Antioxidant Bioactivity of Extracts from Beach Cast Leaves of Posidonia oceanica (L.) Delile
Abstract
1. Introduction
2. Results
2.1. Characterization of the Antioxidant Power
2.1.1. Total Phenolic Content
2.1.2. HPLC Analysis of Phenolic Compounds
2.1.3. Antioxidant Power—DPPH Assay
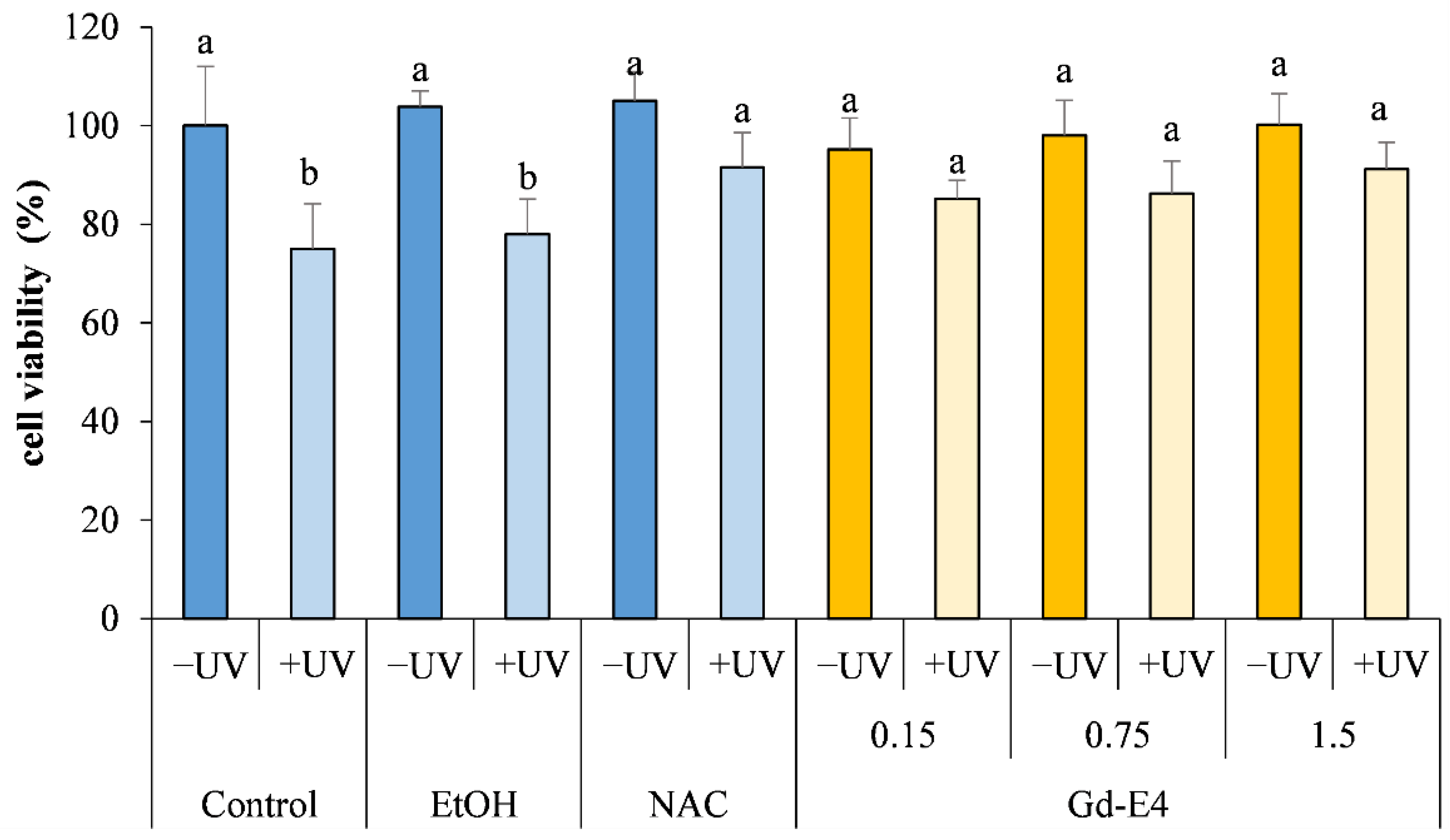
2.2. In Vitro Photo Protective Effect Evaluation of Posidonia Oceanica Extracts
3. Discussion
4. Materials and Methods
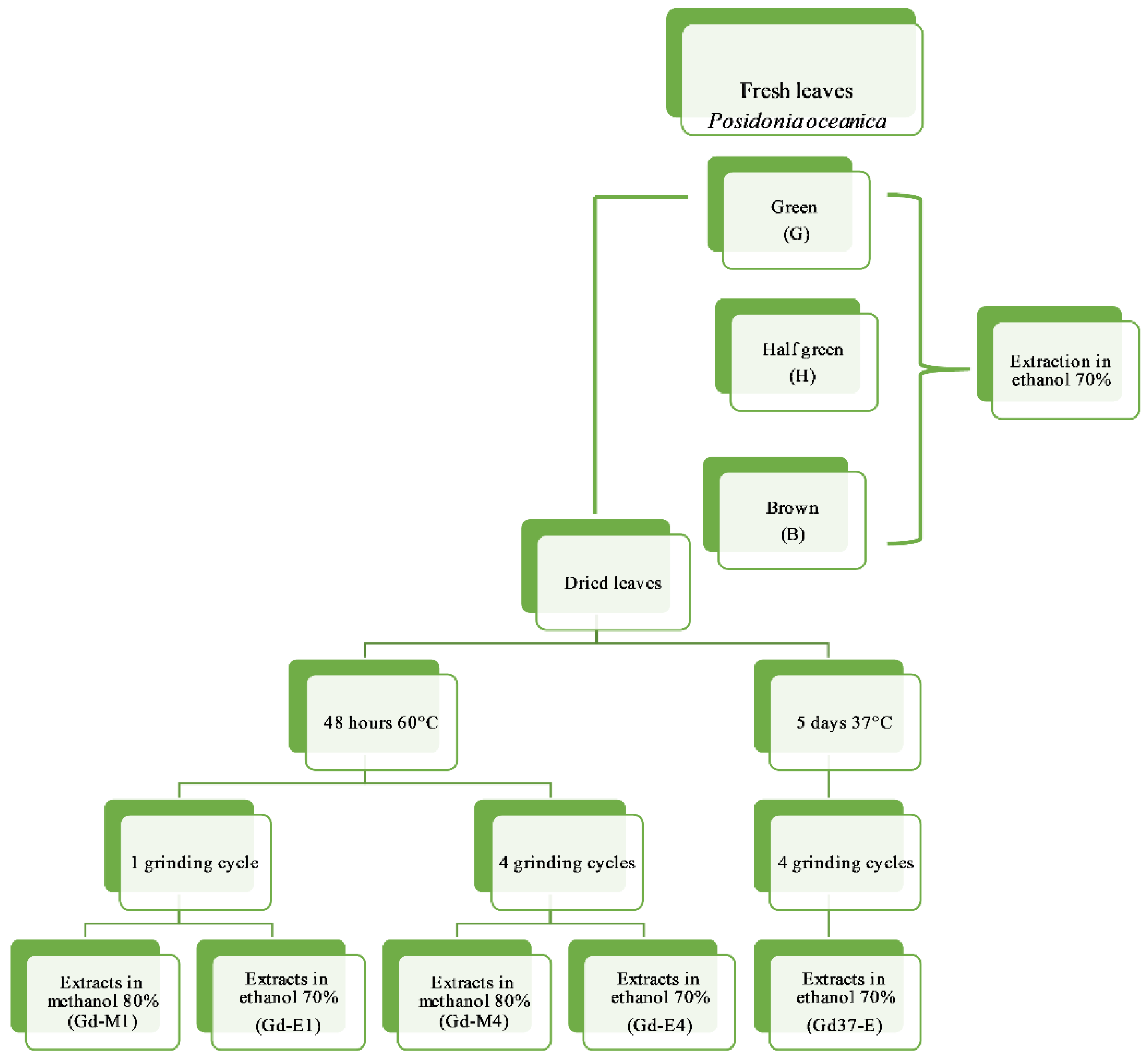
4.1. Samples Collection, Processing and Preparation of Plant Extract
Extraction
4.2. Characterization of the Antioxidant Power
4.2.1. Total Polyphenols Contents
4.2.2. HPLC Analysis of Posidonia Leaf Extracts
4.2.3. DPPH Assay
4.3. Evaluation of Bioactive Properties In Vitro
4.3.1. Cell Culture
4.3.2. Evaluation of Photo-Protective Effect in Fibroblast Cell Line HS-68
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Benito-González, I.; López-Rubio, A.; Martínez-Abad, A.; Ballester, A.R.; Falcó, I.; González-Candelas, L.; Sánchez, G.; Lozano-Sánchez, J.; Borrás-Linares, I.; Segura-Carretero, A.; et al. In-depth characterization of bioactive extracts from posidonia oceanica waste biomass. Mar. Drugs 2019, 17, 409. [Google Scholar] [CrossRef]
- Rotter, A.; Barbier, M.; Bertoni, F.; Bones, A.M.; Cancela, M.L.; Carlsson, J.; Carvalho, M.F.; Cegłowska, M.; Chirivella-Martorell, J.; Conk Dalay, M.; et al. The Essentials of Marine Biotechnology. Front. Mar. Sci. 2021, 8, 158. [Google Scholar] [CrossRef]
- Cornara, L.; Pastorino, G.; Borghesi, B.; Salis, A.; Clericuzio, M.; Marchetti, C.; Damonte, G.; Burlando, B. Posidonia oceanica (L.) delile ethanolic extract modulates cell activities with skin health applications. Mar. Drugs 2018, 16, 21. [Google Scholar] [CrossRef]
