Botryllus schlosseri as a Unique Colonial Chordate Model for the Study and Modulation of Innate Immune Activity
Abstract
1. Introduction
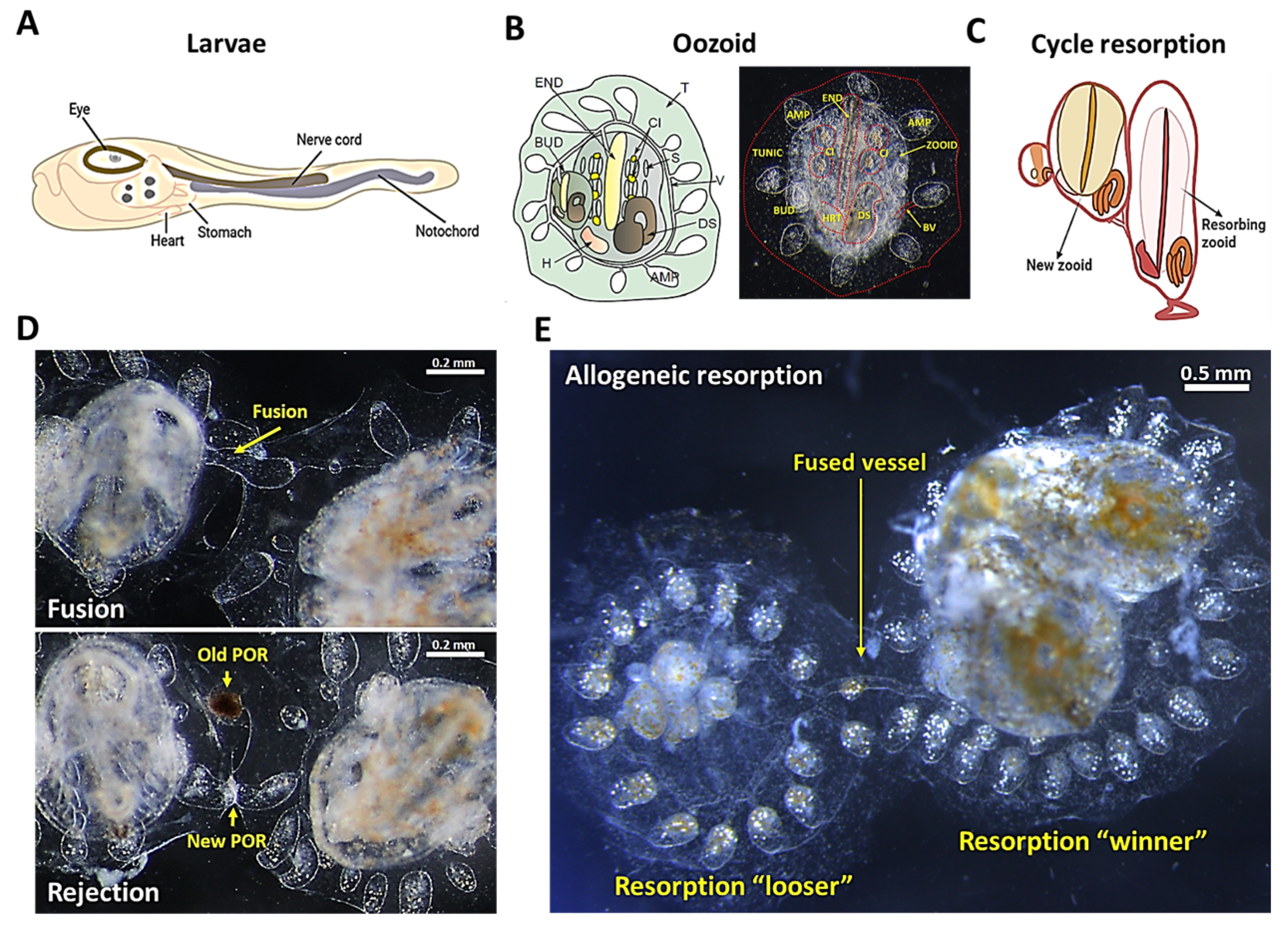
Botryllus schlosseri
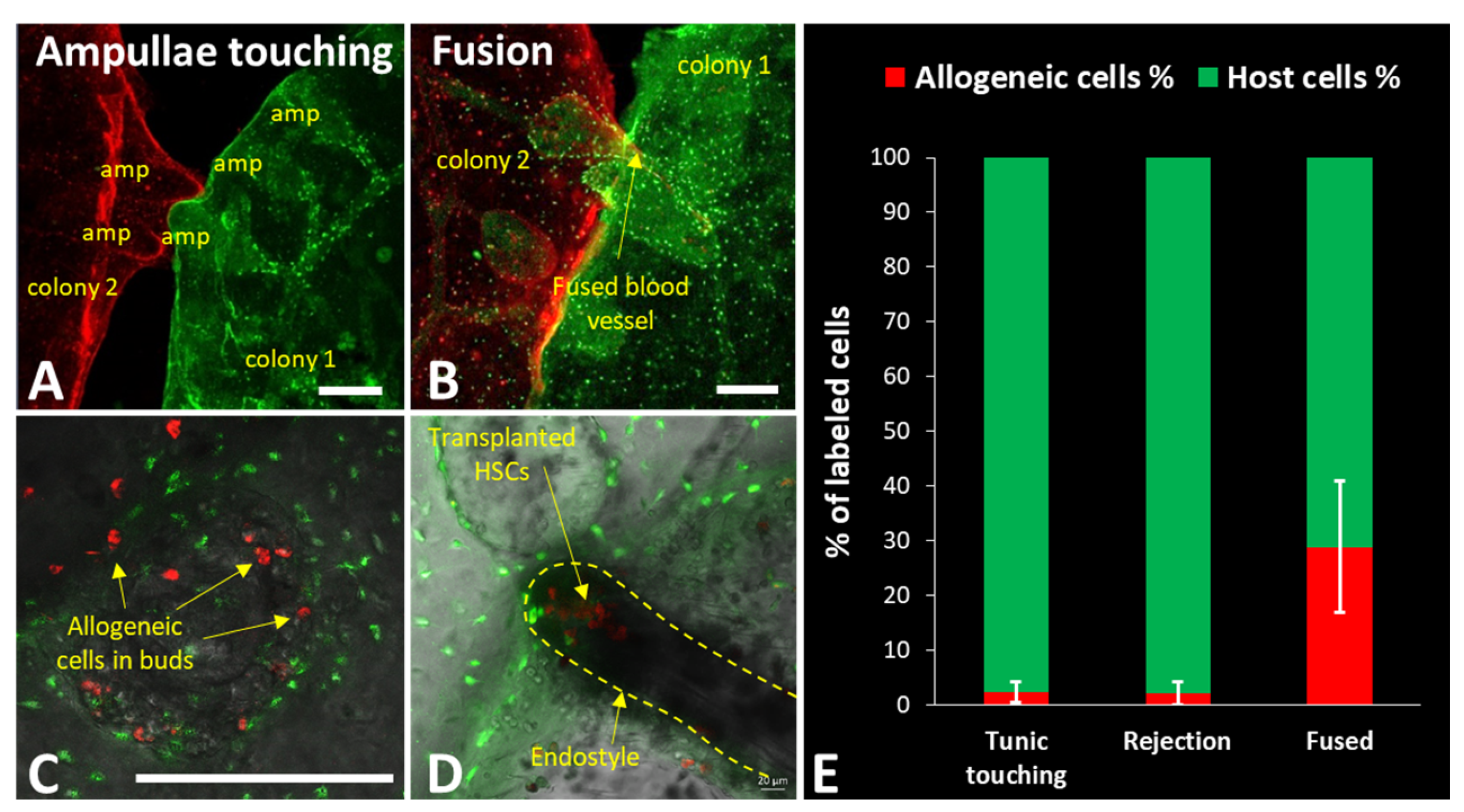
2. Natural Transplantation Phenomena (Fusion/Rejection Mechanisms)
3. Zooid Resorption and Regeneration as a Model for Programed Cell Removal
4. Botryllus schlosseri as a Model to Study the Innate Immunity
5. Botryllus as a Model for HSC Transplantation
6. Allogeneic Resorption as a Model for Chronic Rejection
7. Prospect of a General Allogeneic Model
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Oberbarnscheidt, M.H.; Lakkis, F.G. Innate allorecognition. Immunol. Rev. 2014, 258, 145–149. [Google Scholar] [CrossRef]
- Zecher, D.; Li, Q.; Williams, A.L.; Walters, J.T.; Baddoura, F.K.; Chalasani, G.; Rothstein, D.M.; Shlomchik, W.D.; Demetris, A.J.; Lakkis, F.G. Innate immunity alone is not sufficient for chronic rejection but predisposes healed allografts to T cell-mediated pathology. Transpl. Immunol. 2012, 26, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Ghadially, H.; Ohana, M.; Elboim, M.; Gazit, R.; Gur, C.; Nagler, A.; Mandelboim, O. NK cell receptor NKp46 regulates graft-versus-host disease. Cell Rep. 2014, 7, 1809–1814. [Google Scholar] [CrossRef]
- Simonetta, F.; Alvarez, M.; Negrin, R.S. Natural Killer Cells in Graft-versus-Host-Disease after Allogeneic Hematopoietic Cell Transplantation. Front. Immunol. 2017, 8, 465. [Google Scholar] [CrossRef]
- Geldenhuys, J.; Rossouw, T.M.; Lombaard, H.A.; Ehlers, M.M.; Kock, M.M. Disruption in the Regulation of Immune Responses in the Placental Subtype of Preeclampsia. Front. Immunol. 2018, 9, 1659. [Google Scholar] [CrossRef]
- Gomez-Lopez, N.; Stlouis, D.; Lehr, M.A.; Sanchez-Rodriguez, E.N.; Arenas-Hernandez, M. Immune cells in term and preterm labor. Cell. Mol. Immunol. 2014, 11, 571–581. [Google Scholar] [CrossRef]
- Kwak-Kim, J.; Bao, S.; Lee, S.K.; Kim, J.W.; Gilman-Sachs, A. Immunological Modes of Pregnancy Loss: Inflammation, Immune Effectors, and Stress. Am. J. Reprod. Immunol. 2014, 72, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Moffett, A.; Chazara, O.; Colucci, F. Maternal allo-recognition of the fetus. Fertil. Steril. 2017, 107, 1269–1272. [Google Scholar] [CrossRef]
- Rosental, B.; Brusilovsky, M.; Hadad, U.; Oz, D.; Appel, M.Y.; Afergan, F.; Yossef, R.; Rosenberg, L.A.; Aharoni, A.; Cerwenka, A.; et al. Proliferating Cell Nuclear Antigen Is a Novel Inhibitory Ligand for the Natural Cytotoxicity Receptor NKp44. J. Immunol. 2011, 187, 5693–5702. [Google Scholar] [CrossRef] [PubMed]
- Shemesh, A.; Kugel, A.; Steiner, N.; Yezersky, M.; Tirosh, D.; Edri, A.; Teltsh, O.; Rosental, B.; Sheiner, E.; Rubin, E.; et al. NKp44 and NKp30 splice variant profiles in decidua and tumor tissues: A comparative viewpoint. Oncotarget 2016, 7, 70912–70923. [Google Scholar] [CrossRef] [PubMed]
