Marine Algae-Derived Bioactive Compounds: A New Wave of Nanodrugs?
Abstract
1. Introduction
2. Algal-Sourced Compounds of Medical Interest
2.1. Fatty Acid Content
2.2. Protein Content
2.3. Carbohydrate Content
2.4. Mineral Content
2.5. Vitamin Content
2.6. Pigments
2.7. Polyphenols
3. Qualitative and Quantitative Aspects of Algal-Derived Biocompounds
4. Influence of Biotic and Abiotic Factors in the Production of Algal Biocompounds
5. From Basic Research to Translational Nanomedicine: Advancements and Prospects
5.1. Algal-Derived Compounds and Derived Nanotheranostics for Diabetes
5.2. Algal-Derived Compounds and Nanotheranostics for Neurodegenerative Disorders
6. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AChE | Acetylcholesterinase |
| AD | Alzheimer’s disease |
| As | Arsenic |
| Ca | Calcio |
| CE | Capillary electrophesis |
| CNS | Central nervous system |
| Cu | Copper |
| CVD | Cardiovascular disease |
| DAD | Diode array detection |
| DHA | Docosahexanoic acid |
| DM | Diabetes mellitus |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| ESI | Electrospray ionisation |
| EPA | Eicosapentaenoic acid |
| FAs | Fatty acids |
| FAME | FA methyl esther |
| FID | Flame ionization detector |
| FDA | Food and Drug Administration |
| Fe | Iron |
| FTIR | Fourrier-transform infrared |
| GC | Gas chromatography |
| HPLC | High-performance liquid chromatography |
| I | Iodine |
| K | Potassium |
| LDL | Low-density lipoprotein |
| LPS | Lipopolysaccharides |
| MAA | Mycosporine-like amino acid |
| MAPK | Mitogen-activated protein kinase |
| MDR | Multidrug resistance |
| Mg | Magnesium |
| Mn | Manganese |
| MMPs | Matrix metalloproteinases |
| MNPs | Metallic NPs |
| MS | Mass spectrometry |
| Na | Sodium |
| NMR | Nuclear magnetic resonance |
| NPs | Nanoparticles |
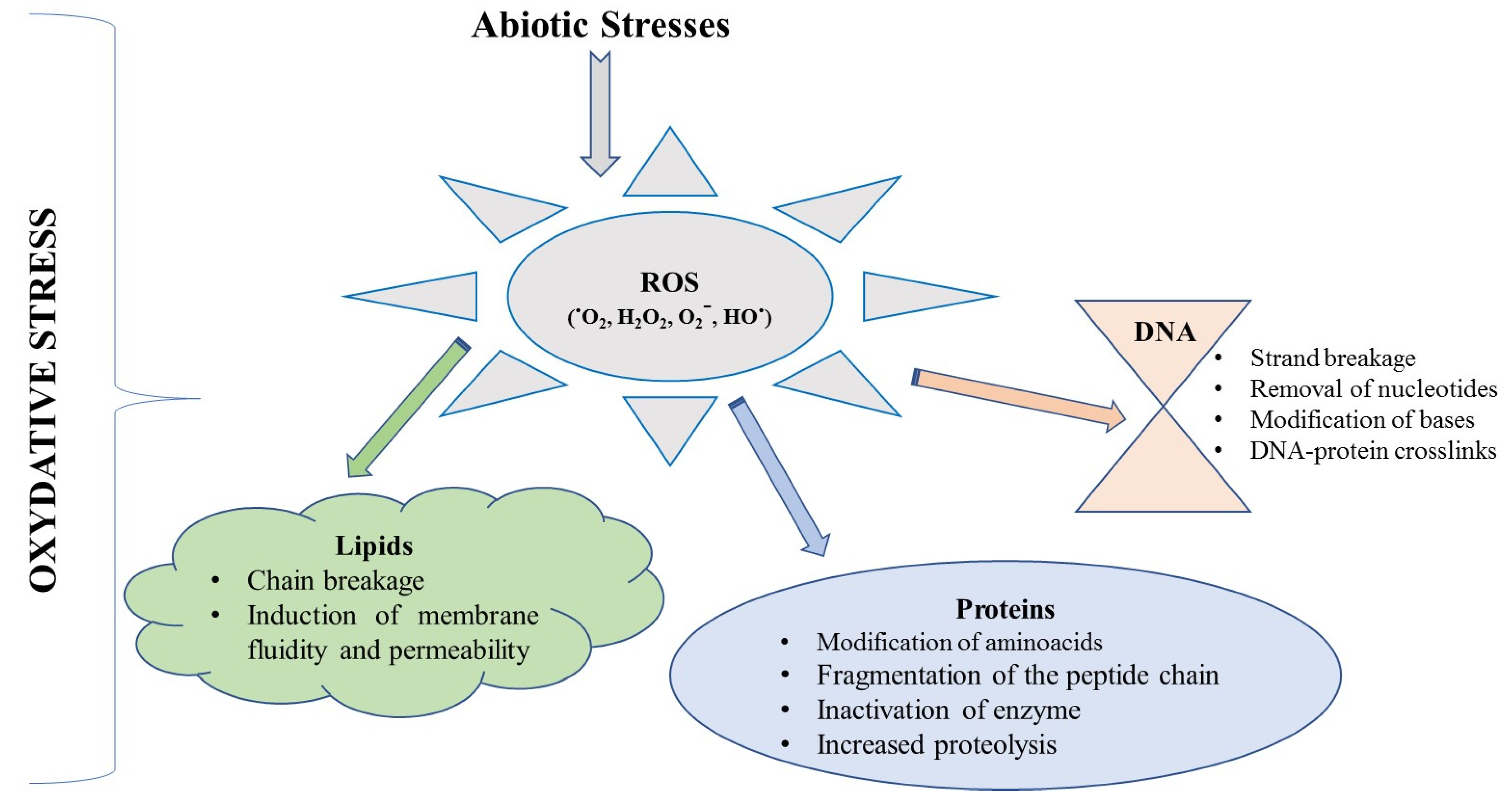
| OS | Oxidative stress |
| P | Phosphorus |
| PC | Phycocyanin |
| PD | Parkinson’s disease |
| PE | Phycoerythrin |
| PUFAs | Polyunsaturaed fatty acids |
| ROS | Reactive oxygen species |
| SFAs | Saturated FAs |
| TLC | Thin-layer chromatography |
| UV–Vis | Ultraviolet–visible |
| VEGF | Vascular endothelial growth factor |
| WHO | World Health Organization |
| Zn | Zinc |
References
- Silva, C.O.; Pinho, J.O.; Lopes, J.M.; Almeida, A.J.; Gaspar, M.M.; Reis, C. Current trends in cancer nanotheranostics: Metallic, polymeric, and lipid-based systems. Pharmaceutics 2019, 11, 22. [Google Scholar] [CrossRef]
- Ventola, C.L.; Bharali, D.J.; Mousa, S.A. The Nanomedicine Revolution: Part 1: Emerging Concepts. Pharmacy and Therapeutics. Pharmacol. Ther. 2010, 128, 512–525. [Google Scholar]
- Khanna, P.; Kaur, A.; Goyal, D. Algae-based metallic nanoparticles: Synthesis, characterization and applications. J. Microbiol. Methods 2019, 163, 105656. [Google Scholar] [CrossRef]
- Menaa, F. When Pharma Meets Nano or The Emerging Era of Nano-Pharmaceuticals. Pharm. Anal. Acta 2013, 4, 223. [Google Scholar] [CrossRef]
- Razzaq, A.; Khan, Z.U.; Saeed, A.; Shah, K.A.; Khan, N.U.; Menaa, B.; Iqbal, H.; Menaa, F. Development of cephradine-loaded gelatin/polyvinyl alcohol electrospun nanofibers for effective diabetic wound healing: In-vitro and in-vivo assessments. Pharmaceutics 2021, 13, 349. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, H.; Khan, B.A.; Khan, Z.U.; Razzaq, A.; Khan, N.U.; Menaa, B.; Menaa, F. Fabrication, physical characterizations and in vitro antibacterial activity of cefadroxil-loaded chitosan/poly(vinyl alcohol) nanofibers against Staphylococcus aureus clinical isolates. Int. J. Biol. Macromol. 2020, 144, 921–931. [Google Scholar] [CrossRef] [PubMed]
- Uzair, B.; Liaqat, A.; Iqbal, H.; Menaa, B.; Razzaq, A.; Thiripuranathar, G.; Rana, N.F.; Menaa, F. Green and cost-effective synthesis of metallic nanoparticles by algae: Safe methods for translational medicine. Bioengineering 2020, 7, 129. [Google Scholar] [CrossRef]
- Riaz, S.; Rana, N.F.; Hussain, I.; Tanweer, T.; Nawaz, A.; Menaa, F.; Janjua, H.A.; Alam, T.; Batool, A.; Naeem, A.; et al. Effect of flavonoid-coated gold nanoparticles on bacterial colonization in mice organs. Nanomaterials 2020, 10, 1769. [Google Scholar] [CrossRef]
- Venkatesan, J.; Kim, S.K.; Shim, M.S. Antimicrobial, antioxidant, and anticancer activities of biosynthesized silver nanoparticles using marine algae ecklonia cava. Nanomaterials 2016, 6, 235. [Google Scholar] [CrossRef]
- Batool, A.; Menaa, F.; Uzair, B.; Khan, B.A.; Menaa, B. Progress and Prospects in Translating Nanobiotechnology in Medical Theranostics. Curr. Nanosci. 2019, 16, 685–707. [Google Scholar] [CrossRef]
- Sathishkumar, R.S.; Sundaramanickam, A.; Srinath, R.; Ramesh, T.; Saranya, K.; Meena, M.; Surya, P. Green synthesis of silver nanoparticles by bloom forming marine microalgae Trichodesmium erythraeum and its applications in antioxidant, drug-resistant bacteria, and cytotoxicity activity. J. Saudi Chem. Soc. 2019, 23, 1180–1191. [Google Scholar] [CrossRef]
- Dubowy, C.; Sehgal, A. Circadian rhythms and sleep in Drosophila melanogaster. Genetics 2017, 205, 1373–1397. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Shukla, S.; Kang, S.M.; Hwang, S.K.; Song, X.; Huh, Y.S.; Han, Y.K. Developments of cyanobacteria for nano-marine drugs: Relevance of nanoformulations in cancer therapies. Mar. Drugs 2018, 16, 179. [Google Scholar] [CrossRef]
- Soares, S.; Sousa, J.; Pais, A.; Vitorino, C. Nanomedicine: Principles, properties, and regulatory issues. Front. Chem. 2018, 6, 360. [Google Scholar] [CrossRef]
- Saadaoui, I.; Rasheed, R.; Abdulrahman, N.; Bounnit, T.; Cherif, M.; Al Jabri, H.; Mraiche, F. Algae-derived bioactive compounds with anti-lung cancer potential. Mar. Drugs 2020, 18, 197. [Google Scholar] [CrossRef] [PubMed]
- Menaa, F.; Fatemeh, Y.; Vashist, S.K.; Iqbal, H.; Sharts, O.N.; Menaa, B. Graphene, an Interesting Nanocarbon Allotrope for Biosensing Applications: Advances, Insights, and Prospects. Biomed. Eng. Comput. Biol. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Priya, P.; Sivamurugan, V.; Murugesan, S.; Shanthi, N. Biosynthesized Nanomaterials as Nanomedicine; JPS Scientific Publications: Tamil Nadu, India, 2018; p. 58. ISBN 978-81-935636-6-3. [Google Scholar]
- Riehemann, K.; Schneider, S.W.; Luger, T.A.; Godin, B.; Ferrari, M.; Fuchs, H. Nanomedicine—Challenge and perspectives. Angew. Chem. Int. Ed. 2009, 48, 872–897. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, S.; Petry, F.C.; Mercadante, A.Z.; Jacob-lopes, E.; Zepka, L.Q. Current Research in Food Science HPLC-PDA-MS/MS as a strategy to characterize and quantify natural pigments from microalgae. Curr. Res. Food Sci. 2020, 3, 100–112. [Google Scholar] [CrossRef]
- Zafar, N.; Uzair, B.; Niazi, M.B.K.; Samin, G.; Bano, A.; Jamil, N.; Sajjad, S.; Menaa, F. Erratum: Zafar et al. Synthesis and characterization of potent and safe ciprofloxacin-loaded Ag/TiO2/Cs nanohybrid against mastitis-causing E. coli. Crystals 2021, 11, 319. [Google Scholar] [CrossRef]
- Fawcett, D.; Verduin, J.J.; Shah, M.; Sharma, S.B.; Poinern, G.E.J. A Review of Current Research into the Biogenic Synthesis of Metal and Metal Oxide Nanoparticles via Marine Algae and Seagrasses. J. Nanosci. 2017, 2017, 8013850. [Google Scholar] [CrossRef]
- Vadlapudi, V.; Kaladhar, D.S.V.G.K.; Naidu, G.K.; Sujatha, B. International Journal of Chemical Studies Review: Green Synthesis of Silver and Gold nanoparticles. Chemijournal 2013, 1, 22–31. [Google Scholar]
- Sharma, A.; Sharma, S.; Sharma, K.; Chetri, S.P.K.; Vashishtha, A.; Singh, P.; Kumar, R.; Rathi, B.; Agrawal, V. Algae as crucial organisms in advancing nanotechnology: A systematic review. J. Appl. Phycol. 2016, 28, 1759–1774. [Google Scholar] [CrossRef]
- Menaa, F.; Wijesinghe, P.A.U.I.; Thiripuranathar, G.; Uzair, B.; Iqbal, H.; Khan, B.A.; Menaa, B. Ecological and Industrial Implications of Dynamic Seaweed-Associated Microbiota Interactions. Mar. Drugs 2020, 18, 641. [Google Scholar] [CrossRef]
- Nasab, S.B.; Homaei, A.; Pletschke, B.I.; Salinas-Salazar, C.; Castillo-Zacarias, C.; Parra-Saldívar, R. Marine resources effective in controlling and treating diabetes and its associated complications. Process Biochem. 2020, 92, 313–342. [Google Scholar] [CrossRef]
- Leandro, A.; Pereira, L.; Gonçalves, A.M.M. Diverse applications of marine macroalgae. Mar. Drugs 2020, 18, 17. [Google Scholar] [CrossRef]
- Myklestad, S.M.; Granum, E. Biology of (1,3)-β-Glucans and Related Glucans in Protozoans and Chromistans; Elsevier Inc.: Amsterdam, The Netherlands, 2009; pp. 353–385. [Google Scholar]
- Miazek, K.; Iwanek, W.; Remacle, C.; Richel, A.; Goffin, D. Effect of Metals, Metalloids and Metallic Nanoparticles on Microalgae Growth and Industrial Product Biosynthesis: A Review. Int. J. Mol. Sci. 2015, 16, 23929–23969. [Google Scholar] [CrossRef]
- Heffernan, N.; Smyth, T.J.; Fitzgerald, R.J.; Soler-Vila, A.; Brunton, N. Antioxidant activity and phenolic content of pressurised liquid and solid-liquid extracts from four Irish origin macroalgae. Int. J. Food Sci. Technol. 2014, 49, 1765–1772. [Google Scholar] [CrossRef]
- Olasehinde, T.A.; Olaniran, A.O.; Okoh, A.I.; Koulen, P. Therapeutic potentials of microalgae in the treatment of Alzheimer’s disease. Molecules 2017, 22, 480. [Google Scholar] [CrossRef]
- Couteau, C.; Coiffard, L. Seaweed Application in Cosmetics. In Seaweed in Health and Disease Prevention; Academic Press: Cambridge, MA, USA, 2016; pp. 423–441. [Google Scholar]
