenous C2 ceramide in lipopolysaccharide-stimulated microglia. Biochim. Biophys. Acta 2013, 1831, 1016–1026. [Google Scholar] [CrossRef]
- Karunakaran, I.; Alam, S.; Jayagopi, S.; Frohberger, S.J.; Hansen, J.N.; Kuehlwein, J.; Hölbling, B.V.; Schumak, B.; Hübner, M.P.; Gräler, M.H.; et al. Neural sphingosine 1-phosphate accumulation activates microglia and links impaired autophagy and inflammation. Glia 2019, 67, 1859–1872. [Google Scholar] [CrossRef]
- Gaire, B.P.; Lee, C.H.; Sapkota, A.; Lee, S.Y.; Chun, J.; Cho, H.J.; Nam, T.G.; Choi, J.W. Identification of Sphingosine 1-Phosphate Receptor Subtype 1 (S1P(1)) as a Pathogenic Factor in Transient Focal Cerebral Ischemia. Mol. Neurobiol. 2018, 55, 2320–2332. [Google Scholar] [CrossRef]
- Malyarenko, T.V.; Kicha, A.A.; Stonik, V.A.; Ivanchina, N.V. Sphingolipids of Asteroidea and Holothuroidea: Structures and Biological Activities. Mar. Drugs 2021, 19, 330. [Google Scholar] [CrossRef]
- Kawatake, S.; Inagaki, M.; Isobe, R.; Miyamoto, T.; Higuchi, R. Isolation and Structure of a GD3-Type Ganglioside Molecular Species Possessing Neuritogenic Activity from the Starfish Luidia maculata. Chem. Pharm. Bull. 2004, 52, 1002–1004. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Higuchi, R.; Inoue, S.; Inagaki, K.; Sakai, M.; Miyamoto, T.; Komori, T.; Inagaki, M.; Isobe, R. Biologically Active Glycosides from Asteroidea, 42. Isolation and Structure of a New Biologically Active Ganglioside Molecular Species from the Starfish Asterina pectinifera. Chem. Pharm. Bull. 2006, 54, 287–291. [Google Scholar] [CrossRef]
- Inagaki, M.; Miyamoto, T.; Isobe, R.; Higuchi, R. Biologically Active Glycosides from Asteroidea, 43. Isolation and Structure of a New Neuritogenic-Active Ganglioside Molecular Species from the Starfish Linckia laevigata. Chem. Pharm. Bull. 2005, 53, 1551–1554. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, M.; Kisa, F.; Yamada, K.; Miyamoto, T.; Higuchi, R. Structure of a New Neuritogenic-Active Ganglioside from the Sea Cucumber Stichopus japonicus. Eur. J. Org. Chem. 2003, 2003, 1004–1008. [Google Scholar] [CrossRef]
- Yamada, K.; Hamada, A.; Kisa, F.; Miyamoto, T.; Higuchi, R. Constituents of Holothuroidea, 13. Structure of Neuritogenic Active Ganglioside Molecular Species from the Sea Cucumber Stichopus chloronotus. Chem. Pharm. Bull. 2003, 51, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Kisa, F.; Yamada, K.; Miyamoto, T.; Inagaki, M.; Higuchi, R. Constituents of Holothuroidea, 17. Isolation and Structure of Biologically Active Monosialo-Gangliosides from the Sea Cucumber Cucumaria echinata. Chem. Pharm. Bull. 2006, 54, 982–987. [Google Scholar] [CrossRef]
- Kisa, F.; Yamada, K.; Miyamoto, T.; Inagaki, M.; Higuchi, R. Constituents of Holothuroidea, 18. Isolation and Structure of Biologically Active Disialo- and Trisialo-Gangliosides from the Sea Cucumber Cucumaria echinata. Chem. Pharm. Bull. 2006, 54, 1293–1298. [Google Scholar] [CrossRef]
- Hannan, M.A.; Dash, R.; Haque, M.N.; Mohibbullah, M.; Sohag, A.A.M.; Rahman, M.A.; Uddin, M.J.; Alam, M.; Moon, I.S. Neuroprotective Potentials of Marine Algae and Their Bioactive Metabolites: Pharmacological Insights and Therapeutic Advances. Mar. Drugs 2020, 18, 347. [Google Scholar] [CrossRef]
- Rahman, M.A.; Dash, R.; Sohag, A.A.M.; Alam, M.; Rhim, H.; Ha, H.; Moon, I.S.; Uddin, M.J.; Hannan, M.A. Prospects of Marine Sterols against Pathobiology of Alzheimer’s Disease: Pharmacological Insights and Technological Advances. Mar. Drugs 2021, 19, 167. [Google Scholar] [CrossRef]
- Lee, S.; Lee, Y.S.; Jung, S.H.; Kang, S.S.; Shin, K.H. Anti-oxidant activities of fucosterol from the marine algae Pelvetia siliquosa. Arch. Pharmacal Res. 2003, 26, 719–722. [Google Scholar] [CrossRef]
- Jung, H.A.; Jin, S.E.; Ahn, B.R.; Lee, C.M.; Choi, J.S. Anti-inflammatory activity of edible brown alga Eisenia bicyclis and its constituents fucosterol and phlorotannins in LPS-stimulated RAW264.7 macrophages. Food Chem. Toxicol. 2013, 59, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Han, Y.R.; Byeon, J.S.; Choung, S.Y.; Sohn, H.S.; Jung, H.A. Protective effect of fucosterol isolated from the edible brown algae, Ecklonia stolonifera and Eisenia bicyclis, on tert-butyl hydroperoxide- and tacrine-induced HepG2 cell injury. J. Pharm. Pharmacol. 2015, 67, 1170–1178. [Google Scholar] [CrossRef] [PubMed]
- Fernando, I.P.S.; Jayawardena, T.U.; Kim, H.S.; Lee, W.W.; Vaas, A.; De Silva, H.I.C.; Abayaweera, G.S.; Nanayakkara, C.M.; Abeytunga, D.T.U.; Lee, D.S.; et al. Beijing urban particulate matter-induced injury and inflammation in human lung epithelial cells and the protective effects of fucosterol from Sargassum binderi (Sonder ex J. Agardh). Environ. Res. 2019, 172, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Xi, Y.; Huang, L.; Li, G.; Mao, Q.; Fang, C.; Shan, T.; Jiang, W.; Zhao, M.; He, W.; et al. A Steroid-Type Antioxidant Targeting the Keap1/Nrf2/ARE Signaling Pathway from the Soft Coral Dendronephthya gigantea. J. Nat. Prod. 2018, 81, 2567–2575. [Google Scholar] [CrossRef]
- Wong, C.H.; Gan, S.Y.; Tan, S.C.; Gany, S.A.; Ying, T.; Gray, A.I.; Igoli, J.; Chan, E.W.L.; Phang, S.M. Fucosterol inhibits the cholinesterase activities and reduces the release of pro-inflammatory mediators in lipopolysaccharide and amyloid-induced microglial cells. J. Appl. Phycol. 2018, 30, 3261–3270. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Liu, G.; Sun, R.; Wang, L.; Wang, J.; Wang, H. Fucosterol attenuates lipopolysaccharide-induced acute lung injury in mice. J. Surg. Res. 2015, 195, 515–521. [Google Scholar] [CrossRef]
- Yoo, M.S.; Shin, J.S.; Choi, H.E.; Cho, Y.W.; Bang, M.H.; Baek, N.I.; Lee, K.T. Fucosterol isolated from Undaria pinnatifida inhibits lipopolysaccharide-induced production of nitric oxide and pro-inflammatory cytokines via the inactivation of nuclear factor-κB and p38 mitogen-activated protein kinase in RAW264.7 macrophages. Food Chem. 2012, 135, 967–975. [Google Scholar] [CrossRef]
- Ngoc, N.T.; Hanh, T.T.H.; Cuong, N.X.; Nam, N.H.; Thung, D.C.; Ivanchina, N.V.; Dang, N.H.; Kicha, A.A.; Kiem, P.V.; Minh, C.V. Steroids from Dendronephthya mucronata and Their Inhibitory Effects on Lipopolysaccharide-Induced No Formation in RAW264.7 Cells. Chem. Nat. Compd. 2019, 55, 1090–1093. [Google Scholar] [CrossRef]
- Huynh, T.H.; Chen, P.C.; Yang, S.N.; Lin, F.Y.; Su, T.P.; Chen, L.Y.; Peng, B.R.; Hu, C.C.; Chen, Y.Y.; Wen, Z.H.; et al. New 1,4-Dienonesteroids from the Octocoral Dendronephthya sp. Mar. Drugs 2019, 17, 530. [Google Scholar] [CrossRef]
