An Overview of New Insights into the Benefits of the Seagrass Posidonia oceanica for Human Health
Abstract
:1. Introduction
2. Ecological Significance of P. oceanica Seagrass Ecosystem
3. Historical and Traditional Medical Uses of P. oceanica
4. Phytochemical Compounds of P. oceanica Leaves
5. P. oceanica Bioactive Properties: From Tradition to New Horizons
5.1. Antibacterial, Antifungal, and Antiviral Role
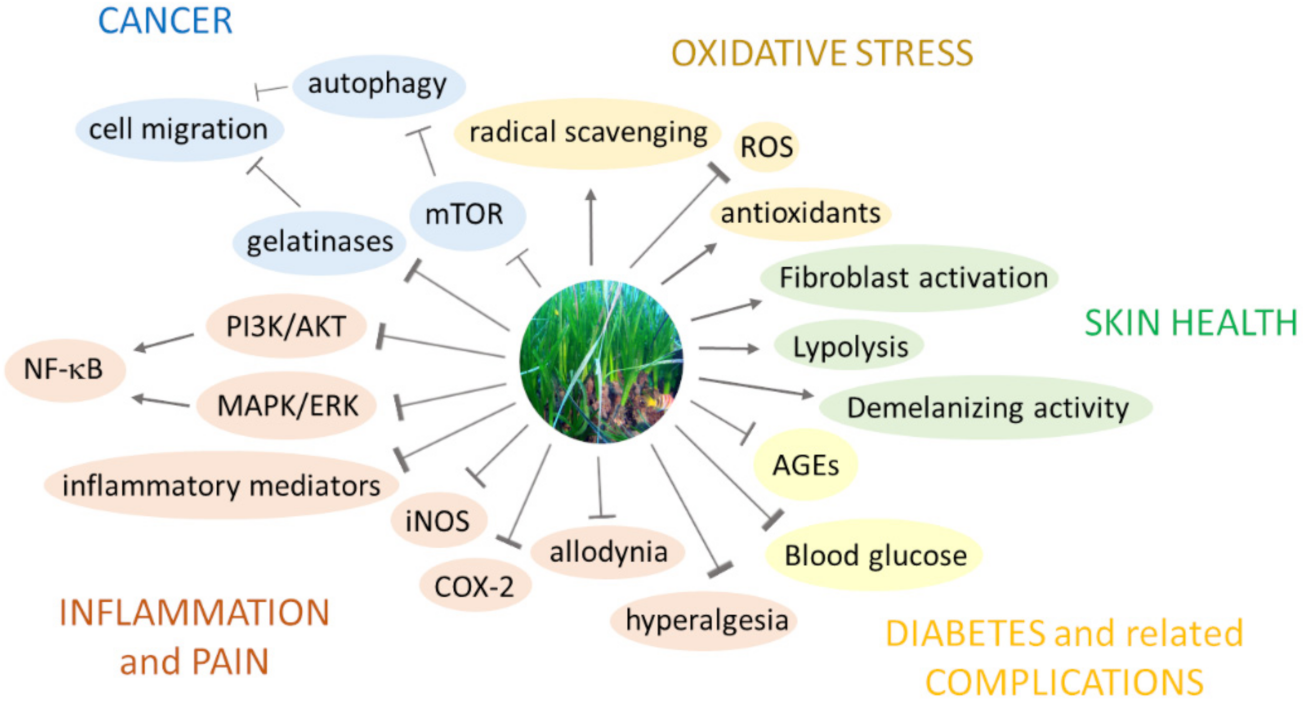
5.2. Insights into the Bioactivities of P. oceanica with Potential Human Health Applications
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- The Angiosperm Phylogeny Group. An update of the angiosperm phylogeny group classification for the orders and families of flowering plants: APG III. Bot. J. Linn. Soc. 2009, 161, 105–121. [Google Scholar] [CrossRef] [Green Version]
- den Hartog, C.; Kuo, J.J. Taxonomy and biogeography in seagrasses. In Seagrasses: Biology, Ecology and Conservation; Larkum, A.W.D., Orth, R.J., Duarte, C.M., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 1–23. [Google Scholar]
- Papenbrock, J. Highlights in seagrasses’ phylogeny, physiology, and metabolism: What makes them special? ISRN Bot. 2012, 2012, 103892. [Google Scholar] [CrossRef] [Green Version]
- Procaccini, G.; Buia, M.C.; Gambi, M.C.; Perez, M.; Pergent, G.; Pergent-Martini, C.; Romer, J. The seagrasses of western Mediterranean. In World Atlas of Seagrasses; Green, E.P., Short, F.T., Eds.; University of California Press: Berkeley, CA, USA, 2003; pp. 48–58. [Google Scholar]
- Gobert, S.; Cambridge, M.T.; Velimirov, B.; Pergent, G.; Lepoint, G.; Bouquegneau, J.M.; Walker, D.I. Biology of Posidonia. In Seagrasses: Biology, Ecology and Conservation; Larkum, A.W.D., Orth, R.J., Duarte, C.M., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 387–408. [Google Scholar]
- Vacchi, M.; De Falco, G.; Simeone, S.; Montefalcone, M.; Morri, C.; Ferrari, M.; Bianchi, C.N. Biogeomorphology of the Mediterranean Posidonia oceanica sea grass meadows. Earth Surf. Process. Landf. 2016, 42, 42–54. [Google Scholar] [CrossRef] [Green Version]
- Boudouresque, C.F.; Bernard, G.; Bonhomme, P.; Charbonnel, E.; Diviacco, G.; Meinesz, A.; Pergent, G.; Pergent-Martini, C.; Ruitton, S.; Tunesi, L. Protection and Conservation of Posidonia Oceanica Meadows; RAMOGE and RAC/SPA Publishers: Tunis, Tunisia, 2012; pp. 1–202. [Google Scholar]
- Pasqualini, V.; Pergent-Martini, C.; Clabaut, P.; Pergent, G. Mapping of Posidonia oceanica using aerial photographs and side scan sonar: Application off the island of Corsica (France). Estuar. Coast. Shelf Sci. 1998, 47, 359–367. [Google Scholar] [CrossRef]
- Bonhomme, D.; Boudouresque, C.F.; Astruch, P.; Bonhomme, J.; Bonhomme, P.; Goujard, A.; Thibaut, T. Typology of the reef formations of the Mediterranan seagrass Posidonia oceanica, and the discovery of extensive reefs in the Gulf of Hyères (Pro-vence, Mediterranean). Sci. Rep. Port-Cros Natl. Park 2015, 29, 41–73. [Google Scholar]