- Gegg, P.; Wells, V. The development of seaweed-derived fuels in the UK: An analysis of stakeholder issues and public perceptions. Energy Policy 2019, 133, 110924. [Google Scholar] [CrossRef]
- Pérez, M.J.; Falqué, E.; Domínguez, H. Antimicrobial action of compounds from marine seaweed. Mar. Drugs 2016, 14, 52. [Google Scholar] [CrossRef] [PubMed]
- Darki, B.Z.; Krakhmalnyi, A.F. Biotic and abiotic factors affecting the population dynamics of Ceratium hirundinella, Peridinium cinctum, and Peridiniopsis elpatiewskyi. Diversity 2019, 11, 137. [Google Scholar] [CrossRef]
- Cheong, K.L.; Qiu, H.M.; Du, H.; Liu, Y.; Khan, B.M. Oligosaccharides derived from red seaweed: Production, properties, and potential health and cosmetic applications. Molecules 2018, 23, 2451. [Google Scholar] [CrossRef]
- Alassali, A.; Cybulska, I. Methods for Upstream Extraction and Chemical Characterization of Secondary Metabolites from Algae Biomass. Adv. Tech. Biol. Med. 2015, 4, 163. [Google Scholar] [CrossRef]
- Khalid, M.; Khalid, N.; Ahmed, I.; Hanif, R. Comparative studies of three novel freshwater microalgae strains for synthesis of silver nanoparticles: Insights of characterization, antibacterial, cytotoxicity and antiviral activities. J. Appl. Phycol. 2017, 29, 1851–1863. [Google Scholar] [CrossRef]
- Probst, Y. A review of the nutrient composition of selected rubus berries. Nutr. Food Sci. 2015, 45, 242–254. [Google Scholar] [CrossRef]
- Mazarrasa, I.; Olsen, Y.S.; Mayol, E.; Marbà, N.; Duarte, C.M. Global unbalance in seaweed production, research effort and biotechnology markets. Biotechnol. Adv. 2014, 32, 1028–1036. [Google Scholar] [CrossRef]
- Gupta, S.; Abu-ghannam, N. Recent developments in the application of seaweeds or seaweed extracts as a means for enhancing the safety and quality attributes of foods. Innov. Food Sci. Emerg. Technol. 2011, 12, 600–609. [Google Scholar] [CrossRef]
- Jiménez-Escrig, A.; Gómez-Ordóñez, E.; Rupérez, P. Seaweed as a source of novel nutraceuticals: Sulfated polysaccharides and peptides. Adv. Food Nutr. Res. 2011, 64, 325–337. [Google Scholar] [PubMed]
- Ramos-Romero, S.; Torrella, J.R.; Pagès, T.; Viscor, G.; Torres, J.L. Edible microalgae and their bioactive compounds in the prevention and treatment of metabolic alterations. Nutrients 2021, 13, 563. [Google Scholar] [CrossRef] [PubMed]
- Rocha, D.H.A.; Seca, A.M.L.; Pinto, D.C.G.A. Seaweed secondary metabolites in vitro and in vivo anticancer activity. Mar. Drugs 2018, 16, 410. [Google Scholar] [CrossRef]
- Iwai, K. Antidiabetic and antioxidant effects of polyphenols in brown alga Ecklonia stolonifera in genetically diabetic KK-Ay mice. Plant Foods Hum. Nutr. 2008, 63, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Bălașa, A.F.; Chircov, C.; Grumezescu, A.M. Marine Biocompounds for Neuroprotection-A Review. Mar. Drugs 2020, 18, 290. [Google Scholar] [CrossRef]
- Olasehinde, T.A.; Olaniran, A.O.; Okoh, A.I. Macroalgae as a valuable source of naturally occurring bioactive compounds for the treatment of Alzheimer’s disease. Mar. Drugs 2019, 17, 609. [Google Scholar] [CrossRef]
- Ernst, A.U.; Bowers, D.T.; Wang, L.H.; Shariati, K.; Plesser, M.D.; Brown, N.K.; Mehrabyan, T.; Ma, M. Nanotechnology in cell replacement therapies for type 1 diabetes. Adv. Drug Deliv. Rev. 2019, 139, 116–138. [Google Scholar] [CrossRef]
- Hamid, N.; Ma, Q.; Boulom, S.; Liu, T.; Zheng, Z.; Balbas, J.; Robertson, J. Seaweed Minor Constituents; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 193–242. [Google Scholar]
- Macartain, P.; Gill, C.I.R.; Brooks, M.; Campbell, R.; Rowland, I.R. Special Article Nutritional Value of Edible Seaweeds. Nutr. Rev. 2007, 65, 535–543. [Google Scholar] [CrossRef] [PubMed]
- da Vaz, B.S.; Moreira, J.B.; De Morais, M.G.; Costa, J.A.V. Microalgae as a new source of bioactive compounds in food supplements. Curr. Opin. Food Sci. 2016, 7, 73–77. [Google Scholar] [CrossRef]
- Peng, Y.; Hu, J.; Yang, B.; Lin, X.P.; Zhou, X.F.; Yang, X.W.; Liu, Y. Chemical Composition of Seaweeds; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 79–124. [Google Scholar]
- Qi, H.; Zhang, Q.; Zhao, T.; Chen, R.; Zhang, H.; Niu, X.; Li, Z. Antioxidant activity of different sulfate content derivatives of polysaccharide extracted from Ulva pertusa (Chlorophyta) in vitro. Int. J. Biol. Macromol. 2005, 37, 195–199. [Google Scholar] [CrossRef]
- Yan, X.; Chuda, Y.; Suzuki, M.; Nagata, T. Fucoxanthin as the major antioxidant in hijikia fusiformis, a common edible seaweed. Biosci. Biotechnol. Biochem. 1999, 63, 605–607. [Google Scholar] [CrossRef]
- Cunha, L.; Grenha, A. Sulfated seaweed polysaccharides as multifunctional materials in drug delivery applications. Mar. Drugs 2016, 14, 42. [Google Scholar] [CrossRef]
- Jia, X.; Yang, J.; Wang, Z.; Liu, R.; Xie, R. Polysaccharides from Laminaria japonica show hypoglycemic and hypolipidemic activities in mice with experimentally induced diabetes. Exp. Biol. Med. 2014, 239, 1663–1670. [Google Scholar] [CrossRef]
- Aryee, A.N.; Agyei, D.; Akanbi, T.O. Recovery and utilization of seaweed pigments in food processing. Curr. Opin. Food Sci. 2018, 19, 113–119. [Google Scholar] [CrossRef]
- Cherry, P.; O’hara, C.; Magee, P.J.; Mcsorley, E.M.; Allsopp, P.J. Risks and benefits of consuming edible seaweeds. Nutr. Rev. 2019, 77, 307–329. [Google Scholar] [CrossRef]
- Pangestuti, R.; Kim, S. Seaweed Proteins, Peptides, and Amino Acids; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 125–140. [Google Scholar]
- Mohamed, S.; Hashim, S.N.; Rahman, A. Seaweeds: A sustainable functional food for complementary and alternative therapy. Trends Food Sci. Technol. 2012, 23, 83–96. [Google Scholar] [CrossRef]
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Pereira, L. Seaweeds as source of bioactive substances and skin care therapy–Cosmeceuticals, algotheraphy, and thalassotherapy. Cosmetics 2018, 5, 68. [Google Scholar] [CrossRef]
- Cherry, P.; Yadav, S.; Strain, C.R.; Allsopp, P.J.; Mcsorley, E.M.; Ross, R.P.; Stanton, C. Prebiotics from seaweeds: An ocean of opportunity? Mar. Drugs 2019, 17, 327. [Google Scholar] [CrossRef]
- Venugopal, V. Sulfated and non-sulfated polysaccharides from seaweeds and their uses: An overview. ECronicon Nutr. 2019, 2, 126–141. [Google Scholar]
- Damonte, E.; Matulewicz, M.; Cerezo, A. Sulfated Seaweed Polysaccharides as Antiviral Agents. Curr. Med. Chem. 2012, 11, 2399–2419. [Google Scholar] [CrossRef] [PubMed]
- De Morais, M.G.; Vaz, B.D.S.; De Morais, E.G.; Costa, J.A.V. Biologically Active Metabolites Synthesized by Microalgae. Biomed Res. Int. 2015, 2015, 835761. [Google Scholar] [CrossRef] [PubMed]
- Cian, R.E.; Drago, S.R.; De Medina, F.S.; Martínez-Augustin, O. Proteins and carbohydrates from red seaweeds: Evidence for beneficial effects on gut function and microbiota. Mar. Drugs 2015, 13, 5358–5383. [Google Scholar] [CrossRef] [PubMed]
- Yoon, N.Y.; Lee, S.H.; Yong, L.; Kim, S.K. Phlorotannins from Ishige okamurae and their acetyl- and butyrylcholinesterase inhibitory effects. J. Funct. Foods 2009, 1, 331–335. [Google Scholar] [CrossRef]
- Janarthanan, M.; Kumar, M.S. Qualitative and Quantitative Analysis of Phytochemical Studies on Selected Seaweeds Acanthopora Spicifera and Sargassum Wightii. Int. J. Eng. Res. Dev. 2013, 7, 11–15. [Google Scholar]
- Imran, M.A.S.; Bhuiyan, F.R.; Ahmed, S.R.; Shanzana, P.; Moli, M.A.; Foysal, S.H.; Dabi, S.B.; Hasan, M. Phytochemical constituency profiling and antimicrobial activity screening of seaweeds extracts collected from the Bay of Bengal sea coasts. J. Adv. Biotechnol. Exp. Ther. 2021, 4, 25–34. [Google Scholar] [CrossRef]
- Dias, V.; Bandeira, S.; Chaúque, E.; Lipassula, M.; Mussagy, A. Evaluation of Phytocompounds and Chemical Elements Present In Selected Species of Seaweeds, to Sustain Future Quantitative Analysis for Bioactive Compounds. J. Drug Deliv. Ther. 2020, 10, 232–239. [Google Scholar] [CrossRef]
- Campbell, A.H.; Harder, T.; Nielsen, S.; Kjelleberg, S.; Steinberg, P.D. Climate change and disease: Bleaching of a chemically defended seaweed. Glob. Chang. Biol. 2011, 17, 2958–2970. [Google Scholar] [CrossRef]
- Harrison, P.J.; Hurd, C.L. Nutrient physiology of seaweeds: Application of concepts to aquaculture. Cah. Biol. Mar. 2001, 42, 71–82. [Google Scholar]
- Marinho, G.S.; Sørensen, A.D.M.; Safafar, H.; Pedersen, A.H.; Holdt, S.L. Antioxidant content and activity of the seaweed Saccharina latissima: A seasonal perspective. J. Appl. Phycol. 2019, 31, 1343–1354. [Google Scholar] [CrossRef]
- Marinho-Soriano, E.; Fonseca, P.C.; Carneiro, M.A.A.; Moreira, W.S.C. Seasonal variation in the chemical composition of two tropical seaweeds. Bioresour. Technol. 2006, 97, 2402–2406. [Google Scholar] [CrossRef]
- De Jesus Raposo, M.F.; De Morais, A.M.M.B.; De Morais, R.M.S.C. Emergent sources of prebiotics: Seaweeds and microalgae. Mar. Drugs 2016, 14, 27. [Google Scholar] [CrossRef] [PubMed]
- Olasehinde, T.A.; Olaniran, A.O.; Okoh, A.I. Phenolic composition, antioxidant activity, anticholinesterase potential and modulatory effects of aqueous extracts of some seaweeds on β-amyloid aggregation and disaggregation. Pharm. Biol. 2019, 57, 460–469. [Google Scholar] [CrossRef]
- Astorga-España, M.S.; Rodríguez Galdón, B.; Rodríguez Rodríguez, E.M.; Díaz Romero, C. Mineral and trace element concentrations in seaweeds from the sub-Antarctic ecoregion of Magallanes (Chile). J. Food Compos. Anal. 2015, 39, 69–76. [Google Scholar] [CrossRef]