- Wijesinghe, W.A.J.P.; Jeon, Y.J. Biological activities and potential cosmeceutical applications of bioactive components from brown seaweeds: A review. Phytochem. Rev. 2011, 10, 431–443. [Google Scholar] [CrossRef]
- Thiyagarasaiyar, K.; Goh, B.-H.; Jeon, Y.-J.; Yow, Y.-Y. Algae Metabolites in Cosmeceutical: An Overview of Current Applications and Challenges. Mar. Drugs 2020, 18, 323. [Google Scholar] [CrossRef] [PubMed]
- Jahan, A.; Ahmad, I.Z.; Fatima, N.; Ansari, V.A.; Akhtar, J. Algal bioactive compounds in the cosmeceutical industry: A review. Phycologia 2019, 56, 410–422. [Google Scholar] [CrossRef]
- Vasarri, M.; De Biasi, A.M.; Barletta, E.; Pretti, C.; Degl’Innocenti, D. An Overview of New Insights into the Benefits of the Seagrass Posidonia oceanica for Human Health. Mar. Drugs 2021, 19, 476. [Google Scholar] [CrossRef] [PubMed]
- Duarte, C.M.; Losada, I.J.; Hendriks, I.; Mazarrasa, I. The role of coastal plant communities for climate change mitigation and adaptation. Nat. Clim. Chang. 2013, 3, 961–968. [Google Scholar] [CrossRef]
- Bharathi, N.P.; Amudha, P.; Vanitha, V. Sea grasses—Novel marine nutraceuticals. Int. J. Pharma Bio. Sci. 2016, 7, 567–573. [Google Scholar] [CrossRef]
- Choi, H.-G.; Lee, J.-H.; Park, H.-H.; Sayegh, F.A.Q. Antioxidant and Antimicrobial Activity of Zostera marina L. Extract. ALGAE 2009, 24, 179–184. [Google Scholar] [CrossRef]
- Kannan, R.R.R.; Arumugam, R.; Anantharaman, P. In vitro antioxidant activities of ethanol extract from Enhalus acoroides (L.F.) Royle. Asian Pac. J. Trop. Med. 2010, 3, 898–901. [Google Scholar] [CrossRef]