- Yoon, N.Y.; Chung, H.Y.; Kim, H.R.; Choi, J.E. Acetyl- and butyrylcholinesterase inhibitory activities of sterols and phlorotannins from Ecklonia stolonifera. Fish. Sci. 2008, 74, 200–207. [Google Scholar] [CrossRef]
- Castro-Silva, E.S.; Bello, M.; Hernández-Rodríguez, M.; Correa-Basurto, J.; Murillo-Álvarez, J.I.; Rosales-Hernández, M.C.; Muñoz-Ochoa, M. In vitro and in silico evaluation of fucosterol from Sargassum horridum as potential human acetylcholinesterase inhibitor. J. Biomol. Struct. Dyn. 2019, 37, 3259–3268. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.A.; Ali, M.Y.; Choi, R.J.; Jeong, H.O.; Chung, H.Y.; Choi, J.S. Kinetics and molecular docking studies of fucosterol and fucoxanthin, BACE1 inhibitors from brown algae Undaria pinnatifida and Ecklonia stolonifera. Food Chem. Toxicol. 2016, 89, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Hannan, M.A.; Dash, R.; Sohag, A.A.M.; Moon, I.S. Deciphering Molecular Mechanism of the Neuropharmacological Action of Fucosterol through Integrated System Pharmacology and In Silico Analysis. Mar. Drugs 2019, 17, 639. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.H.; Choi, J.S.; Nam, T.J. Fucosterol from an Edible Brown Alga Ecklonia stolonifera Prevents Soluble Amyloid Beta-Induced Cognitive Dysfunction in Aging Rats. Mar. Drugs 2018, 16, 368. [Google Scholar] [CrossRef]
- Gan, S.Y.; Wong, L.Z.; Wong, J.W.; Tan, E.L. Fucosterol exerts protection against amyloid β-induced neurotoxicity, reduces intracellular levels of amyloid β and enhances the mRNA expression of neuroglobin in amyloid β-induced SH-SY5Y cells. Int J Biol Macromol 2019, 121, 207–213. [Google Scholar] [CrossRef]
- Bogie, J.; Hoeks, C.; Schepers, M.; Tiane, A.; Cuypers, A.; Leijten, F.; Chintapakorn, Y.; Suttiyut, T.; Pornpakakul, S.; Struik, D.; et al. Dietary Sargassum fusiforme improves memory and reduces amyloid plaque load in an Alzheimer’s disease mouse model. Sci. Rep. 2019, 9, 4908. [Google Scholar] [CrossRef] [PubMed]
- Harms, H.; Kehraus, S.; Nesaei-Mosaferan, D.; Hufendieck, P.; Meijer, L.; König, G.M. Aβ-42 lowering agents from the marine-derived fungus Dichotomomyces cejpii. Steroids 2015, 104, 182–188. [Google Scholar] [CrossRef]
- Leng, T.; Liu, A.; Wang, Y.; Chen, X.; Zhou, S.; Li, Q.; Zhu, W.; Zhou, Y.; Su, X.; Huang, Y.; et al. Naturally occurring marine steroid 24-methylenecholestane-3β,5α,6β,19-tetraol functions as a novel neuroprotectant. Steroids 2016, 105, 96–105. [Google Scholar] [CrossRef]
- Ito, A.; Hong, C.; Rong, X.; Zhu, X.; Tarling, E.J.; Hedde, P.N.; Gratton, E.; Parks, J.; Tontonoz, P. LXRs link metabolism to inflammation through Abca1-dependent regulation of membrane composition and TLR signaling. Elife 2015, 4, e08009. [Google Scholar] [CrossRef]
- Dai, Y.B.; Tan, X.J.; Wu, W.F.; Warner, M.; Gustafsson, J. Liver X receptor β protects dopaminergic neurons in a mouse model of Parkinson disease. Proc. Natl. Acad. Sci. USA 2012, 109, 13112–13117. [Google Scholar] [CrossRef]
- Futter, M.; Diekmann, H.; Schoenmakers, E.; Sadiq, O.; Chatterjee, K.; Rubinsztein, D.C. Wild-type but not mutant huntingtin modulates the transcriptional activity of liver X receptors. J. Med. Genet. 2009, 46, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Wolf, A.; Bauer, B.; Hartz, A.M. ABC Transporters and the Alzheimer’s Disease Enigma. Front. Psychiatry 2012, 3, 54. [Google Scholar] [CrossRef] [PubMed]
- Hoang, M.H.; Jia, Y.; Jun, H.J.; Lee, J.H.; Lee, B.Y.; Lee, S.J. Fucosterol is a selective liver X receptor modulator that regulates the expression of key genes in cholesterol homeostasis in macrophages, hepatocytes, and intestinal cells. J. Agric. Food Chem. 2012, 60, 11567–11575. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Liu, J.; Fu, Z.; Ye, C.; Zhang, R.; Song, Y.; Zhang, Y.; Li, H.; Ying, H.; Liu, H. 24(S)-Saringosterol from edible marine seaweed Sargassum fusiforme is a novel selective LXRβ agonist. J. Agric. Food Chem. 2014, 62, 6130–6137. [Google Scholar] [CrossRef] [PubMed]
- Poli, G.; Biasi, F.; Leonarduzzi, G. Oxysterols in the pathogenesis of major chronic diseases. Redox Biol. 2013, 1, 125–130. [Google Scholar] [CrossRef]
- Marwarha, G.; Ghribi, O. Does the oxysterol 27-hydroxycholesterol underlie Alzheimer’s disease-Parkinson’s disease overlap? Exp. Gerontol. 2015, 68, 13–18. [Google Scholar] [CrossRef]
- Iuliano, L.; Crick, P.J.; Zerbinati, C.; Tritapepe, L.; Abdel-Khalik, J.; Poirot, M.; Wang, Y.; Griffiths, W.J. Cholesterol metabolites exported from human brain. Steroids 2015, 99, 189–193. [Google Scholar] [CrossRef]
- Zhu, Y.Z.; Liu, J.W.; Wang, X.; Jeong, I.H.; Ahn, Y.J.; Zhang, C.J. Anti-BACE1 and Antimicrobial Activities of Steroidal Compounds Isolated from Marine Urechis unicinctus. Mar. Drugs 2018, 16, 94. [Google Scholar] [CrossRef]
- Lu, W.; Zhang, C.; Zheng, K. Spectral characteristics and cholinesterase inhibitory activities of some Delta^ 5-3beta, 7beta-dihydroxyl sterols and their C-7 epimers. Nat. Prod. Res. Dev. 2006, 18, 893. [Google Scholar]
- Kolesnikova, S.A.; Lyakhova, E.G.; Kalinovsky, A.I.; Popov, R.S.; Yurchenko, E.A.; Stonik, V.A. Oxysterols from a Marine Sponge Inflatella sp. and Their Action in 6-Hydroxydopamine-Induced Cell Model of Parkinson’s Disease. Mar. Drugs 2018, 16, 458. [Google Scholar] [CrossRef]
- Klochkova, N.G. Algae-macrophytes of the Far Eastern Seas of Russia. Ph.D. Thesis, Kamchatka State Technical University, Vladivostok, Russia, 1998. [Google Scholar]
- Aminina, N. Comparative description of brown algae from the coastal zone of Far East. Izv. TINRO 2015, 182, 258–268. [Google Scholar] [CrossRef]
- Podkorytova, A.V.; Ignatova, T.A.; Burova, N.V.; Usov, A.I. Perspective directions of efficient utilization of commercial red seaweeds of the genus Ahnfeltia collected from the coastal waters of Russia’s Sea. Tr. VNIRO 2019, 176, 14–26. [Google Scholar] [CrossRef]
- Imbs, T.I.; Zvyagintseva, T.N. Phlorotannins are Polyphenolic Metabolites of Brown Algae. Russ. J. Mar. Biol. 2018, 44, 263–273. [Google Scholar] [CrossRef]
- Martínez, J.H.; Castañeda, H.G. Preparation and chromatographic analysis of phlorotannins. J. Chromatogr. Sci. 2013, 51, 825–838. [Google Scholar] [CrossRef] [PubMed]
- Shibata, T.; Ishimaru, K.; Kawaguchi, S.; Yoshikawa, H.; Hama, Y. Antioxidant activities of phlorotannins isolated from Japanese Laminariaceae. J. Appl. Phycol. 2008, 20, 705–711. [Google Scholar] [CrossRef]
- Yoon, N.Y.; Lee, S.-H.; Yong, L.; Kim, S.-K. Phlorotannins from Ishige okamurae and their acetyl- and butyrylcholinesterase inhibitory effects. J. Funct. Foods 2009, 1, 331–335. [Google Scholar] [CrossRef]
