Intratympanic Administration of Dieckol Prevents Ototoxic Hearing Loss
Abstract
1. Introduction
2. Results
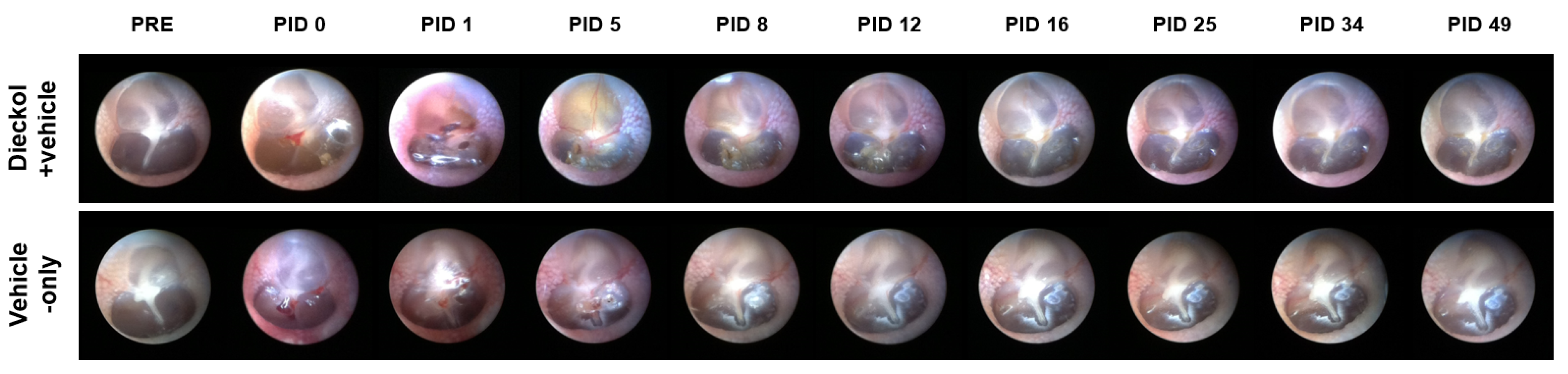
2.1. TM Endoscopy
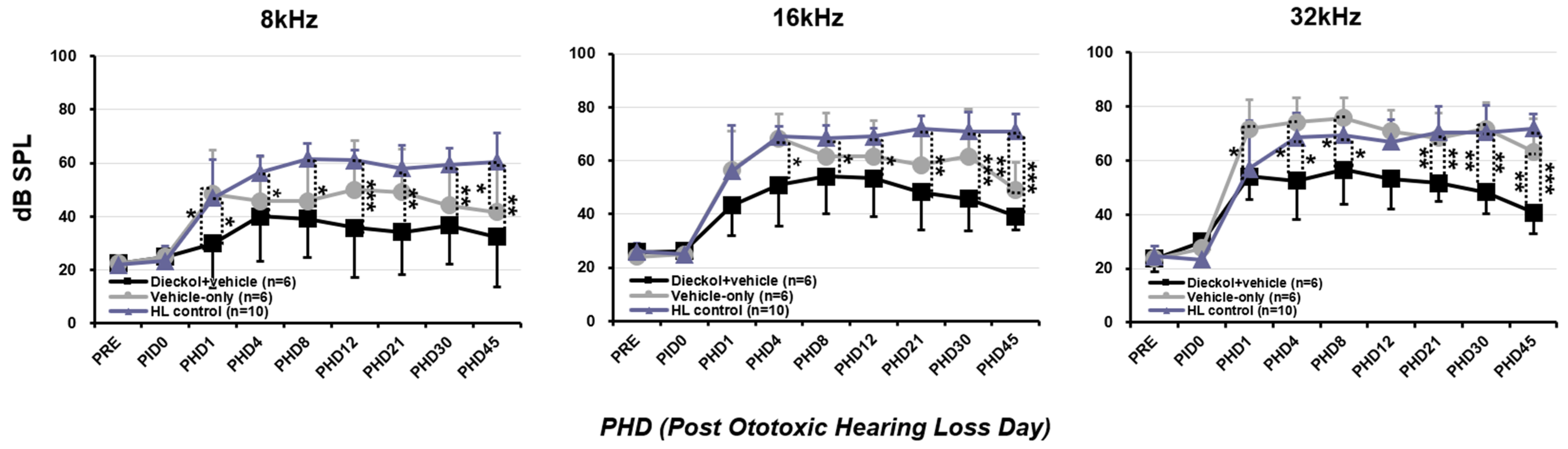
2.2. Hearing Threshold Based on the ABR
2.3. Organ of Corti Surface Preparation and Hair Cell Number
2.4. Middle Ear Histology
3. Discussion
4. Materials and Methods
4.1. Experimental Animals
4.2. IT Drug Delivery
4.3. Induction of Ototoxic Hearing Loss
4.4. Preparation of Dieckol and Vehicle
4.5. Endoscopy of the Tympanic Membrane (TM)
4.6. Assessment of Hearing Threshold Based on the Auditory Brainstem Response
4.7. Organ of Corti Surface Preparation and Hair Cell Enumeration
4.8. Middle Ear Histology
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oeffinger, K.C.; Mertens, A.C.; Sklar, C.A.; Kawashima, T.; Hudson, M.M.; Meadows, A.T.; Friedman, D.L.; Marina, N.; Hobbie, W.; Kadan-Lottick, N.S.; et al. Chronic health conditions in adult survivors of childhood cancer. N. Engl. J. Med. 2006, 355, 1572–1582. [Google Scholar] [CrossRef] [PubMed]
- Freyer, D.R.; Brock, P.R.; Chang, K.W.; Dupuis, L.L.; Epelman, S.; Knight, K.; Mills, D.; Phillips, R.; Potter, E.; Risby, D.; et al. Prevention of cisplatin-induced ototoxicity in children and adolescents with cancer: A clinical practice guideline. Lancet Child Adolesc. Health 2020, 4, 141–150. [Google Scholar] [CrossRef]
- Wei, B.P.; Stathopoulos, D.; O’Leary, S. Steroids for idiopathic sudden sensorineural hearing loss. Cochrane Database Syst. Rev. 2013, 7, CD003998. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekhar, S.S.; Tsai Do, B.S.; Schwartz, S.R.; Bontempo, L.J.; Faucett, E.A.; Finestone, S.A.; Hollingsworth, D.B.; Kelley, D.M.; Kmucha, S.T.; Moonis, G.; et al. Clinical Practice Guideline: Sudden Hearing Loss (Update). Otolaryngol. Head Neck Surg. 2019, 161 (Suppl. S1), S1–S45. [Google Scholar] [CrossRef] [PubMed]
- Shafik, A.G.; Elkabarity, R.H.; Thabet, M.T.; Soliman, N.B.; Kalleny, N.K. Effect of intratympanic dexamethasone administration on cisplatin-induced ototoxicity in adult guinea pigs. Auris Nasus Larynx 2013, 40, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Hill, G.W.; Morest, D.K.; Parham, K. Cisplatin-induced ototoxicity: Effect of intratympanic dexamethasone injections. Otol. Neurotol. 2008, 29, 1005–1011. [Google Scholar] [CrossRef]
- Bird, P.A.; Begg, E.J.; Zhang, M.; Keast, A.T.; Murray, D.P.; Balkany, T.J. Intratympanic versus intravenous delivery of methylprednisolone to cochlear perilymph. Otol. Neurotol. 2007, 28, 1124–1130. [Google Scholar] [CrossRef]