- Hua, K.F.; Hsu, H.Y.; Su, Y.C.; Lin, I.F.; Yang, S.S.; Chen, Y.M.; Chao, L.K. Study on the antiinflammatory activity of methanol extract from seagrass Zostera japonica. J. Agric. Food Chem. 2006, 54, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Menajang, F.S.I.; Mahmudi, M.; Yanuhar, U.; Herawati, E.Y. Evaluation of phytochemical and superoxide dismutase activities of Enhalus acoroides (L.f.) Royle from coastal waters of North Sulawesi, Indonesia. Vet World. 2020, 13, 676–680. [Google Scholar] [CrossRef]
- Boudouresque, C.F.; Bernard, G.; Bonhomme, P.; Charbonnel, E.; Diviacco, G.; Meinesz, A.; Pergent, G.; Pergent-Martini, C.; Ruitton, S.; Tunesi, L. Préservation et Conservation des Herbiers à Posidonia Oceanica; Ramoge Pub: Marseille, France, 2006; pp. 1–202. ISBN 2905540303. [Google Scholar]
- European Community. Council Directive 92/43/EEC of 21st May 1992 on the Conservation of Natural Habitats and of Wild Fauna and Flora. Off. J.Eur.Union 1992, L206, 7–50. [Google Scholar]
- Salomidi, M.; Katsanevakis, S.; Borja, Á.; Braeckman, U.; Damalas, D.; Galparsoro, I.; Mifsud, R.; Mirto, S.; Pascual, M.; Pipitone, C.; et al. Assessment of goods and services, vulnerability, and conservation status of European seabed biotopes: A stepping stone towards ecosystem-based marine spatial management. Mediterr. Mar. Sci. 2012, 13, 49–88. [Google Scholar] [CrossRef]
- Rotini, A.; Chiesa, S.; Manfra, L.; Borrello, P.; Piermarini, R.; Silvestri, C.; Cappucci, S.; Parlagreco, L.; Devoti, S.; Pisapia, M.; et al. Effectiveness of the “ecological beach” model: Beneficial management of posidonia beach casts and banquette. Water 2020, 12, 3238. [Google Scholar] [CrossRef]
- Legislative Decree of 29 April 2010, n. 75 Riordino e Revisione Della Disciplina in Materia di Fertilizzanti, a Norma Dell’articolo 13 Della Legge 7 Luglio 2009, n. 88 In GU N. 121 DEL 26 MAGGIO 2010. Available online: https://www.gazzettaufficiale.it/eli/id/2010/05/26/010G0096/sg (accessed on 10 September 2020).