- Shemesh, A.; Tirosh, D.; Sheiner, E.; Tirosh, N.B.; Brusilovsky, M.; Segev, R.; Rosental, B.; Porgador, A. First Trimester Pregnancy Loss and the Expression of Alternatively Spliced NKp30 Isoforms in Maternal Blood and Placental Tissue. Front. Immunol. 2015, 6, 189. [Google Scholar] [CrossRef][Green Version]
- Azumi, K.; De Santis, R.; De Tomaso, A.; Rigoutsos, I.; Yoshizaki, F.; Pinto, M.R.; Marino, R.; Shida, K.; Ikeda, M.; Ikeda, M.; et al. Genomic analysis of immunity in a Urochordate and the emergence of the vertebrate immune system: “waiting for Godot”. Immunogenetics 2003, 55, 570–581. [Google Scholar] [CrossRef]
- Flajnik, M.F. A cold-blooded view of adaptive immunity. Nat. Rev. Immunol. 2018, 18, 438–453. [Google Scholar] [CrossRef]
- Hirano, M.; Das, S.; Guo, P.; Cooper, M.D. Chapter 4—The Evolution of Adaptive Immunity in Vertebrates. In Advances in Immunology; Frederick, W., Ed.; Academic Press: Cambridge, MA, USA, 2011; Volume 109, pp. 125–157. [Google Scholar] [CrossRef]
- Voskoboynik, A.; Neff, N.F.; Sahoo, D.; Newman, A.M.; Pushkarev, D.; Koh, W.; Passarelli, B.; Fan, H.C.; Mantalas, G.L.; Palmeri, K.J.; et al. The genome sequence of the colonial chordate, Botryllus schlosseri. eLife 2013, 2, e00569. [Google Scholar] [CrossRef]
- Corey, D.M.; Rosental, B.; Kowarsky, M.; Sinha, R.; Ishizuka, K.J.; Palmeri, K.J.; Quake, S.R.; Voskoboynik, A.; Weissman, I.L. Developmental cell death programs license cytotoxic cells to eliminate histocompatible partners. Proc. Natl. Acad. Sci. USA 2016, 113, 6520–6525. [Google Scholar] [CrossRef]
- Rosental, B.; Kowarsky, M.; Seita, J.; Corey, D.M.; Ishizuka, K.J.; Palmeri, K.J.; Chen, S.-Y.; Sinha, R.; Okamoto, J.; Mantalas, G.; et al. Complex mammalian-like haematopoietic system found in a colonial chordate. Nature 2018, 564, 425. [Google Scholar] [CrossRef]
- Ben-Shlomo, R.; Reem, E.; Douek, J.; Rinkevich, B. Population genetics of the invasive ascidian Botryllus schlosseri from South American coasts. Mar. Ecol. Prog. Ser. 2010, 412, 85. [Google Scholar] [CrossRef]
- Stoner, D.S.; Ben-Shlomo, R.; Rinkevich, B.; Weissman, I.L. Genetic variability of Botryllus schlosseri invasions to the east and west coasts of the USA. Mar. Ecol. Prog. Ser. 2002, 243, 93. [Google Scholar] [CrossRef]
- Darwin, C.; Kebler, L. On the Origin of Sspecies by Means of Natural Selection, or, The Preservation of Favoured Races in the Struggle for Life; J. Murray: London, UK, 1859; Volume 1, p. 502. [Google Scholar]
- Delsuc, F.; Brinkmann, H.; Chourrout, D.; Philippe, H. Tunicates and not cephalochordates are the closest living relatives of vertebrates. Nature 2006, 439, 965. [Google Scholar] [CrossRef] [PubMed]
