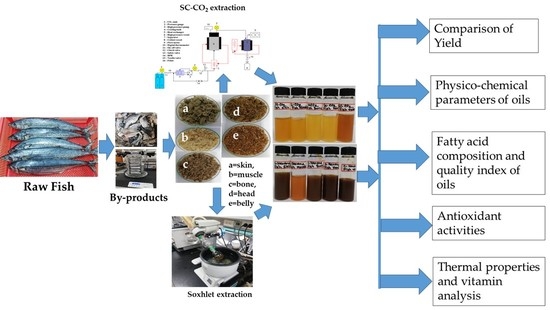
Lipid Indexes and Quality Evaluation of Omega-3 Rich Oil from the Waste of Japanese Spanish Mackerel Extracted by Supercritical CO2
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of the Wastes
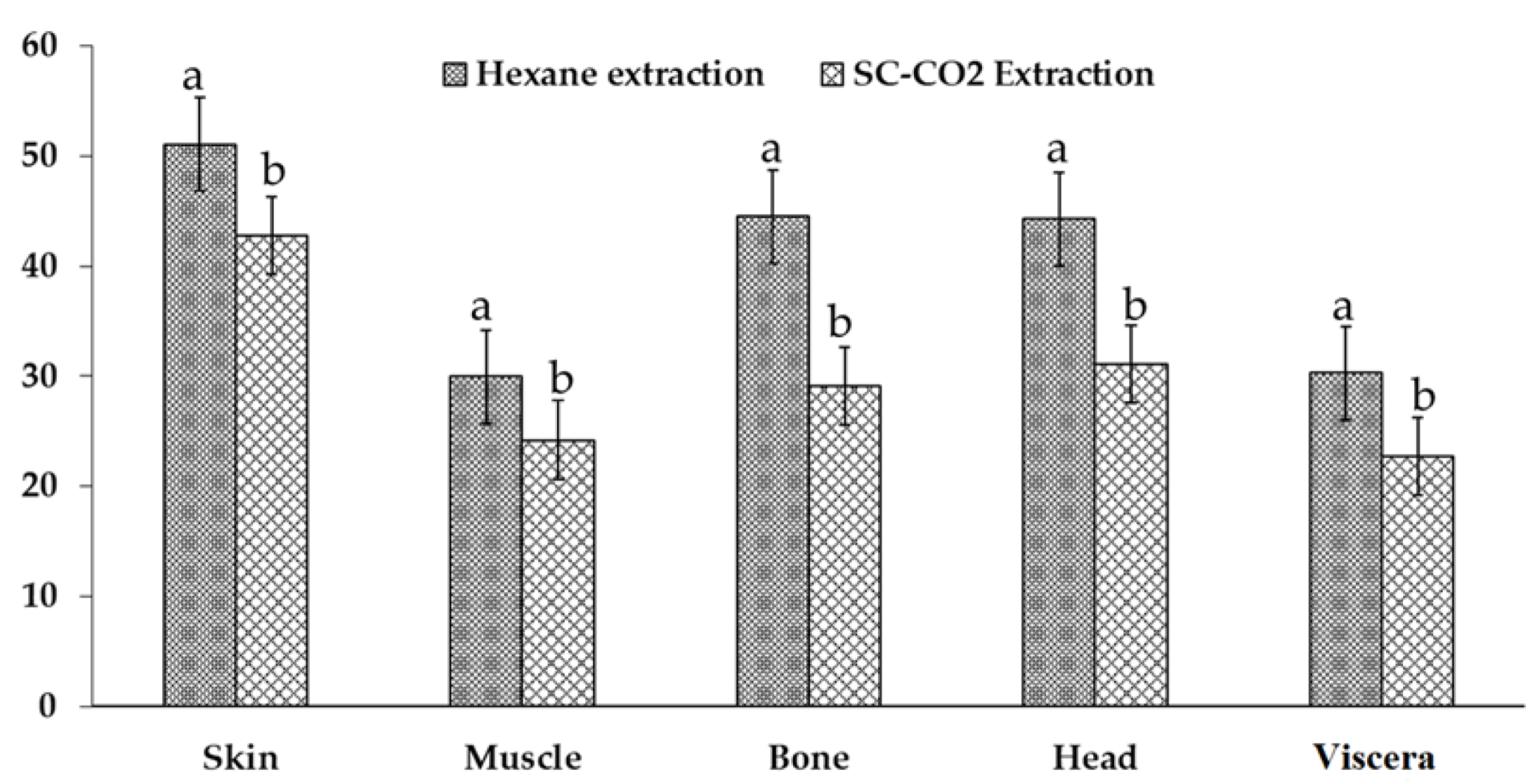
2.2. Comparison of the Yield
2.3. Colour and Viscosity
2.4. Oxidative Quality of the Oils
2.5. Antioxidant Activities
2.5.1. ABTS+ Radical Scavenging Activity
2.5.2. DPPH Radical Scavenging Activity
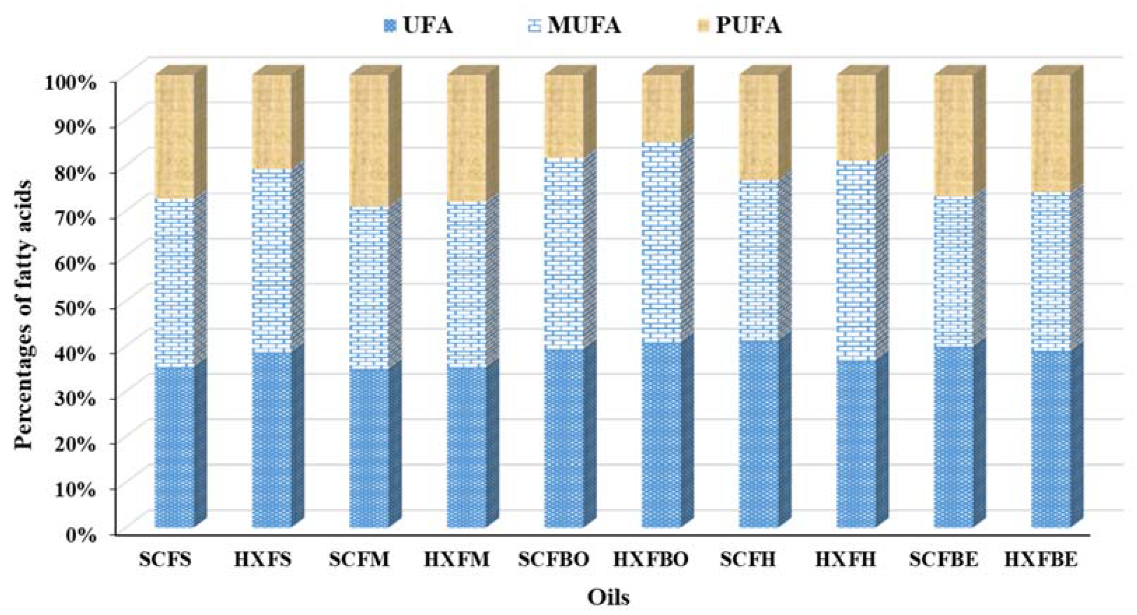
2.6. Fatty Acid Composition of the Oils
2.7. Lipid Quality of the Extracted Oils
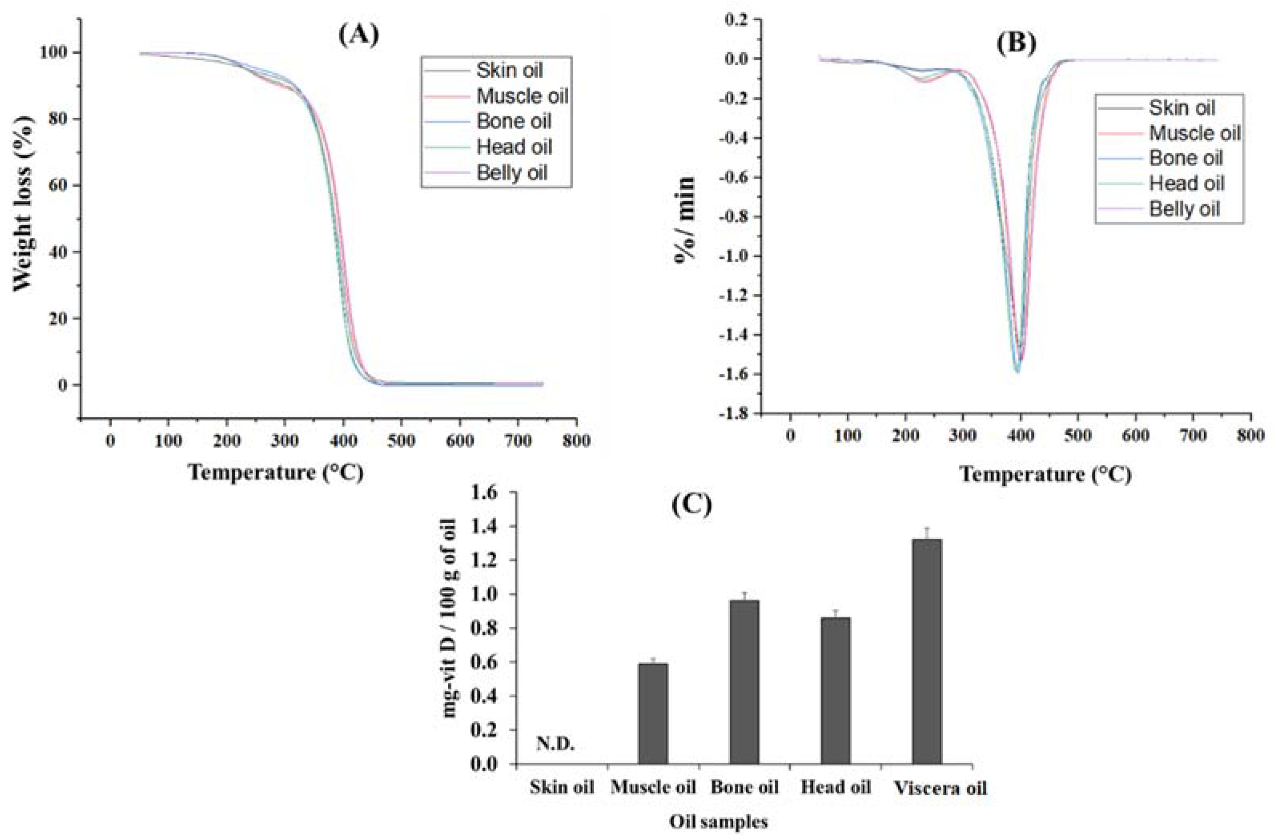
2.8. Thermal Property of the Oil
2.9. Analysis of Vitamin D
3. Materials and Methods
3.1. Sample Preparation
3.2. Reagents and Chemicals
3.3. Characterization of the Raw Materials
3.4. Soxhlet Extraction
3.5. Supercritical Carbon Dioxide Extraction
3.6. Quality Properties of Oils
3.6.1. Colour and Viscosity
3.6.2. Oxidative Quality of Oils
3.7. Antioxidant Activities
3.7.1. ABTS+ Scavenging Activity
3.7.2. DPPH Radical Scavenging Activity
3.8. Fatty Acid Analysis
3.9. Quality Indexes of Oils
3.10. Vitamin-D Analysis
3.11. Thermogravimetric Analysis (TGA)
3.12. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yuvaraj, D.; Bharathiraja, B.; Rithika, J.; Dhanasree, S.; Ezhilarasi, V.; Lavanya, A.; Praveenkumar, R. Production of biofuels from fish wastes: An overview. Biofuels 2019, 10, 301–307. [Google Scholar] [CrossRef]
- Kim, S.-K.; Mendis, E. Bioactive compounds from marine processing byproducts—A review. Food Res. Int. 2006, 39, 383–393. [Google Scholar] [CrossRef]
- Franklin, E.C.; Haq, M.; Roy, V.C.; Park, J.; Chun, B. Supercritical CO2 extraction and quality comparison of lipids from Yellowtail fish (Seriola quinqueradiata) waste in different conditions. J. Food Process. Preserv. 2020, 44, e14892. [Google Scholar] [CrossRef]
- Park, J.; Kim, S.; Lee, S.; Jeong, Y.; Roy, V.C.; Rizkyana, A.D.; Chun, B. Edible oil extracted from anchovies using supercritical CO 2: Availability of fat-soluble vitamins and comparison with commercial oils. J. Food Process. Preserv. 2021, 45, e15441. [Google Scholar] [CrossRef]
- Roy, V.C.; Getachew, A.T.; Cho, Y.-J.; Park, J.-S.; Chun, B.-S. Recovery and bio-potentialities of astaxanthin-rich oil from shrimp (Penaeus monodon) waste and mackerel (Scomberomous niphonius) skin using concurrent supercritical CO2 extraction. J. Supercrit. Fluids 2020, 159, 104773. [Google Scholar] [CrossRef]