- Yu, D.; Gu, J.; Chen, Y.; Kang, W.; Wang, X.; Wu, H. Current Strategies to Combat Cisplatin-Induced Ototoxicity. Front. Pharmacol. 2020, 11, 999. [Google Scholar] [CrossRef]
- Hazlitt, R.A.; Min, J.; Zuo, J. Progress in the Development of Preventative Drugs for Cisplatin-Induced Hearing Loss. J. Med. Chem. 2018, 61, 5512–5524. [Google Scholar] [CrossRef]
- Callejo, A.; Sedo-Cabezon, L.; Juan, I.D.; Llorens, J. Cisplatin-Induced Ototoxicity: Effects, Mechanisms and Protection Strategies. Toxics 2015, 3, 268–293. [Google Scholar] [CrossRef]
- Dickey, D.T.; Wu, Y.J.; Muldoon, L.L.; Neuwelt, E.A. Protection against cisplatin-induced toxicities by N-acetylcysteine and sodium thiosulfate as assessed at the molecular, cellular, and in vivo levels. J. Pharmacol. Exp. Ther. 2005, 314, 1052–1058. [Google Scholar] [CrossRef] [PubMed]
- Chirtes, F.; Albu, S. Prevention and restoration of hearing loss associated with the use of cisplatin. BioMed Res. Int. 2014, 2014, 925485. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.J.; Muldoon, L.L.; Neuwelt, E.A. The chemoprotective agent N-acetylcysteine blocks cisplatin-induced apoptosis through caspase signaling pathway. J. Pharmacol. Exp. Ther. 2005, 312, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Choe, W.T.; Chinosornvatana, N.; Chang, K.W. Prevention of cisplatin ototoxicity using transtympanic N-acetylcysteine and lactate. Otol. Neurotol. 2004, 25, 910–915. [Google Scholar] [CrossRef]
- Haq, S.H.; Al-Ruwaished, G.; Al-Mutlaq, M.A.; Naji, S.A.; Al-Mogren, M.; Al-Rashed, S.; Ain, Q.T.; Al-Amro, A.A.; Al-Mussallam, A. Antioxidant, anticancer activity and phytochemical analysis of green algae, Chaetomorpha collected from the Arabian Gulf. Sci. Rep. 2019, 9, 18906. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Lee, D.Y.; Jung, W.K.; Kim, J.H.; Choi, I.; Park, S.G.; Seo, S.K.; Lee, S.W.; Lee, C.M.; Yea, S.S.; et al. Effects of Ecklonia cava ethanolic extracts on airway hyperresponsiveness and inflammation in a murine asthma model: Role of suppressor of cytokine signaling. Biomed. Pharmacother. 2008, 62, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fu, X.; Duan, D.; Liu, X.; Xu, J.; Gao, X. Extraction and Identification of Phlorotannins from the Brown Alga, Sargassum fusiforme (Harvey) Setchell. Mar. Drugs 2017, 15, 49. [Google Scholar] [CrossRef]
- Athukorala, Y.; Jung, W.-K.; Vasanthan, T.; Jeon, Y.-J. An anticoagulative polysaccharide from an enzymatic hydrolysate of Ecklonia cava. Carbohydr. Polym. 2006, 66, 184–191. [Google Scholar] [CrossRef]