- Buia, M.C.; Gambi, M.C.; Zupo, V. Structure and functioning of Mediterranean seagrass ecosystems: An overview. Biol. Mar. Mediterr. 2000, 7, 167–190. [Google Scholar]
- Lepoint, G.; Havelange, S.; Gobert, S.; Bouquegneau, J.M. Fauna vs. flora contribution to the leaf epiphytes biomass in a Po-sidonia oceanica seagrassbed (Revellata Bay, Corsica). Hydrobiologia 1999, 394, 63–67. [Google Scholar] [CrossRef]
- Boudouresque, C.F. Marine biodiversity in the Mediterranean: Status of species, populations and communities. Trav. Sci. Parc Natl. Port-Cros 2004, 20, 97–146. [Google Scholar]
- Pergent, G. La protection légale de la Posidonie en France: Un outil efficace. Nécessité de son extension a d’autres pays Médi-terranéens. In Les Espèces Marines a Protéger en Méditerranée: Rencontres scientifiques de la Cote Bleue; Boudouresque, C.F., Avon, M., Gravez, V., Eds.; GIS Posidonie: Marseille, France, 1991; pp. 29–34. [Google Scholar]
- European Commission. Directive 2008/56/EC of the European Parliament and of the Council of 17 June 2008 establishing a framework for community actions in the field of marine environmental policy (marine strategy framework directive). O. J. Eur. Communities 2008, 164, 19–40. [Google Scholar]
- Cornara, L.; Pastorino, G.; Borghesi, B.; Salis, A.; Clericuzio, M.; Marchetti, C.; Damonte, G.; Burlando, B. Posidonia oceanica (L.) Delile ethanolic extract modulates cell activities with skin health applications. Mar. Drugs 2018, 16, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leri, M.; Ramazzotti, M.; Vasarri, M.; Peri, S.; Barletta, E.; Pretti, C.; Degl’Innocenti, D. Bioactive compounds from Posidonia oceanica (L.) Delile impair malignant cell migration through autophagy modulation. Mar. Drugs 2018, 16, 137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasarri, M.; Leri, M.; Barletta, E.; Ramazzotti, M.; Marzocchini, R.; Degl’Innocenti, D. Anti-inflammatory properties of the marine plant Posidonia oceanica (L.) Delile. J. Ethnopharmacol. 2019, 247, 112252. [Google Scholar] [CrossRef]
- Piazzini, V.; Vasarri, M.; Degl’Innocenti, D.; Guastini, A.; Barletta, E.; Salvatici, M.C.; Bergonzi, M.C. Comparison of chitosan nanoparticles and soluplus micelles to optimize the bioactivity of Posidonia oceanica extract on human neuroblastoma cell migration. Pharmaceutics 2019, 11, 655. [Google Scholar] [CrossRef] [Green Version]
- Barletta, E.; Ramazzotti, M.; Fratianni, F.; Pessani, D.; Degl’Innocenti, D. Hydrophilic extract from Posidonia oceanica inhibits activity and expression of gelatinases and prevents HT1080 human fibrosarcoma cell line invasion. Cell Adhes. Migr. 2015, 9, 422–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasarri, M.; Barletta, E.; Ramazzotti, M.; Degl’Innocenti, D. In vitro anti-glycation activity of the marine plant Posidonia oceanica (L.) Delile. J. Ethnopharmacol. 2020, 259, 112960. [Google Scholar] [CrossRef]