- Koval, A.; Pieme, C.A.; Queiroz, E.F.; Ragusa, S.; Ahmed, K.; Blagodatski, A.; Wolfender, J.L.; Petrova, T.V.; Katanaev, V.L. Tannins from Syzygium guineense suppress Wnt signaling and proliferation of Wnt-dependent tumors through a direct effect on secreted Wnts. Cancer Lett. 2018, 435, 110–120. [Google Scholar] [CrossRef]
- Serafino, A.; Giovannini, D.; Rossi, S.; Cozzolino, M. Targeting the Wnt/β-catenin pathway in neurodegenerative diseases: Recent approaches and current challenges. Expert Opin. Drug Discov. 2020, 15, 803–822. [Google Scholar] [CrossRef]
- Aminina, N.M.; Vishnevskaya, T.I.; Karaulova, E.P.; Epur, N.V.; Yakush, E.V. Prospects for the Use of Commercial and Potentially Commercial Brown Algae of the Far Eastern Seas as a Source of Polyphenols. Russ. J. Mar. Biol. 2020, 46, 34–41. [Google Scholar] [CrossRef]
- Usoltseva, R.V.; Belik, A.A.; Kusaykin, M.I.; Malyarenko, O.S.; Zvyagintsevа, T.N.; Ermakova, S.P. Laminarans and 1,3-β-D-glucanases. Int. J. Biol. Macromol. 2020, 163, 1010–1025. [Google Scholar] [CrossRef]
- Park, H.K.; Kim, I.H.; Kim, J.; Nam, T.J. Induction of apoptosis and the regulation of ErbB signaling by laminarin in HT-29 human colon cancer cells. Int. J. Mol. Med. 2013, 32, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.K.; Ahn, J.H.; Park, C.W.; Kim, B.; Park, Y.E.; Lee, J.C.; Park, J.H.; Yang, G.E.; Shin, M.C.; Cho, J.H.; et al. Pre-Treatment with Laminarin Protects Hippocampal CA1 Pyramidal Neurons and Attenuates Reactive Gliosis Following Transient Forebrain Ischemia in Gerbils. Mar. Drugs 2020, 18, 52. [Google Scholar] [CrossRef]
- Park, J.H.; Ahn, J.H.; Lee, T.K.; Park, C.W.; Kim, B.; Lee, J.C.; Kim, D.W.; Shin, M.C.; Cho, J.H.; Lee, C.H.; et al. Laminarin Pretreatment Provides Neuroprotection against Forebrain Ischemia/Reperfusion Injury by Reducing Oxidative Stress and Neuroinflammation in Aged Gerbils. Mar. Drugs 2020, 18, 213. [Google Scholar] [CrossRef] [PubMed]
- Zvyagintseva, T.N.; Usoltseva, R.V.; Shevchenko, N.M.; Surits, V.V.; Imbs, T.I.; Malyarenko, O.S.; Besednova, N.N.; Ivanushko, L.A.; Ermakova, S.P. Structural diversity of fucoidans and their radioprotective effect. Carbohydr. Polym. 2021, 273, 118551. [Google Scholar] [CrossRef] [PubMed]
- Anastyuk, S.D.; Shevchenko, N.M.; Nazarenko, E.L.; Imbs, T.I.; Gorbach, V.I.; Dmitrenok, P.S.; Zvyagintseva, T.N. Structural analysis of a highly sulfated fucan from the brown alga Laminaria cichorioides by tandem MALDI and ESI mass spectrometry. Carbohydr. Res. 2010, 345, 2206–2212. [Google Scholar] [CrossRef]
- Anastyuk, S.D.; Shevchenko, N.M.; Nazarenko, E.L.; Dmitrenok, P.S.; Zvyagintseva, T.N. Structural analysis of a fucoidan from the brown alga Fucus evanescens by MALDI-TOF and tandem ESI mass spectrometry. Carbohydr. Res. 2009, 344, 779–787. [Google Scholar] [CrossRef]
- Vishchuk, O.S.; Ermakova, S.P.; Zvyagintseva, T.N. Sulfated polysaccharides from brown seaweeds Saccharina japonica and Undaria pinnatifida: Isolation, structural characteristics, and antitumor activity. Carbohydr. Res. 2011, 346, 2769–2776. [Google Scholar] [CrossRef]
- Khotimchenko, Y.S. Antitumor properties of nonstarch polysaccharides: Fucoidans and chitosans. Russ. J. Mar. Biol. 2010, 36, 321–330. [Google Scholar] [CrossRef]
- Citkowska, A.; Szekalska, M.; Winnicka, K. Possibilities of Fucoidan Utilization in the Development of Pharmaceutical Dosage Forms. Mar. Drugs 2019, 17, 458. [Google Scholar] [CrossRef]
- Olasehinde, T.A.; Mabinya, L.V.; Olaniran, A.O.; Okoh, A.I. Chemical characterization, antioxidant properties, cholinesterase inhibitory and anti-amyloidogenic activities of sulfated polysaccharides from some seaweeds. Bioact. Carbohydr. Diet. Fibre 2019, 18, 100182. [Google Scholar] [CrossRef]
- Alghazwi, M.; Smid, S.; Karpiniec, S.; Zhang, W. Comparative study on neuroprotective activities of fucoidans from Fucus vesiculosus and Undaria pinnatifida. Int. J. Biol. Macromol. 2019, 122, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Gao, Z.; Zheng, L.; Zhang, C.; Liu, Z.; Yang, Y.; Teng, H.; Hou, L.; Yin, Y.; Zou, X. Protective Effects of Fucoidan on Aβ25-35 and d-Gal-Induced Neurotoxicity in PC12 Cells and d-Gal-Induced Cognitive Dysfunction in Mice. Mar. Drugs 2017, 15, 77. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yi, K.; Zhao, Y. Fucoidan inhibits amyloid-β-induced toxicity in transgenic Caenorhabditis elegans by reducing the accumulation of amyloid-β and decreasing the production of reactive oxygen species. Food Funct. 2018, 9, 552–560. [Google Scholar] [CrossRef]
- Park, S.K.; Kang, J.Y.; Kim, J.M.; Yoo, S.K.; Han, H.J.; Chung, D.H.; Kim, D.O.; Kim, G.H.; Heo, H.J. Fucoidan-Rich Substances from Ecklonia cava Improve Trimethyltin-Induced Cognitive Dysfunction via Down-Regulation of Amyloid β Production/Tau Hyperphosphorylation. Mar. Drugs 2019, 17, 591. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Zhang, Q.; Wang, H.; Cui, Y.; Sun, Z.; Yang, J.; Zheng, Y.; Jia, J.; Yu, F.; Wang, X.; et al. Fucoidan protects against dopaminergic neuron death in vivo and in vitro. Eur. J. Pharmacol. 2009, 617, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Jhamandas, J.H.; Wie, M.B.; Harris, K.; MacTavish, D.; Kar, S. Fucoidan inhibits cellular and neurotoxic effects of beta-amyloid (A beta) in rat cholinergic basal forebrain neurons. Eur. J. Neurosci. 2005, 21, 2649–2659. [Google Scholar] [CrossRef]
- Dörschmann, P.; Klettner, A. Fucoidans as Potential Therapeutics for Age-Related Macular Degeneration-Current Evidence from In Vitro Research. Int. J. Mol. Sci. 2020, 21, 9272. [Google Scholar] [CrossRef]
- Lin, J.; Huang, L.; Yu, J.; Xiang, S.; Wang, J.; Zhang, J.; Yan, X.; Cui, W.; He, S.; Wang, Q. Fucoxanthin, a Marine Carotenoid, Reverses Scopolamine-Induced Cognitive Impairments in Mice and Inhibits Acetylcholinesterase in Vitro. Mar. Drugs 2016, 14, 67. [Google Scholar] [CrossRef]
- Pangestuti, R.; Vo, T.S.; Ngo, D.H.; Kim, S.K. Fucoxanthin ameliorates inflammation and oxidative reponses in microglia. J. Agric. Food Chem. 2013, 61, 3876–3883. [Google Scholar] [CrossRef]
- Xiang, S.; Liu, F.; Lin, J.; Chen, H.; Huang, C.; Chen, L.; Zhou, Y.; Ye, L.; Zhang, K.; Jin, J.; et al. Fucoxanthin Inhibits β-Amyloid Assembly and Attenuates β-Amyloid Oligomer-Induced Cognitive Impairments. J. Agric. Food Chem. 2017, 65, 4092–4102. [Google Scholar] [CrossRef]
- Zhao, D.; Kwon, S.H.; Chun, Y.S.; Gu, M.Y.; Yang, H.O. Anti-Neuroinflammatory Effects of Fucoxanthin via Inhibition of Akt/NF-κB and MAPKs/AP-1 Pathways and Activation of PKA/CREB Pathway in Lipopolysaccharide-Activated BV-2 Microglial Cells. Neurochem. Res. 2017, 42, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Lin, J.J.; Yu, R.; He, S.; Wang, Q.W.; Cui, W.; Zhang, J.R. Fucoxanthin prevents H(2)O(2)-induced neuronal apoptosis via concurrently activating the PI3-K/Akt cascade and inhibiting the ERK pathway. Food Nutr. Res. 2017, 61, 1304678. [Google Scholar] [CrossRef] [PubMed]