- Augustine, R.; Hasan, A. Journal of Drug Delivery Science and Technology Emerging applications of biocompatible phytosynthesized metal / metal oxide nanoparticles in healthcare. J. Drug Deliv. Sci. Technol. 2020, 56, 101516. [Google Scholar] [CrossRef]
- Usov, A.I. Polysaccharides of the Red Algae, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2011; Volume 65, pp. 115–217. [Google Scholar]
- Rodríguez-Meizoso, I.; Jaime, L.; Santoyo, S.; Cifuentes, A.; García-Blairsy Reina, G.; Señoráns, F.J.; Ibáñez, E. Pressurized fluid extraction of bioactive compounds from Phormidium species. J. Agric. Food Chem. 2008, 56, 3517–3523. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.; Manning, S.; Montoya, M.; Keller, K.; Poenie, M. Extraction of algal lipids and their analysis by HPLC and mass spectrometry. J. Am. Oil Chem. Soc. 2012, 89, 1371–1381. [Google Scholar] [CrossRef]
- Souza, B.W.S.; Cerqueira, M.A.; Martins, J.T.; Quintas, M.A.C.; Ferreira, A.C.S.; Teixeira, J.A.; Vicente, A.A. Antioxidant potential of two red seaweeds from the Brazilian coasts. J. Agric. Food Chem. 2011, 59, 5589–5594. [Google Scholar] [CrossRef]
- Norziah, M.H.; Ching, C.Y. Nutritional composition of edible seaweed Gracilaria changgi. Food Chem. 2000, 68, 69–76. [Google Scholar] [CrossRef]
- Plaza, M.; Santoyo, S.; Jaime, L.; García-Blairsy Reina, G.; Herrero, M.; Señoráns, F.J.; Ibáñez, E. Screening for bioactive compounds from algae. J. Pharm. Biomed. Anal. 2010, 51, 450–455. [Google Scholar] [CrossRef]
- Halim, R.; Gladman, B.; Danquah, M.K.; Webley, P.A. Oil extraction from microalgae for biodiesel production. Bioresour. Technol. 2011, 102, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.; Caridade, S.G.; Mano, J.F.; Sousa, R.A.; Reis, R.L. Extraction and physico-chemical characterization of a versatile biodegradable polysaccharide obtained from green algae. Carbohydr. Res. 2010, 345, 2194–2200. [Google Scholar] [CrossRef] [PubMed]
- Mendiola, J.A.; Jaime, L.; Santoyo, S.; Reglero, G.; Cifuentes, A.; Ibañez, E.; Señoráns, F.J. Screening of functional compounds in supercritical fluid extracts from Spirulina platensis. Food Chem. 2007, 102, 1357–1367. [Google Scholar] [CrossRef]
- Khan, M.I.; Shin, J.H.; Kim, J.D. The promising future of microalgae: Current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb. Cell Fact. 2018, 17, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, M.T.S.D.; Leal, M.F.C. Seasonal variability in the kinetics of Cu, Pb, Cd and Hg accumulation by macroalgae. Mar. Chem. 2001, 74, 65–85. [Google Scholar] [CrossRef]
- Kroeker, K.J.; Micheli, F.; Gambi, M.C. Ocean acidification causes ecosystem shifts via altered competitive interactions. Nat. Clim. Chang. 2013, 3, 156–159. [Google Scholar] [CrossRef]
- Zozaya-Valdés, E.; Roth-Schulze, A.J.; Thomas, T. Effects of temperature stress and aquarium conditions on the red macroalga Delisea pulchra and its associated microbial community. Front. Microbiol. 2016, 7, 1–10. [Google Scholar] [CrossRef]
- Buchholz, C.M.; Krause, G.; Buck, B.H. Seaweed Biology. Seaweed Biol. 2012, 219, 471–493. [Google Scholar]
- Wernberg, T.; Smale, D.A.; Tuya, F.; Thomsen, M.S.; Langlois, T.J.; De Bettignies, T.; Bennett, S.; Rousseaux, C.S. An extreme climatic event alters marine ecosystem structure in a global biodiversity hotspot. Nat. Clim. Chang. 2013, 3, 78–82. [Google Scholar] [CrossRef]
- Schaffelke, B.; Hewitt, C.L. Impacts of Introduced Seaweeds. In Seaweed Invasions: A Synthesis of Ecological, Economic and Legal Imperatives; De Gruyter: Berlin, Germany, 2008; pp. 77–97. [Google Scholar]
- Guerra-Rivas, G.; Gómez-Gutiérrez, C.M.; Alarcón-Arteaga, G.; Soria-Mercado, I.E.; Ayala-Sánchez, N.E. Screening for anticoagulant activity in marine algae from the Northwest Mexican Pacific coast. J. Appl. Phycol. 2011, 23, 495–503. [Google Scholar] [CrossRef]
- Khairy, H.M.; El-Shafay, S.M. Seasonal variations in the biochemical composition of some common seaweed species from the coast of Abu Qir Bay, Alexandria, Egypt. Oceanologia 2013, 55, 435–452. [Google Scholar] [CrossRef]
- Shafey, A.M. El Green synthesis of metal and metal oxide nanoparticles from plant leaf extracts and their applications: A review. Green Process. Synth. 2020, 9, 304–339. [Google Scholar] [CrossRef]
- Ruocco, N.; Costantini, S.; Guariniello, S.; Costantini, M. Polysaccharides from the marine environment with pharmacological, cosmeceutical and nutraceutical potential. Molecules 2016, 21, 551. [Google Scholar] [CrossRef]
- Serebryakova, A.; Aires, T.; Viard, F.; Serrão, E.A.; Engelen, A.H. Summer shifts of bacterial communities associated with the invasive brown seaweed Sargassum muticum are location and tissue dependent. PLoS ONE 2018, 13, e0206734. [Google Scholar] [CrossRef]
- Guariguata, L.; Whiting, D.R.; Hambleton, I.; Beagley, J.; Linnenkamp, U.; Shaw, J.E. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res. Clin. Pract. 2014, 103, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.M.M.; Zimmet, P.Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diabet. Med. 1998, 15, 539–553. [Google Scholar] [CrossRef]
- Noble, J.A.; Valdes, A.M.; Varney, M.D.; Carlson, J.A.; Moonsamy, P.; Fear, A.L.; Lane, J.A.; Lavant, E.; Rappner, R.; Louey, A.; et al. HLA class I and genetic susceptibility to type 1 diabetes: Results from the type 1 diabetes genetics consortium. Diabetes 2010, 59, 2972–2979. [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.J.; Morgan, N.G.; Foulis, A.K. Pancreatic pathology in type 1 diabetes mellitus. Endocr. Pathol. 2014, 25, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Forbes, J.M.; Cooper, M.E. Mechanisms of diabetic complications. Physiol. Rev. 2013, 93, 137–188. [Google Scholar] [CrossRef]
- Szablewski, L. Role of immune system in type 1 diabetes mellitus pathogenesis. Int. Immunopharmacol. 2014, 22, 182–191. [Google Scholar] [CrossRef]
- Morran, M.P.; Vonberg, A.; Khadra, A.; Pietropaolo, M. Immunogenetics of type 1 diabetes mellitus. Mol. Aspects Med. 2015, 42, 42–60. [Google Scholar] [CrossRef] [PubMed]
- Ounissi-Benkalha, H.; Polychronakos, C. The molecular genetics of type 1 diabetes: New genes and emerging mechanisms. Trends Mol. Med. 2008, 14, 268–275. [Google Scholar] [CrossRef]
- Fonseca, V.A. Defining and characterizing the progression of type 2 diabetes. Diabetes Care 2009, 32, 151–156. [Google Scholar] [CrossRef]
- Lin, Y.; Sun, Z. Current views on type 2 diabetes. J. Endocrinol. 2010, 204, 1–11. [Google Scholar] [CrossRef]
- Prokopenko, I.; McCarthy, M.I.; Lindgren, C.M. Type 2 diabetes: New genes, new understanding. Trends Genet. 2008, 24, 613–621. [Google Scholar] [CrossRef]
- Salonen, J.T.; Uimari, P.; Aalto, J.M.; Pirskanen, M.; Kaikkonen, J.; Todorova, B.; Hyppönen, J.; Korhonen, V.P.; Asikainen, J.; Devine, C.; et al. Type 2 diabetes whole-genome association study in four populations: The DiaGen consortium. Am. J. Hum. Genet. 2007, 81, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Nolan, C.J.; Damm, P.; Prentki, M. Type 2 diabetes across generations: From pathophysiology to prevention and management. Lancet 2011, 378, 169–181. [Google Scholar] [CrossRef]
- Buchanan, T.A.; Xiang, A.H. Gestational diabetes mellitus. J. Clin. Investig. 2005, 115, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Craig, M.E.; Hattersley, A.; Donaghue, K.C. Definition, epidemiology and classification of diabetes in children and adolescents. Pediatr. Diabetes 2009, 10, 3–12. [Google Scholar] [CrossRef]
- Fantonalgo, R.N. Hypoglycemic and Laxative Activities of Crude Ethanolic Extracts of Brown Seaweed Sargassum Oligocystum. J. Nat. Sci. Res. 2017, 7, 45–52. [Google Scholar]
- Faerch, K.; Hulman, A.; Solomon, T.P.J. Heterogeneity of Pre-diabetes and Type 2 Diabetes: Implications for Prediction, Prevention and Treatment Responsiveness. Curr. Diabetes Rev. 2015, 12, 30–41. [Google Scholar] [CrossRef]
- Motshakeri, M.; Ebrahimi, M.; Goh, Y.M.; Matanjun, P.; Mohamed, S. Sargassum polycystum reduces hyperglycaemia, dyslipidaemia and oxidative stress via increasing insulin sensitivity in a rat model of type 2 diabetes. J. Sci. Food Agric. 2013, 93, 1772–1778. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Yang, C.; Liu, B.; Lin, L.; Sarker, S.D.; Nahar, L.; Yu, H.; Cao, H.; Xiao, J. Bioactive compounds from marine macroalgae and their hypoglycemic benefits. Trends Food Sci. Technol. 2018, 72, 1–12. [Google Scholar] [CrossRef]
- Anand, K.; Tiloke, C.; Naidoo, P.; Chuturgoon, A.A. Phytonanotherapy for management of diabetes using green synthesis nanoparticles. J. Photochem. Photobiol. B Biol. 2017, 173, 626–639. [Google Scholar] [CrossRef]
- Shah, M.; Fawcett, D.; Sharma, S.; Tripathy, S.K.; Poinern, G.E.J. Green synthesis of metallic nanoparticles via biological entities. Materials 2015, 8, 7278–7308. [Google Scholar] [CrossRef]
- Ogunwa, T.H.; Adeyelu, T.T.; Fasimoye, R.Y.; Ayenitaju, F.C. In Silico Analysis of Interaction between Seaweed-Derived Bioactive Compounds and Selected Diabetes-Related Targets. Biomed. Chem. Res. Methods 2018, 1, e00074. [Google Scholar] [CrossRef]
- Lee, Y.S.; Shin, K.H.; Kim, B.K.; Lee, S. Anti-diabetic activities of fucosterol from Pelvetia siliquosa. Arch. Pharm. Res. 2004, 27, 1120–1122. [Google Scholar] [CrossRef] [PubMed]