- Bay, D. A field study of the growth dynamics and productivity of Posidonia oceanica (L.) Delile in Calvi Bay, Corsica. Aquat. Bot. 1984, 20, 43–64. [Google Scholar] [CrossRef]
- Micheli, C.; D’Esposito, D.; Belmonte, A.; Peirano, A.; Valiante, L.M.; Procaccini, G. Genetic diversity and structure in two protected Posidonia oceanica meadows. Mar. Environ. Res. 2015, 109, 124–131. [Google Scholar] [CrossRef]
- Vassallo, P.; Paoli, C.; Rovere, A.; Montefalcone, M.; Morri, C.; Bianchi, C.N. The value of the seagrass Posidonia oceanica: A natural capital assessment. Mar. Pollut. Bull. 2013, 75, 157–167. [Google Scholar] [CrossRef]
- Campagne, C.S.; Salles, J.-M.; Boissery, P.; Deter, J. The seagrass Posidonia oceanica: Ecosystem services identification and economic evaluation of goods and benefits. Mar. Pollut. Bull. 2015, 97, 391–400. [Google Scholar] [CrossRef]
- Personnic, S.; Boudouresque, C.F.; Astruch, P.; Ballesteros, E.; Blouet, S.; Bellan-Santini, D.; Bonhomme, P.; Thibault-Botha, D.; Feunteun, E.; Harmelin-Vivien, M.; et al. An ecosystem-based approach to assess the status of a Mediterranean ecosystem, the Posidonia oceanica seagrass meadow. PLoS ONE 2014, 9, e98994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellisario, B.; Camisa, F.; Abbattista, C.; Cimmaruta, R. A network approach to identify bioregions in the distribution of Mediterranean amphipods associated with Posidonia oceanica meadows. PeerJ 2019, 7, e6786. [Google Scholar] [CrossRef] [Green Version]
- El Din, N.G.S.; El-Sherif, Z.M. Nutritional value of Cymodocea nodosa and Posidonia oceanica along the western Egyptian Mediterranean coast. Egypt. J. Aquat. Res. 2013, 39, 153–165. [Google Scholar] [CrossRef] [Green Version]
- Bay, D. Etude in Situ de la Production Primaire d’un Herbier de Posidonies Posidonia oceanica (L.) Delile de la Baie de Cal-vi-Corse. Ph.D. Thesis, University of Liège, Liège, Belgium, 1978; p. 251. [Google Scholar]
- Boudouresque, C.F.; Bernard, G.; Bonhomme, P.; Charbonnel, E.G.; Diviacco, E.; Meinesz, A.; Pergent, G.; Pergent-Martini, C.; Ruitton, S.; Tunesi, L. Préservation et Conservation des Herbiers à Posidonia Oceanica; RaMoGe Publishing: Monaco, 2006; pp. 1–202. [Google Scholar]
- Dauby, P.; Bale, A.J.; Bloomer, N.; Canon, C.; Ling, R.D.; Norro, A.; Robertson, J.E.; Simon, A.; Théate, J.M.; Watson, A.J.; et al. Particle fluxes over a Mediterranean seagrass bed: A one-year sediment trap experiment. Mar. Ecol. Prog. Ser. 1995, 126, 233–246. [Google Scholar] [CrossRef]
- Fonseca, M.S.; Koehl, M.; Kopp, B.S. Biomechanical factors contributing to self-organization in seagrass landscapes. J. Exp. Mar. Biol. Ecol. 2007, 340, 227–246. [Google Scholar] [CrossRef]
- Mateo, M.; Lizaso, J.L.S.; Romero, J. Posidonia oceanica “banquettes”: A preliminary assessment of the relevance for meadow carbon and nutrients budget. Estuar. Coast. Shelf Sci. 2003, 56, 85–90. [Google Scholar] [CrossRef]