- Anderson, E.J.; Thayne, K.A.; Harris, M.; Shaikh, S.R.; Darden, T.M.; Lark, D.S.; Williams, J.M.; Chitwood, W.R.; Kypson, A.P.; Rodriguez, E. Do Fish Oil Omega-3 Fatty Acids Enhance Antioxidant Capacity and Mitochondrial Fatty Acid Oxidation in Human Atrial Myocardium via PPARγ Activation? Antioxid. Redox Signal. 2014, 21, 1156–1163. [Google Scholar] [CrossRef]
- Takeungwongtrakul, S.; Benjakul, S.; Santoso, J.; Trilaksani, W.; Nurilmala, M. Extraction and Stability of Carotenoid-Containing Lipids from Hepatopancreas of Pacific White Shrimp (Litopenaeus vannamei). J. Food Process. Preserv. 2015, 39, 10–18. [Google Scholar] [CrossRef]
- Innis, S.M. Dietary (n-3) Fatty Acids and Brain Development. J. Nutr. 2007, 137, 855–859. [Google Scholar] [CrossRef]
- Sahena, F.; Zaidul, I.S.M.; Jinap, S.; Jahurul, M.H.A.; Khatib, A.; Norulaini, N.A.N. Extraction of fish oil from the skin of Indian mackerel using supercritical fluids. J. Food Eng. 2010, 99, 63–69. [Google Scholar] [CrossRef]
- Sahena, F.; Zaidul, I.S.M.; Jinap, S.; Yazid, A.M.; Khatib, A.; Norulaini, N.A.N. Fatty acid compositions of fish oil extracted from different parts of Indian mackerel (Rastrelliger kanagurta) using various techniques of supercritical CO2 extraction. Food Chem. 2010, 120, 879–885. [Google Scholar] [CrossRef]
- Temelli, F.; Leblanc, E.; Fu, L. Supercritical CO2 Extraction of Oil from Atlantic Mackerel (Scomber scombrus) and Protein Functionality. J. Food Sci. 1995, 60, 703–706. [Google Scholar] [CrossRef]
- Li, Z.; Ye, Z.; Wan, R.; Zhang, C. Model selection between traditional and popular methods for standardizing catch rates of target species: A case study of Japanese Spanish mackerel in the gillnet fishery. Fish. Res. 2015, 161, 312–319. [Google Scholar] [CrossRef]
- Kang, Y.M.; Park, S.Y.; Lee, S.G.; Lee, J.S.; Heu, M.S.; Kim, J.-S. Chemical Characterization of Commercial Dark-fleshed Fishes (Mackerel Scomber japonicus, Japanese Spanish mackerel Scomberomorus niphonius, Pacific herring Clupea pallasii) as a Raw Material for Seafood Products. Korean J. Fish. Aquat. Sci. 2017, 50, 130–138. [Google Scholar] [CrossRef][Green Version]
- Leu, S.-S.; Jhaveri, S.N.; Karakoltsidis, P.A.; Constantinides, S.M. Atlantic Mackerel (Scomber scombrus, L): Seasonal Variation in Proximate Composition and Distribution of Chemical Nutrients. J. Food Sci. 1981, 46, 1635–1638. [Google Scholar] [CrossRef]