- Ohta, T.; Sasaki, S.; Oohori, T.; Yoshikawa, S.; Kurihara, H. A-Glucosidase Inhibitory Activity of a 70% Methanol Extract From Ezoishige (Pelvetia Babingtonii De Toni) and Its Effect on the Elevation of Blood Glucose Level in Rats. Biosci. Biotechnol. Biochem. 2002, 66, 1552–1554. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Yin, X.; Zeng, Q.; Zhu, L.; Chen, J. Isolation, structure, and surfactant properties of polysaccharides from Ulva lactuca L. from South China Sea. Int. J. Biol. Macromol. 2015, 79, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Belhadj, S.; Hentati, O.; Elfeki, A.; Hamden, K. Inhibitory activities of Ulva lactuca polysaccharides on digestive enzymes related to diabetes and obesity. Arch. Physiol. Biochem. 2013, 119, 81–87. [Google Scholar] [CrossRef]
- Tas, S.; Celikler, S.; Ziyanok-Ayvalik, S.; Sarandol, E.; Dirican, M. Ulva rigida improves carbohydrate metabolism, hyperlipidemia, and oxidative stress in streptozotocin-induced diabetic rats. Cell Biochem. Funct. 2011, 29, 108–113. [Google Scholar] [CrossRef]
- Celikler, S.; Tas, S.; Vatan, O.; Ziyanok-Ayvalik, S.; Yildiz, G.; Bilaloglu, R. Anti-hyperglycemic and antigenotoxic potential of Ulva rigida ethanolic extract in the experimental diabetes mellitus. Food Chem. Toxicol. 2009, 47, 1837–1840. [Google Scholar] [CrossRef]
- Lee, S.H.; Jeon, y.j. Anti-diabetic effects of brown algae derived phlorotannins, marine polyphenols through diverse mechanisms. Fitoterapia 2013, 86, 129–136. [Google Scholar] [CrossRef]
- Lee, S.H.; Yong, L.; Karadeniz, F.; Kim, M.M.; Kim, S.K. α-Glucosidase and α-amylase inhibitory activities of phloroglucinal derivatives from edible marine brown alga, Ecklonia cava. J. Sci. Food Agric. 2009, 89, 1552–1558. [Google Scholar] [CrossRef]
- Lee, S.H.; Park, M.H.; Heo, S.J.; Kang, S.M.; Ko, S.C.; Han, J.S.; Jeon, Y.J. Dieckol isolated from Ecklonia cava inhibits α-glucosidase and α-amylase in vitro and alleviates postprandial hyperglycemia in streptozotocin-induced diabetic mice. Food Chem. Toxicol. 2010, 48, 2633–2637. [Google Scholar] [CrossRef]
- Moon, H.E.; Islam, M.N.; Ahn, B.R.; Chowdhury, S.S.; Sohn, H.S.; Jung, H.A.; Choi, J.S. Protein tyrosine phosphatase 1B and α-glucosidase inhibitory phlorotannins from edible brown algae, Ecklonia stolonifera and Eisenia bicyclis. Biosci. Biotechnol. Biochem. 2011, 75, 1472–1480. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Han, J.S.; Heo, S.J.; Hwang, J.Y.; Jeon, Y.J. Protective effects of dieckol isolated from Ecklonia cava against high glucose-induced oxidative stress in human umbilical vein endothelial cells. Toxicol. In Vitro 2010, 24, 375–381. [Google Scholar] [CrossRef]
- Heo, S.J.; Hwang, J.Y.; Choi, J.I.; Han, J.S.; Kim, H.J.; Jeon, Y.J. Diphlorethohydroxycarmalol isolated from Ishige okamurae, a brown alga, a potent α-glucosidase and α-amylase inhibitor, alleviates postprandial hyperglycemia in diabetic mice. Eur. J. Pharmacol. 2009, 615, 252–256. [Google Scholar] [CrossRef]
- Heo, S.-J.; Hwang, J.-Y.; Choi, J.-I.; Lee, S.-H.; Park, P.-J.; Kang, D.-H.; Oh, C.; Kim, D.-W.; Han, J.-S.; Jeon, Y.-J.; et al. Protective effect of diphlorethohydroxycarmalol isolated from Ishige okamurae against high glucose-induced-oxidative stress in human umbilical vein endothelial cells. Food Chem. Toxicol. 2009, 48, 1448–1454. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kang, S.M.; Ko, S.C.; Lee, D.H.; Jeon, Y.J. Octaphlorethol A, a novel phenolic compound isolated from a brown alga, Ishige foliacea, increases glucose transporter 4-mediated glucose uptake in skeletal muscle cells. Biochem. Biophys. Res. Commun. 2012, 420, 576–581. [Google Scholar] [CrossRef]
- Nwosu, F.; Morris, J.; Lund, V.A.; Stewart, D.; Ross, H.A.; McDougall, G.J. Anti-proliferative and potential anti-diabetic effects of phenolic-rich extracts from edible marine algae. Food Chem. 2011, 126, 1006–1012. [Google Scholar] [CrossRef]
- Roy, M.C.; Anguenot, R.; Fillion, C.; Beaulieu, M.; Bérubé, J.; Richard, D. Effect of a commercially available algal phlorotannins extract on digestive enzymes and carbohydrate absorption in vivo. Food Res. Int. 2011, 44, 3026–3029. [Google Scholar] [CrossRef]
- Kang, C.; Jin, Y.B.; Lee, H.; Cha, M.; Sohn, E.T.; Moon, J.; Park, C.; Chun, S.; Jung, E.S.; Hong, J.S.; et al. Brown alga Ecklonia cava attenuates type 1 diabetes by activating AMPK and Akt signaling pathways. Food Chem. Toxicol. 2010, 48, 509–516. [Google Scholar] [CrossRef]
- Lee, S.-H.; Min, K.-H.; Han, J.-S.; Lee, D.-H.; Park, D.-B.; Jung, W.-K.; Park, P.-J.; Jeon, B.-T.; Kim, S.-K.; Jeon, Y.-J. Effects of brown alga, Ecklonia cava on glucose and lipid metabolism in C57BL/KsJ-db/db mice, a model of type 2 diabetes mellitus. Food Chem. Toxicol. 2012, 50, 575–582. [Google Scholar] [CrossRef]
- Min, K.H.; Kim, H.J.; Jeon, Y.J.; Han, J.S. Ishige okamurae ameliorates hyperglycemia and insulin resistance in C57BL/KsJ-db/db mice. Diabetes. Res. Clin. Pract. 2011, 93, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.A.; Islam, M.N.; Lee, C.M.; Oh, S.H.; Lee, S.; Jung, J.H.; Choi, J.S. Kinetics and molecular docking studies of an anti-diabetic complication inhibitor fucosterol from edible brown algae Eisenia bicyclis and Ecklonia stolonifera. Chem. Biol. Interact. 2013, 206, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.L.; Kitajima, C.; Ito, H.; Miyazaki, T.; Baba, M.; Okuyama, T.; Okada, Y. Antidiabetic effect of polyphenols from brown alga Ecklonia kurome in genetically diabetic KK-A y mice. Pharm. Biol. 2012, 50, 393–400. [Google Scholar] [CrossRef]
- Bu, T.; Liu, M.; Zheng, L.; Guo, Y.; Lin, X. α-glucosidase inhibition and the in vivo hypoglycemic effect of butyl-isobutyl-phthalate derived from the Laminaria japonica rhizoid. Phyther. Res. 2010, 24, 1588–1591. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.W.; Han, J.S. Hypoglycemic Effect of Sargassum ringgoldianum Extract in STZ-induced Diabetic Mice. Prev. Nutr. Food Sci. 2012, 17, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.N.; Lee, W.; Bae, G.U.; Kim, Y.K. Anti-diabetic and hypolipidemic effects of Sargassum yezoense in db/db mice. Biochem. Biophys. Res. Commun. 2012, 424, 675–680. [Google Scholar] [CrossRef]
- Kim, S.N.; Choi, H.Y.; Lee, W.; Park, G.M.; Shin, W.S.; Kim, Y.K. Sargaquinoic acid and sargahydroquinoic acid from Sargassum yezoense stimulate adipocyte differentiation through PPARα/γ activation in 3T3-L1 cells. FEBS Lett. 2008, 582, 3465–3472. [Google Scholar] [CrossRef] [PubMed]
- Vinoth Kumar, T.; Lakshmanasenthil, S.; Geetharamani, D.; Marudhupandi, T.; Suja, G.; Suganya, P. Fucoidan—A α-d-glucosidase inhibitor from Sargassum wightii with relevance to type 2 diabetes mellitus therapy. Int. J. Biol. Macromol. 2015, 72, 1044–1047. [Google Scholar] [CrossRef]
- Hwang, P.A.; Hung, Y.L.; Tsai, Y.K.; Chien, S.Y.; Kong, Z.L. The brown seaweed Sargassum hemiphyllum exhibits α-amylase and α-glucosidase inhibitory activity and enhances insulin release in vitro. Cytotechnology 2015, 67, 653–660. [Google Scholar] [CrossRef]
- He, W.F.; Yao, L.G.; Liu, H.L.; Guo, Y.W. Thunberol, a new sterol from the Chinese brown alga Sargassum thunbergii. J. Asian Nat. Prod. Res. 2014, 16, 685–689. [Google Scholar] [CrossRef]
- Park, M.H.; Nam, Y.H.; Han, J.S. Sargassum coreanum extract alleviates hyperglycemia and improves insulin resistance in db/db diabetic mice. Nutr. Res. Pract. 2015, 9, 472–479. [Google Scholar] [CrossRef]
- Jung, H.A.; Islam, M.N.; Lee, C.M.; Jeong, H.O.; Chung, H.Y.; Woo, H.C.; Choi, J.S. Promising antidiabetic potential of fucoxanthin isolated from the edible brown algae Eisenia bicyclis and Undaria pinnatifida. Fish. Sci. 2012, 78, 1321–1329. [Google Scholar] [CrossRef]
- Okada, Y.; Ishimaru, A.; Suzuki, R.; Okuyama, T. A New Phloroglucinol Derivative from the Brown Alga Eisenia bicyclis: Potential for the Effective Treatment of Diabetic Complications. J. Nat. Prod. 2004, 67, 103–105. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.T.; Rioux, L.E.; Turgeon, S.L. Alpha-amylase and alpha-glucosidase inhibition is differentially modulated by fucoidan obtained from Fucus vesiculosus and Ascophyllum nodosum. Phytochemistry 2014, 98, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Ko, S.C.; Kang, M.C.; Lee, D.H.; Jeon, Y.J. Octaphlorethol A, a marine algae product, exhibits antidiabetic effects in type 2 diabetic mice by activating AMP-activated protein kinase and upregulating the expression of glucose transporter 4. Food Chem Toxicol. 2016, 91, 58–64. [Google Scholar] [CrossRef]
- Balasubramaniam, V.; Mustar, S.; Mustafa Khalid, N.; Abd Rashed, A.; Mohd Noh, M.F.; Wilcox, M.D.; Chater, P.I.; Brownlee, I.A.; Pearson, J.P. Inhibitory activities of three Malaysian edible seaweeds on lipase and α-amylase. J. Appl. Phycol. 2013, 25, 1405–1412. [Google Scholar] [CrossRef]
- Liao, X.; Yang, L.; Chen, M.; Yu, J.; Zhang, S.; Ju, Y. The hypoglycemic effect of a polysaccharide (GLP) from Gracilaria lemaneiformis and its degradation products in diabetic mice. Food Funct. 2015, 6, 2542–2549. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Oh, H.; Jung, J.; Park, S.; Park, Y.I.; Bak, S.; Lee, M. Effect of Agar-free Gelidium Amansii on Obesity in DIO C57BL/6J Mice Model. FASEB J. 2015, 29, 750.2. [Google Scholar] [CrossRef]
- Lee, Y.; Oh, H.; Lee, M. Anti-inflammatory effects of Agar free-Gelidium amansii (GA) extracts in high-fat diet-induced obese mice. Nutr. Res. Pract. 2018, 12, 479–485. [Google Scholar] [CrossRef]