- Benoit, G.; Comeau, A. A sustainable future for the Mediterranean. In The Blue Plan’s Environment and Development Outlook, 1st ed.; Benoit, G., Comeau, A., Eds.; Earth Scan: London, UK, 2005; p. 462. [Google Scholar]
- Orth, R.; Carruthers, T.J.B.; Dennison, W.C.; Duarte, C.M.; Fourqurean, J.W., Jr.; Hughes, A.R.; Kendrick, G.; Kenworthy, W.J.; Olyarnik, S. A global crisis for seagrass ecosystems. BioScience 2006, 56, 987–996. [Google Scholar] [CrossRef] [Green Version]
- Waycott, M.; Duarte, C.M.; Carruthers, T.J.B.; Orth, R.J.; Dennison, W.C.; Olyarnik, S.; Calladine, A.; Fourqurean, J.W.; Heck, K.L.; Hughes, A.R.; et al. Accelerating loss of seagrasses across the globe threatens coastal ecosystems. Proc. Natl. Acad. Sci. USA 2009, 106, 12377–12381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bianchi, C.N.; Morri, C. Climate change and biological response in Mediterranean Sea ecosystems: A need for broad-scale and long-term research. Ocean. Chall. 2004, 13, 32–36. [Google Scholar]
- Marbà, N.; Diaz-Almela, E.; Duarte, C.M. Mediterranean seagrass (Posidonia oceanica) loss between 1842 and 2009. Biol. Conserv. 2014, 176, 183–190. [Google Scholar] [CrossRef]
- Gera, A.; Alcoverro, T.; Mascaró, O.; Pérez, M.; Romero, J. Exploring the utility of Posidonia oceanica chlorophyll fluorescence as an indicator of water quality within the European water framework directive. Environ. Monit. Assess. 2011, 184, 3675–3686. [Google Scholar] [CrossRef]
- Pergent-Martini, C.; Leoni, V.; Pasqualini, V.; Ardizzone, G.; Balestri, E.; Bedini, R.; Belluscio, A.; Belsher, T.; Borg, J.; Boudouresque, C.; et al. Descriptors of Posidonia oceanica meadows: Use and application. Ecol. Indic. 2005, 5, 213–230. [Google Scholar] [CrossRef]
- Carrasco-Acosta, M.; Garcia-Jimenez, P. Maintaining and storing encapsulated cells for propagation of Posidonia oceanica (L.) Delile. Aquat. Biol. 2021, 30, 47–57. [Google Scholar] [CrossRef]
- De Lumley, H.; Pillard, B.; Pillard, F. L’habitat et les activités de l’homme du Lazaret. Une cabanne acheuléenne de la grotte du Lazaret (Nice). Mém. Soc. Préhist. Fr. 1969, 7, 183–222. [Google Scholar]
- Weddell, H.A. Note sur les aegagropiles de mer. In Proceedings of the Actes du Congrès International de Botanique, Amsterdam, The Netherlands, 13–17 April 1877; pp. 58–62. [Google Scholar]
- Tackolom, V.; Drar, M. Flora of Egypt: Angiospermae, part Monocotyledones: Liliaceae musaceae. Bull. Fac. Sci. 1954, 30, 1–648. [Google Scholar]
- Boudouresque, C.F.; Meinesz, A. Découverte de l’herbier de Posidonie. Cah. Parc Natl. Port-Cros 1982, 4, 1–79. [Google Scholar]
- Le Floch, E. Contribution à Une Étude Ethnobotanique de la Flore Tunisienne; Ministère de l’Education Nationale et de la Recherche Scientifique: Tunis, Tunisia, 1983; pp. 1–402. [Google Scholar]
- De Saint-Pierre, M.E.G. Sur la germination et le mode de développement du Posidonia caulini. Bull. Société Bot. Fr. 1857, 4, 575–577. [Google Scholar] [CrossRef] [Green Version]