- Olsen, R.L.; Toppe, J.; Karunasagar, I. Challenges and realistic opportunities in the use of by-products from processing of fish and shellfish. Trends Food Sci. Technol. 2014, 36, 144–151. [Google Scholar] [CrossRef]
- Haq, M.; Ahmed, R.; Cho, Y.-J.; Chun, B.-S. Quality Properties and Bio-potentiality of Edible Oils from Atlantic Salmon By-products Extracted by Supercritial Carbon Dioxide and Conventional Methods. Waste Biomass Valoriz. 2017, 8, 1953–1967. [Google Scholar] [CrossRef]
- Ivanovs, K.; Blumberga, D. Extraction of fish oil using green extraction methods: A short review. Energy Procedia 2017, 128, 477–483. [Google Scholar] [CrossRef]
- Getachew, A.T.; Saravana, P.S.; Cho, Y.J.; Woo, H.C.; Chun, B.S. Concurrent extraction of oil from roasted coffee (Coffea arabica) and fucoxanthin from brown seaweed (Saccharina japonica) using supercritical carbon dioxide. J. CO2 Util. 2018, 25, 137–146. [Google Scholar] [CrossRef]
- Zhang, C.; Ye, Z.; Li, Z.; Wan, R.; Ren, Y.; Dou, S. Population structure of Japanese Spanish mackerel Scomberomorus niphonius in the Bohai Sea, the Yellow Sea and the East China Sea: Evidence from random forests based on otolith features. Fish. Sci. 2016, 82, 251–256. [Google Scholar] [CrossRef]
- Alfio, V.G.; Manzo, C.; Micillo, R. From Fish Waste to Value: An Overview of the Sustainable Recovery of Omega-3 for Food Supplements. Molecules 2021, 26, 1002. [Google Scholar] [CrossRef]
- Bonilla, J.R.; Hoyos Concha, J.L. Métodos de extracción, refinación y concentración de aceite de pescado como fuente de ácidos grasos omega-3. Cienc. Tecnol. Agropecu. 2018, 19, 645–668. [Google Scholar] [CrossRef]
- Sathivel, S. Thermal and flow properties of oils from salmon heads. J. Am. Oil Chem. Soc. 2005, 82, 147–152. [Google Scholar] [CrossRef]
- Zahir, E.; Saeed, R.; Hameed, M.A.; Yousuf, A. Study of physicochemical properties of edible oil and evaluation of frying oil quality by Fourier Transform-Infrared (FT-IR) Spectroscopy. Arab. J. Chem. 2017, 10, S3870–S3876. [Google Scholar] [CrossRef]
- García-Moreno, P.J.; Khanum, M.; Guadix, A.; Guadix, E.M. Optimization of biodiesel production from waste fish oil. Renew. Energy 2014, 68, 618–624. [Google Scholar] [CrossRef]
- Rubio-Rodríguez, N.; de Diego, S.M.; Beltrán, S.; Jaime, I.; Sanz, M.T.; Rovira, J. Supercritical fluid extraction of fish oil from fish by-products: A comparison with other extraction methods. J. Food Eng. 2012, 109, 238–248. [Google Scholar] [CrossRef]