- Manni, L.; Lane, N.J.; Joly, J.S.; Gasparini, F.; Tiozzo, S.; Caicci, F.; Zaniolo, G.; Burighel, P. Neurogenic and non-neurogenic placodes in ascidians. J. Exp. Zoology. Part B Mol. Dev. Evol. 2004, 302, 483. [Google Scholar] [CrossRef]
- Kowarsky, M.; Anselmi, C.; Hotta, K.; Burighel, P.; Zaniolo, G.; Caicci, F.; Rosental, B.; Neff, N.F.; Ishizuka, K.J.; Palmeri, K.J.; et al. Sexual and asexual development: Two distinct programs producing the same tunicate. Cell Rep. 2021, 34, 108681. [Google Scholar] [CrossRef]
- Manni, L.; Gasparini, F.; Hotta, K.; Ishizuka, K.J.; Ricci, L.; Tiozzo, S.; Voskoboynik, A.; Dauga, D. Ontology for the Asexual Development and Anatomy of the Colonial Chordate Botryllus schlosseri. PLoS ONE 2014, 9, e96434. [Google Scholar] [CrossRef]
- Manni, L.; Burighel, P. Common and divergent pathways in alternative developmental processes of ascidians. BioEssays 2006, 28, 902–912. [Google Scholar] [CrossRef]
- Milkman, R. Genetic and developmental studies on Botryllus schlosseri. Biol. Bull. 1967, 132, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Cima, F.; Basso, G.; Ballarin, L. Apoptosis and phosphatidylserine-mediated recognition during the take-over phase of the colonial life-cycle in the ascidian Botryllus schlosseri. Cell Tissue Res. 2003, 312, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Lauzon, R.J.; Ishizuka, K.J.; Weissman, I.L. A cyclical, developmentally-regulated death phenomenon in a colonial urochordate. Dev. Dyn. 1992, 194, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Lauzon, R.J.; Patton, C.W.; Weissman, I.L. A morphological and immunohistochemical study of programmed cell death in Botryllus schlosseri (Tunicata, Ascidiacea). Cell Tissue Res. 1993, 272, 115–127. [Google Scholar] [CrossRef]
- Laird, D.J.; De Tomaso, A.W.; Cooper, M.D.; Weissman, I.L. 50 million years of chordate evolution: Seeking the origins of adaptive immunity. Proc. Natl. Acad. Sci. USA 2000, 97, 6924–6926. [Google Scholar] [CrossRef] [PubMed]
- Laird, D.J.; De Tomaso, A.W.; Weissman, I.L. Stem Cells are Units of Natural Selection in a Colonial Ascidian. Cell 2005, 123, 1351–1360. [Google Scholar] [CrossRef]
- Rinkevich, Y.; Voskoboynik, A.; Rosner, A.; Rabinowitz, C.; Paz, G.; Oren, M.; Douek, J.; Alfassi, G.; Moiseeva, E.; Ishizuka, K.J.; et al. Repeated, Long-Term Cycling of Putative Stem Cells between Niches in a Basal Chordate. Dev. Cell 2013, 24, 76–88. [Google Scholar] [CrossRef]
- Stoner, D.S.; Rinkevich, B.; Weissman, I.L. Heritable germ and somatic cell lineage competitions in chimeric colonial protochordates. Proc. Natl. Acad. Sci. USA 1999, 96, 9148–9153. [Google Scholar] [CrossRef] [PubMed]
- Stoner, D.S.; Weissman, I.L. Somatic and germ cell parasitism in a colonial ascidian: Possible role for a highly polymorphic allorecognition system. Proc. Natl. Acad. Sci. USA 1996, 93, 15254–15259. [Google Scholar] [CrossRef] [PubMed]