- Li, Y.; Qian, Z.-J.; Ryu, B.; Lee, S.-H.; Kim, M.-M.; Kim, S.-K. Chemical components and its antioxidant properties in vitro: An edible marine brown alga, Ecklonia cava. Bioorg. Med. Chem. 2009, 17, 1963–1973. [Google Scholar] [CrossRef]
- Kang, I.-J.; Jeon, Y.E.; Yin, X.F.; Nam, J.-S.; You, S.G.; Hong, M.S.; Jang, B.G.; Kim, M.-J. Butanol extract of Ecklonia cava prevents production and aggregation of beta-amyloid, and reduces beta-amyloid mediated neuronal death. Food Chem. Toxicol. 2011, 49, 2252–2259. [Google Scholar] [CrossRef]
- Yoon, J.-S.; Yadunandam, A.K.; Kim, S.-J.; Woo, H.-C.; Kim, H.-R.; Kim, G.-D. Dieckol, isolated from Ecklonia stolonifera, induces apoptosis in human hepatocellular carcinoma Hep3B cells. J. Nat. Med. 2013, 67, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.-J.; Park, J.-H.; Lee, B.H.; Chee, H.Y.; Lee, K.B.; Oh, S.-M. Suppression of NF-κB by dieckol extracted from Ecklonia cava negatively regulates LPS induction of inducible nitric oxide synthase gene. Appl. Biochem. Biotechnol. 2014, 173, 957–967. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.A.; Jung, H.J.; Jeong, H.Y.; Kwon, H.J.; Ali, M.Y.; Choi, J.S. Phlorotannins isolated from the edible brown alga Ecklonia stolonifera exert anti-adipogenic activity on 3T3-L1 adipocytes by downregulating C/EBPα and PPARγ. Fitoterapia 2014, 92, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.Y.; Han, S.Y.; Shin, H.-C.; Byun, J.Y.; Rah, Y.C.; Park, M.K. Protective effect of a purified polyphenolic extract from Ecklonia cava against noise-induced hearing loss: Prevention of temporary threshold shift. Int. J. Pediatr. Otorhinolaryngol. 2016, 87, 178–184. [Google Scholar] [CrossRef]
- Woo, H.; Kim, M.-K.; Park, S.; Han, S.-H.; Shin, H.-C.; Kim, B.-g.; Oh, S.-H.; Suh, M.-W.; Lee, J.-H.; Park, M.-K. Effect of Phlorofucofuroeckol A and Dieckol Extracted from Ecklonia cava on Noise-induced Hearing Loss in a Mouse Model. Mar. Drugs 2021, 19, 443. [Google Scholar] [CrossRef]
- Huang, T.; Cheng, A.G.; Stupak, H.; Liu, W.; Kim, A.; Staecker, H.; Lefebvre, P.P.; Malgrange, B.; Kopke, R.; Moonen, G.; et al. Oxidative stress-induced apoptosis of cochlear sensory cells: Otoprotective strategies. Int. J. Dev. Neurosci. 2000, 18, 259–270. [Google Scholar] [CrossRef]
- Balaban, R.S.; Nemoto, S.; Finkel, T. Mitochondria, oxidants, and aging. Cell 2005, 120, 483–495. [Google Scholar] [CrossRef]