- Boran, G.; Karaçam, H.; Boran, M. Changes in the quality of fish oils due to storage temperature and time. Food Chem. 2006, 98, 693–698. [Google Scholar] [CrossRef]
- Aryee, A.N.A.; Simpson, B.K. Comparative studies on the yield and quality of solvent-extracted oil from salmon skin. J. Food Eng. 2009, 92, 353–358. [Google Scholar] [CrossRef]
- Chantachum, S. Separation and quality of fish oil from precooked and non-precooked tuna heads. Food Chem. 2000, 69, 289–294. [Google Scholar] [CrossRef]
- Guseva, D.A.; Prozorovskaya, N.N.; Shironin, A.V.; Sanzhakov, M.A.; Evteeva, N.M.; Rusina, I.F.; Kasaikina, O.T. Antioxidant activity of vegetable oils with different omega-6/omega-3 fatty acids ratio. Biochem. Suppl. Ser. B Biomed. Chem. 2010, 4, 366–371. [Google Scholar] [CrossRef]
- Zuta, C.P.; Simpson, B.K.; Chan, H.M.; Phillips, L. Concentrating PUFA from Mackerel Processing Waste. JAOCS J. Am. Oil Chem. Soc. 2003, 80, 933–936. [Google Scholar] [CrossRef]
- Czernichow, S.; Thomas, D.; Bruckert, E. Acides gras oméga-6 et maladies cardiovasculaires. Méd./Sci. 2011, 27, 614–618. [Google Scholar] [CrossRef]
- Celik, M.; Diler, A.; Kucukgulmez, A. A comparison of the proximate compositions and fatty acid profiles of zander (Sander lucioperca) from two different regions and climatic conditions. Food Chem. 2005, 92, 637–641. [Google Scholar] [CrossRef]
- Justi, K.; Hayashi, C.; Visentainer, J.; de Souza, N.; Matsushita, M. The influence of feed supply time on the fatty acid profile of Nile tilapia (Oreochromis niloticus) fed on a diet enriched with n-3 fatty acids. Food Chem. 2003, 80, 489–493. [Google Scholar] [CrossRef]
- Schmid, M.; Stengel, D.B. Intra-thallus differentiation of fatty acid and pigment profiles in some temperate Fucales and Laminariales. J. Phycol. 2015, 51, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Milićević, D.; Vranić, D.; Mašić, Z.; Parunović, N.; Trbović, D.; Nedeljković-Trailović, J.; Petrović, Z. The role of total fats, saturated/unsaturated fatty acids and cholesterol content in chicken meat as cardiovascular risk factors. Lipids Health Dis. 2014, 13, 42. [Google Scholar] [CrossRef]
- Łuczyńska, J.; Paszczyk, B.; Nowosad, J.; Łuczyński, M. Mercury, Fatty Acids Content and Lipid Quality Indexes in Muscles of Freshwater and Marine Fish on the Polish Market. Risk Assessment of Fish Consumption. Int. J. Environ. Res. Public Health 2017, 14, 1120. [Google Scholar] [CrossRef] [PubMed]