- Voskoboynik, A.; Soen, Y.; Rinkevich, Y.; Rosner, A.; Ueno, H.; Reshef, R.; Ishizuka, K.J.; Palmeri, K.J.; Moiseeva, E.; Rinkevich, B.; et al. Identification of the Endostyle as a Stem Cell Niche in a Colonial Chordate. Cell Stem Cell 2008, 3, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Sabbadin, A. Le basi genetiche della capacita di fusione fra colonie in Botryllus schlosseri (Ascidiacea). Rend Accad Naz Lincei Ser VIII 1962, 32, 1031–1035. [Google Scholar]
- Scofield, V. Allorecognition and microbial infection: Roles in the evolution of sex and immunity. In The Origin and Evolution of Sex; Halvorson, H., Monroy, A., Eds.; Alan R. Liss: New York, NY, USA, 1985; Volume 7, p. 213. [Google Scholar]
- Voskoboynik, A.; Newman, A.M.; Corey, D.M.; Sahoo, D.; Pushkarev, D.; Neff, N.F.; Passarelli, B.; Koh, W.; Ishizuka, K.J.; Palmeri, K.J.; et al. Identification of a Colonial Chordate Histocompatibility Gene. Science 2013, 341, 384. [Google Scholar] [CrossRef]
- Rinkevich, B.; Tartakover, S.; Gershon, H. Contribution of morula cells to allogeneic responses in the colonial urochordate Botryllus schlosseri. Mar. Biol. 1998, 131, 227–236. [Google Scholar] [CrossRef]
- Ueno, H.; Weissman, I.L. Clonal Analysis of Mouse Development Reveals a Polyclonal Origin for Yolk Sac Blood Islands. Dev. Cell 2006, 11, 519. [Google Scholar] [CrossRef]
- Ueno, H.; Turnbull, B.B.; Weissman, I.L. Two-step oligoclonal development of male germ cells. Proc. Natl. Acad. Sci. USA 2009, 106, 175–180. [Google Scholar] [CrossRef]
- Weissman, I.L. Stem cells are units of natural selection for tissue formation, for germline development, and in cancer development. Proc. Natl. Acad. Sci. USA 2015, 112, 8922–8928. [Google Scholar] [CrossRef]
- Beerman, I.; Bhattacharya, D.; Zandi, S.; Sigvardsson, M.; Weissman, I.L.; Bryder, D.; Rossi, D.J. Functionally distinct hematopoietic stem cells modulate hematopoietic lineage potential during aging by a mechanism of clonal expansion. Proc. Natl. Acad. Sci. USA 2010, 107, 5465–5470. [Google Scholar] [CrossRef]
- Pang, W.W.; Price, E.A.; Sahoo, D.; Beerman, I.; Maloney, W.J.; Rossi, D.J.; Schrier, S.L.; Weissman, I.L. Human bone marrow hematopoietic stem cells are increased in frequency and myeloid-biased with age. Proc. Natl. Acad. Sci. USA 2011, 108, 20012–20017. [Google Scholar] [CrossRef]
- Rossi, D.J.; Bryder, D.; Seita, J.; Nussenzweig, A.; Hoeijmakers, J.; Weissman, I.L. Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nat. Cell Biol. 2007, 447, 725–729. [Google Scholar] [CrossRef]
- Rossi, D.J.; Bryder, D.; Zahn, J.M.; Ahlenius, H.; Sonu, R.; Wagers, A.J.; Weissman, I.L. Cell intrinsic alterations underlie hematopoietic stem cell aging. Proc. Natl. Acad. Sci. USA 2005, 102, 9194–9199. [Google Scholar] [CrossRef]
- Rossi, D.J.; Jamieson, C.H.; Weissman, I.L. Stems Cells and the Pathways to Aging and Cancer. Cell 2008, 132, 681–696. [Google Scholar] [CrossRef]