- Mangiardi, D.A.; McLaughlin-Williamson, K.; May, K.E.; Messana, E.P.; Mountain, D.C.; Cotanche, D.A. Progression of hair cell ejection and molecular markers of apoptosis in the avian cochlea following gentamicin treatment. J. Comp. Neurol. 2004, 475, 1–18. [Google Scholar] [CrossRef]
- Coffin, A.B.; Rubel, E.W.; Raible, D.W. Bax, Bcl2, and p53 differentially regulate neomycin- and gentamicin-induced hair cell death in the zebrafish lateral line. J. Assoc. Res. Otolaryngol. 2013, 14, 645–659. [Google Scholar] [CrossRef]
- Sun, S.; Yu, H.; Yu, H.; Honglin, M.; Ni, W.; Zhang, Y.; Guo, L.; He, Y.; Xue, Z.; Ni, Y.; et al. Inhibition of the activation and recruitment of microglia-like cells protects against neomycin-induced ototoxicity. Mol. Neurobiol. 2015, 51, 252–267. [Google Scholar] [CrossRef]
- Liu, L.; Chen, Y.; Qi, J.; Zhang, Y.; He, Y.; Ni, W.; Li, W.; Zhang, S.; Sun, S.; Taketo, M.M.; et al. Wnt activation protects against neomycin-induced hair cell damage in the mouse cochlea. Cell Death Dis. 2016, 7, e2136. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Chen, Z.; Su, K.; Yin, S.; Wang, J. Comparison of cochlear cell death caused by cisplatin, alone and in combination with furosemide. Toxicol. Pathol. 2014, 42, 376–385. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, J.H.; Kim, S.J.; Oh, G.S.; Moon, H.D.; Kwon, K.B.; Park, C.; Park, B.H.; Lee, H.K.; Chung, S.Y.; et al. Roles of NADPH oxidases in cisplatin-induced reactive oxygen species generation and ototoxicity. J. Neurosci. 2010, 30, 3933–3946. [Google Scholar] [CrossRef] [PubMed]
- Sha, S.H.; Schacht, J. Antioxidants attenuate gentamicin-induced free radical formation in vitro and ototoxicity in vivo: D-methionine is a potential protectant. Hear. Res. 2000, 142, 34–40. [Google Scholar] [CrossRef]
- Kawamoto, K.; Sha, S.H.; Minoda, R.; Izumikawa, M.; Kuriyama, H.; Schacht, J.; Raphael, Y. Antioxidant gene therapy can protect hearing and hair cells from ototoxicity. Mol. Ther. 2004, 9, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Park, C.; Han, A.L.; Youn, M.J.; Lee, J.H.; Kim, Y.; Kim, E.S.; Kim, H.J.; Kim, J.K.; Lee, H.K.; et al. Ebselen attenuates cisplatin-induced ROS generation through Nrf2 activation in auditory cells. Hear. Res. 2009, 251, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.; Qi, W.; Yu, D.; Jiang, H.; Han, C.; Kim, M.J.; Katsuno, K.; Hsieh, Y.H.; Miyakawa, T.; Salvi, R.; et al. Addition of exogenous NAD+ prevents mefloquine-induced neuroaxonal and hair cell degeneration through reduction of caspase-3-mediated apoptosis in cochlear organotypic cultures. PLoS ONE 2013, 8, e79817. [Google Scholar] [CrossRef]