- Horst, R.L.; Goff, J.P.; Reinhardt, T.A. Role of vitamin D in calcium homeostasis and its use in prevention of bovine periparturient paresis. Acta Vet. Scand. Suppl. 2004, 97, 35–50. [Google Scholar]
- Yoshizawa, T.; Handa, Y.; Uematsu, Y.; Takeda, S.; Sekine, K.; Yoshihara, Y.; Kawakami, T.; Arioka, K.; Sato, H.; Uchiyama, Y.; et al. Mice lacking the vitamin D receptor exhibit impaired bone formation, uterine hypoplasia and growth retardation after weaning. Nat. Genet. 1997, 16, 391–396. [Google Scholar] [CrossRef]
- Sun, J.; Kong, J.; Duan, Y.; Szeto, F.L.; Liao, A.; Madara, J.L.; Li, Y.C. Increased NF-κB activity in fibroblasts lacking the vitamin D receptor. Am. J. Physiol. Metab. 2006, 291, E315–E322. [Google Scholar] [CrossRef]
- Lee, H.-J.; Roy, V.C.; Ho, T.C.; Park, J.-S.; Jeong, Y.-R.; Lee, S.-C.; Kim, S.-Y.; Chun, B.-S. Amino Acid Profiles and Biopotentiality of Hydrolysates Obtained from Comb Penshell (Atrina pectinata) Viscera Using Subcritical Water Hydrolysis. Mar. Drugs 2021, 19, 137. [Google Scholar] [CrossRef]
- Ulbricht, T.L.V.; Southgate, D.A.T. Coronary heart disease: Seven dietary factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef]
- Jeong, B.Y.; Choi, B.D.; Moon, S.K.; Lee, J.S. Fatty acid composition of 72 species of Korean fish. Fish. Aquatic Sci. 1998, 1, 129–146. [Google Scholar]

| Colour | SC-CO2 Extracted Oils | n-Hexane Extracted Oils | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Skin | Muscle | Bone | Head | Viscera | Skin | Muscle | Bone | Head | Viscera | |
| L* | 16.40 ± 0.69 f | 25.85 ± 0.79 b | 25.56 ± 0.51 b | 32.06 ± 0.12 a | 18.71 ± 0.32 e | 16.35 ± 1.05 f | 19.95 ± 0.43 d | 21.63 ± 0.58 c | 17.00 ± 0.61 f | 16.61 ± 0.47 f |
| a* | 3.38 ± 0.42 e | 1.12 ± 0.12 f | −2.11 ± 0.13 h | −1.16 ± 0.14 g | 3.31 ± 0.15 e | 5.23 ± 0.15 d | 3.77 ± 0.47 e | 9.04 ± 0.15 b | 7.78 ± 0.47 c | 9.65 ± 0.38 a |
| b* | 19.39 ± 0.42 b | 10.22 ± 0.19 h | 19.85 ± 0.43 b | 11.23 ± 0.23 g | 13.25 ± 0.25 f | 17.32 ± 0.34 d | 18.48 ± 0.28 c | 22.36 ± 0.42 a | 15.53 ± 0.48 e | 17.50 ± 0.47 d |
| Viscosity | ||||||||||
| cP | 37.97 ± 0.87 g | 20.20 ± 0.46 i | 106.40 ± 1.15 b | 68.60 ± 0.30 d | 8.60 ± 0.10 j | 58.70 ± 0.70 e | 57.20 ± 0.56 f | 145.33 ± 1.20 a | 96.13 ± 1.08 c | 25.73 ± 0.42 h |