- Corces, M.; Hong, W.-J.; Weissman, I.L.; Medeiros, B.C.; Majeti, R. Preleukemic mutations in human acute myeloid leukemia affect epigenetic regulators and persist in remission. Proc. Natl. Acad. Sci. USA 2014, 111, 2548–2553. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, S.; Jamieson, C.H.; Pang, W.W.; Park, C.Y.; Chao, M.P.; Majeti, R.; Traver, D.; Van Rooijen, N.; Weissman, I.L. CD47 Is Upregulated on Circulating Hematopoietic Stem Cells and Leukemia Cells to Avoid Phagocytosis. Cell 2009, 138, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, C.H.; Ailles, L.E.; Dylla, S.J.; Muijtjens, M.; Jones, C.; Zehnder, J.L.; Gotlib, J.; Li, K.; Manz, M.G.; Keating, A.; et al. Granulocyte-macrophage progenitors as candidate leukemic stem cells in blast-crisis CML. N. Engl. J. Med. 2004, 351, 657–667. [Google Scholar] [CrossRef] [PubMed]
- Jan, M.; Snyder, T.M.; Corces-Zimmerman, M.R.; Vyas, P.; Weissman, I.L.; Quake, S.R.; Majeti, R. Clonal Evolution of Preleukemic Hematopoietic Stem Cells Precedes Human Acute Myeloid Leukemia. Sci. Transl. Med. 2012, 4, 149ra118. [Google Scholar] [CrossRef]
- Miyamoto, T.; Weissman, I.L.; Akashi, K. AML1/ETO-expressing nonleukemic stem cells in acute myelogenous leukemia with 8;21 chromosomal translocation. Proc. Natl. Acad. Sci. USA 2000, 97, 7521–7526. [Google Scholar] [CrossRef]
- Sykes, S.M.; Kokkaliaris, K.D.; Milsom, M.D.; Levine, R.L.; Majeti, R. Clonal evolution of preleukemic hematopoietic stem cells in acute myeloid leukemia. Exp. Hematol. 2015, 43, 989–992. [Google Scholar] [CrossRef] [PubMed]
- Kojima, Y.; Volkmer, J.-P.; McKenna, K.; Civelek, M.; Lusis, A.J.; Miller, C.L.; Direnzo, D.; Nanda, V.; Ye, J.; Connolly, A.J.; et al. CD47-blocking antibodies restore phagocytosis and prevent atherosclerosis. Nature 2016, 536, 86. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, F.; Wang, S.; Yang, R.; Chen, C.; Zhao, W. Imaging endogenous HClO in atherosclerosis using a novel fast-response fluorescence probe. Chem. Commun. 2020, 56, 2598. [Google Scholar] [CrossRef] [PubMed]
- Scofield, V.L.; Schlumpberger, J.M.; West, L.A.; Weissman, I.L. Protochordate allorecognition is controlled by a MHC-like gene system. Nature 1982, 295, 499. [Google Scholar] [CrossRef] [PubMed]
- Burighel, P.; Schiavinato, A. Degenerative regression of the digestive tract in the colonial ascidian Botryllus schlosseri (Pallas). Cell Tissue Res. 1984, 235, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Ballarin, L.; Burighel, P.; Cima, F. A Tale of Death and Life: Natural Apoptosis in the Colonial Ascidian Botryllus schlosseri (Urochordata, Ascidiacea). Curr. Pharm. Des. 2008, 14, 138. [Google Scholar] [CrossRef] [PubMed]
- Ballarin, L.; Schiavon, F.; Manni, L. Natural Apoptosis during the Blastogenetic Cycle of the Colonial Ascidian Botryllus schlosseri: A Morphological Analysis. Zoöl. Sci. 2010, 27, 96. [Google Scholar] [CrossRef]