- Ministry Decree/Decreto Ministeriale del Ministero Delle Politiche Agricole e Forestali del 22 Gennaio 2009 Aggiornamento Degli Allegati al Decreto Legislativo 29 Aprile 2006, n. 217, Concernente la Revisione Della Disciplina in Materia di Fertilizzanti in GU n.88 del 16-04-2009. Available online: www.gazzettaufficiale.it/eli/id/2009/04/16/09A03940/sg (accessed on 10 September 2020).
- Leri, M.; Ramazzotti, M.; Vasarri, M.; Peri, S.; Barletta, E.; Pretti, C.; Degl’Innocenti, D. Bioactive compounds from Posidonia oceanica (L.) delile impair malignant cell migration through autophagy modulation. Mar. Drugs 2018, 16, 137. [Google Scholar] [CrossRef] [PubMed]
- Heglmeier, A.; Zidorn, C. Secondary metabolites of Posidonia oceanica (Posidoniaceae). Biochem. Syst. Ecol. 2010, 38, 964–970. [Google Scholar] [CrossRef]
- Subhashini, P.; Dilipan, E.; Thangaradjou, T.; Papenbrock, J. Bioactive natural products from marine angiosperms: Abundance and functions. Rev. Nat. Prod. Bioprospect 2013, 3, 129–136. [Google Scholar] [CrossRef]
- Messina, C.M.; Troia, A.; Arena, R.; Manuguerra, S.; Ioannou, T.; Curcuraci, E.; Renda, G.; Hellio, C.; Santulli, A. Species-specific antioxidant power and bioactive properties of the extracts obtained from wild mediterranean Calendula spp. (Asteraceae). Appl. Sci. 2019, 9, 4627. [Google Scholar] [CrossRef]
- Messina, C.M.; Manuguerra, S.; Catalano, G.; Arena, R.; Cocchi, M.; Morghese, M.; Montenegro, L.; Santulli, A. Green biotechnology for valorisation of residual biomasses in nutraceutic sector: Characterization and extraction of bioactive compounds from grape pomace and evaluation of the protective effects in vitro. Nat. Prod. Res. 2021, 35, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Manuguerra, S.; Caccamo, L.; Mancuso, M.; Arena, R.; Rappazzo, A.C.; Genovese, L.; Santulli, A.; Messina, C.M.; Maricchiolo, G. The antioxidant power of horseradish, Armoracia rusticana, underlies antimicrobial and antiradical effects, exerted in vitro. Nat. Prod. Res. 2020, 34, 1567–1570. [Google Scholar] [CrossRef] [PubMed]
- Messina, C.M.; Renda, G.; Laudicella, V.A.; Trepos, R.; Fauchon, M.; Hellio, C.; Santulli, A. From ecology to biotechnology, study of the defense strategies of algae and halophytes (from trapani saltworks, NW sicily) with a focus on antioxidants and antimicrobial properties. Int. J. Mol. Sci. 2019, 20, 881. [Google Scholar] [CrossRef] [PubMed]
- Carocho, M.; Ferreira, I.C.F.R. A review on antioxidants, prooxidants and related controversy: Natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem. Toxicol. 2013, 51, 15–25. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, S.Y. Antioxidant activity and phenolic compounds in selected herbs. J. Agric. Food Chem. 2001, 49, 5165–5170. [Google Scholar] [CrossRef]
- Bibi, F.; Naseer, M.I.; Hassan, A.M.; Yasir, M.; Al-Ghamdi, A.A.K.; Azhar, E.I. Diversity and antagonistic potential of bacteria isolated from marine grass Halodule uninervis. 3 Biotech 2018, 8, 48. [Google Scholar] [CrossRef]
- Pinazo-Durán, M.D.; Gallego-Pinazo, R.; García-Medina, J.J.; Zanón-Moreno, V.; Nucci, C.; Dolz-Marco, R.; Martínez-Castillo, S.; Galbis-Estrada, C.; Marco-Ramírez, C.; López-Gálvez, M.I.; et al. Clinical Interventions in Aging Dovepress Oxidative stress and its downstream signaling in aging eyes. Clin. Interv. Aging 2014, 2014, 637–652. [Google Scholar] [CrossRef]
- Vasarri, M.; Leri, M.; Barletta, E.; Ramazzotti, M.; Marzocchini, R.; Degl’Innocenti, D. Anti-inflammatory properties of the marine plant Posidonia oceanica (L.) Delile. J. Ethnopharmacol. 2020, 247, 112252. [Google Scholar] [CrossRef]