- Kitano, Y.; Murazumi, K.; Duan, J.; Kurose, K.; Kobayashi, S.; Sugawara, T.; Hirata, T. Effect of dietary porphyran from the red alga, Porphyra yezoensis, on glucose metabolism in diabetic KK-Ay mice. J. Nutr. Sci. Vitaminol. 2012, 58, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Abirami, S.G.; Kowsalya, S. Antidiabetic activity of Ulva fasciata and its impact on carbohydrate metabolism enzymes in alloxan induced diabetic rats. Int. J. Res. Phytochem. Pharmacol. 2013, 3, 136–141. [Google Scholar]
- Qin, J.; Su, H.; Zhang, Y.; Gao, J.; Zhu, L.; Wu, X.; Pan, H.; Li, X. Highly brominated metabolites from marine red alga Laurencia similis inhibit protein tyrosine phosphatase 1B. Bioorg. Med. Chem. Lett. 2010, 20, 7152–7154. [Google Scholar] [CrossRef]
- Kurihara, H.; Mitani, T.; Kawabata, J. Inhibitory potencies of bromophenol from Rhodomelaceae algae against α-glucosidase activity. Fish. Sci. 1999, 65, 300–303. [Google Scholar] [CrossRef]
- Kim, K.Y.; Nguyen, T.H.; Kurihara, H.; Kim, S.M. α-Glucosidase inhibitory activity of bromophenol purified from the red alga polyopes lancifolia. J. Food Sci. 2010, 75, H145–H150. [Google Scholar] [CrossRef]
- Kurihari, H.; Mitani, T.; Kawabata, J.; Takahashi, K. Two new bromophenols from the red alga Odonth coralia. J. Nat. Prod. 1999, 62, 882–884. [Google Scholar] [CrossRef]
- Li, J.; Guo, S.J.; Su, H.; Han, L.J.; Shi, D.Y. Total synthesis of bis-(2,3-dibromo-4,5-dihydroxyphenyl)-methane as potent PTP1B inhibitor. Chin. Chem. Lett. 2008, 19, 1290–1292. [Google Scholar] [CrossRef]
- Shi, D.; Guo, S.; Jiang, B.; Guo, C.; Wang, T.; Zhang, L.; Li, J. HPN, a synthetic analogue of bromophenol from red alga Rhodomela confervoides: Synthesis and anti-diabetic effects in C57BL/KsJ-db/db mice. Mar. Drugs 2013, 11, 350–362. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Xu, F.; He, J.; Li, J.; Fan, X.; Han, L. Inhibition of bromophenols against PTP1B and anti-hyperglycemic effect of Rhodomela confervoides extract in diabetic rats. Chin. Sci. Bull. 2008, 53, 2476–2479. [Google Scholar] [CrossRef]
- Liu, X.; Li, X.; Gao, L.; Cui, C.; Li, C.; Li, J.; Wang, B. Extraction and PTP1B inhibitory activity of bromophenols from the marine red alga Symphyocladia latiuscula. Chin. J. Oceanol. Limnol. 2011, 29, 686–690. [Google Scholar] [CrossRef]
- Vishnu Kiran, M.; Murugesan, S. Biogenic silver nanoparticles by Halymenia poryphyroides and its in vitro anti-diabetic efficacy. J. Chem. Pharm. Res. 2013, 5, 1001–1008. [Google Scholar]
- Gammon, K. Neurodegenerative disease: Brain windfall. Nature 2014, 515, 299–300. [Google Scholar] [CrossRef] [PubMed]
- Barbalace, M.C.; Malaguti, M.; Giusti, L.; Lucacchini, A.; Hrelia, S.; Angeloni, C. Anti-inflammatory activities of marine algae in neurodegenerative diseases. Int. J. Mol. Sci. 2019, 20, 3061. [Google Scholar] [CrossRef]
- Han, Z.; Tian, R.; Ren, P.; Zhou, W.; Wang, P.; Luo, M.; Jin, S.; Jiang, Q. Parkinson’s disease and Alzheimer’s disease: A Mendelian randomization study. BMC Med. Genet. 2018, 19, 215. [Google Scholar] [CrossRef]
- Gaugler, J.; James, B.; Johnson, T.; Scholz, K.; Weuve, J. 2016 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2016, 12, 459–509. [Google Scholar]
- Barbosa, M.; Valentão, P.; Andrade, P.B. Bioactive compounds from macroalgae in the new millennium: Implications for neurodegenerative diseases. Mar. Drugs 2014, 12, 4934–4972. [Google Scholar] [CrossRef] [PubMed]
- Biundo, R.; Weis, L.; Facchini, S.; Formento-Dojot, P.; Vallelunga, A.; Pilleri, M.; Antonini, A. Cognitive profiling of Parkinson disease patients with mild cognitive impairment and dementia. Park. Relat. Disord. 2014, 20, 394–399. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M.T. Parkinson’s Disease and Parkinsonism. Am. J. Med. 2019, 132, 802–807. [Google Scholar] [CrossRef] [PubMed]
- Jellinger, K.A. Basic mechanisms of neurodegeneration: A critical update. J. Cell. Mol. Med. 2010, 14, 457–487. [Google Scholar] [CrossRef]
- Pangestuti, R.; Kim, S.K. Neuroprotective effects of marine algae. Mar. Drugs 2011, 9, 803–818. [Google Scholar] [CrossRef]
- Hannan, M.A.; Dash, R.; Haque, M.N.; Mohibbullah, M.; Sohag, A.A.M.; Rahman, M.A.; Uddin, M.J.; Alam, M.; Moon, I.S. Neuroprotective Potentials of Marine Algae and Their Bioactive Metabolites: Pharmacological Insights and Therapeutic Advances. Mar. Drugs 2020, 18, 347. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Dzhemileva, L.; Gloriozova, T.; D’yakonov, V. Natural and synthetic drugs used for the treatment of the dementia. Biochem. Biophys. Res. Commun. 2020, 524, 772–783. [Google Scholar] [CrossRef]
- Polidori, M.C. Preventive benefits of natural nutrition and lifestyle counseling against alzheimer’s disease onset. J. Alzheimer’s Dis. 2014, 42, S475–S482. [Google Scholar] [CrossRef]
- Noori, T.; Dehpour, A.R.; Sureda, A.; Sobarzo-Sanchez, E.; Shirooie, S. Role of natural products for the treatment of Alzheimer’s disease. Eur. J. Pharmacol. 2021, 898, 145576. [Google Scholar] [CrossRef] [PubMed]
- Menaa, F. Tapping into Deep-Water Reservoirs to Overcome Antibiotic Resistance through Bacteria-Producing Unique Secondary Metabolites. Pharm. Anal. Acta 2015, 06, 4172. [Google Scholar]
- Lordan, S.; Ross, R.P.; Stanton, C. Marine bioactives as functional food ingredients: Potential to reduce the incidence of chronic diseases. Mar. Drugs 2011, 9, 1056–1100. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.M.; Allsopp, P.J.; Magee, P.J.; Gill, C.I.; Nitecki, S.; Strain, C.R.; Mcsorley, E.M. Seaweed and human health. Nutr. Rev. 2014, 72, 205–216. [Google Scholar] [CrossRef]
- Wijesekara, I.; Senevirathne, M.; Li, Y.X.; Kim, S.K. Functional Ingredients from Marine Algae as Potential Antioxidants in the Food Industry. In Handbook of Marine Macroalgae: Biotechnology and Applied Phycology; John Wiley & Sons: Hoboken, NJ, USA, 2011; pp. 398–402. [Google Scholar]
- Grozdanic, N.; Stanojkovic, T.P.; Kljajic, Z.; Etahiri, S.; Assobhei, O.; Konic-Ristic, A.; Srdic-Rajic, T.; Kardum, N.; Backovic, S. In Vitro Evaluation of Antioxidant and Antitumoral Activities of Marine Algae Gelidium Sesquipedale and Fucus Spiralis. Eur. J. Cancer 2012, 48, S26. [Google Scholar] [CrossRef]
- Vo, T.S.; Ngo, D.H.; Kim, S.K. Potential targets for anti-inflammatory and anti-allergic activities of marine algae: An overview. Inflamm. Allergy Drug Targets 2012, 11, 90–101. [Google Scholar] [CrossRef]
- Matsubara, K.; Matsuura, Y.; Hori, K.; Miyazawa, K. An anticoagulant proteoglycan from the marine green alga, Codium pugniformis. J. Appl. Phycol. 2000, 12, 9–14. [Google Scholar] [CrossRef]
- Huheihel, M.; Ishanu, V.; Tal, J.; Arad, S. Activity of Porphyridium sp. polysaccharide against herpes simplex viruses in vitro and in vivo. J. Biochem. Biophys. Methods 2002, 50, 189–200. [Google Scholar] [CrossRef]
- Kong, C.S.; Kim, J.A.; Yoon, N.Y.; Kim, S.K. Induction of apoptosis by phloroglucinol derivative from Ecklonia cava in MCF-7 human breast cancer cells. Food Chem. Toxicol. 2009, 47, 1653–1658. [Google Scholar] [CrossRef]
- Li, Y.; Lee, S.H.; Le, Q.T.; Kim, M.M.; Kim, S.K. Anti-allergic effects of phlorotannins on histamine release via binding inhibition between IgE and FcεRI. J. Agric. Food Chem. 2008, 56, 12073–12080. [Google Scholar] [CrossRef]
- Syad, A.N.; Rajamohamed, B.S.; Shunmugaiah, K.P.; Kasi, P.D. Neuroprotective effect of the marine macroalga Gelidiella acerosa: Identification of active compounds through bioactivity-guided fractionation. Pharm. Biol. 2016, 54, 2073–2081. [Google Scholar] [CrossRef] [PubMed]
- Rengasamy, K.R.R.; Kulkarni, M.G.; Stirk, W.A.; Van Staden, J. Advances in algal drug research with emphasis on enzyme inhibitors. Biotechnol. Adv. 2014, 32, 1364–1381. [Google Scholar] [CrossRef]
- Emerit, J.; Edeas, M.; Bricaire, F. Neurodegenerative diseases and oxidative stress. Biomed. Pharmacother. 2004, 58, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Akyol, A.; Herken, H.; Uz, E.; Fadıllıoǧlu, E.; Ünal, S.; Söǧüt, S.; Özyurt, H.; Savaş, H.A. The indices of endogenous oxidative and antioxidative processes in plasma from schizophrenic patients: The possible role of oxidant/antioxidant imbalance. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2002, 26, 995–1005. [Google Scholar] [CrossRef]
- Castelli, V.; Benedetti, E.; Antonosante, A.; Catanesi, M.; Pitari, G.; Ippoliti, R.; Cimini, A.; D’Angelo, M. Neuronal cells rearrangement during aging and neurodegenerative disease: Metabolism, oxidative stress and organelles dynamic. Front. Mol. Neurosci. 2019, 12, 132. [Google Scholar] [CrossRef]
- Moosmann, B.; Behl, C. Antioxidants as treatment for neurodegenerative disorders. Expert Opin. Investig. Drugs 2002, 11, 1407–1435. [Google Scholar] [CrossRef] [PubMed]
- Fallarero, A.; Loikkanen, J.J.; Männistö, P.T.; Castañeda, O.; Vidal, A. Effects of aqueous extracts of Halimeda incrassata (Ellis) lamouroux and Bryothamnion triquetrum (S.G.Gmelim) Howe on hydrogen peroxide and methyl mercury-induced oxidative stress in GT1-7 mouse hypothalamic immortalized cells. Phytomedicine 2003, 10, 39–47. [Google Scholar] [CrossRef]
- Lim, C.S.; Jin, D.Q.; Sung, J.Y.; Lee, J.H.; Choi, H.G.; Ha, I.; Han, J.S. Antioxidant and anti-inflammatory activities of the methanolic extract of Neorhodomela aculeate in hippocampal and microglial cells. Biol. Pharm. Bull. 2006, 29, 1212–1216. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Spencer, J.P.E.; Vafeiadou, K.; Williams, R.J.; Vauzour, D. Neuroinflammation: Modulation by flavonoids and mechanisms of action. Mol. Asp. Med. 2012, 33, 83–97. [Google Scholar] [CrossRef]