- Voskoboynik, A.; Rinkevich, B.; Weiss, A.; Moiseeva, E.; Reznick, A.Z. Macrophage involvement for successful degeneration of apoptotic organs in the colonial urochordate Botryllus schlosseri. J. Exp. Biol. 2004, 207, 2409. [Google Scholar] [CrossRef]
- Lauzon, R.J.; Ishizuka, K.J.; Weissman, I.L. Cyclical Generation and Degeneration of Organs in a Colonial Urochordate Involves Crosstalk between Old and New: A Model for Development and Regeneration. Dev. Biol. 2002, 249, 333–348. [Google Scholar] [CrossRef]
- Rinkevich, B.; Weissman, I.L. Botryllus schlosseri (tunicata) whole colony irradiation: Do senescent zooid resorption and immunological resorption involve similar recognition events? J. Exp. Zoöl. 1990, 253, 189. [Google Scholar] [CrossRef]
- Rosental, B.; Raveh, T.; Voskoboynik, A.; Weissman, I.L. Evolutionary perspective on the hematopoietic system through a colonial chordate: Allogeneic immunity and hematopoiesis. Curr. Opin. Immunol. 2020, 62, 91. [Google Scholar] [CrossRef]
- Franchi, N.; Ballarin, L. Immunity in Protochordates: The Tunicate Perspective. Front. Immunol. 2017, 8, 674. [Google Scholar] [CrossRef] [PubMed]
- Lauzon, R.J.; Brown, C.; Kerr, L.; Tiozzo, S. Phagocyte dynamics in a highly regenerative urochordate: Insights into development and host defense. Dev. Biol. 2013, 374, 357–373. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Peronato, A.; Franchi, N.; Loriano, B. BsTLR1: A new member of the TLR family of recognition proteins from the colonial ascidian Botryllus schlosseri. Fish Shellfish. Immunol. 2020, 106, 967. [Google Scholar] [CrossRef] [PubMed]
- Khalturin, K.; Becker, M.; Rinkevich, B.; Bosch, T.C. Urochordates and the origin of natural killer cells: Identification of a CD94/NKR-P1-related receptor in blood cells of Botryllus. Proc. Natl. Acad. Sci. USA 2003, 100, 622–627. [Google Scholar] [CrossRef] [PubMed]
- Franchi, N.; Schiavon, F.; Carletto, M.; Gasparini, F.; Bertoloni, G.; Tosatto, S.C.; Ballarin, L. Immune roles of a rhamnose-binding lectin in the colonial ascidian Botryllus schlosseri. Immunobiolgy 2011, 216, 725–736. [Google Scholar] [CrossRef]
- Franchi, N.; Schiavon, F.; Betti, M.; Canesi, L.; Ballarin, L. Insight on signal transduction pathways involved in phagocytosis in the colonial ascidian Botryllus schlosseri. J. Invertebr. Pathol. 2013, 112, 260–266. [Google Scholar] [CrossRef]
- Ballarin, L. Immunobiology of compound ascidians, with particular reference to Botryllus schlosseri: State of art. Invertebr. Surviv. J. 2008, 5, 54. [Google Scholar]
- Cheung, R.C.F.; Wong, J.H.; Pan, W.; Chan, Y.S.; Yin, C.M.; Dan, X.L.; Ng, T.B. Marine lectins and their medicinal applications. Appl. Microbiol. Biotechnol. 2015, 99, 3755. [Google Scholar] [CrossRef] [PubMed]