- Astudillo-Pascual, M.; Domínguez, I.; Aguilera, P.A.; Garrido Frenich, A. New phenolic compounds in posidonia oceanica seagrass: A comprehensive array using high resolution mass spectrometry. Plants 2021, 10, 864. [Google Scholar] [CrossRef] [PubMed]
- Ammar, N.M.; Hassan, H.A.; Mohammed, M.A.; Serag, A.; Abd El-Alim, S.H.; Elmotasem, H.; El Raey, M.; El Gendy, A.N.; Sobeh, M.; Abdel-Hamid, A.H.Z. Metabolomic profiling to reveal the therapeutic potency ofPosidonia oceanicananoparticles in diabetic rats. RSC Adv. 2021, 11, 8398–8410. [Google Scholar] [CrossRef]
- Aseervatham, G.S.B.; Sivasudha, T.; Jeyadevi, R.; Arul Ananth, D. Environmental factors and unhealthy lifestyle influence oxidative stress in humans--an overview. Environ. Sci. Pollut. Res. Int. 2013, 20, 4356–4369. [Google Scholar] [CrossRef]
- Al-Gubory, K.H. Environmental pollutants and lifestyle factors induce oxidative stress and poor prenatal development. Reprod. Biomed. Online 2014, 29, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Corinaldesi, C.; Barone, G.; Marcellini, F.; Dell’Anno, A.; Danovaro, R. Marine microbial-derived molecules and their potential use in cosmeceutical and cosmetic products. Mar. Drugs 2017, 15, 118. [Google Scholar] [CrossRef] [PubMed]
- Zidorn, C. Secondary metabolites of seagrasses (Alismatales and Potamogetonales; Alismatidae): Chemical diversity, bioactivity, and ecological function. Phytochemistry 2016, 124, 5–28. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C. Plant polyphenols: Free radical scavengers or chain-breaking antioxidants? Biochem. Soc. Symp. 1995, 61, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Michalak, A. Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress. Pol. J. Environ. Stud. 2006, 15, 523–530. [Google Scholar]
- Gião, M.S.; Pereira, C.I.; Fonseca, S.C.; Pintado, M.E.; Malcata, F.X. Effect of particle size upon the extent of extraction of antioxidant power from the plants Agrimonia eupatoria, Salvia sp. and Satureja montana. Food Chem. 2009, 117, 412–416. [Google Scholar] [CrossRef]
- Franco, D.; Sineiro, J.; Rubilar, M.; Sánchez, M.; Jerez, M.; Pinelo, M.; Costoya, N.; Núñez, M.J. Polyphenols from plant materials: Extraction and antioxidant power. Electron. J. Environ. Agric. Food Chem. 2008, 7, 3210–3216. [Google Scholar]
- Alothman, M.; Bhat, R.; Karim, A.A. Antioxidant capacity and phenolic content of selected tropical fruits from Malaysia, extracted with different solvents. Food Chem. 2009, 115, 785–788. [Google Scholar] [CrossRef]
- Shi, J.; Yu, J.; Pohorly, J.; Young, J.C.; Bryan, M.; Wu, Y. Optimization of the extraction of polyphenols from grape seed meal by aqueous ethanol solution. Food Agric. Environ. 2003, 1, 42–47. [Google Scholar]
- Anwar, F.; Kalsoom, U.; Sultana, B.; Mushtaq, M.; Mehmood, T.; Arshad, H.A. Effect of drying method and extraction solvent on the total phenolics and antioxidant activity of cauliflower (Brassica oleracea L.) Extracts. Int. Food Res. J. 2013, 20, 653–659. [Google Scholar]
- Grignon-Dubois, M.; Rezzonico, B. Phenolic fingerprint of the seagrass Posidonia oceanica from four locations in the Mediterranean Sea: First evidence for the large predominance of chicoric acid. Bot. Mar. 2015, 58, 379–391. [Google Scholar] [CrossRef]
- Lim, Y.Y.; Murtijaya, J. Antioxidant properties of Phyllanthus amarus extracts as affected by different drying methods. LWT Food Sci. Technol. 2007, 40, 1664–1669. [Google Scholar] [CrossRef]
- Chan, E.W.C.; Lim, Y.Y.; Wong, S.K.; Lim, K.K.; Tan, S.P.; Lianto, F.S.; Yong, M.Y. Effects of different drying methods on the antioxidant properties of leaves and tea of ginger species. Food Chem. 2009, 113, 166–172. [Google Scholar] [CrossRef]