- Braun-Blanquet, J.; Roussine, N.; Negre, R. Les Groupements Végétaux de la France Méditerranéenne; Macabet Frères Publishing: Vaison-la-Romaine, France, 1952; pp. 1–297. [Google Scholar]
- A&L Canada Laboratories. Compost Management Program—Compost Analysis for Available Nutrients and Soil Suitability Criteria and Evaluation. 2012. Available online: https://www.alcanada.com/pdf/technical/compost/Compost_Handbook.pdf (accessed on 19 July 2021).
- Sauvageau, C. Observations sur la structure des feuilles des plantes aquatiques (suite). J. Bot. Paris 1890, 4, 181–192. [Google Scholar]
- Lami, R. L’utilisation des végétaux marins des côtes de France. Rev. Bot. Appl. Agric. Tropic. 1941, 21, 653–670. [Google Scholar] [CrossRef]
- Karleskint, G.; Turner, R.; Small, J. Introduction to Marine Biology, 3rd ed.; Cengage Learning Customer and Sales Support: Belmont, CA, USA, 2009; p. 573. [Google Scholar]
- Batanouny, K.H. Wild Medicinal Plants in Egypt; Academy of Scientific Research and Technology: Cairo, Egypt, 1999. [Google Scholar]
- Cazzuola, F. Le Piante Utili e Nocive Agli Uomini e Agli Animali Che Crescono Spontanee e Coltivate in Italia con Brevi Cenni Sopra la Coltura, Sopra i Prodotti e Sugli Usi Che se ne Fanno; Loescher Editore: Torino, Italy, 1880; pp. 1–217. [Google Scholar]
- El-Mokasabi, F.M. Floristic composition and traditional uses of plant species at Wadi Alkuf, Al-Jabal Al-Akhder, Libya. Am. Eur. J. Agric. Environ. Sci. 2014, 14, 685–697. [Google Scholar]
- Gokce, G.; Haznedaroglu, M.Z. Evaluation of antidiabetic, antioxidant and vasoprotective effects of Posidonia oceanica extract. J. Ethnopharmacol. 2008, 115, 122–130. [Google Scholar] [CrossRef]
- Agostini, S.; Desjobert, J.-M.; Pergent, G. Distribution of phenolic compounds in the seagrass Posidonia oceanica. Phytochemistry 1998, 48, 611–617. [Google Scholar] [CrossRef]
- Costa, J.; Desjobert, J.; Pergent, G. Variations in the concentration of phenolic compounds in the seagrass Posidonia oceanica under conditions of competition. Phytochemistry 2004, 65, 3211–3220. [Google Scholar] [CrossRef]
- Cuny, P.; Serve, L.; Jupin, H.; Boudouresque, C.-F. Water soluble phenolic compounds of the marine phanerogam Posidonia oceanica in a Mediterranean area colonised by the introduced chlorophyte Caulerpa taxifolia. Aquat. Bot. 1995, 52, 237–242. [Google Scholar] [CrossRef]
- Haznedaroglu, M.Z.; Zeybek, U. HPLC determination of chicoric acid in leaves of Posidonia oceanica. Pharm. Biol. 2007, 45, 745–748. [Google Scholar] [CrossRef]
- Cariello, L.; Zanetti, L. Distribution of chicoric acid during leaf development of Posidonia oceanica. Bot. Mar. 1979, 22, 359–360. [Google Scholar] [CrossRef]
- Grignon-Dubois, M.; Rezzonico, B. Phenolic fingerprint of the seagrass Posidonia oceanica from four locations in the Mediterranean Sea: First evidence for the large predominance of chicoric acid. Bot. Mar. 2015, 58. [Google Scholar] [CrossRef]
