Alkyl Glycerol Ethers as Adaptogens
Abstract
1. Introduction
2. About Our Experiments
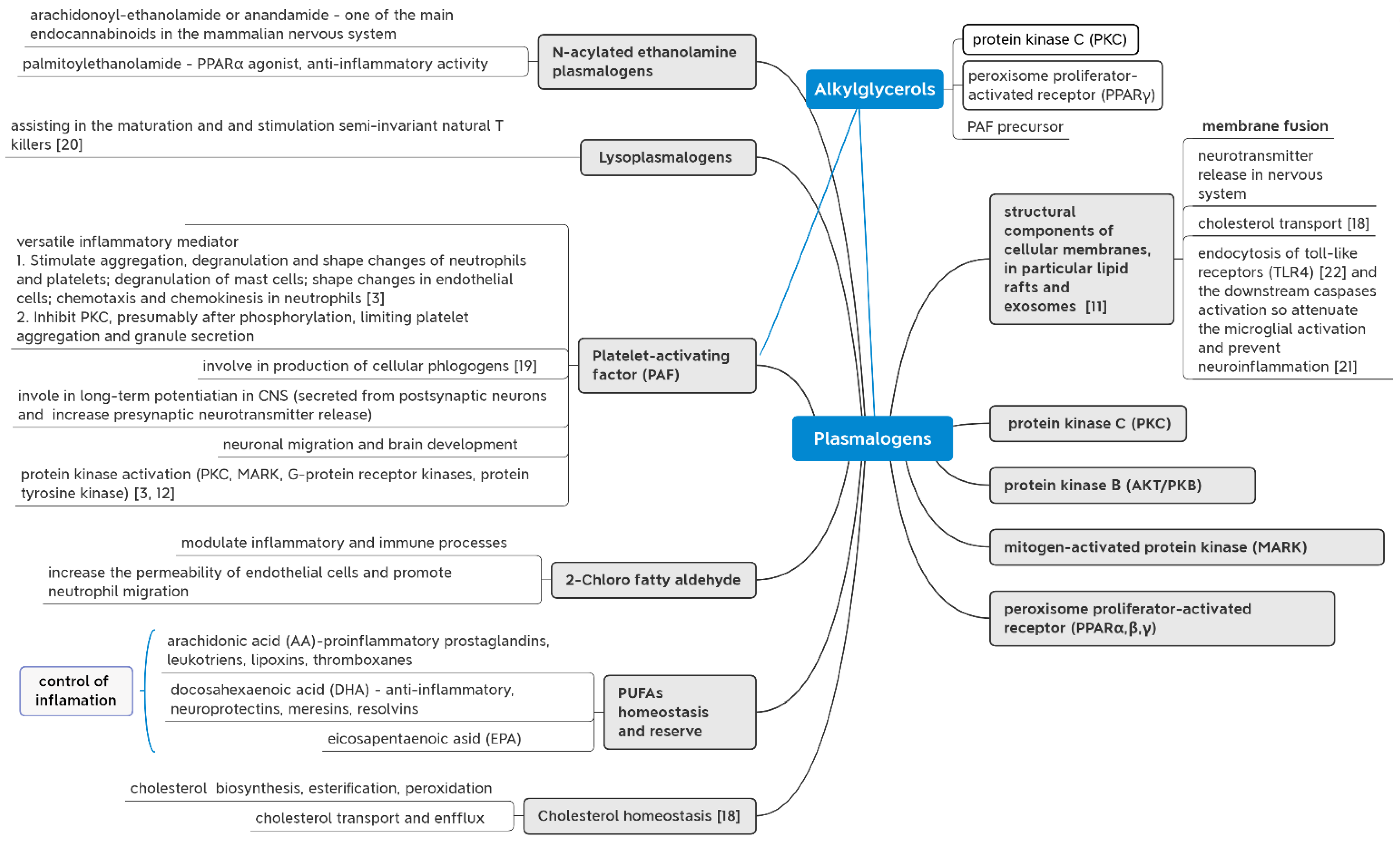
3. Mechanisms of AGs Action
4. Oxidative Stress
5. Aging
6. Nerve System
7. Hematopoiesis, Immunity and Inflammation
8. Other Properties of AGs
9. Conclusions
- AGs prevent many of the negative effects of aging.