- Le Lann, K.; Jégou, C.; Stiger-Pouvreau, V. Effect of different conditioning treatments on total phenolic content and antioxidant activities in two Sargassacean species: Comparison of the frondose Sargassum muticum (Yendo) Fensholt and the cylindrical Bifurcaria bifurcata R. Ross. Phycol. Res. 2008, 56, 238–245. [Google Scholar] [CrossRef]
- Cannac, M.; Ferrat, L.; Barboni, T.; Pergent, G.; Pasqualini, V. The influence of tissue handling on the flavonoid content of the aquatic plant Posidonia oceanica. J. Chem. Ecol. 2007, 33, 1083–1088. [Google Scholar] [CrossRef] [PubMed]
- Liazid, A.; Palma, M.; Brigui, J.; Barroso, C.G. Investigation on phenolic compounds stability during microwave-assisted extraction. J. Chromatogr. A 2007, 1140, 29–34. [Google Scholar] [CrossRef]
- Mrkìc, V.; Cocci, E.; Rosa, M.D.; Sacchetti, G. Effect of drying conditions on bioactive compounds and antioxidant activity of broccoli (Brassica oleracea L.). J. Sci. Food Agric. 2006, 86, 1559–1566. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Maity, N.; Nema, N.K.; Sarkar, B.K. Bioactive compounds from natural resources against skin aging. Phytomedicine 2011, 19, 64–73. [Google Scholar] [CrossRef]
- Pallela, R.; Na-Young, Y.; Kim, S.K. Anti-photoaging and photoprotective compounds derived from marine organisms. Mar. Drugs 2010, 8, 1189–1202. [Google Scholar] [CrossRef]
- Edreva, A.; Velikova, V.; Tsonev, T.; Dagnon, S.; Gesheva, E. Stress-protective role of secondary metabolites: Diversity of functions and mechanisms. Gen. Appl. Plant. Physiol. 2008, 34, 67–68. [Google Scholar]
- Svobodová, A.; Psotová, J.; Walterová, D. Natural phenolics in the prevention of UV-induced skin damage. A review. Biomed. Pap. Med. Fac. Univ. Palacky. Olomouc. Czech. Repub. 2003, 147, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Lu, S.; Kivlin, R.; Wallin, B.; Card, E.; Bagdasarian, A.; Tamakloe, T.; Wang, W.-J.; Song, X.; Chu, W.M.; et al. SIRT1 confers protection against UVB- and H2O2-induced cell death via modulation of p53 and JNK in cultured skin keratinocytes. J. Cell. Mol. Med. 2009, 13, 3632–3643. [Google Scholar] [CrossRef]
- Masaki, H. Role of antioxidants in the skin: Anti-aging effects. J. Dermatol. Sci. 2010, 58, 85–90. [Google Scholar] [CrossRef]
- Barletta, E.; Ramazzotti, M.; Fratianni, F.; Pessani, D.; Degl’Innocenti, D. Hydrophilic extract from Posidonia oceanic inhibits activity and expression of gelatinases and prevents HT1080 human fibrosarcoma cell line invasion. Cell Adhes. Migr. 2015, 9, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Debacq-Chainiaux, F.; Leduc, C.; Verbeke, A.; Toussaint, O. UV, stress and aging. Dermatoendocrinology 2012, 4, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, L.; Messina, C.M.; Manuguerra, S.; Santagati, L.M.; Pasquinucci, L.; Turnaturi, R.; Parenti, C.; Arena, R.; Santulli, A. In vitro antioxidant activity and in vivo topical efficacy of lipid nanoparticles co-loadingm idebenone and tocopheryl acetate. Appl. Sci. 2019, 9, 845. [Google Scholar] [CrossRef]
- Lee, S.-G.; Kang, H. Evaluation of Antioxidant and Anti-neuroinflammatory Activities of Hizikia fusiformis (Harvey) Okamura Extract. Trop. J. Pharm. Res. 2015, 14, 463–468. [Google Scholar] [CrossRef]
- Arena, R.; Manuguerra, S.; Collins, E.; Mahdhi, A.; Renda, G.; Messina, C.M.; Santulli, A. Antioxidant properties of a supercritical fluid extract of the halophyte Mesembryanthemum nodiflorum L. from sicilian coasts: Nutraceutical and cosmeceutical applications. Appl. Sci. 2020, 10, 2374. [Google Scholar] [CrossRef]