- Natarajan, S.; Shanmugiahthevar, K.P.; Kasi, P.D. Cholinesterase inhibitors from Sargassum and Gracilaria gracilis: Seaweeds inhabiting South Indian coastal areas (Hare Island, Gulf of Mannar). Nat. Prod. Res. 2009, 23, 355–369. [Google Scholar] [CrossRef]
- Pangestuti, R.; Kim, S.K. Neuroprotective properties of chitosan and its derivatives. Mar. Drugs 2010, 8, 2117–2128. [Google Scholar] [CrossRef] [PubMed]
- Fernando, I.P.S.; Nah, J.W.; Jeon, Y.J. Potential anti-inflammatory natural products from marine algae. Environ. Toxicol. Pharmacol. 2016, 48, 22–30. [Google Scholar] [CrossRef] [PubMed]
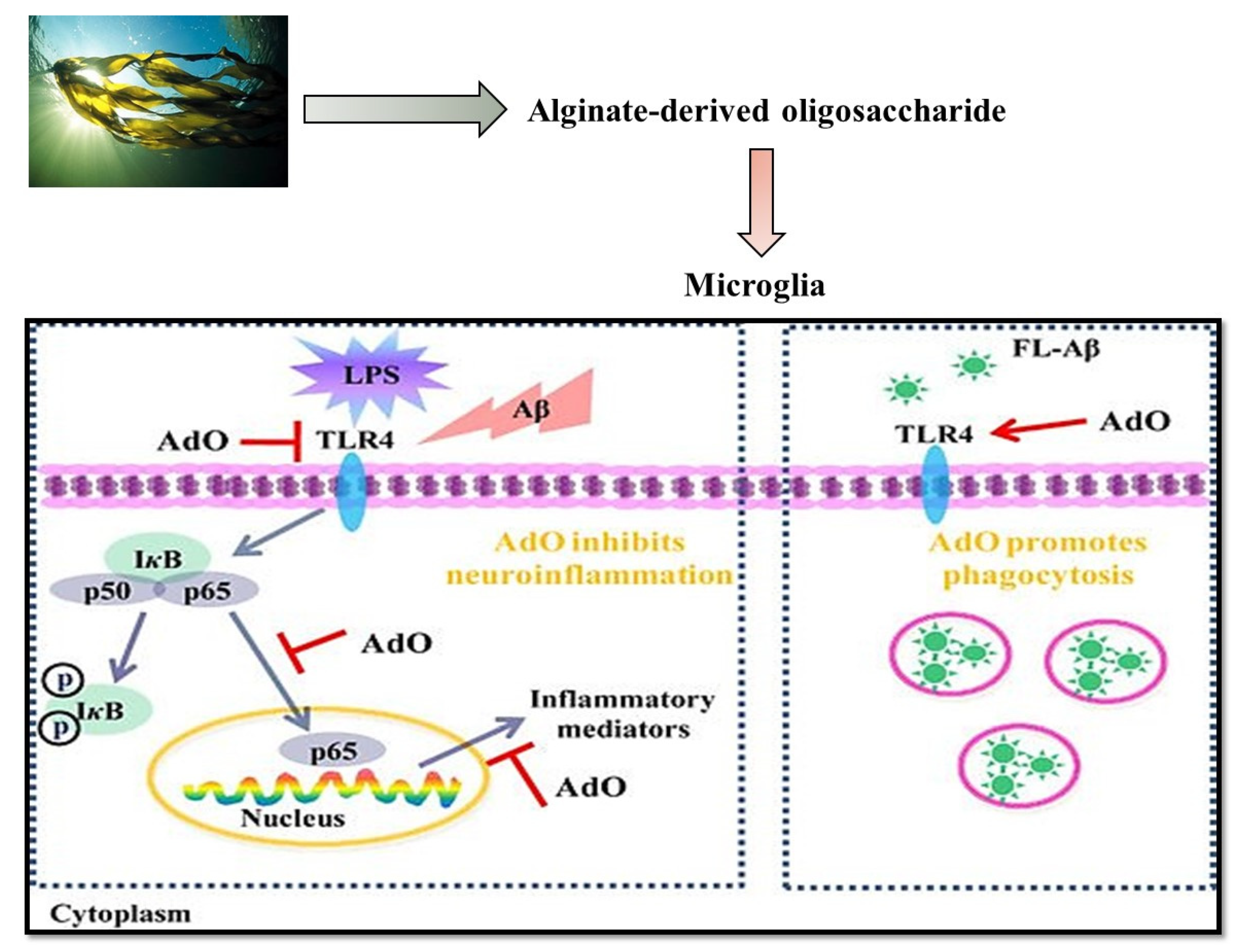
- Zhou, R.; Shi, X.Y.; Bi, D.C.; Fang, W.S.; Wei, G.B.; Xu, X. Alginate-derived oligosaccharide inhibits neuroinflammation and promotes microglial phagocytosis of β-amyloid. Mar. Drugs 2015, 13, 5828–5846. [Google Scholar] [CrossRef] [PubMed]
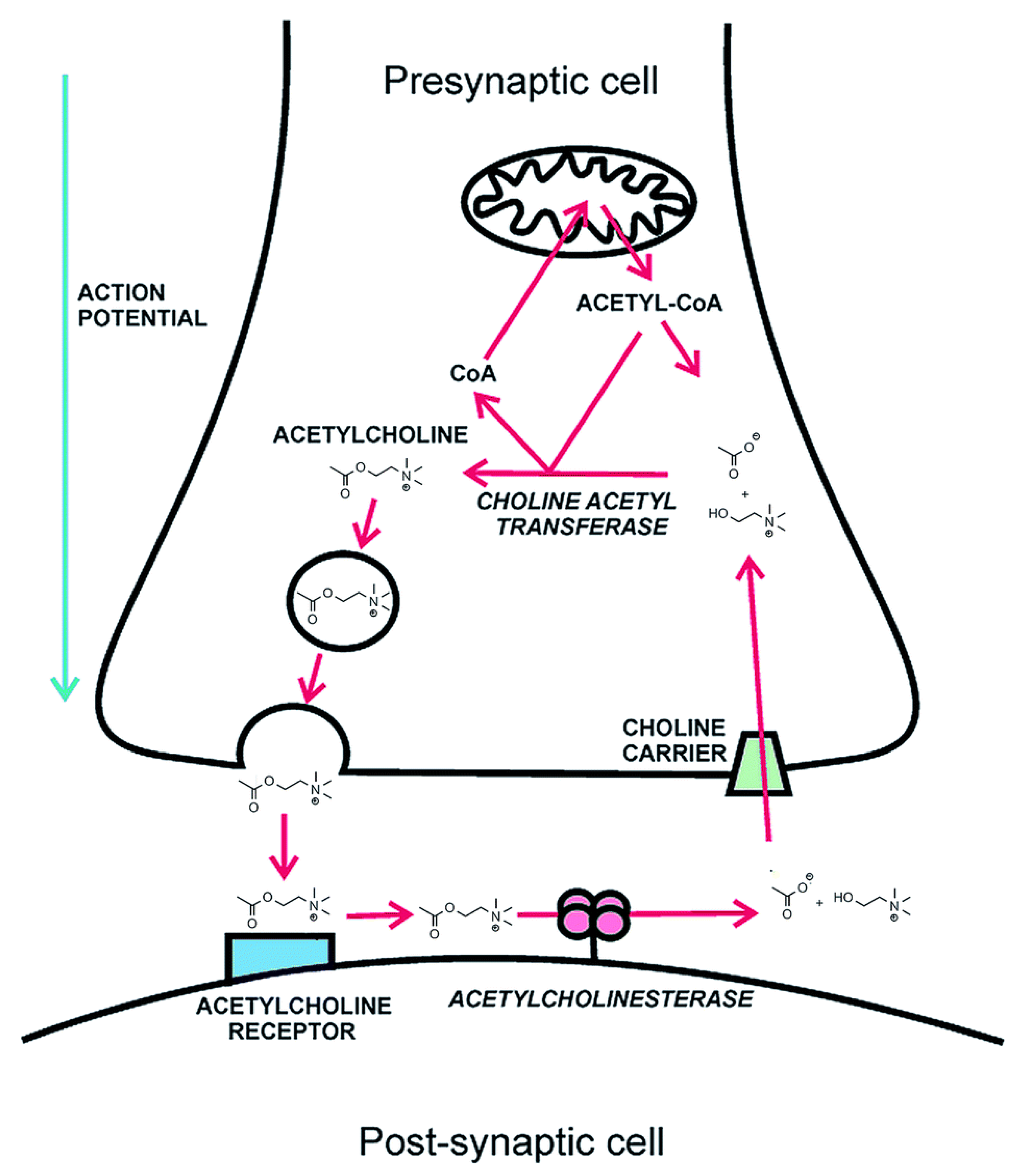
- Myung, C.S.; Shin, H.C.; Hai, Y.B.; Soo, J.Y.; Bong, H.L.; Jong, S.K. Improvement of memory by dieckol and phlorofucofuroeckol in ethanol-treated mice: Possible involvement of the inhibition of acetylcholinesterase. Arch. Pharm. Res. 2005, 28, 691–698. [Google Scholar] [CrossRef]
- Custódio, L.; Justo, T.; Silvestre, L.; Barradas, A.; Duarte, C.V.; Pereira, H.; Barreira, L.; Rauter, A.P.; Alberício, F.; Varela, J. Microalgae of different phyla display antioxidant, metal chelating and acetylcholinesterase inhibitory activities. Food Chem. 2012, 131, 134–140. [Google Scholar] [CrossRef]
- Igarashi, M.; Kim, H.W.; Chang, L.; Ma, K.; Rapoport, S.I. Dietary n-6 polyunsaturated fatty acid deprivation increases docosahexaenoic acid metabolism in rat brain. J. Neurochem. 2012, 120, 985–997. [Google Scholar] [CrossRef] [PubMed]
- Moodie, L.W.K.; Sepcic, K.; Turk, T.; Frangez, R.; Svenson, J. Natural cholinesterase inhibitors from marine organisms. Nat. Prod. Rep. 2019, 36, 1053–1092. [Google Scholar] [CrossRef]
- Shimizu, H.; Koyama, T.; Yamada, S.; Lipton, S.A.; Satoh, T. Zonarol, a sesquiterpene from the brown algae Dictyopteris undulata, provides neuroprotection by activating the Nrf2/ARE pathway. Biochem. Biophys. Res. Commun. 2015, 457, 718–722. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.A.; Oh, S.H.; Choi, J.S. Molecular docking studies of phlorotannins from Eisenia bicyclis with BACE1 inhibitory activity. Bioorg. Med. Chem. Lett. 2010, 20, 3211–3215. [Google Scholar] [CrossRef] [PubMed]
- Ahn, B.R.; Moon, H.E.; Kim, H.R.; Jung, H.A.; Choi, J.S. Neuroprotective effect of edible brown alga Eisenia bicyclis on amyloid beta peptide-induced toxicity in PC12 cells. Arch. Pharm. Res. 2012, 35, 1989–1998. [Google Scholar] [CrossRef]
- Kim, K.A.; Kim, S.M.; Kang, S.W.; Jeon, S.I.I.; Um, B.H.; Jung, S.H. Edible Seaweed, Eisenia bicyclis, Protects Retinal Ganglion Cells Death Caused by Oxidative Stress. Mar. Biotechnol. 2012, 14, 383–395. [Google Scholar] [CrossRef]
- Li, Y.; Qian, Z.J.; Ryu, B.M.; Lee, S.H.; Kim, M.M.; Kim, S.K. Chemical components and its antioxidant properties in vitro: An edible marine brown alga, Ecklonia cava. Bioorg. Med. Chem. 2009, 17, 1963–1973. [Google Scholar] [CrossRef]
- Cho, S.; Han, D.; Kim, S.B.; Yoon, M.; Yang, H.; Jin, Y.H.; Jo, J.; Yong, H.; Lee, S.H.; Jeon, Y.J.; et al. Depressive effects on the central nervous system and underlying mechanism of the enzymatic extract and its phlorotannin-rich fraction from ecklonia cava edible brown seaweed. Biosci. Biotechnol. Biochem. 2012, 76, 163–168. [Google Scholar] [CrossRef]
- Kang, S.M.; Cha, S.H.; Ko, J.Y.; Kang, M.C.; Kim, D.; Heo, S.J.; Kim, J.S.; Heu, M.S.; Kim, Y.T.; Jung, W.K.; et al. Neuroprotective effects of phlorotannins isolated from a brown alga, Ecklonia cava, against H2O2-induced oxidative stress in murine hippocampal HT22 cells. Environ. Toxicol. Pharmacol. 2012, 34, 96–105. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, K.; Kang, K.A.; Lee, N.H.; Hyun, J.W.; Kim, H.S. Phloroglucinol exerts protective effects against oxidative stress-induced cell damage in SH-SY5Y cells. J. Pharmacol. Sci. 2012, 119, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.W.; Lee, H.S.; Shin, H.C.; Lee, B.H. Multifunctional activity of polyphenolic compounds associated with a potential for alzheimer’s disease therapy from Ecklonia cava. Phyther. Res. 2015, 29, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Geng, M.; Li, J.; Xin, X.; Wang, J.; Tang, M.; Zhang, J.; Zhang, X.; Ding, J. Acidic oligosaccharide sugar chain, a marine-derived acidic oligosaccharide, inhibits the cytotoxicity and aggregation of amyloid beta protein. J. Pharmacol. Sci. 2004, 95, 248–255. [Google Scholar] [CrossRef]
- Kannan, R.R.; Aderogba, M.A.; Ndhlala, A.R.; Stirk, W.A.; Van Staden, J. Acetylcholinesterase inhibitory activity of phlorotannins isolated from the brown alga, Ecklonia maxima (Osbeck) Papenfuss. Food Res. Int. 2013, 54, 1250–1254. [Google Scholar] [CrossRef]
- Yoon, N.Y.; Chung, H.Y.; Kim, H.R.; Choi, J.E. Acetyl- and butyrylcholinesterase inhibitory activities of sterols and phlorotannins from Ecklonia stolonifera. Fish. Sci. 2008, 74, 200–207. [Google Scholar] [CrossRef]
- Rocha De Souza, M.C.; Marques, C.T.; Guerra Dore, C.M.; Ferreira Da Silva, F.R.; Oliveira Rocha, H.A.; Leite, E.L. Antioxidant activities of sulfated polysaccharides from brown and red seaweeds. J. Appl. Phycol. 2007, 19, 153–160. [Google Scholar] [CrossRef]
- Lee, H.R.; Do, H.; Lee, S.R.; Sohn, E.S.; Pyo, S.; Son, E. Effects of fucoidan on neuronal cell proliferation: Association with NO production through the iNOS pathway. J. Food Sci. Nutr. 2007, 12, 74–78. [Google Scholar] [CrossRef]
- Do, H.; Pyo, S.; Sohn, E.H. Suppression of iNOS expression by fucoidan is mediated by regulation of p38 MAPK, JAK/STAT, AP-1 and IRF-1, and depends on up-regulation of scavenger receptor B1 expression in TNF-α- and IFN-γ-stimulated C6 glioma cells. J. Nutr. Biochem. 2010, 21, 671–679. [Google Scholar] [CrossRef]
- Huang, W.C.; Yen, F.C.; Shiao, Y.J.; Shie, F.S.; Chan, J.L.; Yang, C.N.; Sung, Y.J.; Huang, F.L.; Tsay, H.J. Enlargement of Aβ aggregates through chemokine-dependent microglial clustering. Neurosci. Res. 2009, 63, 280–287. [Google Scholar] [CrossRef]
- Liu, H.; Gu, L. Phlorotannins from brown algae (Fucus vesiculosus) inhibited the formation of advanced glycation endproducts by scavenging reactive carbonyls. J. Agric. Food Chem. 2012, 60, 1326–1334. [Google Scholar] [CrossRef]
- Wozniak, M.; Bell, T.; Dénes, Á.; Falshaw, R.; Itzhaki, R. Anti-HSV1 activity of brown algal polysaccharides and possible relevance to the treatment of Alzheimer’s disease. Int. J. Biol. Macromol. 2015, 74, 530–540. [Google Scholar] [CrossRef]
- Sangeetha, R.K.; Bhaskar, N.; Baskaran, V. Comparative effects of β-carotene and fucoxanthin on retinol deficiency induced oxidative stress in rats. Mol. Cell. Biochem. 2009, 331, 59–67. [Google Scholar] [CrossRef]
- Gao, Y.; Dong, C.; Yin, J.; Shen, J.; Tian, J.; Li, C. Neuroprotective effect of fucoidan on H2O2-induced apoptosis in PC12 cells via activation of PI3K/Akt pathway. Cell. Mol. Neurobiol. 2012, 32, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.Q.; Zhang, L.J.; Zhang, T.; Luo, D.Z.; Jia, Y.J.; Guo, Z.X.; Zhang, Q.B.; Wang, X.; Wang, X.M. Inhibitory effect of fucoidan on nitric oxide production in lipopolysaccharide-activated primary microglia. Clin. Exp. Pharmacol. Physiol. 2010, 37, 422–428. [Google Scholar] [CrossRef]
- Ina, A.; Hayashi, K.I.; Nozaki, H.; Kamei, Y. Pheophytin a, a low molecular weight compound found in the marine brown alga Sargassum fulvellum, promotes the differentiation of PC12 cells. Int. J. Dev. Neurosci. 2007, 25, 63–68. [Google Scholar] [CrossRef]
- Mori, J.; Iwashima, M.; Wakasugi, H.; Saito, H.; Matsunaga, T.; Ogasawara, M.; Takahashi, S.; Suzuki, H.; Hayashi, T. New plastoquinones isolated from the brown alga, Sargassum micracanthum. Chem. Pharm. Bull. 2005, 53, 1159–1163. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Li, Z.; Chen, M.; Sun, Z.; Ling, Y.; Jiang, J.; Huang, C. Structural elucidation and protective role of a polysaccharide from Sargassum fusiforme on ameliorating learning and memory deficiencies in mice. Carbohydr. Polym. 2016, 139, 150–158. [Google Scholar] [CrossRef]
- Zhao, D.; Zheng, L.; Qi, L.; Wang, S.; Guan, L.; Xia, Y.; Cai, J. Structural features and potent antidepressant effects of total sterols and β-sitosterol extracted from Sargassum horneri. Mar. Drugs 2016, 14, 123. [Google Scholar] [CrossRef] [PubMed]
- Byoung, W.C.; Ryu, G.; Soo, H.P.; Eun, S.K.; Shin, J.; Seok, S.R.; Hyeon, C.S.; Bong, H.L. Anticholinesterase activity of plastoquinones from Sargassum sagamianum: Lead compounds for Alzheimer’s disease therapy. Phyther. Res. 2007, 21, 423–426. [Google Scholar]
- Jung, M.; Kyoung, H.J.; Kim, B.; Bong, H.L.; Byoung, W.C.; Oh, K.B.; Shin, J. Meroditerpenoids from the brown alga Sargassum siliquastrum. J. Nat. Prod. 2009, 72, 1723. [Google Scholar] [CrossRef]
- Meenakshi, S.; Umayaparvathi, S.; Saravanan, R.; Manivasagam, T.; Balasubramanian, T. Neuroprotective effect of fucoidan from Turbinaria decurrens in MPTP intoxicated Parkinsonic mice. Int. J. Biol. Macromol. 2016, 86, 425–433. [Google Scholar] [CrossRef]