- Plasmalogens are regulators of the normal activity of the nervous tissue and the brain as a whole and prevent the development of dementias.
- AGs stimulate hematopoiesis, which contributes to the body adaptation to various conditions, including extreme ones.
- AGs improve the body immune status.
- A novel approach to AGs as adaptogens seems promising and creates multiple opportunities for their potential application.
10. Outlooks
Supplementary Materials
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iannitti, T.; Palmieri, B. An update on the therapeutic role of alkylglycerols. Mar. Drugs 2010, 8, 2267–2300. [Google Scholar] [CrossRef]
- Deniau, A.-L.; Mosset, P.; Pédrono, F.; Mitre, R.; Le Bot, D.; Legrand, A.B. Multiple Beneficial Health Effects of Natural Alkylglycerols from Shark Liver Oil. Mar. Drugs 2010, 8, 2175–2184. [Google Scholar] [CrossRef]
- Snyder, F.; Lee, T.; Wykle, R. Ether-linked lipids and their bioactive species. In Biochemistry of Lipids, Lipoproteins and Membranes; Vance, D.E., Vance, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2002; pp. 233–262. [Google Scholar] [CrossRef]
- Bakes, M.J.; Nichols, P.D. Lipid, fatty acid and squalene composition of liver oil from six species of deep-sea sharks collected in southern Australian waters. Comp. Biochem. Physiol. B 1995, 110, 267–275. [Google Scholar] [CrossRef]
- Magnusson, C.D.; Haraldsson, G.G. Ether lipids. Chem. Phys. Lipids 2011, 164, 315–340. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Kishimura, H. Amount and composition of diacyl glyceryl ethers in various tissue lipids of the deep-sea squid Berryteuthis magister. J. Oleo Sci. 2002, 51, 523–529. [Google Scholar] [CrossRef][Green Version]
- Hayashi, K.; Kishimura, H. Content and composition of diacyl glyceryl ethers in the pyloric ceca and ovaries of the asteroids Solaster paxillatus and Asterias amurensis. Fish. Sci. 1997, 63, 945–949. [Google Scholar] [CrossRef]
- Phleger, C.; Nichols, P.D.; Virtue, P. Lipids and buoyancy in southern ocean pteropods. Lipids 1997, 32, 1093–1100. [Google Scholar] [CrossRef]
- Poleschuk, T.S.; Sultanov, R.M.; Ermolenko, E.V.; Shulgina, L.V.; Kasyanov, S.P. Protective action of alkylglycerols under stress. Stress 2020, 23, 213–220. [Google Scholar] [CrossRef]
- Sultanov, R.M.; Ermolenko, E.V.; Poleschuk, T.S.; Denisenko, Y.K.; Kasyanov, S.P. Action of alkyl glycerol ethers and n-3 polyunsaturated fatty acids diet on hematological parameters of blood and liver plasmalogen level in aged rats. J. Food Sci. 2021, 86, 2727–2735. [Google Scholar] [CrossRef]
- Pugliese, P.T.; Jordan, K.; Cederberg, H.; Brohult, J. Some biological actions of alkylglycerols from shark liver oil. J. Altern. Complement. Med. 1998, 4, 87–99. [Google Scholar] [CrossRef]
- Dorninger, F.; Forss-Petter, S.; Wimmerb, I.; Berger, J. Plasmalogens, platelet-activating factor and beyond—Ether lipids in signaling and neurodegeneration. Neurobiol. Dis. 2020, 145, 105061. [Google Scholar] [CrossRef] [PubMed]
- Pedrono, F.; Khan, N.; Legrand, A. Regulation of calcium signalling by 1-O-alkylglycerols in human Jurkat T lymphocytes. Life Sci. 2004, 74, 2793–2801. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.E.; Park, H.S.; Yoo, H.J.; Baek, I.-J.; Yoon, J.E.; Ko, M.S.; Kim, A.-R.; Kim, R.S.; Park, H.-S.; Lee, S.E.; et al. Protective role of endogenous plasmalogens against hepatic steatosis and steatohepatitis. Hepatology 2017, 66, 416–431. [Google Scholar] [CrossRef]