- Maqsood, S.; Benjakul, S.; Abushelaibi, A.; Alam, A. Phenolic Compounds and Plant Phenolic Extracts as Natural Antioxidants in Prevention of Lipid Oxidation in Seafood: A Detailed Review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 1125–1140. [Google Scholar] [CrossRef]
- Dhouibi, N.; Manuguerra, S.; Arena, R.; Mahdhi, A.; Messina, C.M.; Santulli, A.; Dhaouadi, H. Screening of Antioxidant Potentials and Bioactive Properties of the Extracts Obtained from Two Centaurea L. Species (C. kroumirensis Coss. and C. sicula L. subsp sicula). Appl. Sci. 2020, 10, 2267. [Google Scholar] [CrossRef]
- Poljšak, B.; Dahmane, R. Free radicals and extrinsic skin aging. Dermatol. Res. Pract. 2012, 2012, 135206. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Yan, Y.; Li, L.; Peng, S.; Qu, T.; Wang, B. Ultraviolet B-induced apoptosis of human skin fibroblasts involves activation of caspase-8 and -3 with increased expression of vimentin. Photodermatol. Photoimmunol. Photomed. 2010, 26, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, S. Dandelion Extracts Protect Human Skin Fibroblasts from UVB Damage and Cellular Senescence. Oxid. Med. Cell. Longev. 2015, 2015, 619560. [Google Scholar] [CrossRef] [PubMed]
- Pacheco-Palencia, L.A.; Noratto, G.; Hingorani, L.; Talcott, S.T.; Mertens-Talcott, S.U. Protective Effects of Standardized Pomegranate (Punica granatum L.) Polyphenolic Extract in Ultraviolet-Irradiated Human Skin Fibroblasts. J. Agric. Food Chem. 2008, 56, 8434–8441. [Google Scholar] [CrossRef]
- Deidun, A.; Saliba, S.; Schembri, P.J. Quantitative assessment and physical characterisation of Posidonia oceanica wrack beached along the Maltese coastline. Biol. Mar. Mediterr. 2011, 18, 307–308. [Google Scholar]
- Guala, I.; Simone, S.; Cristina, B.M.; Flagella, S.; Baroli, M.; De Falco, G. Posidonia Oceanica ‘banquettes’ removal: Environmental Impact and Management implications. Biol. Mar. Mediterr. 2006, 13, 149–153. [Google Scholar]
- Simeone, S.; De Falco, G. Morphology and composition of beach-cast Posidonia oceanica litter on beaches with different exposures. Geomorphology 2012, 151–152, 224–233. [Google Scholar] [CrossRef]
- Gharbi, S.; Renda, G.; La Barbera, L.; Amri, M.; Messina, C.M.; Santulli, A. Tunisian tomato by-products, as a potential source of natural bioactive compounds. Nat. Prod. Res. 2017, 31, 626–631. [Google Scholar] [CrossRef]
- Fish, W.W.; Perkins-Veazie, P.; Collins, J.K. A Quantitative Assay for Lycopene That Utilizes Reduced Volumes of Organic Solvents. J. Food Compos. Anal. 2002, 15, 309–317. [Google Scholar] [CrossRef]
- Musa, K.H.; Abdullah, A.; Jusoh, K.; Subramaniam, V. Antioxidant Activity of Pink-Flesh Guava (Psidium guajava L.): Effect of Extraction Techniques and Solvents. Food Anal. Methods 2011, 4, 100–107. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Krishnaiah, D.; Sarbatly, R.; Nithyanandam, R. A review of the antioxidant potential of medicinal plant species. Food Bioprod. Process. 2011, 89, 217–233. [Google Scholar] [CrossRef]
- Oki, T.; Masuda, M.; Furuta, S.; Nishiba, Y.; Terahara, N.; Suda, I. Involvement of anthocyanins and other phenolic compounds in radical-scavenging activity of purple-fleshed sweet potato cultivars. J. Food Sci. 2002, 67, 1752–1756. [Google Scholar] [CrossRef]
- Puigventós, L.; Navarro, M.; Alechaga, É.; Núñez, O.; Saurina, J.; Hernández-Cassou, S.; Puignou, L. Determination of polyphenolic profiles by liquid chromatography-electrospray-tandem mass spectrometry for the authentication of fruit extracts. Anal. Bioanal. Chem. 2015, 407, 597–608. [Google Scholar] [CrossRef] [PubMed]