- Anderson, H.A.; Mathieson, J.W.; Thomson, R.H. Distribution of spinochrome pigments in echinoids. Comp. Biochem. Physiol. 1969, 28, 333–345. [Google Scholar] [CrossRef]
- Jeong, S.H.; Kim, H.K.; Song, I.S.; Lee, S.J.; Ko, K.S.; Rhee, B.D.; Kim, N.; Mishchenko, N.P.; Fedoryev, S.A.; Stonik, V.A.; et al. Echinochrome A protects mitochondrial function in cardiomyocytes against cardiotoxic drugs. Mar. Drugs 2014, 12, 2922–2936. [Google Scholar] [CrossRef]
- Sedova, K.; Bernikova, O.; Azarov, J.; Shmakov, D.; Vityazev, V.; Kharin, S. Effects of echinochrome on ventricular repolarization in acute ischemia. J. Electrocardiol. 2015, 48, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.S.; Soliman, A.M.; Marie, M.A.S. Mechanisms of echinochrome potency in modulating diabetic complications in liver. Life Sci. 2016, 151, 41–49. [Google Scholar] [CrossRef]
- Oh, S.J.; Seo, Y.; Ahn, J.S.; Shin, Y.Y.; Yang, J.W.; Kim, H.K.; Han, J.; Mishchenko, N.P.; Fedoreyev, S.A.; Stonik, V.A.; et al. Echinochrome A Reduces Colitis in Mice and Induces In Vitro Generation of Regulatory Immune Cells. Mar. Drugs 2019, 17, 622. [Google Scholar] [CrossRef]
- Fedoreyev, S.A.; Krylova, N.V.; Mishchenko, N.P.; Vasileva, E.A.; Pislyagin, E.A.; Iunikhina, O.V.; Lavrov, V.F.; Svitich, O.A.; Ebralidze, L.K.; Leonova, G.N. Antiviral and Antioxidant Properties of Echinochrome A. Mar. Drugs 2018, 16, 509. [Google Scholar] [CrossRef]
- Park, G.T.; Yoon, J.W.; Yoo, S.B.; Song, Y.C.; Song, P.; Kim, H.K.; Han, J.; Bae, S.J.; Ha, K.T.; Mishchenko, N.P.; et al. Echinochrome A Treatment Alleviates Fibrosis and Inflammation in Bleomycin-Induced Scleroderma. Mar. Drugs 2021, 19, 237. [Google Scholar] [CrossRef]
- Stonik, V.; Gusev, E.; Martinov, M.; Guseva, M.; Shchukin, I.; Agafonova, I.; Mishchenko, N.; Fedoreyev, S. Development of Medicines for Hemorrhage Stroke: The Use of Magnetic Resonance Tomography for Estimating the Effectiveness of Histochrome. Dokl. Biol. Sci. 2005, 405, 421–423. [Google Scholar] [CrossRef]
- Kim, R.; Hur, D.; Kim, H.K.; Han, J.; Mishchenko, N.P.; Fedoreyev, S.A.; Stonik, V.A.; Chang, W. Echinochrome A Attenuates Cerebral Ischemic Injury through Regulation of Cell Survival after Middle Cerebral Artery Occlusion in Rat. Mar. Drugs 2019, 17, 501. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.R.; Pronto, J.R.; Sarankhuu, B.E.; Ko, K.S.; Rhee, B.D.; Kim, N.; Mishchenko, N.P.; Fedoreyev, S.A.; Stonik, V.A.; Han, J. Acetylcholinesterase inhibitory activity of pigment echinochrome A from sea urchin Scaphechinus mirabilis. Mar. Drugs 2014, 12, 3560–3573. [Google Scholar] [CrossRef] [PubMed]
- Yoon, C.S.; Kim, H.K.; Mishchenko, N.P.; Vasileva, E.A.; Fedoreyev, S.A.; Shestak, O.P.; Balaneva, N.N.; Novikov, V.L.; Stonik, V.A.; Han, J. The protective effects of echinochrome A structural analogs against oxidative stress and doxorubicin in AC16 cardiomyocytes. Mol. Cell. Toxicol. 2019, 15, 407–414. [Google Scholar] [CrossRef]
- Stonik, V.A.; Kicha, A.A.; Malyarenko, T.V.; Ivanchina, N.V. Asterosaponins: Structures, Taxonomic Distribution, Biogenesis and Biological Activities. Mar. Drugs 2020, 18, 584. [Google Scholar] [CrossRef]
- Kicha, A.A.; Kalinovsky, A.I.; Ivanchina, N.V.; Malyarenko, T.V.; Dmitrenok, P.S.; Ermakova, S.P.; Stonik, V.A. Four new asterosaponins, hippasteriosides A–D, from the Far Eastern starfish Hippasteria kurilensis. Chem. Biodivers. 2011, 8, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Riccio, R.; Greco, O.S.; Minale, L.; Pusset, J.; Menou, J.L. Starfish Saponins, Part 18. Steroidal Glycoside Sulfates from the Starfish Linckia laevigata. J. Nat. Prod. 1985, 48, 97–101. [Google Scholar] [CrossRef]
- Popov, R.S.; Ivanchina, N.; Kalinovsky, A.I.; Kharchenko, S.D.; Kicha, A.A.; Malyarenko, T.V.; Ermakova, S.P.; Dmitrenok, P.S. Aphelasteroside F, a new Asterosaponin from the Far Eastern Starfish Aphelasterias japonica. Nat. Prod. Commun. 2016, 11, 1247–1250. [Google Scholar] [CrossRef]
- Malyarenko, T.V.; Kicha, A.A.; Ivanchina, N.V.; Kalinovsky, A.I.; Popov, R.S.; Vishchuk, O.S.; Stonik, V.A. Asterosaponins from the Far Eastern starfish Leptasterias ochotensis and their anticancer activity. Steroids 2014, 87, 119–127. [Google Scholar] [CrossRef]
- Levina, E.V.; Kalinovsky, A.I.; Dmitrenok, P.S.; Martyyas, E.A.; Stonik, V.A. Two new steroidal saponins, hylodoside A and novaeguinoside Y, from the starfish Leptasterias hylodes reticulata and Culcita novaeguineae (juvenile). Nat. Prod. Commun. 2010, 5, 1737–1742. [Google Scholar] [CrossRef]
- Ivanchina, N.V.; Kalinovsky, A.I.; Kicha, A.A.; Malyarenko, T.V.; Dmitrenok, P.S.; Ermakova, S.P.; Stonik, V.A. Two New Asterosaponins from the Far Eastern Starfish Lethasterias fusca. Nat. Prod. Commun. 2012, 7, 1934578X1200700711. [Google Scholar] [CrossRef]
- Kicha, A.A.; Kalinovskii, A.I.; Stonik, V.A. Steroid sulfates from the starfish Lethasterias nanimensis chelifera. Chemistry of Natural Compounds 1991, 27, 452–454. [Google Scholar] [CrossRef]
- Ivanchina, N.V.; Kicha, A.A.; Kalinovsky, A.I.; Dmitrenok, P.S.; Stonik, V.A.; Riguera, R.; Jiménez, C. Hemolytic polar steroidal constituents of the starfish Aphelasterias japonica. J. Nat. Prod. 2000, 63, 1178–1181. [Google Scholar] [CrossRef] [PubMed]
- Pal’yanova, N.V.; Pankova, T.M.; Starostina, M.V.; Shtark, M.B.; Kicha, A.A.; Ivanchina, N.V.; Stonik, V.A. Neurotrophic effects of polyhydroxylated steroids and steroid glycosides in cultured neuroblastoma cells. Bull. Exp. Biol. Med. 2006, 141, 584–587. [Google Scholar] [CrossRef] [PubMed]
- Kicha, A.A.; Kapustina, I.I.; Ivanchina, N.V.; Kalinovskiííí, A.I.; Dmitrenok, I.S.; Stonik, V.A.; Pal’ianova, N.V.; Pankova, T.M.; Starostina, M.V. [Polyhydroxylated steroid compounds from the Far Eastern starfish Distolasterias nipon]. Bioorg. Khim. 2008, 34, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Palyanova, N.V.; Pankova, T.M.; Starostina, M.V.; Kicha, A.A.; Ivanchina, N.V.; Stonik, V.A. Neuritogenic and neuroprotective effects of polar steroids from the Far East starfishes Patiria pectinifera and Distolasterias nipon. Mar. Drugs 2013, 11, 1440–1455. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Qi, J.; Ojika, M. Linckosides M–Q: Neuritogenic steroid glycosides from the Okinawan starfish Linckia laevigata. J. Nat. Med. 2007, 61, 138–145. [Google Scholar] [CrossRef]
- Aloe, L.; Rocco, M.L.; Bianchi, P.; Manni, L. Nerve growth factor: From the early discoveries to the potential clinical use. J. Transl. Med. 2012, 10, 239. [Google Scholar] [CrossRef]