- Wynalda, K.M.; Murphy, R.C. Low concentration ozone reacts with plasmalogen glycerophosphoethanolamine lipids in lung surfactant. Chem. Res. Toxicol. 2010, 23, 108–117. [Google Scholar] [CrossRef]
- Broniec, A.; Klosinski, R.; Pawlak, A.; Wrona-Krol, M.; Thompson, D.; Sarna, T. Interactions of plasmalogens and their diacyl analogs with singlet oxygen in selected model systems. Free Radic. Biol. Med. 2011, 50, 892–898. [Google Scholar] [CrossRef] [PubMed]
- Honsho, M.; Fujiki, Y. Plasmalogen homeostasis—Regulation of plasmalogen biosynthesis and its physiological consequence in mammals. FEBS Lett. 2017, 591, 2720–2729. [Google Scholar] [CrossRef]
- Paul, S.; Lancaster, G.I.; Meikle, P.J. Plasmalogens: A potential therapeutic target for neurodegenerative and cardiometabolic disease. Prog. Lipid Res. 2019, 74, 186–195. [Google Scholar] [CrossRef]
- Kulikov, V.I.; Muzy, G. Bioregulatory role of platelet activating factor in intracellular processes and cell-cell interactions. Biochemistry 1998, 63, 57–66. [Google Scholar]
- Facciotti, F.; Ramanjaneyulu, G.S.; Lepore, M.; Sansano, S.; Cavallari, M.; Kistowska, M.; Forss-Petter, S.; Ni, G.; Colone, A.; Singhal, A.; et al. Peroxisome-derived lipids are self antigens that stimulate invariant natural killer T cells in the thymus. Nat. Immunol. 2012, 13, 474–480. [Google Scholar] [CrossRef]
- Tyrtyshnaia, A.; Manzhulo, I.; Sultanov, R.M.; Ermolenko, E.V. Adult hippocampal neurogenesis in neuropathic pain and alkyl glycerol ethers treatment. Acta Histochem. 2017, 119, 812–821. [Google Scholar] [CrossRef]
- Ali, F.; Hossain, S.; Sejimo, S.; Akashi, K. Plasmalogens inhibit endocytosis of toll-like receptor 4 to attenuate the inflammatory signal in microglial cells. Mol. Neurobiol. 2019, 56, 3404–3419. [Google Scholar] [CrossRef]
- Braverman, N.E.; Moser, A.B. Functions of plasmalogen lipids in health and disease. Biochim. Biophys. Acta 2012, 1822, 1442–1452. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, C.M.; Yang, K.; Liu, G.; Moon, S.H.; Dilthey, B.G.; Gross, R.W. Cytochrome C is an oxidative stress-activated plasmalogenase that cleaves plasmenylcholine and plasmenylethanolamine at the sn-1vinyl ether linkage. J. Biol. Chem. 2018, 293, 8693–8709. [Google Scholar] [CrossRef] [PubMed]
- Zoeller, R.A.; Morand, O.H.; Raetz, C.R. A possible role for plasmalogens in protecting animal cells against photosensitized killing. J. Biol. Chem. 1988, 263, 11590–11596. [Google Scholar] [CrossRef] [PubMed]
- Karaman, Y.K.; Novgorodtseva, T.P.; Gvozdenko, T.A.; Kasyanov, S.P. Effect of 1-O-Alkylglycerols from sea hydrobionts on the metabolic status of rats with alimentary dyslipidemia. Funct. Foods Health Dis. 2013, 3, 103–110. [Google Scholar] [CrossRef]
- Dean, J.M.; Lodhi, I.J. Structural and functional roles of ether lipids. Protein Cell 2018, 9, 196–206. [Google Scholar] [CrossRef]
- Naudí, A.; Jové, M.; Ayala, V.; Portero-Otín, M.; Barja, G.; Pamplona, R. Membrane lipid unsaturation as physiological adaptation to animal longevity. Front. Physiol. 2013, 4, 372–385. [Google Scholar] [CrossRef]