- Cannac, M.; Ferrat, L.; Pergent-Martini, C.; Pergent, G.; Pasqualini, V. Effects of fish farming on flavonoids in Posidonia oceanica. Sci. Total Environ. 2006, 370, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Heglmeier, A.; Zidorn, C. Secondary metabolites of Posidonia oceanica (Posidoniaceae). Biochem. Syst. Ecol. 2010, 38, 964–970. [Google Scholar] [CrossRef]
- Viso, A.-C.; Pesando, D.; Bernard, P.; Marty, J.-C. Lipid components of the Mediterranean seagrass Posidonia oceanica. Phytochemistry 1993, 34, 381–387. [Google Scholar] [CrossRef]
- Klap, V.; Hemminga, M.; Boon, J. Retention of lignin in seagrasses: Angiosperms that returned to the sea. Mar. Ecol. Prog. Ser. 2000, 194, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Hammami, S.; Ben Salem, A.; Ashour, M.L.; Cheriaa, J.; Graziano, G.; Mighri, Z.; Mohamed, L.A. A novel methylated sesquiterpene from seagrass Posidonia oceanica (L.) Delile. Nat. Prod. Res. 2013, 27, 1265–1270. [Google Scholar] [CrossRef]
- Bernard, P.; Pesando, D. Antibacterial and antifungal activity of extracts from the rhizomes of the Mediterranean seagrass Posidonia oceanica (L.) Delile. Bot. Mar. 1989, 32. [Google Scholar] [CrossRef]
- Berfad, M.A.; Alnour, T.M.S. Phytochemical analysis and antibacterial activity of the 5 different extracts from the seagrasses Posidonia oceanica. J. Med. Plant. Stud. 2014, 2, 15–18. [Google Scholar]
- Farid, M.M.; Marzouk, M.M.; Hussein, S.R.; Elkhateeb, A.; Abdel-Hameed, E.S. Comparative study of Posidonia oceanica L.: LC/ESI/MS analysis, cytotoxic activity and chemosystematic significance. J. Mater. Environ. Sci. 2018, 9, 1676–1682. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Munekata, P.E.; Gomez, B.; Barba, F.J.; Mora, L.; Perez-Santaescolastica, C.; Toldra, F. Bioactive peptides as natural antioxidants in foods—A review. Trends Food Sci. Technol. 2018, 79, 136–147. [Google Scholar] [CrossRef]
- Baptista, R.C.; Horita, C.N.; Sant’Ana, A.S. Natural products with preservative properties for enhancing the microbiological safety and extending the shelf-life of seafood: A review. Food Res. Int. 2019, 127, 108762. [Google Scholar] [CrossRef]
- Kourkoutas, Y.; Proestos, C. Food Preservation: Challenges and Efforts for the Future. Foods 2020, 9, 391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piva, G.; Fracassetti, D.; Tirelli, A.; Mascheroni, E.; Musatti, A.; Inglese, P.; Piergiovanni, L.; Rollini, M. Evaluation of the an-tioxidant/antimicrobial performance of Posidonia oceanica in comparison with three commercial natural extracts and as a treatment on fresh-cut peaches (Prunus persica Batsch). Postharvest. Biol. Tec. 2017, 124, 54–61. [Google Scholar] [CrossRef] [Green Version]