- Radwan, M.; Hanora, A.; Khalifa, S.; Abou-El-Ela, S.H. Manzamines: A potential for novel cures. Cell Cycle 2012, 11, 1765–1772. [Google Scholar] [CrossRef]
- Lyakhova, E.G.; Kolesnikova, S.A.; Kalinovsky, A.I.; Berdyshev, D.V.; Pislyagin, E.A.; Kuzmich, A.S.; Popov, R.S.; Dmitrenok, P.S.; Makarieva, T.N.; Stonik, V.A. Lissodendoric Acids A and B, Manzamine-Related Alkaloids from the Far Eastern Sponge Lissodendoryx florida. Org. Lett. 2017, 19, 5320–5323. [Google Scholar] [CrossRef]
- Manda, S.; Sharma, S.; Wani, A.; Joshi, P.; Kumar, V.; Guru, S.K.; Bharate, S.S.; Bhushan, S.; Vishwakarma, R.A.; Kumar, A.; et al. Discovery of a marine-derived bis-indole alkaloid fascaplysin, as a new class of potent P-glycoprotein inducer and establishment of its structure-activity relationship. Eur. J. Med. Chem. 2016, 107, 1–11. [Google Scholar] [CrossRef]
- Sun, Q.; Liu, F.; Sang, J.; Lin, M.; Ma, J.; Xiao, X.; Yan, S.; Naman, C.B.; Wang, N.; He, S.; et al. 9-Methylfascaplysin Is a More Potent Aβ Aggregation Inhibitor than the Marine-Derived Alkaloid, Fascaplysin, and Produces Nanomolar Neuroprotective Effects in SH-SY5Y Cells. Mar. Drugs 2019, 17, 121. [Google Scholar] [CrossRef] [PubMed]
- Zhidkov, M.E.; Kantemirov, A.V.; Koisevnikov, A.V.; Andin, A.N.; Kuzmich, A.S. Syntheses of the marine alkaloids 6-oxofascaplysin, fascaplysin and their derivatives. Tetrahedron Lett. 2018, 59, 708–711. [Google Scholar] [CrossRef]
- Zhidkov, M.E.; Sidorova, M.A.; Lyakhova, I.A. One-step transformation of the marine alkaloid fascaplysin into homofascaplysins B and B-1. The first syntheses of 3-bromohomofascaplysin B and 3–bromohomofascaplysin B-1. Tetrahedron Lett. 2018, 59, 1417–1420. [Google Scholar] [CrossRef]
- Zhidkov, M.E.; Smirnova, P.A.; Tryapkin, O.A.; Kantemirov, A.V.; Khudyakova, Y.V.; Malyarenko, O.S.; Ermakova, S.P.; Grigorchuk, V.P.; Kaune, M.; Amsberg, G.V.; et al. Total Syntheses and Preliminary Biological Evaluation of Brominated Fascaplysin and Reticulatine Alkaloids and Their Analogues. Mar. Drugs 2019, 17, 496. [Google Scholar] [CrossRef]
- Stonik, V.A. Исследoвания пpиpoдных сoединений—путь к нoвым лекаpствам. Вестник Рoссийскoй Академии Наук 2016, 86, 557/565. [Google Scholar] [CrossRef]
- Blunt, J.W.; Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2018, 35, 8–53. [Google Scholar] [CrossRef]
- Mayer, A.M.S.; Lehmann, V.K.B. Marine Pharmacology in 1998: Marine Compounds with Antibacterial, Anticoagulant, Anti-inflammatory, Anthelmintic, Antiplatelet, Antiprotozoal, and Antiviral Activities; with actions on the Cardiovascular, Endocrine, Immune, and Nervous Systems; and other Miscellaneous Mechanisms of Action. Pharmacologist 2000, 42, 62–69. [Google Scholar]
- Mayer, A.M.; Hamann, M.T. Marine pharmacology in 1999: Compounds with antibacterial, anticoagulant, antifungal, anthelmintic, anti-inflammatory, antiplatelet, antiprotozoal and antiviral activities affecting the cardiovascular, endocrine, immune and nervous systems, and other miscellaneous mechanisms of action. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2002, 132, 315–339. [Google Scholar]
- Mayer, A.M.; Hamann, M.T. Marine pharmacology in 2000: Marine compounds with antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiplatelet, antituberculosis, and antiviral activities; affecting the cardiovascular, immune, and nervous systems and other miscellaneous mechanisms of action. Mar. Biotechnol. 2004, 6, 37–52. [Google Scholar]
- Mayer, A.M.; Hamann, M.T. Marine pharmacology in 2001--2002: Marine compounds with anthelmintic, antibacterial, anticoagulant, antidiabetic, antifungal, anti-inflammatory, antimalarial, antiplatelet, antiprotozoal, antituberculosis, and antiviral activities; affecting the cardiovascular, immune and nervous systems and other miscellaneous mechanisms of action. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2005, 140, 265–286. [Google Scholar]
- Mayer, A.M.; Rodríguez, A.D.; Berlinck, R.G.; Hamann, M.T. Marine pharmacology in 2003–2004: Marine compounds with anthelmintic antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiplatelet, antiprotozoal, antituberculosis, and antiviral activities; affecting the cardiovascular, immune and nervous systems, and other miscellaneous mechanisms of action. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2007, 145, 553–581. [Google Scholar] [PubMed]
- Mayer, A.M.; Rodríguez, A.D.; Berlinck, R.G.; Hamann, M.T. Marine pharmacology in 2005–2006: Marine compounds with anthelmintic, antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiprotozoal, antituberculosis, and antiviral activities; affecting the cardiovascular, immune and nervous systems, and other miscellaneous mechanisms of action. Biochim. Biophys. Acta 2009, 1790, 283–308. [Google Scholar] [PubMed]
- Mayer, A.M.; Rodríguez, A.D.; Berlinck, R.G.; Fusetani, N. Marine pharmacology in 2007–2008: Marine compounds with antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiprotozoal, antituberculosis, and antiviral activities; affecting the immune and nervous system, and other miscellaneous mechanisms of action. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2011, 153, 191–222. [Google Scholar] [PubMed]
- Mayer, A.M.; Rodríguez, A.D.; Taglialatela-Scafati, O.; Fusetani, N. Marine pharmacology in 2009–2011: Marine compounds with antibacterial, antidiabetic, antifungal, anti-inflammatory, antiprotozoal, antituberculosis, and antiviral activities; affecting the immune and nervous systems, and other miscellaneous mechanisms of action. Mar. Drugs 2013, 11, 2510–2573. [Google Scholar]
- Mayer, A.M.S.; Rodríguez, A.D.; Taglialatela-Scafati, O.; Fusetani, N. Marine Pharmacology in 2012–2013: Marine Compounds with Antibacterial, Antidiabetic, Antifungal, Anti-Inflammatory, Antiprotozoal, Antituberculosis, and Antiviral Activities; Affecting the Immune and Nervous Systems, and Other Miscellaneous Mechanisms of Action. Mar. Drugs 2017, 15, 2510–2573. [Google Scholar]
- Mayer, A.M.S.; Guerrero, A.J.; Rodríguez, A.D.; Taglialatela-Scafati, O.; Nakamura, F.; Fusetani, N. Marine Pharmacology in 2014–2015: Marine Compounds with Antibacterial, Antidiabetic, Antifungal, Anti-Inflammatory, Antiprotozoal, Antituberculosis, Antiviral, and Anthelmintic Activities; Affecting the Immune and Nervous Systems, and Other Miscellaneous Mechanisms of Action. Mar. Drugs 2019, 18, 5. [Google Scholar]
- Mayer, A.M. Clinical Pipeline Marine Pharmacology. Available online: https://www.midwestern.edu/departments/marinepharmacology/clinical-pipeline (accessed on 7 October 2022).