| SC-CO2 Extracted Oils | n-Hexane Extracted Oils | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Skin | Muscle | Bone | Head | Viscera | Skin | Muscle | Bone | Head | Viscera | |
| AV (mg KOH/g) | 7.76 ± 0.18 d | 7.13 ± 0.08 f | 7.31 ± 0.18 ef | 7.63 ± 0.17 de | 7.36 ± 0.15 ef | 9.96 ± 0.17 c | 10.84 ± 0.25 a | 10.73 ± 0.22 ab | 10.37 ± 0.25 b | 9.66 ± 0.35 c |
| PV (meq/kg) | 1.14 ± 0.12 f | 1.26 ± 0.06 de | 1.16 ± 0.04 ef | 1.28 ± 0.04 cd | 1.06 ± 0.04 f | 1.61 ± 0.10 ab | 1.65 ± 0.06 a | 1.53 ± 0.06 b | 1.53 ± 0.07 b | 1.39 ± 0.04 d |
| FFA (%) | 4.03 ± 0.09 d | 3.75 ± 0.09 e | 3.41 ± 0.10 f | 3.45 ± 0.04 fg | 3.29 ± 0.06 g | 4.69 ± 0.11 a | 4.47 ± 0.09 b | 4.20 ± 0.04 c | 4.26 ± 0.06 c | 4.17 ± 0.05 c |
| Name of the Fatty Acids | Supercritical CO2 Extracted By-Product Oil | n-Hexane Extracted By-Product Oil | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Skin | Muscle | Bone | Head | Viscera | Skin | Muscle | Bone | Head | Viscera | |
| Saturated fatty acids (SFA) | ||||||||||
| Myristic | 4.58 ± 0.04 de | 4.06 ± 0.07 g | 4.84 ± 0.08 cd | 5.03 ± 0.09 bc | 4.38 ± 0.49 efg | 5.05 ± 0.07 bc | 4.47 ± 0.05 ef | 5.33 ± 0.21 ab | 4.21 ± 0.09 fgh | 3.51 ± 0.08 a |
| Pentadaecanoic | 0.78 ± 0.07 de | 0.72 ± 0.02 e | 0.89 ± 0.05 ab | 0.88 ± 0.03 abc | 0.83 ± 0.07 bcd | 0.84 ± 0.04 bcd | 0.80 ± 0.05 def | 0.95 ± 0.05 a | 0.77 ± 0.07 de | 0.75 ± 0.08 de |
| Palmitic | 23.90 ± 0.22 de | 23.73 ± 0.38 e | 26.17 ± 0.15 b | 26.26 ± 1.30 b | 25.87 ± 0.30 b | 25.65 ± 0.46 bc | 24.76 ± 0.57 cd | 27.48 ± 0.41 a | 22.49 ± 0.42 f | 24.29 ± 0.29 de |
| Stearic | 5.10 ± 0.10 d | 5.25 ± 0.14 cd | 5.94 ± 0.03 c | 5.53 ± 0.27 c | 7.54 ± 0.35 a | 5.37 ± 0.18 cd | 5.28 ± 0.14 cd | 5.89 ± 0.35 c | 5.18 ± 0.08 cd | 7.14 ± 0.15 b |
| Arachidic | 0.64 ± 0.04 ab | 0.53 ± 0.02 bcd | 0.62 ± 0.03 abc | 0.48 ± 0.09 cd | 0.51 ± 0.05 bcd | 0.68 ± 0.15 a | 0.47 ± 0.05 d | 0.54 ± 0.09 bcd | 0.54 ± 0.05 bcd | 0.54 ± 0.04 bcd |
| Behenic | 0.25 ± 0.06 a | 0.27 ± 0.03 a | 0.28 ± 0.08 a | 0.25 ± 0.03 a | 0.24 ± 0.03 a | 0.24 ± 0.02 a | 0.22 ± 0.03 a | 0.28 ± 0.07 a | 0.22 ± 0.03 a | 0.25 ± 0.04 a |
| Heneicosanoic | 0.29 ± 0.01 ab | 0.29 ± 0.02 ab | 0.27 ± 0.02 ab | 0.26 ± 0.05 ab | 0.23 ± 0.03 b | 0.32 ± 0.05 a | ND | 0.29 ± 0.02 ab | 0.27 ± 0.03 ab | 0.28 ± 0.08 ab |
| Lignoceric | 0.22 ± 0.03 ab | 0.22 ± 0.03 ab | 0.25 ± 0.05 ab | 0.24 ± 0.03 bc | 0.24 ± 0.03 ab | 0.13 ± 0.02 c | 0.18 ± 0.01 bc | 0.22 ± 0.03 ab | 0.24 ± 0.06 ab | 0.26 ± 0.02 a |