- Benito-González, I.; López-Rubio, A.; Martínez-Abad, A.; Ballester, A.-R.; Falcó, I.; González-Candelas, L.; Sánchez, G.; Lozano-Sánchez, J.; Borrás-Linares, I.; Segura-Carretero, A.; et al. In-depth characterization of bioactive extracts from Posidonia oceanica waste biomass. Mar. Drugs 2019, 17, 409. [Google Scholar] [CrossRef] [Green Version]
- Catanesi, M.; Caioni, G.; Castelli, V.; Benedetti, E.; D’Angelo, M.; Cimini, A. Benefits under the sea: The role of marine compounds in neurodegenerative disorders. Mar. Drugs 2021, 19, 24. [Google Scholar] [CrossRef] [PubMed]
- Sureda, A.; Box, A.; Terrados, J.; Deudero, S.; Pons, A. Antioxidant response of the seagrass Posidonia oceanica when epiphytized by the invasive macroalgae Lophocladia lallemandii. Mar. Environ. Res. 2008, 66, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Tutar, O.; Marín-Guirao, L.; Ruiz, J.; Procaccini, G. Antioxidant response to heat stress in seagrasses. A gene expression study. Mar. Environ. Res. 2017, 132, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Holmer, M.; Marbà, N.; Lamote, M.; Duarte, C.M. Deterioration of sediment quality in seagrass meadows (Posidonia oceanica) invaded by macroalgae (Caulerpa sp.). Chesap. Sci. 2009, 32, 456–466. [Google Scholar] [CrossRef] [Green Version]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive oxygen species in metabolic and inflammatory signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal. Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Micheli, L.; Vasarri, M.; Barletta, E.; Lucarini, E.; Ghelardini, C.; Degl’Innocenti, D.; Mannelli, L.D.C. Efficacy of Posidonia oceanica extract against inflammatory pain: In vivo studies in mice. Mar. Drugs 2021, 19, 48. [Google Scholar] [CrossRef]
- Khandia, R.; Dadar, M.; Munjal, A.; Dhama, K.; Karthik, K.; Tiwari, R.; Yatoo, M.I.; Iqbal, H.M.; Singh, K.P.; Joshi, S.K.; et al. A Comprehensive review of autophagy and its various roles in infectious, non-infectious, and lifestyle diseases: Current knowledge and prospects for disease prevention, novel drug design, and therapy. Cells 2019, 8, 674. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Li, Q.; Hu, J.; Wang, H.; Xu, F.; Bian, Q. Application of natural products derivatization method in the design of targeted anticancer agents from 2000 to 2018. Bioorg. Med. Chem. 2019, 27, 115150. [Google Scholar] [CrossRef]
- Colone, M.; Calcabrini, A.; Stringaro, A. Drug delivery systems of natural products in oncology. Molecules 2020, 25, 4560. [Google Scholar] [CrossRef]
- Petrovska, B.B. Historical review of medicinal plants′ usage. Pharmacogn. Rev. 2012, 6, 1–5. [Google Scholar] [CrossRef] [Green Version]



| Compound | Molecular Formula | Structure | References |
|---|---|---|---|
| Chicoric Acid | C22H18O12 | 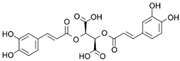 | [15,59,60,61] |
| Caftaric Acid | C13H12O9 | 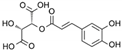 | [15,19,56,59,61] |
| Gentisic Acid | C7H6O4 |  | [15,19,56,59,61] |
| Chlorogenic Acid | C16H18O9 | 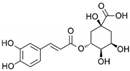 | [15,19,56,59,61] |