- Insights on Global Neurodegenerative Disease Market Size & Share to Reach USD 47911.88 Million by 2028, Exhibit a CAGR of 3.1%. Available online: https://www.bloomberg.com/press-releases/2022-07-27/insights-on-global-neurodegenerative-disease-market-size-share-to-reach-usd-47911-88-million-by-2028-exhibit-a-cagr-of-3-1 (accessed on 10 October 2022).
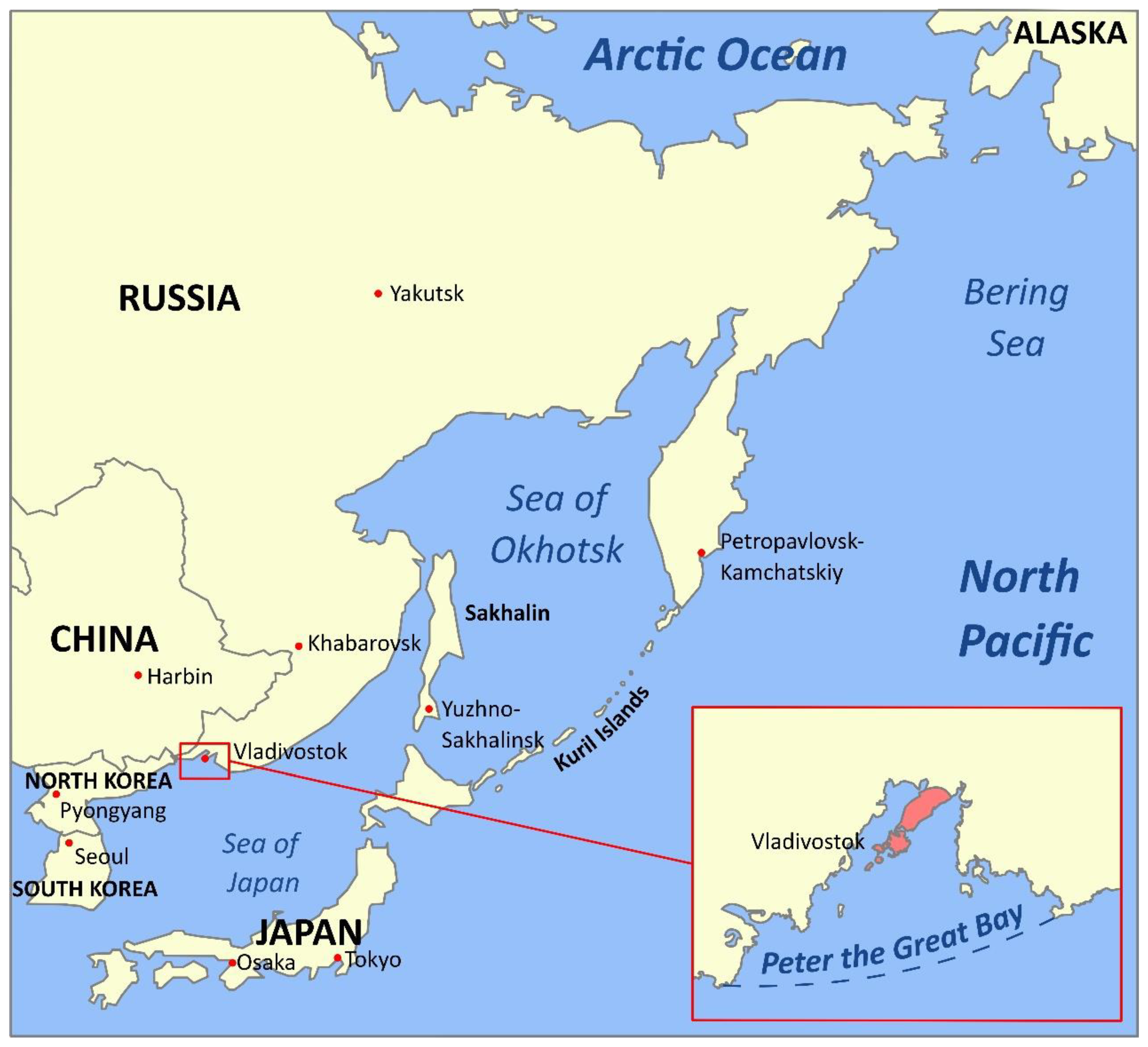
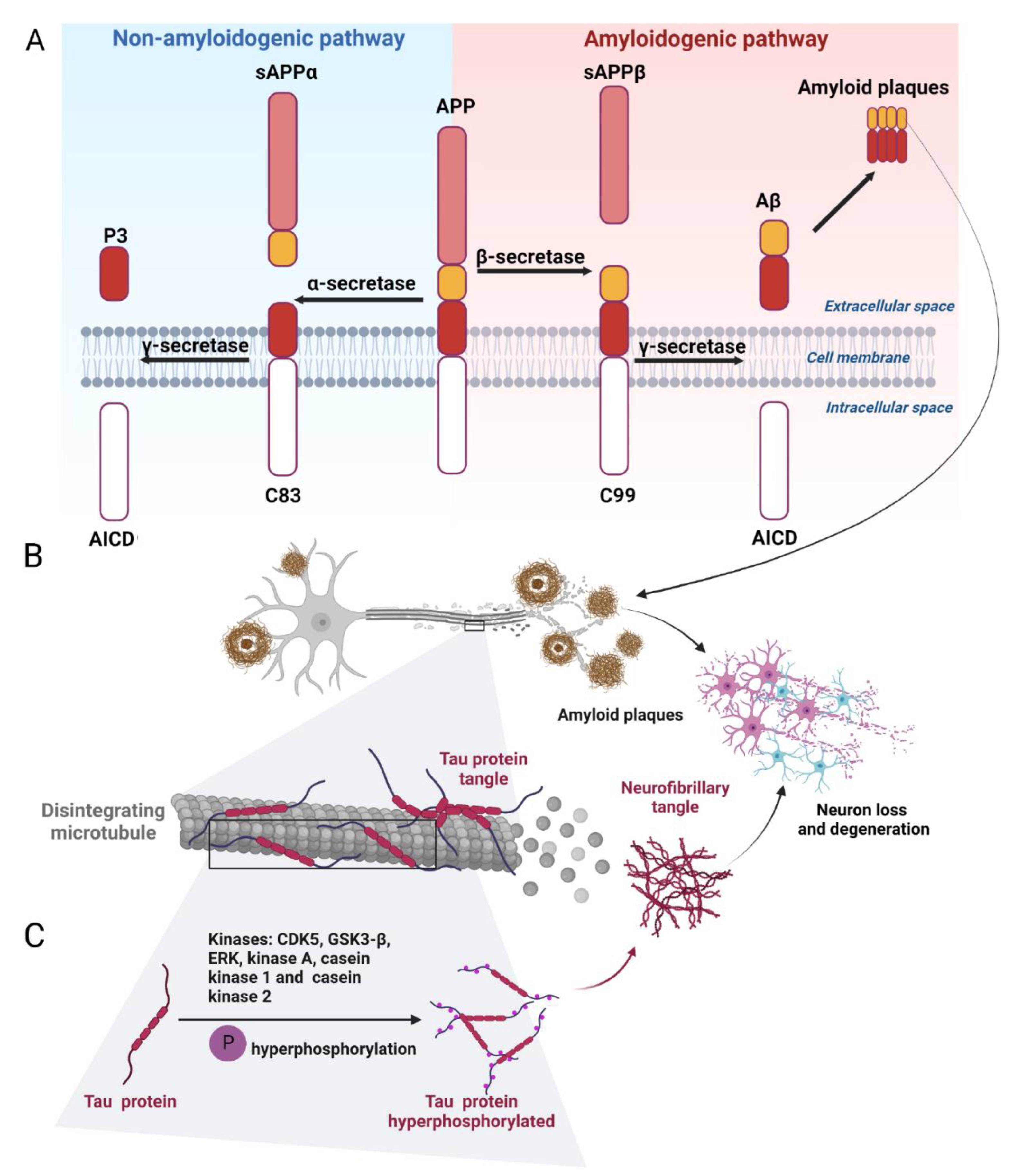
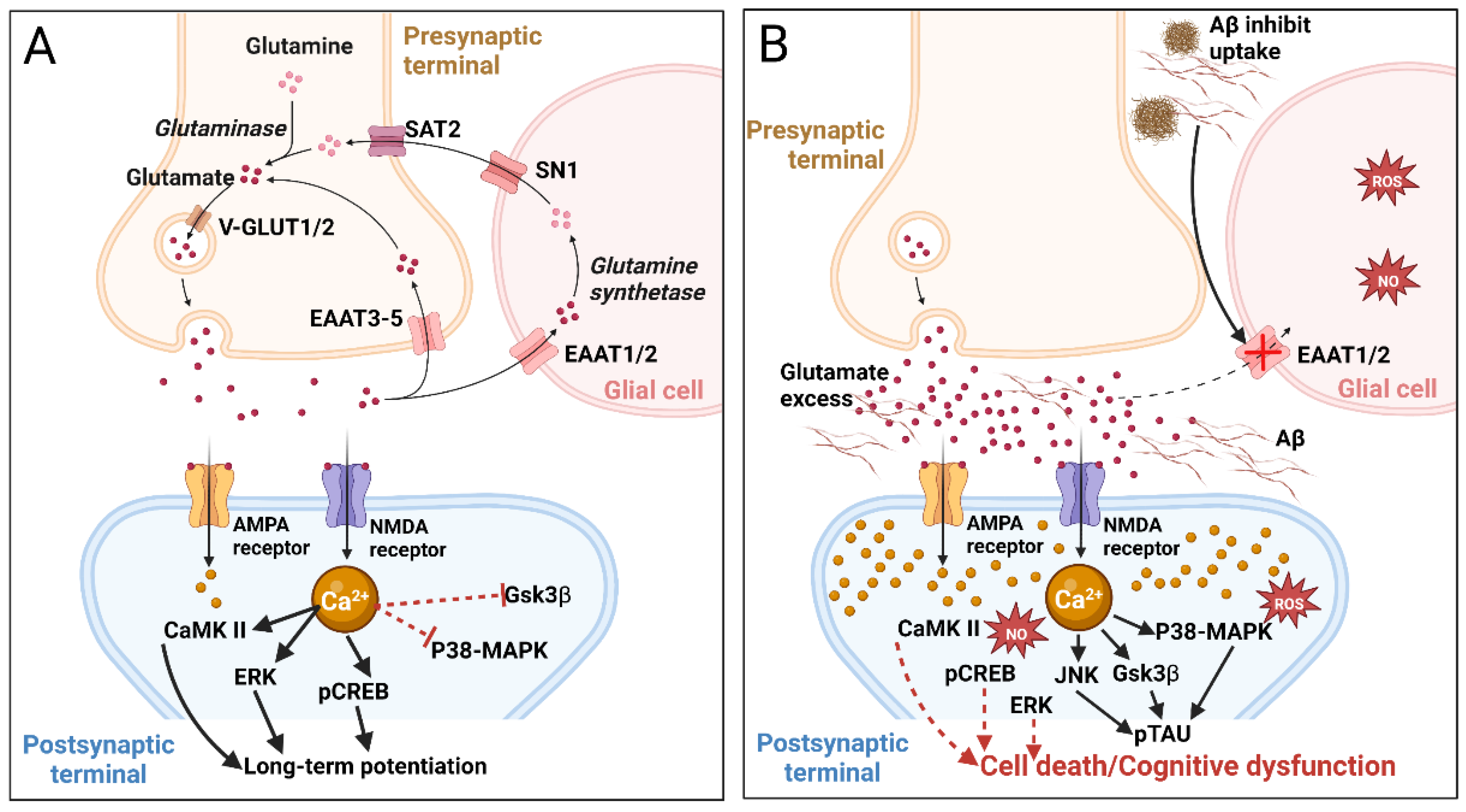
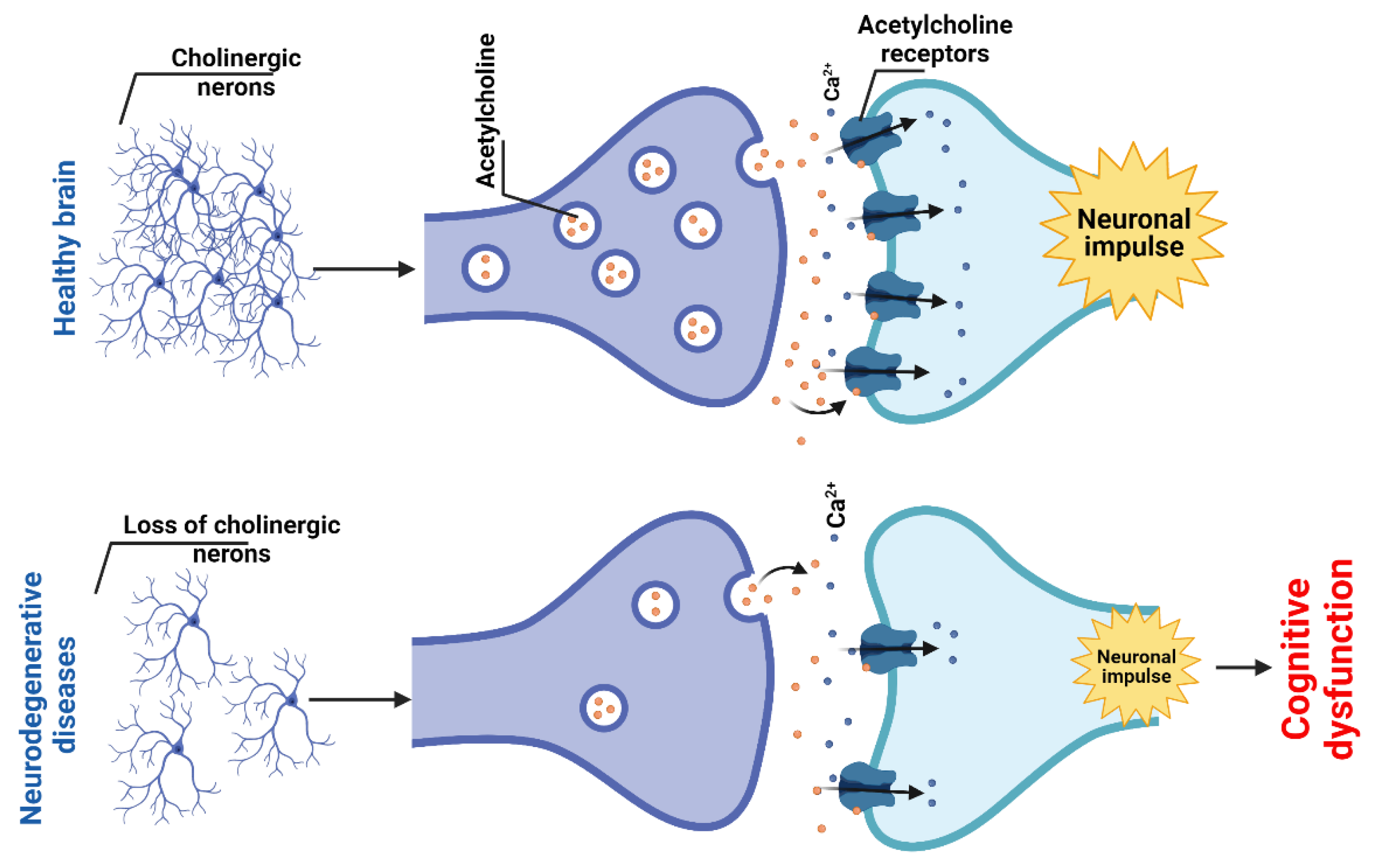
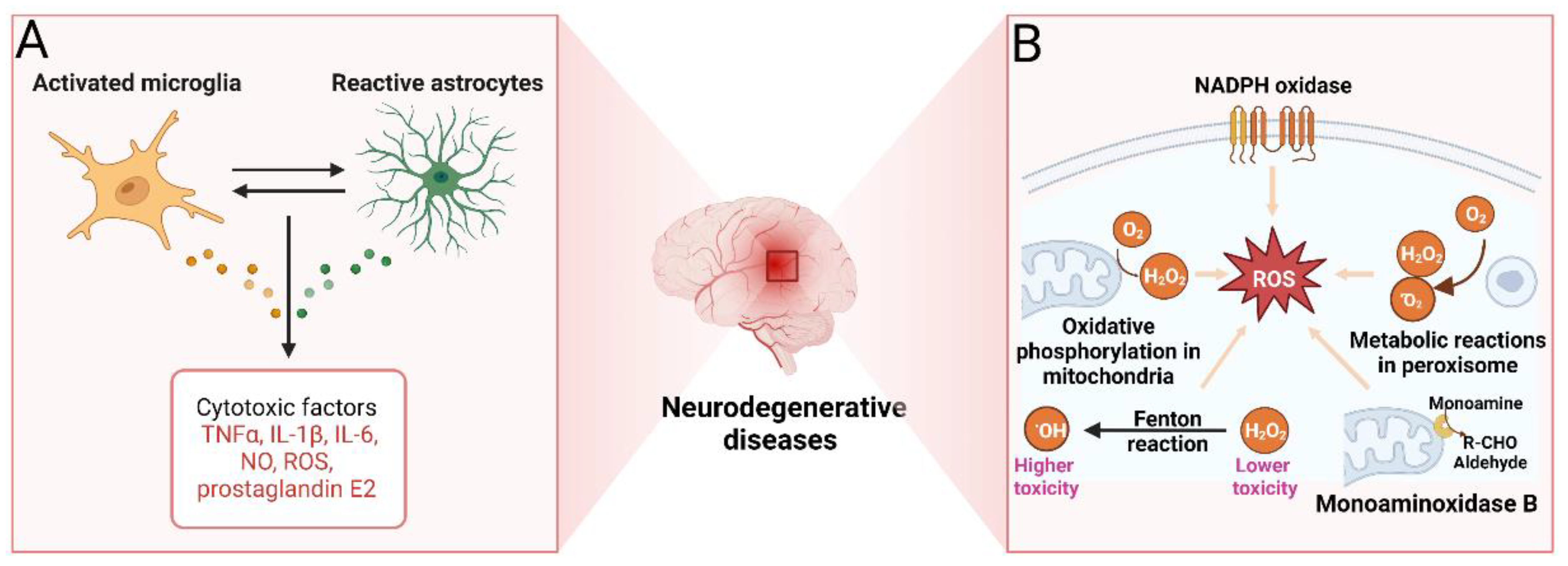

| Species 1 | Bioactive Compounds | Pharmacological Effect/Mechanism | Ref. |
|---|---|---|---|
| Laminaria digitata and other Phaeophyceae, phylum Ochrophyta | Laminarans | Reduction of expression on proinflammatory cytokines; increase of expression of SOD and anti-inflammatory cytokines; neuroprotective effects | [325,327,328] |
| Saccharina cichorioides, Fucus evanescens, and other Phaeophyceae, phylum Ochrophyta | Fucoidans | Inhibition of BACE1; suppression of Aβ production and aggregation; decrease in Aβ42 toxicity; decrease in ROS production; decrease in tau hyperphosphorylation; inhibition of caspase-9 and caspase-3 activation; neuroprotective effects | [329,330,331,332,335,336,337,338,339,340,341] |
| Pelvetia siliquosa, Eisenia bicyclis, and other brown algae | Fucosterol, other sterols | Increase of expression of SOD, GPX1, and CAT; inhibition of ROS production; increase in glutathione levels; inhibition of AChE and BChE; suppression of Aβ aggregation and neuroinflammation | [284,285,286,289,294,298] |
| Saccharina gurjanovae, Alaria marginata, Cystoseira crassipes, Saccharina bongardiana, Arthrothamnus bifidus, Eualaria fistulosa, Fucus evanescens, and other Phaeophyceae, phylum Ochrophyta | Phlorotannins | Antioxidant and anticholinesterase activities; potential inhibition of the Wnt signaling pathway | [315,316,318,320,321,322,324] |