| Monounsaturated fatty acid (MUFA) | ||||||||||
| Myristoleic | ND | ND | ND | 0.12 ± 0.01 a | ND | ND | 0.13 ± 0.02 a | ND | ND | ND |
| Palmitoleic | 6.78 ± 0.22 c | 5.66 ± 0.10 e | 7.43 ± 0.43 b | 7.57 ± 0.17 b | 5.07 ± 0.16 f | 7.22 ± 0.05 b | 6.16 ± 0.05 d | 7.98 ± 0.25 a | 7.36 ± 0.22 b | 5.06 ± 0.08 f |
| Cis-10 heptadecanoic | 0.36 ± 0.05 c | 0.31 ± 0.10 c | 0.32 ± 0.03 c | 0.69 ± 0.04 a | 0.28 ± 0.06 c | 0.33 ± 0.02 c | 0.51 ± 0.04 b | 0.33 ± 0.07 c | 0.27 ± 0.06 c | 0.26 ± 0.03 c |
| Oleic | 26.46 ± 0.45 f | 26.37 ± 0.44 f | 30.51 ± 0.25 b | 27.51 ± 0.40 e | 24.14 ± 0.14 g | 28.61 ± 0.43 d | 27.23 ± 0.82 e | 32.14 ± 0.27 a | 29.43 ± 0.46 c | 24.22 ± 0.31 g |
| Elaidic | 0.19 ± 0.05 ab | 0.15 ± 0.05 b | 0.19 ± 0.06 ab | 0.19 ± 0.05 ab | 0.22 ± 0.03 ab | 0.23 ± 0.05 ab | 0.15 ± 0.02 b | 0.21 ± 0.04 ab | 0.17 ± 0.02 ab | 0.24 ± 0.05 a |
| Eicosenoic | 3.26 ± 0.08 a | 3.15 ± 0.08 ab | 3.02 ± 0.08 abc | 2.94 ± 0.04 bc | 2.85 ± 0.09 c | 3.25 ± 0.04 a | 2.97 ± 0.06 bc | 3.15 ± 0.26 ab | 2.97 ± 0.24 bc | 2.89 ± 0.21 bc |
| Nervonic | 0.77 ± 0.02 bcd | 0.69 ± 0.05 cd | 0.89 ± 0.09 ab | 0.75 ± 0.03 bcd | 0.81 ± 0.07 abc | 0.73 ± 0.03 cd | 0.63 ± 0.02 d | 0.79 ± 0.15 abcd | 0.76 ± 0.13 bcd | 0.93 ± 0.07 a |
| Polyunsaturated fatty acid (PUFA) | ||||||||||
| Linoleic | 1.82 ± 0.06 bcd | 1.66 ± 0.09 f | 1.88 ± 0.10 abc | 2.02 ± 0.08 bcd | 3.25 ± 0.07 a | 1.93 ± 0.04 ab | 1.71 ± 0.03 ef | 1.83 ± 0.09 b | 1.74 ± 0.20 def | 3.01 ± 0.15 b |
| Linolelaidic | 0.16 ± 0.04 c | 0.19 ± 0.02 bc | 0.21 ± 0.04 bc | 0.22 ± 0.03 b | 0.17 ± 0.04 bc | 0.16 ± 0.02 c | 0.28 ± 0.02 a | 0.17 ± 0.03 bc | 0.17 ± 0.03 bc | 0.15 ± 0.04 c |
| r-Linolenic | 0.15 ± 0.03 b | 0.37 ± 0.05 a | 0.39 ± 0.09 a | 0.12 ± 0.02 b | 0.39 ± 0.05 a | 0.13 ± 0.01 b | 0.14 ± 0.01 b | 0.42 ± 0.03 a | 0.37 ± 0.05 a | 0.39 ± 0.06 a |
| Linolenic | 1.08 ± 0.11 b | 1.10 ± 0.07 b | 0.93 ± 0.02 c | 1.10 ± 0.13 b | 1.45 ± 0.05 a | 0.97 ± 0.07 bc | 1.10 ± 0.08 b | 0.90 ± 0.02 c | 0.93 ± 0.13 c | 1.40 ± 0.05 a |
| Ecosadienoic | 1.50 ± 0.04 ab | 1.66 ± 0.25 a | 1.52 ± 0.11 ab | 1.51 ± 0.55 ab | 1.30 ± 0.07 bc | 1.28 ± 0.17 bc | 1.60 ± 0.08 ab | 1.34 ± 0.04 abc | 1.45 ± 0.37 abc | 1.15 ± 0.18 c |
| Eicosatrienoic | 0.19 ± 0.03 c | 0.16 ± 0.04 c | ND | 2.32 ± 0.02 a | 0.29 ± 0.05 b | 0.14 ± 0.02 c | 0.15 ± 0.03 c | ND | 0.15 ± 0.03 c | 0.25 ± 0.04 b |