- Pradas, I.; Jové, M.; Huynh, K.; Puig, J.; Ingles, M.; Borras, C.; Vicña, J.; Meikle, P.J.; Pamplona, R. Exceptional human longevity is associated with a specific plasma phenotype of ether lipids. Redox Biol. 2019, 21, 101–127. [Google Scholar] [CrossRef]
- Blank, M.L.; Cress, E.A.; Smith, Z.L.; Snyder, F. Meats and fish consumed in the American diet contain substantial amounts of ether-linked phospholipids. J. Nutr. 1992, 122, 1656–1661. [Google Scholar] [CrossRef]
- Wood, P.L.; Khan, M.A.; Mankidy, R.; Smith, T.; Goodenowe, D.B. Plasmalogen deficit: A new and testable hypothesis for the etiology of Alzheimer’s disease. In Alzheimer’s Disease Pathogenesis-Core Concepts, Shifting Paradigms and Therapeutic Targets; De La Monte, S., Ed.; IntechOpen: London, UK, 2011; pp. 561–588. [Google Scholar] [CrossRef]
- Chen, S.; Liu, C. Ether glycerophospholipids and their potential as therapeutic agents. Curr. Org. Chem. 2013, 17, 802–811. [Google Scholar] [CrossRef]
- Perichon, R.; Bourre, J.M.; Kelly, J.F.; Roth, G. The role of peroxisomes in aging. Cell Mol. Life Sci. 1998, 54, 641–652. [Google Scholar] [CrossRef] [PubMed]
- Goodenowe, D.B.; Cook, L.L.; Liu, J.; Lu, J.L.Y.; Jayasinghe, D.A.; Ahiahonu, W.K.; Heath, D.; Yamazaki, Y.; Flax, J.; Krenitsky, K.F.; et al. Peripheral ethanolamine plasmalogen deficiency: A logical causative factor in Alzheimer’s disease and dementia. J. Lipid Res. 2007, 48, 2485–2498. [Google Scholar] [CrossRef] [PubMed]
- Cunnane, S.C.; Chouinard-Watkins, R.; Castellano, C.A.; Barberger-Gateau, P. Docosahexaenoic acid homeostasis, brain aging and Alzheimer’s disease: Can we reconcile the evidence? Prostaglandins Leukot. Essent. Fat. Acids 2013, 88, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Lizard, G.; Rouaud, O.; Demarquoy, J.; Cherkaoui-Malki, M.; Iuliano, L. Potential roles of peroxisomes in Alzheimer’s disease and in dementia of the Alzheimer’s type. J. Alzheimers Dis. 2012, 29, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Zhanga, L.; Shia, H.; Xuea, C.; Yanagitac, T.; Zhanga, T.; Wanga, Y. EPA-enriched ethanolamine plasmalogen alleviates atherosclerosis via mediating bile acids metabolism. J. Funct. Foods 2020, 66, 103824. [Google Scholar] [CrossRef]
- Zhou, Y.; Yu, N.; Zhao, J.; Xie, Z.; Yang, Z.; Tian, B. Advances in the Biosynthetic Pathways and Application Potential of Plasmalogens in Medicine. Front. Cell Dev. Biol. 2020, 8, 765. [Google Scholar] [CrossRef]
- Latyshev, N.A.; Kasyanov, S.P.; Blinov, Y.G. Alkyl glycerol ethers of marine organisms: Structure, distribution, and biological activity. Izv. TINRO 2012, 169, 261–277. [Google Scholar]
- Reznichenko, A.; Korstanje, R. The Role of platelet-activating factor in mesangial pathophysiology. Am. J. Pathol. 2015, 185, 888e896. [Google Scholar] [CrossRef]
- Mitre, R.; Etienne, M.; Martinais, S.; Salmon, H.; Allaume, P.; Legrand, P.; Legrand, A.B. Humoral defence improvement and haematopoiesis stimulation in sows and offspring by oral supply of shark-liver oil to mothers during gestation and lactation. Br. J. Nutr. 2005, 94, 753–762. [Google Scholar] [CrossRef]