- Rafiquzzaman, S.M.; Kim, E.Y.; Lee, J.M.; Mohibbullah, M.; Alam, M.B.; Soo Moon, I.; Kim, J.M.; Kong, I.S. Anti-Alzheimers and anti-inflammatory activities of a glycoprotein purified from the edible brown alga Undaria pinnatifida. Food Res. Int. 2015, 77, 118–124. [Google Scholar] [CrossRef]
- Bauer, S.; Jin, W.; Zhang, F.; Linhardt, R.J. The Application of Seaweed Polysaccharides and Their Derived Products with Potential for the Treatment of Alzheimer’s Disease. Mar. Drugs 2021, 19, 89. [Google Scholar] [CrossRef]
- Kim, M.J.; Li, Y.X.; Dewapriya, P.; Ryu, B.; Kim, S.K. Floridoside suppresses pro-inflammatory responses by blocking MAPK signaling in activated microglia. BMB Rep. 2013, 46, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Machado, L.P.; Carvalho, L.R.; Young, M.C.M.; Cardoso-Lopes, E.M.; Centeno, D.C.; Zambotti-Villela, L.; Colepicolo, P.; Yokoya, N.S. Evaluation of acetylcholinesterase inhibitory activity of Brazilian red macroalgae organic extracts. Rev. Bras. Farmacogn. 2015, 25, 657–662. [Google Scholar] [CrossRef]
- Yabuta, Y.; Fujimura, H.; Kwak, C.S.; Enomoto, T.; Watanabe, F. Antioxidant activity of the phycoerythrobilin compound formed from a dried Korean purple laver (Porphyra sp.) during in vitro digestion. Food Sci. Technol. Res. 2010, 16, 347–351. [Google Scholar] [CrossRef]
- Grisante, A.I.; Stanich, P. Esclerose múltipla: Aspectos nutricionais e o papel dos nutrientes específicos. ConScientiae Saude 2008, 5, 67–74. [Google Scholar] [CrossRef][Green Version]
- Yang, P.; Liu, D.Q.; Liang, T.J.; Li, J.; Zhang, H.Y.; Liu, A.H.; Guo, Y.W.; Mao, S.C. Bioactive constituents from the green alga Caulerpa racemosa. Bioorg. Med. Chem. 2015, 23, 38–45. [Google Scholar] [CrossRef]
- Rengasamy, K.R.R.; Amoo, S.O.; Aremu, A.O.; Stirk, W.A.; Gruz, J.; Šubrtová, M.; Doležal, K.; Van Staden, J. Phenolic profiles, antioxidant capacity, and acetylcholinesterase inhibitory activity of eight South African seaweeds. J. Appl. Phycol. 2015, 27, 1599–1605. [Google Scholar] [CrossRef]
- Lee, C.; Park, G.H.; Ahn, E.M.; Kim, B.A.; Park, C.I.; Jang, J.H. Protective effect of Codium fragile against UVB-induced pro-inflammatory and oxidative damages in HaCaT cells and BALB/c mice. Fitoterapia 2013, 86, 54–63. [Google Scholar] [CrossRef]
| Phlorotannins | Anti-Diabetic Effects | Sources | References |
|---|---|---|---|
| Dieckol | α-Glucosidase inhibitor | Ecklonia cava | [129] |
| Postprandial-hyperglycemia-lowering | [130] | ||
| PTP 1B inhibition | [131] | ||
| Protective effect against diabetes complication | [132] | ||
| Fucodiphloroethol G | α-Glucosidase inhibitor | E. cava | [129] |
| 6,6′-Bieckol | α-Glucosidase inhibitor | E. cava | [129] |
| 7-Phloroeckol | α-Glucosidase inhibitor | E. cava | [129] |
| PTP 1B inhibition | [131] | ||
| Phlorofucofuroeckol A | α-Glucosidase inhibitor | E. cava | [129] |
| PTP 1B inhibition | [131] | ||
| Phloroglucinol | α-Glucosidase inhibitor | E. stolonifera | [131] |
| PTP 1B inhibition | E. bicyclis | [131] | |
| Dioxinodehydroeckol | α-Glucosidase inhibitor | E. stolonifera | [131] |
| PTP 1B inhibition | E. bicyclis | [131] | |
| Diphlorethohydroxycarmalol | α-Glucosidase inhibition | Ishige okamurae | [133] |
| Postprandial-hyperglycemia-lowering | [133] | ||
| Protective effect against diabetes complications | [134] | ||
| Eckol | α-Glucosidase inhibitor | E. stolonifera | [131] |
| PTP 1B inhibition | E. bicyclis | [131] | |
| Octaphlorethol A | Glucose uptake effect in skeletal muscle | I. foliacea | [135] |
| Polyphenolic-rich extract | α-Glucosidase inhibitor | Ascophyllum nodosum | [136] |
| Phlorotannin-rich extract | Postprandial hyperglycemia-lowering | A. nodosum | [137] |
| Fucus vesiculosus | [137] | ||
| Polyphenolic-rich extract | Glucose uptake in skeletal muscle | E. cava | [138] |
| Dieckol-rich extract | Improvement of insulin sensitivity | E. cava | [139] |
| Polyphenolic-rich extract | Improvement of insulin sensitivity | I. okamurae | [140] |
| Macroalgae | Major Compound | Effects | References |
|---|---|---|---|
| Brown Algae | |||
| Pelvetia siliquosa | Fucosterol | Inhibition of blood glucose level and glycogen degradation | [122] |
| Pelvetia babingtonii | Methanol extract | α-Glucosidase inhibition and suppression of postprandial hyperglycemia | [123] |
| Ecklonia stolonifera | Polyphenols | α-Glucosidase inhibition; suppression of the increase in plasma glucose | [44] |
| Phlorotannins | PTP1B and α-glucosidase inhibition | [131] | |
| Fucosterol | RLAR, HRAR, PTP1B, α-glucosidase activities, and AGE formation inhibition | [141] | |
| Eisenia bicyclis Ecklonia stolonifera | Dieckol Eckol 7-Phloroeckol Phlorofucofuroeckol-A | α-Glucosidase, and PTP1B | [131] |
| Ecklonia cava | Dieckol 7-Phloroeckol Phlorofucofuroeckol-A 6,6-Bieckol Fucodiphloroethol-G | Activation of both AMPK and Akt signal pathways; improvement of insulin sensitivity; α-Glucosidase and α-amylase inhibition | [130] |
| Ecklonia kurome | Phlorotannins | α-Amylase inhibition; amelioration of hyperinsulinemia | [142] |
| Laminaria japonica | Polysaccharides | Reduced fasting blood glucose; increased the levels of insulin and amylin | [55] |
| Butyl-isobutyl-phthalate | α-Glucosidase inhibition | [143] | |
| Sargassum ringgoldianum | Polyphenols | α-Amylase and α-glucosidase inhibition | [144] |
| Sargassum yezoense | Sargaquinoic acid Sargahydroquinoic acid | Enhances the transcriptional activities of PPARα and PPARγ | [145] |
| Amelioration of insulin resistance | [146] | ||
| Sargassum wightii | Fucoidan | α-d-glucosidase inhibition | [147] |
| Sargassum polycystum | Extract | Increasing insulin sensitivity | [117] |
| Sargassum hemiphyllum | Fucoxanthin | α-Amylase and α-glucosidase inhibition, and insulin release enhancement | [148] |
| Sargassum thunbergii | Thunberol | PTP1B inhibition | [149] |
| Sargassum coreanum | Extract | Alteration of the hepatic glucose metabolic enzyme activities and improvement of insulin resistance | [150] |
| Undaria pinnatifida | Fucoxanthin | HRAR, RLAR, PTP1B inhibition, and AGE formation | [151] |
| Improve insulin signaling | |||
| Eisenia bicyclis | Phlorotannins | Inhibition of AGEs and α-amylase | [152] |
| Fucoxanthin | Inhibition of RLAR, HRAR, PTP1B activities and AGE formation | [151] | |
| Fucosterol | Inhibition of RLAR, HRAR, PTP1B, α-glucosidase activities, and AGE formation | [141] | |
| Ascophyllum nodosum | PhlorotanninsFucoidan | α-Amylase and α-glucosidase inhibition | [153] |
| Ishige okamurae | Diphlorethohydroxycarmalol | α-Amylase and α-glucosidase inhibition | [133] |
| Extract | Alteraation of the hepatic glucose metabolic enzyme activities, and improvement of insulin resistance | [140] | |
| Ishige foliacea | Octaphlorethol A | Increase in GLUT4-mediated glucose utilization via activation of AMPK in muscle | [154] |
| Red Algae | |||
| Kappaphycus alvarezii, Eucheuma denticulatum | Extract | Inhibitory activity towards α-amylase | [155] |
| Gracilaria lemaneiformis | Polysaccharide | Inhibitory activity towards α-glucosidase | [156] |
| Gelidim amansii | Ethanol extract | Significant decrease of plasma glucose | [157,158] |
| Porphyra yezoensis | Porphyran | Increase of adiponectin levels | [159] |
| Green Algae | |||
| Ulva rigida | Ethanol extract | Regeneration of β-cells and/or potentiate the insulin resistance | [127] |
| Ulva fasciata | Sulfated polysaccharides | Reduce blood glucose level, and restore hepatic glycogen content | [160] |
| Ulva lactula | Polysaccharides | α-amylase, maltase, and sucrase inhibition; Delay glucose absorption | [125] |
| Grateloupia elliptica | 2,4,6-Tribromophenol | α-Glucosidase inhibition | [146] |
| 2,4-Dibromophenol | |||
| Laurencia similis | 3′,5′,6′,6-Tetrabromo-2,4-dimethyldiphenyl ether | PTP1B inhibition | [161] |
| 1,2,5-Tribromo-3-bromoamino-7-bromomethylnaphthalene | |||
| 2,5,8-Tribromo-3-bromoamino-7-bromomethylnaphthalene | |||
| 2,5,6-Tribromo-3-bromoamino-7-bromomethylnaphthalene | |||
| 2′,5′,6′,5,6-Pentabromo-3′,4′,3,4-tetramethoxybenzo-phenone | |||
| Bis-(2,3-dibromo-4,5-dihydroxybenzyl) ether | |||
| Odonthalia corymbifera | Bis-(2,3-dibromo-4,5-dihydroxybenzyl) ether | α-Glucosidase inhibition | [162] |
| 2,3-Dibromo-4,5-dihydroxybenzyl alcohol | |||
| 2,3-Dibromo-4,5-dimethoxybenzyl methyl ether | |||
| 4-Bromo-2,3-dihydroxy-6-hydroxymethylphenyl 2,5-dibromo-6-hydroxy-3-hydroxymethylphenyl ether | |||
| 4-Bromo-2,3-dimethoxy-6-methoxymethylphenyl 2,5-dibromo-6-methoxy-3-methoxymethylphenyl ether | |||
| 4-Bromo-2,3-dimethoxy-6-methoxymethylphenyl 2,5-dibromo-6-methoxy-3-methoxymethylphenyl ether | |||
| 3-Bromo-4,5-dimethoxybenzyl methyl ether | |||
| Polyopes lancifolia | Bis-(2,3-dibromo-4,5-dihydroxybenzyl) ether | α-Glucosidase inhibition | [163] |
| Polysiphonia morrowii | 3-Bromo-4,5-dihydroxybenzyl alcohol | α-Glucosidase inhibition | [164] |
| 3-Bromo-4,5-dihydroxybenzyl methyl ether | |||
| Rhodomela confervoides | Bis-(2,3-dibromo-4,5-dihydroxybenzyl) methane | Potent PTP1B inhibition | [165] |
| 3-Bromo-4,5-bis(2,3-dibromo-4,5-dihydroxybenzyl)-1,2-benzene-diol | [166] | ||
| 3,4-Dibromo-5-(2-bromo-3,4-dihydroxy-6-(isopropoxymethyl)benzyl)benzene-1,2-diol | |||
| 2,2′,3,3′-Tetrabromo-4,4′,5,5′-tetra-hydroxydiphenyl methane | [167] | ||
| 2,2′,3-Tribromo-3′,4,4′,5-tetrahydroxy-6′-ethyloxy-methyldiphenyl methane | |||
| Symphylocladia latiuscula | 2,3-Dibromo-4,5-dihydroxybenzyl methyl ether | PTP1B inhibition | [168] |