| Elcosatrienoic | 2.81 ± 0.09 a | 2.65 ± 0.04 ab | 2.26 ± 0.05 cd | 2.35 ± 0.15 cd | 2.76 ± 0.07 a | 2.78 ± 0.16 a | 2.70 ± 0.10 ab | 2.28 ± 0.08 cd | 2.49 ± 0.30 bc | 2.19 ± 0.17 d |
| Arachidonic | 0.91 ± 0.04 cd | 0.91 ± 0.05 cd | 0.79 ± 0.06 def | 0.88 ± 0.03 de | 1.76 ± 0.07 a | 1.05 ± 0.14 c | 0.72 ± 0.08 ef | 0.65 ± 0.06 g | 0.74 ± 0.12 def | 1.56 ± 0.16 b |
| Docosadienoic | 0.55 ± 0.08 b | 0.51 ± 0.05 bcd | 0.41 ± 0.06 def | 0.45 ± 0.04 bcd | 0.76 ± 0.07 a | 0.41 ± 0.08 de | 0.52 ± 0.04 bc | 0.34 ± 0.05 e | 0.35 ± 0.07 e | 0.51 ± 0.05 bcd |
| Eicosapentaenoic | 5.81 ± 0.69 a | 4.93 ± 0.06 a | 3.40 ± 0.06 bc | 3.57 ± 0.02 bc | 3.81 ± 0.15 b | 3.53 ± 0.08 bc | 5.14 ± 0.12 a | 2.63 ± 0.21 c | 2.81 ± 0.29 bc | 3.70 ± 0.22 b |
| Docosahexaenoic | 12.56 ± 0.38 c | 15.01 ± 0.28 a | 6.55 ± 0.19 h | 7.34 ± 0.16 g | 10.81 ± 0.15 d | 8.16 ± 0.04 f | 14.51 ± 0.45 b | 4.43 ± 0.09 i | 6.19 ± 0.17 h | 10.27 ± 0.34 e |
| ΣSFA | 35.72 | 35.07 | 39.26 | 38.93 | 39.84 | 38.28 | 36.18 | 40.98 | 33.92 | 37.02 |
| ΣMUFA | 37.82 | 36.33 | 42.36 | 33.77 | 33.37 | 40.37 | 37.78 | 44.60 | 40.96 | 33.6 |
| ΣPUFA | 27.54 | 29.15 | 18.34 | 21.88 | 26.75 | 20.56 | 28.57 | 14.99 | 17.39 | 24.58 |
| ω- 3/ω- 6 | 2.97 | 3.51 | 1.83 | 1.25 | 2.21 | 2.10 | 3.55 | 1.23 | 1.63 | 2.43 |
| AI | 0.77 | 0.72 | 0.87 | 0.99 | 0.88 | 0.87 | 0.76 | 0.93 | 0.77 | 0.80 |
| TI | 0.76 | 0.75 | 0.90 | 0.81 | 0.90 | 0.85 | 0.78 | 0.92 | 0.78 | 0.90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roy, V.C.; Park, J.-S.; Ho, T.C.; Chun, B.-S. Lipid Indexes and Quality Evaluation of Omega-3 Rich Oil from the Waste of Japanese Spanish Mackerel Extracted by Supercritical CO2. Mar. Drugs 2022, 20, 70. https://doi.org/10.3390/md20010070
Roy VC, Park J-S, Ho TC, Chun B-S. Lipid Indexes and Quality Evaluation of Omega-3 Rich Oil from the Waste of Japanese Spanish Mackerel Extracted by Supercritical CO2. Marine Drugs. 2022; 20(1):70. https://doi.org/10.3390/md20010070
Chicago/Turabian StyleRoy, Vikash Chandra, Jin-Seok Park, Truc Cong Ho, and Byung-Soo Chun. 2022. "Lipid Indexes and Quality Evaluation of Omega-3 Rich Oil from the Waste of Japanese Spanish Mackerel Extracted by Supercritical CO2" Marine Drugs 20, no. 1: 70. https://doi.org/10.3390/md20010070
APA StyleRoy, V. C., Park, J.-S., Ho, T. C., & Chun, B.-S. (2022). Lipid Indexes and Quality Evaluation of Omega-3 Rich Oil from the Waste of Japanese Spanish Mackerel Extracted by Supercritical CO2. Marine Drugs, 20(1), 70. https://doi.org/10.3390/md20010070