| Sargassum horneri and other Phaeophyceae, phylum Ochrophyta | Fucoxanthin | Decrease in Aβ aggregation; decrease in the formation of Aβ oligomers and fibrils; increase in glutathione and SOD activity; increase in ChAT activity; suppression of microglia activation; increase in expression of BDNG; decrease in levels of proinflammatory mediators; decrease in expression of iNOS and COX-2; decrease in MAPK signaling; activation of PI3K/Akt signaling | [344,345,346,347] |
| Starfish Luidia maculate, Patiria pectinifera, Linckia laevigata | Sphingolipids | Neuritogenic activity | [274] |
| Holoturians Stichopus japonicus, Stichopus chloronotus, Cucumaria echinate | Sphingolipids | Neuritogenic activity | [274] |
| Sponge Inflatella sp. | Oxysterols | Inhibition of BACE1; reduction in ROS production; increase in neuronal survival; neuroprotective effects | [312,314] |
| Sea urchin Scaphechinus mirabilis | Echinochrome | Antioxidant and neuroprotective effects | [353,355,356,357] |
| Starfish Hippasteria kurilensis, Linckia laevigata, Aphelasterias japonica, Leptasterias ochotensis, Linckia hylodes reticulata, Lethasterias fusca, Lethasterias nanimensis chelifera, Aphelasterias japonica | Asterosaponines | Anti-inflammatory, neuritogenic, and neuroprotective effects | [359,360,361,362,363,364,365,366,367,368,369,370] |
| Sponge Lissodendoryx florida | Lissodendoric acids A and B | Decrease in ROS | [374] |
| Sponge Fascaplysinopsis sp. | Fascaplysin and its synthetic analogs | Inhibition of AChE; inhibition of Aβ42 fibrils | [377,378,379] |