| Caffeic Acid | C9H8O4 |  | [15,19,56,59,61] |
| Ferulic Acid | C10H10O4 | 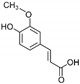 | [15,19,56,59,61] |
| Cinnamic Acid | C9H8O2 |  | [15,19,56,59,61] |
| Gallic Acid | C7H6O5 | 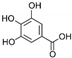 | [15,19,56,59,61] |
| p-Coumaric Acid | C9H8O3 | 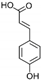 | [15,19,56,59,61] |
| Quercitin | C15H10O7 |  | [62,63] |
| Myricetin | C15H10O8 | 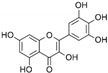 | [62,63] |
| Kaempferol | C15H10O6 |  | [62,63] |
| Isorhamnetin | C16H12O7 | 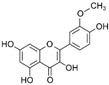 | [62,63] |
| Phloroglucinol | C6H6O3 | 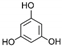 | [56,59,62,63] |
| Pyrocatechol | C6H6O2 | 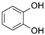 | [56,59,62,63] |
| Pyrogallol | C6H3(OH)3 |  | [56,59,62,63] |
| Vanillin | C8H8O3 |  | [56,59,62,63] |
| 4-Hydroxybenzaldehyde | C7H6O2 |  | [56,59,62,63] |
| 3,4-Dihydroxybenzaldehyde | C7H6O3 |  | [56,59,62,63] |
| Benzoic acid | C6H5COOH |  | [56,59,62,63] |
| 4-Hydroxybenzoic acid | C7H6O3 |  | [56,59,62,63] |
| p-Anisic acid | C8H8O3 | 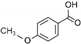 | [56,59,62,63] |
| Vanillic acid | C8H8O4 |  | [56,59,62,63] |
| Syringic acid | C9H10O5 | 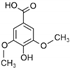 | [56,59,62,63] |
| Phloretin | C15H14O5 | 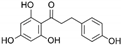 | [56,59,62,63] |
| Phlorizin | C21H24O10 | 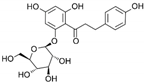 | [56,59,62,63] |
| Palmitic acid | C16H32O2 |  | [64] |
| Palmitoleic acid | C16H30O2 |  | [64] |
| Oleic acid | C18H34O2 |  | [64] |
| Linoleic acid | C18H32O2 |  | [64] |
| Campesterol | C28H48O |  | [64] |
| Stigmasterol | C29H48O | 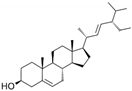 | [64] |
| β-Sitosterol | C29H50O | 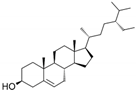 | [64] |
| Posidozinol | C16H32 |  | [66] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasarri, M.; De Biasi, A.M.; Barletta, E.; Pretti, C.; Degl’Innocenti, D. An Overview of New Insights into the Benefits of the Seagrass Posidonia oceanica for Human Health. Mar. Drugs 2021, 19, 476. https://doi.org/10.3390/md19090476
Vasarri M, De Biasi AM, Barletta E, Pretti C, Degl’Innocenti D. An Overview of New Insights into the Benefits of the Seagrass Posidonia oceanica for Human Health. Marine Drugs. 2021; 19(9):476. https://doi.org/10.3390/md19090476
Chicago/Turabian StyleVasarri, Marzia, Anna Maria De Biasi, Emanuela Barletta, Carlo Pretti, and Donatella Degl’Innocenti. 2021. "An Overview of New Insights into the Benefits of the Seagrass Posidonia oceanica for Human Health" Marine Drugs 19, no. 9: 476. https://doi.org/10.3390/md19090476
APA StyleVasarri, M., De Biasi, A. M., Barletta, E., Pretti, C., & Degl’Innocenti, D. (2021). An Overview of New Insights into the Benefits of the Seagrass Posidonia oceanica for Human Health. Marine Drugs, 19(9), 476. https://doi.org/10.3390/md19090476