- Kang, M.C.; Kang, S.M.; Ahn, G.; Kim, K.N.; Kang, N.; Samarakoon, K.W.; Oh, M.C.; Lee, J.S.; Jeon, Y.J. Protective effect of a marine polyphenol, dieckol against carbon tetrachloride-induced acute liver damage in mouse. Environ. Toxicol. Pharmacol. 2013, 35, 517–523. [Google Scholar] [CrossRef]
- Lee, S.H.; Han, J.S.; Heo, S.J.; Hwang, J.Y.; Jeon, Y.J. Protective effects of dieckol isolated from Ecklonia cava against high glucose-induced oxidative stress in human umbilical vein endothelial cells. Toxicol. In Vitro 2010, 24, 375–381. [Google Scholar] [CrossRef]
- Yang, E.-J.; Moon, J.-Y.; Kim, M.-J.; Kim, D.S.; Kim, C.-S.; Lee, W.J.; Lee, N.H.; Hyun, C.-G. Inhibitory effect of Jeju endemic seaweeds on the production of pro-inflammatory mediators in mouse macrophage cell line RAW 264.7. J. Zhejiang Univ. Sci. B 2010, 11, 315–322. [Google Scholar] [CrossRef]
- Lopes, G.; Sousa, C.; Silva, L.R.; Pinto, E.; Andrade, P.B.; Bernardo, J.; Mouga, T.; Valentão, P. Can phlorotannins purified extracts constitute a novel pharmacological alternative for microbial infections with associated inflammatory conditions? PLoS ONE 2012, 7, e31145. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.R.; Shin, T.S.; Lee, M.S.; Park, J.Y.; Park, K.E.; Yoon, N.Y.; Kim, J.S.; Choi, J.S.; Jang, B.C.; Byun, D.S.; et al. Isolation and identification of phlorotannins from Ecklonia stolonifera with antioxidant and anti-inflammatory properties. J. Agric. Food Chem. 2009, 57, 3483–3489. [Google Scholar] [CrossRef]
- Yotsu-Yamashita, M.; Kondo, S.; Segawa, S.; Lin, Y.C.; Toyohara, H.; Ito, H.; Konoki, K.; Cho, Y.; Uchida, T. Isolation and structural determination of two novel phlorotannins from the brown alga Ecklonia kurome Okamura, and their radical scavenging activities. Mar. Drugs 2013, 11, 165–183. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.-K.; Heo, S.-J.; Jeon, Y.-J.; Lee, C.-M.; Park, Y.-M.; Byun, H.-G.; Choi, Y.H.; Park, S.-G.; Choi, I.-W. Inhibitory effects and molecular mechanism of dieckol isolated from marine brown alga on COX-2 and iNOS in microglial cells. J. Agric. Food Chem. 2009, 57, 4439–4446. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Dellamary, L.; Fernandez, R.; Ye, Q.; LeBel, C.; Piu, F. Principles of inner ear sustained release following intratympanic administration. Laryngoscope 2011, 121, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Shi, X. Pathophysiology of the cochlear intrastrial fluid-blood barrier. Hear. Res. 2016, 338, 52–63. [Google Scholar] [CrossRef]