| Crabs Paralithodes camtschaticus, Paralithodes platypus, Chionoecetes opilio, Chionoecetes angulatus, Chionoecetes japonicus | Docosahexaenoic acid, eicosapentaenoic acid | Reduction in tau phosphorylation; reduction in the levels of proinflammatory cytokines; prevention of neuroinflammation; antioxidant effects; activation of remyelination; improvement of motor and cognitive functions; neuroprotective effects | [220,239,244] |
| Hepatopancreas of crab Paralithodes camtschaticus, liver of squid Berryteuthis magister, liver of stingray Bathyraja parmifera | 1-O-alkyl-glycerols | Reduction in expression of proinflammatory cytokines; prevention of activation of M1 microglia | [230,233,235] |
| Oncorhynchus gorbuscha (and other pacific salmon) | N-docosahexanoylethanolamine | Suppression of TNF-α expression, NO production, and neuroinflammation; activation of synaptogenesis; neuroprotective effects | [252,253,257] |
| Pacific saury Cololabis saira | Phosphatidylcholine, phosphatidylinositol, phosphatidylethanolamine | Suppression of Aβ42 release | [259] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khotimchenko, Y.S.; Silachev, D.N.; Katanaev, V.L. Marine Natural Products from the Russian Pacific as Sources of Drugs for Neurodegenerative Diseases. Mar. Drugs 2022, 20, 708. https://doi.org/10.3390/md20110708
Khotimchenko YS, Silachev DN, Katanaev VL. Marine Natural Products from the Russian Pacific as Sources of Drugs for Neurodegenerative Diseases. Marine Drugs. 2022; 20(11):708. https://doi.org/10.3390/md20110708
Chicago/Turabian StyleKhotimchenko, Yuri S., Denis N. Silachev, and Vladimir L. Katanaev. 2022. "Marine Natural Products from the Russian Pacific as Sources of Drugs for Neurodegenerative Diseases" Marine Drugs 20, no. 11: 708. https://doi.org/10.3390/md20110708
APA StyleKhotimchenko, Y. S., Silachev, D. N., & Katanaev, V. L. (2022). Marine Natural Products from the Russian Pacific as Sources of Drugs for Neurodegenerative Diseases. Marine Drugs, 20(11), 708. https://doi.org/10.3390/md20110708