- Franchi, N.; Ballarin, L. Preliminary characterization of complement in a colonial tunicate: C3, Bf and inhibition of C3 opsonic activity by compstatin. Dev. Comp. Immunol. 2014, 46, 430. [Google Scholar] [CrossRef]
- Peronato, A.; Drago, L.; Rothbacher, U.; Macor, P.; Ballarin, L.; Franchi, N. Complement system and phagocytosis in a colonial protochordate. Dev. Comp. Immunol. 2020, 103, 103530. [Google Scholar] [CrossRef]
- Nicola, F.; Loriano, B. Morula cells as key hemocytes of the lectin pathway of complement activation in the colonial tunicate Botryllus schlosseri. Fish Shellfish. Immunol. 2017, 63, 157. [Google Scholar] [CrossRef]
- Ballarin, L.; Menin, A.; Franchi, N.; Bertoloni, G.; Cima, F. Morula cells and non-self recognition in the compound ascidian Botryllus schlosseri. Invertebr. Surviv. J. 2005, 2, 1. [Google Scholar]
- Palanisamy, S.K.; Rajendran, N.M.; Marino, A. Natural Products Diversity of Marine Ascidians (Tunicates; Ascidiacea) and Successful Drugs in Clinical Development. Nat. Prod. Bioprosp. 2017, 7, 1–111. [Google Scholar] [CrossRef]
- Ramesh, C.; Tulasi, B.R.; Raju, M.; Thakur, N.; Dufosse, L. Marine Natural Products from Tunicates and Their Associated Microbes. Mar. Drugs 2021, 19, 308. [Google Scholar] [CrossRef]
- Copelan, E.A. Hematopoietic Stem-Cell Transplantation. N. Engl. J. Med. 2006, 354, 1813. [Google Scholar] [CrossRef] [PubMed]
- Doulatov, S.; Notta, F.; Laurenti, E.; Dick, J.E. Hematopoiesis: A Human Perspective. Cell Stem Cell 2012, 10, 120. [Google Scholar] [CrossRef] [PubMed]
- Osawa, M.; Hanada, K.-I.; Hamada, H.; Nakauchi, H. Long-Term Lymphohematopoietic Reconstitution by a Single CD34-Low/Negative Hematopoietic Stem Cell. Science 1996, 273, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Spangrude, G.J.; Heimfeld, S.; Weissman, I.L. Purification and characterization of mouse hematopoietic stem cells. Science 1988, 241, 58–62. [Google Scholar] [CrossRef]
- Weaver, C.T.; Hatton, R.D.; Mangan, P.R.; Harrington, L.E. IL-17 Family Cytokines and the Expanding Diversity of Effector T Cell Lineages. Annu. Rev. Immunol. 2007, 25, 821. [Google Scholar] [CrossRef] [PubMed]
- Miossec, P.; Kolls, J.K. Targeting IL-17 and TH17 cells in chronic inflammation. Nat. Rev. Drug Discov. 2012, 11, 763. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Qian, Y. IL-17/IL-17 receptor system in autoimmune disease: Mechanisms and therapeutic potential. Clin. Sci. 2012, 122, 487–511. [Google Scholar] [CrossRef]
- van der Waart, A.B.; van der Velden, W.J.; Blijlevens, N.M.; Dolstra, H. Targeting the IL17 Pathway for the Prevention of Graft-Versus-Host Disease. Biol. Blood Marrow Transpl. 2014, 20, 752. [Google Scholar] [CrossRef]