| 3,5-Dibromo-4-hydroxybenzoic acid | |||
| 2,3,6-Tribromo-4,5-dihydroxymethylbenzene | |||
| 2,3,6-Tribromo-4,5-dihydroxybenzaldehyde | |||
| 2,3,6-Tribromo-4,5-dihydroxybenzyl methyl ether | |||
| Bis-(2,3,6-tribromo-4,5-dihydroxyphenyl) methane | |||
| 1,2-Bis-(2,3,6-tribromo-4,5-dihydroxyphenyl)-ethane | |||
| 1-(2,3,6-Tribromo-4,5-dihydroxybenzyl)-pyrrolidin-2-one | |||
| 2,3,6-Tribromo-4,5-dihydroxybenzyl alcohol | α-Glucosidase inhibition | [164] |
| Marine Algae Species | Compounds of Interest | Model | Pharmacological Effects | References |
|---|---|---|---|---|
| Brown Algae | ||||
| Dictyopteris undulata | Sesquiterpene, zonarol | In vitro | Activates the Nrf2/ARE pathway, induces phase-2 enzymes, and protects neuronal cells from oxidative stress | [211] |
| Eisenia bicyclis | Phlorotannins | In vitro | Inhibits AChE at IC50 = 4.8 mg.mL−1 | [212] |
| Suppression of BACE-1 enzyme activity at IC50 = 5.35 µM | [213] | |||
| Decreased Aβ-induced cell death at IC50 = 800 µM | [214] | |||
| Ecklonia cava | Dieckol, phlorofucofuroeckol | In vivo | Improvement of memory, and possible involvement in AChE inhibition | [207] |
| Triphlorethol-A | Anti-oxidative activity, scavenging activity against ROS and DPPH via activation of ERK protein | [207] | ||
| Phlorotannins | In vitro | Scavenging activity against hydroxyl, superoxide, and peroxyl radicals at IC50 = 392.5, 115.2, and 128.9 µM, respectively | [215] | |
| In vivo | Potentiated pentobarbital-induced sleep at >50 mg.kg−1 | [216] | ||
| Neuroprotective effects against H2O2-induced oxidative stress in murine hippocampal HT22 cells at IC50 = 50 µM | [217] | |||
| Phloroglucinol | In vivo | Reduces the toxicity ROS induced by hydrogen peroxide at IC50 = 10 µg.mL−1 | [218] | |
| Eckol | In vitro, In vivo | Inhibits BChE IC50 = 29 µM | [219] | |
| 7-phloroeckol) | In vitro, In vivo | Inhibits BChE at IC50 = 0.95 µM | [219] | |
| Ecklonia kurome | Acidic oligosaccharide sugar chain (AOSC) | In vitro | Blocks the fibril formation of Aβ at IC50 = 100 µg.mL−1 | [220] |
| Ecklonia maxima | Phlorotannins | In vitro | Inhibits AChE at IC50 = 62.61 to 150.80 µg.mL−1 | [221] |
| Ecklonia stolonifera | Phlorotannins (dieckol, eckstolonol, eckol 2-phloroeckol, 7-phloroeckol, phlorofucofuroeckol A) | In vitro | Inhibits AChE at IC50 = 4.89 to 42.66 µM Inhibits BuChE at IC50 = 136.71 to 230.27 µM | [222] |
| Sterol (fucosterol) | In vitro | Inhibits BChE at IC50 = 421.72 µM | [222] | |
| Fucus vesiculosus | Fucoidan | In vitro | Blocks microglial uptake of fDNA at only 40 ng.mL−1 | [200] |
| In vivo | Inhibits superoxide radicals, hydroxyl radicals, and lipid peroxidation at IC50 = 0.058, 0.157, and 1.250 mg.mL−1 | [223] | ||
| Neuroprotective through iNOS | [224] | |||
| Inhibits TNF-α and IFN-γ-stimulated NO production via p38 MAPK, AP-1, JAK/STAT, and IRF-1 | [225] | |||
| Inhibits beta-amyloid induced microglial clustering at IC50 = 10 µM | [226] | |||
| Phlorotannins | In vivo | Suppresses the overproduction of intracellular ROS induced by hydrogen peroxide at IC50 = 0.068 mg.mL−1 | [227] | |
| Marginariella boryana | Sulfated fucans | In vitro | Prevents the accumulation of Aβ | [228] |
| Ishige okamurae | Diphlorethohydroxycarmalol (DPHC) | In vivo | Neuroprotection against hydrogen peroxide (H2O2)-induced oxidative stress in murine hippocampal neuronal cells at IC50 = 50 µM | [67] |
| Phlorotannins | In vitro | Inhibits AChE at IC50 = 46.42 µM Inhibits BChE at IC50 = 110.83 µM | [67] | |
| Padina gymnospora | Fucoxanthin | In vivo | Anti-oxidative activity, reduces lipid peroxidation in rats at IC50 = 0.83 µM | [229] |
| Papenfussiella lutea | Sesquiterpenes | In vivo | Inhibiting AChE at IC50 = 65 µM | [228] |
| Saccharina japonica | Fucoidan | In vivo | Reduces the toxicity of H2O2 in PC12 cells via activation of PI3K/Akt pathway | [230] |
| S. japonica | Fucoidan | In vivo | Inhibits microglia, inhibits LPS-induced NO production via suppression of p38 MAPK and ERK phosphorylation at IC50 = 125 µg.mL−1 | [231] |
| Sargassum fulvellum | Pheophytin A | In vivo | Produce neurite outgrowth, at IC50 = 3.9 µg.mL−1 in PC12 cells | [232] |
| Sargassum macrocarpum | Carotenoids, sargaquinoic acid, and sargachromenol | In vivo | Promotes neurite outgrowth activity and survival of PC-12 cells and neurite outgrowth through activation of cAMP and MAP kinase pathways at IC50 = 9 µM | [223] |
| Sargassum micracanthum | Plastoquinones | In vivo | Anti-oxidative activity, lipid peroxidation at IC50 = 0.95–44.3 µg.mL−1 | [233] |
| Hijikia fusiformis | Fucoxanthin | In vitro | Anti-oxidative activity, DPPH radical scavenging | [53] |
| Sargassum fusiforme | Fucoidan | In vivo | Ameliorates learning and memory deficiencies, and potential ingredient for treatment of Alzheimer’s disease | [234] |
| Sargassum horneri | Total sterols, β-sitosterol | In vivo | Antidepressant effect | [235] |
| Sargassum sagamianum | Sargaquinoic acid, sargachromenol | In vitro | Inhibits AChE IC50 = 23.2 and 32.7 µM, respectively, inhibits BuChE at IC50 = 26 µM (for sargaquinoic acid) | [236] |
| Sargassum siliquastrum | Meroditerpenoids | In vitro | Radical-scavenging activity as well as weak inhibitory activities against sortase A and isocitrate lyase | [237] |
| Scytothamnus australis | Sulfated fucans | In vivo | Prevents the accumulation of Aβ | [228] |
| Splachnidium rugosum | Sulfated fucans | In vivo | Prevents the accumulation of Aβ | [228] |
| Turbinaria decurrens | Fucoidan | In vivo | Potential neuroprotective effects in Parkinson’s disease | [238] |
| Undaria pinnatifida | Glycoprotein | In vivo | AChE, BChE, and BACE1 inhibitory activities with IC50 values of 63.56, 99.03, and 73.35 µg.mL−1, respectively | [239] |
| Zonaria spiralis | Spiralisone A, Chromone 6 | In vitro | Kinases inhibitory to CDK5/p25, CK1δ, and GSK3β at IC50 = 10.0, <10 µM, and <10 µM, respectively | [240] |
| Red Algae | ||||
| Chondracanthus acicularis | Lambda-carrageenan | In vitro | Inhibits superoxide radicals, hydroxyl radicals, and lipid peroxidation at IC50 = 0.046, 0.357, and 2.267 mg.mL−1, respectively | [223] |
| Chondrophycus undulatus | Floridoside | In vivo | Suppresses pro- inflammatory responses in microglia by markedly inhibiting the production of nitric oxide (NO) and reactive oxygen species (ROS) at IC50 = 10 µM | [241] |
| Eucheuma denticulatum | Iota-carrageenan | In vitro | Inhibits superoxide radicals, hydroxyl radicals, and lipid peroxidation at IC50 = 0.332, 0.281, and 0.830 mg.mL−1, respectively | [223] |
| Gelidiella acerosa | Phytol | In vitro, in vivo | Antioxidant activities at IC50 = 25–125 µg.mL−1 | [193] |
| Kappaphycus alvarezii | Kappa-carrageenan | In vitro | Inhibits superoxide radicals, hydroxyl radicals, and lipid peroxidation at IC50 = 0.112, 0.335, and 0.323 mg.mL−1, respectively | [223] |
| Ochtodes secundiramea | Halogenated monoterpenes | In vitro | Inhibits AChE at IC50 = 400 µg mL−1 | [242] |
| Porphyra/Pyropia sp. | Phycoerythrobilin | In vitro | Antioxidant activity at IC50 = 0.048 mmol.g−1 | [243] |
| Rhodomela confervoides | Bromophenols | In vitro | Antioxidant activity at IC50 = 5.22–23.60 µM | [166] |
| Rhodomelopsis africana | Phenolic compounds, Flavonoids | In vitro | Inhibits AChE at IC50 = 0.12 mg.mL−1 | [244] |
| Green Algae | ||||
| Caulerpa racemosa | Bisindole alkaloid (A and B), α-tocospirone, Sterol (23E)-3βhydroxystigmasta-5,23dien28-one | In vivo | Increase 5.5% of cell viability in SH-SY5Y cells, inhibits AChE at IC50 = 5.5 mg.mL−1 | [245] |
| Codium capitatum | Phenolic compounds, Flavonoids | In vitro | Inhibits AChE at IC50 = 0.11 mg.mL−1 | [246] |
| Codium duthieae | Phenolic compounds, Flavonoids | In vitro | Inhibits AChE at IC50 = 0.14 mg.mL−1 | [246] |
| Codium fragile | Clerosterol | In vivo, in vitro | Exhibits reducing activity to COX-2, iNOS, and TNF-α at IC50 = 3 µg.mL−1 | [247] |
| Halimeda cuneata | Phenolic compounds, Flavonoids | In vitro | Inhibits AChE at IC50 = 0.07 mg.mL−1 | [246] |
| Ulva pertusa | Sulfated polysaccharides | In vitro | Scavenging activity for superoxide radicals | [52] |
| Ulva fasciata | Phenolic compounds, Flavonoids | In vitro | Inhibits AChE at IC50 = 0.07 mg.mL−1 | [246] |
| Ulva prolifera | Pheophorbide A | In vitro | Antioxidant activity at IC50 = 71.9 µM | [52] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menaa, F.; Wijesinghe, U.; Thiripuranathar, G.; Althobaiti, N.A.; Albalawi, A.E.; Khan, B.A.; Menaa, B. Marine Algae-Derived Bioactive Compounds: A New Wave of Nanodrugs? Mar. Drugs 2021, 19, 484. https://doi.org/10.3390/md19090484
Menaa F, Wijesinghe U, Thiripuranathar G, Althobaiti NA, Albalawi AE, Khan BA, Menaa B. Marine Algae-Derived Bioactive Compounds: A New Wave of Nanodrugs? Marine Drugs. 2021; 19(9):484. https://doi.org/10.3390/md19090484
Chicago/Turabian StyleMenaa, Farid, Udari Wijesinghe, Gobika Thiripuranathar, Norah A. Althobaiti, Aishah E. Albalawi, Barkat Ali Khan, and Bouzid Menaa. 2021. "Marine Algae-Derived Bioactive Compounds: A New Wave of Nanodrugs?" Marine Drugs 19, no. 9: 484. https://doi.org/10.3390/md19090484
APA StyleMenaa, F., Wijesinghe, U., Thiripuranathar, G., Althobaiti, N. A., Albalawi, A. E., Khan, B. A., & Menaa, B. (2021). Marine Algae-Derived Bioactive Compounds: A New Wave of Nanodrugs? Marine Drugs, 19(9), 484. https://doi.org/10.3390/md19090484