- Cho, J.-A.; Kim, B.J.; Hwang, Y.-J.; Woo, S.-W.; Noh, T.-S.; Suh, M.-W. Effect and Biocompatibility of a Cross-Linked Hyaluronic Acid and Polylactide-co-glycolide Microcapsule Vehicle in Intratympanic Drug Delivery for Treating Acute Acoustic Trauma. Int. J. Mol. Sci. 2021, 22, 5720. [Google Scholar] [CrossRef]
- Park, M.; Hwang, Y.J.; Noh, T.S.; Woo, S.W.; Park, J.H.; Park, S.H.; Kim, M.S.; Suh, M.W. Biocompatibility and Therapeutic Effect of 3 Intra-Tympanic Drug Delivery Vehicles in Acute Acoustic Trauma. Audiol. Neurootol. 2020, 25, 291–296. [Google Scholar] [CrossRef]
- Li, H.; Suh, M.W.; Oh, S.H. Dual Viscosity Mixture Vehicle for Intratympanic Dexamethasone Delivery Can Block Ototoxic Hearing Loss. Front. Pharmacol. 2021, 12, 701002. [Google Scholar] [CrossRef]
- Sadreev, I.I.; Burwood, G.W.S.; Flaherty, S.M.; Kim, J.; Russell, I.J.; Abdullin, T.I.; Lukashkin, A.N. Drug Diffusion Along an Intact Mammalian Cochlea. Front. Cell. Neurosci. 2019, 13, 161. [Google Scholar] [CrossRef]
- Plontke, S.K.; Biegner, T.; Kammerer, B.; Delabar, U.; Salt, A.N. Dexamethasone concentration gradients along scala tympani after application to the round window membrane. Otol. Neurotol. 2008, 29, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Yüksel Aslıer, N.G.; Tağaç, A.A.; Durankaya, S.M.; Çalışır, M.; Ersoy, N.; Kırkım, G.; Yurdakoç, K.; Bağrıyanık, H.A.; Yılmaz, O.; Sütay, S.; et al. Dexamethasone-loaded chitosan-based genipin-cross-linked hydrogel for prevention of cisplatin induced ototoxicity in Guinea pig model. Int. J. Pediatr. Otorhinolaryngol. 2019, 122, 60–69. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Oh, S.H.; Shin, H.-C.; Suh, M.-W. Intratympanic Administration of Dieckol Prevents Ototoxic Hearing Loss. Mar. Drugs 2022, 20, 622. https://doi.org/10.3390/md20100622
Li H, Oh SH, Shin H-C, Suh M-W. Intratympanic Administration of Dieckol Prevents Ototoxic Hearing Loss. Marine Drugs. 2022; 20(10):622. https://doi.org/10.3390/md20100622
Chicago/Turabian StyleLi, Hui, Seung Ha Oh, Hyeon-Cheol Shin, and Myung-Whan Suh. 2022. "Intratympanic Administration of Dieckol Prevents Ototoxic Hearing Loss" Marine Drugs 20, no. 10: 622. https://doi.org/10.3390/md20100622
APA StyleLi, H., Oh, S. H., Shin, H.-C., & Suh, M.-W. (2022). Intratympanic Administration of Dieckol Prevents Ototoxic Hearing Loss. Marine Drugs, 20(10), 622. https://doi.org/10.3390/md20100622