- Carnevale-Schianca, F.; Leisenring, W.; Martin, P.J.; Furlong, T.; Schoch, G.; Anasetti, C.; Appelbaum, F.R.; Carpenter, P.A.; Deeg, H.J.; Kiem, H.-P.; et al. Longitudinal Assessment of Morbidity and Acute Graft-versus-Host Disease after Allogeneic Hematopoietic Cell Transplantation: Retrospective Analysis of a Multicenter Phase III Study. Biol. Blood Marrow Transpl. 2009, 15, 749. [Google Scholar] [CrossRef]
- Holt, C.D. Overview of Immunosuppressive Therapy in Solid Organ Transplantation. Anesthesiol. Clin. 2017, 35, 365. [Google Scholar] [CrossRef]
- Ustun, C.; Jillella, A.; Shah, R.; Sterling, K.; DeRemer, D.; Savage, N.; Awan, F.; Gossage, J.R.; Dillard, T.; Martin, P.J. Second allo-SCT from a different donor can improve severe steroid-resistant gut GVHD. Bone Marrow Transpl. 2010, 45, 1658. [Google Scholar] [CrossRef] [PubMed]
- Lindahl, K.F.; Wilson, D.B. Histocompatibility antigen-activated cytotoxic T lymphocytes. II. Estimates of the frequency and specificity of precursors. J. Exp. Med. 1977, 145, 508. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, W.A.; Chen, Z.; Gras, S.; Archbold, J.; Tynan, F.E.; Clements, C.S.; Bharadwaj, M.; Kjer-Nielsen, L.; Saunders, P.M.; Wilce, M.C.; et al. T Cell Allorecognition via Molecular Mimicry. Immunity 2009, 31, 897. [Google Scholar] [CrossRef]
- Taketa, D.A.; De Tomaso, A.W. Botryllus schlosseri allorecognition: Tackling the enigma. Dev. Comp. Immunol. 2015, 48, 254. [Google Scholar] [CrossRef]
- De Tomaso, A.W.; Nyholm, S.V.; Palmeri, K.J.; Ishizuka, K.J.; Ludington, W.B.; Mitchel, K.; Weissman, I.L. Isolation and characterization of a protochordate histocompatibility locus. Nature 2005, 438, 454. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goldstein, O.; Mandujano-Tinoco, E.A.; Levy, T.; Talice, S.; Raveh, T.; Gershoni-Yahalom, O.; Voskoboynik, A.; Rosental, B. Botryllus schlosseri as a Unique Colonial Chordate Model for the Study and Modulation of Innate Immune Activity. Mar. Drugs 2021, 19, 454. https://doi.org/10.3390/md19080454
Goldstein O, Mandujano-Tinoco EA, Levy T, Talice S, Raveh T, Gershoni-Yahalom O, Voskoboynik A, Rosental B. Botryllus schlosseri as a Unique Colonial Chordate Model for the Study and Modulation of Innate Immune Activity. Marine Drugs. 2021; 19(8):454. https://doi.org/10.3390/md19080454
Chicago/Turabian StyleGoldstein, Oron, Edna Ayerim Mandujano-Tinoco, Tom Levy, Shani Talice, Tal Raveh, Orly Gershoni-Yahalom, Ayelet Voskoboynik, and Benyamin Rosental. 2021. "Botryllus schlosseri as a Unique Colonial Chordate Model for the Study and Modulation of Innate Immune Activity" Marine Drugs 19, no. 8: 454. https://doi.org/10.3390/md19080454
APA StyleGoldstein, O., Mandujano-Tinoco, E. A., Levy, T., Talice, S., Raveh, T., Gershoni-Yahalom, O., Voskoboynik, A., & Rosental, B. (2021). Botryllus schlosseri as a Unique Colonial Chordate Model for the Study and Modulation of Innate Immune Activity. Marine Drugs, 19(8), 454. https://doi.org/10.3390/md19080454