- Osmond, D.G.; Roylance, P.J.; Webb, A.J.; Yoffey, J.M. The action of batyl alcohol and selachyl alcohol on the bone marrow of the guinea pig. Acta Haemat. 1963, 29, 180–186. [Google Scholar] [CrossRef]
- Pedrono, F.; Cheminade, C.; Legrand, A. Natural 1-O-alkylglycerols reduce platelet-activating factor-induced release of [3H]-serotonin in rabbit platelets. Prostaglandins Leukot. Essent. Fat. Acids 2004, 71, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, B.; Pennelli, A.; Di Cerbo, A. Jurassic surgery and immunity enhancement by alkyglycerols of shark liver oil. Lipids Health Dis. 2014, 13, 178. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Zhang, M.; Wu, S.; Zhong, Y.; Van Tol, E.; Cai, W. Alkylglycerols modulate the proliferation and differentiation of non-specific agonist and specific antigen-stimulated splenic lymphocytes. PLoS ONE 2014, 9, e96207. [Google Scholar] [CrossRef] [PubMed]
- Manzhulo, I.V.; Tyrtyshnaia, A.A.; Mischenko, P.V.; Egoraeva, A.; Belova, A.S.; Kasyanov, S.P.; Sultanov, R.M.; Pislyagin, E.A. Alkyl glycerols activate RAW264.7 macrophage cell line. Nat. Prod. Commun. 2019, 2019, 1934578X19858516. [Google Scholar] [CrossRef]
- Vitorino, D.C.; Buzzachera, C.F.; Curi, R.; Fernandes, L.C. Effect of chronic supplementation with shark liver oil on immune responses of exercise-trained rats. Eur. J. Appl. Physiol. 2010, 108, 1125–1132. [Google Scholar] [CrossRef]
- Benzoni, G.; Foresti, F.; Archetti, I.L.; Coceva, G.; Guyonvarch, A.; Alborali, L. Specific and non-specific immunity of piglets from sows fed diets containing specific fatty acids in field conditions. J. Anim. Physiol. Anim. Nutr. 2013, 97, 996–1005. [Google Scholar] [CrossRef]
- Oh, S.Y.; Jadhav, L.S. Effects of dietary alkylglycerols in lactating rats on immune responses in pups. Pediatr. Res. 1994, 36, 300–305. [Google Scholar] [CrossRef]
- Bozelli, J.C.; Azher, S.; Epand, R.M. Plasmalogens and Chronic Inflammatory Diseases. Front. Physiol. 2021, 12, 730829. [Google Scholar] [CrossRef]
- Samimi, N.; Sepehrimanesh, M.; Koohi-Hosseinabadi, O.; Homayounfar, R.; Mokhtari, M.; Farjam, M. The therapeutic effect of shark liver oil in a rat model of acetic acid-induced ulcerative colitis. Evid.-Based Complement. Altern. Med. 2020, 2020, 2419230. [Google Scholar] [CrossRef]
- Selye, H. The Physiology and Pathology of Exposure to Stress; ACTA, INC.: Monreal, QC, Canada, 1950. [Google Scholar]
- Rahal, A.; Kumar, A.; Singh, V.; Yadav, B.; Tiwari, R.; Chakraborty, S.; Dhama, K. Oxidative stress, prooxidants, and antioxidants: The interplay. BioMed. Res. Int. 2014, 2014, 761264. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sultanov, R.; Ermolenko, E.; Poleshchuk, T.; Kasyanov, S. Alkyl Glycerol Ethers as Adaptogens. Mar. Drugs 2023, 21, 4. https://doi.org/10.3390/md21010004
Sultanov R, Ermolenko E, Poleshchuk T, Kasyanov S. Alkyl Glycerol Ethers as Adaptogens. Marine Drugs. 2023; 21(1):4. https://doi.org/10.3390/md21010004
Chicago/Turabian StyleSultanov, Ruslan, Ekaterina Ermolenko, Tatiana Poleshchuk, and Sergey Kasyanov. 2023. "Alkyl Glycerol Ethers as Adaptogens" Marine Drugs 21, no. 1: 4. https://doi.org/10.3390/md21010004
APA StyleSultanov, R., Ermolenko, E., Poleshchuk, T., & Kasyanov, S. (2023). Alkyl Glycerol Ethers as Adaptogens. Marine Drugs, 21(1), 4. https://doi.org/10.3390/md21010004