- Robbins, R.J.; Bean, S.R. Development of a quantitative high-performance liquid chromatography- photodiode array detection measurement system for phenolic acids. J. Chromatogr. A 2004, 1038, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Yeddes, N.; Chérif, J.; Guyot, S.; Sotin, H.; Ayadi, M. Comparative Study of Antioxidant Power, Polyphenols, Flavonoids and Betacyanins of the Peel and Pulp of Three Tunisian Opuntia Forms. Antioxidants 2013, 2, 37–51. [Google Scholar] [CrossRef]
- Abbes, M.; Baati, H.; Guermazi, S.; Messina, C.; Santulli, A.; Gharsallah, N.; Ammar, E. Biological properties of carotenoids extracted from Halobacterium halobium isolated from a Tunisian solar saltern. BMC Complement. Altern. Med. 2013, 13, 255. [Google Scholar] [CrossRef] [PubMed]
- Saija, A.; Tomaino, A.; Trombetta, D.; Pellegrino, M.L.; Tita, B.; Messina, C.; Bonina, F.P.; Rocco, C.; Nicolosi, G.; Castelli, F. “In vitro” antioxidant and photoprotective properties and interaction with model membranes of three new quercetin esters. Eur. J. Pharm. Biopharm. 2003, 56, 167–174. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Underwood, A.J. Experiments in Ecology: Their Logical Design and Interpretation using Analysis of Variance; Cambridge University Press: Cambridge, UK, 1997; ISBN 0521556961. [Google Scholar]


| Phenolic Compounds | G | H | B | Gd-M1 | Gd-M4 | Gd-E1 | Gd-E4 | Gd37-E |
|---|---|---|---|---|---|---|---|---|
| Phloroglucinol | 25.606 | 4.131 | 15.831 | 95.95 | 104.603 | 82.159 | 102.085 | 117.296 |
| Gallic Acid | 103.159 | 25.572 | 29.57 | 299.883 | 271.192 | 199.412 | 411.845 | 472.836 |
| ρ-hydroxybenzoic Acid | 208.729 | 6.675 | 7.91 | 0 | 0 | 2.714 | 519.272 | 110.807 |
| Vanillic Acid | 76.269 | 3.857 | 21.942 | 111.614 | 78.035 | 66.206 | 131.375 | 161.822 |
| Caffeic Acid | 36.889 | 5.012 | 12.869 | 153.203 | 128.947 | 87.069 | 193.761 | 120.565 |
| ρ-coumaric Acid | 25.237 | 6.749 | 13.033 | 92.729 | 39.48 | 35.685 | 87.501 | 150.997 |
| Ferulic Acid | 23.335 | 9.32 | 15.635 | 86.098 | 60.814 | 46.106 | 83.422 | 82.53 |
| Chicoric Acid | 48.746 | 4.385 | 12.704 | 1873.188 | 2270.211 | 1208.203 | 4991.813 | 2843.772 |
| Quercetin | 26.993 | 1.992 | 9.71 | 65.891 | 57.743 | 46.599 | 105.185 | 135.457 |
| Total | 574.963 | 67.693 | 139.204 | 2778.556 | 3011.025 | 1774.153 | 6626.259 | 4196.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Messina, C.M.; Arena, R.; Manuguerra, S.; Pericot, Y.; Curcuraci, E.; Kerninon, F.; Renda, G.; Hellio, C.; Santulli, A. Antioxidant Bioactivity of Extracts from Beach Cast Leaves of Posidonia oceanica (L.) Delile. Mar. Drugs 2021, 19, 560. https://doi.org/10.3390/md19100560
Messina CM, Arena R, Manuguerra S, Pericot Y, Curcuraci E, Kerninon F, Renda G, Hellio C, Santulli A. Antioxidant Bioactivity of Extracts from Beach Cast Leaves of Posidonia oceanica (L.) Delile. Marine Drugs. 2021; 19(10):560. https://doi.org/10.3390/md19100560
Chicago/Turabian StyleMessina, Concetta Maria, Rosaria Arena, Simona Manuguerra, Yann Pericot, Eleonora Curcuraci, Fanny Kerninon, Giuseppe Renda, Claire Hellio, and Andrea Santulli. 2021. "Antioxidant Bioactivity of Extracts from Beach Cast Leaves of Posidonia oceanica (L.) Delile" Marine Drugs 19, no. 10: 560. https://doi.org/10.3390/md19100560
APA StyleMessina, C. M., Arena, R., Manuguerra, S., Pericot, Y., Curcuraci, E., Kerninon, F., Renda, G., Hellio, C., & Santulli, A. (2021). Antioxidant Bioactivity of Extracts from Beach Cast Leaves of Posidonia oceanica (L.) Delile. Marine Drugs, 19(10), 560. https://doi.org/10.3390/md19100560







